Multi-Omics Analysis of Chronic Heat Stress-Induced Biological Effects, Liver Injury, and Heat Tolerance Mechanisms via Oxidative and Anti-Inflammatory Pathways in Early-Pregnancy Sows
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Environmental Conditions
2.2. Sample Collection and Processing
2.3. Detection of Hematological, Biochemical, and Endocrine Parameters in Blood
2.4. Histopathological and Immunofluorescence Analysis of Liver
2.5. Transcriptome, Proteome, and Metabolome Analysis
2.6. Correlation Analysis of Proteomics, Transcriptomics, and Metabolomics
2.7. Data Analysis
3. Results
3.1. Environmental Control, Body Temperature, and Respiratory Rate
3.2. Changes in Hematological Parameters, Blood Acid–Base Balance, and Oxygen Metabolism Under HS
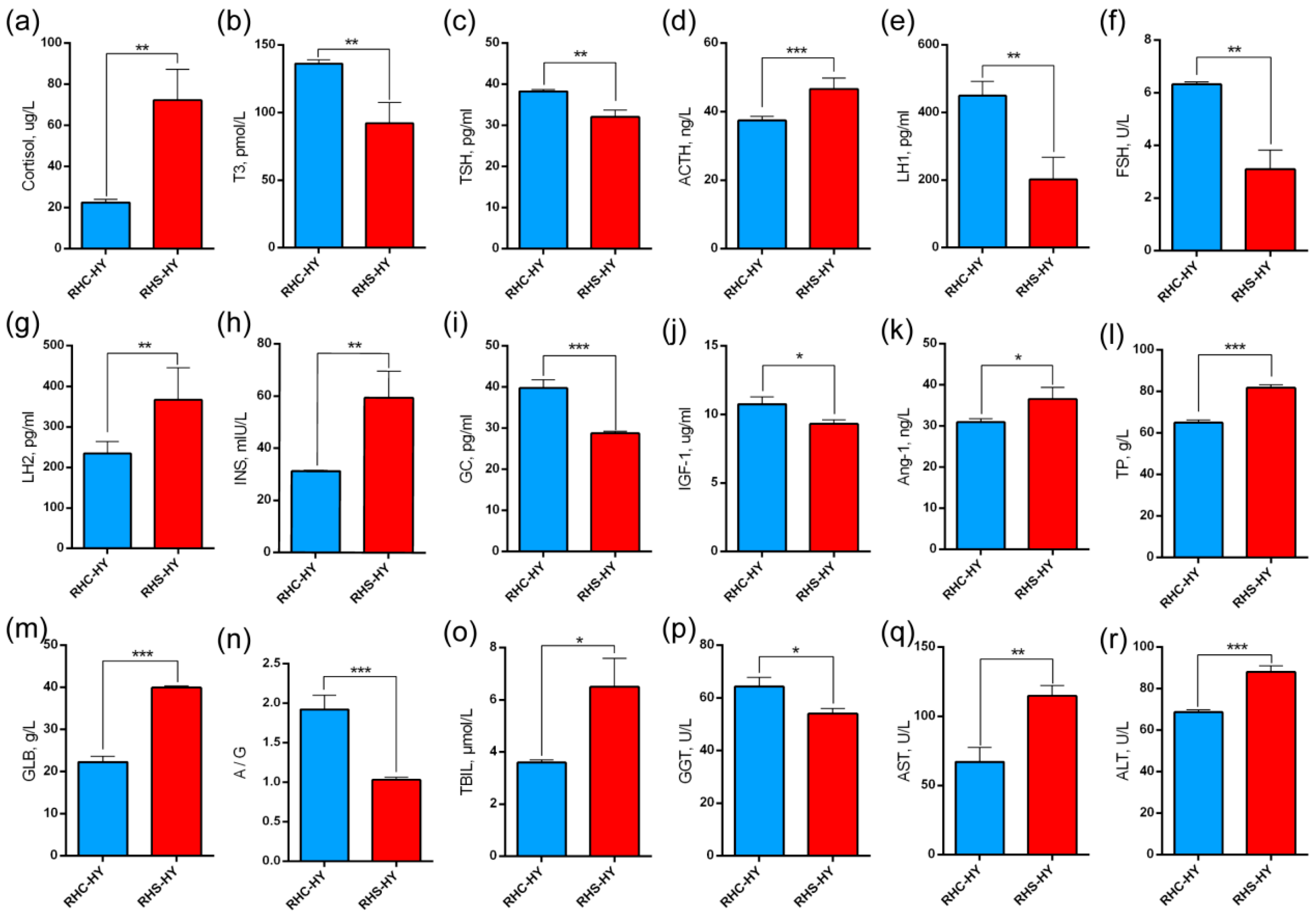
3.3. Analysis of Serum Hormone Levels and Liver Function Indices
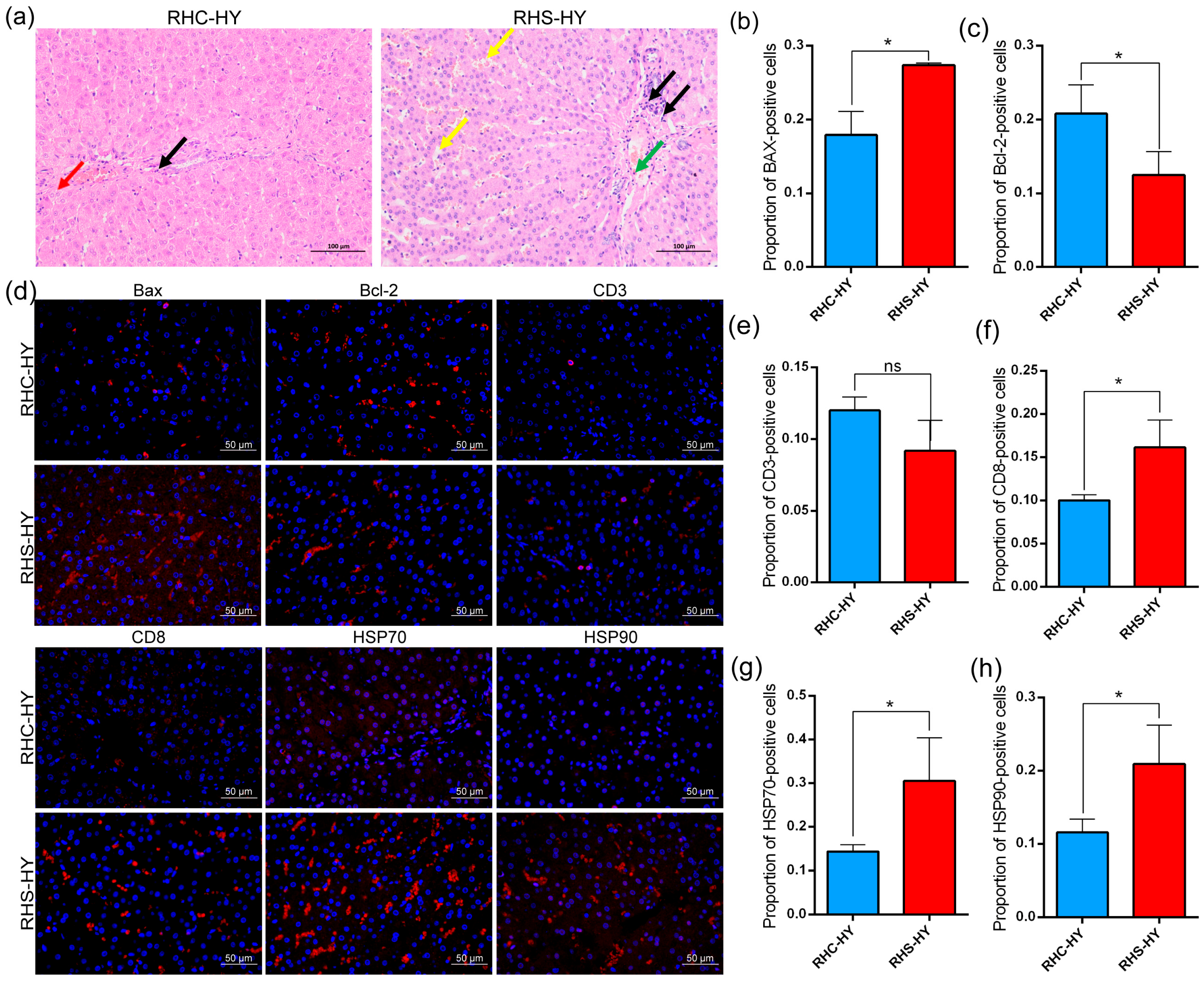
3.4. Analysis of Liver Histological Features and Labeled Proteins
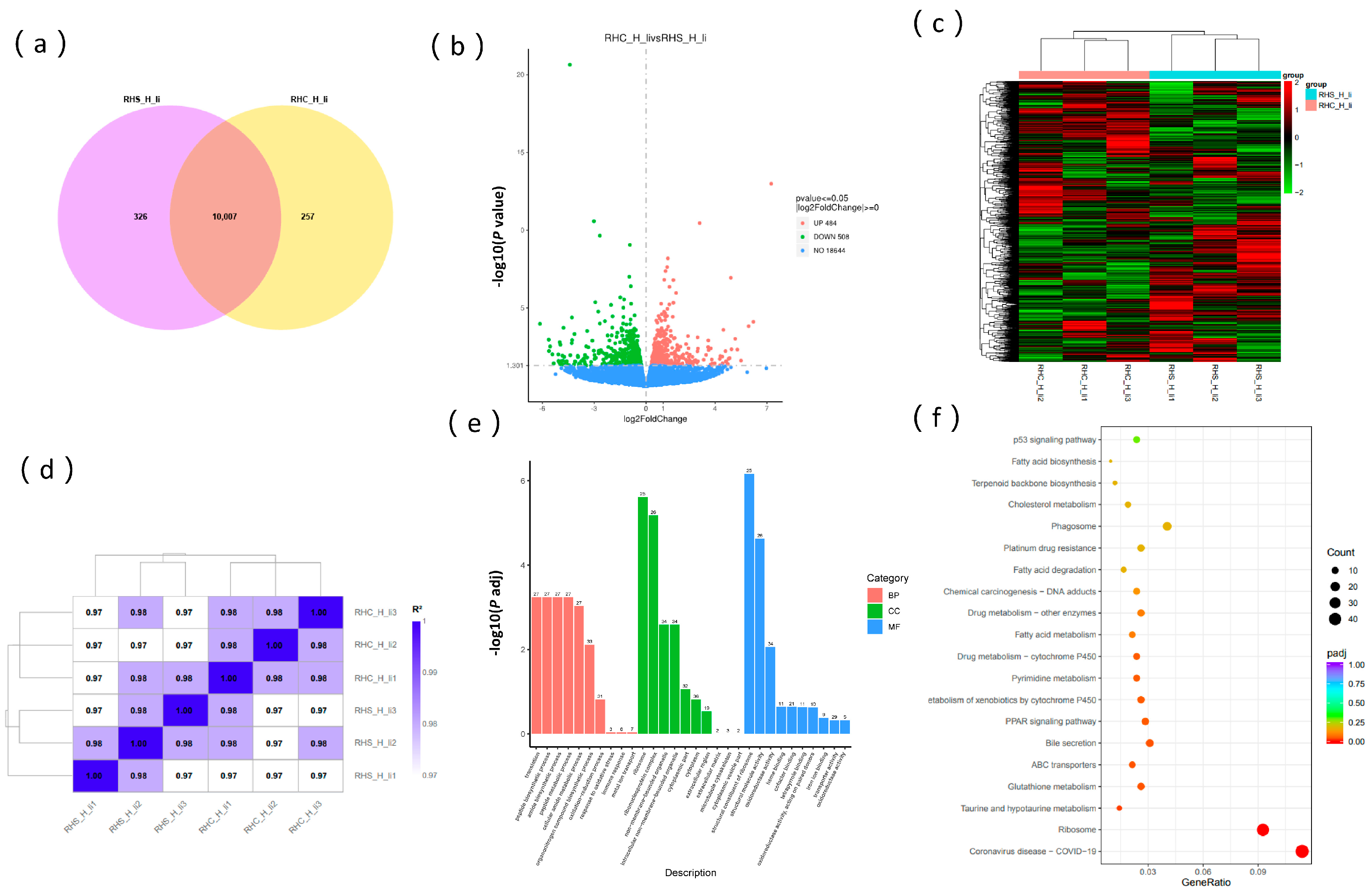
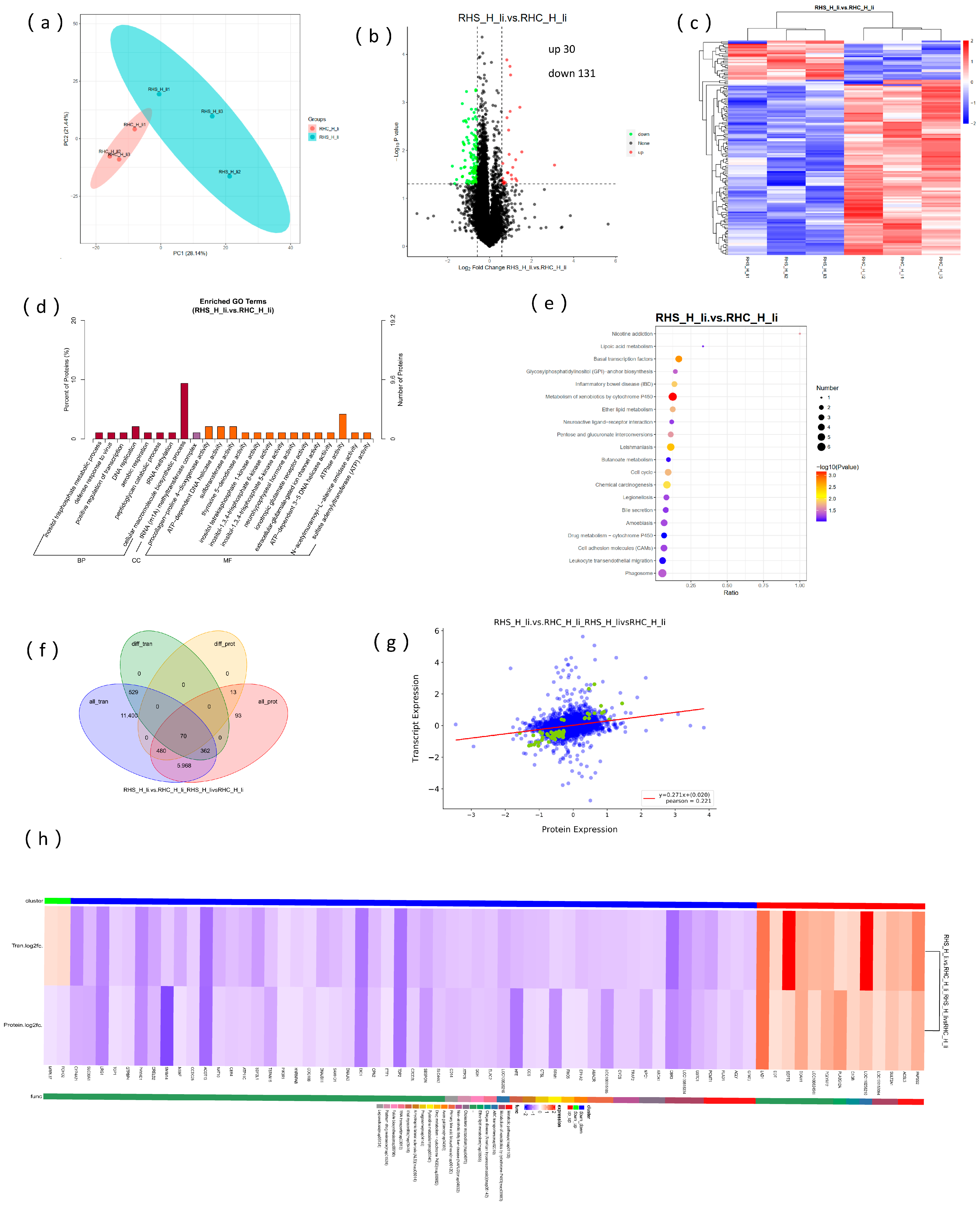
3.5. Transcriptome Analysis of Sows During Early Pregnancy Under HS
3.6. Overview of the Proteome Data and Correlation Analysis with Transcriptome
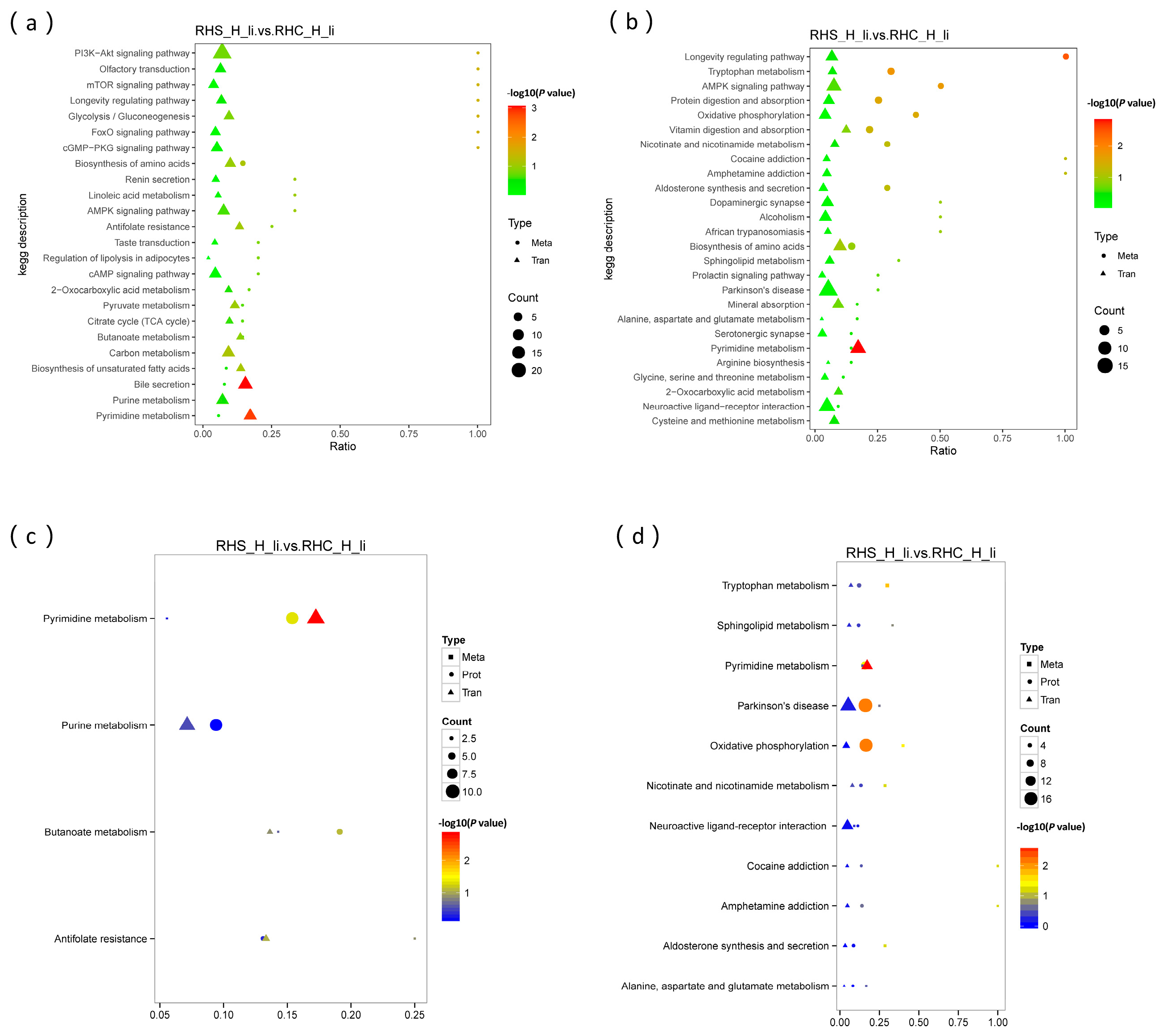
3.7. Analysis of Liver Metabolic Characteristics and the Association with Transcriptome and Proteome
3.8. Focus on Oxidation Resistance Through Multi-Omics Analysis
4. Discussion
4.1. Establish the Heat Stress Environment for Early Gestation Sows
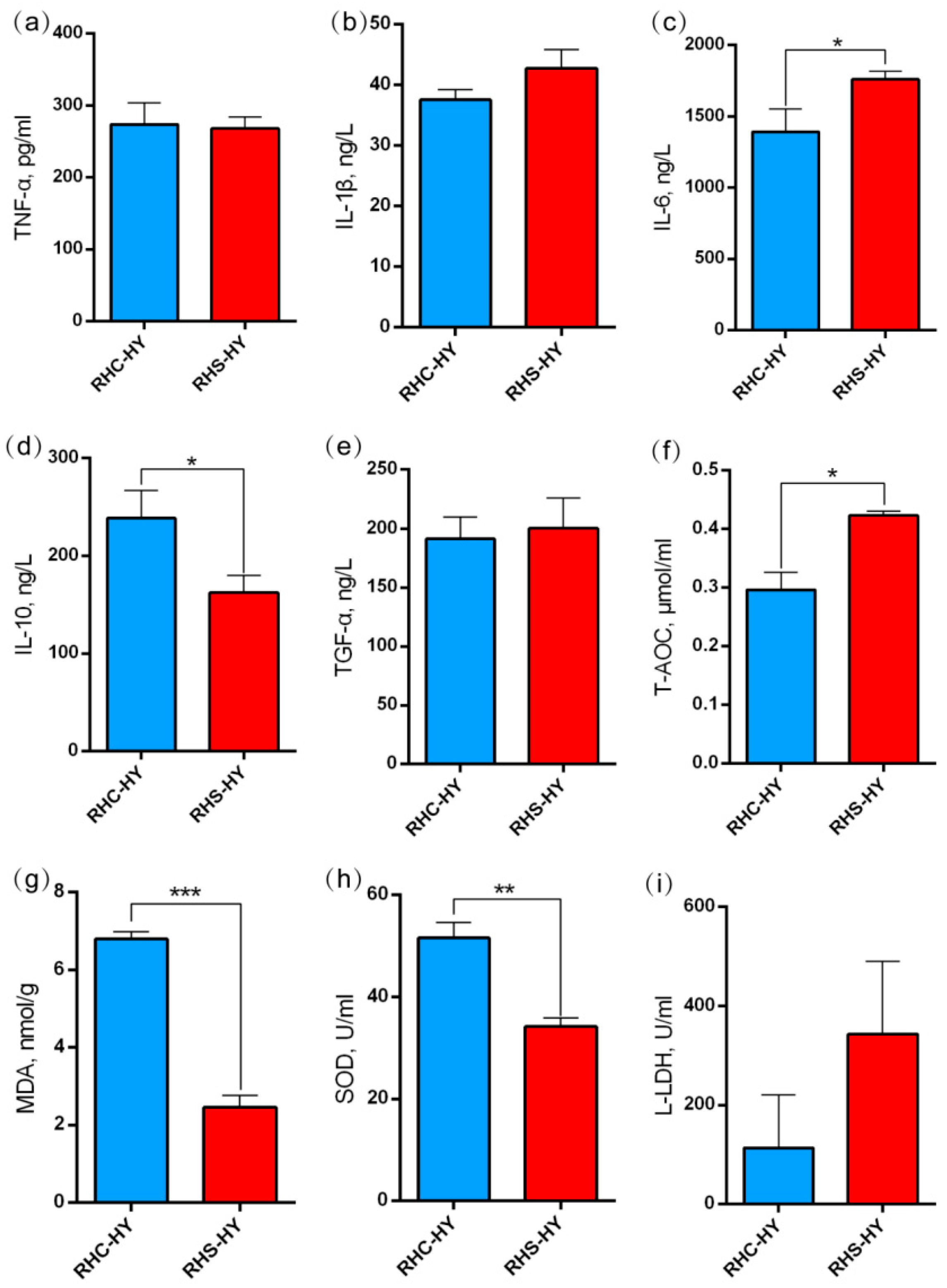
4.2. Blood Oxygen Levels Decrease, Leading to Inflammation
4.3. Endocrine Disturbances Adversely Affect Embryonic Development
4.4. Liver Injury Induces Oxidative Stress and Inflammatory Responses
4.5. Multi-Omics Analysis of Liver Oxidative Stress and Inflammation Regulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Guillén Bolaños, T.; Bindi, M.; Brown, S.; Camilloni, I.A.; Diedhiou, A.; Djalante, R.; Ebi, K.; et al. The human imperative of stabilizing global climate change at 1.5 °C. Science 2019, 365, eaaw6974. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Miao, C.; Hanel, M.; Borthwick, A.G.; Duan, Q.; Ji, D.; Li, H. Global heat stress on health, wildfires, and agricultural crops under different levels of climate warming. Environ. Int. 2019, 128, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Bonell, A.; Sonko, B.; Badjie, J.; Samateh, T.; Saidy, T.; Sosseh, F.; Sallah, Y.; Bajo, K.; Murray, K.A.; Hirst, J.; et al. Environmental heat stress on maternal physiology and fetal blood flow in pregnant subsistence farmers in The Gambia, west Africa: An observational cohort study. Lancet Planet Health 2022, 6, e968–e976. [Google Scholar] [CrossRef]
- Roos, N.; Kovats, S.; Hajat, S.; Filippi, V.; Chersich, M.; Luchters, S.; Scorgie, F.; Nakstad, B.; Stephansson, O.; Chamnha Consortium Hess, J. Maternal and newborn health risks of climate change: A call for awareness and global action. Acta Obs. Gynecol. Scand. 2021, 100, 566–570. [Google Scholar] [CrossRef]
- Victoria Sanz Fernandez, M.; Johnson, J.S.; Abuajamieh, M.; Stoakes, S.K.; Seibert, J.T.; Cox, L.; Kahl, S.; Elsasser, T.H.; Ross, J.W.; Clay Isom, S.; et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 2015, 3, e12315. [Google Scholar] [CrossRef]
