Abstract
The widespread use of lead (Pb) has led to serious environmental and human health problems worldwide. The application of oxide nanoparticles (CeO2 NPs) in alleviating abiotic stress in plants has received extensive attention. In this study, 50 mg·L−1 CeO2 NPs can improve Pb resistance and promote rice growth. Specifically, this study observed that CeO2 NPs increased the activity of antioxidant enzymes peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX), but the difference did not reach a significant level. At the same time, CeO2 NPs upregulated antioxidant metabolites alpha-linolenic acid, linoleic acid, ferulic acid, and kaempferol under Pb stress. In addition, CeO2 NPs upregulated multiple defense response-related genes, such as OsOPR1 and OsPR10a; RPR10a, and improved rice carbon flow and energy supply by upregulating sucrose and D-glucose. The results of this study provided technical support for alleviating Pb stress in rice.
1. Introduction
Soil can act as a pollution absorber and retain various pollutants, such as heavy metals [1]. Heavy metal pollution in soil systems results from natural processes and human factors [2,3,4] and is widespread worldwide [5]. Over the past 40 years, China’s rapid economic development has negatively impacted the ecological environment, and soil pollution has become increasingly serious [6,7,8]. According to reports, cadmium (Cd), lead (Pb), and arsenic (As) are the most serious pollutants in China’s industrial and agricultural regions in terms of soil pollution and health risks [9,10].
The widespread use of Pb has caused serious environmental and human health problems worldwide, and no known beneficial biological functions of Pb have been found [11,12]. Pb pollution affects seed germination, plant growth, and metabolism [13,14,15]. Plants growing in Pb-contaminated soil absorb Pb through roots and transport it to leaves. Unbound Pb ions in plants become biohazards, directly damaging photosynthetic function [16,17]. High concentrations of Pb may inhibit the enzyme activities associated with chlorophyll biosynthesis, CO2 fixation, and the aggregation of the pigment protein complexes in photosystems [18,19]. In addition, plants exposed to Pb stress produce reactive oxygen species (ROS). These ROS deplete plant cell antioxidants and damage cell membrane structure and lipid composition [20]. Pb has been reported to affect the activities of various enzymes in plants. With the increase of Pb2+ concentration (5–50 mg·L−1), peroxidase (POD) activity and malonaldehyde (MDA) content peaked at 20 mg·L−1, while superoxide dismutase (SOD) and catalase (CAT) activities decreased first and then increased in Potamogeton crispus [21,22]. These results indicated that plants exposed to Pb stress have complex physiological response characteristics, and the dynamic changes in the activities of different antioxidant enzymes may be closely related to the degree of plant cell damage and stress regulation mechanisms.
The application of nanoparticles in agriculture has been widely reported. Some inorganic forms of nanoparticles have been shown to alleviate plant abiotic stress by enhancing antioxidant defense enzymes [23,24]. Cerium oxide nanoparticles (CeO2 NPs) have been reported to exhibit antioxidant behavior and suppress nitric oxide and hydroxyl radicals in biological systems [25]. As a nanozyme, CeO2 NPs play an essential role in alleviating abiotic stress factors such as salt, drought, and heavy metals [24]. According to the report, the application of CeO2 NPs had an overall positive effect on wheat growth by controlling the transfer of Cd from soil into plant tissues under Cd stress [26]. Therefore, CeO2 NPs have great potential as nanozymes to alleviate plant heavy metal stress and improve stress resistance. Up to now, there are no reports on the research of CeO2 NPs in alleviating Pb stress in rice.
Based on previous reports, we speculated that applying CeO2 NPs may positively regulate Pb resistance in rice. Therefore, this study attempted to verify the hypothesis from multiple perspectives, such as physiology, transcriptomics, and metabolomics, which are conducive to ensuring food security and sustainable agricultural development.
2. Materials and Methods
2.1. Synthesis of CeO2 NPs
CeO2 NPs were provided by the College of Agriculture, South China Agricultural University (particle size 5.92 nm; potential −45.07 mV). The synthesis of CeO2 NPs was based on the method of An et al. [27].
2.2. Experimental Design
This study was conducted in 2024. The test site was an outdoor artificial climate greenhouse at Guangdong Ocean University (natural light, day/night temperatures of 31/25 ± 2 °C, and relative humidity of 60%). The rice test variety was Huanghuazhan, provided by the Physiology and Biochemistry Laboratory of Guangdong Ocean University.
The sterilized rice seeds were soaked in water for 24 h and germinated for 24 h. The germinated seeds were sown in 20.4 cm × 16.9 cm × 14.5 cm (upper diameter/height/lower diameter) sealed-bottom pots, with 57 seeds per pot. Each pot contained 2.5 kg of brick laterite (0.21 g urea, 0.19 g potassium chloride, and 0.25 g diammonium phosphate as base fertilizer). Thirteen days after sowing, 50 mg·L−1 CeO2 NPs aqueous solution was sprayed on the leaves (4 mL per pot), and water was used as a control. Seven hours later, 583 mg of lead acetate trihydrate was applied to each pot. Twelve days after the first Pb stress treatment, 583 mg of lead acetate trihydrate was added to each pot again (the final concentration of Pb in each pot was 254.79 mg·kg−1 (DW)).
In this study, three treatments were set up, namely control (CK); Pb treatment (Pb); and Pb treatment + 50 mg·L−1 CeO2 NPs (Ce50).
