Biosynthetic Machinery to Abiotic Stress-Driven Emission: Decoding Multilayer Regulation of Volatile Terpenoids in Plants
Abstract
1. Introduction
2. Biosynthesis and Key Regulatory Networks of Volatile Terpenoids
2.1. Biosynthetic Pathways of Volatile Terpenoids
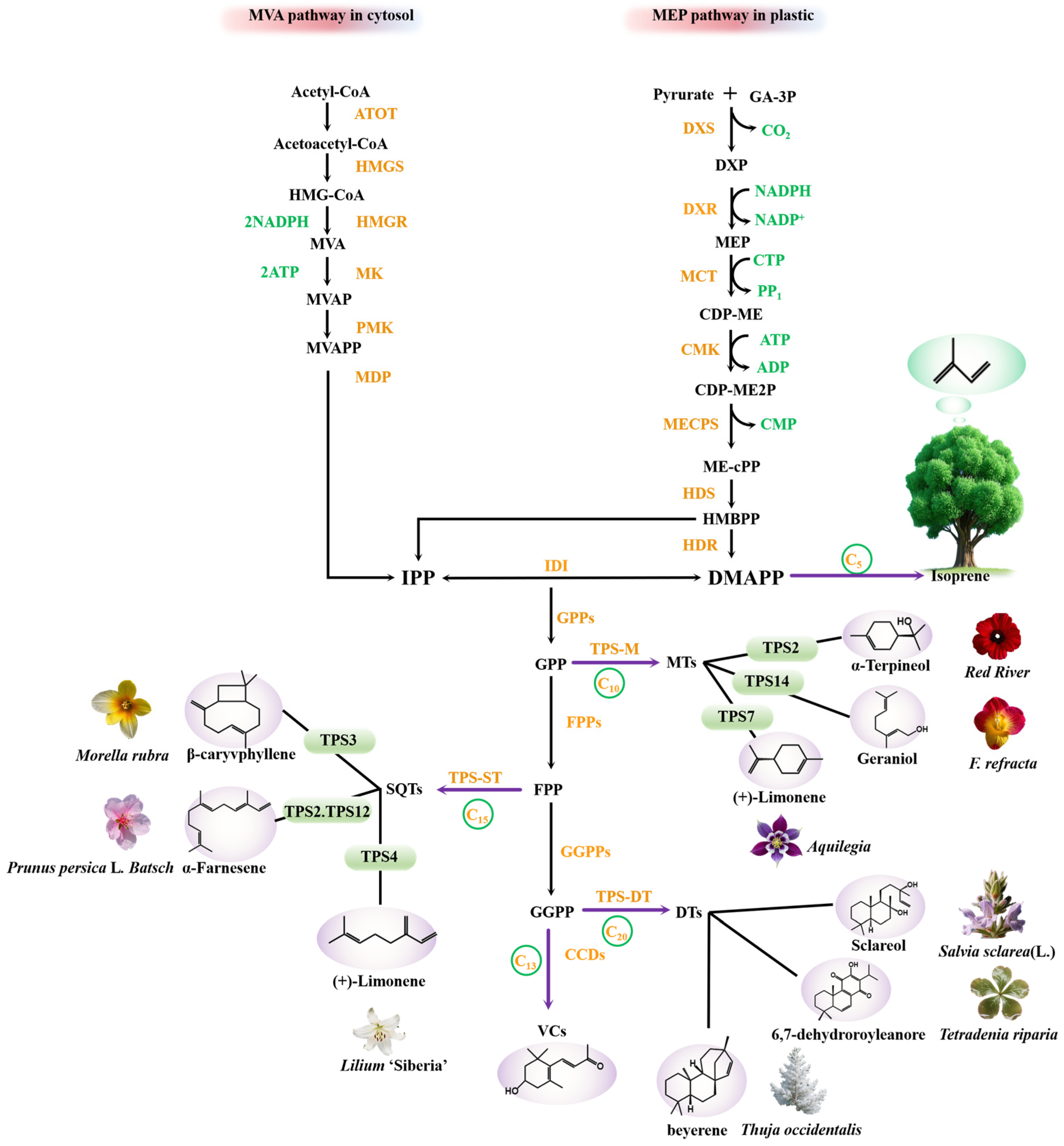
2.2. Regulatory Determinants of Volatile Terpenoid Biosynthesis
2.2.1. Key Enzymes
2.2.2. Transcription Factors
2.2.3. Plant Hormones
| Transcription Factor | Gene Name | Species Name | Regulatory Substance | Reference |
|---|---|---|---|---|
| MYB | MsMYB | Mentha spicata | Inhibits limonene and carvone biosynthesis | [92] |
| AlMYB59 | Atractylodes lancea | Promotes β-eudesmol, atractylon, and atractylone biosynthesis | [93] | |
| FhMYB21L1, FhMYB21L2 | Freesia hybrida | Involved in monoterpene and sesquiterpene biosynthesis | [82] | |
| LiMYB108 | Lilium | Promotes ocimene and linalool biosynthesis | [94] | |
| AmMYB24 | Antirrhinum majus | Promotes ocimene biosynthesis | [95] | |
| JsMYB108, JsMYB305 | Jasminum sambac | Involved in monoterpene and sesquiterpene biosynthesis | [96] | |
| MYB | Saussurea lappa | Involved in sesquiterpene lactone biosynthesis | [97] | |
| HcMYB1, HcMYB2 | Hedychium coronarium | Promotes linalool biosynthesis | [98] | |
| SlMYB75 | Solanum lycopersicum L. | Promotes terpene volatile biosynthesis | [66] | |
| HcMYB | Hedychium coronarium | Promotes terpene biosynthesis | [99] | |
| OfMYB1R114, 70, 201 | Osmanthus fragrans | Promotes β-ionone biosynthesis | [65] | |
| CsMYB68, 147, 148, 193 | Camellia sinensis | Promotes monoterpene and sesquiterpene biosynthesis | [100] | |
| MYB24 | Vitis vinifera cv. ‘Béquignol’ | Involved in monoterpene biosynthesis | [101] | |
| MYB5 | Rosa rugosa | Involved in sesquiterpene biosynthesis | [102] | |
| bHLH | MYC2 | Arabidopsis thaliana | Promotes sesquiterpene biosynthesis | [69] |
| PbbHLH4 | Phalaenopsis orchids | Promotes monoterpene biosynthesis | [103] | |
| CpMYC2, CpbHLH13 | Chimonanthus praecox L. | Promotes β-caryophyllene and linalool biosynthesis | [74] | |
| bHLH35 | Osmanthus fragrans | Promotes linalool and linalool oxide biosynthesis | [104] | |
| AabHLH2, 3 | Artemisia annua L. | Promotes sesquiterpene lactone biosynthesis | [105] | |
| LibHLH22, 63 | Lilium ‘Siberia’ | Promotes linalool and ocimene biosynthesis | [106] | |
| SlMYC1 | Solanum lycopersicum | Promotes monoterpene biosynthesis in leaves while inhibiting sesquiterpene biosynthesis in stem trichomes | [107] | |
| PpbHLH1 | Prunus persica L. | Promotes linalool biosynthesis | [108] | |
| LaMYC4 | L. angustifolia | Promotes volatile organic compound biosynthesis | [109] | |
| AP2/ERF | MdERF3 | Malus domestica | Promotes α-farnesene biosynthesis | [110] |
| CitERF71 | Citrus sinensis Osbeck | Promotes E-geraniol biosynthesis | [59] | |
| PpERF5,7 | Prunus persica | Promotes linalool biosynthesis | [111] | |
| WRKY | AaWRKY40 | Artemisia annua | Promotes terpene biosynthesis | [112] |
| CrWRKY1 | Catharanthus roseus | Promotes terpene indole alkaloid biosynthesis | [79] | |
| OfWRKY139 | Sweet Osmanthus | Promotes monoterpene biosynthesis | [113] | |
| NAC | AaNAC2, 3, 4 | Actinidia chinensis Planch | Promotes monoterpene biosynthesis | [114] |
| GoNAC42 | Gossypium hirsutum | Promotes monoterpene biosynthesis | [115] | |
| NAC-NOR | Solanum lycopersicum | Promotes volatile organic compound biosynthesis | [116] |
3. Regulatory Mechanisms of Volatile Terpenoids in Abiotic Stress Responses
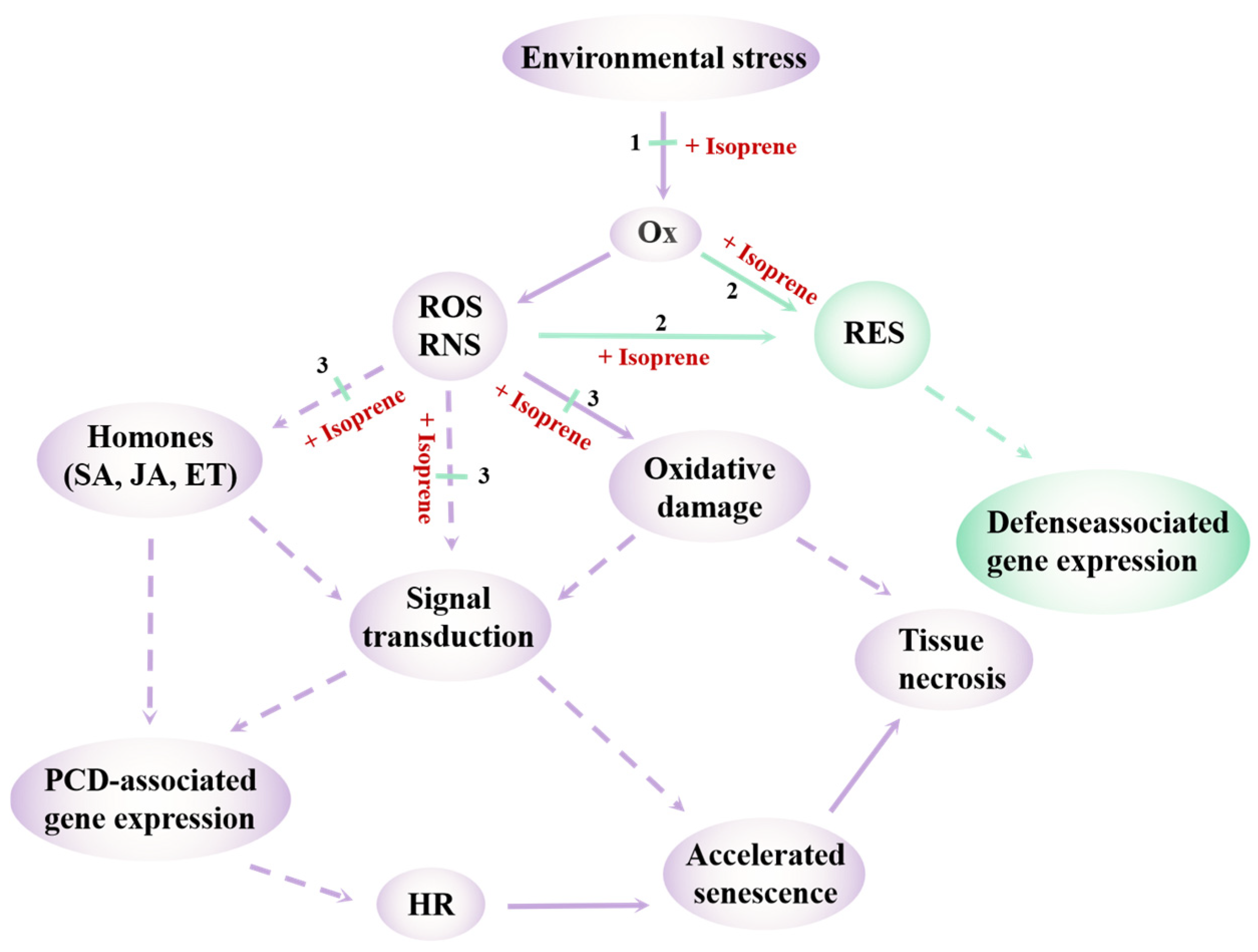
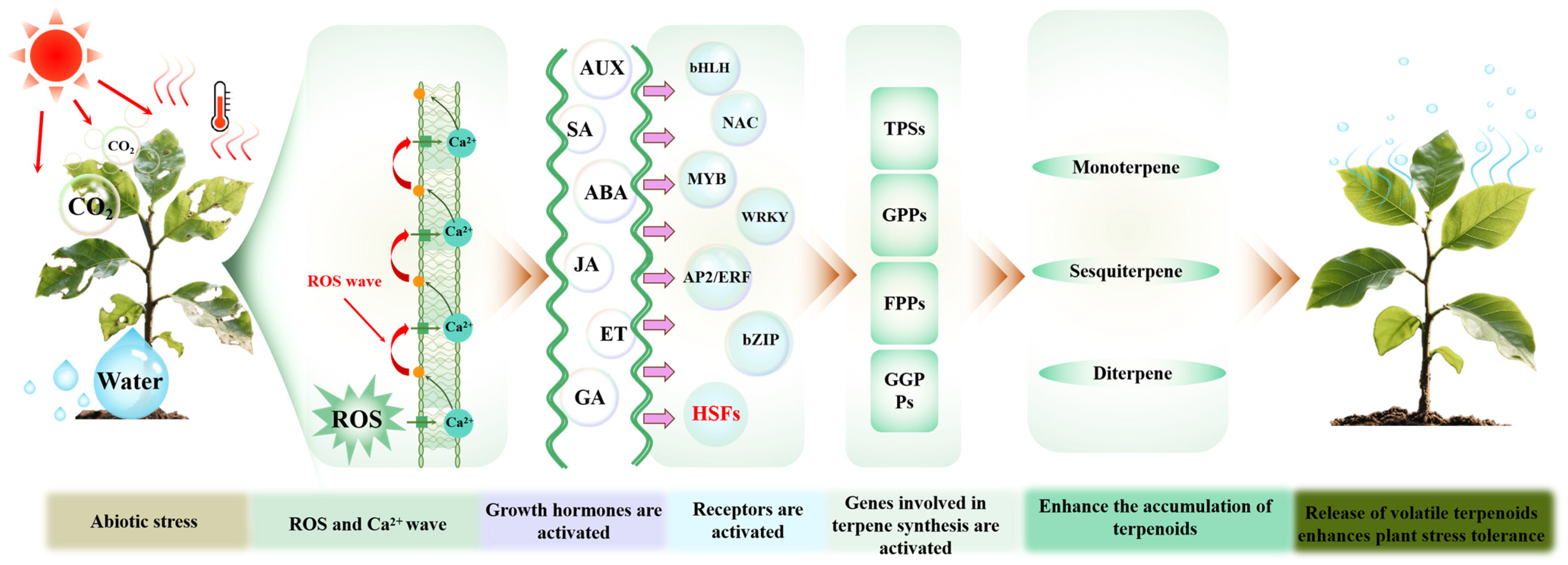
3.1. Protective Roles and Mechanisms of Volatile Terpenoids Under Abiotic Stress
3.2. Temperature Stress
3.3. CO2 Concentration
3.4. Light
3.5. Water
3.6. Mechanical Damage
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdallah, I.I.; Quax, W.J. A Glimpse into the Biosynthesis of Terpenoids. In Proceedings of the International Conference on Natural Resources and Life Sciences (NRLS-2016); KnE Life Sciences: Dubai, UAE, 2017; pp. 81–98. [Google Scholar]
- Reynolds, W.F.; Enriquez, R.G. Chapter 7 Terpenes: Mono-, sesqui-, and higher terpenes. In Modern NMR Approaches to the Atructure Elucidation of Natural Products; Williams, A.J., Martin, G.E., Rovnyak, D., Eds.; The Royal Society of Chemistry: London, UK, 2017; Volume 2, pp. 251–274. [Google Scholar]
- Holopainen, J.K.; Himanen, S.J.; Yuan, J.; Chen, F.; Stewart, C.N. Ecological functions of terpenoids in changing climates. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2913–2940. [Google Scholar]
- Buchmann, S.L.; Nabhan, G.P. The Forgotten Pollinators; Island Press: Washington, DC, USA, 1996. [Google Scholar]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A.; Pichersky, E. Floral volatiles from Clarkia breweri and C. concinna (Onagraceae): Recent evolution of floral scent and moth pollination. Plant Syst. Evol. 1995, 194, 55–67. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plantbiol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, X.; Campos-Herrera, R.; Jaffuel, G.; Röder, G.; Turlings, T.C. Diffusion of the maize root signal (E)-β-caryophyllene in soils of different textures and the effects on the migration of the entomopathogenic nematode Heterorhabditis megidis. Rhizosphere 2017, 3, 53–59. [Google Scholar] [CrossRef]
- Arimura, G.-i.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Chen, X.; Yeh, S. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 2001, 125, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Arimura, G.; Ohnishi, T. ‘Hidden’terpenoids in plants: Their biosynthesis, localization and ecological roles. Plant Cell Physiol. 2017, 58, 1615–1621. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Pulido, P.; Perello, C.; Rodriguez-Concepcion, M. New insights into plant isoprenoid metabolism. Mol. Plant 2012, 5, 964–967. [Google Scholar] [CrossRef]
- Newman, J.D.; Chappell, J. Isoprenoid biosynthesis in plants: Carbon partitioning within the cytoplasmic pathway. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Schauvinhold, I.; Larson, M.; Xu, R.; Charbonneau, A.L.; Schmidt, A.; Wilkerson, C.; Last, R.L.; Pichersky, E. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc. Natl. Acad. Sci. USA 2009, 106, 10865–10870. [Google Scholar] [CrossRef] [PubMed]
