Protective Effects of Fish Oil Against Brain Impairment in Rats with Chronic Ethanol-Induced Liver Damage Involving the NRF2 Pathway and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study Protocol
2.2. Blood Biochemical Examination
2.3. Behavioral Tests
2.3.1. Y Maze Spontaneous Alternation
2.3.2. Novel Object Recognition (NOR) Test
2.3.3. Behavioral Analysis
2.4. Antioxidant and Antioxidative Enzyme Activities
2.5. Histopathological Evaluation and Immunohistochemical (IHC) Staining
2.6. Western Blotting Analysis of the NRF2 Signaling Pathway
2.7. Reverse-Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Ethanol Consumption, Body Weights (BWs), and Tissue Weights
3.2. Hepatic Damage
3.3. Hepatic Oxidative Hemostasis Shifts
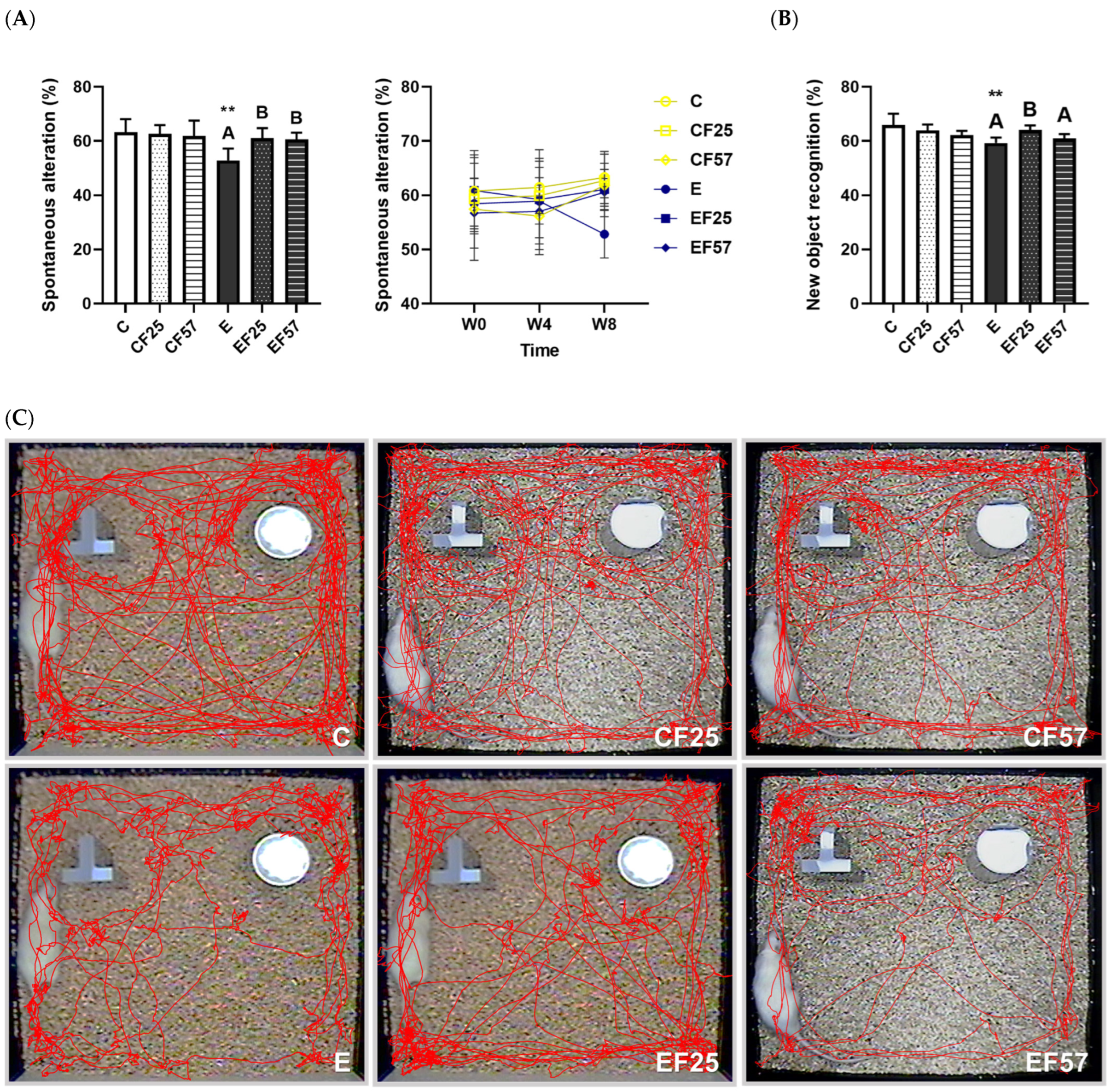
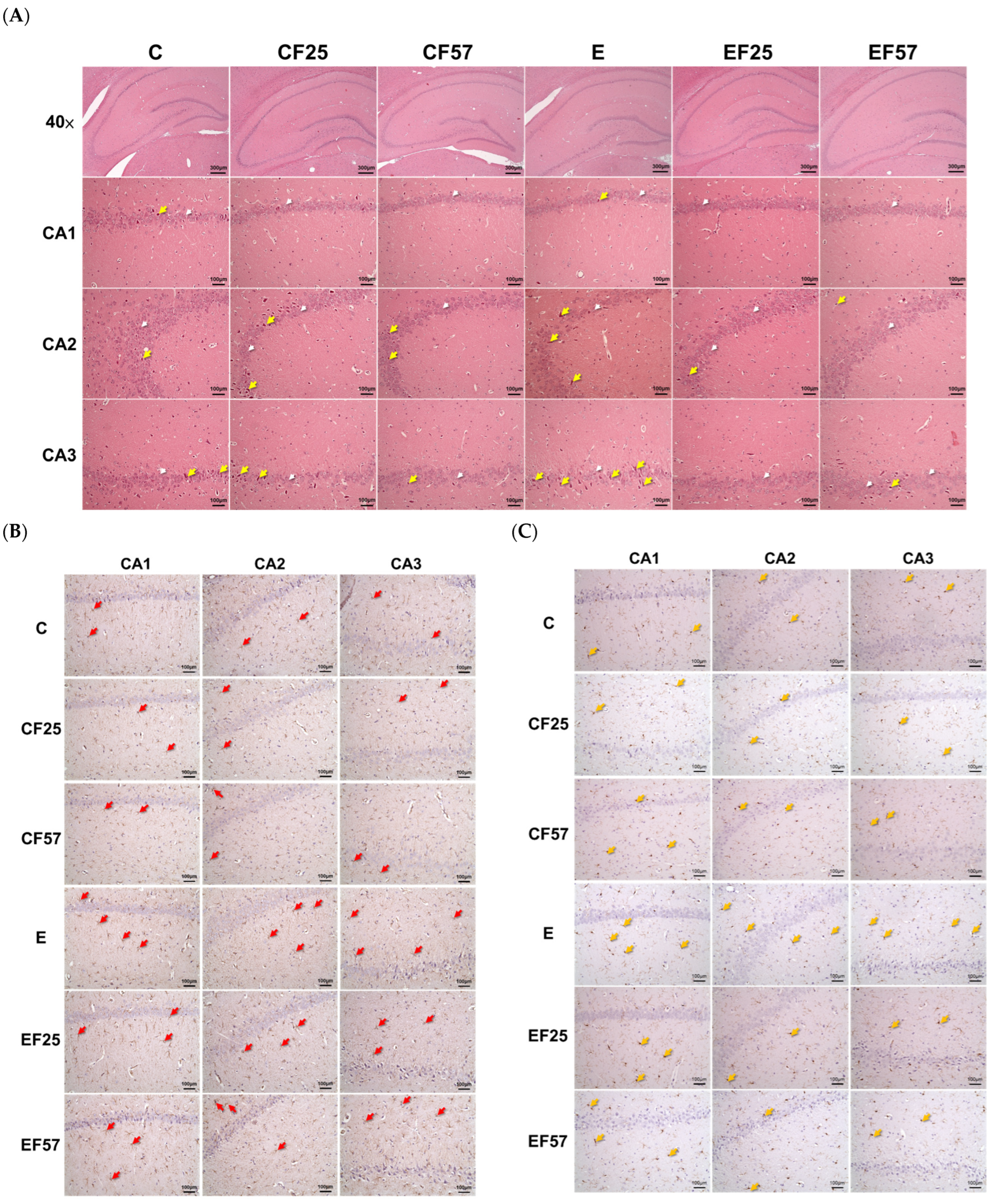
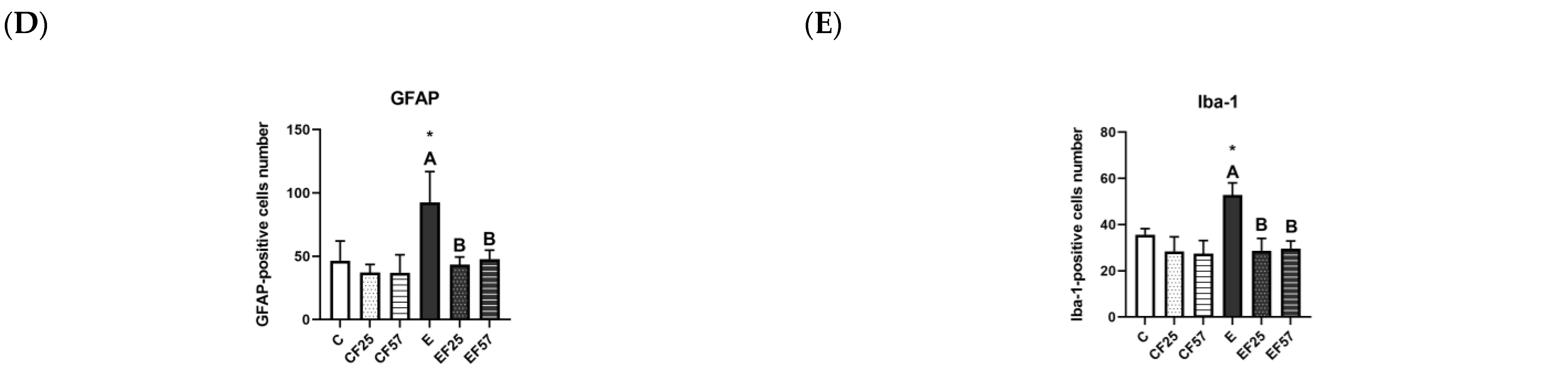
3.4. Behavioral and Histopathological Changes in the Brain
3.5. Antioxidant Defense in the Prefrontal Cortex
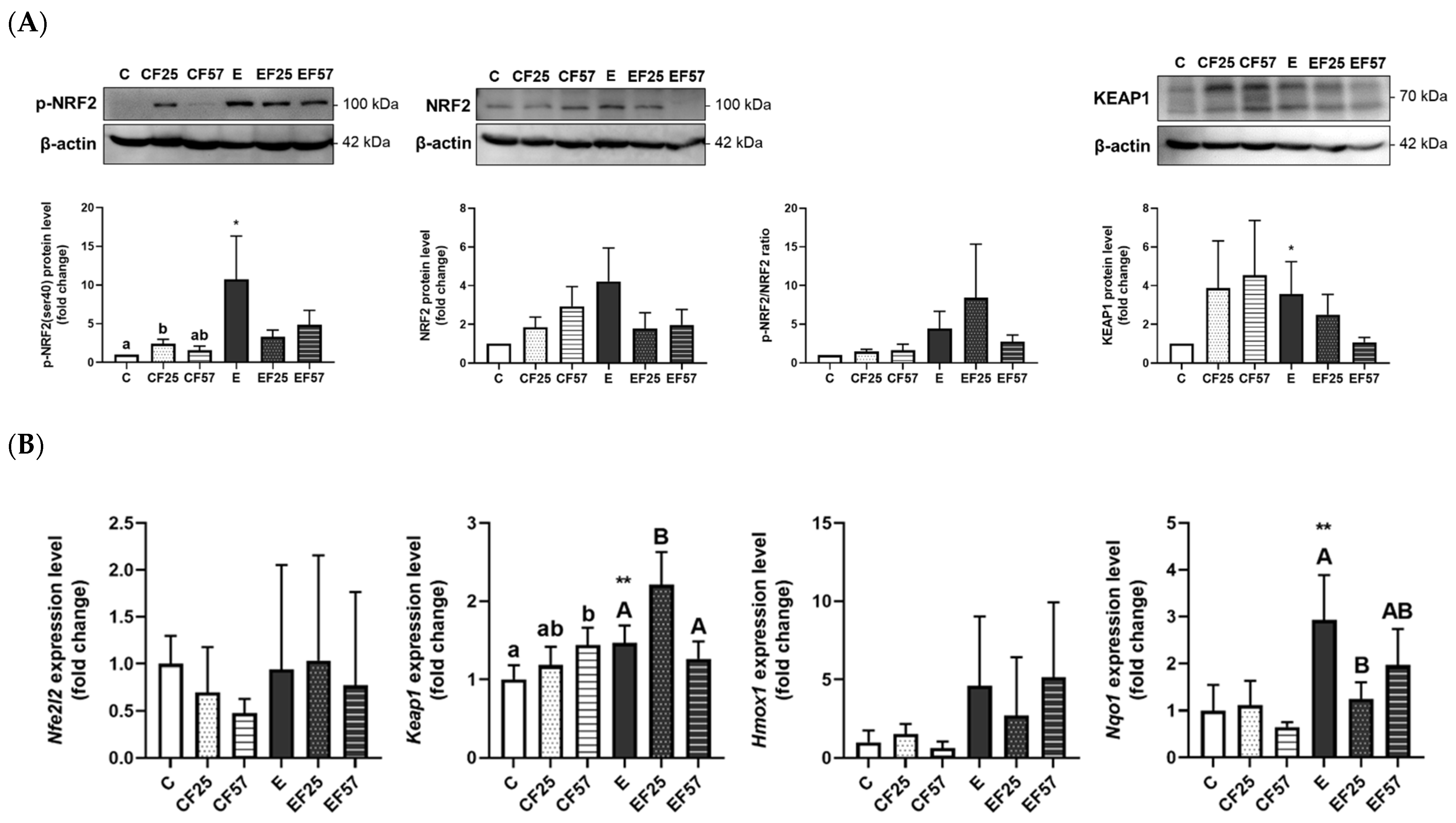
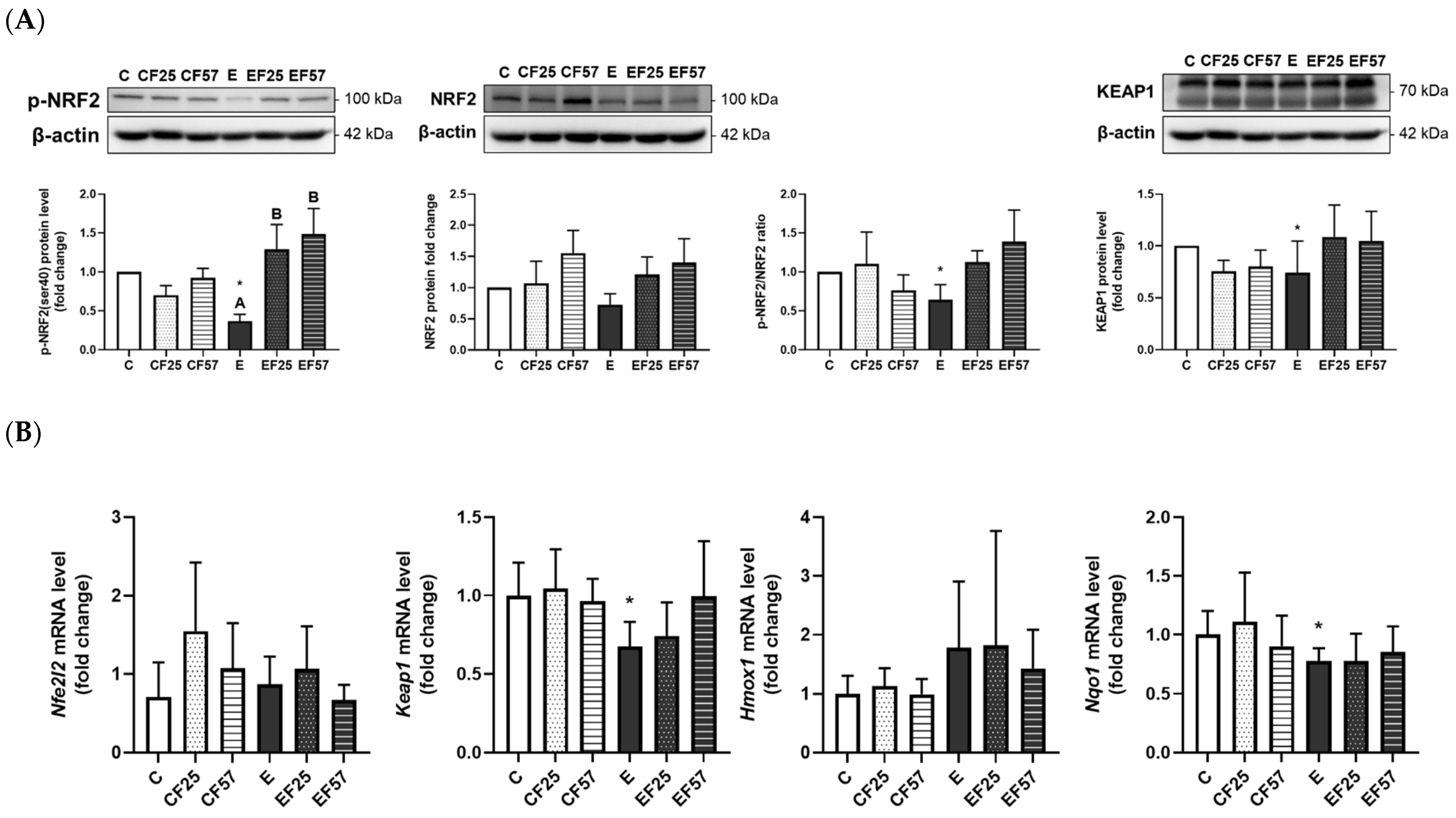
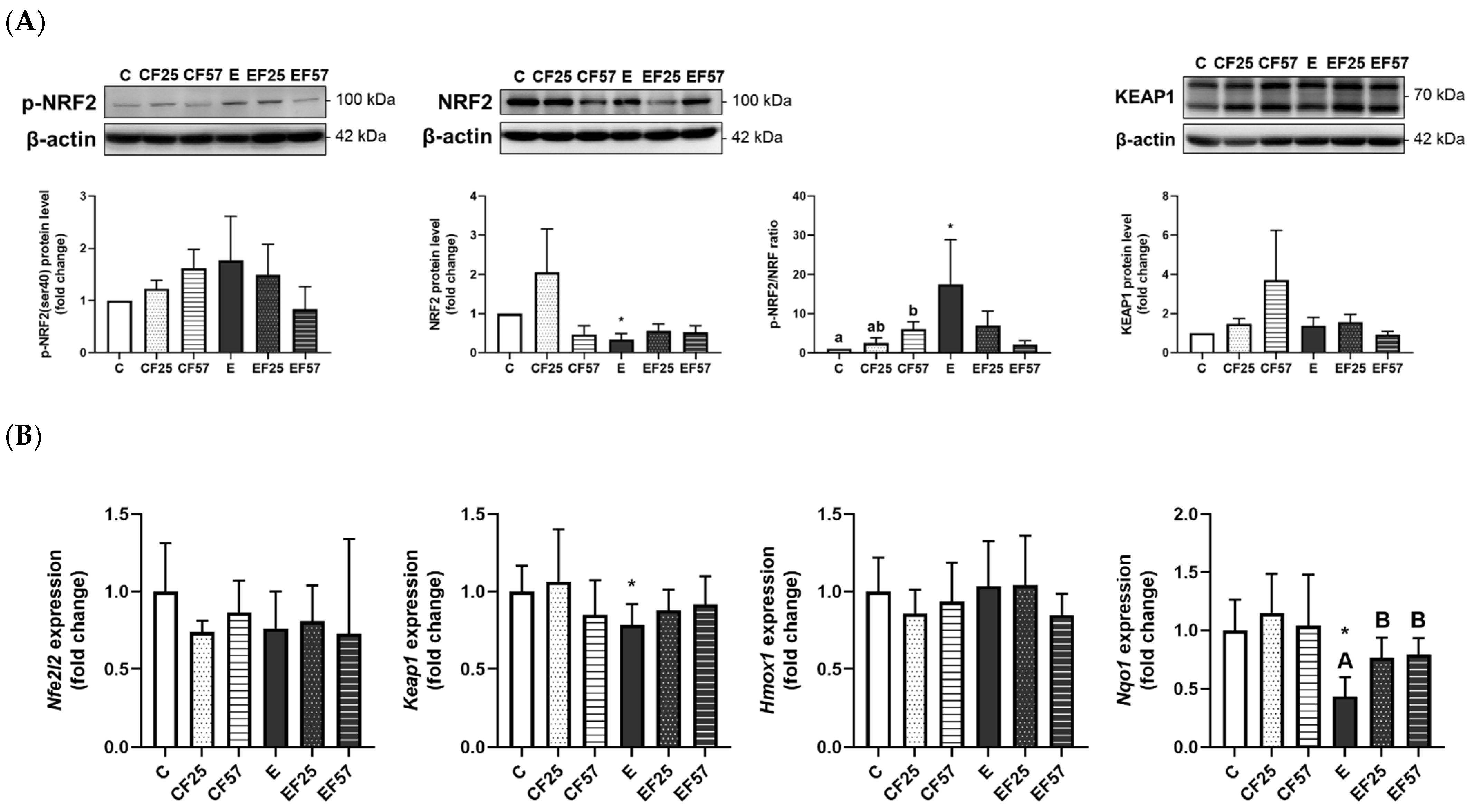
3.6. The NRF2/KEAP1 Pathway in the Prefrontal Cortex and Hippocampus
4. Discussion
4.1. Ethanol Administration Amount
4.2. Ethanol-Induced Hepatic Injury and Fish Oil
4.2.1. Ethanol and Hepatic Injury
4.2.2. Ethanol-Induced Hepatic Injury, Oxidative Stress, and the NRF2/KEAP1 Pathway
4.2.3. Fish Oil and Ethanol-Induced Hepatic Injury
4.3. Ethanol-Induced Brain Damage and Fish Oil
4.3.1. Ethanol-Induced Behavioral Impairment and Brain Damage
4.3.2. Ethanol-Induced Brain Oxidative Stress and the NRF2/KEAP1 Pathway
4.3.3. Dosage of Fish Oil Substitution
4.3.4. Research Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HHE | 4-hydroxy-2E-hexenal |
| AD | Alzheimer’s disease |
| ADH | alcohol dehydrogenase |
| ALD | alcoholic liver disease |
| ALDH | aldehyde dehydrogenase |
| ALT | alanine aminotransferase |
| ANOVA | analysis of variance |
| ARBD | alcoholic-related brain disease |
| ARE | antioxidant response element |
| AST | aspartate aminotransferase |
| Aβ | β-amyloid |
| BW | body weight |
| CAT | catalase |
| CNS | central nervous system |
| CPT | carnitine palmitoyl transferase |
| CYP2E1 | cytochrome P450 2E1 |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GFAP | glial fibrillary acidic protein |
| GPx | glutathione peroxidase |
| GRd | glutathione reductase |
| GSH | glutathione |
| GSK3β | glycogen synthase kinase-3β |
| HIF | hypoxia-inducible factor |
| hmox1 | heme oxygenase 1 |
| HO | heme oxygenase |
| Iba-1 | ionized calcium-binding adapter molecule 1 |
| IHC | histopathological evaluation and immunohistochemical |
| IRS-1 | insulin receptor substrate 1 |
| KEAP1 | kelch-like ECH-associated protein 1 |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MCAD | medium-chain acyl-coenzyme A dehydrogenase |
| MEOS | microsomal ethanol oxidation system |
