Endosomal H2O2 Molecules Act as Signaling Mediators in Akt/PKB Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Transfection
2.3. Plasmids and siRNAs
2.4. Confocal Microscopy and Immunofluorescence
2.5. Immunoblot Analysis
2.6. Endocytosis Analysis
2.7. Statistical Analysis
3. Results
3.1. H2O2 Production in Early Endosomes During Epidermal Growth Factor (EGF) Activation Is Demonstrated via HyPer-Endo, an Endosomal-Targeting H2O2 Reporter
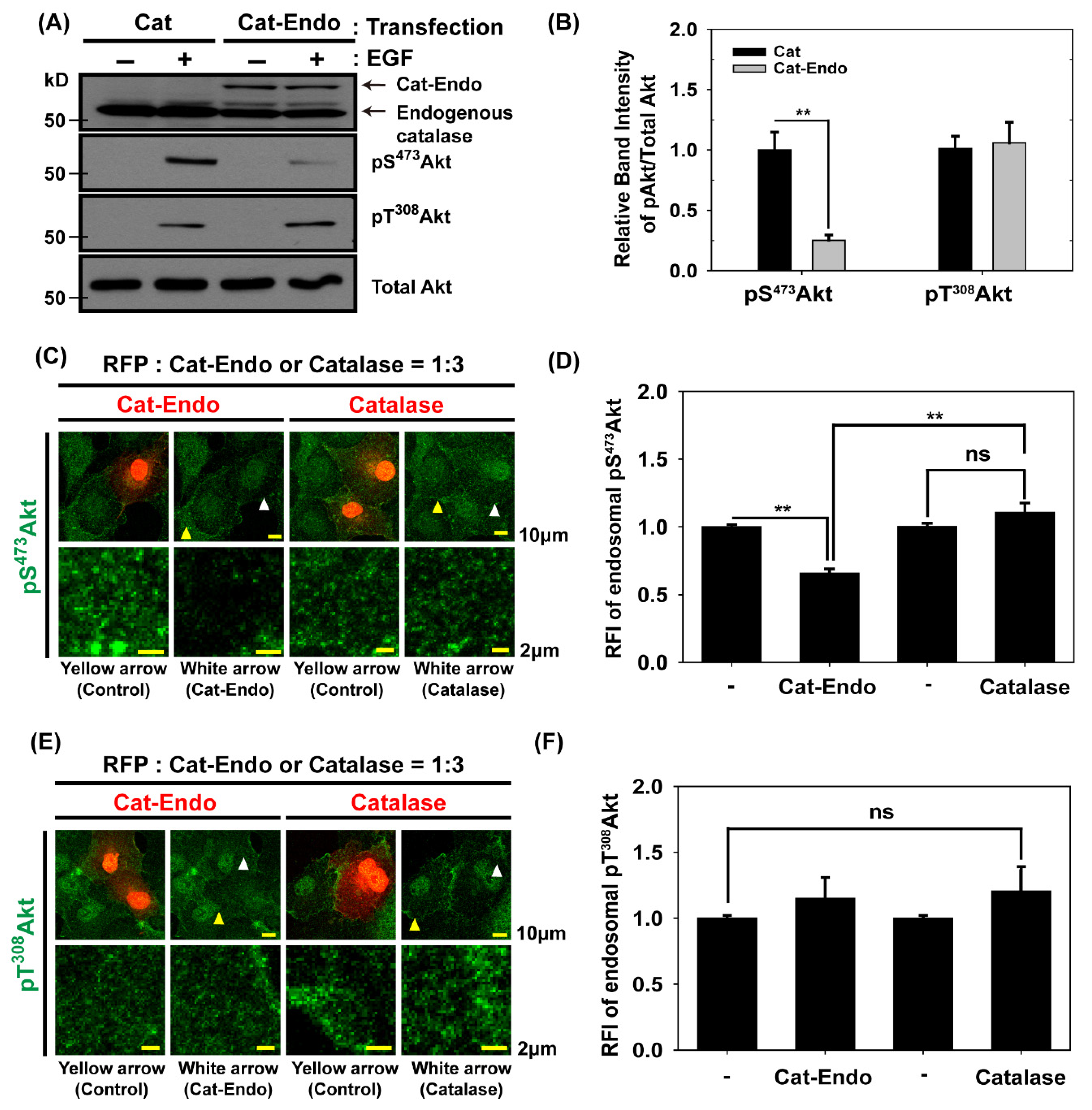
3.2. Akt Activation by H2O2 Production in Cells Activated by Growth Factors
3.3. Endosomal H2O2 Production Is Required for Akt Activation Through the Phosphorylation of Akt on Ser473
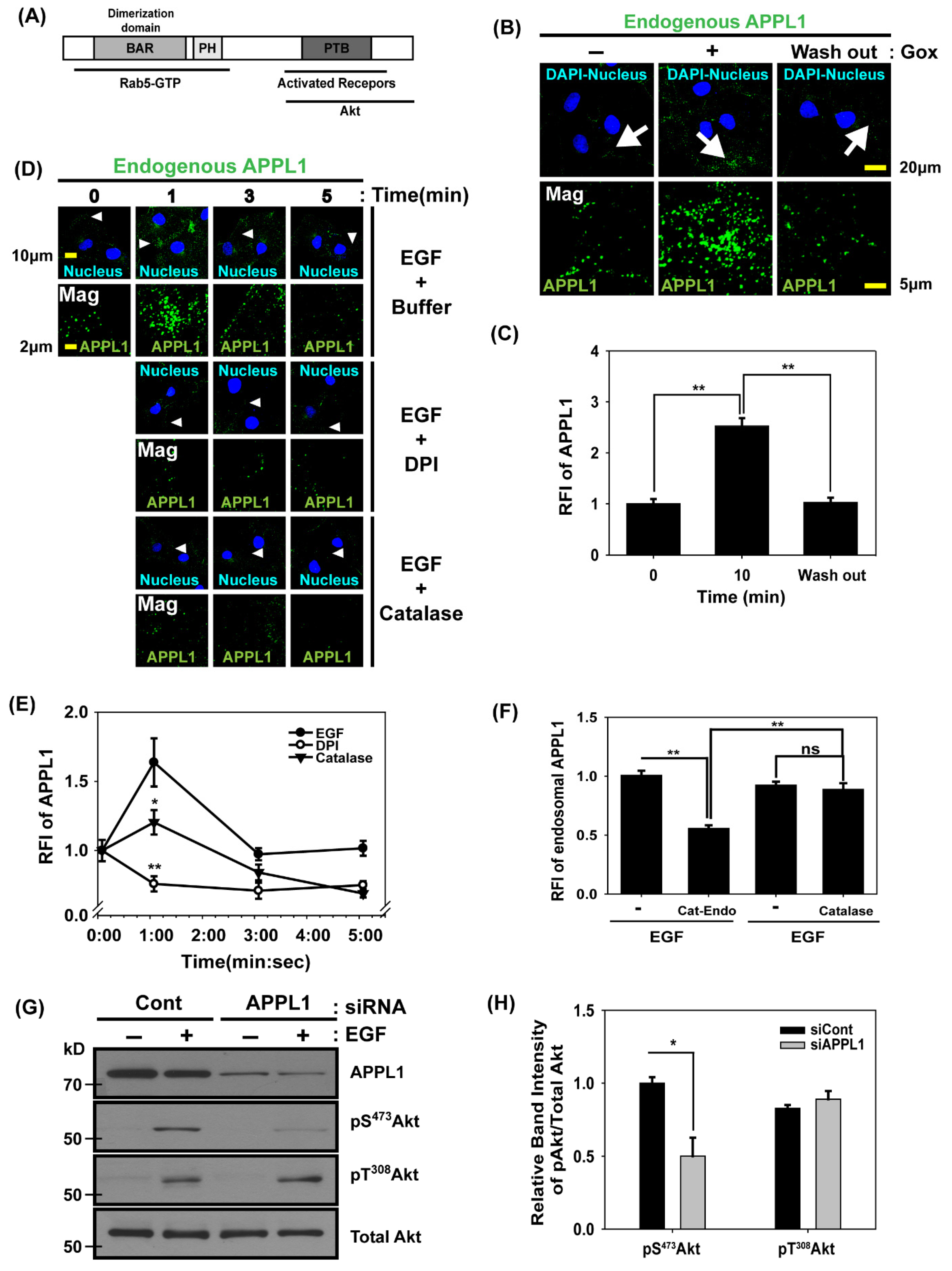
3.4. APPL1, the Rab5 Effector and Akt Binding Protein, Is Localized in Early Endosomes Dependent on H2O2 Production
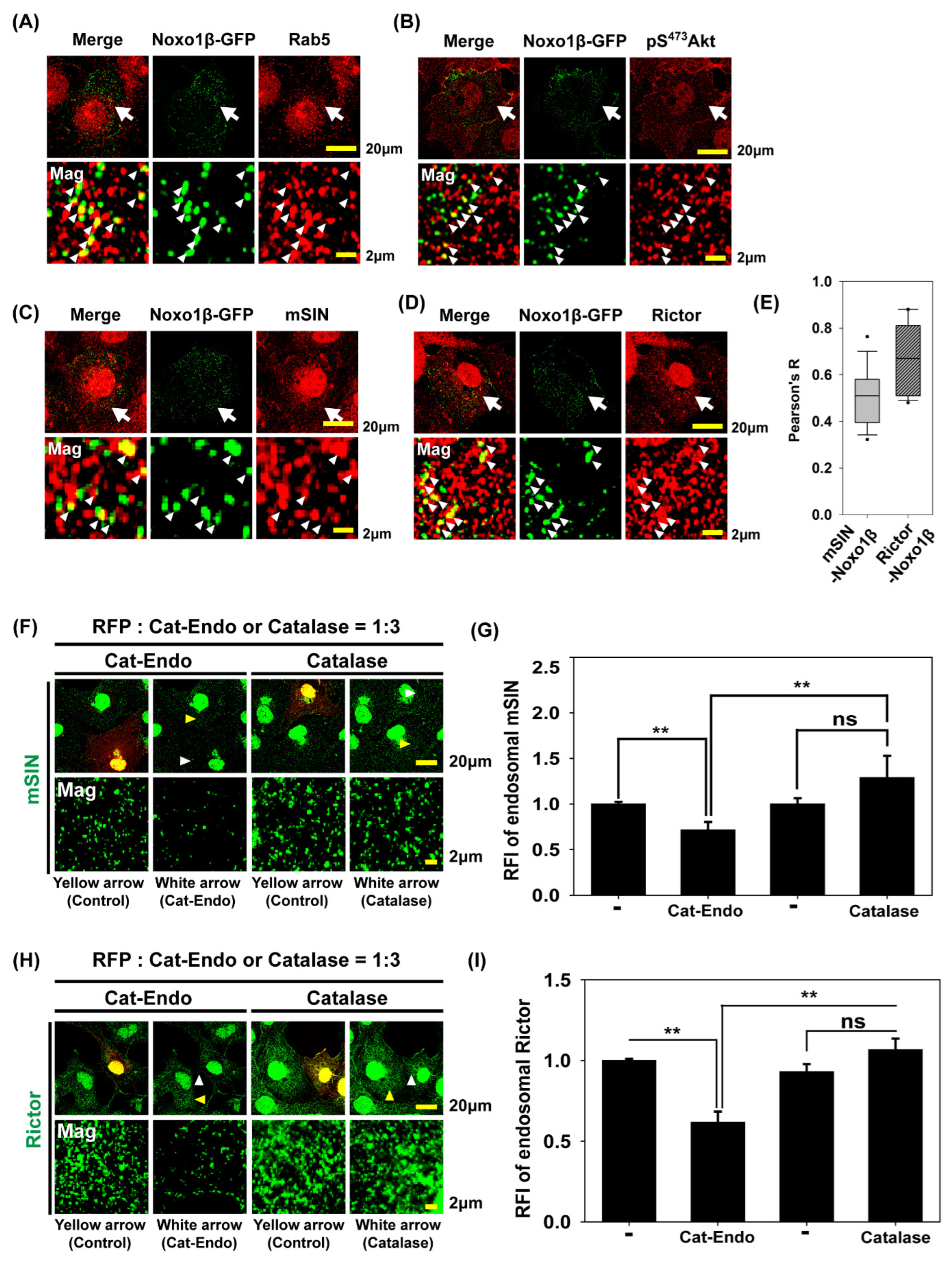
3.5. Endosomal H2O2 via NADPH Oxidase (Nox) Complex Is Responsible for Akt Phosphorylation at S473 and mTORC2 Localization into Early Endosomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APPL1 | Adaptor protein containing PH domain, PTB domain, and Leucine zipper motif 1 |
| CFP | Cyan fluorescent protein |
| DPI | Diphenyleneiodonium chloride |
| EEA1 | Early endosome antigen 1 |
| EGF | Epidermal growth factor |
| FOXO1/3 | Forkhead box O1/O3 |
| GFP | Green fluorescent protein |
| GOx | Glucose oxidase |
| GSK 3 | Glycogen synthase kinase 3 |
| H2O2 | Hydrogen peroxide |
| IGF | Insulin-like growth factor |
| mSIN | Mammalian Sty1/Spc1-interacting protein |
| mTORC2 | Mechanistic target of rapamycin (mTor) complex 2 |
| Nox | NADPH oxidase |
| Noxo1 | NADPH oxidase organizer 1 |
| PDGF | Platelet-derived growth factor |
| PDK1 | Phosphoinositide-dependent protein kinase 1 |
| PI3K | Phosphoinositide 3-kinase |
| Prx | Peroxiredoxin |
| PtdIns | Phosphatidylinositol |
| PTEN | Phosphatase and tensin homolog |
| PTP | Protein tyrosine phosphatase |
| Redoxosome | Redox-active endosome |
| Rictor | Rapamycin-insensitive companion of mTOR |