- Kuzuyama, T. Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci. Biotechnol. Biochem. 2002, 66, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Schwender, J.; Disch, A.; Rohmer, M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997, 400, 271–274. [Google Scholar] [CrossRef]
- Barja, M.V.; Rodriguez-Concepcion, M. Plant geranylgeranyl diphosphate synthases: Every (gene) family has a story. Abiotech 2021, 2, 289–298. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identifcation and characterization of terpene synthase genes accounting for volatile terpene emissions in flowers of Freesia x hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef]
- Bao, T.; Kimani, S.; Li, Y.; Li, H.; Yang, S.; Zhang, J.; Wang, Q.; Wang, Z.; Ning, G.; Wang, L. Allelic variation of terpene synthases drives terpene diversity in the wild species of the Freesia genus. Plant Physiol. 2023, 192, 2419–2435. [Google Scholar] [CrossRef]
- Yang, S.; Wang, N.; Kimani, S.; Li, Y.; Bao, T.; Ning, G.; Li, L.; Liu, B.; Wang, L.; Gao, X. Characterization of Terpene synthase variation in flowers of wild Aquilegia species from Northeastern Asia. Hortic. Res. 2022, 9, uhab020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Zhu, Y.; Zhao, L.; Ju, P.; Wang, G.; Zhou, C.; Zhu, C.; Jia, H.; Jiao, Y. MrTPS3 and MrTPS20 are responsible for β-caryophyllene and α-pinene production, respectively, in red bayberry (Morella rubra). Front. Plant Sci. 2022, 12, 798086. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Li, R.; Su, Y.; Li, X.; Zhang, B. Functional genomics reveals functions of terpene synthases for volatile terpene formation in peach. Food Qual. Saf. 2024, 8, fyae027. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Zhou, Y.; Ashraf, U.; Li, X.; Yu, Y.; Yue, Y.; Ahmad, K.W.; Yu, R.; Fan, Y. Molecular cloning, characterization and expression analysis of LoTPS2 and LoTPS4 involved in floral scent formation in oriental hybrid Lilium variety ‘Siberia’. Phytochemistry 2020, 173, 112294. [Google Scholar] [CrossRef]
- Caniard, A.; Zerbe, P.; Legrand, S.; Cohade, A.; Valot, N.; Magnard, J.-L.; Bohlmann, J.; Legendre, L. Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant Biol. 2012, 12, 119. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Rodrigues, F.; Amorin, A.C.L.; de Rezende, C.M.; Soković, M.; Tešević, V.; Vučković, I.; Krstić, G.; Cortez, L.E.R.; Colauto, N.B. New natural diterpene-type abietane from Tetradenia riparia essential oil with cytotoxic and antioxidant activities. Molecules 2014, 19, 514–524. [Google Scholar] [CrossRef]
- Tsiri, D.; Graikou, K.; Pobłocka-Olech, L.; Krauze-Baranowska, M.; Spyropoulos, C.; Chinou, I.J.M. Chemosystematic value of the essential oil composition of Thuja species cultivated in Poland—Antimicrobial activity. Molecules 2009, 14, 4707–4715. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Suzuki, M.; Kamide, Y.; Nagata, N.; Seki, H.; Ohyama, K.; Kato, H.; Masuda, K.; Sato, S.; Kato, T.; Tabata, S. Loss of function of 3-hydroxy-3-methylglutaryl coenzyme A reductase 1 (HMG1) in Arabidopsis leads to dwarfing, early senescence and male sterility, and reduced sterol levels. Plant J. 2004, 37, 750–761. [Google Scholar] [CrossRef]
- Dai, Z.; Cui, G.; Zhou, S.-F.; Zhang, X.; Huang, L. Cloning and characterization of a novel 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Salvia miltiorrhiza involved in diterpenoid tanshinone accumulation. J. Plant Physiol. 2011, 168, 148–157. [Google Scholar] [CrossRef]
- Rao, S.; Meng, X.; Liao, Y.; Yu, T.; Cao, J.; Tan, J.; Xu, F.; Cheng, S. Characterization and functional analysis of two novel 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes (GbHMGR2 and GbHMGR3) from Ginkgo biloba. Sci. Rep. 2019, 9, 14109. [Google Scholar] [CrossRef]
- Wei, H.; Xu, C.; Movahedi, A.; Sun, W.; Li, D.; Zhuge, Q. Characterization and function of 3-hydroxy-3-methylglutaryl-CoA reductase in Populus trichocarpa: Overexpression of PtHMGR enhances terpenoids in transgenic poplar. Front. Plant Sci. 2019, 10, 1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, Y.; Lou, Y.; Niu, J.; Yin, C.; Zhao, J.; Du, W.; Yue, A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase genes from Glycine max regulate plant growth and isoprenoid biosynthesis. Sci. Rep. 2023, 13, 3902. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Guan, L.; Yu, K.; Haider, M.S.; Nasim, M.; Liu, Z.; Li, T.; Zhang, K.; Jiu, S.; Jia, H. Expressional diversity of grapevine 3-Hydroxy-3-methylglutaryl-CoA reductase (VvHMGR) in different grapes genotypes. BMC Plant Biol. 2021, 21, 279. [Google Scholar] [CrossRef]
- Shang, J.; Feng, D.; Liu, H.; Niu, L.; Li, R.; Li, Y.; Chen, M.; Li, A.; Liu, Z.; He, Y. Evolution of the biosynthetic pathways of terpene scent compounds in roses. Curr. Biol. 2024, 34, 3550–3563.8. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lyu, S.; Chen, D.; Lin, Y.; Chen, J.; Chen, G.; Ye, N. Volatiles emitted at different flowering stages of Jasminum sambac and expression of genes related to α-farnesene biosynthesis. Molecules 2017, 22, 546. [Google Scholar] [CrossRef]
- Carretero-Paulet, L.; Ahumada, I.; Cunillera, N.; Rodrıguez-Concepción, M.; Ferrer, A.; Boronat, A.; Campos, N. Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Plant Physiol. 2002, 129, 1581–1591. [Google Scholar] [CrossRef]
- Cao, P.-H.; Di, W.; Su, G.; Xi, L.; Qiao, Z.-y.; Xie, Y.-L.; Dong, M.-h.; Du, T.-x.; Zhang, X.; Zhang, R. OsDXR interacts with OsMORF1 to regulate chloroplast development and the RNA editing of chloroplast genes in rice. J. Integr. Agric. 2023, 22, 669–678. [Google Scholar] [CrossRef]
- Carretero-Paulet, L.; Cairo, A.; Botella-Pavía, P.; Besumbes, O.; Campos, N.; Boronat, A.; Rodríguez-Concepción, M. Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol. Biol. 2006, 62, 683–695. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Chen, Y.; Gao, M.; Wu, L.; Wang, Y. Overexpression of 1-deoxy-D-xylulose-5-phosphate reductoisomerase enhances the monoterpene content in Litsea cubeba. For. Res. 2023, 3, 11. [Google Scholar] [CrossRef]