| MUFA | monounsaturated fatty acid |
| nfe2l2 | nuclear factor, erythroid 2 like 2 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIAAA | National Institute on Alcohol Abuse and Alcoholism |
| NOR | novel object-recognition |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| PKC | protein kinase C |
| PUFAs | polyunsaturated fatty acids |
| PVDF | polyvinylidene difluoride |
| RIPA | radioimmunoprecipitation assay |
| ROS | reactive oxygen species |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SOD | superoxide dismutase |
| WHO | World Health Organization |
Appendix A

| Antibody | Type | Product No. | Source | Dilution | |
|---|---|---|---|---|---|
| Primary antibody | CYP2E1 | polyclonal | AB1252 | Millipore | 1:10,000 |
| p-NRF2(ser40) | polyclonal | bs-2013R | Bioss Antibodies | 1:1000 | |
| NRF2 | polyclonal | 16396-1-AP | Proteintech Group | 1:1000 | |
| KEAP1 | polyclonal | 10503-2-AP | Proteintech Group | 1:2000 | |
| Internal controls | β-actin | monoclonal | sc-47778 | Santa Cruz Biotechnology | 1:1000 |
| GAPDH | monoclonal | MAB374 | Millipore | 1:600 | |
| Secondary antibody | anti-mouse IgG | HRP conjugated | #405306 | BioLegend | 1:5000 |
| anti-rabbit IgG | HRP conjugated | #406401 | BioLegend | 1:5000 |
| Forward 5′→3′ | Reverse 3′→5′ | GenBank No. | |
|---|---|---|---|
| NFE2L2 | CTCTCTGGAGACGGCCATGACT | CTGGGCTGGGGACAGTGGTAGT | NM_001399173.1 |
| KEAP1 | TGGGTCAAATACGACTGCCC | GCAGGATCTCGCACTTTTGC | NM_057152.2 |
| HMOX1 | GAGCGAAACAAGCAGAACCC | ACCTCGTGGAGACGCTTTAC | NM_012580.2 |
| NQO1 | TCCGAAGCATTTCAGGGTCG | TCTGCGTGGGCCAATACAAT | NM_017000.3 |
| GAPDH | AGTGCCAGCCTCGTCTCATA | GATGGTGATGGGTTTCCCGT | NM_017008.4 |
References
- World Health Organization. Global Status Report on Alcohol and Health and Treatment of Substance Use Disorders; World Health Organization: Geneva, Switzerland, 2024; pp. 23–64. [Google Scholar]
- No Level of Alcohol Consumption Is Safe for Our Health. Available online: https://www.who.int/europe/news/item/04-01-2023-no-level-of-alcohol-consumption-is-safe-for-our-health (accessed on 10 April 2025).
- Anderson, B.O.; Berdzuli, N.; Ilbawi, A.; Kestel, D.; Kluge, H.P.; Krech, R.; Mikkelsen, B.; Neufeld, M.; Poznyak, V.; Rekve, D. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 2023, 8, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Tsermpini, E.E.; Plemenitaš Ilješ, A.; Dolžan, V. Alcohol-induced oxidative stress and the role of antioxidants in alcohol use disorder: A systematic review. Antioxidants 2022, 11, 1374. [Google Scholar] [CrossRef]
- Evert, D.L.; Oscar-Berman, M. Alcohol-Related Cognitive Impairments: An Overview of How Alcoholism May Affect the Workings of the Brain. Alcohol. Health Res. World 1995, 19, 89–96. [Google Scholar]