- Renaudeau, D.; Leclercq-Smekens, M.; Herin, M. Differences in skin characteristics in European (Large White) and Caribbean (Creole) growing pigs with reference to thermoregulation. Anim. Res. 2006, 55, 209–217. [Google Scholar] [CrossRef]
- Cui, C.; Song, H.; Han, Y.; Yu, H.; Li, H.; Yang, Y.; Zhang, B. Gut microbiota-associated taurine metabolism dysregulation in a mouse model of Parkinson’s disease. mSphere 2023, 8, e0043123. [Google Scholar] [CrossRef]
- Ravanelli, N.; Casasola, W.; English, T.; Edwards, K.M.; Jay, O. Heat stress and fetal risk. Environmental limits for exercise and passive heat stress during pregnancy: A systematic review with best evidence synthesis. Br. J. Sports Med. 2019, 53, 799–805. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Cao, X.; Guo, X.; Wang, J.; Liu, Y.; Zhou, H.; Ma, B.; Peng, S. Modulation of liver metabolism and gut microbiota by Alhagi-honey alleviated heat stress-induced liver damage. Stress Biol. 2024, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Shen, C.; Dong, X.; Li, J.; Liu, J. Effects of isorhamnetin on liver injury in heat stroke-affected rats under dry-heat environments via oxidative stress and inflammatory response. Sci. Rep. 2024, 14, 7476. [Google Scholar] [CrossRef]
- Guo, B.; Yan, L.; Tang, Y.; Du, J.; Dai, Z.; Liu, J.; Lei, M.; Hou, Z.; Zhu, H. Green Light Mitigates Cyclic Chronic Heat-Stress-Induced Liver Oxidative Stress and Inflammation via NF-κB Pathway Inhibition in Geese. Antioxidants 2024, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ding, K.N.; Shen, X.L.; Liu, H.X.; Zhang, Y.A.; Liu, Y.Q.; He, Y.M.; Tang, L.P. Chronic heat stress promotes liver inflammation in broilers via enhancing NF-κB and NLRP3 signaling pathway. BMC Vet. Res. 2022, 18, 289. [Google Scholar] [CrossRef]
- E Lagoda, M.; O’driscoll, K.; Galli, M.C.; Cerón, J.J.; Ortín-Bustillo, A.; Marchewka, J.; A Boyle, L. Indicators of improved gestation housing of sows. Part II: Effects on physiological measures, reproductive performance and health of the offspring. Anim. Welf. 2023, 32, e52. [Google Scholar] [CrossRef]
- Lucy, M.C.; Safranski, T.J. Heat stress in pregnant sows: Thermal responses and subsequent performance of sows and their offspring. Mol. Reprod. Dev. 2017, 84, 946–956. [Google Scholar] [CrossRef]
- Knox, R.V. Swine fertility in a changing climate. Anim. Reprod. Sci. 2024, 269, 107537. [Google Scholar] [CrossRef]
- Merks, J.W.M.; Mathur, P.K.; Knol, E.F. Mathur and E.F.; Knol, New phenotypes for new breeding goals in pigs. Animal 2012, 6, 535–543. [Google Scholar] [CrossRef]
- Harlizius, B.; Mathur, P.; Knol, E.F. Breeding for resilience: New opportunities in a modern pig breeding program. J. Anim. Sci. 2020, 98 (Suppl. S1), S150–S154. [Google Scholar] [CrossRef]
- McConn, B.R.; Schinckel, A.P.; Robbins, L.; Gaskill, B.N.; Green-Miller, A.R.; Lay, D.C.; Johnson, J.S. A behavior and physiology-based decision support tool to predict thermal comfort and stress in non-pregnant, mid-gestation, and late-gestation sows. J Anim. Sci. Biotechnol. 2022, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Grossi, G.; Goglio, P.; Vitali, A.; Williams, A.G. Livestock and climate change: Impact of livestock on climate and mitigation strategies. Anim. Front. 2019, 9, 69–76. [Google Scholar] [CrossRef]
- Niu, K.; Zhong, J.; Hu, X. Impacts of climate change-induced heat stress on pig productivity in China. Sci. Total Environ. 2023, 908, 168215. [Google Scholar] [CrossRef]