| RME | Receptor-mediated endocytosis |
| ROS | Reactive oxygen species |
| SHIP | The SH2-containing inositol 5′-phosphatase |
| TSC2 | Tuberous sclerosis 2 |
References
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Lim, J.M.; Lee, K.S.; Woo, H.A.; Kang, D.; Rhee, S.G. Control of the pericentrosomal H2O2 level by peroxiredoxin I is critical for mitotic progression. J. Cell Biol. 2015, 210, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Oakley, F.D.; Abbott, D.; Li, Q.; Engelhardt, J.F. Signaling components of redox active endosomes: The redoxosomes. Antioxid. Redox Signal. 2009, 11, 1313–1333. [Google Scholar] [CrossRef]
- Rhee, S.G.; Kil, I.S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2017, 86, 749–775. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M. Localizing NADPH oxidase-derived ROS. Sci. STKE Signal Transduct. Knowl. Environ. 2006, 2006, re8. [Google Scholar] [CrossRef]
- Woo, H.A.; Yim, S.H.; Shin, D.H.; Kang, D.; Yu, D.Y.; Rhee, S.G. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 2010, 140, 517–528. [Google Scholar] [CrossRef]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C.; Toker, A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 1997, 275, 665–668. [Google Scholar] [CrossRef]
- Guilherme, A.; Klarlund, J.K.; Krystal, G.; Czech, M.P. Regulation of phosphatidylinositol 3,4,5-trisphosphate 5′-phosphatase activity by insulin. J. Biol. Chem. 1996, 271, 29533–29536. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Stokoe, D.; Stephens, L.R.; Copeland, T.; Gaffney, P.R.; Reese, C.B.; Painter, G.F.; Holmes, A.B.; McCormick, F.; Hawkins, P.T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 1997, 277, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, S.R.; Yang, K.S.; Ahn, Y.; Kim, Y.J.; Stadtman, E.R.; Rhee, S.G. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA 2004, 101, 16419–16424. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Yang, K.S.; Kwon, J.; Lee, C.; Jeong, W.; Rhee, S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002, 277, 20336–20342. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Kim, S.; Heo, S.; Brzostowski, J.; Kang, D. Endosomal mTORC2 Is Required for Phosphoinositide-Dependent AKT Activation in Platelet-Derived Growth Factor-Stimulated Glioma Cells. Cancers 2021, 13, 2405. [Google Scholar] [CrossRef]
- Braccini, L.; Ciraolo, E.; Campa, C.C.; Perino, A.; Longo, D.L.; Tibolla, G.; Pregnolato, M.; Cao, Y.; Tassone, B.; Damilano, F.; et al. PI3K-C2gamma is a Rab5 effector selectively controlling endosomal Akt2 activation downstream of insulin signalling. Nat. Commun. 2015, 6, 7400. [Google Scholar] [CrossRef]
- Li Chew, C.; Lunardi, A.; Gulluni, F.; Ruan, D.T.; Chen, M.; Salmena, L.; Nishino, M.; Papa, A.; Ng, C.; Fung, J.; et al. In Vivo Role of INPP4B in Tumor and Metastasis Suppression through Regulation of PI3K-AKT Signaling at Endosomes. Cancer Discov. 2015, 5, 740–751. [Google Scholar] [CrossRef]
- Menon, S.; Dibble, C.C.; Talbott, G.; Hoxhaj, G.; Valvezan, A.J.; Takahashi, H.; Cantley, L.C.; Manning, B.D. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 2014, 156, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Perera, R.M.; Balkin, D.M.; Pirruccello, M.; Toomre, D.; De Camilli, P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 2009, 136, 1110–1121. [Google Scholar] [CrossRef]
- Di Fiore, P.P.; De Camilli, P. Endocytosis and signaling. an inseparable partnership. Cell 2001, 106, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Padilla, B.E.; Hasdemir, B.; Cottrell, G.S.; Bunnett, N.W. Endosomes: A legitimate platform for the signaling train. Proc. Natl. Acad. Sci. USA 2009, 106, 17615–17622. [Google Scholar] [CrossRef] [PubMed]
- von Zastrow, M.; Sorkin, A. Signaling on the endocytic pathway. Curr. Opin. Cell Biol. 2007, 19, 436–445. [Google Scholar] [CrossRef]
- Cheng, K.K.; Iglesias, M.A.; Lam, K.S.; Wang, Y.; Sweeney, G.; Zhu, W.; Vanhoutte, P.M.; Kraegen, E.W.; Xu, A. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009, 9, 417–427. [Google Scholar] [CrossRef]