- Ariesen, A.D.; Neubert, J.H.; Gaastra, G.F.; Tucha, O.; Koerts, J. Risky Decision-Making in Adults with Alcohol Use Disorder-A Systematic and Meta-Analytic Review. J. Clin. Med. 2023, 12, 2943. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, C.; Pandey, A.K.; Chorlian, D.B.; Meyers, J.L.; Kinreich, S.; Pandey, G.; Subbie-Saenz de Viteri, S.; Zhang, J.; Kuang, W.; Barr, P.B.; et al. Predicting Alcohol-Related Memory Problems in Older Adults: A Machine Learning Study with Multi-Domain Features. Behav. Sci. 2023, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Topiwala, A.; Ebmeier, K.P.; Maullin-Sapey, T.; Nichols, T.E. Alcohol consumption and MRI markers of brain structure and function: Cohort study of 25,378 UK Biobank participants. NeuroImage Clin. 2022, 35, 103066. [Google Scholar] [CrossRef]
- King, J.A.; Nephew, B.C.; Choudhury, A.; Poirier, G.L.; Lim, A.; Mandrekar, P. Chronic alcohol-induced liver injury correlates with memory deficits: Role for neuroinflammation. Alcohol 2020, 83, 75–81. [Google Scholar] [CrossRef]
- Waddell, J.; McKenna, M.C.; Kristian, T. Brain ethanol metabolism and mitochondria. Curr. Top. Biochem. Res. 2022, 23, 1–13. [Google Scholar]
- Kamal, H.; Tan, G.C.; Ibrahim, S.F.; Shaikh, M.F.; Mohamed, I.N.; Mohamed, R.M.P.; Hamid, A.A.; Ugusman, A.; Kumar, J. Alcohol Use Disorder, Neurodegeneration, Alzheimer’s and Parkinson’s Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity. Front. Cell Neurosci. 2020, 14, 282. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Disco 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Sun, J.; Fu, J.; Zhong, Y.; Li, L.; Chen, C.; Wang, X.; Wang, L.; Hou, Y.; Wang, H.; Zhao, R. NRF2 mitigates acute alcohol-induced hepatic and pancreatic injury in mice. Food Chem. Toxicol. 2018, 121, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, H.; Zou, L.; Yang, B.; Chen, W.; Rong, X.; Zhang, X.; He, L.; Li, X.; Peng, Y. The nrf2 activator rta-408 ameliorates chronic alcohol exposure-induced cognitive impairment and nlrp3 inflammasome activation by modulating impaired mitophagy initiation. Free Radic. Biol. Med. 2024, 220, 15–27. [Google Scholar] [CrossRef]
- Venkataraman, A.; Kalk, N.; Sewell, G.; Ritchie, C.W.; Lingford-Hughes, A. Alcohol and Alzheimer’s disease—Does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s disease? Alcohol. Alcohol. 2017, 52, 151–158. [Google Scholar]
- Ozyurt, G.; Ekmen, D.; Durmuş, M.; Ucar, Y. Assessment of the safety of dietary fish oil supplements in terms of content and quality. Env. Sci. Pollut. Res. Int. 2022, 29, 25006–25019. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, D.; Bai, H. Efficacy of fish oil supplementation on metabolic dysfunction-associated steatotic liver disease: A meta-analysis. Front. Nutr. 2025, 12, 1524830. [Google Scholar] [CrossRef]
- Venturini, D.; Simão, A.N.C.; Urbano, M.R.; Dichi, I. Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition 2015, 31, 834–840. [Google Scholar] [CrossRef]