2.3. Morphology and Fresh Weight Determination
After the second stress treatment, plant height, shoot fresh weight, root fresh weight, and leaf area were measured on the 4th (first sampling) and 8th (second sampling) days.
The leaf area was measured by a Yaxin-1241 portable leaf area meter (Beijing Yaxin LIYI Technology Co., LTD, China).
2.4. Photosynthesis-Related Index Detection
The transpiration rate (Tr), stomatal conductance (Gs), net photosynthetic rate (Pn), and intercellular carbon dioxide concentration (Ci) of the latest fully expanded leaf were measured by LI-6800 portable photosynthetic system (Li-Cor Inc., Lincoln, NE, USA) on the 4th day (first sampling) after the second stress treatment.
2.5. Antioxidant Enzyme Activity Detection
The activities of antioxidant enzymes POD, CAT, and ascorbate peroxidase (APX) in leaves were detected on the 4th day (first sampling) after the second stress treatment according to Zhang et al.’s method [28].
2.6. Transcriptome Detection
The latest fully expanded leaves were collected for transcriptome sequencing on the 4th day (first sampling) after the second stress treatment. The collected samples were placed in pre-cooled test tubes and temporarily stored in liquid nitrogen. The test tubes containing the samples were then stored in a −80 °C freezer for future use. ShanghaiMajorbio Bio-pharm Biotechnology Co., Ltd. (Shanghai, China) performed RNA purification, reverse transcription, library construction, and sequencing.
2.7. Metabolome Detection
The latest fully expanded leaves were collected for metabolome detection on the 4th day (first sampling) after the second stress treatment. The collected samples were placed in pre-cooled test tubes and temporarily stored in liquid nitrogen. The test tubes containing the samples were then stored in a −80 °C freezer for future use. ShanghaiMajorbio Bio-pharm Biotechnology Co., Ltd. (Shanghai, China) performed LC-MS analysis.
2.8. Real-Time Quantitative PCR
The latest fully expanded leaves were collected on the 4th day (first sampling) after the second stress treatment. The primers used for RT-qPCR were listed in Table S1. The OsActin gene was used as a reference. The relative expression levels were calculated using the 2−∆∆CT method.
2.9. Statistical Analysis
SPSS 22 was used to conduct one-way ANOVA. Origin 2021 was used to create figures. This study used Endnote 20 to introduce literature and Grammarly to correct grammatical errors.
3. Results
3.1. Morphology and Fresh Weight
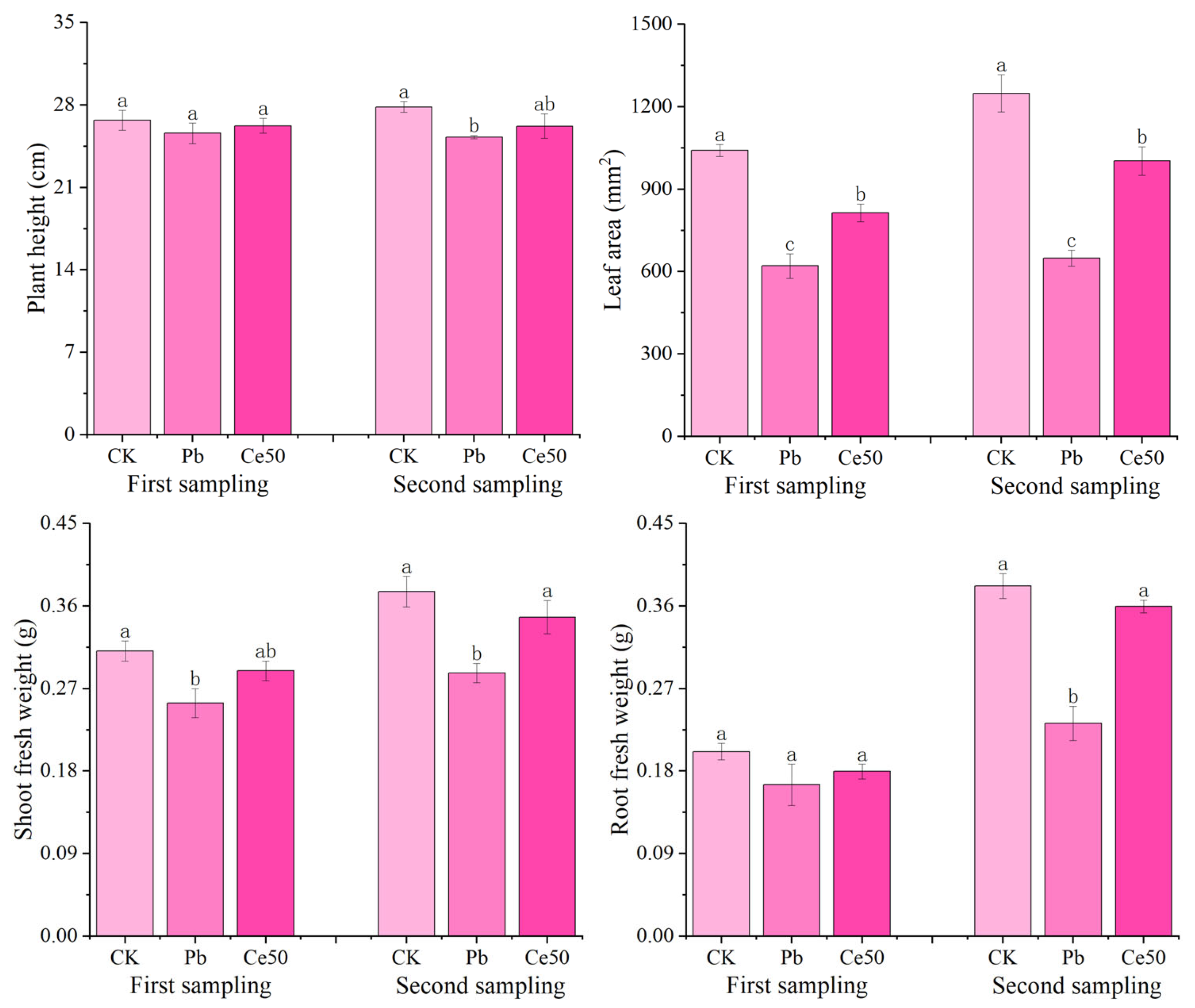
As shown in Figure 1, there was no significant difference in plant height among the different treatments at the first sampling. The shoot fresh weight and leaf area of CK were significantly higher than those of Pb by 22.44% and 67.85%, respectively; the leaf area of Ce50 was significantly higher than that of Pb by 31.16%, respectively. At the second sampling, the plant height, root fresh weight, shoot fresh weight, and leaf area of CK were significantly higher than those of Pb by 10.16%, 64.51%, 30.89%, and 92.50%, respectively. The root fresh weight, shoot fresh weight, and leaf area of Ce50 were significantly higher than those of Pb by 54.88%, 21.14%, and 54.60%, respectively.