- Lange, B.M.; Croteau, R. Isoprenoid biosynthesis via a mevalonate-independent pathway in plants: Cloning and heterologous expression of 1-deoxy-D-xylulose-5-phosphate reductoisomerase from peppermint. Arch. Biochem. Biophys. 1999, 365, 170–174. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Croteau, R.B. Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc. Natl. Acad. Sci. USA 2001, 98, 8915–8920. [Google Scholar] [CrossRef]
- García-Alcázar, M.; Giménez, E.; Pineda, B.; Capel, C.; García-Sogo, B.; Sánchez, S.; Yuste-Lisbona, F.J.; Angosto, T.; Capel, J.; Moreno, V. Albino T-DNA tomato mutant reveals a key function of 1-deoxy-D-xylulose-5-phosphate synthase (DXS1) in plant development and survival. Sci. Rep. 2017, 7, 45333. [Google Scholar] [CrossRef]
- Enfissi, E.M.; Fraser, P.D.; Lois, L.M.; Boronat, A.; Schuch, W.; Bramley, P.M. Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol. J. 2005, 3, 17–27. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.J.; Green, S.; Atkinson, R.G. Floral sesquiterpenes and their synthesis in dioecious kiwifruit. Plant Signal. Behav. 2010, 5, 61–63. [Google Scholar] [CrossRef]
- Emanuelli, F.; Battilana, J.; Costantini, L.; Le Cunff, L.; Boursiquot, J.-M.; This, P.; Grando, M.S. A candidate gene association study on muscat flavor in grapevine (Vitis vinifera L.). BMC Plant Biol. 2010, 10, 241. [Google Scholar] [CrossRef]
- Gong, Y.-f.; Liao, Z.-h.; Guo, B.-h.; Sun, X.-f.; Tang, K.-x. Molecular cloning and expression profile analysis of Ginkgo biloba DXS gene encoding 1-deoxy-D-xylulose 5-phosphate synthase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Planta Medica 2006, 72, 329–335. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, D.; Wang, H.; Wu, B.; Cheng, J.; Zhang, B. Identification of UGT85A glycosyltransferases associated with volatile conjugation in grapevine (Vitis vinifera× Vitis labrusca). Hortic. Plant J. 2023, 9, 1095–1107. [Google Scholar] [CrossRef]
- Caputi, L.; Lim, E.K.; Bowles, D.J. Discovery of new biocatalysts for the glycosylation of terpenoid scaffolds. Chem.-A Eur. J. 2008, 14, 6656–6662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2020, 226, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, C.; Zhang, G.; Teixeira da Silva, J.A.; Duan, J. Genome-wide identification and expression profile of TPS gene family in Dendrobium officinale and the role of DoTPS10 in linalool biosynthesis. Int. J. Mol. Sci. 2020, 21, 5419. [Google Scholar] [CrossRef]
- Wei, C.; Li, M.; Cao, X.; Jin, Z.; Zhang, C.; Xu, M.; Chen, K.; Zhang, B. Linalool synthesis related PpTPS1 and PpTPS3 are activated by transcription factor PpERF61 whose expression is associated with DNA methylation during peach fruit ripening. Plant Sci. 2022, 317, 111200. [Google Scholar] [CrossRef]
- Yang, S.-M.; Chu, H.-Y.; Wang, Y.-X.; Guo, B.-L.; An, T.-Y.; Shen, Q. Analysis of monoterpene biosynthesis and functional TPSs of Perilla frutescens based on transcriptome and metabolome. Med. Plant Biol. 2024, 3, e017. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Shen, S.; Yin, X.; Klee, H.; Zhang, B.; Chen, K. Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit. J. Exp. Bot. 2017, 68, 4929–4938. [Google Scholar] [CrossRef] [PubMed]
- Vom Endt, D.; Kijne, J.W.; Memelink, J. Transcription factors controlling plant secondary metabolism: What regulates the regulators? Phytochemistry 2002, 61, 107–114. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.-Z.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.; Samaha, R. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, B.-K.; Yoon, I.-S.; Kim, K.-H.; Kwon, T.-R. Salt tolerance in rice: Focus on mechanisms and approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Gibalová, A.; Reňák, D.; Matczuk, K.; Dupl’áková, N.; Cháb, D.; Twell, D.; Honys, D. AtbZIP34 is required for Arabidopsis pollen wall patterning and the control of several metabolic pathways in developing pollen. Plant Mol. Biol. 2009, 70, 581–601. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Mol. Biol. Of Plants 2013, 19, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ding, W.; Wu, X.; Wang, L.; Yang, X.; Yue, Y. Insights into the MYB-related transcription factors involved in regulating floral aroma synthesis in Sweet Osmanthus. Front. Plant Sci. 2022, 13, 765213. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Di, T.; Chen, X.; Zheng, T.; Sun, W.; Yang, M.; Zhou, M.; Shen, Z.; Chen, H.; Su, N. CbMYB108 integrates the regulation of diterpene biosynthesis and trichome development in Conyza blinii against UV-B. Plant Cell Environ. 2024, 47, 1300–1318. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Ma, B.; Li, Y.-Y.; Han, M.-Z.; Wu, J.; Zhou, X.-F.; Tian, J.; Wang, W.-H.; Leng, P.-S.; Hu, Z.-H. Transcriptome analysis identifies key gene LiMYB305 involved in monoterpene biosynthesis in Lilium ‘Siberia’. Front. Plant Sci. 2022, 13, 1021576. [Google Scholar] [CrossRef]
- Hong, G.-J.; Xue, X.-Y.; Mao, Y.-B.; Wang, L.-J.; Chen, X.-Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef]
- Guo, A.; He, K.; Liu, D.; Bai, S.; Gu, X.; Wei, L.; Luo, J. DATF: A database of Arabidopsis transcription factors. Bioinformatics 2005, 21, 2568–2569. [Google Scholar] [CrossRef]
- Du, M.; Zhao, J.; Tzeng, D.T.; Liu, Y.; Deng, L.; Yang, T.; Zhai, Q.; Wu, F.; Huang, Z.; Zhou, M. MYC2 orchestrates a hierarchical transcriptional cascade that regulates jasmonate-mediated plant immunity in tomato. Plant Cell 2017, 29, 1883–1906. [Google Scholar] [CrossRef]
- Ji, Y.; Xiao, J.; Shen, Y.; Ma, D.; Li, Z.; Pu, G.; Li, X.; Huang, L.; Liu, B.; Ye, H. Cloning and characterization of AabHLH1, a bHLH transcription factor that positively regulates artemisinin biosynthesis in Artemisia annua. Plant Cell Physiol. 2014, 55, 1592–1604. [Google Scholar] [CrossRef]
- Xu, Q.; He, Y.; Yan, X.; Zhao, S.; Zhu, J.; Wei, C. Unraveling a crosstalk regulatory network of temporal aroma accumulation in tea plant (Camellia sinensis) leaves by integration of metabolomics and transcriptomics. Environ. Exp. Bot. 2018, 149, 81–94. [Google Scholar] [CrossRef]
