ELONGATED HYPOCOTYL5 Regulates Resistance to Root-Knot Nematode by Modulating Antioxidant System and Jasmonic Acid in Cucumis sativus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cucumber Transformation
2.2. Plant Material and Growth Condition
2.3. Propagation and Inoculation of Meloidogyne Incognita
2.4. Susceptibility Assessment
2.5. RNA-Seq Experimental Method
2.6. RNA Isolation and Complementary DNA Synthesis
2.7. Quantitative Real-Time PCR
2.8. Determination of Antioxidant Enzyme Activity
2.9. Determination of Plant Hormones
2.10. Statistical Analysis
3. Results
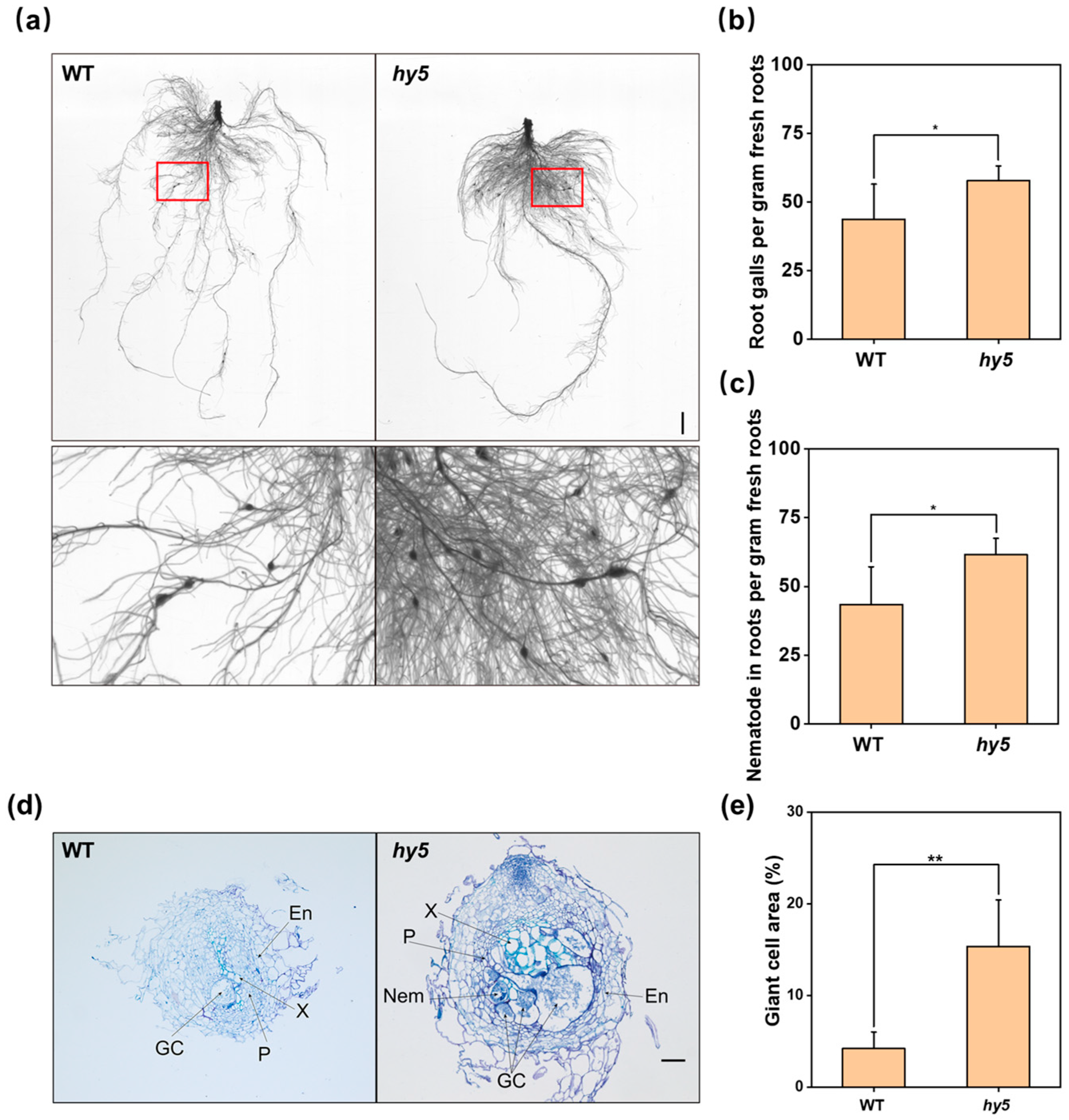
3.1. Loss of CsHY5 Reduced Plant Resistance to Root-Knot Nematodes
3.2. The Nematodes That Invaded Cshy5 Mutants Developed to the Female Stage More Quickly
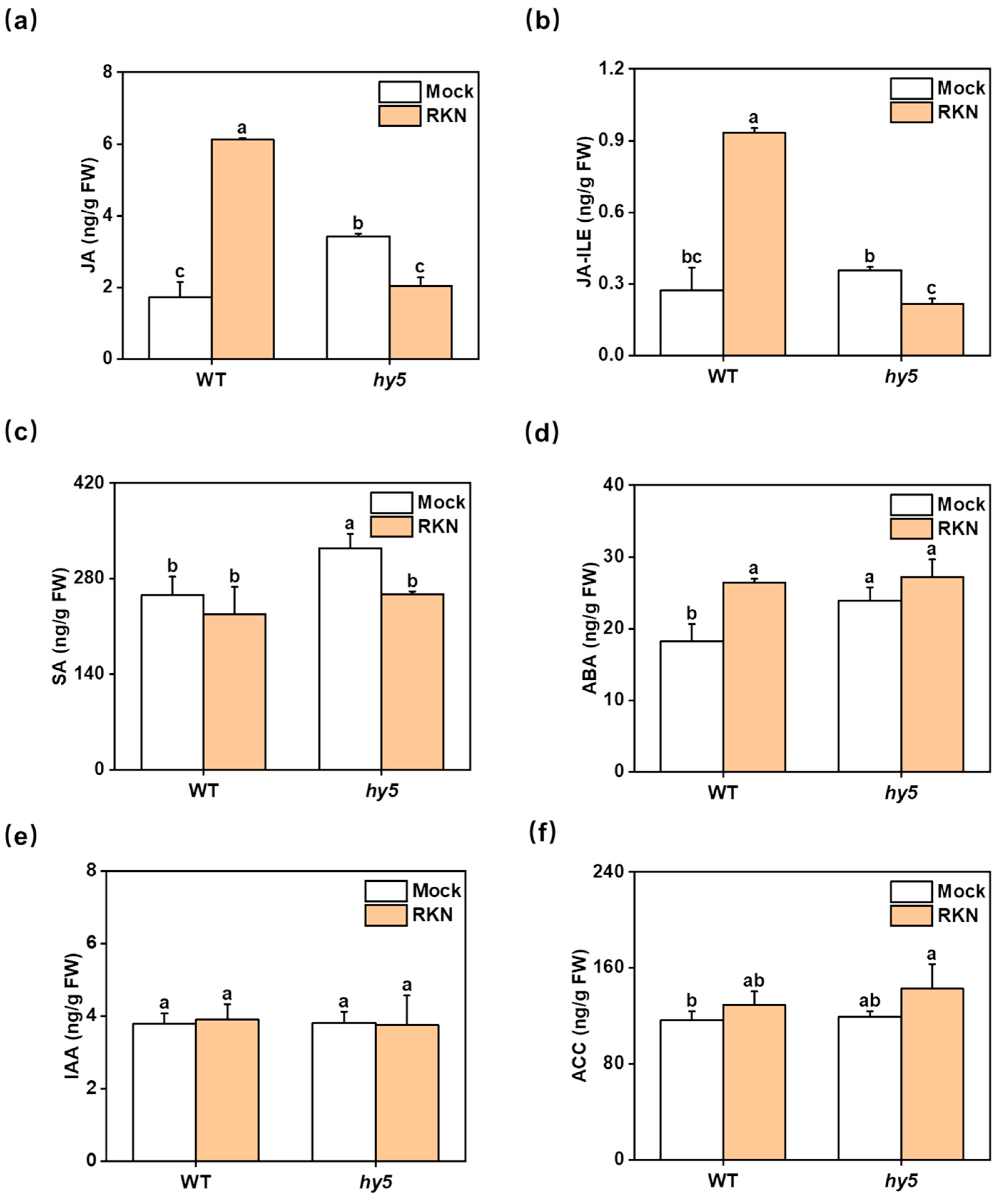
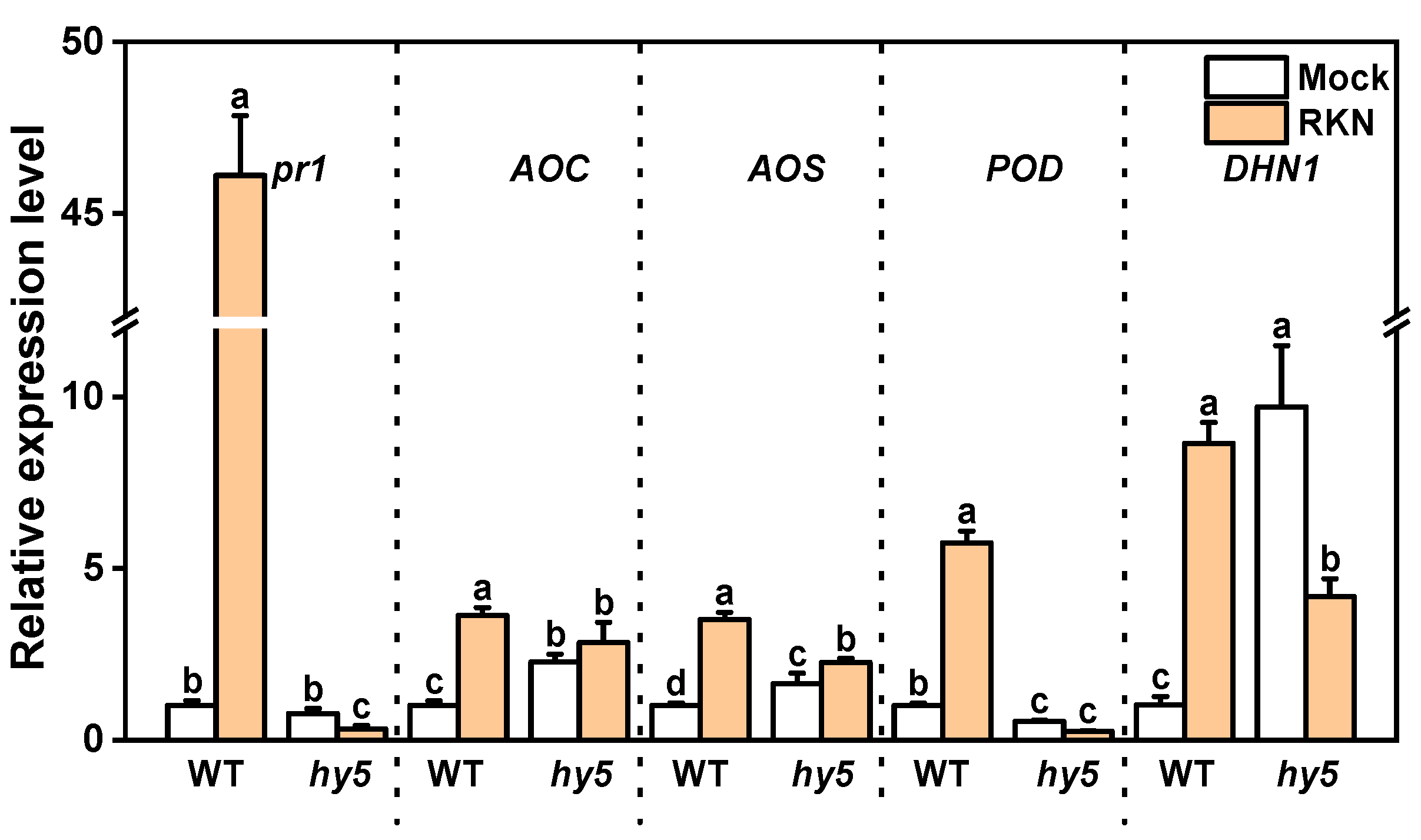
3.3. Changes in Antioxidant Enzyme Activity and Hormones
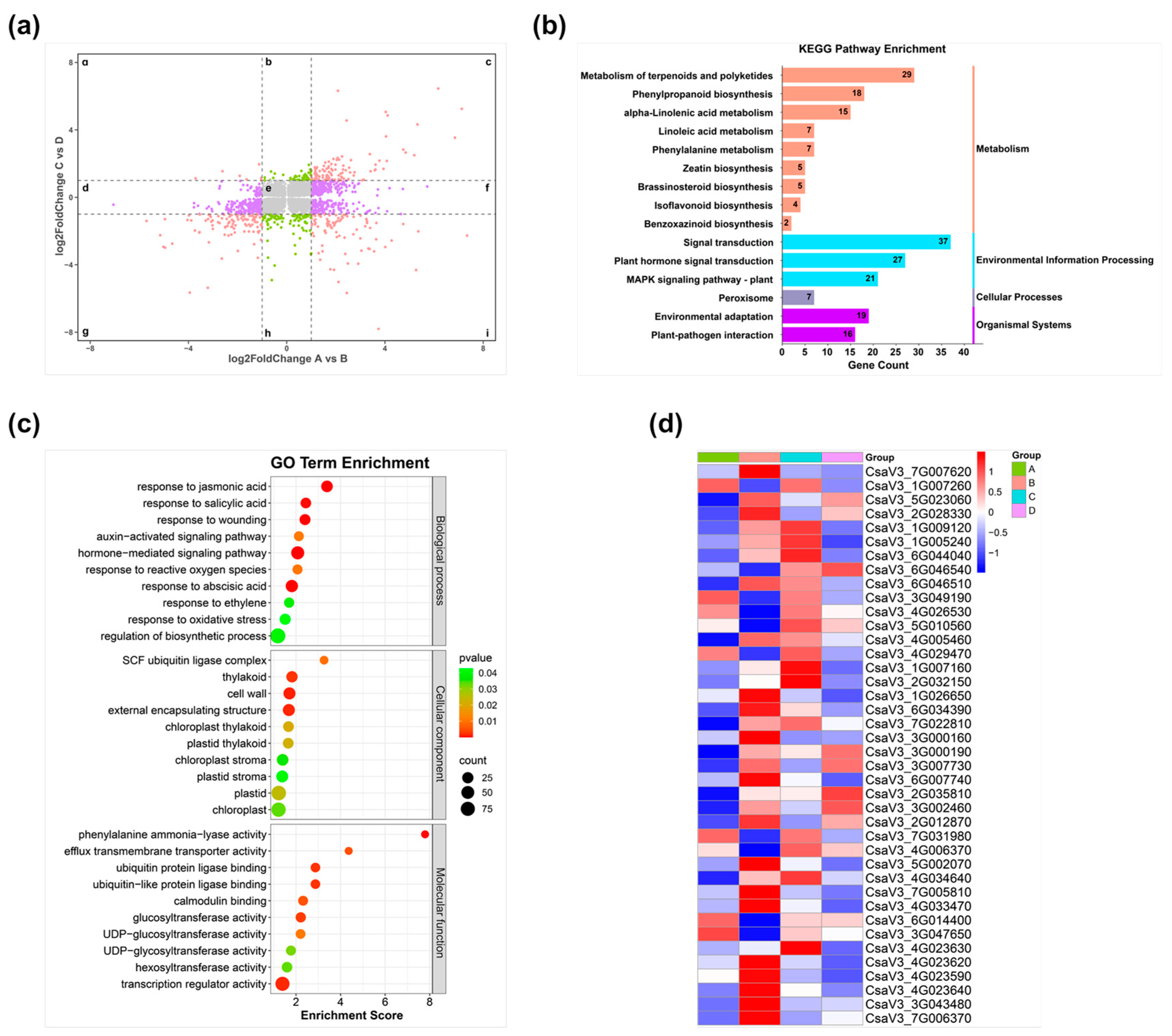
3.4. Transcriptome Analysis of WT and Cshy5 Infected with RKNs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elling, A.A. Major Emerging Problems with minor Meloidogyne Species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.L.; Hussey, R.S.; Mitchum, M.G.; Baum, T.J. Parasitism proteins in nematode-plant interactions. Curr. Opin. Plant Biol. 2008, 11, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, S.; Sterken, M.G.; van Schaik, C.; Oortwijn, M.E.P.; Sukarta, O.C.A.; Lozano-Torres, J.L.; Dicke, M.; Helder, J.; Kammenga, J.E.; Goverse, A.; et al. Genome-wide association mapping of the architecture of susceptibility to the root-knot nematode Meloidogyne incognita in Arabidopsis thaliana. New Phytol. 2018, 218, 724–737. [Google Scholar] [CrossRef]
- De Waele, D.; Elsen, A. Challenges in tropical plant nematology. Annu. Rev. Phytopathol. 2007, 45, 457–485. [Google Scholar] [CrossRef]
- Guan, W.; Zhao, X.; Dickson, D.W.; Mendes, M.L. Root-knot Nematode Resistance, Yield, and Fruit Quality of Specialty Melons Grafted onto Cucumis metulifer. Hortscience A Publ. Am. Soc. Hortic. Sci. 2014, 49, 1046–1051. [Google Scholar] [CrossRef]