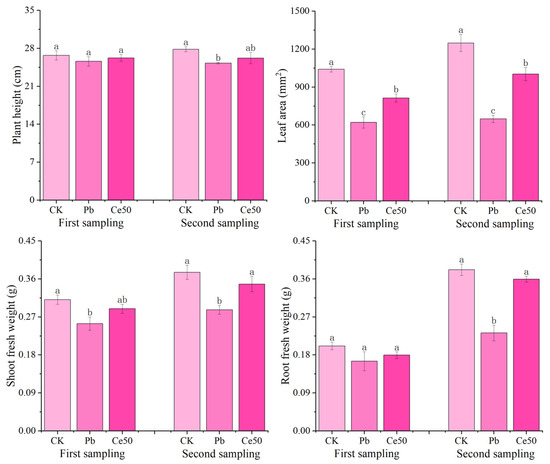
Figure 1.
The changes in rice morphology and fresh weight. The different letters indicate significant differences (p < 0.05). The value represents the average ± standard error (three replicates).
3.2. Antioxidant Enzyme Activity and Antioxidant Metabolite Abundance
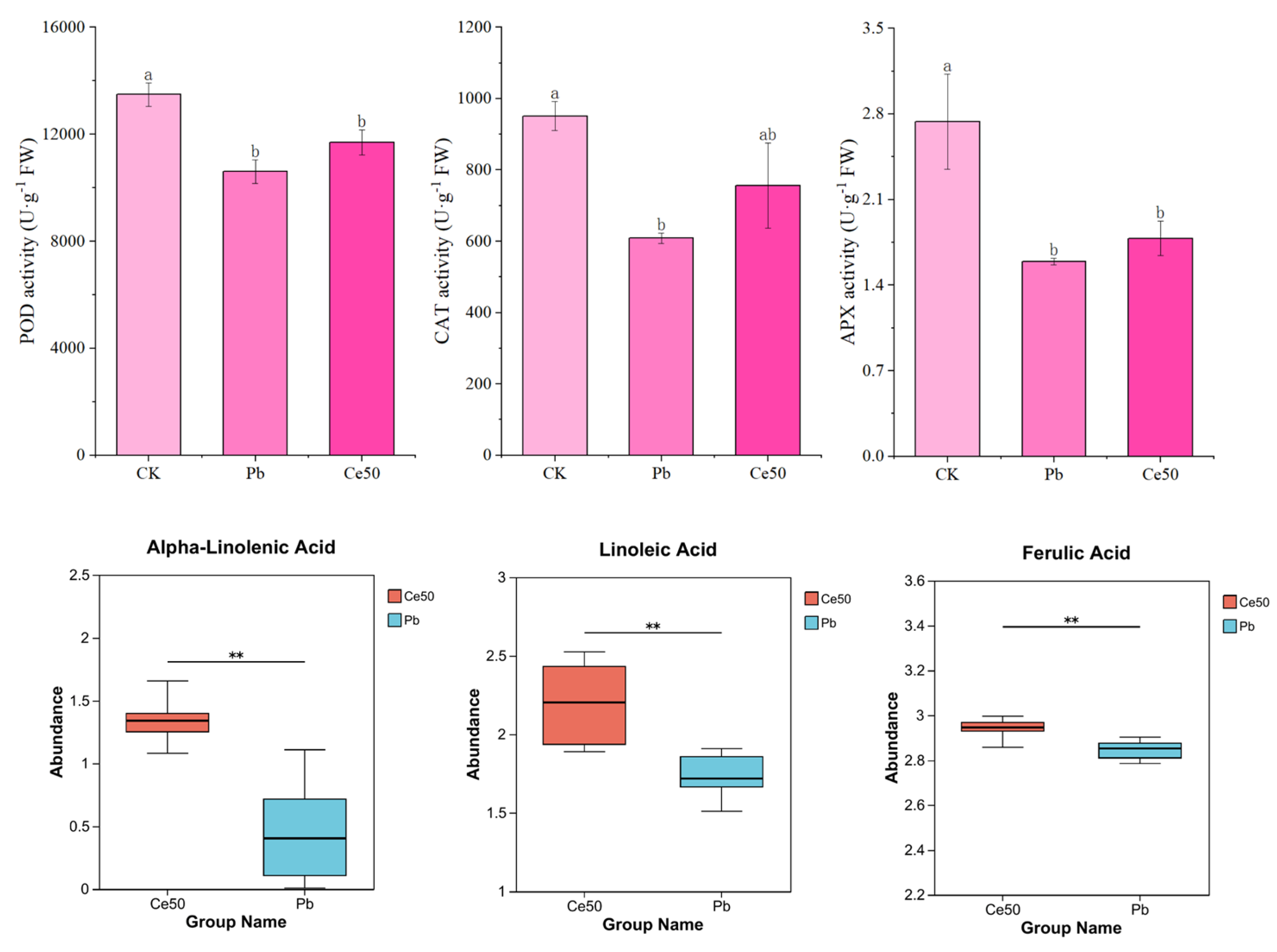
As shown in Figure 2, POD, CAT, and APX activities significantly decreased by 27.15%, 56.31%, and 71.88% under Pb stress, respectively, compared with CK. Compared with Pb, the activities of Ce50 of these three enzymes were slightly increased. In addition, this study found that 50 mg·L−1 CeO2 NPs upregulated alpha-linolenic acid, linoleic acid, and ferulic acid under Pb stress.
