Culture Medium Enriched with Ultrafine Carbon Monoxide Bubbles Enhances In Vitro Blastocyst Formation of In Vivo-Fertilized Mouse Zygotes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of UFB Culture Medium
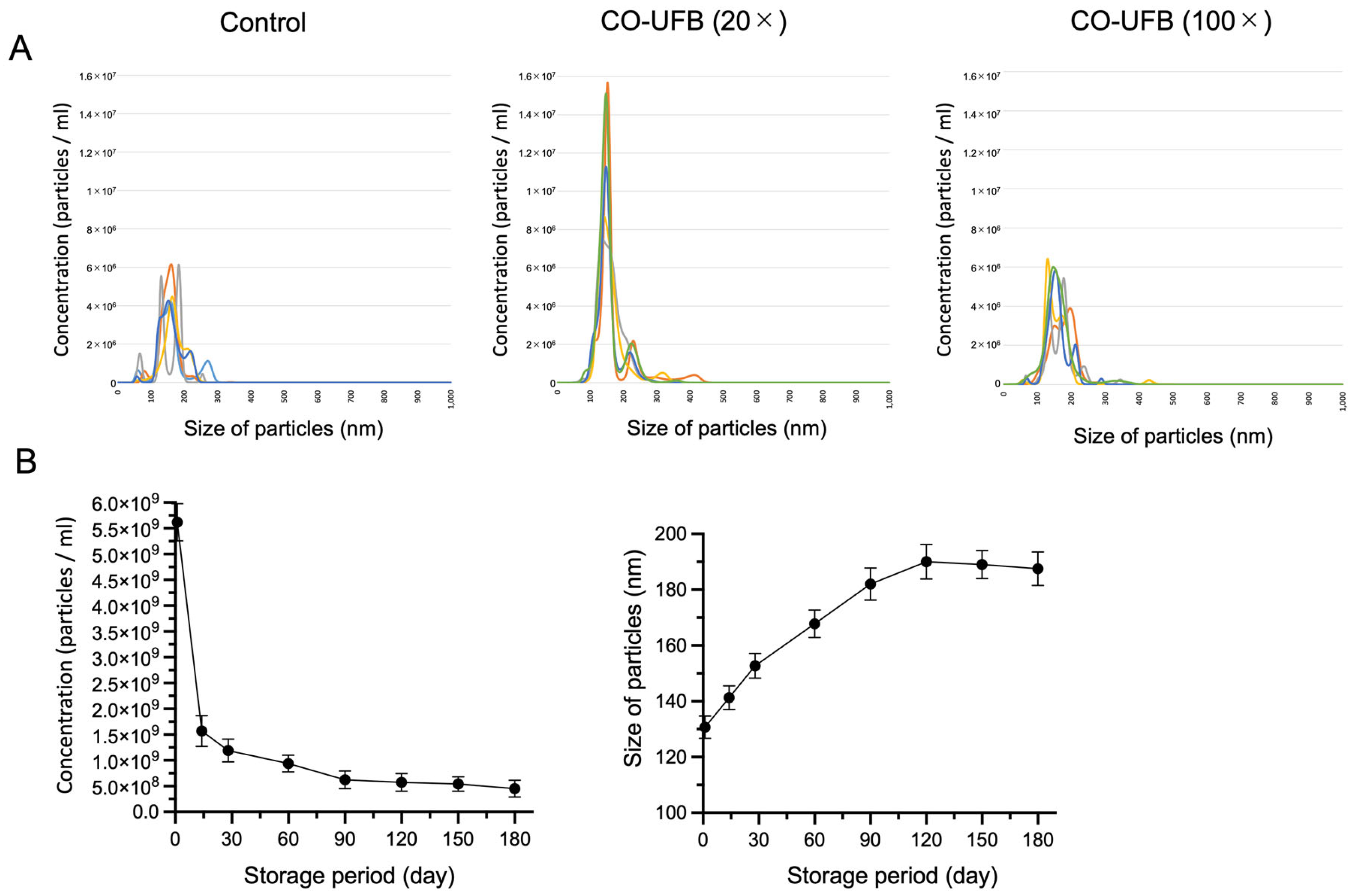
2.2. Nanoparticle Tracking Analysis (NTA) Measurement of UFB
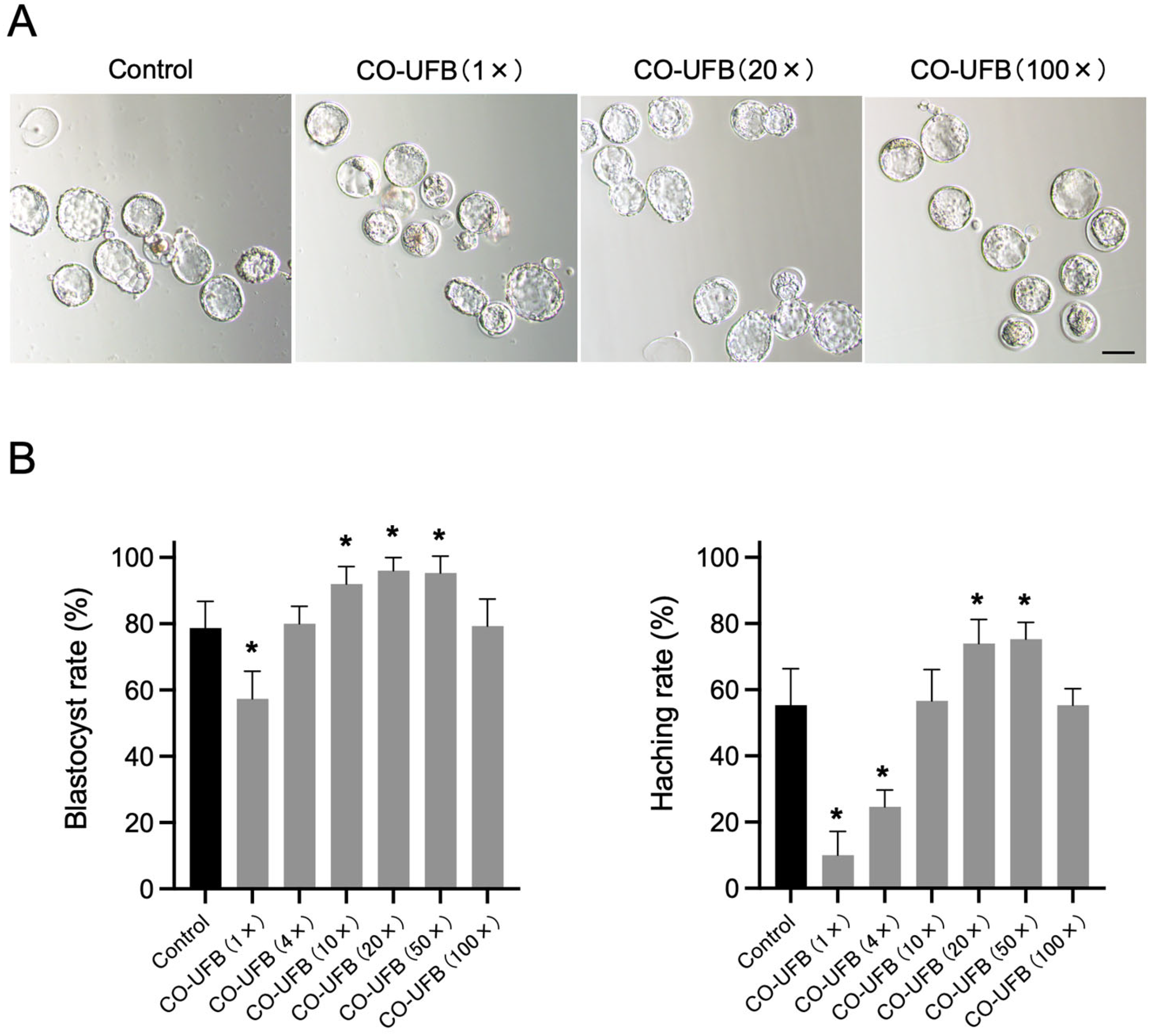
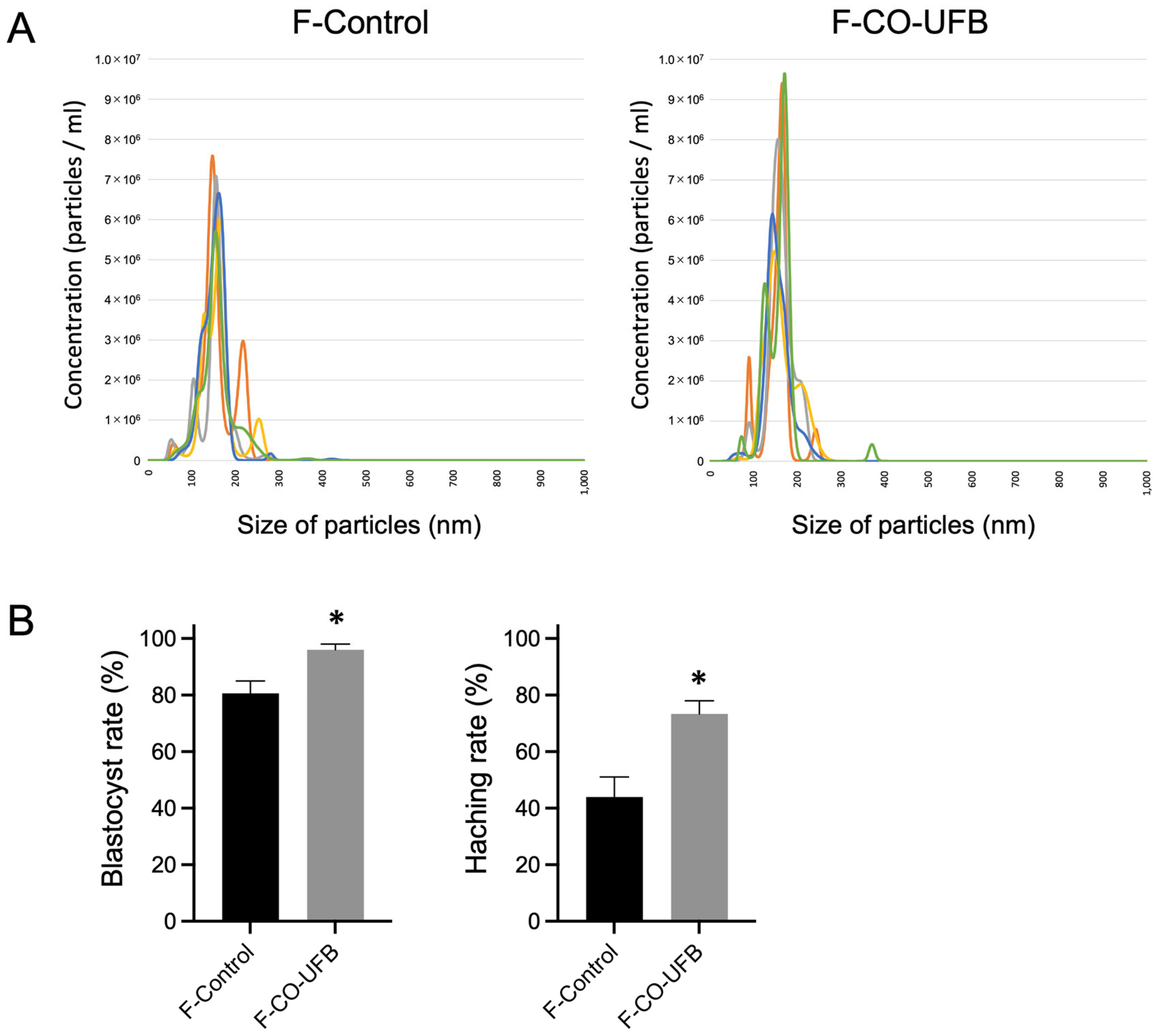
2.3. In Vitro Culture of Embryos
2.4. cDNA Library Preparation and RNA Sequencing of Blastocysts
2.5. RNA-Sequencing Data Analysis of Blastocysts
2.6. Statistical Analysis
3. Results
3.1. Comparison of CO-UFB Size and Abundance
3.2. Effect of CO-UFB on Embryonic Development
3.3. Effect of CO-UFB (Filtration) on Embryonic Development
3.4. Effect of UFB on the Gene of Blastocysts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thoma, M.E.; McLain, A.C.; Louis, J.F.; King, R.B.; Trumble, A.C.; Sundaram, R.; Germaine, M.; Louis, B. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013, 99, 1324–1331.e1. