Role and Functions of Irisin: A Perspective on Recent Developments and Neurodegenerative Diseases
Abstract
1. Introduction
Irisin: Biogenesis, Structure and Tissue Distribution
2. Irisin’s Function and Systemic Effects
2.1. Impact on Muscle, Bone and Other Peripheral Tissues
2.2. Irisin’s Neuroprotective Role and CNS Relevance
2.3. Protective Effects of Irisin: Apoptosis
2.4. Protective Effects of Irisin: Autophagy
2.5. Protective Effects of Irisin: Mitochondrial Homeostasis
2.6. Protective Effects of Irisin: Anti-Inflammatory and Antioxidant Actions
3. Molecular Cascades and Receptor Interactions
3.1. FNDC5 Expression and Cleavage
3.2. Receptor Identification: The Role of Integrins
3.3. Downstream Signaling Pathways
3.3.1. AMPK Pathway
3.3.2. MAPK/ERK Pathway
3.3.3. PI3K/AKT Pathway
3.3.4. cAMP/PKA/CREB Pathways
3.3.5. NRF2 and NF-κB Pathways
3.3.6. Other Pathways
4. Irisin and Neurodegenerative Diseases
4.1. Irisin and Alzheimer’s Disease
4.2. Irisin and Parkinson’s Disease
4.3. Irisin and Amyotrophic Lateral Sclerosis
4.4. Irisin and Frontotemporal Dementia
4.5. Irisin and Multiple Sclerosis
5. Potential Applications of Irisin
5.1. Irisin as a Biomarker and Therapeutic Target: Potential and Challenges
5.2. Irisin Diagnostic Potential in Metabolic Diseases
5.3. Challenges in Irisin Detection and Standardization
6. Conclusions: Present and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-syn | α-synuclein |
| AβPP | amyloid-β precursor protein |
| ACSL4 | Acyl-CoA Synthetase Long-Chain Family Member 4 |
| AD | Alzheimer’s Disease |
| ADAM10 | ADAM Metallopeptidase Domain 10 |
| AKT | AKT Serine/Threonine Kinase |
| ALS | Amyotrophic lateral sclerosis |
| AMPK | AMP-activated protein kinase |
| ATF4 | Activating Transcription Factor 4 |
| Aβ | amyloid beta |
| BAX | BCL2 Associated X, Apoptosis Regulator |
| BBB | Blood-Brain Barrier |
| BCL2 | BCL2 Apoptosis Regulator |
| BDNF | Brain Derived Neurotrophic Factor |
| BMI | body-mass index |
| bvFTD | behavioral variant FTD |
| cAMP | Cyclic adenosine monophosphate |
| CASP3/9 | Caspase 3/9 |
| CAV1 | caveolin 1 |
| CMA | chaperone-mediated autophagy |
| CNS | Central Nervous System |
| CREB | cAMP response element-binding protein |
| DDIT3 | DNA Damage Inducible Transcript 3 |
| DG | dentate gyrus |
| DOAJ | Directory of open access journals |
| EIF2A | Eukaryotic Translation Initiation Factor 2A |
| FAK | focal adhesion kinase |
| FNDC5 | fibronectin type III domain-containing protein 5 |
| FNIII | fibronectin type III |
| FOXO1 | Forkhead box protein O1 |
| FTD | Frontotemporal dementia |
| G6Pase | Glucose-6-Phosphatase |
| GPX4 | Glutathione Peroxidase 4 |
| GSDMD | Gasdermin D |
| GSH | glutathione |
| GSK3 | Glycogen Synthase Kinase 3 |
| GYS1 | Glycogen synthase |
| HIF1α | hypoxia-inducible factor 1 subunit α |
| HMOX1 | heme oxygenase-1 |
| Hsp90α | Heat Shock Protein 90 Alpha |
| IGF1 | Insulin Like Growth Factor 1 |
| IGF1R | Insulin Like Growth Factor 1 Receptor |
| IL1β | Interleukin 1 Beta |
| IL6 | Interleukin 6 |
| iNOS | inducible nitric oxide synthase |
| IRF3 | interferon regulatory |
| IRS1 | Insulin Receptor Substrate 1 |
| IRS2 | Insulin Receptor Substrate 2 |
| IκB | Inhibitor of Nuclear Factor Kappa B Kinase |
| JAK2 | Janus Kinase 2 |
| LDH | lactate dehydrogenase |
| LD | Linear dichroism |
| LPS | lipopolysaccharide |
| MAP1LC3B | Microtubule Associated Protein 1 Light Chain 3 Beta |
| MAPK | Mitogen-Activated Protein Kinase |
| MAPK1 or ERK2 | Mitogen-Activated Protein Kinase 1 |
| MAPK14 or p38 | Mitogen-Activated Protein Kinase 14 |
| MAPK3 or ERK1 | Mitogen-Activated Protein Kinase 3 |
| MAPK8 | Mitogen-Activated Protein Kinase 8 |
| MBA | muscle-brain axis |
| MCT2 | monocarboxylate transporter 2 |
| MDA | malondialdehyde |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MFN2 | Mitofusin 2 |
| MS | Multiple sclerosis |
| MTOR | Mechanistic Target of Rapamycin Kinase |
| MT-CO2 | cyclooxygenase 2 |
| MYD88 | MYD88 Innate Immune Signal Transduction Adaptor |
| NCD | non-communicable disease |
| ncRNA | Non-coding RNA |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | nerve growth factor |
| NICD | Active Notch intracellular domain |
| NLRP3 | NLR Family Pyrin Domain Containing 3 |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| NRF1 | NFE1 Like BZIP Transcription Factor 1 |
| NRF2 or NFE2L2 | NFE2 Like BZIP Transcription Factor 2 |
| NTRK2 | Neurotrophic Receptor Tyrosine Kinase 2 |
| NURR1 | nuclear receptor related 1 protein |
| OPA1 | OPA1 Mitochondrial Dynamin Like GTPase |
| PCK | Phosphoenolpyruvate Carboxykinase |
| PD | Parkinson’s disease |
| PFFs | Preformed fibrils |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase |
| PINK1 | PTEN Induced Kinase 1 |
| PKA | Protein Kinase cAMP-Activated |
| POCD | Postoperative cognitive dysfunction |
| PPARγ | Peroxisome Proliferator Activated Receptor Gamma |
| PRKN | Parkin |
| ROS | reactive oxygen species |
| RRMS | relapsing-remitting MS |
| SIRT1 | Sirtuin 1 |
| SLC2A or GLUT4 | Solute Carrier Family 2 (Facilitated Glucose Transporter), Member 4 |
| SMAD3 | SMAD Family Member 3 |
| SNAI1 | Snail Family Transcriptional Repressor 1 |
| SOD | superoxide dismutase |
| SOD1 | superoxide dismutase type 1 |
| SQSTM1 | Sequestosome 1 |
| SREBF2 | Sterol Regulatory Element Binding Transcription Factor 2 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STAT6 | Signal Transducer and Activator of Transcription 6 |
| TCA | tricarboxylic acid cycle |
| TFAM | Transcription Factor A, Mitochondrial |
| TGFβ | Transforming growth factor β |
| TLA | Three letter acronym |
| TLR4 | Toll-like receptor 4 |
| TNFα | Tumor Necrosis Factor |
| UCP1 | uncoupling protein 1 |
| ULK1 | Unc-51 Like Autophagy Activating Kinase 1 |
| VEGF | vascular endothelial growth factor |
| VO2max | maximal oxygen uptake |
References
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Alkadhi, K.A.; Dao, A.T. Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 86, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.C.; Grabowska, W.A.; Chun, Y.; Risacher, S.L.; Philip, V.M.; Saykin, A.J.; Rizzo, S.J.S.; Howell, G.R. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol. Aging 2019, 80, 154–172. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.A.L.; Harmer, A.R.; Freitas, D.A.; Mendonça, V.A.; Lacerda, A.C.R.; Leite, H.R. An update on potential links between type 2 diabetes mellitus and Alzheimer's disease. Mol. Biol. Rep. 2020, 47, 6347–6356. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Diniz-Magalhaes, C.O.; Cruz, P.P.; de Oliveira, G.H.B.; Prates, J.T.A.C.; de Azevedo Ferreira, C.M.; Silva, R.R.; Cassilhas, R.C. Physical Exercise Inhibits Cognitive Impairment and Memory Loss in Aged Mice, and Enhances Pre- and Post-Synaptic Proteins in the Hippocampus of Young and Aged Mice. Neuromol. Med. 2024, 26, 31. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Pahlavani, H.A.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and mitochondrial mechanisms in patients with sarcopenia. Front. Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Zunner, B.E.M.; Wachsmuth, N.B.; Eckstein, M.L.; Scherl, L.; Schierbauer, J.R.; Haupt, S.; Stumpf, C.; Reusch, L.; Moser, O. Myokines and Resistance Training: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3501. [Google Scholar] [CrossRef]
- Vints, W.A.J.; Gökçe, E.; Langeard, A.; Pavlova, I.; Çevik, Ö.S.; Ziaaldini, M.M.; Todri, J.; Lena, O.; Sakkas, G.K.; Jak, S.; et al. Myokines as mediators of exercise-induced cognitive changes in older adults: Protocol for a comprehensive living systematic review and meta-analysis. Front. Aging Neurosci. 2023, 15, 1213057. [Google Scholar] [CrossRef]
- Oudbier, S.J.; Goh, J.; Looijaard, S.M.L.M.; Reijnierse, E.M.; Meskers, C.G.M.; Maier, A.B. Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 77, 1959–1968. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.-Y.; Moon, S.; Park, D.-H.; Kwak, H.-B.; Kang, J.-H. Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflug. Arch. 2019, 471, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Vints, W.A.; Levin, O.; Fujiyama, H.; Verbunt, J.; Masiulis, N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front. Neuroendocr. 2022, 66, 100993. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Elbelt, U.; Hofmann, T.; Stengel, A. Irisin: What promise does it hold? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 541–547. [Google Scholar] [CrossRef]
- Irving, B.A.; Still, C.D.; Argyropoulos, G. Does IRISIN Have a BRITE Future as a Therapeutic Agent in Humans? Curr. Obes. Rep. 2014, 3, 235–241. [Google Scholar] [CrossRef]
- Chen, N.; Li, Q.; Liu, J.; Jia, S. Irisin, an exercise-induced myokine as a metabolic regulator: An updated narrative review. Diabetes Metab. Res. Rev. 2015, 32, 51–59. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Improta-Caria, A.C.; Jesus-Silva, F.M.; Magalhães, C.O.D.E.; Freitas, D.A.; Lacerda, A.C.R.; Mendonça, V.A.; Cassilhas, R.C.; Leite, H.R. High-intensity resistance training induces changes in cognitive function, but not in locomotor activity or anxious behavior in rats induced to type 2 diabetes. Physiol. Behav. 2020, 223, 112998. [Google Scholar] [CrossRef]
- Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Cadena-Ullauri, S.; Frias-Toral, E.; Guevara-Ramírez, P.; Paz-Cruz, E.; Chapela, S.; Montalván, M.; Morales-López, T.; Simancas-Racines, D.; et al. The Molecular Mechanisms of the Relationship between Insulin Resistance and Parkinson’s Disease Pathogenesis. Nutrients 2023, 15, 3585. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Lin, S. Irisin: A bridge between exercise and neurological diseases. Heliyon 2022, 8, e12352. [Google Scholar] [CrossRef] [PubMed]
- Küster, O.C.; Laptinskaya, D.; Fissler, P.; Schnack, C.; Zügel, M.; Nold, V.; Thurm, F.; Pleiner, S.; Karabatsiakis, A.; von Einem, B.; et al. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J. Alzheimers Dis. 2017, 59, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.A.L.; Lima, N.S.; Amorim, F.T.; Gripp, F.; Magalhães, C.O.D.E.; Pinto, S.H.; Dias-Peixoto, M.F.; Monteiro-Junior, R.S.; Bourbeau, K.; Cassilhas, R.C. Endurance and high-intensity interval training improve the levels of anxiety and quality of life in overweight men. Rev. Assoc. Med. Bras. (1992) 2021, 67, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Bos, I.; De Boever, P.; Panis, L.I.; Meeusen, R. Physical Activity, Air Pollution and the Brain. Sports Med. 2014, 44, 1505–1518. [Google Scholar] [CrossRef]
- Gripp, F.; de Jesus Gomes, G.; De Sousa, R.A.L.; Alves de Andrade, J.; Pinheiro Queiroz, I.; Diniz Magalhães, C.O.; Cassilhas, R.C.; de Castro Magalhães, F.; Amorim, F.T.; Dias-Peixoto, M.F. A Real-World High-Intensity Interval Training Protocol for Cardiorespiratory Fitness Improvement. J.Vis. Exp. 2022, 180, e63708. [Google Scholar] [CrossRef]