- Lin, K.-H.; Flowers, B.; Knauer, M.; Lin, E.-C. Estimation of genotype by environmental interaction for litter traits by reaction norm model in Taiwan Landrace sows. J. Anim. Sci. 2024, 102, skae189. [Google Scholar] [CrossRef] [PubMed]
- Robbins, L.A.; Green-Miller, A.R.; Lay, D.C.; Schinckel, A.P.; Johnson, J.S.; Gaskill, B.N. Evaluation of sow thermal pref-erence across three stages of reproduction. J. Anim. Sci. 2021, 99, skab202. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.; Lambertz, C.; Das, G.; Reiner, G.; Gauly, M. Effects of temperature and temperature-humidity index on the reproductive performance of sows during summer months under a temperate climate. Anim. Sci. J. 2016, 87, 1334–1339. [Google Scholar] [CrossRef]
- Baert, S.; Aubé, L.; Haley, D.B.; Bergeron, R.; Devillers, N. The protective role of wallowing against heat stress in gestating and lactating sows housed outdoors. Physiol. Behav. 2022, 254, 113898. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.H.F.; Johnson, J.S.; Wen, H.; Maskal, J.M.; Tiezzi, F.; Maltecca, C.; Huang, Y.; DeDecker, A.E.; Schinckel, A.P.; Brito, L.F. Genetic parameters for automatically-measured vaginal temperature, respiration efficiency, and other thermotolerance indicators measured on lactating sows under heat stress conditions. Genet. Sel. Evol. 2023, 55, 65. [Google Scholar] [CrossRef]
- Pörtner, H. Climate change and temperature-dependent biogeography: Oxygen limitation of thermal tolerance in animals. Sci. Nat. 2001, 88, 137–146. [Google Scholar] [CrossRef]
- Thompson, L.P.; Pence, L.; Pinkas, G.; Song, H.; Telugu, B.P. Placental Hypoxia During Early Pregnancy Causes Maternal Hypertension and Placental Insufficiency in the Hypoxic Guinea Pig Model. Biol. Reprod. 2016, 95, 128. [Google Scholar] [CrossRef]
- Turan, S.; Aberdeen, G.W.; Thompson, L.P. Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full-term pregnant guinea pig. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R330–R339. [Google Scholar] [CrossRef]
- Lee, A.M.C.; Morrison, J.L.; Botting, K.J.; Shandala, T.; Xian, C.J. Effects of Maternal Hypoxia during Pregnancy on Bone Development in Offspring: A Guinea Pig Model. Int. J. Endocrinol. 2014, 2014, 916918. [Google Scholar] [CrossRef]
- Lagoda, M.E.; Marchewka, J.; O’Driscoll, K.; Boyle, L.A. Risk Factors for Chronic Stress in Sows Housed in Groups, and Associated Risks of Prenatal Stress in Their Offspring. Front. Veter. Sci. 2022, 9, 883154. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, C.; Hao, Y.; Gu, X.; Wang, H. Chronic Heat Stress Induces Acute Phase Responses and Serum Metabolome Changes in Finishing Pigs. Animals 2019, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.V.N.; Singh, G.; Varshney, V.P. Antioxidants Supplementation on Acid Base Balance during Heat Stress in Goats. Asian-Australas. J. Anim. Sci. 2010, 23, 1462–1468. [Google Scholar] [CrossRef]
- Borges, S.A.; da Silva, A.V.F.; Majorka, A.; Hooge, D.M.; Cummings, K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult. Sci. 2004, 83, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Al-Saiady, M.; Al-Shaikh, M.; Al-Mufarrej, S.; Al-Showeimi, T.; Mogawer, H.; Dirrar, A. Effect of chelated chromium supplementation on lactation performance and blood parameters of Holstein cows under heat stress. Anim. Feed. Sci. Technol. 2004, 117, 223–233. [Google Scholar] [CrossRef]