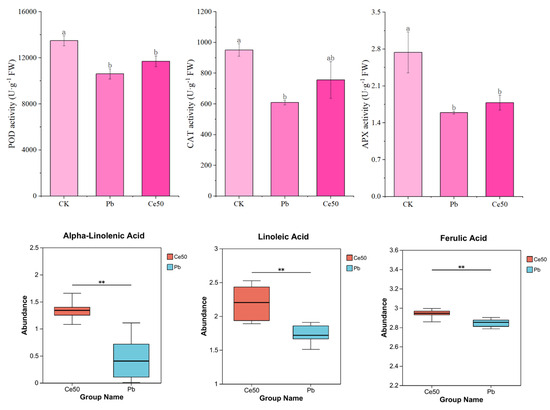
Figure 2.
The antioxidant enzyme activity and antioxidant metabolite abundance. For antioxidant enzymes, the different letters indicate significant differences (p < 0.05); the value represents the average ± standard error (three replicates). For antioxidant metabolites, ** 0.001 < p ≤ 0.01.
3.3. Photosynthetic Pigment Content
As shown in Table 1, the chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents of Pb at the first sampling were significantly higher than those of CK by 3.24%, 14.07%, 6.35%, and 6.80%, respectively. At the second sampling, the contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids in CK were significantly higher than those of Pb by 4.65%, 21.58%, 9.50%, and 4.12%. The contents of chlorophyll a, chlorophyll b, and total chlorophyll in Ce50 were slightly higher than those in Pb, and the content of carotenoids was significantly higher than that in Pb by 3.47%.

Table 1.
Changes in photosynthetic pigment content of rice.