- Nie, Y.; Dai, B.; Guo, X.; Liu, D. Cleavage of FNDC5 and insights into its maturation process. Mol. Cell. Endocrinol. 2020, 510, 110840. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef]
- Panati, K.; Narala, V.R.; Narasimha, V.R.; Derangula, M.; Tatireddigari, V.R.A.; Yeguvapalli, S. Expression, purification and biological characterisation of recombinant human irisin (12.5 kDa). J. Genet. Eng. Biotechnol. 2018, 16, 459–466. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; D’Souza, C.; Al Darmaki, R.S.M.H.; Baniyas, M.M.Y.H.; Adeghate, E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides 2018, 104, 15–23. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belén Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, I.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.; Drott, C.J.; Carlsson, P.O.; Espes, D. Irisin-A Pancreatic Islet Hormone. Biomedicines 2022, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Dun, S.L.; Lyu, R.M.; Chen, Y.H.; Chang, J.K.; Luo, J.J.; Dun, N.J. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Gür, F.; Timurkaan, S.; Yalcin, M.; Girgin, A.; Tarakçı, B.G. Immunohistochemical localization of irisin in mole rats (Spalax leucodon). Biotech. Histochem. 2017, 92, 245–251. [Google Scholar] [CrossRef]
- Teufel, A.; Malik, N.; Mukhopadhyay, M.; Westphal, H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene 2002, 297, 79–83. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Sun, Y.; Xu, H.; Li, N.; Wang, Y.; Tian, X.; Zhao, C.; Wang, B.; Zhu, B.; et al. Exercise ameliorates muscular excessive mitochondrial fission, insulin resistance and inflammation in diabetic rats via irisin/AMPK activation. Sci. Rep. 2024, 14, 10658. [Google Scholar] [CrossRef]
- Choi, J.-W.; Balakrishnan, R. Aerobic exercise–induced myokine irisin release: A novel strategy to promote neuroprotection and improve cognitive function. Neural Regen. Res. 2024, 21, 306–307. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Fu, S.; Li, Y.; Su, L.; Li, X.; Wu, G.; Jiang, J.; Zhao, Z.; Yang, C.; et al. Irisin reprograms microglia through activation of STAT6 and prevents cognitive dysfunction after surgery in mice. Brain Behav. Immun. 2024, 125, 68–91. [Google Scholar] [CrossRef]
- Witmer, N.H.; Linzer, C.R.; Boudreau, R.L. Fndc5 is translated from an upstream ATG start codon and cleaved to produce irisin myokine precursor protein in humans and mice. Cell Metab. 2024, 36, 879–881. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Alis, R.; Lippi, G. Circulating irisin detection: Does it really work? Trends Endocrinol. Metab. 2015, 26, 335–336. [Google Scholar] [CrossRef]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A myth rather than an exercise-inducible myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, M.; Tan, J.; Pei, X.; Lu, C.; Xin, Y.; Deng, S.; Zhao, F.; Gao, Y.; Gong, Y. Irisin ameliorates neuroinflammation and neuronal apoptosis through integrin alphaVbeta5/AMPK signaling pathway after intracerebral hemorrhage in mice. J. Neuroinflammation 2022, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Suresh, C.; Mottla, J.; Hamarneh, S.R.; Irazoqui, J.E.; Frontera, W.; Torriani, M.; Stanley, T.; Makimura, H. FNDC5 relates to skeletal muscle IGF-I and mitochondrial function and gene expression in obese men with reduced growth hormone. Growth Horm. IGF Res. 2015, 26, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.F.; She, Q.Y.; Hu, P.P.; Jia, N.; Li, A. Irisin, a fascinating field in our times. Trends Endocrinol. Metab. 2022, 33, 601–613. [Google Scholar] [CrossRef]
- Ellefsen, S.; Vikmoen, O.; Slettaløkken, G.; Whist, J.E.; Nygaard, H.; Hollan, I.; Rauk, I.; Vegge, G.; Strand, T.A.; Raastad, T.; et al. Irisin and FNDC5: Effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur. J. Appl. Physiol. 2014, 114, 1875–1888. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Zhu, H.-L.; Chang, W.; Zhang, Y.-F.; Ling, Q.; Wang, K.-W.; Zhang, J.; Zhang, Q.-B.; Kan, X.-L.; Wang, Q.-N.; et al. Maternal prednisone exposure during pregnancy elevates susceptibility to osteoporosis in female offspring: The role of mitophagy/FNDC5 alteration in skeletal muscle. J. Hazard. Mater. 2024, 469, 133997. [Google Scholar] [CrossRef]
- Shang, X.; Hao, X.; Hou, W.; Liu, J.; Chi, R.; Deng, X.; Pan, C.; Xu, T. Exercise-induced modulation of myokine irisin on muscle-bone unit in the rat model of post-traumatic osteoarthritis. J. Orthop. Surg. Res. 2024, 19, 49. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Z.; Wang, W.; Cui, P.; Kong, C.; Chen, X.; Lu, S. Irisin as an agent for protecting against osteoporosis: A review of the current mechanisms and pathways. J. Adv. Res. 2023, 62, 175–186. [Google Scholar] [CrossRef]
- Deng, W.; Xu, M.; Dong, R.; Yan, Y.; Jiang, Q. Irisin promotes tilapia muscle cell growth and amino acid uptake via IGF-1 signaling. J. Endocrinol. 2024, 262, e240122. [Google Scholar] [CrossRef]
- Xin, C.; Liu, J.; Zhang, J.D.; Zhu, D.; Wang, H.; Xiong, L.; Lee, Y.; Ye, J.; Lian, K.; Xu, C.; et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int. J. Obes. 2016, 40, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.-I.; Oh, Y.; Kim, J.H.; et al. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-Induced Irisin Secretion Is Independent of Age or Fitness Level and Increased Irisin May Directly Modulate Muscle Metabolism Through AMPK Activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wang, J.; Wang, K.; Liu, Q.; Li, H.; Xu, S.; Gu, C.; Zhu, Y. Exerkine FNDC5/irisin-enriched exosomes promote proliferation and inhibit ferroptosis of osteoblasts through interaction with Caveolin. Aging Cell 2024, 23, e14181. [Google Scholar] [CrossRef]
- Colaianni, G.; Errede, M.; Sanesi, L.; Notarnicola, A.; Celi, M.; Zerlotin, R.; Storlino, G.; Pignataro, P.; Oranger, A.; Pesce, V.; et al. Irisin Correlates Positively With BMD in a Cohort of Older Adult Patients and Downregulates the Senescent Marker p21 in Osteoblasts. J. Bone Miner. Res. 2021, 36, 305–314. [Google Scholar] [CrossRef]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2019, 35, 766–775. [Google Scholar] [CrossRef]
- Pesce, M.; Ballerini, P.; Paolucci, T.; Puca, I.; Farzaei, M.H.; Patruno, A. Irisin and Autophagy: First Update. Int. J. Mol. Sci. 2020, 21, 7587. [Google Scholar] [CrossRef]
- Xing, S.; Ma, Y.; Song, B.; Bai, M.; Wang, K.; Song, W.; Cao, T.; Guo, C.; Zhang, Y.; Wang, Z.; et al. Irisin reshapes bone metabolic homeostasis to delay age-related osteoporosis by regulating the multipotent differentiation of BMSCs via Wnt pathway. Front. Mol. Biosci. 2025, 11, 1524978. [Google Scholar] [CrossRef]
- Li, H.; Luo, D.; Xie, W.; Ye, W.; Chen, J.; Alberton, P.; Zhang, M.; Feng, E.; Docheva, D.; Lin, D. Irisin reduces senile osteoporosis by inducing osteocyte mitophagy through Ampk activation. iScience 2024, 27, 111042. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Baamonde, I.; Baltar, J.; Castro, A.I.; Domínguez, E.; Collado, M.; Casanueva, F.F.; Pardo, M. Lack of Adipocyte-Fndc5/Irisin Expression and Secretion Reduces Thermogenesis and Enhances Adipogenesis. Sci. Rep. 2017, 7, 16289. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin Stimulates Browning of White Adipocytes Through Mitogen-Activated Protein Kinase p38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-Q.; Chen, D.; Sun, H.-J.; Ding, L.; Wang, J.-J.; Chen, Q.; Li, Y.-H.; Zhou, Y.-B.; Han, Y.; Zhang, F.; et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta 2015, 1852, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell 2018, 175, 1756–1768.e17, Erratum in Cell 2019, 178, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Gheit, R.E.A.E.; Younis, R.L.; El-Saka, M.H.; Emam, M.N.; Soliman, N.A.; El-Sayed, R.M.; Hafez, Y.M.; AbuoHashish, N.A.; Radwan, D.A.; Khaled, H.E.; et al. Irisin improves adiposity and exercise tolerance in a rat model of postmenopausal obesity through enhancing adipo-myocyte thermogenesis. J. Physiol. Biochem. 2022, 78, 897–913. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, Q.; Wang, Y.; Wei, S.; Yang, L.; Zhang, J.; Liu, C.; et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019, 20, 296–306. [Google Scholar] [CrossRef]
- Liu, J.-F.; Su, G.; Chen, L.-X.; Zhou, J.-P.; Gao, J.; Zhang, J.-J.; Wu, Q.-H.; Chen, W.; Chen, D.-Y.; Zhang, Z.-C. Irisin Attenuates Apoptosis Following Ischemia–Reperfusion Injury Through Improved Mitochondria Dynamics and ROS Suppression Mediated Through the PI3K/Akt/mTOR Axis. Mol. Neurobiol. 2023, 60, 4261–4272. [Google Scholar] [CrossRef]
- Li, H.; Qin, S.; Liang, Q.; Xi, Y.; Bo, W.; Cai, M.; Tian, Z. Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice. Biomedicines 2021, 9, 701. [Google Scholar] [CrossRef]
- Bonfante, I.L.; Chacon-Mikahil, M.P.; Brunelli, D.T.; Gáspari, A.F.; Duft, R.G.; Lopes, W.A.; Bonganha, V.; Libardi, C.A.; Cavaglieri, C.R. Combined training, FNDC5/irisin levels and metabolic markers in obese men: A randomised controlled trial. Eur. J. Sport Sci. 2017, 17, 629–637. [Google Scholar] [CrossRef]
- Staiger, H.; Böhm, A.; Scheler, M.; Berti, L.; Machann, J.; Schick, F.; Machicao, F.; Fritsche, A.; Stefan, N.; Weigert, C.; et al. Common genetic variation in the human FNDC5 locus, encoding the novel muscle-derived ‘browning’ factor irisin, determines insulin sensitivity. PLoS ONE 2013, 8, e61903. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, R.; Jiang, F.; Wang, J.; Chen, M.; Peng, D.; Yan, J.; Bao, Y.; Hu, C.; Jia, W. An Interaction between a FNDC5 Variant and Obesity Modulates Glucose Metabolism in a Chinese Han Population. PLoS ONE 2014, 9, e109957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef]
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Zecca, C.; Dell'Abate, M.T.; Buccoliero, C.; Logroscino, G.; Colucci, S.; Grano, M. FNDC5/Irisin System in Neuroinflammation and Neurodegenerative Diseases: Update and Novel Perspective. Int. J. Mol. Sci. 2021, 22, 1605. [Google Scholar] [CrossRef]
- Lima-Filho, R.A.S.; Benedet, A.L.; De Bastiani, M.A.; Povala, G.; Cozachenco, D.; Ferreira, S.T.; De Felice, F.G.; Rosa-Neto, P.; Zimmer, E.R.; Lourenco, M.V.; et al. Association of the fibronectin type III domain–containing protein 5 rs1746661 single nucleotide polymorphism with reduced brain glucose metabolism in elderly humans. Brain Commun. 2023, 5, fcad216. [Google Scholar] [CrossRef]
- de Freitas, G.B.; Lourenco, M.V.; De Felice, F.G. Protective actions of exercise-related FNDC5/Irisin in memory and Alzheimer’s disease. J. Neurochem. 2020, 155, 602–611. [Google Scholar] [CrossRef]