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Makker, K.; Desai, N.R.; Agarwal, A. Impact of oxidative stress on IVF. Expert Rev. Obstet. Gynecol. 2008, 3, 539–554. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S.S.R. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod. Biomed. Online 2004, 9, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Oyawoye, O.; Abdel Gadir, A.; Garner, A.; Constantinovici, N.; Perrett, C.; Hardiman, P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: Relationship to outcome. Hum. Reprod. 2003, 18, 2270–2274. [Google Scholar] [CrossRef]
- Ra, K.; Oh, H.J.; Kim, E.Y.; Kang, S.K.; Ra, J.C.; Kim, E.H.; Lee, B.C. Anti-oxidative effects of human adipose stem cell conditioned medium with different basal medium during mouse embryo in vitro culture. Animals 2020, 10, 1414. [Google Scholar] [CrossRef]
- Johnson, M.H.; Nasr-Esfahani, M.H. Radical solutions and cultural problems: Could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? BioEssays 1994, 16, 31–38. [Google Scholar] [CrossRef]
- Cambra, J.M.; Martinez, C.A.; Rodriguez-Martinez, H.; Martinez, E.A.; Cuello, C.; Gil, M.A. N-(2-mercaptopropionyl)-glycine enhances in vitro pig embryo production and reduces oxidative stress. Sci. Rep. 2020, 10, 18632. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; He, C.; Ji, P.; Li, Y.; Tan, D.; Liu, G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014, 101, 577–586. [Google Scholar] [CrossRef]
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413. [Google Scholar] [CrossRef]
- Zarbakhsh, S. Effect of antioxidants on preimplantation embryo development in vitro: A review. Zygote 2021, 29, 179–193. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Gaseotransmitters: New frontiers for translational science. Sci. Transl. Med. 2010, 2, 59ps54. [Google Scholar] [CrossRef] [PubMed]
- Peers, C.; Steele, D.S. Carbon monoxide: A vital signalling molecule and potent toxin in the myocardium. J. Mol. Cell. Cardiol. 2012, 52, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation 2010, 122, 11–19. [Google Scholar] [CrossRef]
- Nakao, A.; Toyoda, Y. Application of carbon monoxide for transplantation. Curr. Pharm. Biotechnol. 2012, 13, 827–836. [Google Scholar] [CrossRef]
- Nakao, A.; Yamada, T.; Kohama, K.; Yoshie, N.; Fujisaki, N.; Kotani, J. Application of carbon monoxide for treatment of acute kidney injury. Acute Med. Surg. 2014, 1, 127–134. [Google Scholar] [CrossRef]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-pressure solubility of gases in liquid water. Chem. Rev. 1977, 77, 219–262. [Google Scholar] [CrossRef]
- Mann, B.E. Carbon monoxide: An essential signalling molecule. Top. Organomet. Chem. 2010, 32, 247–285. [Google Scholar] [CrossRef]
- Romão, C.C.; Blättler, W.A.; Seixas, J.D.; Bernardes, G.J.L. Developing drug molecules for therapy with carbon monoxide. Chem. Soc. Rev. 2012, 41, 3571–3583. [Google Scholar] [CrossRef]
- ISO 20480-1; Fine Bubble Technology—General Principles for Usage and Measurement of Fine Bubbles—Part 1: Terminology. International Organization for Standardization: Geneva, Switzerland, 2017.