- Miaczynska, M.; Christoforidis, S.; Giner, A.; Shevchenko, A.; Uttenweiler-Joseph, S.; Habermann, B.; Wilm, M.; Parton, R.G.; Zerial, M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 2004, 116, 445–456. [Google Scholar] [CrossRef]
- Schenck, A.; Goto-Silva, L.; Collinet, C.; Rhinn, M.; Giner, A.; Habermann, B.; Brand, M.; Zerial, M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 2008, 133, 486–497. [Google Scholar] [CrossRef]
- Mitsuuchi, Y.; Johnson, S.W.; Sonoda, G.; Tanno, S.; Golemis, E.A.; Testa, J.R. Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 1999, 18, 4891–4898. [Google Scholar] [CrossRef]
- Lin, D.C.; Quevedo, C.; Brewer, N.E.; Bell, A.; Testa, J.R.; Grimes, M.L.; Miller, F.D.; Kaplan, D.R. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol. Cell. Biol. 2006, 26, 8928–8941. [Google Scholar] [CrossRef]
- Varsano, T.; Dong, M.Q.; Niesman, I.; Gacula, H.; Lou, X.; Ma, T.; Testa, J.R.; Yates, J.R., 3rd; Farquhar, M.G. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol. Cell. Biol. 2006, 26, 8942–8952. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Lam, K.S.; Wang, Y.; Huang, Y.; Carling, D.; Wu, D.; Wong, C.; Xu, A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 2007, 56, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Kikani, C.K.; Riojas, R.A.; Langlais, P.; Wang, L.; Ramos, F.J.; Fang, Q.; Christ-Roberts, C.Y.; Hong, J.Y.; Kim, R.Y.; et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 2006, 8, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, J.M.; Park, S.H.; Kim, S.; Heo, S.; Balla, T.; Jeong, W.; Rhee, S.G.; Kang, D. Inactivation of the PtdIns(4)P phosphatase Sac1 at the Golgi by H2O2 produced via Ca2+-dependent Duox in EGF-stimulated cells. Free Radic. Biol. Med. 2019, 131, 40–49. [Google Scholar] [CrossRef]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chang, T.S.; Jeong, W.; Kang, D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef]