- Kobilo, T.; Liu, Q.-R.; Gandhi, K.; Mughal, M.; Shaham, Y.; van Praag, H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn. Mem. 2011, 18, 605–609. [Google Scholar] [CrossRef]
- Sumsuzzman, D.M.; Jin, Y.; Choi, J.; Yu, J.-H.; Lee, T.H.; Hong, Y. Pathophysiological role of endogenous irisin against tumorigenesis and metastasis: Is it a potential biomarker and therapeutic? Tumor Biol. 2019, 41. [Google Scholar] [CrossRef]
- Guo, P.; Jin, Z.; Wu, H.; Li, X.; Ke, J.; Zhang, Z.; Zhao, Q. Effects of irisin on the dysfunction of blood–brain barrier in rats after focal cerebral ischemia/reperfusion. Brain Behav. 2019, 9, e01425. [Google Scholar] [CrossRef]
- Yu, Q.; Li, G.; Ding, Q.; Tao, L.; Li, J.; Sun, L.; Sun, X.; Yang, Y. Irisin Protects Brain against Ischemia/Reperfusion Injury through Suppressing TLR4/MyD88 Pathway. Cerebrovasc. Dis. 2020, 49, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Asadi, Y.; Gorjipour, F.; Behrouzifar, S.; Vakili, A. Irisin Peptide Protects Brain Against Ischemic Injury Through Reducing Apoptosis and Enhancing BDNF in a Rodent Model of Stroke. Neurochem. Res. 2018, 43, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Song, F.; Xu, K.; Liu, Z.; Han, S.; Li, F.; Sun, Y. Irisin Attenuates Neuroinflammation and Prevents the Memory and Cognitive Deterioration in Streptozotocin-Induced Diabetic Mice. Mediat. Inflamm. 2019, 2019, 1567179. [Google Scholar] [CrossRef]
- Wrann, C.D. FNDC5/irisin—Their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015, 1, 55–61. [Google Scholar] [CrossRef]
- Guo, P.; Jin, Z.; Wang, J.; Sang, A.; Wu, H. Irisin Rescues Blood-Brain Barrier Permeability following Traumatic Brain Injury and Contributes to the Neuroprotection of Exercise in Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2021, 2021, 1118981. [Google Scholar] [CrossRef]
- Cheng, Y.; Cui, Y.; Zhai, Y.; Xin, W.; Yu, Y.; Liang, J.; Li, S.; Sun, H. Neuroprotective Effects of Exogenous Irisin in Kainic Acid-Induced Status Epilepticus. Front. Cell. Neurosci. 2021, 15, 738533. [Google Scholar] [CrossRef]
- Lourenco, M.V.; de Freitas, G.B.; Raony, Í.; Ferreira, S.T.; De Felice, F.G. Irisin stimulates protective signaling pathways in rat hippocampal neurons. Front. Cell. Neurosci. 2022, 16, 953991. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Mikami, T. Exercise hormone irisin prevents physical inactivity-induced cognitive decline in mice. Behav. Brain Res. 2022, 433, 114008. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Ding, L.-N.; Zeng, N.-X.; Liu, H.-M.; Zheng, S.-H.; Xu, J.-W.; Li, R.-M. Icariin induces irisin/FNDC5 expression in C2C12 cells via the AMPK pathway. Biomed. Pharmacother. 2019, 115, 108930. [Google Scholar] [CrossRef]
- Androutsellis-Theotokis, A.; Leker, R.R.; Soldner, F.; Hoeppner, D.J.; Ravin, R.; Poser, S.W.; Rueger, M.A.; Bae, S.-K.; Kittappa, R.; McKay, R.D.G. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 2006, 442, 823–826. [Google Scholar] [CrossRef]
- O’Callaghan, J.P.; Kelly, K.A.; VanGilder, R.L.; Sofroniew, M.V.; Miller, D.B. Early Activation of STAT3 Regulates Reactive Astrogliosis Induced by Diverse Forms of Neurotoxicity. PLoS ONE 2014, 9, e102003. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Harada, C.; Namekata, K.; Matsuzawa, A.; Camps, M.; Ji, H.; Swinnen, D.; Jorand-Lebrun, C.; Muzerelle, M.; Vitte, P.; et al. Regulation of the severity of neuroinflammation and demyelination by TLR-ASK1-p38 pathway. EMBO Mol. Med. 2010, 2, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Q.; Wang, Y.; Peng, J.; Shao, L.; Li, X. Irisin protects against sepsis-associated encephalopathy by suppressing ferroptosis via activation of the Nrf2/GPX4 signal axis. Free Radic. Biol. Med. 2022, 187, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dong, Y.; Tucker, D.; Wang, R.; Ahmed, M.E.; Brann, D.; Zhang, Q. Treadmill Exercise Exerts Neuroprotection and Regulates Microglial Polarization and Oxidative Stress in a Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 56, 1469–1484. [Google Scholar] [CrossRef]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Zhang, D.; Bae, C.; Lee, J.; Lee, J.; Jin, Z.; Kang, M.; Cho, Y.S.; Kim, J.-H.; Lee, W.; Lim, S.-K. The bone anabolic effects of irisin are through preferential stimulation of aerobic glycolysis. Bone 2018, 114, 150–160. [Google Scholar] [CrossRef]
- Medel, V.; Crossley, N.; Gajardo, I.; Muller, E.; Barros, L.F.; Shine, J.M.; Sierralta, J. Whole-brain neuronal MCT2 lactate transporter expression links metabolism to human brain structure and function. Proc. Natl. Acad. Sci. USA 2022, 119, e2204619119. [Google Scholar] [CrossRef]
- Netzahualcoyotzi, C.; Pellerin, L. Neuronal and astroglial monocarboxylate transporters play key but distinct roles in hippocampus-dependent learning and memory formation. Prog. Neurobiol. 2020, 194, 101888. [Google Scholar] [CrossRef]
- Yang, C.; Pan, R.-Y.; Guan, F.; Yuan, Z. Lactate metabolism in neurodegenerative diseases. Neural Regen. Res. 2024, 19, 69–74. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Zhao, L. L-Lactate Administration Improved Synaptic Plasticity and Cognition in Early 3xTg-AD Mice. Int. J. Mol. Sci. 2025, 26, 1486. [Google Scholar] [CrossRef]
- Han, H.; Zhao, Y.; Du, J.; Wang, S.; Yang, X.; Li, W.; Song, J.; Zhang, S.; Zhang, Z.; Tan, Y.; et al. Exercise improves cognitive dysfunction and neuroinflammation in mice through Histone H3 lactylation in microglia. Immun. Ageing 2023, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, H.; Liu, W.; Zhao, Y.; Xie, F.; Sun, Z.; Zhang, L.; Dong, H.; Wang, X.; Qian, L. Hippocampal Lactate-Infusion Enhances Spatial Memory Correlated with Monocarboxylate Transporter 2 and Lactylation. Brain Sci. 2024, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Zhang, J.; Yin, X.; Wang, J.; Li, L.; Ju, Q.; Wang, Y.; Jiang, Y.; Liu, X.; Chen, Y.; et al. SIRT1 improves lactate homeostasis in the brain to alleviate parkinsonism via deacetylation and inhibition of PKM. Cell Rep. Med. 2024, 5, 101684. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Fan, S.; Dykstra, H.; Rom, S.; Mercer, A.; Reichenbach, N.L.; Gofman, L.; Persidsky, Y. Inhibition of Glycogen Synthase Kinase 3β Promotes Tight Junction Stability in Brain Endothelial Cells by Half-Life Extension of Occludin and Claudin. PLoS ONE 2013, 8, e55972. [Google Scholar] [CrossRef]
- Isla, A.G.; Vázquez-Cuevas, F.G.; Peña-Ortega, F. Exercise Prevents Amyloid-β-Induced Hippocampal Network Disruption by Inhibiting GSK3β Activation. J. Alzheimer’s Dis. 2016, 52, 333–343. [Google Scholar] [CrossRef]
- Souza, P.S.; Gonçalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.L.; Santos, A.R.; et al. Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption. Mol. Neurobiol. 2016, 54, 4723–4737. [Google Scholar] [CrossRef]
- Zarbakhsh, S.; Safari, M.; Aldaghi, M.R.; Sameni, H.; Ghahari, L.; Lagmouj, Y.K.; Jaberi, K.R.; Parsaie, H. Irisin protects the substantia nigra dopaminergic neurons in the rat model of Parkinson’s disease. Iran. J. Basic Med. Sci. 2019, 22, 722–728. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Zhang, L.J.; Zhao, N.; Chang, S.H.; Yang, L. FNDC5/Irisin protects neurons through Caspase3 and Bax pathways. Cell. Biochem. Funct. 2024, 42, e3912. [Google Scholar] [CrossRef]
- Sadier, N.S.; El Hajjar, F.; Sabouri, A.A.K.A.; Abou-Abbas, L.; Siomava, N.; Almutary, A.G.; Tambuwala, M.M. Irisin: An unveiled bridge between physical exercise and a healthy brain. Life Sci. 2024, 339, 122393. [Google Scholar] [CrossRef]
- Pang, Q.; Wang, P.; Pan, Y.; Dong, X.; Zhou, T.; Song, X.; Zhang, A. Irisin protects against vascular calcification by activating autophagy and inhibiting NLRP3-mediated vascular smooth muscle cell pyroptosis in chronic kidney disease. Cell Death Dis. 2022, 13, 283. [Google Scholar] [CrossRef]
- Trojani, M.-C.; Santucci-Darmanin, S.; Breuil, V.; Carle, G.F.; Pierrefite-Carle, V. Autophagy and bone diseases. Jt. Bone Spine 2022, 89, 105301. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, A.; Li, T.; Chen, H. Irisin alleviates the pyroptosis of beta cells in T2DM by inhibiting NLRP3 inflammasome through regulating miR-19b-3p/SOCS3/STAT3 axis mediated autophagy. IUBMB Life 2024, 76, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Bi, J.; Yang, L.; Zhang, J.; Wan, Y.; Chen, X.; Wang, Y.; Wu, Z.; Lv, Y.; Wu, R. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin. Transl. Med. 2020, 10, e173. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Han, Z.; Gao, M.; Tian, L. FNDC5/irisin ameliorates bone loss of type 1 diabetes by suppressing endoplasmic reticulum stress-mediated ferroptosis. J. Orthop. Surg. Res. 2024, 19, 205. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, X.; Heng, T.; Zhu, Y.; Gong, L.; Liu, N.; Yao, X.; Luo, Y. FNDC5/Irisin mitigates high glucose-induced neurotoxicity in HT22 cell via ferroptosis. Biosci. Trends 2024, 18, 465–475. [Google Scholar] [CrossRef]
- Ibrahim, R.R.; Shafik, N.M.; El-Esawy, R.O.; El-Sakaa, M.H.; Arakeeb, H.M.; El-Sharaby, R.M.; Ali, D.A.; El-Deeb, O.S.; El-Khalik, S.R.A. The emerging role of irisin in experimentally induced arthritis: A recent update involving HMGB1/MCP1/Chitotriosidase I–mediated necroptosis. Redox Rep. 2022, 27, 21–31. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2017, 14, 207–215. [Google Scholar] [CrossRef]
- Wang, L.; Klionsky, D.J.; Shen, H.-M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2022, 24, 186–203. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Wu, S.; Wu, Y.; Chen, H.; Xin, J.; Li, H.; Lan, J.; Xue, K.; Li, X.; et al. Irisin ameliorates angiotensin II-induced cardiomyocyte apoptosis through autophagy. J. Cell. Physiol. 2019, 234, 17578–17588. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, H.; Zhang, H.; Wang, T.; Zheng, Q.; Li, Z. Controlled Activation of TRPV1 Channels on Microglia to Boost Their Autophagy for Clearance of Alpha-Synuclein and Enhance Therapy of Parkinson’s Disease. Adv. Mater. 2022, 34, e2108435. [Google Scholar] [CrossRef] [PubMed]
- Moors, T.E.; Hoozemans, J.J.M.; Ingrassia, A.; Beccari, T.; Parnetti, L.; Chartier-Harlin, M.-C.; van de Berg, W.D.J. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol. Neurodegener. 2017, 12, 11. [Google Scholar] [CrossRef]
- Kam, T.I.; Park, H.; Chou, S.C.; Van Vranken, J.G.; Mittenbühler, M.J.; Kim, H.A.M.; Choi, Y.R.; Biswas, D.; Wang, J.; Shin, Y.; et al. Amelioration of pathologic alpha-synuclein-induced Parkinson’s disease by irisin. Proc. Natl. Acad. Sci. USA 2022, 119, e2204835119. [Google Scholar] [CrossRef]
- Bi, J.; Yang, L.; Wang, T.; Zhang, J.; Li, T.; Ren, Y.; Wang, M.; Chen, X.; Lv, Y.; Wu, R. Irisin Improves Autophagy of Aged Hepatocytes via Increasing Telomerase Activity in Liver Injury. Oxidative Med. Cell. Longev. 2020, 2020, 6946037. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Zhu, S.; Liu, H.; Xu, S. Irisin Protects Musculoskeletal Homeostasis via a Mitochondrial Quality Control Mechanism. Int. J. Mol. Sci. 2024, 25, 10116. [Google Scholar] [CrossRef]