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii, a Major Threat to Tomato Production: Current Status and Future Prospects for Its Management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Barcala, M.; Cabrera, J.; Fenoll, C. Chapter One—Overview of Root-Knot Nematodes and Giant Cells. Adv. Bot. Res. 2015, 73, 1–32. [Google Scholar]
- Rutter, W.B.; Franco, J.; Gleason, C. Rooting out the Mechanisms of Root-Knot Nematode-Plant Interactions. Annu. Rev. Phytopathol. 2022, 60, 43–76. [Google Scholar] [CrossRef]
- Calderón-Urrea, A.; Vanholme, B.; Vangestel, S.; Kane, S.M.; Bahaji, A.; Pha, K.; Garcia, M.; Snider, A.; Gheysen, G. Early development of the root-knot nematode Meloidogyne incognita. BMC Dev. Biol. 2016, 16, 10. [Google Scholar] [CrossRef]
- Jones, M.G.; Payne, H.L. Early stages of nematode-induced giant-cell formation in roots of Impatiens balsamina. J. Nematol. 1978, 10, 70–84. [Google Scholar] [PubMed]
- Rodiuc, N.; Vieira, P.; Banora, M.Y.; de Almeida Engler, J. On the track of transfer cell formation by specialized plant-parasitic nematodes. Front. Plant Sci. 2014, 5, 160. [Google Scholar] [CrossRef]
- Bartlem, D.G.; Jones, M.G.K.; Hammes, U.Z. Vascularization and nutrient delivery at root-knot nematode feeding sites in host roots. J. Exp. Bot. 2013, 65, 1789–1798. [Google Scholar] [CrossRef]
- Absmanner, B.; Stadler, R.; Hammes, U.Z. Phloem development in nematode-induced feeding sites: The implications of auxin and cytokinin. Front. Plant Sci. 2013, 4, 241. [Google Scholar] [CrossRef]
- Siddique, S.; Grundler, F.M. Parasitic nematodes manipulate plant development to establish feeding sites. Curr. Opin. Microbiol. 2018, 46, 102–108. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Jones, J.D.G. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, F.M. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 2005, 6, 973–979. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-Microbe Interactions: Shaping the Evolution of the Plant Immune Response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Li, R.; Rashotte, A.M.; Singh, N.K.; Weaver, D.B.; Lawrence, K.S.; Locy, R.D. Integrated signaling networks in plant responses to sedentary endoparasitic nematodes: A perspective. Plant Cell Rep. 2015, 34, 5–22. [Google Scholar] [CrossRef]