- Damaiyanti, D.W.; Tsai, Z.-Y.; Masbuchin, A.N.; Huang, C.-Y.; Liu, P.-Y. Interplay between fish oil, obesity and cardiometabolic diabetes. J. Formos. Med. Assoc. 2023, 122, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Abrescia, P.; Treppiccione, L.; Rossi, M.; Bergamo, P. Modulatory role of dietary polyunsaturated fatty acids in Nrf2-mediated redox homeostasis. Prog. Lipid Res. 2020, 80, 101066. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Medoro, A.; Intrieri, M.; Saso, L.; Scapagnini, G.; Kang, J.X. Targeting NRF2-KEAP1 axis by Omega-3 fatty acids and their derivatives: Emerging opportunities against aging and diseases. Free Radic. Biol. Med. 2022, 193, 736–750. [Google Scholar] [CrossRef]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Power, R.; Howard, A.N.; Bergin, P.; Roche, W.; Prado-Cabrero, A.; Pope, G.; Cooke, J.; Power, T.; Mulcahy, R. Supplementation with Carotenoids, Omega-3 Fatty Acids, and Vitamin E Has a Positive Effect on the Symptoms and Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2022, 90, 233–249. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Xiao, Q.; Chen, Y.-H.; Shirakawa, H.; Lai, J.-H.; Chiang, Y.-H.; Yang, S.-C. Effects of fish oil on insulin resistance in the brains of rats with alcoholic liver damage. J. Funct. Foods 2024, 120, 106369. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Shirakawa, H.; Lu, N.-S.; Peng, H.-C.; Xiao, Q.; Yang, S.-C. Impacts of fish oil on the gut microbiota of rats with alcoholic liver damage. J. Nutr. Biochem. 2020, 86, 108491. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H. The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Res. 2018, 148, 8–16. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, Y.-H.; Chen, Y.-L.; Chien, Y.-S.; Hsieh, L.-H.; Shirakawa, H.; Yang, S.-C. Potential Benefits of Epidermal Growth Factor for Inhibiting Muscle Degrative Markers in Rats with Alcoholic Liver Damage. Int. J. Mol. Sci. 2023, 24, 8845. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Lee, Y.-B.; Lee, B.; Lee, D. A quantitative analysis of spontaneous alternation behaviors on a Y-maze reveals adverse effects of acute social isolation on spatial working memory. Sci. Rep. 2023, 13, 14722. [Google Scholar] [CrossRef]
- Grayson, B.; Leger, M.; Piercy, C.; Adamson, L.; Harte, M.; Neill, J.C. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res. 2015, 285, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Sturdivant, N.; Venier, S.; Ali, S.; Wolchok, J.; Balachandran, K. Blood-Brain Barrier Breakdown and Astrocyte Reactivity Evident in the Absence of Behavioral Changes after Repeated Traumatic Brain Injury. Neurotrauma Rep. 2021, 2, 399–410. [Google Scholar] [CrossRef]
- Understanding Alcohol Drinking Patterns. Available online: https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-drinking-patterns (accessed on 10 April 2025).