- Tan, Y.; Ouyang, H.; Xiao, X.; Zhong, J.; Dong, M. Irisin ameliorates septic cardiomyopathy via inhibiting DRP1-related mitochondrial fission and normalizing the JNK-LATS2 signaling pathway. Cell Stress Chaperon. 2019, 24, 595–608. [Google Scholar] [CrossRef]
- Chu, C.T. Mechanisms of selective autophagy and mitophagy: Implications for neurodegenerative diseases. Neurobiol. Dis. 2019, 122, 23–34. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; He, Y.; Luo, X.; Zhang, W.; Yu, L.; Chen, X.; He, X.; Yuan, Y.; Wang, X.; et al. Qiliqiangxin reduced cardiomyocytes apotosis and improved heart function in infarcted heart through Pink1/Parkin-mediated mitochondrial autophagy. BMC Complement. Med. Ther. 2020, 20, 203. [Google Scholar] [CrossRef]
- Jiang, H.-K.; Wang, Y.-H.; Sun, L.; He, X.; Zhao, M.; Feng, Z.-H.; Yu, X.-J.; Zang, W.-J. Aerobic Interval Training Attenuates Mitochondrial Dysfunction in Rats Post-Myocardial Infarction: Roles of Mitochondrial Network Dynamics. Int. J. Mol. Sci. 2014, 15, 5304–5322. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, Y.; Ogasawara, R.; Tamura, Y.; Fujita, S.; Hatta, H. Effect of electrical stimulation-induced resistance exercise on mitochondrial fission and fusion proteins in rat skeletal muscle. Appl. Physiol. Nutr. Metab. 2015, 40, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Raefsky, S.M.; Mattson, M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free. Radic. Biol. Med. 2017, 102, 203–216. [Google Scholar] [CrossRef]
- Dionísio, P.; Amaral, J.; Rodrigues, C. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Adam-Vizi, V.; Chinopoulos, C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006, 27, 639–645. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Chesselet, M.-F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Wang, K.; Xue, Y.; Hu, S.; Jin, Y.; Zhu, G.; Shi, Q.; Rui, Y. Irisin alleviates obesity-induced bone loss by inhibiting interleukin 6 expression via TLR4/MyD88/NF-κB axis in adipocytes. J. Adv. Res. 2024, 69, 343–359. [Google Scholar] [CrossRef]
- Qiu, R.; Sun, W.; Su, Y.; Sun, Z.; Fan, K.; Liang, Y.; Lin, X.; Zhang, Y. Irisin’s emerging role in Parkinson’s disease research: A review from molecular mechanisms to therapeutic prospects. Life Sci. 2024, 357, 123088. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J. Recept. Signal Transduct. 2020, 41, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yu, X.; Zhang, H.; Dai, H.; Huang, Y.; Wu, F. Irisin alleviates chronic constriction injury-induced hyperalgesia and affective disorders in mice through NF-κB and Nrf2 signaling pathways. IBRO Neurosci. Rep. 2024, 17, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.J.; Alviña, K. Multiple Roles in Neuroprotection for the Exercise Derived Myokine Irisin. Front. Aging Neurosci. 2021, 13, 649929. [Google Scholar] [CrossRef]
- Horwitz, A.; Birk, R. Irisin Ameliorate Acute Pancreatitis and Acinar Cell Viability through Modulation of the Unfolded Protein Response (UPR) and PPARγ-PGC1α-FNDC5 Pathways. Biomolecules 2024, 14, 643. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- Cardanho-Ramos, C.; Morais, V.A. Mitochondrial Biogenesis in Neurons: How and Where. Int. J. Mol. Sci. 2021, 22, 13059. [Google Scholar] [CrossRef]
- Choi, J.-W.; Jo, S.-W.; Kim, D.-E.; Paik, I.-Y.; Balakrishnan, R. Aerobic exercise attenuates LPS-induced cognitive dysfunction by reducing oxidative stress, glial activation, and neuroinflammation. Redox Biol. 2024, 71, 103101. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, Y.J.; Song, I.-S.; Noh, Y.H.; Seo, K.W.; Kim, M.; Han, J. Glucocorticoid receptor positively regulates transcription of FNDC5 in the liver. Sci. Rep. 2017, 7, 43296. [Google Scholar] [CrossRef]
- Huerta, A.E.; Prieto-Hontoria, P.L.; Fernández-Galilea, M.; Sáinz, N.; Cuervo, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Circulating irisin and glucose metabolism in overweight/obese women: Effects of α-lipoic acid and eicosapentaenoic acid. J. Physiol. Biochem. 2015, 71, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Popov, D.V.; Lysenko, E.A.; Kuzmin, I.V.; Vinogradova, O.L.; Grigoriev, A.I. Regulation of PGC-1α Isoform Expression in Skeletal Muscles. Acta Naturae 2015, 7, 48–59. [Google Scholar] [CrossRef]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Zhou, X.; Song, J.; Feng, Y.; Qiu, T.; Sheng, S.; Zhang, M.; Zhang, X.; Hao, J.; et al. Foxj3 Regulates Thermogenesis of Brown and Beige Fat Via Induction of PGC-1α. Diabetes 2023, 73, 178–196. [Google Scholar] [CrossRef]
- Small, D.M.; Morais, C.; Coombes, J.S.; Bennett, N.C.; Johnson, D.W.; Gobe, G.C. Oxidative stress-induced alterations in PPAR-γ and associated mitochondrial destabilization contribute to kidney cell apoptosis. Am. J. Physiol. Physiol. 2014, 307, F814–F822. [Google Scholar] [CrossRef]
- Cao, R.Y.; Zheng, H.; Redfearn, D.; Yang, J. FNDC5: A novel player in metabolism and metabolic syndrome. Biochimie 2019, 158, 111–116. [Google Scholar] [CrossRef]
- Wille, E.; Scholze, J.; Alegria, E.; Ferri, C.; Langham, S.; Stevens, W.; Jeffries, D.; Uhl-Hochgraeber, K. Modelling the costs of care of hypertension in patients with metabolic syndrome and its consequences, in Germany, Spain and Italy. Eur. J. Health Econ. 2010, 12, 205–218. [Google Scholar] [CrossRef]
- Pang, M.; Yang, J.; Rao, J.; Wang, H.; Zhang, J.; Wang, S.; Chen, X.; Dong, X. Time-Dependent Changes in Increased Levels of Plasma Irisin and Muscle PGC-1α and FNDC5 after Exercise in Mice. Tohoku J. Exp. Med. 2018, 244, 93–103. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Panati, K.; Suneetha, Y.; Narala, V.R. Irisin/FNDC5—An updated review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 689–697. [Google Scholar] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P. Irisin and FNDC5 in retrospect: An exercise hormone or a transmembrane receptor? Adipocyte 2013, 2, 289–293. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Donelan, W.; Li, S.; Tang, D. Expression of Recombinant Rat Secretable FNDC5 in Pichia Pastoris and Detection of Its Biological Activity. Front. Endocrinol. 2022, 13, 852015. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.-S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.; Baharvand, H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef]
- Ghahrizjani, F.A.; Ghaedi, K.; Salamian, A.; Tanhaei, S.; Nejati, A.S.; Salehi, H.; Nabiuni, M.; Baharvand, H.; Nasr-Esfahani, M.H. Enhanced expression of FNDC5 in human embryonic stem cell-derived neural cells along with relevant embryonic neural tissues. Gene 2015, 557, 123–129. [Google Scholar] [CrossRef]
- Tanhaei, S.; Nikpour, P.; Ghaedi, K.; Rabiee, F.; Moghadam, F.H.; Nasr-Esfahani, M.H. RNA/Protein Discordant Expression of Fndc5 in Central Nervous System Is Likely to Be Mediated Through microRNAs. DNA Cell Biol. 2018, 37, 373–380. [Google Scholar] [CrossRef]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J.; et al. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. eLife 2020, 9, e58172. [Google Scholar] [CrossRef]
- Oguri, Y.; Shinoda, K.; Kim, H.; Alba, D.L.; Bolus, W.R.; Wang, Q.; Brown, Z.; Pradhan, R.N.; Tajima, K.; Yoneshiro, T.; et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 2020, 182, 563–577.e20. [Google Scholar] [CrossRef]
- Mu, A.; Wales, T.E.; Zhou, H.; Draga-Coletă, S.-V.; Gorgulla, C.; Blackmore, K.A.; Mittenbühler, M.J.; Kim, C.R.; Bogoslavski, D.; Zhang, Q.; et al. Irisin acts through its integrin receptor in a two-step process involving extracellular Hsp90α. Mol. Cell 2023, 83, 1903–1920.e12. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin reverses intestinal epithelial barrier dysfunction during intestinal injury via binding to the integrin αVβ5 receptor. J. Cell. Mol. Med. 2019, 24, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; He, Z.; Du, J.; Xu, M.; Cui, J.; Han, X.; Tong, D.; Li, H.; Yan, M.; Yu, Z. Irisin promotes fracture healing by improving osteogenesis and angiogenesis. J. Orthop. Transl. 2022, 37, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-Q.; Geng, Z.; Zhou, B.; Zhang, F.; Han, Y.; Zhou, Y.-B.; Wang, J.-J.; Gao, X.-Y.; Chen, Q.; Li, Y.-H.; et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism 2018, 83, 31–41. [Google Scholar] [CrossRef]
- Hussey, S.E.; McGee, S.L.; Garnham, A.; McConell, G.K.; Hargreaves, M. Exercise increases skeletal muscle GLUT4 gene expression in patients with type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 768–771. [Google Scholar] [CrossRef]
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020, 10, 51. [Google Scholar] [CrossRef]
- Yano, N.; Zhang, L.; Wei, D.; Dubielecka, P.M.; Wei, L.; Zhuang, S.; Zhu, P.; Qin, G.; Liu, P.Y.; Chin, Y.E.; et al. Irisin counteracts high glucose and fatty acid-induced cytotoxicity by preserving the AMPK-insulin receptor signaling axis in C2C12 myoblasts. Am. J. Physiol. Metab. 2020, 318, E791–E805. [Google Scholar] [CrossRef]
- Greenhill, C. Irisin receptor in osteocytes identified. Nat. Rev. Endocrinol. 2019, 15, 63. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/Irisin: Physiology and Pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef]
- Peng, J.; Wu, J. Effects of the FNDC5/Irisin on Elderly Dementia and Cognitive Impairment. Front. Aging Neurosci. 2022, 14, 863901. [Google Scholar] [CrossRef]
- Li, R.-L.; Wu, S.-S.; Wu, Y.; Wang, X.-X.; Chen, H.-Y.; Xin, J.-J.; Li, H.; Lan, J.; Xue, K.-Y.; Li, X.; et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J. Mol. Cell. Cardiol. 2018, 121, 242–255. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Xiong, X.-Q.; Ren, X.-S.; Zhao, M.-X.; Shi, C.-X.; Wang, J.-J.; Zhou, Y.-B.; Zhang, F.; Han, Y.; Gao, X.-Y.; et al. FNDC5 Alleviates Hepatosteatosis by Restoring AMPK/mTOR-Mediated Autophagy, Fatty Acid Oxidation, and Lipogenesis in Mice. Diabetes 2016, 65, 3262–3275. [Google Scholar] [CrossRef]