- Melillo, M.T.; Leonetti, P.; Bongiovanni, M.; Castagnone-Sereno, P.; Bleve-Zacheo, T. Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol. 2006, 170, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Matera, C.; Radakovic, Z.S.; Hasan, M.S.; Gutbrod, P.; Rozanska, E.; Sobczak, M.; Torres, M.A.; Grundler, F.M. Parasitic worms stimulate host NADPH oxidases to produce reactive oxygen species that limit plant cell death and promote infection. Sci. Signal. 2014, 7, ra33. [Google Scholar] [CrossRef]
- Nahar, K.; Kyndt, T.; De Vleesschauwer, D.; Höfte, M.; Gheysen, G. The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol. 2011, 157, 305–316. [Google Scholar] [CrossRef]
- Nahar, K.; Kyndt, T.; Hause, B.; Höfte, M.; Gheysen, G. Brassinosteroids suppress rice defense against root-knot nematodes through antagonism with the jasmonate pathway. Mol. Plant Microbe Interact. 2013, 26, 106–115. [Google Scholar] [CrossRef]
- Kyndt, T.; Nahar, K.; Haeck, A.; Verbeek, R.; Demeestere, K.; Gheysen, G. Interplay between Carotenoids, Abscisic Acid and Jasmonate Guides the Compatible Rice-Meloidogyne graminicola Interaction. Front. Plant Sci. 2017, 8, 951. [Google Scholar] [CrossRef]
- Yimer, H.Z.; Nahar, K.; Kyndt, T.; Haeck, A.; Van Meulebroek, L.; Vanhaecke, L.; Demeestere, K.; Hofte, M.; Gheysen, G. Gibberellin antagonizes jasmonate-induced defense against Meloidogyne graminicola in rice. New Phytol. 2018, 218, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, G.; Mitchum, M.G. Phytoparasitic Nematode Control of Plant Hormone Pathways. Plant Physiol. 2019, 179, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Lahari, Z.; Ullah, C.; Kyndt, T.; Gershenzon, J.; Gheysen, G. Strigolactones enhance root-knot nematode (Meloidogyne graminicola) infection in rice by antagonizing the jasmonate pathway. New Phytol. 2019, 224, 454–465. [Google Scholar] [CrossRef]