3.4. Photosynthesis
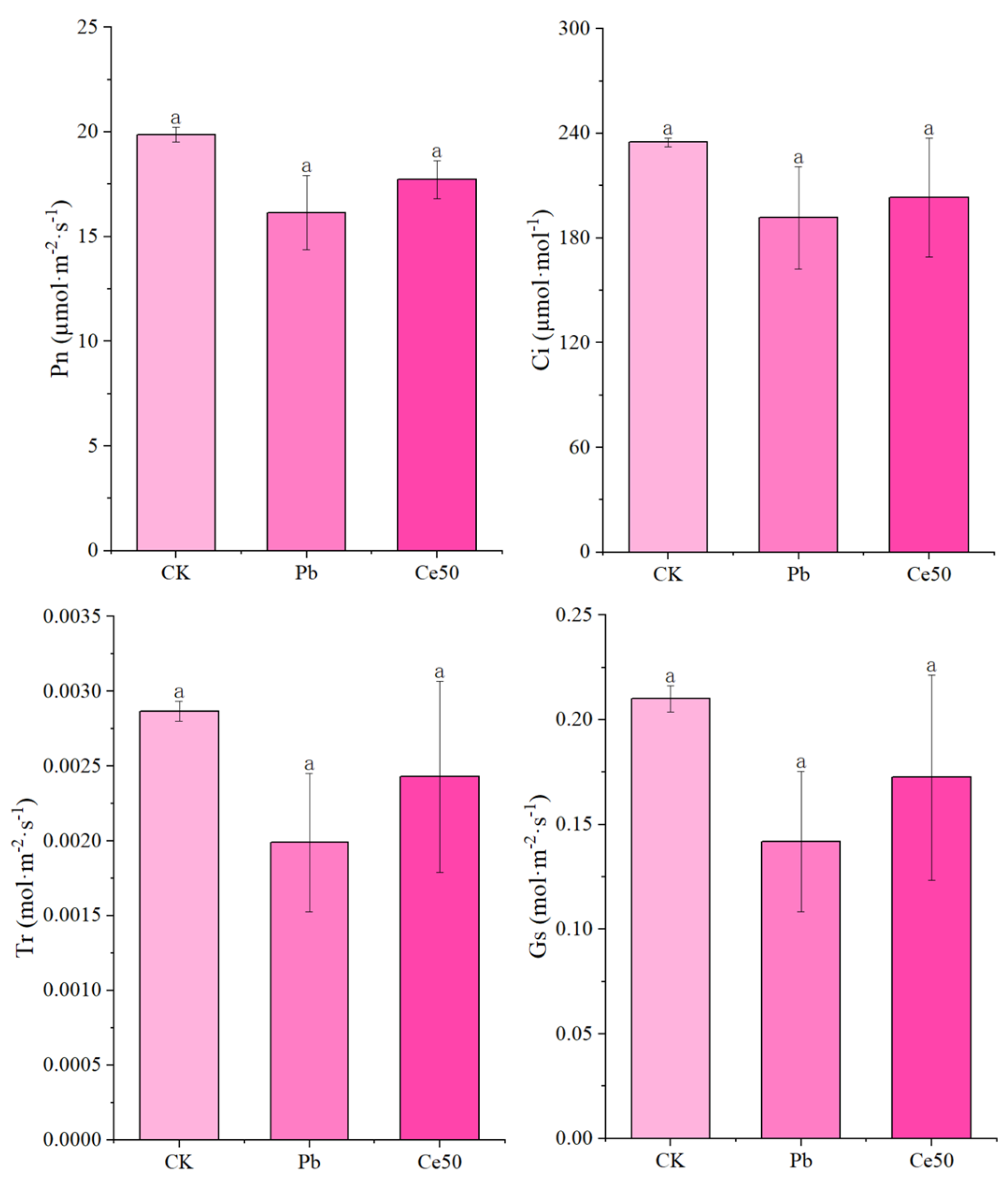
As shown in Figure 3, Pb stress caused a decrease in Pn, Gs, Tr, and Ci, but the differences were not significant. 50 mg·L−1 CeO2 NPs had a certain effect on improving Pn, Gs, and Tr under Pb stress (not significant).
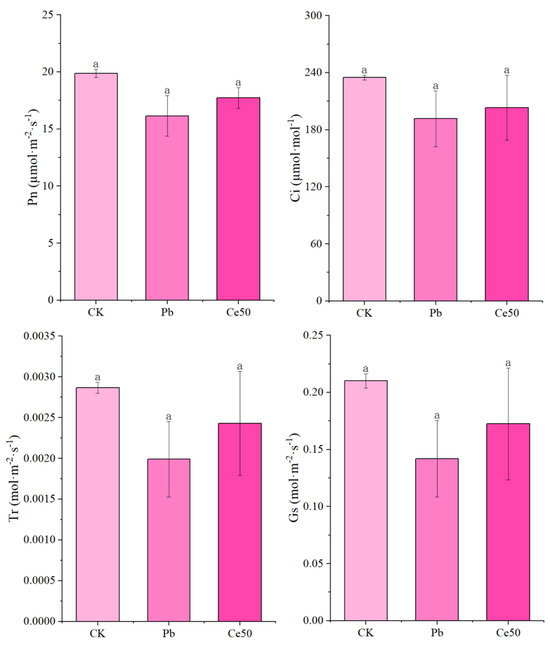
Figure 3.
The changes in the photosynthetic parameters of rice. The different letters indicate significant differences (p < 0.05). The value represents the average ± standard error (three replicates).
3.5. Transcriptome
3.5.1. Quality Control
This study completed a transcriptome analysis of nine samples. The Q30 base percentage was more than 96.47%. The clean reads of each sample were aligned with the specified reference genome, and the alignment rates ranged from 92.24% to 94% (Tables S2 and S3).
3.5.2. Differentially Expressed Gene (DEG) Statistics
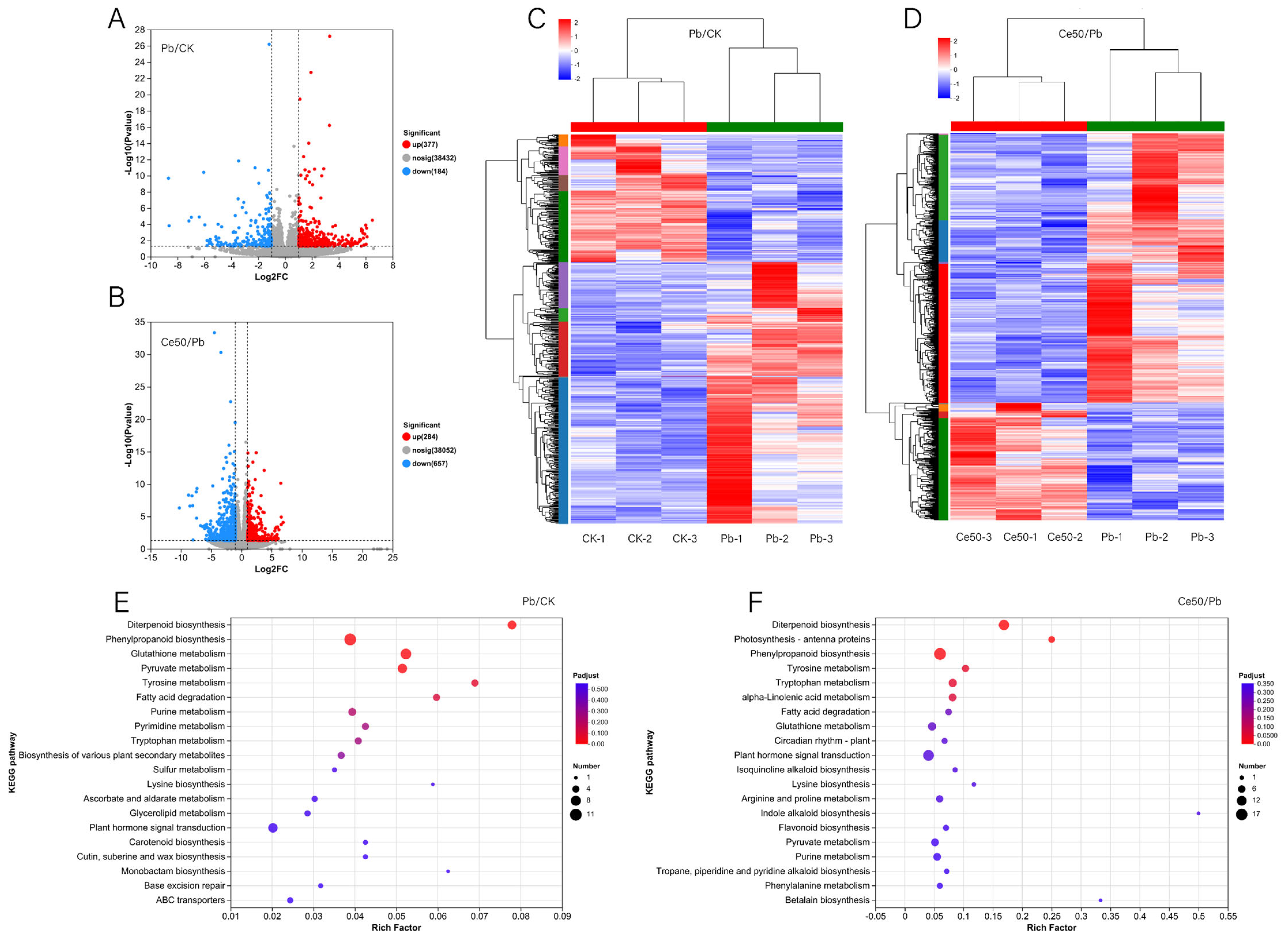