- Qi, F.; Deng, Y.; Huang, W.; Cai, Y.; Hong, K.; Xiang, S. Irisin suppresses PDGF-BB-induced proliferation of vascular smooth muscle cells in vitro by activating AMPK/mTOR-mediated autophagy. Eur. J. Histochem. 2024, 68, 4104. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yu, R.; Liu, S.; Huwatibieke, B.; Li, Z.; Zhang, W. Irisin Inhibits Hepatic Cholesterol Synthesis via AMPK-SREBP2 Signaling. EBioMedicine 2016, 6, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, M.; Zhao, Y.; Dong, M. Irisin Protects Against LPS-Stressed Cardiac Damage Through Inhibiting Inflammation, Apoptosis, and Pyroptosis. Shock 2021, 56, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Han, Z.; Jiao, R.; Yuan, G.; Ma, C.; Yan, X.; Meng, A. Irisin Ameliorates PM2.5-Induced Acute Lung Injury by Regulation of Autophagy Through AMPK/mTOR Pathway. J. Inflamm. Res. 2023, 16, 1045–1057. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Rodrigues, V.D.; Cherain, L.M.A.; de Lima, E.P.; Boaro, B.L.; Oliveira, J.d.S.C.; Chagas, E.F.B.; Catharin, V.C.S.; Haber, J.F.d.S.; Bueno, P.C.d.S.; et al. Targeting AMPK with Irisin: Implications for metabolic disorders, cardiovascular health, and inflammatory conditions—A systematic review. Life Sci. 2024, 360, 123230. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2012, 76, 496. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Chen, Y.; Zhao, Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int. J. Clin. Exp. Pathol. 2015, 8, 6490–6497. [Google Scholar]
- Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Zhang, J.; Luo, Y.; Nie, Y.; Hu, Y.; Yang, Z.; Zhang, J.; et al. Irisin promotes proliferation but inhibits differentiation in osteoclast precursor cells. FASEB J. 2018, 32, 5813–5823. [Google Scholar] [CrossRef]
- Qiao, X.Y.; Nie, Y.; Ma, Y.X.; Chen, Y.; Cheng, R.; Yinrg, W.Y.; Hu, Y.; Xu, W.M.; Xu, L.Z. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin enhances osteoblast differentiation in vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Spiegelman, B.M. Irisin ERKs the fat. Diabetes 2014, 63, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Farahabadi, S.S.; Ghaedi, K.; Ghazvini Zadegan, F.; Karbalaie, K.; Rabiee, F.; Nematollahi, M.; Baharvand, H.; Nasr-Esfahani, M.H. ERK1/2 is a key regulator of Fndc5 and PGC1alpha expression during neural differentiation of mESCs. Neuroscience 2015, 297, 252–261. [Google Scholar] [CrossRef]
- Paoletti, I.; Coccurello, R. Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology. Int. J. Mol. Sci. 2024, 25, 13480. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Shi, C.-X.; Gao, R.; Sun, H.-J.; Xiong, X.-Q.; Ding, L.; Chen, Q.; Li, Y.-H.; Wang, J.-J.; Kang, Y.-M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, N.; Kang, X.; Hu, Y.; Shi, S. Irisin alleviates FFA induced β-cell insulin resistance and inflammatory response through activating PI3K/AKT/FOXO1 signaling pathway. Endocrine 2021, 75, 740–751. [Google Scholar] [CrossRef]
- Draznin, B. Molecular mechanisms of insulin resistance: Serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: The two sides of a coin. Diabetes 2006, 55, 2392–2397. [Google Scholar] [CrossRef]
- Tarassishin, L.; Suh, H.-S.; Lee, S.C. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J. Neuroinflamm. 2011, 8, 187. [Google Scholar] [CrossRef]
- Smith, M.V.; Lee, M.J.; Islam, A.S.; Rohrer, J.L.; Goldberg, V.M.; Beidelschies, M.A.; Greenfield, E.M. Inhibition of the PI3K-Akt signaling pathway reduces tumor necrosis factor-alpha production in response to titanium particles in vitro. J. Bone Jt. Surg. Am. 2007, 89, 1019–1027. [Google Scholar]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Tang, N.; Qiu, J.; Zhang, D.; Huang, F.; Cheng, Y.; Ding, K.; Li, W.; Zhang, P.; Tan, X. Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2017, 493, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Teng, L.; Zhong, Y.; Ma, P.; Xu, L.; Xiao, P. Neuroprotection of isookanin against MPTP-induced cell death of SH-SY5Y cells via BCL2/BAX and PI3K/AKT pathways. Psychopharmacology 2023, 240, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.P.; Budni, J.; Ludka, F.K.; Pazini, F.L.; Rosa, J.M.; Oliveira, Á.; Lopes, M.W.; Tasca, C.I.; Leal, R.B.; Rodrigues, A.L.S. Involvement of PI3K/Akt Signaling Pathway and Its Downstream Intracellular Targets in the Antidepressant-Like Effect of Creatine. Mol. Neurobiol. 2015, 53, 2954–2968. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; De Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Moon, H.-S.; Dincer, F.; Mantzoros, C.S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 2013, 62, 1131–1136. [Google Scholar] [CrossRef]
- Li, P.; Hu, Y.; Tong, L.; Bi, X. High-intensity training on CREB activation for improving brain health: A narrative review of possible molecular talks. Front. Endocrinol. 2025, 15, 1498495. [Google Scholar] [CrossRef]
- van Zundert, B.; Montecino, M. Epigenetics in Learning and Memory. Subcell Biochem. 2025, 108, 51–71. [Google Scholar]
- Yang, X.-Y.; Tse, M.C.; Hu, X.; Jia, W.-H.; Du, G.-H.; Chan, C.B. Interaction of CREB and PGC-1α Induces Fibronectin Type III Domain-Containing Protein 5 Expression in C2C12 Myotubes. Cell. Physiol. Biochem. 2018, 50, 1574–1584. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, J.; Kong, D.; Guo, X.; Li, J.; Long, Z.; Peng, J.; Wang, Z.; Wu, H.; Liu, P.; et al. Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPARγ and Nrf2 signaling. Free. Radic. Biol. Med. 2023, 201, 98–110. [Google Scholar] [CrossRef]
- Costa, R.M.; Drew, C.; Silva, A.J. Notch to remember. Trends Neurosci. 2005, 28, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, X.; Shu, J.; Li, H.; Yang, T.; Li, N.; Yang, P. Irisin Alleviates Autoimmune Uveitis Through Promoting Retinal Microglial M1 to M2 Phenotypic Polarization Mediated by HIF-1α Pathway. Inflammation 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Sun, S.; Chang, Y.; Lian, P.; Guo, H.; Zheng, S.; Ma, R.; Li, G. Irisin inhibits microglial senescence via TFAM-mediated mitochondrial metabolism in a mouse model of tauopathy. Immun. Ageing 2024, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Dicarlo, M.; Pignataro, P.; Zerlotin, R.; Suriano, C.; Zecca, C.; Dell’abate, M.T.; Storlino, G.; Oranger, A.; Sanesi, L.; Mori, G.; et al. Short-Term Irisin Treatment Enhanced Neurotrophin Expression Differently in the Hippocampus and the Prefrontal Cortex of Young Mice. Int. J. Mol. Sci. 2023, 24, 9111. [Google Scholar] [CrossRef] [PubMed]
- Siteneski, A.; Cunha, M.P.; Lieberknecht, V.; Pazini, F.L.; Gruhn, K.; Brocardo, P.S.; Rodrigues, A.L.S. Central irisin administration affords antidepressant-like effect and modulates neuroplasticity-related genes in the hippocampus and prefrontal cortex of mice. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2018, 84, 294–303. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New Insights in the Biology of BDNF Synthesis and Release: Implications in CNS Function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.-M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2012, 14, 7–23. [Google Scholar] [CrossRef]
- Kuipers, S.D.; Bramham, C.R. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: New insights and implications for therapy. Curr. Opin. Drug Discov. Devel. 2006, 9, 580–586. [Google Scholar]
- Li, M.; Dai, F.-R.; Du, X.-P.; Yang, Q.-D.; Zhang, X.; Chen, Y. Infusion of BDNF into the nucleus accumbens of aged rats improves cognition and structural synaptic plasticity through PI3K-ILK-Akt signaling. Behav. Brain Res. 2012, 231, 146–153. [Google Scholar] [CrossRef]
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin reverses the IL-6 induced epithelial-mesenchymal transition in osteosarcoma cell migration and invasion through the STAT3/Snail signaling pathway. Oncol. Rep. 2017, 38, 2647–2656. [Google Scholar] [CrossRef]
- Chen, X.; Sun, K.; Zhao, S.; Geng, T.; Fan, X.; Sun, S.; Zheng, M.; Jin, Q. Irisin promotes osteogenic differentiation of bone marrow mesenchymal stem cells by activating autophagy via the Wnt//β-catenin signal pathway. Cytokine 2020, 136, 155292. [Google Scholar] [CrossRef]
- Chen, Y.; Sha, W.; Zhang, Y.; Kou, W.; Yang, L.; Guo, R.; Li, C.; Zhao, J.; Wang, Z. Irisin-regulated lncRNAs and their potential regulatory functions in chondrogenic differentiation of human mesenchymal stem cells. Open Med. 2024, 19, 20241073. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-Y.; Chiu, K.-P.; Tsai, M.-L.; Yeh, J.-K.; Huang, Y.-C.; Li, Y.-R.; Wang, H.-D.; Hsieh, I.-C.; Wen, M.-S.; Wang, C.-Y. MicroRNA dynamics in irisin-mediated signaling pathways within adipose tissue. J. Biosci. 2024, 49, 89. [Google Scholar] [CrossRef]
- Li, B.; Dong, Y.; Hu, S.; Liu, T. MiR-143-3p/FNDC5 axis: A novel regulator of insulin sensitivity. Endocrine 2023, 83, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.L.; Zhang, Z.D.; Dong, Z.; Shan, T.; Yin, Z.S. mir-150-5p inhibits the osteogenic differentiation of bone marrow-derived mesenchymal stem cells by targeting irisin to regulate the p38/MAPK signaling pathway. J. Orthop. Surg. Res. 2024, 19, 190. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, F.; Fan, J.; Wu, T.; Jia, S.; Li, J.; Chen, N. Swimming alleviates myocardial fibrosis of type II diabetic rats through activating miR-34a-mediated SIRT1/PGC-1α/FNDC5 signal pathway. PLoS ONE 2024, 19, e0310136. [Google Scholar] [CrossRef]
- Thau, L.; Reddy, V.; Singh, P. Anatomy, Central Nervous System; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Xu, W.; Huang, Y.; Zhou, R. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell. Mol. Immunol. 2025, 22, 341–355. [Google Scholar] [CrossRef]
- Rana, S.; Wahab, N.A.; Shahidan, W.N.S.; Atif, S.; Fahim, A. Irisin as a novel diagnostic biomarker for inflammatory diseases: A review. J. Ayub Med. Coll. Abbottabad 2024, 36, 636–641. [Google Scholar] [CrossRef]
- Tan, M.M.J.; Han, E.; Shrestha, P.; Wu, S.; Shiraz, F.; Koh, G.C.-H.; McKee, M.; Legido-Quigley, H. Framing global discourses on non-communicable diseases: A scoping review. BMC Health Serv. Res. 2021, 21, 20. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell. Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF—A Key Transducer of Antidepressant Effects. Neuropharmacology 2015, 102, 72–79. [Google Scholar] [CrossRef]
- Dicarlo, M.; Pignataro, P.; Zecca, C.; Dell’Abate, M.T.; Urso, D.; Gnoni, V.; Giugno, A.; Borlizzi, F.; Zerlotin, R.; Oranger, A.; et al. Irisin Levels in Cerebrospinal Fluid Correlate with Biomarkers and Clinical Dementia Scores in Alzheimer Disease. Ann. Neurol. 2024, 96, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Song, J. The Role of Irisin in Alzheimer’s Disease. J. Clin. Med. 2018, 7, 407. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pai, M.C. Circulating levels of Irisin in obese individuals at genetic risk for Alzheimer’s disease: Correlations with amyloid-beta, metabolic, and neurocognitive indices. Behav. Brain. Res. 2021, 400, 113013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, S.; Hu, Y.; Liu, Q.; Liu, C.; Chai, H.; Luo, Y.; Jin, L.; Li, S. Irisin exhibits neuroprotection by preventing mitochondrial damage in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hou, G.; Hou, G.; Wang, C.; Shi, B.; Zheng, Y. Serum Irisin as a Potential Biomarker for Cognitive Decline in Vascular Dementia. Front. Neurol. 2021, 12, 755046. [Google Scholar] [CrossRef]
- Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695.