- Wang, G.T.; Hu, C.Y.; Zhou, J.; Liu, Y.; Cai, J.X.; Pan, C.Z.; Wang, Y.; Wu, X.D.; Shi, K.; Xia, X.J.; et al. Systemic Root-Shoot Signaling Drives Jasmonate-Based Root Defense against Nematodes. Curr. Biol. 2019, 29, 3430–3438.e4. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, F.F.; Sheo, S.J.; Zhang, H.; Li, G.P.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q.; Shil, K. Involvement of nitric oxide in the jasmonate-dependent basal defense against root-knot nematode in tomato plants. Front. Plant Sci. 2015, 6, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.C.; Liang, J.J.; Huang, H.; Yang, J.S.; Feng, J.P.; Sun, L.L.; Yang, R.; Zhao, M.J.; Wang, J.L.; Wang, S.H. Tomato defence against Meloidogyne incognita by jasmonic acid-mediated fine-tuning of kaempferol homeostasis. New Phytol. 2023, 238, 1651–1670. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Cao, H.F.; Yin, J.; Kang, L.; Ge, F. Elevated CO2 changes the interactions between nematode and tomato genotypes differing in the JA pathway. Plant Cell Environ. 2010, 33, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Wu, C.; Ahammed, G.J.; Wu, C.; Wan, C.; Chen, J. Red Light-Induced Systemic Resistance Against Root-Knot Nematode Is Mediated by a Coordinated Regulation of Salicylic Acid, Jasmonic Acid and Redox Signaling in Watermelon. Front. Plant Sci. 2018, 9, 899. [Google Scholar] [CrossRef]
- Griebel, T.; Zeier, J. Light Regulation and Daytime Dependency of Inducible Plant Defenses in Arabidopsis: Phytochrome Signaling Controls Systemic Acquired Resistance Rather Than Local Defense. Plant Physiol. 2008, 147, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.D.; Liu, M.J.; Sun, X.B.; Zhao, M.; Chow, W.S.; Sun, G.Y.; Zhang, Z.S.; Hu, Y.B. Light Suppresses Bacterial Population through the Accumulation of Hydrogen Peroxide in Tobacco Leaves Infected with Pseudomonas syringae pv. tabaci. Front. Plant Sci. 2016, 7, 512. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, M.M.; Ren, Y.; Onac, E.; Zhou, G.F.; Peng, S.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Light-induced systemic resistance in tomato plants against root-knot nematode Meloidogyne incognita. Plant Growth Regul. 2015, 76, 167–175. [Google Scholar] [CrossRef]