- Vásquez, N.; Cervantes, M.; Bernal-Barragán, H.; Rodríguez-Tovar, L.E.; Morales, A. Short- and Long-Term Exposure to Heat Stress Differently Affect Performance, Blood Parameters, and Integrity of Intestinal Epithelia of Growing Pigs. Animals 2022, 12, 2529. [Google Scholar] [CrossRef]
- Park, S.D.; Gu, B.-H.; Park, Y.J.; Joo, S.S.; Lee, S.-S.; Kim, S.-H.; Kim, E.T.; Kim, D.H.; Lee, S.S.; Lee, S.J.; et al. Dynamic changes in blood immune cell composition and function in Holstein and Jersey steers in response to heat stress. Cell Stress Chaperones 2021, 26, 705–720. [Google Scholar]
- Terry, R.; Nowland, T.L.; van Wettere, W.H.E.J.; Plush, K.J. Synthetic Olfactory Agonist Use in the Farrowing House to Reduce Sow Distress and Improve Piglet Survival. Animals 2021, 11, 2613. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Lu, M.; Yang, X.; Xue, Y.; Yao, W. A controlled heat stress during late gestation affects thermoregulation, productive performance, and metabolite profiles of primiparous sow. J. Therm. Biol. 2019, 81, 33–40. [Google Scholar] [CrossRef]
- Zhao, W.; Artaiz, O.; Iqbal, Y.; Le, H.; DiGiacomo, K.; Leury, B.; Fothergill, L.; Furness, J.; Liu, F.; Green, M.; et al. Heat stress of gilts around farrowing causes oxygen insufficiency in the umbilical cord and reduces piglet survival. Animal 2022, 16, 100668. [Google Scholar] [CrossRef]
- Ciechanowska, M.; Łapot, M.; Mateusiak, K.; Przekop, F. Neuroendocrine regulation of GnRH release and expression of GnRH and GnRH receptor genes in the hypothalamus-pituitary unit in different physiological states. Reprod. Biol. 2010, 10, 85–124. [Google Scholar] [CrossRef]
- Bonilla, A.; Oliveira, L.; Ozawa, M.; Newsom, E.; Lucy, M.; Hansen, P. Developmental changes in thermoprotective actions of insulin-like growth factor-1 on the preimplantation bovine embryo. Mol. Cell. Endocrinol. 2011, 332, 170. [Google Scholar] [CrossRef] [PubMed]
- Ouhilal, S.; Vuguin, P.; Cui, L.; Du, X.-Q.; Gelling, R.W.; Reznik, S.E.; Russell, R.; Parlow, A.F.; Karpovsky, C.; Santoro, N.; et al. Hypoglycemia, hyperglucagonemia, and fetoplacental defects in glucagon receptor knockout mice: A role for glucagon action in pregnancy maintenance. Am. J. Physiol. Metab. 2012, 302, E522–E531. [Google Scholar] [CrossRef]
- E Rudolph, T.; Roths, M.; Freestone, A.D.; White-Springer, S.H.; Rhoads, R.P.; Baumgard, L.H.; Selsby, J.T. Heat stress alters hematological parameters in barrows and gilts. J. Anim. Sci. 2024, 102, skae123. [Google Scholar] [CrossRef]
- Huau, G.; Liaubet, L.; Gourdine, J.-L.; Riquet, J.; Renaudeau, D. Multi-tissue metabolic and transcriptomic responses to a short-term heat stress in swine. BMC Genom. 2024, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Choi, Y.; Kim, D.; Min, Y.; Cho, E.; Kim, J. Effects of cooling systems on physiological responses and intestinal microflora in early gestating sows exposed to high-temperature stress. J. Anim. Sci. Technol. 2021, 63, 904. [Google Scholar] [CrossRef]
- Ko, S.-H. Effects of Heat Stress-Induced Sex Hormone Dysregulation on Reproduction and Growth in Male Adolescents and Beneficial Foods. Nutrients 2024, 16, 3032. [Google Scholar] [CrossRef]
- Lu, B.; Liang, W.; Liang, C.; Yu, Z.; Xie, X.; Chen, Z. Effect of Heat Stress on Expression of Main Reproductive Hormone in Hypothalamic-Pituitary-Gonadal Axis of Wenchang Chicks. Braz. J. Poult. Sci. 2021, 23, eRBCA-2019. [Google Scholar] [CrossRef]