In the Pb/CK comparison group, 377 upregulated DEGs and 184 downregulated DEGs were detected (Figure 4A,C). In the Ce50/Pb comparison group, 284 upregulated and 657 downregulated DEGs were detected (Figure 4B,D).
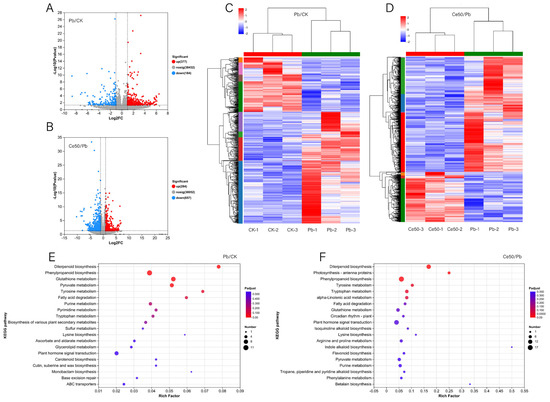
Figure 4.
DEGs volcano plot, cluster analysis, and KEGG enrichment analysis. (A,B) DEGs volcano plot; (C,D) cluster analysis; (E,F) KEGG enrichment analysis.
3.5.3. KEGG Annotation, KEGG Enrichment Analysis, and GO Enrichment Analysis
KEGG annotation analysis found that many DEGs in the Pb/CK comparison group were classified into biosynthesis of other secondary metabolites (17 DEGs), carbohydrate metabolism (15 DEGs), and amino acid metabolism (13 DEGs), indicating that Pb stress may trigger the responses in secondary metabolism and carbohydrate metabolism (Figure S1A). Similarly, in the Ce50/Pb comparison group, most DEGs were attributed to carbohydrate metabolism (34 DEGs), followed by biosynthesis of other secondary metabolites (31 DEGs) and amino acid metabolism (26 DEGs) (Figure S1B).