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). Obes. Facts 2024, 17, 374–443. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zheng, K.; Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. The Lieber-DeCarli Diet-A Flagship Model for Experimental Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2018, 42, 1828–1840. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Agudo, M.; Luque-Tévar, M.; Cucarella, C.; Martín-Sanz, P.; Casado, M. Advances in Understanding the Role of NRF2 in Liver Pathophysiology and Its Relationship with Hepatic-Specific Cyclooxygenase-2 Expression. Antioxidants 2023, 12, 1491. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 Pathway in Liver Diseases. Front. Cell Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef]
- Li, H.; Xie, Z.; Zhang, Y.; Liu, Y.; Niu, A.; Liu, Y.; Zhang, L.; Guan, L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 2021, 44, 101385. [Google Scholar] [CrossRef]
- Song, G.; Han, H.; Park, S.; Sa, S.; Chung, W.; Lee, B.Y. Effects of GSH on Alcohol Metabolism and Hangover Improvement in Humans: A Randomized Double-Blind Placebo-Controlled Crossover Clinical Trial. Nutrients 2024, 16, 3262. [Google Scholar] [CrossRef]
- Contreras-Zentella, M.L.; Villalobos-García, D.; Hernández-Muñoz, R. Ethanol Metabolism in the Liver, the Induction of Oxidant Stress, and the Antioxidant Defense System. Antioxidants 2022, 11, 1258. [Google Scholar] [CrossRef]
- Xiong, W.; Garfinkel, A.E.M.; Li, Y.; Benowitz, L.I.; Cepko, C.L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Investig. 2015, 125, 1433–1445. [Google Scholar] [CrossRef]
- Vell, M.S.; Creasy, K.T.; Scorletti, E.; Seeling, K.S.; Hehl, L.; Rendel, M.D.; Schneider, K.M.; Schneider, C.V. Omega-3 intake is associated with liver disease protection. Front. Public Health 2023, 11, 1192099. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Koh, J.M.; Im, D.S. N-3 Polyunsaturated Fatty Acids Protect against Alcoholic Liver Steatosis by Activating FFA4 in Kupffer Cells. Int. J. Mol. Sci. 2024, 25, 5476. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Peng, H.C.; Chien, Y.W.; Chen, Y.L.; Lu, N.S.; Yang, S.C. Effects of Fish Oil on Lipid Metabolism and Its Molecular Biological Regulators in Chronic Ethanol-Fed Rats. Nutrients 2018, 10, 802. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Gustafson, D.L.; Dehn, D.L.; Han, J.Y.; Boonchoong, P.; Berliner, L.J.; Ross, D. NAD (P) H: Quinone oxidoreductase 1: Role as a superoxide scavenger. Mol. Pharmacol. 2004, 65, 1238–1247. [Google Scholar] [CrossRef]
- Yue, R.; Chen, G.-Y.; Xie, G.; Hao, L.; Guo, W.; Sun, X.; Jia, W.; Zhang, Q.; Zhou, Z.; Zhong, W. Activation of PPARα-catalase pathway reverses alcoholic liver injury via upregulating NAD synthesis and accelerating alcohol clearance. Free Radic. Biol. Med. 2021, 174, 249–263. [Google Scholar] [CrossRef]