- Jin, Y.; Sumsuzzman, D.M.; Choi, J.; Kang, H.; Lee, S.-R.; Hong, Y. Molecular and Functional Interaction of the Myokine Irisin with Physical Exercise and Alzheimer’s Disease. Molecules 2018, 23, 3229. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Lin, T.-W.; Tsai, S.-F.; Kuo, Y.-M. Physical Exercise Enhances Neuroplasticity and Delays Alzheimer’s Disease. Brain Plast. 2018, 4, 95–110. [Google Scholar] [CrossRef]
- Belviranlı, M.; Okudan, N. Exercise Training Protects Against Aging-Induced Cognitive Dysfunction via Activation of the Hippocampal PGC-1α/FNDC5/BDNF Pathway. Neuromol. Med. 2018, 20, 386–400. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Okudan, N.; Kabak, B.; Erdoğan, M.; Karanfilci, M. The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. Phys. Sportsmed. 2016, 44, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef]
- Kuwano, T.; Nakao, S.; Yamamoto, H.; Tsuneyoshi, M.; Yamamoto, T.; Kuwano, M.; Ono, M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2003, 18, 300–310. [Google Scholar] [CrossRef]
- Samad, T.A.; Moore, K.A.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001, 410, 471–475. [Google Scholar] [CrossRef]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S.T. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef][Green Version]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, W.; Zhang, M.; Tian, X.; Li, Y.; Lü, Y. Imbalance of Microglial TLR4/TREM2 in LPS-Treated APP/PS1 Transgenic Mice: A Potential Link Between Alzheimer’s Disease and Systemic Inflammation. Neurochem. Res. 2019, 44, 1138–1151. [Google Scholar] [CrossRef]
- Kuhn, P.H.; Wang, H.; Dislich, B.; Colombo, A.; Zeitschel, U.; Ellwart, J.W.; Kremmer, E.; Rossner, S.; Lichtenthaler, S.F. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010, 29, 3020–3032. [Google Scholar] [CrossRef]
- Jorissen, E.; Prox, J.; Bernreuther, C.; Weber, S.; Schwanbeck, R.; Serneels, L.; Snellinx, A.; Craessaerts, K.; Thathiah, A.; Tesseur, I.; et al. The Disintegrin/Metalloproteinase ADAM10 Is Essential for the Establishment of the Brain Cortex. J. Neurosci. 2010, 30, 4833–4844. [Google Scholar] [CrossRef]
- Chen, K.; Wang, K.; Wang, T. Protective effect of irisin against Alzheimer’s disease. Front. Psychiatry 2022, 13, 967683. [Google Scholar] [CrossRef] [PubMed]
- Bellettini-Santos, T.; Batista-Silva, H.; Marcolongo-Pereira, C.; Quintela-Castro, F.C.d.A.; Barcelos, R.M.; Chiepe, K.C.M.B.; Rossoni, J.V.; Passamani-Ambrosio, R.; da Silva, B.S.; Chiarelli-Neto, O.; et al. Move Your Body toward Healthy Aging: Potential Neuroprotective Mechanisms of Irisin in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 12440. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-T.; Uruno, A.; Katsuoka, F.; Yamamoto, M. Role of NRF2 in Pathogenesis of Alzheimer’s Disease. Antioxidants 2024, 13, 1529. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.S.; Arif, M.; Hill, E.J.; Aldred, S.; Nagel, D.A.; Nevill, A.; Randeva, H.S.; Bailey, C.J.; Bellary, S.; Brown, J.E. Plasma irisin levels predict telomere length in healthy adults. Age 2014, 36, 995–1001. [Google Scholar] [CrossRef]
- Sung, V.W.; Nicholas, A.P. Nonmotor symptoms in Parkinson’s disease: Expanding the view of Parkinson’s disease beyond a pure motor, pure dopaminergic problem. Neurol. Clin. 2013, 31 (Suppl. S3), S1–S16. [Google Scholar] [CrossRef]
- Harms, A.S.; Ferreira, S.A.; Romero-Ramos, M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol. 2021, 141, 527–545. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Walzik, D.; Chirino, T.Y.W.; Zimmer, P.; Joisten, N. Molecular insights of exercise therapy in disease prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 138. [Google Scholar] [CrossRef]
- Shi, X.; Gu, Q.; Fu, C.; Ma, J.; Li, D.; Zheng, J.; Chen, S.; She, Z.; Qi, X.; Li, X.; et al. Relationship of irisin with disease severity and dopamine uptake in Parkinson’s disease patients. NeuroImage Clin. 2023, 41, 103555. [Google Scholar] [CrossRef]
- Zhu, M.; Peng, Q.; Li, S.; Zhang, G.; Zhang, Z. Irisin promotes autophagy and attenuates NLRP3 inflammasome activation in Parkinson’s disease. Int. Immunopharmacol. 2025, 149, 114201. [Google Scholar] [CrossRef]
- Xu, X.; He, X.; Ma, S.; Li, M.; Huang, Q. Nurr1 downregulation is caused by CREB inactivation in a Parkinson’s disease mouse model. Neurosci. Lett. 2021, 759, 136045. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular Mechanisms and Therapeutic Implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef]
- Petri, S.; Korner, S.; Kiaei, M. Nrf2/ARE Signaling Pathway: Key Mediator in Oxidative Stress and Potential Therapeutic Target in ALS. Neurol. Res. Int. 2012, 2012, 878030. [Google Scholar] [CrossRef] [PubMed]
- Bayer, H.; Lang, K.; Buck, E.; Higelin, J.; Barteczko, L.; Pasquarelli, N.; Sprissler, J.; Lucas, T.; Holzmann, K.; Demestre, M.; et al. ALS-causing mutations differentially affect PGC-1alpha expression and function in the brain vs. peripheral tissues. Neurobiol. Dis. 2017, 97 Pt A, 36–45. [Google Scholar] [CrossRef]
- Lunetta, C.; Lizio, A.; Tremolizzo, L.; Ruscica, M.; Macchi, C.; Riva, N.; Weydt, P.; Corradi, E.; Magni, P.; Sansone, V. Serum irisin is upregulated in patients affected by amyotrophic lateral sclerosis and correlates with functional and metabolic status. J. Neurol. 2018, 265, 3001–3008. [Google Scholar] [CrossRef]
- Boeve, B.F.; Boxer, A.L.; Kumfor, F.; Pijnenburg, Y.; Rohrer, J.D. Advances and controversies in frontotemporal dementia: Diagnosis, biomarkers, and therapeutic considerations. Lancet Neurol. 2022, 21, 258–272. [Google Scholar] [CrossRef]
- Fraga, V.G.; Magalhães, C.A.; Loures, C.d.M.G.; de Souza, L.C.; Guimarães, H.C.; Zauli, D.A.G.; Carvalho, M.d.G.; Ferreira, C.N.; Caramelli, P.; de Sousa, L.P.; et al. Inflammatory and Pro-resolving Mediators in Frontotemporal Dementia and Alzheimer’s Disease. Neuroscience 2019, 421, 123–135. [Google Scholar] [CrossRef]
- Bright, F.; Werry, E.L.; Dobson-Stone, C.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kassiou, M.; et al. Neuroinflammation in frontotemporal dementia. Nat. Rev. Neurol. 2019, 15, 540–555. [Google Scholar] [CrossRef]
- Fraga, V.G.; Ferreira, C.N.; Oliveira, F.R.; Cândido, A.L.; Carvalho, M.d.G.; Reis, F.M.; Caramelli, P.; De Souza, L.C.; Gomes, K.B. Irisin levels are correlated with inflammatory markers in frontotemporal dementia. J. Clin. Neurosci. 2021, 93, 92–95. [Google Scholar] [CrossRef]
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Machado-Santos, J.; Saji, E.; Tröscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef] [PubMed]
- Thebault, S.; Abdoli, M.; Fereshtehnejad, S.-M.; Tessier, D.; Tabard-Cossa, V.; Freedman, M.S. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci. Rep. 2020, 10, 10381. [Google Scholar] [CrossRef]
- Altaş, M.; Uca, A.U.; Akdağ, T.; Odabaş, F.Ö.; Tokgöz, O.S. Serum levels of irisin and nesfatin-1 in multiple sclerosis. Arq. Neuro Psiquiatr. 2022, 80, 161–167. [Google Scholar] [CrossRef]
- Algul, S.; Ozcelik, O. Evaluating the energy regulatory hormones of nesfatin-1, irisin, adropin and preptin in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 68, 104221. [Google Scholar] [CrossRef]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef]
- Bilek, F.; Cetisli-Korkmaz, N.; Ercan, Z.; Deniz, G.; Demir, C.F. Aerobic exercise increases irisin serum levels and improves depression and fatigue in patients with relapsing remitting multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2022, 61, 103742. [Google Scholar] [CrossRef]
- Osama, A.; Zhang, J.; Yao, J.; Yao, X.; Fang, J. Nrf2: A dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 2020, 64, 101206. [Google Scholar] [CrossRef]