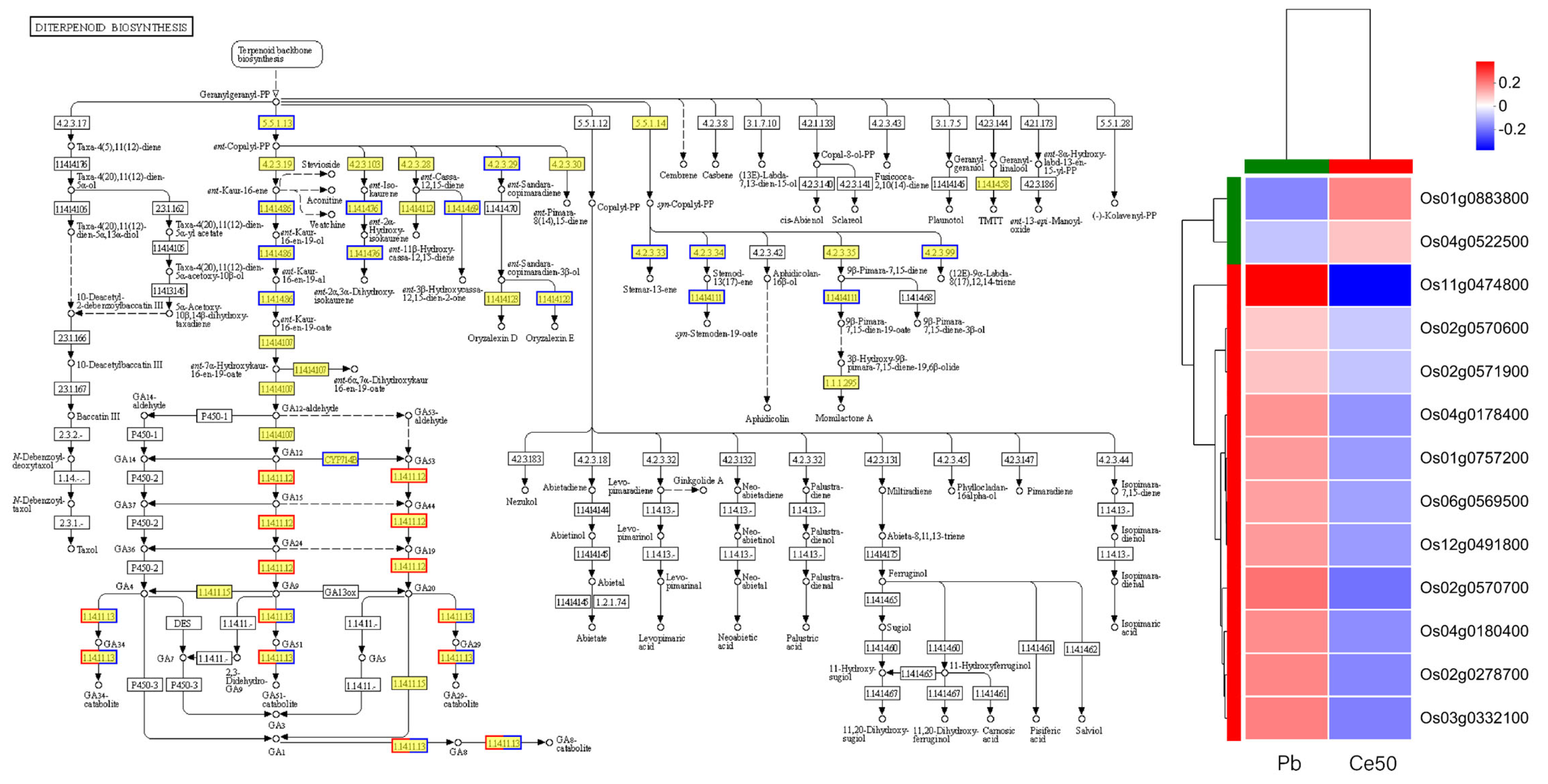
KEGG enrichment analysis found that the P-adjust of the diterpenoid biosynthesis pathway was the smallest in both comparison groups, reflecting the core role of diterpenoid metabolism in Pb stress response and CeO2 NPs may mediate the process of rice encountering Pb stress by regulating diterpenoid metabolism (Figure 4E,F, Figure 5 and Figure S2).
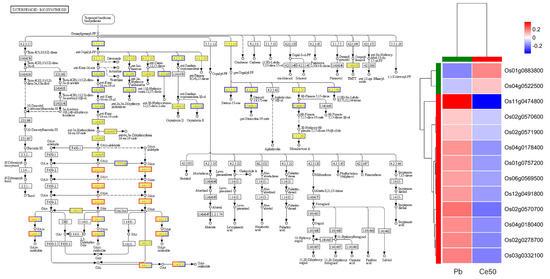
Figure 5.
Diterpenoid biosynthesis pathway in the Ce50/Pb comparison group. In the pathway diagram, red represents upregulated genes; blue represents downregulated genes. The copyright of the KEGG map has been obtained.
GO enrichment analysis found that 17 DEGs were classified into antioxidant activity (Figure S3B), and 58 DEGs were classified into defense response in the Ce50/Pb comparison group (Figure S3A).
3.5.4. TF Gene Statistics
As shown in Figure S1C, in the Pb/CK comparison group, six genes were classified into the NAC family; five were classified into the WRKY family; the ERF family contained four genes; and MYB also had four TF genes. In the Ce50/Pb comparison group, most of the DEGs were classified into the bHLH family, totaling nine; eight were classified into the WRKY family; eight were classified into the MYB family; the NAC family contained seven; and the HB-other family contained five (Figure S1D).
3.5.5. Expression of Stress Resistance-Related Genes
This study screened some essential genes related to rice stress resistance. These genes were upregulated in Pb + CeO2 NP treatment compared with Pb. OsmiR528 and OsHKT1;1 are related to rice salt resistance; OsRCI2-5 and OsLG3; OsERF62 are related to rice drought resistance; SOD1-Fe is a superoxide dismutase gene. In addition, this study selected some DEGs for real-time quantitative PCR detection, and the results obtained were similar to the transcriptome data (Table S4).