- Azevedo, A.; Oliveira, H.; Rubio, J. Bulk nanobubbles in the mineral and environmental areas: Updating research and applications. Adv. Colloid Interface Sci. 2019, 271, 101992. [Google Scholar] [CrossRef]
- Miyamoto, S.; Hirakawa, T.; Noguchi, Y.; Urushiyama, D.; Miyata, K.; Baba, T.; Yotsumoto, F.; Yasunaga, S.; Nakabayashi, K.; Hata, K.; et al. Properties of Ultrafine Bubbles Generated Using a Generator System. In Vivo 2023, 37, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Zeta potential of microbubbles in aqueous solutions: Electrical properties of the gas-water interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef] [PubMed]
- Sueishi, N.; Ohshima, T.; Oikawa, T.; Takemura, H.; Kasai, M.; Kitano, K.; Maeda, N.; Nakamura, Y. Plaque-removal effect of ultrafine bubble water: Oral application in patients undergoing orthodontic treatment. Dent. Mater. J. 2021, 40, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Yamashita, T.; Kaida, R.; Seo, S.; Tanaka, K.; Abe, S.; Nakano, M.; Fujii, Y.; Kuchitsu, K. Ultrafine bubble water mitigates plant growth in damaged soil. Biosci. Biotechnol. Biochem. 2021, 85, 2466–2475. [Google Scholar] [CrossRef]
- Hung, J.C.; Li, N.J.; Peng, C.Y.; Yang, C.C.; Ko, S.S. Safe farming: Ultrafine bubble water reduces insect infestation and improves melon yield and quality. Plants 2024, 13, 537. [Google Scholar] [CrossRef]
- Kugino, K.; Tamaru, S.; Hisatomi, Y.; Sakaguchi, T. Long-duration carbon dioxide anesthesia of fish using ultra fine (nano-scale) bubbles. PLoS ONE 2016, 11, e0153542. [Google Scholar] [CrossRef]
- Matsumoto, A.; Imanishi, H.; Yamanaka-Takaichi, M.; Hirae, M.; Tsuruta, D.; Nakai, K. Beneficial effects of ultrafine bubble shower on a mouse model of atopic dermatitis. Front. Immunol. 2024, 15, 1483000. [Google Scholar] [CrossRef]
- Gombodorj, N.; Yokobori, T.; Mutsuki, N.; Erkhem-Ochir, B.; Okami, H.; Asao, T.; Saeki, H.; Shirabe, K.; Yamanouchi, D. Effects of ultrafine single-nanometer oxygen bubbles on radiation sensitivity in a tumor-bearing mouse model. Int. J. Mol. Sci. 2022, 23, 6838. [Google Scholar] [CrossRef]
- Le, T.H.; Phan, A.H.T.; Le, K.C.M.; Phan, T.D.U.; Nguyen, K.T. Utilizing polymer-conjugate albumin-based ultrafine gas bubbles in combination with ultra-high frequency radiations in drug transportation and delivery. RSC Adv. 2021, 11, 34440–34448. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Qi, N.; Qin, Y.; Zhang, X.; Li, Y. Generation and stability of size-adjustable bulk nanobubbles based on periodic pressure change. Sci. Rep. 2019, 9, 1118. [Google Scholar] [CrossRef]
- Ueda, Y.; Tokuda, Y.; Zushi, T. Electrochemical performance of ultrafine bubble water. ECS Trans. 2014, 58, 11–19. [Google Scholar] [CrossRef]
- Yasuda, K.; Matsushima, H.; Asakura, Y. Generation and reduction of bulk nanobubbles by ultrasonic irradiation. Chem. Eng. Sci. 2019, 195, 455–461. [Google Scholar] [CrossRef]
- Hirakawa, T.; Nakabayashi, K.; Ito, N.; Hata, K.; Imi, S.; Shibata, M.; Urushiyama, D.; Miyata, K.; Yotsumoto, F.; Yasunaga, S.; et al. Transwell culture with adipose tissue-derived stem cells and fertilized eggs mimics the in vivo development of fertilized eggs to blastocysts in the Fallopian tube: An animal study. Antioxidants 2024, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Chen, Y.; Lun, A.T.L.; Smyth, G.K. From reads to genes to pathways: Differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Research 2016, 5, 1438. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Li, L.; Moore, P.K. An overview of the biological significance of endogenous gases: New roles for old molecules. Biochem. Soc. Trans. 2007, 35, 1138–1141. [Google Scholar] [CrossRef]