- Poburko, D.; Santo-Domingo, J.; Demaurex, N. Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J. Biol. Chem. 2011, 286, 11672–11684. [Google Scholar] [CrossRef]
- Li, Q.; Harraz, M.M.; Zhou, W.; Zhang, L.N.; Ding, W.; Zhang, Y.; Eggleston, T.; Yeaman, C.; Banfi, B.; Engelhardt, J.F. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol. Cell. Biol. 2006, 26, 140–154. [Google Scholar] [CrossRef]
- Li, Q.; Spencer, N.Y.; Oakley, F.D.; Buettner, G.R.; Engelhardt, J.F. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid. Redox Signal. 2009, 11, 1249–1263. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Harizanova, J.; Stockert, R.; Schroder, K.; Bastiaens, P.I.H.; Neel, B.G. Assay to visualize specific protein oxidation reveals spatio-temporal regulation of SHP2. Nat. Commun. 2017, 8, 466. [Google Scholar] [CrossRef]
- Calleja, V.; Alcor, D.; Laguerre, M.; Park, J.; Vojnovic, B.; Hemmings, B.A.; Downward, J.; Parker, P.J.; Larijani, B. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007, 5, e95. [Google Scholar] [CrossRef] [PubMed]
- Calleja, V.; Laguerre, M.; Parker, P.J.; Larijani, B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: Structural mechanism for allosteric inhibition. PLoS Biol. 2009, 7, e17. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dibble, C.C.; Matsuzaki, M.; Manning, B.D. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol. Cell. Biol. 2008, 28, 4104–4115. [Google Scholar] [CrossRef]
- Lim, J.M.; Park, S.; Lee, M.S.; Balla, T.; Kang, D.; Rhee, S.G. Accumulation of PtdIns(4)P at the Golgi mediated by reversible oxidation of the PtdIns(4)P phosphatase Sac1 by H2O2. Free Radic. Biol. Med. 2019, 130, 426–435. [Google Scholar] [CrossRef]
- Scheid, M.P.; Huber, M.; Damen, J.E.; Hughes, M.; Kang, V.; Neilsen, P.; Prestwich, G.D.; Krystal, G.; Duronio, V. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: Studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J. Biol. Chem. 2002, 277, 9027–9035. [Google Scholar] [CrossRef]
- Howe, C.L.; Mobley, W.C. Signaling endosome hypothesis: A cellular mechanism for long distance communication. J. Neurobiol. 2004, 58, 207–216. [Google Scholar] [CrossRef]