- Islam, S.Z.; Babadoost, M.; Bekal, S.; Lambert, K. Red Light-induced Systemic Disease Resistance against Root-knot Nematode Meloidogyne javanica and Pseudomonas syringae pv. tomato DC 3000. J. Phytopathol. 2010, 156, 708–714. [Google Scholar] [CrossRef]
- Wang, L.X.; Wu, X.T.; Xing, Q.J.; Zhao, Y.P.; Yu, B.; Ma, Y.; Wang, F.; Qi, H.Y. PIF8-WRKY42-mediated salicylic acid synthesis modulates red light induced powdery mildew resistance in oriental melon. Plant Cell Environ. 2023, 46, 1726–1742. [Google Scholar] [CrossRef]
- Jeong, R.D.; Kachroo, A.; Kachroo, P. Blue light photoreceptors are required for the stability and function of a resistance protein mediating viral defense in Arabidopsis. Plant Signal. Behav. 2010, 5, 1504–1509. [Google Scholar] [CrossRef]
- Wu, L.; Yang, H.Q. CRYPTOCHROME 1 is Implicated in Promoting R Protein-Mediated Plant Resistance to Pseudomonas syringae in Arabidopsis. Mol. Plant 2010, 3, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chu, L.; Zhang, Y.; Bian, Y.; Xiao, J.; Xu, D. HY5: A Pivotal Regulator of Light-Dependent Development in Higher Plants. Front. Plant Sci. 2021, 12, 800989. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; He, K.; Stolc, V.; Lee, H.; Figueroa, P.; Gao, Y.; Tongprasit, W.; Zhao, H.; Lee, I.; Deng, X.W. Analysis of Transcription Factor HY5 Genomic Binding Sites Revealed Its Hierarchical Role in Light Regulation of Development. Plant Cell 2007, 19, 731–749. [Google Scholar] [CrossRef]
- Bursch, K.; Toledo-Ortiz, G.; Pireyre, M.; Lohr, M.; Braatz, C.; Johansson, H. Identification of BBX proteins as rate-limiting cofactors of HY5. Nat. Plants 2020, 6, 921–928. [Google Scholar] [CrossRef]
- Catalá, R.; Medina, J.; Salinas, J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16475–16480. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Chen, X.; Wu, X.; Xiang, X.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Foyer, C.H.; et al. SlHY5 Integrates Temperature, Light, and Hormone Signaling to Balance Plant Growth and Cold Tolerance. Plant Physiol. 2018, 179, 749–760. [Google Scholar] [CrossRef]