- Aslam, M.Z.; Lin, X.; Li, X.; Yang, N.; Chen, L. Molecular cloning and functional characterization of CpMYC2 and CpBHLH13 transcription factors from wintersweet (Chimonanthus praecox L.). Plants 2020, 9, 785. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Zhang, W.; Li, J.; Han, M.; Bai, H.; Li, H.; Shi, L. LaMYC7, a positive regulator of linalool and caryophyllene biosynthesis, confers plant resistance to Pseudomonas syringae. Hortic. Res. 2024, 11, uhae044. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Liao, Y.-C.; Lv, F.-F.; Zhang, Z.; Sun, P.-W.; Gao, Z.-H.; Hu, K.-P.; Sui, C.; Jin, Y.; Wei, J.-H. Transcription factor AsMYC2 controls the jasmonate-responsive expression of ASS1 regulating sesquiterpene biosynthesis in Aquilaria sinensis (Lour.) Gilg. Plant Cell Physiol. 2017, 58, 1924–1933. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 2015, 167, 295–306. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, S.R.; Dwivedi, V.; Rai, A.; Pal, S.; Shasany, A.K.; Nagegowda, D.A. A WRKY transcription factor from Withania somnifera regulates triterpenoid withanolide accumulation and biotic stress tolerance through modulation of phytosterol and defense pathways. New Phytol. 2017, 215, 1115–1131. [Google Scholar] [CrossRef]
- Suttipanta, N.; Pattanaik, S.; Kulshrestha, M.; Patra, B.; Singh, S.K.; Yuan, L. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2011, 157, 2081–2093. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Wang, J.-W.; Wang, S.; Wang, J.-Y.; Chen, X.-Y. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-A. Plant Physiol. 2004, 135, 507–515. [Google Scholar] [CrossRef]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Gao, F.; Jin, W.; Li, S.; Kimani, S.; Yang, S.; Bao, T.; Gao, X.; Wang, L. MYB21 interacts with MYC2 to control the expression of terpene synthase genes in flowers of Freesia hybrida and Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 4140–4158. [Google Scholar] [CrossRef]
- Wang, X.y.; Zhu, N.n.; Yang, J.s.; Zhou, D.; Yuan, S.t.; Pan, X.j.; Jiang, C.x.; Wu, Z.g. Environment. CwJAZ4/9 negatively regulates jasmonate-mediated biosynthesis of terpenoids through interacting with CwMYC2 and confers salt tolerance in Curcuma wenyujin. Plant Cell Environ. 2024, 47, 3090–3110. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, T.; Dong, J.; Li, W.; Ma, X.; Li, J.; Fang, Y.; Chen, K.; Zhang, K. Regulation of the main terpenoids biosynthesis and accumulation in fruit trees. Hortic. Plant J. 2024, in press. [Google Scholar] [CrossRef]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.-F.; Sharon, M.; Browse, J. Jasmonate perception by inositol-phosphate-potentiated COI1–JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, Y.; Zhang, L.; Ji, Y.; Tan, D.; Yuan, H.; Wang, A. The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 2017, 29, 1316–1334. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, S.; Yu, W.; Ehsan, S.; Zhang, Y.; Jia, H.; Fang, J. Jasmonate increases terpene synthase expression, leading to strawberry resistance to Botrytis cinerea infection. Plant Cell Rep. 2022, 41, 1243–1260. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, L.; Ma, K.; Wang, W.; Lv, H.; Gao, M.; Wang, X.; Zhang, X.; Ren, S.; Zhang, N. The jasmonate-induced bHLH gene SlJIG functions in terpene biosynthesis and resistance to insects and fungus. J. Integr. Plant Biol. 2022, 64, 1102–1115. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Caarls, L.; Vos, I.A.; Pieterse, C.M.; Van Wees, S.C. Ethylene: Traffic controller on hormonal crossroads to defense. Plant Physiol. 2015, 169, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Wei, Y.; Li, X.-Y.; Zhang, H.-M.; Meng, X.; Duan, C.-Q.; Pan, Q.-H. Ethylene-responsive VviERF003 modulates glycosylated monoterpenoid synthesis by upregulating VviGT14 in grapes. Hortic. Res. 2024, 11, uhae065. [Google Scholar] [CrossRef]
- Yuan, J.; Sun, K.; Deng-Wang, M.-Y.; Dai, C.-C. The mechanism of ethylene signaling induced by endophytic fungus Gilmaniella sp. AL12 mediating sesquiterpenoids biosynthesis in Atractylodes lancea. Front. Plant Sci. 2016, 7, 361. [Google Scholar] [CrossRef]
- Reddy, V.A.; Wang, Q.; Dhar, N.; Kumar, N.; Venkatesh, P.N.; Rajan, C.; Panicker, D.; Sridhar, V.; Mao, H.Z.; Sarojam, R. Spearmint R2R3-MYB transcription factor Ms MYB negatively regulates monoterpene production and suppresses the expression of geranyl diphosphate synthase large subunit (Ms GPPS. LSU). Plant Biotechnol. J. 2017, 15, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, S.; Sun, J.; Li, X.; Wang, H.; Guo, X.; Wang, Y.; Jiang, D.; Lyu, C.; Kang, C. Genome resequencing reveals the genetic basis of population evolution, local adaptation, and rewiring of the rhizome metabolome in Atractylodes lancea. Hortic. Res. 2024, 11, uhae167. [Google Scholar] [CrossRef]
- Yun-Yao, Y.; Xi, Z.; Ming-Zheng, H.; Zeng-Hui, H.; Jing, W.; Nan, M.; Ping-Sheng, L.; Xiao-Feng, Z. LiMYB108 is involved in floral monoterpene biosynthesis induced by light intensity in Lilium ‘Siberia’. Plant Cell Rep. 2023, 42, 763–773. [Google Scholar] [CrossRef]
- Han, J.; Li, T.; Wang, X.; Zhang, X.; Bai, X.; Shao, H.; Wang, S.; Hu, Z.; Wu, J.; Leng, P. AmMYB24 regulates floral terpenoid biosynthesis induced by blue light in snapdragon flowers. Front. Plant Sci. 2022, 13, 885168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, Y.; He, X.; Wang, Y.; Lv, M.; WU BH, C.Q. Cloning of JsMYB108 and JsMYB305 and analysis of their activation on TPS gene in Jasminum sambac. Chin. J. Trop. Crops 2021, 42, 1539–1548. [Google Scholar]
- Thakur, V.; Bains, S.; Pathania, S.; Sharma, S.; Kaur, R.; Singh, K. Comparative transcriptomics reveals candidate transcription factors involved in costunolide biosynthesis in medicinal plant-Saussurea lappa. Int. J. Biol. Macromol. 2020, 150, 52–67. [Google Scholar] [CrossRef]
- Ke, Y.; Abbas, F.; Zhou, Y.; Yu, R.; Fan, Y. Auxin-responsive R2R3-MYB transcription factors HcMYB1 and HcMYB2 activate volatile biosynthesis in Hedychium coronarium flowers. Front. Plant Sci. 2021, 12, 710826. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Ke, Y.; Zhou, Y.; Yu, Y.; Waseem, M.; Ashraf, U.; Wang, C.; Wang, X.; Li, X.; Yue, Y. Genome-wide analysis reveals the potential role of MYB transcription factors in floral scent formation in Hedychium coronarium. Front. Plant Sci. 2021, 12, 623742. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xia, E.; Fu, J.; Xu, Y.; Zhao, X.; Tong, W.; Tang, Q.; Tadege, M.; Fernie, A.R.; Zhao, J. Diverse roles of MYB transcription factors in regulating secondary metabolite biosynthesis, shoot development, and stress responses in tea plants (Camellia sinensis). Plant J. 2022, 110, 1144–1165. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Z.; Ferrier, T.; Orduña, L.; Santiago, A.; Peris, A.; Wong, D.C.; Kappel, C.; Savoi, S.; Loyola, R. MYB24 orchestrates terpene and flavonol metabolism as light responses to anthocyanin depletion in variegated grape berries. Plant Cell 2023, 35, 4238–4265. [Google Scholar] [CrossRef]