- Yang, X.-X.; Yang, R.; Zhang, F. Role of Nrf2 in Parkinson’s Disease: Toward New Perspectives. Front. Pharmacol. 2022, 13, 919233. [Google Scholar] [CrossRef]
- Arslanbaeva, L.; Bisaglia, M. Activation of the Nrf2 Pathway as a Therapeutic Strategy for ALS Treatment. Molecules 2022, 27, 1471. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; de Lago, E.; Martínez, A.; Fernández-Ruiz, J. New Statement about NRF2 in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Biomolecules 2022, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Pocheć, E. The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis. Antioxidants 2021, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Raghani, N.; Chorawala, M.; Bhattacharya, S.; Prajapati, B.G.; Elossaily, G.M.; Chaiyasut, C. NF-κB Pathway and Its Inhibitors: A Promising Frontier in the Management of Alzheimer’s Disease. Biomedicines 2023, 11, 2587. [Google Scholar] [CrossRef]
- Dolatshahi, M.; Hameghavandi, M.H.R.; Sabahi, M.; Rostamkhani, S. Nuclear factor-kappa B (NF-κB) in pathophysiology of Parkinson disease: Diverse patterns and mechanisms contributing to neurodegeneration. Eur. J. Neurosci. 2021, 54, 4101–4123. [Google Scholar] [CrossRef]
- Källstig, E.; McCabe, B.D.; Schneider, B.L. The Links between ALS and NF-κB. Int. J. Mol. Sci. 2021, 22, 3875. [Google Scholar] [CrossRef]
- Bottero, V.; Alrafati, F.; Santiago, J.A.; Potashkin, J.A. Transcriptomic and Network Meta-Analysis of Frontotemporal Dementias. Front. Mol. Neurosci. 2021, 14, 747798. [Google Scholar] [CrossRef]
- Leibowitz, S.M.; Yan, J. NF-κB Pathways in the Pathogenesis of Multiple Sclerosis and the Therapeutic Implications. Front. Mol. Neurosci. 2016, 9, 84. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Li, Z.-Y.; Jiang, X.-Q.; Wu, F.; Li, Z.-T.; Chen, H.; Xi, D.; Zhang, Y.-Y.; Chen, Z.-Q. Irisin alleviates renal injury caused by sepsis via the NF-κB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6470–6476. [Google Scholar]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Medhi, B.; Batra, G.; Jain, M.; Singh, R.S.; Sharma, A.R.; Singh, A.; Prakash, A. Novel therapeutic targets for amyotrophic lateral sclerosis. Indian J. Pharmacol. 2019, 51, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Noshita, N.; Lewén, A.; Sugawara, T.; Chan, P.H. Evidence of Phosphorylation of Akt and Neuronal Survival after Transient Focal Cerebral Ischemia in Mice. J. Cereb. Blood Flow Metab. 2001, 21, 1442–1450. [Google Scholar] [CrossRef]
- Beard, R.S.; Hoettels, B.A.; Meegan, J.E.; Wertz, T.S.; Cha, B.J.; Yang, X.; Oxford, J.T.; Wu, M.H.; Yuan, S.Y. AKT2 maintains brain endothelial claudin-5 expression and selective activation of IR/AKT2/FOXO1-signaling reverses barrier dysfunction. J. Cereb. Blood Flow Metab. 2018, 40, 374–391. [Google Scholar] [CrossRef]
- Jin, Z.; Guo, P.; Li, X.; Ke, J.; Wang, Y.; Wu, H. Neuroprotective effects of irisin against cerebral ischemia/ reperfusion injury via Notch signaling pathway. Biomed. Pharmacother. 2019, 120, 109452. [Google Scholar] [CrossRef]
- Cai, Z.; Yan, L.-J.; Li, K.; Quazi, S.H.; Zhao, B. Roles of AMP-activated Protein Kinase in Alzheimer’s Disease. Neuromol. Med. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Athari, S.Z.; Farajdokht, F.; Keyhanmanesh, R.; Mohaddes, G. AMPK signaling pathway as a potential therapeutic target for Parkinson’s disease. Adv. Pharm. Bull. 2023, 14, 120–131. [Google Scholar] [CrossRef]
- García-García, R.; Martín-Herrero, L.; Blanca-Pariente, L.; Pérez-Cabello, J.; Roodveldt, C. Immune Signaling Kinases in Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia (FTD). Int. J. Mol. Sci. 2021, 22, 13280. [Google Scholar] [CrossRef]
- Askari, H.; Rabiei, F.; Lohrasbi, F.; Ghadir, S.; Arbastan, A.M.; Ghasemi-Kasman, M. AMP-activated protein kinase as a mediator of mitochondrial dysfunction of multiple sclerosis in animal models: A systematic review. J. Cell. Physiol. 2024, 239, e31230. [Google Scholar] [CrossRef]
- Song, J.H.; Yu, J.T.; Tan, L. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease: Risk, Mechanisms, and Therapy. Mol. Neurobiol. 2015, 52, 1477–1493. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Riolo, G.; Ricci, C.; De Angelis, N.; Marzocchi, C.; Guerrera, G.; Borsellino, G.; Giannini, F.; Battistini, S. BDNF and Pro-BDNF in Amyotrophic Lateral Sclerosis: A New Perspective for Biomarkers of Neurodegeneration. Brain Sci. 2022, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Benussi, L.; Binetti, G.; Ghidoni, R. Loss of Neuroprotective Factors in Neurodegenerative Dementias: The End or the Starting Point? Front. Neurosci. 2017, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Nociti, V.; Romozzi, M. The Role of BDNF in Multiple Sclerosis Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 8447. [Google Scholar] [CrossRef]
- Jo, D.; Song, J. Irisin Acts via the PGC-1α and BDNF Pathway to Improve Depression-like Behavior. Clin. Nutr. Res. 2021, 10, 292–302. [Google Scholar] [CrossRef]
- Huang, I.-H.; Chung, W.-H.; Wu, P.-C.; Chen, C.-B. JAK–STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Lashgari, N.-A.; Roudsari, N.M.; Momtaz, S.; Sathyapalan, T.; Abdolghaffari, A.H.; Sahebkar, A. The involvement of JAK/STAT signaling pathway in the treatment of Parkinson’s disease. J. Neuroimmunol. 2021, 361, 577758. [Google Scholar] [CrossRef]
- Richardson, P.J.; Smith, D.P.; de Giorgio, A.; Snetkov, X.; Almond-Thynne, J.; Cronin, S.; Mead, R.J.; McDermott, C.J.; Shaw, P.J. Janus kinase inhibitors are potential therapeutics for amyotrophic lateral sclerosis. Transl. Neurodegener. 2023, 12, 47. [Google Scholar] [CrossRef]
- Rusek, M.; Smith, J.; El-Khatib, K.; Aikins, K.; Czuczwar, S.J.; Pluta, R. The Role of the JAK/STAT Signaling Pathway in the Pathogenesis of Alzheimer’s Disease: New Potential Treatment Target. Int. J. Mol. Sci. 2023, 24, 864. [Google Scholar] [CrossRef]
- Jain, M.; Singh, M.K.; Shyam, H.; Mishra, A.; Kumar, S.; Kumar, A.; Kushwaha, J. Role of JAK/STAT in the Neuroinflammation and its Association with Neurological Disorders. Ann. Neurosci. 2021, 28, 191–200. [Google Scholar] [CrossRef]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’adamio, L.; Grassi, C.; Devanand, D.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimer’s Dis. 2018, 64, S611–S631. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.B.; Morais, V.A. PINK1: A Bridge between Mitochondria and Parkinson’s Disease. Life 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; Sipos, J.; Thau-Habermann, N.; Körner, S.; Rath, K.J.; Dengler, R.; Petri, S. Altered Expression of DJ-1 and PINK1 in Sporadic ALS and in the SOD1G93AALS Mouse Model. J. Neuropathol. Exp. Neurol. 2013, 72, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Choe, Y.-J.; Bannwarth, S.; Kim, J.; Maitra, S.; Dorn, G.W.; Taylor, J.P.; Paquis-Flucklinger, V.; Kim, N.C. TDP-43 and PINK1 mediate CHCHD10S59L mutation–induced defects in Drosophila and in vitro. Nat. Commun. 2021, 12, 1924. [Google Scholar] [CrossRef]
- Cossu, D.; Yokoyama, K.; Sechi, L.A.; Hattori, N. Potential of PINK1 and PARKIN Proteins as Biomarkers for Active Multiple Sclerosis: A Japanese Cohort Study. Front. Immunol. 2021, 12, 681386. [Google Scholar] [CrossRef]
- Munoz, I.Y.M.; Romero, E.d.S.C.; Garcia, J.d.J.G. Irisin a Novel Metabolic Biomarker: Present Knowledge and Future Directions. Int. J. Endocrinol. 2018, 2018, 7816806. [Google Scholar] [CrossRef]
- Remuzgo-Martínez, S.; Rueda-Gotor, J.; Pulito-Cueto, V.; López-Mejías, R.; Corrales, A.; Lera-Gómez, L.; Pérez-Fernández, R.; Portilla, V.; González-Mazón, Í.; Blanco, R.; et al. Irisin as a Novel Biomarker of Subclinical Atherosclerosis, Cardiovascular Risk and Severe Disease in Axial Spondyloarthritis. Front. Immunol. 2022, 13, 894171. [Google Scholar] [CrossRef]