- Moreau, M.; Degrave, A.; Vedel, R.; Bitton, F.; Patrit, O.; Renou, J.P.; Barny, M.A.; Fagard, M. EDS1 Contributes to Nonhost Resistance of Arabidopsis thaliana Against Erwinia amylovora. Mol. Plant Microbe Interact. 2012, 25, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Li, Y.; Guang, Y.L.; Lin, J.H.; Zhou, Y.; Yu, T.; Ding, F.; Wang, Y.F.; Chen, J.Y.; Zhou, Y.H.; et al. Red light induces salicylic acid accumulation by activating CaHY5 to enhance pepper resistance against Phytophthora capsici. Hortic. Res. 2023, 10, uhad213. [Google Scholar] [CrossRef]
- Kaur, H.; Manchanda, P.; Kumar, P.; Dhall, R.K.; Chhuneja, P.; Weng, Y. Genome-wide identification and characterization of parthenocarpic fruit set-related gene homologs in cucumber (Cucumis sativus L.). Sci. Rep. 2023, 13, 2403. [Google Scholar] [CrossRef]
- Giné, A.; López-ómez, M.; Vela, M.D.; Ornat, C.; Sorribas, F.J. Thermal requirements and population dynamics of root-knot nematodes on cucumber and yield losses under protected cultivation. Plant Pathol. 2015, 63, 1446–1453. [Google Scholar] [CrossRef]
- Expósito, A.; García, S.; Giné, A.; Escudero, N.; Sorribas, F.J. Cucumis metuliferus reduces Meloidogyne incognita virulence against the Mi1.2 resistance gene in a tomato-melon rotation sequence. Pest Manag. Sci. 2019, 75, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, Y.; Liu, X.; Dong, M.; Zhang, Y.; Chen, S.; Zhang, W.; Li, Y.; Tang, M.; Zhai, X.; et al. A CsMYB6-CsTRY module regulates fruit trichome initiation in cucumber. J. Exp. Bot. 2018, 69, 1887–1902. [Google Scholar] [CrossRef] [PubMed]
- Bybd, D.W.; Kirkpatrick, T.; Barker, K.R. An Improved Technique for Clearing and Staining Plant Tissues for Detection of Nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2−ΔΔ CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhao, Z.G.; Si, J.; Di, C.X.; Han, J.; An, L.Z. Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul. 2009, 59, 207–214. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ismail, A.E. Growing Jatropha curcas and Jatropha gossypiifolia as a Interculture with Sunflower for Control of Meloidogyne Javanica in Egypt. Int. J. Sustain. Agric. Res. 2014, 1, 39–44. [Google Scholar]