- Wang, Q.; Du, B.; Bai, Y.; Chen, Y.; Li, F.; Du, J.; Wu, X.; Yan, L.; Bai, Y.; Chai, G. Saline-alkali stress affects the accumulation of proanthocyanidins and sesquiterpenoids via the MYB5-ANR/TPS31 cascades in the rose petals. Hortic. Res. 2024, 11, uhae243. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Hung, Y.-C.; Tsai, W.-C.; Chen, W.-H.; Chen, H.-H. PbbHLH4 regulates floral monoterpene biosynthesis in Phalaenopsis orchids. J. Exp. Bot. 2018, 69, 4363–4377. [Google Scholar] [CrossRef]
- Han, Y.; Lu, M.; Yue, S.; Li, K.; Dong, M.; Liu, L.; Wang, H.; Shang, F. Comparative methylomics and chromatin accessibility analysis in Osmanthus fragrans uncovers regulation of genic transcription and mechanisms of key floral scent production. Hortic. Res. 2022, 9, uhac096. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, H.; Xie, L.; Hao, X.; Kayani, S.-I.; Liu, H.; Qin, W.; Chen, T.; Pan, Q.; Liu, P. Basic helix-loop-helix transcription factors AabHLH2 and AabHLH3 function antagonistically with AaMYC2 and are negative regulators in artemisinin biosynthesis. Front. Plant Sci. 2022, 13, 885622. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guo, Z.; Zhong, J.; Liang, Y.; Zhang, P.; Sun, M. The LibHLH22 and LibHLH63 from Lilium ‘Siberia’can positively regulate volatile terpenoid biosynthesis. Horticulturae 2023, 9, 459. [Google Scholar] [CrossRef]
- Xu, J.; van Herwijnen, Z.O.; Dräger, D.B.; Sui, C.; Haring, M.A.; Schuurink, R.C. SlMYC1 regulates type VI glandular trichome formation and terpene biosynthesis in tomato glandular cells. Plant Cell 2018, 30, 2988–3005. [Google Scholar] [CrossRef]
- Wei, C.; Liu, H.; Cao, X.; Zhang, M.; Li, X.; Chen, K.; Zhang, B. Synthesis of flavour-related linalool is regulated by PpbHLH1 and associated with changes in DNA methylation during peach fruit ripening. Plant Biotechnol. J. 2021, 19, 2082–2096. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, W.; Li, J.; Wang, D.; Bai, H.; Li, H.; Shi, L. The transcription factor LaMYC4 from lavender regulates volatile terpenoid biosynthesis. BMC Plant Biol. 2022, 22, 289. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.; Zhang, M.; Liu, S.; Hao, Y.; Zhang, Y. MdMYC2 and MdERF3 positively co-regulate α-farnesene biosynthesis in apple. Front. Plant Sci. 2020, 11, 512844. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Miao, Y.; Deng, L.; Zhang, B.; Meng, J.; Wang, Y.; Pan, L.; Niu, L.; Liu, H. Interaction between PpERF5 and PpERF7 enhances peach fruit aroma by upregulating PpLOX4 expression. Plant Physiol. Biochem. 2022, 185, 378–389. [Google Scholar] [CrossRef]
- De Paolis, A.; Caretto, S.; Quarta, A.; Di Sansebastiano, G.-P.; Sbrocca, I.; Mita, G.; Frugis, G.J.P. Genome-wide identification of WRKY genes in Artemisia annua: Characterization of a putative ortholog of AtWRKY40. Plants 2020, 9, 1669. [Google Scholar] [CrossRef]
- Ding, W.; Ouyang, Q.; Li, Y.; Shi, T.; Li, L.; Yang, X.; Ji, K.; Wang, L.; Yue, Y. Genome-wide investigation of WRKY transcription factors in sweet osmanthus and their potential regulation of aroma synthesis. Tree Physiol. 2020, 40, 557–572. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.J.; Chen, X.; Wang, M.Y.; Matich, A.J.; Perez, R.L.; Allan, A.C.; Green, S.A.; Atkinson, R.G. Natural variation in monoterpene synthesis in kiwifruit: Transcriptional regulation of terpene synthases by NAC and ETHYLENE-INSENSITIVE3-like transcription factors. Plant Physiol. 2015, 167, 1243–1258. [Google Scholar] [CrossRef]
- Lin, J.-L.; Chen, L.; Wu, W.-K.; Guo, X.-X.; Yu, C.-H.; Xu, M.; Nie, G.-B.; Dun, J.-l.; Li, Y.; Xu, B.; et al. Single-cell RNA sequencing reveals a hierarchical transcriptional regulatory network of terpenoid biosynthesis in cotton secretory glandular cells. Mol. Plant 2023, 16, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lin, Y.; Xu, M.; Bian, H.; Zhang, C.; Wang, J.; Wang, H.; Xu, Y.; Niu, Q.; Zuo, J. The role and interaction between transcription factor NAC-NOR and DNA demethylase SlDML2 in the biosynthesis of tomato fruit flavor volatiles. New Phytol. 2022, 235, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef]
- Pooja, G.; Shweta, S.; Patel, P. Oxidative stress and free radicals in disease pathogenesis: A review. Discov. Med. 2025, 2, 104. [Google Scholar] [CrossRef]
- Ravi, B.; Foyer, C.H.; Pandey, G.K. The integration of reactive oxygen species (ROS) and calcium signalling in abiotic stress responses. Plant Cell Environ. 2023, 46, 1985–2006. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Azarabadi, S.; Abdollahi, H.; Torabi, M.; Salehi, Z.; Nasiri, J. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur. J. Plant Pathol. 2017, 147, 279–294. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Liu, J.; Li, M.; Farooq, T.H.; Ma, X.; Yan, X.; Wu, P. Differential effects of exogenous VOCs on the growth and stress responses of Cunninghamia lanceolata seedlings under low phosphorus. BMC Plant Biol. 2025, 25, 299. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhao, M.; Jing, T.; Zhang, M.; Lu, M.; Yu, G.; Wang, J.; Guo, D.; Pan, Y.; Hoffmann, T.D. Volatile compound-mediated plant–plant interactions under stress with the tea plant as a model. Hortic. Res. 2023, 10, uhad143. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Srikanth, P.; Maxton, A.; Masih, S.A.; Sofo, A.; Khan, N.A. Isoprene: An antioxidant to guard plants against stress. Int. J. Plant Biol. 2024, 15, 161–174. [Google Scholar] [CrossRef]
- Loreto, F.; Mannozzi, M.; Maris, C.; Nascetti, P.; Ferranti, F.; Pasqualini, S. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol. 2001, 126, 993–1000. [Google Scholar] [CrossRef]
- Affek, H.P.; Yakir, D. Protection by isoprene against singlet oxygen in leaves. Plant Physiol. 2002, 129, 269–277. [Google Scholar] [CrossRef]