- Yeligar, S.M.; Machida, K.; Kalra, V.K. Ethanol-induced HO-1 and NQO1 Are Differentially Regulated by HIF-1α and Nrf2 to Attenuate Inflammatory Cytokine Expression. J. Biol. Chem. 2010, 285, 35359–35373. [Google Scholar] [CrossRef]
- Navarro, D.; Gasparyan, A.; Martí Martínez, S.; Díaz Marín, C.; Navarrete, F.; García Gutiérrez, M.S.; Manzanares, J. Methods to Identify Cognitive Alterations from Animals to Humans: A Translational Approach. Int. J. Mol. Sci. 2023, 24, 7653. [Google Scholar] [CrossRef]
- Sachdeva, A.; Chandra, M.; Choudhary, M.; Dayal, P.; Anand, K.S. Alcohol-Related Dementia and Neurocognitive Impairment: A Review Study. Int. J. High. Risk Behav. Addict. 2016, 5, e27976. [Google Scholar] [CrossRef]
- Ge, C.L.; Chen, W.; Zhang, L.N.; Ai, Y.H.; Zou, Y.; Peng, Q.Y. Hippocampus-prefrontal cortex inputs modulate spatial learning and memory in a mouse model of sepsis induced by cecal ligation puncture. CNS Neurosci. Ther. 2023, 29, 390–401. [Google Scholar] [CrossRef]
- García, F.; Torres, M.-J.; Chacana-Véliz, L.; Espinosa, N.; El-Deredy, W.; Fuentealba, P.; Negrón-Oyarzo, I. Prefrontal cortex synchronization with the hippocampus and parietal cortex is strategy-dependent during spatial learning. Commun. Biol. 2025, 8, 79. [Google Scholar] [CrossRef]
- Gong, Y.-S.; Guo, J.; Hu, K.; Gao, Y.-Q.; Hou, F.-L.; Song, F.-L.; Liang, C.-Y. Chronic ethanol consumption and thiamine deficiency modulate β-amyloid peptide level and oxidative stress in the brain. Alcohol. Alcohol. 2017, 52, 159–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fowler, A.K.; Thompson, J.; Chen, L.; Dagda, M.; Dertien, J.; Dossou, K.S.; Moaddel, R.; Bergeson, S.E.; Kruman, I.I. Differential sensitivity of prefrontal cortex and hippocampus to alcohol-induced toxicity. PLoS ONE 2014, 9, e106945. [Google Scholar] [CrossRef] [PubMed]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S. Effects of omega-3 polyunsaturated fatty acids on brain functions: A systematic review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef] [PubMed]
- Haidary, M.; Ahmadi-Soleimani, S.M.; Ghofraninezad, M.; Azhdari-Zarmehri, H.; Beheshti, F. Omega-3 fatty acids supplementation prevents learning and memory impairment induced by chronic ethanol consumption in adolescent male rats through restoration of inflammatory and oxidative responses. Int. J. Dev. Neurosci. 2024, 84, 423–433. [Google Scholar] [CrossRef]
- Byerley, L.O.; Reppel, J.E.; Pahng, A.R.; Edwards, S.R. Omega-3 Dietary Fatty Acids Improve Spatial Learning and Memory but Do Not Alter the Escalation of Alcohol Drinking. FASEB J. 2016, 30, 679. [Google Scholar] [CrossRef]
- Shi, Z.; Xie, Y.; Ren, H.; He, B.; Wang, M.; Wan, J.B.; Yuan, T.F.; Yao, X.; Su, H. Fish oil treatment reduces chronic alcohol exposure induced synaptic changes. Addict. Biol. 2019, 24, 577–589. [Google Scholar] [CrossRef]