- Matta, S.G.; Caldas-Bussiere, M.C.; Viana, K.S.; Faes, M.R.; Paes de Carvalho, C.S.; Dias, B.L.; Quirino, C.R. Effect of inhibition of synthesis of inducible nitric oxide synthase-derived nitric oxide by aminoguanidine on the in vitro maturation of oocyte-cumulus complexes of cattle. Anim. Reprod. Sci. 2009, 111, 189–201. [Google Scholar] [CrossRef]
- Goud, P.T.; Goud, A.P.; Diamond, M.P.; Gonik, B.; Abu-Soud, H.M. Nitric oxide extends the oocyte temporal window for optimal fertilization. Free Radic. Biol. Med. 2008, 45, 453–459. [Google Scholar] [CrossRef]
- Goud, P.T.; Goud, A.P.; Joshi, N.; Puscheck, E.; Diamond, M.P.; Abu-Soud, H.M. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil. Steril. 2014, 102, 151–159.e5. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.R.L.; Pires, P.R.L.; Adona, P.R.; Câmara de Bem, T.H.; Leal, C.L.V. Influence of nitric oxide during maturation on bovine oocyte meiosis and embryo development in vitro. Reprod. Fertil. Dev. 2008, 20, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Khodade, V.S.; Toscano, J.P. Reactive sulfur species in biology and medicine. Antioxidants 2023, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Nevoral, J.; Žalmanová, T.; Zámostná, K.; Kott, T.; Kučerová-Chrpová, V.; Bodart, J.F.; Gelaude, A.; Procházka, R.; Orsák, M.; Šulc, M.; et al. Endogenously produced hydrogen sulfide is involved in porcine oocyte maturation in vitro. Nitric Oxide 2015, 51, 24–35. [Google Scholar] [CrossRef]
- Nevoral, J.; Petr, J.; Gelaude, A.; Bodart, J.F.; Kucerova-Chrpova, V.; Sedmikova, M.; Krejcova, T.; Kolbabova, T.; Dvorakova, M.; Vyskocilova, A.; et al. Dual effects of hydrogen sulfide donor on meiosis and cumulus expansion of porcine cumulus-oocyte complexes. PLoS ONE 2014, 9, e99613. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef]
- Venditti, P.; Napolitano, G. Mitochondrial management of ROS in physiological and pathological conditions. Antioxidants 2025, 14, 43. [Google Scholar] [CrossRef]
- Wang, Y.; Yen, F.S.; Zhu, X.G.; Timson, R.C.; Weber, R.; Xing, C.; Liu, Y.; Allwein, B.; Luo, H.; Yeh, H.W.; et al. SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells. Nature 2021, 599, 136–140. [Google Scholar] [CrossRef]
- Venditti, P.; Napolitano, G.; Di Meo, S. Role of enzymatic and non-enzymatic processes in H2O2 removal by rat liver and heart mitochondria. J. Bioenerg. Biomembr. 2014, 46, 83–91. [Google Scholar] [CrossRef]
- Almeida, A.S.; Figueiredo-Pereira, C.; Vieira, H.L.A. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Front. Physiol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Vyas, H.S.; Jadeja, R.N.; Vohra, A.; Upadhyay, K.K.; Thounaojam, M.C.; Bartoli, M.; Devkar, R.V. CORM-A1 alleviates pro-atherogenic manifestations via miR-34a-5p downregulation and an improved mitochondrial function. Antioxidants 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, P.; Chen, M.; Liu, Y.; Zhou, L.; Fang, X.; Huang, Z. Carbon monoxide-releasing molecules attenuate postresuscitation myocardial injury and protect cardiac mitochondrial function by reducing the production of mitochondrial reactive oxygen species in a rat model of cardiac arrest. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, L.R.; Alexander, M.; Sorensen, A.M.; Sarantopoulos, N.; Lau, H.; Klopfer, M.; Ziegler, M.E.; Banyard, D.A.; Evans, G.R.D.; Lakey, J.R.T.; et al. Micro/nanobubbles: Improving pancreatic islet cell survival for transplantation. Ann. Plast. Surg. 2019, 83, 583–588. [Google Scholar] [CrossRef]