- Hu, C.; Niu, X.; Chen, S.; Wen, J.; Bao, M.; Mohyuddin, S.G.; Yong, Y.; Liu, X.; Wu, L.; Yu, Z.; et al. A Comprehensive Analysis of the Colonic Flora Diversity, Short Chain Fatty Acid Metabolism, Transcripts, and Biochemical Indexes in Heat-Stressed Pigs. Front. Immunol. 2021, 12, 717723. [Google Scholar] [CrossRef]
- Zhao, L.; McMillan, R.P.; Xie, G.; Giridhar, S.G.L.W.; Baumgard, L.H.; El-Kadi, S.; Selsby, J.; Ross, J.; Gabler, N.; Hulver, M.W.; et al. Heat stress decreases metabolic flexibility in skeletal muscle of growing pigs. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R1096–R1106. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Zhou, X.; Cao, Y.; Li, Y.; Li, C. Bama miniature pigs’ liver possess great heat tolerance through upreg-ulation of Nrf2-mediated antioxidative enzymes. J. Therm. Biol. 2017, 67, 15–21. [Google Scholar] [CrossRef]
- Čukić, A.; Rakonjac, S.; Djoković, R.; Cincović, M.; Bogosavljević-Bošković, S.; Petrović, M.; Savić, Ž.; Andjušić, L.; Andjelić, B. Influence of Heat Stress on Body Temperatures Measured by Infrared Thermography, Blood Metabolic Parameters and Its Correlation in Sheep. Metabolites 2023, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Li, B.; Ding, L.; Wei, X.; Wang, P.; Chen, Z.; Han, S.; Huang, T.; Wang, B.; et al. Physiological responses to heat stress in the liver of rainbow trout (Oncorhynchus mykiss) revealed by UPLC-QTOF-MS metabolomics and biochemical assays. Ecotoxicol. Environ. Saf. 2022, 242, 113949. [Google Scholar] [CrossRef] [PubMed]
- Ghulam Mohyuddin, S.; Khan, I.; Zada, A.; Qamar, A.; Arbab, A.A.; Ma, X.B.; Yu, Z.C.; Liu, X.X.; Yong, Y.H.; Ju, X.H.; et al. Influence of Heat Stress on Intestinal Epithelial Barrier Function, Tight Junction Protein, and Immune and Reproductive Physiology. Biomed Res. Int. 2022, 2022, 8547379. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhong, X.; Zhang, L.; Wang, Y.; Wang, T. Heat Shock Protein 70 Expression is Increased in the Liver of Neonatal In-trauterine Growth Retardation Piglets. Asian-Australas. J. Anim. Sci. 2012, 25, 1096–1101. [Google Scholar] [CrossRef][Green Version]
- Tóthová, C.; Link, R.; Kyzeková, P.; Nagy, O. Serum protein electrophoretic pattern in piglets during the early postnatal period. Sci. Rep. 2021, 11, 17539. [Google Scholar] [CrossRef]
- Ijiri, M.; Odo, K.; Sato, M.; Kawaguchi, M.; Fujimoto, Y.; Miura, N.; Matsuo, T.; Hou, D.X.; Yamato, O.; Tanabe, T.; et al. Potential Biomarkers for Chronic Seasonal Heat Stress in Kagoshima Berkshire Pigs Reared in the Subtropical Region. J. Vet. Res. 2022, 66, 209–214. [Google Scholar] [CrossRef]
- Liu, E.; Zhao, X.; Li, C.; Wang, Y.; Li, L.; Zhu, H.; Ling, Q. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Kang, D.; Park, J.; Khan, M.; Shim, K. Chronic heat stress regulates the relation between heat shock protein and immunity in broiler small intestine. Sci. Rep. 2020, 10, 18872. [Google Scholar] [CrossRef]
- Alberghina, D.; Amato, A.; Brancato, G.; Cavallo, C.; Liotta, L.; Lopreiato, V. Impact of Heat Stress on the Balance between Oxidative Markers and the Antioxidant Defence System in the Plasma of Mid-Lactating Modicana Dairy Cows. Animals 2024, 14, 2034. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Hozzein, W.N.; Mousa, A.S.M.; Rabie, W.; Alkhalifah, D.H.M.; Selim, S.; AbdElgawad, H. Heat stress as an innovative approach to enhance the antioxidant production in Pseudooceanicola and Bacillus isolates. Sci. Rep. 2020, 10, 15076. [Google Scholar] [CrossRef]