- Yavari, M.; Khedmatgozar, H.; Ramalingam, L.; Harris, B.; Kahathuduwa, C.N.; Zu, Y.; Moustaid-Moussa, N. Mechanisms Mediating the Protective Effects of Eicosapentaenoic Acid in Brain of the APPswePS1dE9 Alzheimer’s Mouse Model. Curr. Dev. Nutr. 2022, 6, 815. [Google Scholar] [CrossRef]
- Ren, H.; Luo, C.; Feng, Y.; Yao, X.; Shi, Z.; Liang, F.; Kang, J.X.; Wan, J.B.; Pei, Z.; Su, H. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017, 31, 282–293. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Hu, X.; Sun, B.; Cao, G.; Gao, Y. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915. [Google Scholar] [CrossRef]
- Díaz, M.; Valdés-Baizabal, C.; de Pablo, D.P.; Marin, R. Age-dependent changes in Nrf2/Keap1 and target antioxidant protein expression correlate to lipoxidative adducts, and are modulated by dietary N-3 LCPUFA in the hippocampus of mice. Antioxidants 2024, 13, 206. [Google Scholar] [CrossRef]
- Jin, S.; Cao, Q.; Yang, F.; Zhu, H.; Xu, S.; Chen, Q.; Wang, Z.; Lin, Y.; Cinar, R.; Pawlosky, R.J. Brain ethanol metabolism by astrocytic ALDH2 drives the behavioural effects of ethanol intoxication. Nat. Metab. 2021, 3, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, M.; Gandhi, N.; Saiyed, Z.; Pichili, V.; Thangavel, S.; Khatavkar, P.; Yndart-Arias, A.; Nair, M. Effects of alcohol on histone deacetylase 2 (HDAC2) and the neuroprotective role of trichostatin A (TSA). Alcohol. Clin. Exp. Res. 2011, 35, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tao, X.; Xiong, Z.; Wang, H.; Zeng, L. Mechanism of HDAC2 regulating Nrf2 acetylation level in neuronal ferroptosis of neonatal rats with hypoxic-ischemic brain injury. Brain Inj. 2025, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef]
- Dong, H.; Hao, L.; Zhang, W.; Zhong, W.; Guo, W.; Yue, R.; Sun, X.; Zhou, Z. Activation of AhR-NQO1 signaling pathway protects against alcohol-induced liver injury by improving redox balance. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 793–811. [Google Scholar] [CrossRef]
- Lu, N.-S.; Chiu, W.-C.; Chen, Y.-L.; Peng, H.-C.; Shirakawa, H.; Yang, S.-C. Fish oil up-regulates hepatic autophagy in rats with chronic ethanol consumption. J. Nutr. Biochem. 2020, 77, 108314. [Google Scholar] [CrossRef]


| Group 2 | - | F25 | F57 | Interaction (p Value) 3 | |
|---|---|---|---|---|---|
| Ethanol × Fish Oil | |||||
| Initial BW (g) | C | 264.7 ± 13.0 | 271.2 ± 5.9 | 270.7 ± 11.8 | 0.5164 |
| E | 271.4 ± 6.4 | 274.0 ± 9.9 | 267.7 ± 12.8 | ||
| Final BW (g) | C | 449.9 ± 10.9 | 460.4 ± 8.0 | 442.3 ± 25.5 | 0.5559 |
| E | 406.2 ± 21.2 * | 404.2 ± 14.7 | 399.1 ± 11.8 | ||
| Relative liver weight (%) | C | 3.06 ± 0.37 | 3.15 ± 0.19 | 3.15 ± 0.15 | 0.5370 |
| E | 3.46 ± 0.41 | 3.85 ± 0.48 | 3.63 ± 0.30 | ||
| AST (U/L) | C | 59.7 ± 5.8 a | 65.8 ± 5.8 a | 74.5 ± 3.3 b | 0.0058 |
| E | 193.7 ± 61.3 *A | 120.8 ± 38.9 B | 134.3 ± 19.6 A | ||