- Feshitan, J.A.; Legband, N.D.; Borden, M.A.; Terry, B.S. Systemic oxygen delivery by peritoneal perfusion of oxygen microbubbles. Biomaterials 2014, 35, 2600–2606. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Ebina, K.; Hirao, M.; Morimoto, T.; Koizumi, K.; Kitaguchi, K.; Matsuoka, H.; Iwahashi, T.; Yoshikawa, H. Oxygen ultra-fine bubbles water administration prevents bone loss of glucocorticoid-induced osteoporosis in mice by suppressing osteoclast differentiation. Osteoporos. Int. 2017, 28, 1063–1075. [Google Scholar] [CrossRef]
- Matsuoka, H.; Ebina, K.; Tanaka, H.; Hirao, M.; Iwahashi, T.; Noguchi, T.; Suzuki, K.; Nishimoto, S.; Murase, T.; Yoshikawa, H. Administration of oxygen ultra-fine bubbles improves nerve dysfunction in a rat sciatic nerve crush injury model. Int. J. Mol. Sci. 2018, 19, 1395. [Google Scholar] [CrossRef]
- Kida, H.; Feril, L.B.; Irie, Y.; Endo, H.; Itaka, K.; Tachibana, K. Influence of nanobubble size distribution on ultrasound-mediated plasmid DNA and messenger RNA gene delivery. Front. Pharmacol. 2022, 13, 855495. [Google Scholar] [CrossRef]
- Morimoto, Y.; Durante, W.; Lancaster, D.G.; Klattenhoff, J.; Tittel, F.K. Real-time measurements of endogenous CO production from vascular cells using an ultrasensitive laser sensor. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H483–H488. [Google Scholar] [CrossRef]
- Michel, B.W.; Lippert, A.R.; Chang, C.J. A reaction-based fluorescent probe for selective imaging of carbon monoxide in living cells using a palladium-mediated carbonylation. J. Am. Chem. Soc. 2012, 134, 15668–15671. [Google Scholar] [CrossRef]
- Minegishi, S.; Yumura, A.; Miyoshi, H.; Negi, S.; Taketani, S.; Motterlini, R.; Foresti, R.; Kano, K.; Kitagishi, H. Detection and removal of endogenous carbon monoxide by selective and cell-permeable hemoprotein model complexes. J. Am. Chem. Soc. 2017, 139, 5984–5991. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirakawa, T.; Nakabayashi, K.; Ito, N.; Ishiwata, K.; Urushiyama, D.; Miyata, K.; Baba, T.; Hata, K.; Yasunaga, S.; Yotsumoto, F.; et al. Culture Medium Enriched with Ultrafine Carbon Monoxide Bubbles Enhances In Vitro Blastocyst Formation of In Vivo-Fertilized Mouse Zygotes. Antioxidants 2025, 14, 684. https://doi.org/10.3390/antiox14060684
Hirakawa T, Nakabayashi K, Ito N, Ishiwata K, Urushiyama D, Miyata K, Baba T, Hata K, Yasunaga S, Yotsumoto F, et al. Culture Medium Enriched with Ultrafine Carbon Monoxide Bubbles Enhances In Vitro Blastocyst Formation of In Vivo-Fertilized Mouse Zygotes. Antioxidants. 2025; 14(6):684. https://doi.org/10.3390/antiox14060684
Chicago/Turabian StyleHirakawa, Toyofumi, Kazuhiko Nakabayashi, Noriko Ito, Keisuke Ishiwata, Daichi Urushiyama, Kohei Miyata, Tsukasa Baba, Kenichiro Hata, Shin’ichiro Yasunaga, Fusanori Yotsumoto, and et al. 2025. "Culture Medium Enriched with Ultrafine Carbon Monoxide Bubbles Enhances In Vitro Blastocyst Formation of In Vivo-Fertilized Mouse Zygotes" Antioxidants 14, no. 6: 684. https://doi.org/10.3390/antiox14060684
APA StyleHirakawa, T., Nakabayashi, K., Ito, N., Ishiwata, K., Urushiyama, D., Miyata, K., Baba, T., Hata, K., Yasunaga, S., Yotsumoto, F., Tachibana, K., & Miyamoto, S. (2025). Culture Medium Enriched with Ultrafine Carbon Monoxide Bubbles Enhances In Vitro Blastocyst Formation of In Vivo-Fertilized Mouse Zygotes. Antioxidants, 14(6), 684. https://doi.org/10.3390/antiox14060684