- Ma, B.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Chronic heat stress causes liver damage via endoplasmic reticulum stress-induced apoptosis in broilers. Poult. Sci. 2022, 101, 102063. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.N.; Lu, M.H.; Guo, Y.N.; Liang, S.S.; Mou, R.W.; He, Y.M.; Tang, L.P. Resveratrol relieves chronic heat stress-induced liver oxidative damage in broilers by activating the Nrf2-Keap1 signaling pathway. Ecotoxicol. Environ. Saf. 2023, 249, 114411. [Google Scholar] [CrossRef]
- Tang, L.P.; Liu, Y.L.; Zhang, J.X.; Ding, K.N.; Lu, M.H.; He, Y.M. Heat stress in broilers of liver injury effects of heat stress on oxidative stress and autophagy in liver of broilers. Poult. Sci. 2022, 101, 102085. [Google Scholar] [CrossRef] [PubMed]
- Setroikromo, R.; Wierenga, P.; van Waarde, M.; Brunsting, J.; Vellenga, E.; Kampinga, H. Heat shock proteins and Bcl-2 expression and function in relation to the differential hyperthermic sensitivity between leukemic and normal hematopoietic cells. Cell Stress Chaperones 2007, 12, 320–330. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Jacotot, E.; Deniaud, A.; Borgne-Sanchez, A.; Touat, Z.; Briand, J.-P.; Le Bras, M.; Brenner, C. Therapeutic peptides: Targeting the mitochondrion to modulate apoptosis. Biochim. Biophys. Acta (BBA) Bioenerg. 2006, 1757, 1312–1323. [Google Scholar] [CrossRef]
- Wen, C.; Wei, S.; Zong, X.; Wang, Y.; Jin, M. Microbiota-gut-brain axis and nutritional strategy under heat stress. Anim. Nutr. 2021, 7, 1329–1336. [Google Scholar] [CrossRef]
- Prodromou, C. Mechanisms of Hsp90 regulation. Biochem. J. 2016, 473, 2439–2452. [Google Scholar] [CrossRef]
- Kim, J.; Yenari, M.; Lee, J. Regulation of inflammatory transcription factors by heat shock protein 70 in primary cultured astrocytes exposed to oxygen–glucose deprivation. Neuroscience 2015, 286, 272–280. [Google Scholar] [CrossRef]
- Imao, M.; Nagaki, M.; Moriwaki, H. Dual effects of heat stress on tumor necrosis factor-α-induced hepatocyte apoptosis in mice. Mod. Pathol. 2006, 86, 959–967. [Google Scholar] [CrossRef]
- Quan, P.; Guo, P.; He, J.; Liu, X. Heat-stress memory enhances the acclimation of a migratory insect pest to global warming. Mol. Ecol. 2024, 33, e17493. [Google Scholar] [CrossRef] [PubMed]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The Anti-Inflammatory Effect of Taurine on Cardiovascular Disease. Nutrients 2020, 12, 2847. [Google Scholar] [CrossRef] [PubMed]
- Mizota, T.; Hishiki, T.; Shinoda, M.; Naito, Y.; Hirukawa, K.; Masugi, Y.; Itano, O.; Obara, H.; Kitago, M.; Yagi, H.; et al. The hy-potaurine-taurine pathway as an antioxidative mechanism in patients with acute liver failure. J. Clin. Biochem. Nutr. 2022, 70, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Yabunaka, N.; Fujisawa, H.; Watanabe, I.; Agishi, Y. Effect of thermal stress on glutathione metabolism in human erythrocytes. Eur. J. Appl. Physiol. 1994, 68, 87–91. [Google Scholar] [CrossRef]
- Li, N.; Liu, D.; Wang, C.; Yan, G.; Zhang, S.; Jiang, Y.; Shen, M.; Jia, B.; Xu, L.; Huang, B.; et al. Comparison study of protective effects of porcine bile acids and sheep bile acids against heat stress in chickens. J. Sci. Food Agric. 2023, 103, 5687–5696. [Google Scholar] [CrossRef]