| ALT (U/L) | C | 40.0 ± 12.1 | 49.5 ± 5.2 | 49.2 ± 7.8 | 0.6402 |
| E | 96.0 ± 15.8 * | 92.0 ± 32.8 | 93.7 ± 23.4 |
| Group 2 | - | F25 | F57 | Interaction (p Value) 3 | |
|---|---|---|---|---|---|
| Ethanol × Fish Oil | |||||
| GSH (µM/mg) | C | 35.9 ± 18.11 | 23.34 ± 8.4 | 21.19 ± 4.63 | 0.0247 |
| E | 10.20 ± 2.66 * | 12.31 ± 4.07 | 13.93 ± 6.35 | ||
| GPx (nmol/min/mg) | C | 0.225 ± 0.055 | 0.241 ± 0.083 | 0.174 ± 0.054 | 0.3770 |
| E | 0.147 ± 0.035 * | 0.175 ± 0.057 | 0.149 ± 0.027 | ||
| GRd (nmol/min/mg) | C | 12.336 ± 2.393 | 12.2 ± 1.793 | 12.85 ± 1.82 | 0.2203 |
| E | 9.429 ± 2.892 | 12.996 ± 4.46 | 12.188 ± 3.261 | ||
| SOD (U/mg) | C | 13.27 ± 2.97 | 14.98 ± 4.11 | 13.68 ± 4.23 | 0.7607 |
| E | 8.79 ± 2.27 * | 12.15 ± 3.95 | 10.85 ± 3.12 | ||
| CAT (nmol/min/mg) | C | 4705.4 ± 1886.4 | 4638.2 ± 1136.7 | 5344.4 ± 864.6 | 0.4142 |
| E | 2522.3 ± 724.2 *A | 3062.4 ± 458.5 B | 4222.9 ± 921.0 B |
| Group 2 | - | F25 | F57 | Interaction (p Value) 3 | |
|---|---|---|---|---|---|
| Ethanol × Fish Oil | |||||
| GSH (µM/mg) | C | 20.11 ± 4.03 | 13.81 ± 8.11 | 12.05 ± 5.25 | 0.4606 |
| E | 12.31 ± 6.18 * | 11.18 ± 4.37 | 15.27 ± 5.62 | ||
| GPx (nmol/min/mg) | C | 283.89 ± 39.03 | 230.21 ± 52.66 | 240.74 ± 47.79 | 0.0798 |
| E | 279.35 ± 91.85 | 274.37 ± 78.98 | 306.15 ± 87.59 | ||
| SOD (U/mg) | C | 7.96 ± 1.32 | 6.71 ± 1.34 | 5.91 ± 1.38 | 0.7309 |
| E | 7.48 ± 2.89 | 7.03 ± 0.6 | 6.4 ± 1.04 | ||
| CAT (nmol/min/mg) | C | 6.95 ± 3.3 | 9.33 ± 3.25 | 9.64 ± 2.03 | 0.2093 |
| E | 11.60 ± 2.48 *A | 13.19 ± 2.89 AB | 17.79 ± 4.09 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Q.; Chen, Y.-H.; Fu, L.-C.; Nurrahma, H.A.; Lai, J.-H.; Shirakawa, H.; Yang, S.-C. Protective Effects of Fish Oil Against Brain Impairment in Rats with Chronic Ethanol-Induced Liver Damage Involving the NRF2 Pathway and Oxidative Stress. Antioxidants 2025, 14, 704. https://doi.org/10.3390/antiox14060704
Xiao Q, Chen Y-H, Fu L-C, Nurrahma HA, Lai J-H, Shirakawa H, Yang S-C. Protective Effects of Fish Oil Against Brain Impairment in Rats with Chronic Ethanol-Induced Liver Damage Involving the NRF2 Pathway and Oxidative Stress. Antioxidants. 2025; 14(6):704. https://doi.org/10.3390/antiox14060704
Chicago/Turabian StyleXiao, Qian, Yi-Hsiu Chen, Lu-Chi Fu, Herlin Ajeng Nurrahma, Jing-Huei Lai, Hitoshi Shirakawa, and Suh-Ching Yang. 2025. "Protective Effects of Fish Oil Against Brain Impairment in Rats with Chronic Ethanol-Induced Liver Damage Involving the NRF2 Pathway and Oxidative Stress" Antioxidants 14, no. 6: 704. https://doi.org/10.3390/antiox14060704
APA StyleXiao, Q., Chen, Y.-H., Fu, L.-C., Nurrahma, H. A., Lai, J.-H., Shirakawa, H., & Yang, S.-C. (2025). Protective Effects of Fish Oil Against Brain Impairment in Rats with Chronic Ethanol-Induced Liver Damage Involving the NRF2 Pathway and Oxidative Stress. Antioxidants, 14(6), 704. https://doi.org/10.3390/antiox14060704