- Wang, T.J.; Huang, S.; Zhang, A.; Guo, P.; Liu, Y.; Xu, C.; Cong, W.; Liu, B.; Xu, Z.Y. JMJ17-WRKY40 and HY5-ABI5 modules regulate the expression of ABA-responsive genes in Arabidopsis. New Phytol. 2021, 230, 567–584. [Google Scholar] [CrossRef]
- Song, K.; Kim, H.C.; Shin, S.; Kim, K.H.; Moon, J.C.; Kim, J.Y.; Lee, B.M. Transcriptome Analysis of Flowering Time Genes under Drought Stress in Maize Leaves. Front. Plant Sci. 2017, 8, 267. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dwivedi, S.; Bhagavatula, L.; Datta, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef]
- Wu, B.; Jia, X.; Zhu, W.; Gao, Y.; Tan, K.; Duan, Y.; Chen, L.; Fan, H.; Wang, Y.; Liu, X.; et al. Light signaling regulates root-knot nematode infection and development via HY5-SWEET signaling. BMC Plant Biol. 2024, 24, 664. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Tomitaka, Y.; Abe, H.; Tsuda, S.; Futai, K.; Mizukubo, T. Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode (Meloidogyne incognita) in tomato plants (Solanum lycopersicum) after foliar treatment with methyl jasmonate. J. Plant Physiol. 2011, 168, 1084–1097. [Google Scholar] [CrossRef]
- Gleason, C.; Leelarasamee, N.; Meldau, D.; Feussner, I. OPDA Has Key Role in Regulating Plant Susceptibility to the Root-Knot Nematode Meloidogyne hapla in Arabidopsis. Front. Plant Sci. 2016, 7, 1565. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.R.; Jia, L.; Goggin, L. Effects of Jasmonate-Induced Defenses on Root-Knot Nematode Infection of Resistant and Susceptible Tomato Cultivars. J. Chem. Ecol. 2005, 31, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; You, J.; Li, C.J.H.; Hua, C.; Wang, C.L. Exogenous application of methyl jasmonate induces defence against in soybean. Nematology 2017, 19, 293–304. [Google Scholar] [CrossRef]
- Verbeek, R.E.M.; Van Buyten, E.; Alam, M.Z.; De Vleesschauwer, D.; Van Bockhaven, J.; Asano, T.; Kikuchi, S.; Haeck, A.; Demeestere, K.; Gheysen, G.; et al. Jasmonate-Induced Defense Mechanisms in the Belowground Antagonistic Interaction Between Pythium arrhenomanes and Meloidogyne graminicola in Rice. Front. Plant Sci. 2019, 10, 1515. [Google Scholar] [CrossRef]