- Yin, C.; Tang, S.; Liu, L.; Cao, A.; Xie, J.; Zhang, H. Effects of Bile Acids on Growth Performance and Lipid Metabolism during Chronic Heat Stress in Broiler Chickens. Animals 2021, 11, 630. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Peng, H.; Chen, G.; Nie, Q.; Zhang, X.; Luo, W. Effects of long-time and short-time heat stress on the meat quality of geese. Poult. Sci. 2024, 103, 104112. [Google Scholar] [CrossRef]
- Fang, W.; Wen, X.; Meng, Q.; Liu, L.; Xie, J.; Zhang, H.; Everaert, N. Running Head: Heat Affects Cholesterol and Bile Acid Alterations in Cholesterol and Bile Acids Metabolism in Large White Pigs during Short-Term Heat Exposure. Animals 2020, 10, 359. [Google Scholar] [CrossRef]
- McCracken, V.; Xie, G.; Deaver, S.; Baumgard, L.; Rhoads, R.; Rhoads, M. Short communication: Hepatic progesterone-metabolizing enzymes cytochrome P450 2C and 3A in lactating cows during thermoneutral and heat stress conditions. J. Dairy Sci. 2015, 98, 3152–3157. [Google Scholar] [CrossRef]
- Jo, J.-H.; Nejad, J.G.; Peng, D.-Q.; Kim, H.-R.; Kim, S.-H.; Lee, H.-G. Characterization of Short-Term Heat Stress in Holstein Dairy Cows Using Altered Indicators of Metabolomics, Blood Parameters, Milk MicroRNA-216 and Characteristics. Animals 2021, 11, 722. [Google Scholar] [CrossRef]
- Tabiri, H.Y.; Sato, K.; Takahashi, K.; Toyomizu, M.; Akiba, Y. Effects of Heat Stress and Dietary Tryptophan on Performance and Plasma Amino Acid Concentrations of Broiler Chickens. Asian-Australas. J. Anim. Sci. 2002, 15, 247–253. [Google Scholar] [CrossRef]
- Cammisotto, V.; Valeriani, E.; Pignatelli, P.; Violi, F. Nicotinamide Adenine Dinucleotide Phosphate Oxidases and Metabolic Dysfunction-Associated Steatotic Liver Disease. Antioxidants 2025, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P.; Muskhelishvili, L.; Miller, B.J.; James, S.J. Presence and consequence of uracil in preneoplastic DNA from folate/methyl-deficient rats. Carcinogenesis 1997, 18, 2071–2076. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, M.; Wu, X.; Zhang, J. Metabolic Response of Pleurotus ostreatus to Continuous Heat Stress. Front. Microbiol. 2020, 10, 3148. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, J.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Xie, S.; Zhang, W.; Deng, J.; Tan, B.; et al. Protective effect of steroidal saponins on heat stress in the liver of largemouth bass (Micropterus salmoides) revealed by metabolomic analysis. Aquac. Rep. 2023, 33, 101875. [Google Scholar] [CrossRef]
- Koriem, K.M.; Tharwat, H.A. Malic Acid Improves Behavioral, Biochemical, and Molecular Disturbances in the Hy-pothalamus of Stressed Rats. J. Integr. Neurosci. 2023, 22, 98. [Google Scholar] [CrossRef]
- Wang, Y.; Saelao, P.; Kern, C.; Jin, S.; Gallardo, R.A.; Kelly, T.; Dekkers, J.M.; Lamont, S.J.; Zhou, H. Liver Transcriptome Responses to Heat Stress and Newcastle Disease Virus Infection in Genetically Distinct Chicken Inbred Lines. Genes 2020, 11, 1067. [Google Scholar] [CrossRef]
- Venediktova, N.; Solomadin, I.; Nikiforova, A.; Belosludtsev, K.N.; Mironova, G. Effects of the Long-Term Administration of Uridine on the Functioning of Rat Liver Mitochondria in Hyperthyroidism. Int. J. Mol. Sci. 2023, 24, 16730. [Google Scholar] [CrossRef]