- Silva Santos, L.; Dalmázio, I.; Eberlin, M.N.; Claeys, M.; Augusti, R. Mimicking the atmospheric OH-radical-mediated photooxidation of isoprene: Formation of cloud-condensation nuclei polyols monitored by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2104–2108. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F.; Tsonev, T.; Brilli, F.; Edreva, A. Isoprene prevents the negative consequences of high temperature stress in Platanus orientalis leaves. Funct. Plant Biol. 2006, 33, 931–940. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Hashem, H.A. JA-Mediated Defenses to Promote Thermotolerance in Plants: What Is the State of Our Knowledge? In Jasmonates and Plant Defense; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 159–186. [Google Scholar]
- Sauer, F.; Schäfer, C.; Neeb, P.; Horie, O.; Moortgat, G.K. Formation of hydrogen peroxide in the ozonolysis of isoprene and simple alkenes under humid conditions. Atmos. Environ. 1999, 33, 229–241. [Google Scholar] [CrossRef]
- Fares, S.; Loreto, F.; Kleist, E.; Wildt, J. Stomatal uptake and stomatal deposition of ozone in isoprene and monoterpene emitting plants. Plant Biol. 2007, 9, e69–e78. [Google Scholar] [CrossRef] [PubMed]
- Alméras, E.; Stolz, S.; Vollenweider, S.; Reymond, P.; Mène-Saffrané, L.; Farmer, E.E. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 2003, 34, 205–216. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Copolovici, L.O.; Filella, I.; Llusià, J.; Niinemets, U.; Peñuelas, J. The capacity for thermal protection of photosynthetic electron transport varies for different monoterpenes in Quercus ilex. Plant Physiol. 2005, 139, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wang, B.; Ying, B.; Zhou, L.; Zhang, R. Monoterpene emissions contribute to thermotolerance in Cinnamomum camphora. Trees 2017, 31, 1759–1771. [Google Scholar] [CrossRef]
- Paulino, B.N.; Silva, G.N.; Araújo, F.F.; Néri-Numa, I.A.; Pastore, G.M.; Bicas, J.L.; Molina, G. Beyond natural aromas: The bioactive and technological potential of monoterpenes. Trends Food Sci. Technol. 2022, 128, 188–201. [Google Scholar] [CrossRef]
- Bsaibes, S.; Piel, F.; Gros, V.; Truong, F.; Lafouge, F.; Ciuraru, R.; Buysse, P.; Kammer, J.; Loubet, B.; Staudt, M. Monoterpene Chemical Speciation with High Time Resolution Using a FastGC/PTR-MS: Results from the COV3ER Experiment on Quercus ilex. Atmosphere 2020, 11, 690. [Google Scholar] [CrossRef]
- Ryu, D.-H.; Cho, J.-Y.; Yang, S.-H.; Kim, H.-Y. Effects of harvest timing on phytochemical composition in Lamiaceae plants under an environment-controlled system. Antioxidants 2023, 12, 1909. [Google Scholar] [CrossRef]
- Malik, T.G.; Sahu, L.K.; Gupta, M.; Mir, B.A.; Gajbhiye, T.; Dubey, R.; Clavijo McCormick, A.; Pandey, S.K. Environmental factors affecting monoterpene emissions from terrestrial vegetation. Plants 2023, 12, 3146. [Google Scholar] [CrossRef]
- Delfine, S.; Csiky, O.; Seufert, G.; Loreto, F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): Monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 2000, 146, 27–36. [Google Scholar] [CrossRef]
- Wu, J.; Cao, X.; Sun, X.; Chen, Y.; Zhang, P.; Li, Y.; Ma, C.; Wu, L.; Liang, X.; Fu, Q. OsEL2 Regulates Rice Cold Tolerance by MAPK Signaling Pathway and Ethylene Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 1633. [Google Scholar] [CrossRef]
- Zhou, H. The Role of Monoterpenes in Plant Physiological and Antioxidant Responses to Drought Stress. Ph.D. Thesis, Lancaster University (United Kingdom), Lancaster, UK, 2025. [Google Scholar]
- Dani, K.S.; Fineschi, S.; Michelozzi, M.; Trivellini, A.; Pollastri, S.; Loreto, F. Diversification of petal monoterpene profiles during floral development and senescence in wild roses: Relationships among geraniol content, petal colour, and floral lifespan. Oecologia 2021, 197, 957–969. [Google Scholar] [CrossRef]
- Wen, D.; Guan, Y.; Jiang, L.; Chen, S.; Chen, F.; Liu, B.; Niinemets, Ü.; Jiang, Y. Heat-stress induced sesquiterpenes of Chrysanthemum nankingense attract herbivores but repel herbivore feeding. Arthropod-Plant Interact. 2023, 17, 111–122. [Google Scholar] [CrossRef]
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Protection of Artemisia annua roots and leaves against oxidative stress induced by arsenic. Biol. Plant. 2017, 61, 367–377. [Google Scholar] [CrossRef]
- Koç, E.; Karayiğit, B. Plant secondary metabolites in stress tolerance. In Climate-Resilient Agriculture, Vol 1: Crop Responses and Agroecological Perspectives; Springer: Cham, Switzerland, 2023; pp. 379–433. [Google Scholar]
- Liu, B.; Zhang, L.; Rusalepp, L.; Kaurilind, E.; Sulaiman, H.Y.; Püssa, T.; Niinemets, Ü. Heat priming improved heat tolerance of photosynthesis, enhanced terpenoid and benzenoid emission and phenolics accumulation in Achillea millefolium. Plant Cell Environ. 2021, 44, 2365–2385. [Google Scholar] [CrossRef] [PubMed]
- Khakdan, F.; Govahi, M.; Mohebi, Z.; Ranjbar, M. Water deficit stress responses of monoterpenes and sesquiterpenes in different Iranian cultivars of basil. Physiol. Plant. 2021, 173, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Dias, C.; Amaro, A.; Fonseca, A.; Ferrante, A.; Silvestre, A.; Rocha, S.M.; Isidoro, N.; Pintado, M. ß-Farnesene exogenous application as a novel damage induction model to fast explore the effectiveness of postharvest strategies: The case study of the ‘Rocha’pear DOP. Horticulturae 2022, 8, 93. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Ren, A.; Shi, L.; Zhu, J.; Yu, H.; Jiang, A.; Zheng, H.; Zhao, M. Shedding light on the mechanisms underlying the environmental regulation of secondary metabolite ganoderic acid in Ganoderma lucidum using physiological and genetic methods. Fungal Genet. Biol. 2019, 128, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, L. Integration of multiple volatile cues into plant defense responses. New Phytol. 2022, 233, 618–623. [Google Scholar] [CrossRef]
- Loreto, F.; D’Auria, S. How do plants sense volatiles sent by other plants? Trends Plant Sci. 2022, 27, 29–38. [Google Scholar] [CrossRef]