- Naor, N.; Gurung, F.B.; Ozalvo, R.; Bucki, P.; Sanadhya, P.; Miyara, S.B. Tight regulation of allene oxide synthase (AOS) and allene oxide cyclase-3 (AOC3) promote Arabidopsis susceptibility to the root-knot nematode Meloidogyne javanica. Eur. J. Plant Pathol. 2018, 150, 149–165. [Google Scholar] [CrossRef]
- Chopra, D.; Hasan, M.S.; Matera, C.; Chitambo, O.; Mendy, B.; Mahlitz, S.V.; Naz, A.A.; Szumski, S.; Janakowski, S.; Sobczak, M.; et al. Plant parasitic cyst nematodes redirect host indole metabolism via NADPH oxidase-mediated ROS to promote infection. New Phytol. 2021, 232, 318–331. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Wei, L.H.; Kaloshian, I. Root-knot nematodes induce pattern-triggered immunity in Arabidopsis thaliana roots. New Phytol. 2016, 211, 276–287. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Li, J.; Huang, M.; E, M.; Cui, D.; Wu, G.; Li, S.; Li, Y. ELONGATED HYPOCOTYL5 Regulates Resistance to Root-Knot Nematode by Modulating Antioxidant System and Jasmonic Acid in Cucumis sativus. Antioxidants 2025, 14, 679. https://doi.org/10.3390/antiox14060679
Ma F, Li J, Huang M, E M, Cui D, Wu G, Li S, Li Y. ELONGATED HYPOCOTYL5 Regulates Resistance to Root-Knot Nematode by Modulating Antioxidant System and Jasmonic Acid in Cucumis sativus. Antioxidants. 2025; 14(6):679. https://doi.org/10.3390/antiox14060679
Chicago/Turabian StyleMa, Fusheng, Juanqi Li, Mengwei Huang, Mengyan E, Dandan Cui, Guoxiu Wu, Shengli Li, and Yang Li. 2025. "ELONGATED HYPOCOTYL5 Regulates Resistance to Root-Knot Nematode by Modulating Antioxidant System and Jasmonic Acid in Cucumis sativus" Antioxidants 14, no. 6: 679. https://doi.org/10.3390/antiox14060679
APA StyleMa, F., Li, J., Huang, M., E, M., Cui, D., Wu, G., Li, S., & Li, Y. (2025). ELONGATED HYPOCOTYL5 Regulates Resistance to Root-Knot Nematode by Modulating Antioxidant System and Jasmonic Acid in Cucumis sativus. Antioxidants, 14(6), 679. https://doi.org/10.3390/antiox14060679