| Item | Treatments | ||
|---|---|---|---|
| RHC-HY | RHS-HY | p-Value | |
| WBC, 109/L | 13.55 ± 3.72 | 14.06 ± 4.35 | NS |
| Neu% | 40.23 ± 7.68 | 45.57 ± 6.54 | NS |
| Lym% | 41.17 ± 5.08 | 41.57 ± 2.59 | NS |
| Mon% | 6.33 ± 1.91 | 6.27 ± 0.35 | NS |
| Eos% | 11.63 ± 11.52 | 6.43 ± 4.48 | NS |
| Bas% | 0.63 ± 0.15 | 0.17 ± 0.12 | <0.05 |
| Neu#, 109/L | 5.38 ± 1.39 | 6.25 ± 1.21 | NS |
| Lym#, 109/L | 5.46 ± 0.87 | 5.90 ± 2.05 | NS |
| Mon#, 109/L | 0.81 ± 0.07 | 0.87 ± 0.22 | NS |
| Eos#, 109/L | 0.62 ± 0.09 | 1.46 ± 0.53 | NS |
| Bas# | 0.08 ± 0.02 | 0.02 ± 0.01 | <0.05 |
| RBC, 1012/L | 5.64 ± 0.32 | 8.18 ± 1.19 | <0.01 |
| HGB, g/L | 112.33 ± 3.21 | 137.67 ± 3.515 | <0.001 |
| HCT, % | 33.70 ± 31.25 | 44.70 ± 7.03 | <0.001 |
| MCV, fL | 57.07 ± 5.98 | 54.67 ± 2.15 | NS |
| MCH, pg | 19.30 ± 1.51 | 18.70 ± 0.80 | NS |
| MCHC, g/L | 338.67 ± 9.50 | 341.67 ± 9.07 | NS |
| RDW-CV, % | 17.23 ± 0.06 | 16.53 ± 0.40 | <0.05 |
| RDW-SD, fL | 39.83 ± 4.41 | 36.60 ± 0.78 | NS |
| PLT, 109/L | 318.00 ± 6.24 | 302.00 ± 61.00 | NS |
| MPV, fL | 10.47 ± 1.01 | 10.43 ± 1.12 | NS |
| PDW | 19.50 ± 0.75 | 17.87 ± 1.65 | NS |
| PCT, % | 0.19 ± 0.12 | 0.31 ± 0.04 | NS |
| Item | Treatments | ||
|---|---|---|---|
| RHC-HY | RHS-HY | p-Value | |
| Blood pH | 7.53 ± 0.17 | 7.56 ± 0.03 | NS |
| Blood PCO2, mmHg | 23.60 ± 7.56 | 30.70 ± 3.64 | NS |
| Blood PO2, mmHg | 121.67 ± 7.51 | 50.67 ± 8.39 | <0.001 |
| Blood BEecf, mmol/L | 2.33 ± 1.15 | 5.33 ± 1.53 | NS |
| Blood HCO3, mmol/L | 23.50 ± 1.18 | 25.83 ± 3.35 | NS |
| Blood TCO2, mmol/L | 24.00 ± 1.00 | 28.67 ± 1.53 | <0.05 |
| Blood SO2% | 97.67 ± 3.21 | 89.67 ± 5.86 | NS |
| Blood Na, mmol/L | 137.67 ± 2.52 | 144.00 ± 1.00 | <0.05 |
| Blood K, mmol/L | 6.47 ± 0.55 | 5.23 ± 0.91 | NS |
| Blood Ca, mmol/L | 1.18 ± 0.13 | 1.34 ± 0.03 | NS |
| Blood Hct | 31.67 ± 6.51 | 41.00 ± 1.73 | NS |
| Blood Hb, g/dL | 8.83 ± 1.92 | 13.97 ± 0.58 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, J.; Wen, Z.; Chen, L.; Pu, Q.; Luo, T.; Wu, X.; Ma, Z.; Luo, Z.; Luo, J.; Wang, J. Multi-Omics Analysis of Chronic Heat Stress-Induced Biological Effects, Liver Injury, and Heat Tolerance Mechanisms via Oxidative and Anti-Inflammatory Pathways in Early-Pregnancy Sows. Antioxidants 2025, 14, 623. https://doi.org/10.3390/antiox14060623
Chai J, Wen Z, Chen L, Pu Q, Luo T, Wu X, Ma Z, Luo Z, Luo J, Wang J. Multi-Omics Analysis of Chronic Heat Stress-Induced Biological Effects, Liver Injury, and Heat Tolerance Mechanisms via Oxidative and Anti-Inflammatory Pathways in Early-Pregnancy Sows. Antioxidants. 2025; 14(6):623. https://doi.org/10.3390/antiox14060623
Chicago/Turabian StyleChai, Jie, Zhenhao Wen, Li Chen, Qiang Pu, Taorun Luo, Xiaoqian Wu, Zihan Ma, Zonggang Luo, Jia Luo, and Jingyong Wang. 2025. "Multi-Omics Analysis of Chronic Heat Stress-Induced Biological Effects, Liver Injury, and Heat Tolerance Mechanisms via Oxidative and Anti-Inflammatory Pathways in Early-Pregnancy Sows" Antioxidants 14, no. 6: 623. https://doi.org/10.3390/antiox14060623
APA StyleChai, J., Wen, Z., Chen, L., Pu, Q., Luo, T., Wu, X., Ma, Z., Luo, Z., Luo, J., & Wang, J. (2025). Multi-Omics Analysis of Chronic Heat Stress-Induced Biological Effects, Liver Injury, and Heat Tolerance Mechanisms via Oxidative and Anti-Inflammatory Pathways in Early-Pregnancy Sows. Antioxidants, 14(6), 623. https://doi.org/10.3390/antiox14060623