- Sun, Y.; Fernie, A.R. Plant secondary metabolism in a fluctuating world: Climate change perspectives. Trends Plant Sci. 2024, 29, 560–571. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. BVOCs: Plant defense against climate warming? Trends Plant Sci. 2003, 8, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Niinemets, Ü.; Peñuelas, J. Changes in floral bouquets from compound-specific responses to increasing temperatures. Glob. Change Biol. 2014, 20, 3660–3669. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, J.G.; Lichtenthaler, H.K.; May, H.U.; Lichtenthaler, F.W. Is isoprene emitted by plants synthesized via the novel isopentenyl pyrophosphate pathway? Z. für Naturforschung C 1997, 52, 15–23. [Google Scholar] [CrossRef]
- Silver, G.M.; Fall, R. Enzymatic synthesis of isoprene from dimethylallyl diphosphate in aspen leaf extracts. Plant Physiol. 1991, 97, 1588–1591. [Google Scholar] [CrossRef]
- Cinege, G.; Louis, S.; Hänsch, R.; Schnitzler, J.-P. Regulation of isoprene synthase promoter by environmental and internal factors. Plant Mol. Biol. 2009, 69, 593–604. [Google Scholar] [CrossRef]
- Rasulov, B.; Hüve, K.; Bichele, I.; Laisk, A.; Niinemets, Ü. Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: A kinetic analysis. Plant Physiol. 2010, 154, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Li, Z.; Ratliff, E.A.; Sharkey, T.D. Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiol. 2011, 155, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, J.; Fall, R. Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol. 1993, 101, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Singsaas, E.L. Why plants emit isoprene. Nature 1995, 374, 769. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef]
- Pollastri, S.; Jorba, I.; Hawkins, T.J.; Llusià, J.; Michelozzi, M.; Navajas, D.; Peñuelas, J.; Hussey, P.J.; Knight, M.R.; Loreto, F. Leaves of isoprene-emitting tobacco plants maintain PSII stability at high temperatures. New Phytol. 2019, 223, 1307–1318. [Google Scholar] [CrossRef]
- Zheng, T.; Lv, J.; Sadeghnezhad, E.; Cheng, J.; Jia, H. Transcriptomic and metabolomic profiling of strawberry during postharvest cooling and heat storage. Front. Plant Sci. 2022, 13, 1009747. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Zhang, Y.; Du, B.; Feng, C.; Zhou, Y.; Mei, X.; Jiang, Y.; Duan, X.; Yang, Z. Differential responses of four biosynthetic pathways of aroma compounds in postharvest strawberry (Fragaria× ananassa Duch.) under interaction of light and temperature. Food Chem. 2017, 221, 356–364. [Google Scholar] [CrossRef]
- Rosenstiel, T.N.; Potosnak, M.J.; Griffin, K.L.; Fall, R.; Monson, R.K. Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 2003, 421, 256–259. [Google Scholar] [CrossRef]
- Pegoraro, E.; Rey, A.; Bobich, E.G.; Barron-Gafford, G.; Grieve, K.A.; Malhi, Y.; Murthy, R. Effect of elevated CO2 concentration and vapour pressure deficit on isoprene emission from leaves of Populus deltoides during drought. Funct. Plant Biol. 2004, 31, 1137–1147. [Google Scholar] [CrossRef]
- Centritto, M.; Nascetti, P.; Petrilli, L.; Raschi, A.; Loreto, F. Environment. Profiles of isoprene emission and photosynthetic parameters in hybrid poplars exposed to free-air CO2 enrichment. Plant Cell Environ. 2004, 27, 403–412. [Google Scholar] [CrossRef]
- Calfapietra, C.; Scarascia Mugnozza, G.; Karnosky, D.F.; Loreto, F.; Sharkey, T.D. Isoprene emission rates under elevated CO2 and O3 in two field-grown aspen clones differing in their sensitivity to O3. New Phytol. 2008, 179, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Sharkey, T.D. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 1990, 182, 523–531. [Google Scholar] [CrossRef]
- Way, D.A.; Ghirardo, A.; Kanawati, B.; Esperschütz, J.; Monson, R.K.; Jackson, R.B.; Schmitt-Kopplin, P.; Schnitzler, J. Increasing atmospheric CO2 reduces metabolic and physiological differences between isoprene- and non-isoprene-emitting poplars. New Phytol. 2013, 200, 534–546. [Google Scholar] [CrossRef]
- Staudt, M.; Joffre, R.; Rambal, S.; Kesselmeier, J. Effect of elevated CO2 on monoterpene emission of young Quercus ilex trees and its relation to structural and ecophysiological parameters. Tree Physiol. 2001, 21, 437–445. [Google Scholar] [CrossRef]
- Loreto, F.; Fischbach, R.J.; Schnitzler, J.P.; Ciccioli, P.; Brancaleoni, E.; Calfapietra, C.; Seufert, G. Monoterpene emission and monoterpene synthase activities in the Mediterranean evergreen oak Quercus ilex L. grown at elevated CO2 concentrations. Glob. Change Biol. 2001, 7, 709–717. [Google Scholar] [CrossRef]
- Kainulainen, P.; Holopainen, J.; Holopainen, T. The influence of elevated CO2 and O3 concentrations on Scots pine needles: Changes in starch and secondary metabolites over three exposure years. Oecologia 1998, 114, 455–460. [Google Scholar] [CrossRef]
- Constable, J.V.; Litvak, M.E.; Greenberg, J.P.; Monson, R.K. Monoterpene emission from coniferous trees in response to elevated CO2 concentration and climate warming. Glob. Change Biol. 1999, 5, 252–267. [Google Scholar] [CrossRef]
- Lerdau, M.; Gershenzon, J. Allocation theory and chemical defense. In Plant Resource Allocation; Bazzaz, F.A., Grace, J., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 265–277. [Google Scholar]
- Wang, X.; Zhang, Y.; Tan, Y.; Tan, Y.; Bai, J.; Gu, D.; Ma, Z.; Du, J.; Han, Z. Effects of light on the emissions of biogenic isoprene and monoterpenes: A review. Atmos. Pollut. Res. 2022, 13, 101397. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, Z.; Lai, J.; Liu, Y.; Huang, X.; Yi, Z. Effects of temperature and light on isoprene and monoterpene emission from Loropetalum chinense and Nandina domestica. Acta Sci. Circumstantiae 2019, 39, 3126–3133. [Google Scholar]
- Jardine, K.; Chambers, J.; Alves, E.G.; Teixeira, A.; Garcia, S.; Holm, J.; Higuchi, N.; Manzi, A.; Abrell, L.; Fuentes, J.D. Dynamic balancing of isoprene carbon sources reflects photosynthetic and photorespiratory responses to temperature stress. Plant Physiol. 2014, 166, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Van Meeningen, Y.; Schurgers, G.; Rinnan, R.; Holst, T. Isoprenoid emission response to changing light conditions of English oak, European beech and Norway spruce. Biogeosciences 2017, 14, 4045–4060. [Google Scholar] [CrossRef]
- Staudt, M.; Bertin, N.; Frenzel, B.; Seufert, G.J. Seasonal variation in amount and composition of monoterpenes emitted by young Pinus pinea trees–implications for emission modeling. J. Atmos. Chem. 2000, 35, 77–99. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond vegetables: Effects of indoor LED light on specialized metabolite biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J. Sci. Food Agric. 2022, 102, 472–487. [Google Scholar] [CrossRef]