- Miaczynska, M.; Pelkmans, L.; Zerial, M. Not just a sink: Endosomes in control of signal transduction. Curr. Opin. Cell Biol. 2004, 16, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Scita, G.; Di Fiore, P.P. The endocytic matrix. Nature 2010, 463, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.J.; Mitin, N.; Keller, P.J.; Chenette, E.J.; Madigan, J.P.; Currin, R.O.; Cox, A.D.; Wilson, O.; Kirschmeier, P.; Der, C.J. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J. Biol. Chem. 2008, 283, 25150–25163. [Google Scholar] [CrossRef]
- Diggins, N.L.; Webb, D.J. APPL1 is a multifunctional endosomal signaling adaptor protein. Biochem. Soc. Trans. 2017, 45, 771–779. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. Biological roles for the NOX family NADPH oxidases. J. Biol. Chem. 2008, 283, 16961–16965. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Cornu, M.; Cybulski, N.; Polak, P.; Betz, C.; Trapani, F.; Terracciano, L.; Heim, M.H.; Ruegg, M.A.; Hall, M.N. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012, 15, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, E.; Facchinetti, V.; Liu, D.; Soto, N.; Wei, S.; Jung, S.Y.; Huang, Q.; Qin, J.; Su, B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006, 127, 125–137. [Google Scholar] [CrossRef]
- Stephens, L.; Anderson, K.; Stokoe, D.; Erdjument-Bromage, H.; Painter, G.F.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; McCormick, F.; Tempst, P.; et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 1998, 279, 710–714. [Google Scholar] [CrossRef]
- Thapa, N.; Chen, M.; Horn, H.T.; Choi, S.; Wen, T.; Anderson, R.A. Phosphatidylinositol-3-OH kinase signalling is spatially organized at endosomal compartments by microtubule-associated protein 4. Nat. Cell Biol. 2020, 22, 1357–1370. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A.; Kang, D. The role of peroxiredoxins in the transduction of H2O2 signals. Antioxid. Redox Signal. 2017, 28, 537–557. [Google Scholar] [CrossRef]