- Gurger, M.; Telo, S.; Yilmaz, M.; Atescelik, M.; Gul, E.; Goktekin, M. Diagnostic and Prognostic Relevance of Serum Irisin Level in Patients with Pulmonary Embolism. Clin. Lab. 2021, 67, 707–712. [Google Scholar] [CrossRef]
- Gaggini, M.; Cabiati, M.; Del Turco, S.; Navarra, T.; De Simone, P.; Filipponi, F.; Del Ry, S.; Gastaldelli, A.; Basta, G. Increased FNDC5/Irisin expression in human hepatocellular carcinoma. Peptides 2017, 88, 62–66. [Google Scholar] [CrossRef]
- Altay, D.U.; Keha, E.E.; Karagüzel, E.; Menteşe, A.; Yaman, S.O.; Alver, A. The Diagnostic Value of FNDC5/Irisin in Renal Cell Cancer. Int. Braz. J. Urol. 2018, 44, 734–739. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Ghobadian, B.; Ghasemi, N.; Sadeh, H.; Abedimanesh, N.; Rahimlou, M. Relationship of serum irisin levels, physical activity, and metabolic syndrome biomarkers in obese individuals with low-calorie intake and non-obese individuals with high-calorie intake. J. Health Popul. Nutr. 2025, 44, 2. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Maurici, J.; Rosa, A.; Azcona-Granada, N.; Peña, E.; Ricart-Jané, D.; Viñas, A.; López-Tejero, M.D.; Domingo, J.C.; Miñarro, A.; Baena-Fustegueras, J.A.; et al. Irisin as a Novel Biomarker of Subclinical Atherosclerosis in Severe Obesity. Int. J. Mol. Sci. 2023, 24, 8171. [Google Scholar] [CrossRef]
- De Meneck, F.; de Souza, L.V.; Oliveira, V.; Franco, M.D. High irisin levels in overweight/obese children and its positive correlation with metabolic profile, blood pressure, and endothelial progenitor cells. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 756–764. [Google Scholar] [CrossRef]
- Sahin-Efe, A.; Upadhyay, J.; Ko, B.-J.; Dincer, F.; Park, K.H.; Migdal, A.; Vokonas, P.; Mantzoros, C. Irisin and leptin concentrations in relation to obesity, and developing type 2 diabetes: A cross sectional and a prospective case-control study nested in the Normative Aging Study. Metabolism 2018, 79, 24–32. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Zulet, M.A.; Lopez-Legarrea, P.; de la Iglesia, R.; Pardo, M.; Carreira, M.C.; Martínez, J.A.; Casanueva, F.F. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism 2014, 63, 520–531. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Huang, Y.-Y.; Gusdon, A.M.; Qu, S. Irisin: A new molecular marker and target in metabolic disorder. Lipids Health Dis. 2015, 14, 2. [Google Scholar] [CrossRef]
- Dozio, E.; Vianello, E.; Sitzia, C.; Ambrogi, F.; Benedini, S.; Gorini, S.; Rampoldi, B.; Rigolini, R.; Tacchini, L.; Romanelli, M.M.C. Circulating Irisin and esRAGE as Early Biomarkers of Decline of Metabolic Health. J. Clin. Med. 2020, 9, 454. [Google Scholar] [CrossRef]
- Abdu Allah, A.M.; Hammoudah, S.A.; Abd El Gayed, E.M.; El-Attar, L.M.; Shehab-Eldin, W.A. Obesity and its association with irisin level among individuals with FNDC5/irisin gene variants RS16835198 and RS726344. Protein Pept. Lett. 2018, 25, 560–569. [Google Scholar] [CrossRef]
- Brondani, L.A.; Boelter, G.; Assmann, T.S.; Leitão, C.B.; Canani, L.H.; Crispim, D. Irisin-encoding gene (FNDC5) variant is associated with changes in blood pressure and lipid profile in type 2 diabetic women but not in men. Metabolism 2015, 64, 952–957. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Mohammed, A.K.; Al-Attas, O.S.; Amer, O.E.; Clerici, M.; Alenad, A.; Alokail, M.S. SNPs in FNDC5 (irisin) are associated with obesity and modulation of glucose and lipid metabolism in Saudi subjects. Lipids Health Dis. 2016, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisloff, U.; Tjonna, A.E.; Raastad, T.; et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef] [PubMed]
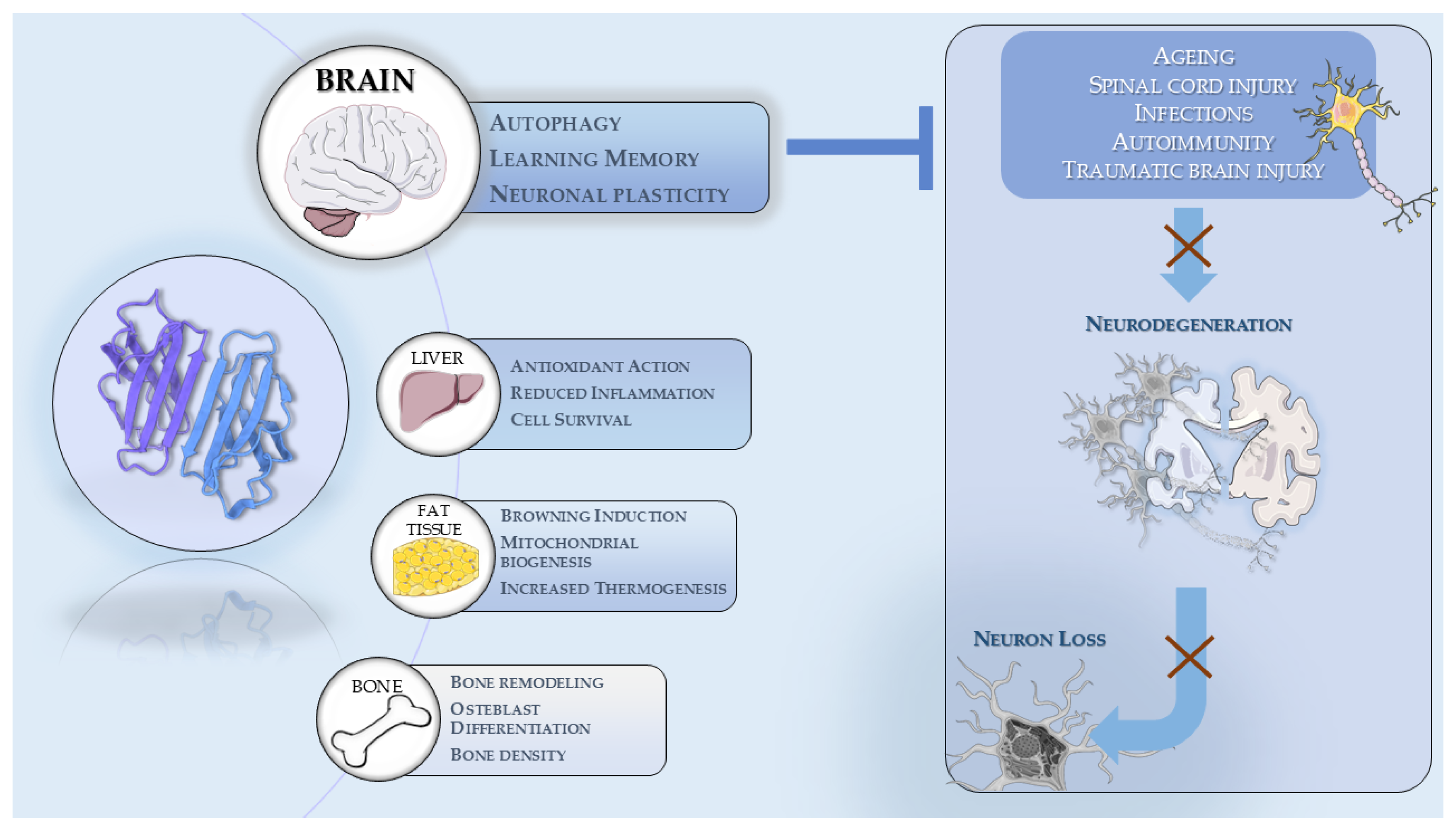
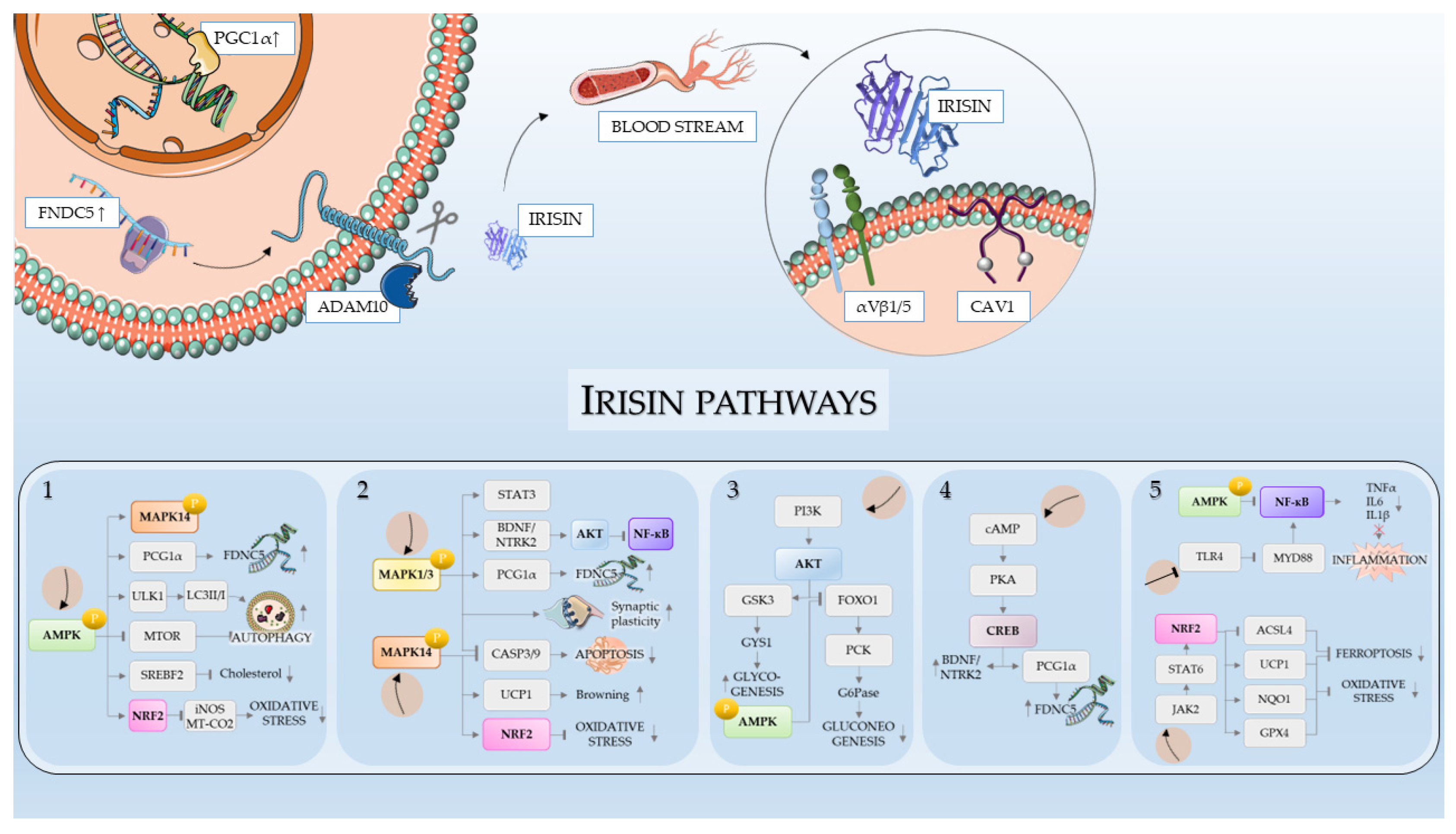
| Pathway | Role in AD | Role in PD | Role in ALS | Role in FTD | Role in MS | Effect of Irisin |
|---|---|---|---|---|---|---|
| NRF2 | Regulates antioxidant defense; reduced activity linked to Aβ accumulation [280] | Protects dopaminergic neurons from oxidative stress [281] | Supports antioxidant gene expression; impaired in ALS [282] | May protect against oxidative damage in neurons [283] | Reduces oxidative stress in oligodendrocytes [284] | Activates NRF2; increases antioxidant enzymes (e.g., SOD, HO1) [285] |
| NF-κB | Promotes neuroinflammation; activated by Aβ and tau [286] | Involved in dopaminergic neuron inflammation [287] | Chronic activation linked to glial inflammation [288] | Associated with TDP-43-mediated inflammation [289] | Drives demyelination and T-cell activation [290] | Inhibits NF-κB activation; reduces pro-inflammatory cytokines (e.g., IL1β, TNFα) [291] |
| PI3K/AKT | Supports neuronal survival; impaired in AD [292] | Protects neurons; promotes dopamine synthesis [293] | Promotes motor neuron survival [294] | Regulates neuronal viability [295] | Maintains BBB and modulates immune response [296] | Activates AKT; promotes cell survival and neurogenesis [297] |
| AMPK | Regulates autophagy; dysfunction linked to tau pathology [298] | Controls mitochondrial biogenesis and autophagy [299] | Maintains energy balance and autophagy in neurons [300] | Modulates metabolism and neuroinflammation [300] | Regulates immune cell energy status and reduces inflammation [301] | Activates AMPK; improves mitochondrial function and autophagy [187] |
| BDNF/CREB | Decreased BDNF levels in AD [302] | Supports dopaminergic neuron survival and plasticity [303] | May maintain neuromuscular synapse function [304] | Reduces synaptic loss and supports behavioral flexibility [305] | Enhances remyelination and synaptic integrity [306] | Increases BDNF expression via CREB; promotes synaptic plasticity [307] |
| JAK/STAT | Involved in cytokine-mediated neuroinflammation [308] | Modulates glial activity and neuron-glia communication [309] | Drives inflammatory glial activation in ALS [310] | Linked to neuroinflammatory signaling [311] | Critical in autoimmune demyelination [312] | May inhibit excessive cytokine production; modulates immune signaling pathways [21] |
| Autophagy (mTOR, PINK1/Parkin) | Impaired clearance of Aβ and tau; contributes to AD pathology [313] | Dysfunction leads to α-syn aggregation [314] | Downregulated in ALS murine models. Crucial for clearance of protein aggregates and damaged mitochondria [315] | Deficits linked to TDP-43 accumulation, which is involved in FTD etiopathogenesis [316] | Enhances myelin repair by clearing damaged organelles [317] | Activates autophagy via AMPK; promotes clearance of protein aggregates [262] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minuti, A.; Raffaele, I.; Scuruchi, M.; Lui, M.; Muscarà, C.; Calabrò, M. Role and Functions of Irisin: A Perspective on Recent Developments and Neurodegenerative Diseases. Antioxidants 2025, 14, 554. https://doi.org/10.3390/antiox14050554
Minuti A, Raffaele I, Scuruchi M, Lui M, Muscarà C, Calabrò M. Role and Functions of Irisin: A Perspective on Recent Developments and Neurodegenerative Diseases. Antioxidants. 2025; 14(5):554. https://doi.org/10.3390/antiox14050554
Chicago/Turabian StyleMinuti, Aurelio, Ivana Raffaele, Michele Scuruchi, Maria Lui, Claudia Muscarà, and Marco Calabrò. 2025. "Role and Functions of Irisin: A Perspective on Recent Developments and Neurodegenerative Diseases" Antioxidants 14, no. 5: 554. https://doi.org/10.3390/antiox14050554
APA StyleMinuti, A., Raffaele, I., Scuruchi, M., Lui, M., Muscarà, C., & Calabrò, M. (2025). Role and Functions of Irisin: A Perspective on Recent Developments and Neurodegenerative Diseases. Antioxidants, 14(5), 554. https://doi.org/10.3390/antiox14050554