- Rasulov, B.; Hüve, K.; Laisk, A.; Niinemets, Ü. Induction of a longer term component of isoprene release in darkened aspen leaves: Origin and regulation under different environmental conditions. Plant Physiol. 2011, 156, 816–831. [Google Scholar] [CrossRef]
- Rasulov, B.; Copolovici, L.; Laisk, A.; Niinemets, U. Postillumination isoprene emission: In vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiol. 2009, 149, 1609–1618. [Google Scholar] [CrossRef]
- Hu, Z.; Li, T.; Zheng, J.; Yang, K.; He, X.; Leng, P. Ca2+ signal contributing to the synthesis and emission of monoterpenes regulated by light intensity in Lilium ‘siberia’. Plant Physiol. Biochem. 2015, 91, 1–9. [Google Scholar] [CrossRef]
- Peng, X.; Wang, B.; Wang, X.; Ni, B.; Zuo, Z. Variations in aroma and specific flavor in strawberry under different colored light-quality selective plastic film. Flavour Fragr. J. 2020, 35, 350–359. [Google Scholar] [CrossRef]
- Staudt, M.; Lhoutellier, L. Monoterpene and sesquiterpene emissions from Quercus coccifera exhibit interacting responses to light and temperature. Biogeosciences 2011, 8, 2757–2771. [Google Scholar] [CrossRef]
- Sun, Z.; Shen, Y.; Niinemets, Ü. Responses of isoprene emission and photochemical efficiency to severe drought combined with prolonged hot weather in hybrid Populus. J. Exp. Bot. 2020, 71, 7364–7381. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Arve, L.E.; Torre, S.; Olsen, J.E.; Tanino, K.K. Stomatal responses to drought stress and air humidity. In Abiotic Stress in Plants-Mechanisms and Adaptations; Shanker, A., Venkateswarlu, B., Eds.; IntechOpen: London, UK, 2011; pp. 267–280. [Google Scholar]
- Pantin, F.; Blatt, M.R. Stomatal response to humidity: Blurring the boundary between active and passive movement. Plant Physiol. 2018, 176, 485–488. [Google Scholar] [CrossRef]
- Parveen, S.; Rashid, M.H.U.; Inafuku, M.; Iwasaki, H.; Oku, H. Molecular regulatory mechanism of isoprene emission under short-term drought stress in the tropical tree Ficus septica. Tree Physiol. 2019, 39, 440–453. [Google Scholar] [CrossRef]
- Zhou, H.; Ashworth, K.; Dodd, I.C. Exogenous monoterpenes mitigate H2O2-induced lipid damage but do not attenuate photosynthetic decline during water deficit in tomato. J. Exp. Bot. 2023, 74, 5327–5340. [Google Scholar] [CrossRef] [PubMed]
- Ormeno, E.; Mévy, J.-P.; Vila, B.; Bousquet-Mélou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water deficit stress induces different monoterpene and sesquiterpene emission changes in Mediterranean species. Relationship between terpene emissions and plant water potential. Chemosphere 2007, 67, 276–284. [Google Scholar] [CrossRef]
- Haberstroh, S.; Kreuzwieser, J.; Lobo-do-Vale, R.; Caldeira, M.C.; Dubbert, M.; Werner, C. Terpenoid emissions of two Mediterranean woody species in response to drought stress. Front. Plant Sci. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Mancini, I.; Domingo, G.; Bracale, M.; Loreto, F.; Pollastri, S. Isoprene emission influences the proteomic profile of Arabidopsis plants under well-watered and drought-stress conditions. Int. J. Mol. Sci. 2022, 23, 3836. [Google Scholar] [CrossRef]
- Loreto, F.; Sharkey, T. Environment. Isoprene emission by plants is affected by transmissible wound signals. Plant Cell Environ. 1993, 16, 563–570. [Google Scholar] [CrossRef]
- Jardine, K.J.; Meyers, K.; Abrell, L.; Alves, E.G.; Serrano, A.M.Y.; Kesselmeier, J.; Karl, T.; Guenther, A.; Vickers, C.; Chambers, J.Q. Emissions of putative isoprene oxidation products from mango branches under abiotic stress. J. Exp. Bot. 2013, 64, 3669. [Google Scholar] [CrossRef]
- Velikova, V.; Sharkey, T.D.; Loreto, F. Stabilization of thylakoid membranes in isoprene-emitting plants reduces formation of reactive oxygen species. Plant Signal. Behav. 2012, 7, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Davoine, C. Reactive electrophile species. Curr. Opin. Plant Biol. 2007, 10, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, S.H.; Hua, J.; Li, D.S.; Ling, Y.; Luo, Q.; Li, S.H. Characterization of defensive cadinenes and a novel sesquiterpene synthase responsible for their biosynthesis from the invasive Eupatorium adenophorum. New Phytol. 2021, 229, 1740–1754. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Galbally, I.E.; Porter, N.; Weeks, I.A.; Lawson, S. BVOC emissions from mechanical wounding of leaves and branches of Eucalyptus sideroxylon (red ironbark). J. Atmos. Chem. 2011, 68, 265–279. [Google Scholar] [CrossRef]
- Loreto, F.; Nascetti, P.; Graverini, A.; Mannozzi, M. Emission and content of monoterpenes in intact and wounded needles of the Mediterranean pine, Pinus pinea. Funct. Ecol. 2000, 14, 589–595. [Google Scholar] [CrossRef]
- Zeng, L.; Jin, S.; Xu, Y.-Q.; Granato, D.; Fu, Y.-Q.; Sun, W.-J.; Yin, J.-F.; Xu, Y.-Q. Exogenous stimulation-induced biosynthesis of volatile compounds: Aroma formation of oolong tea at postharvest stage. Crit. Rev. Food Sci. Nutr. 2024, 64, 76–86. [Google Scholar] [CrossRef]
- Ton, J.; D’Alessandro, M.; Jourdie, V.; Jakab, G.; Karlen, D.; Held, M.; Mauch-Mani, B.; Turlings, T.C. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007, 49, 16–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Y.; Jin, S. Biosynthetic Machinery to Abiotic Stress-Driven Emission: Decoding Multilayer Regulation of Volatile Terpenoids in Plants. Antioxidants 2025, 14, 673. https://doi.org/10.3390/antiox14060673
Shan Y, Jin S. Biosynthetic Machinery to Abiotic Stress-Driven Emission: Decoding Multilayer Regulation of Volatile Terpenoids in Plants. Antioxidants. 2025; 14(6):673. https://doi.org/10.3390/antiox14060673
Chicago/Turabian StyleShan, Yingying, and Songheng Jin. 2025. "Biosynthetic Machinery to Abiotic Stress-Driven Emission: Decoding Multilayer Regulation of Volatile Terpenoids in Plants" Antioxidants 14, no. 6: 673. https://doi.org/10.3390/antiox14060673
APA StyleShan, Y., & Jin, S. (2025). Biosynthetic Machinery to Abiotic Stress-Driven Emission: Decoding Multilayer Regulation of Volatile Terpenoids in Plants. Antioxidants, 14(6), 673. https://doi.org/10.3390/antiox14060673