- Tonks, N.K. Redox redux: Revisiting PTPs and the control of cell signaling. Cell 2005, 121, 667–670. [Google Scholar] [CrossRef]
- Stone, J.R.; Yang, S. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef]
- Jo, S.I.; Kim, S.; Lim, J.M.; Rhee, S.G.; Jeong, B.G.; Cha, S.S.; Chang, J.B.; Kang, D. Control of the signaling role of PtdIns(4)P at the plasma membrane through H2O2-dependent inactivation of synaptojanin 2 during endocytosis. Redox Biol. 2024, 71, 103097. [Google Scholar] [CrossRef]
- Shahin, W.S.; Engelhardt, J.F. Isolation of Redox-Active Endosomes (Redoxosomes) and Assessment of NOX Activity. Methods Mol. Biol. 2019, 1982, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Hebchen, D.M.; Schroder, K. Redox Signaling in Endosomes Using the Example of EGF Receptors: A Graphical Review. Antioxidants 2024, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.Z.; Su, Q.P.; Cui, Y.; Bahnson, E.M.; Li, G.; Wang, M.; Yang, Y.; Collins, J.A.; Wu, D.; Gu, Q.; et al. Redox-active endosomes mediate alpha5beta1 integrin signaling and promote chondrocyte matrix metalloproteinase production in osteoarthritis. Sci. Signal. 2023, 16, eadf8299. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, C.; Heo, S.; Kang, D. Endosomal H2O2 Molecules Act as Signaling Mediators in Akt/PKB Activation. Antioxidants 2025, 14, 594. https://doi.org/10.3390/antiox14050594
Park S, Kim C, Heo S, Kang D. Endosomal H2O2 Molecules Act as Signaling Mediators in Akt/PKB Activation. Antioxidants. 2025; 14(5):594. https://doi.org/10.3390/antiox14050594
Chicago/Turabian StylePark, Sujin, Chaewon Kim, Sukyeong Heo, and Dongmin Kang. 2025. "Endosomal H2O2 Molecules Act as Signaling Mediators in Akt/PKB Activation" Antioxidants 14, no. 5: 594. https://doi.org/10.3390/antiox14050594
APA StylePark, S., Kim, C., Heo, S., & Kang, D. (2025). Endosomal H2O2 Molecules Act as Signaling Mediators in Akt/PKB Activation. Antioxidants, 14(5), 594. https://doi.org/10.3390/antiox14050594