3.6. Metabolome
3.6.1. Differential Metabolite Statistics, OPLS-DA, and OPLS-DA Permutation Test
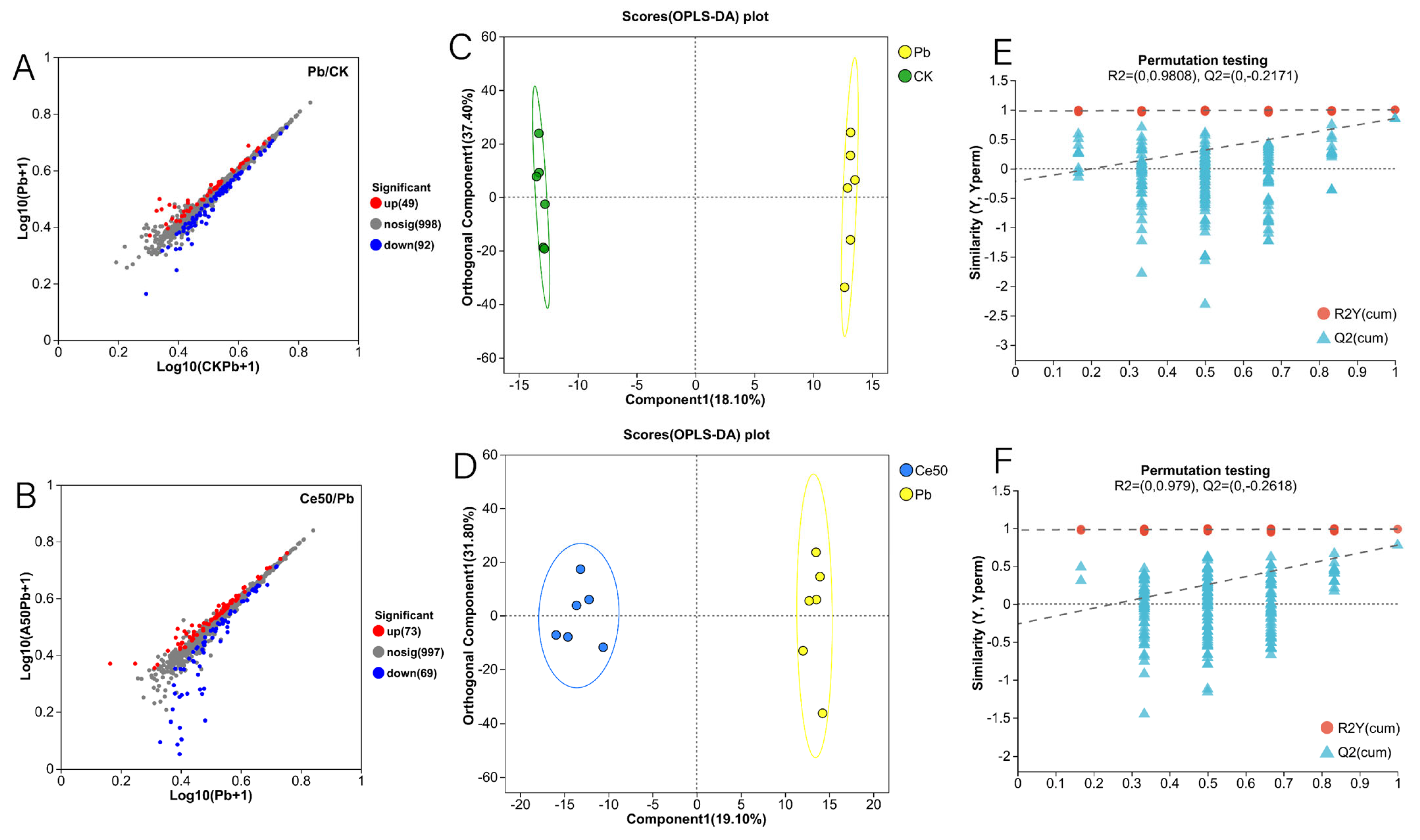
In this study, the six samples of each treatment in the OPLS-DA score plot were distributed in the same area, and the different treatments were separated (Figure 6C,D). The OPLS-DA permutation test showed that Q2 < 0, indicating that the model was stable and not overfitting (Figure 6E,F). A total of 141 differential metabolites were detected in the Pb/CK comparison group, of which 49 were upregulated, and 92 were downregulated (Figure 6A and Figure 7A). In the Ce50/Pb comparison group, 142 differential metabolites were detected, of which 73 were upregulated, and 69 were downregulated (Figure 6B and Figure 7B).
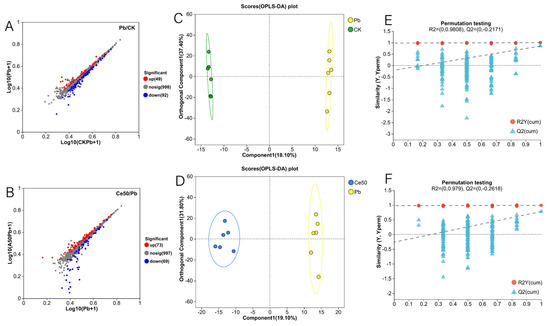
Figure 6.
Volcano plot of differential metabolites, OPLS-DA, and OPLS-DA permutation test. (A,B) Volcano plot of differential metabolites; (C,D) OPLS-DA; (E,F) OPLS-DA permutation test ((E) in the Pb/CK comparison group; (F) in the Ce50/Pb comparison group).
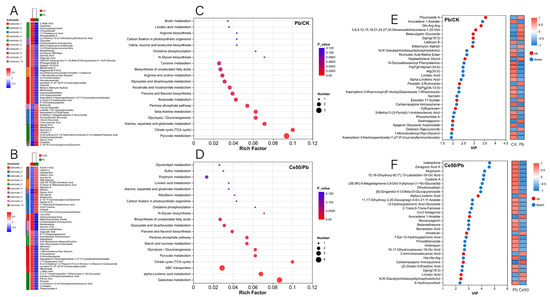
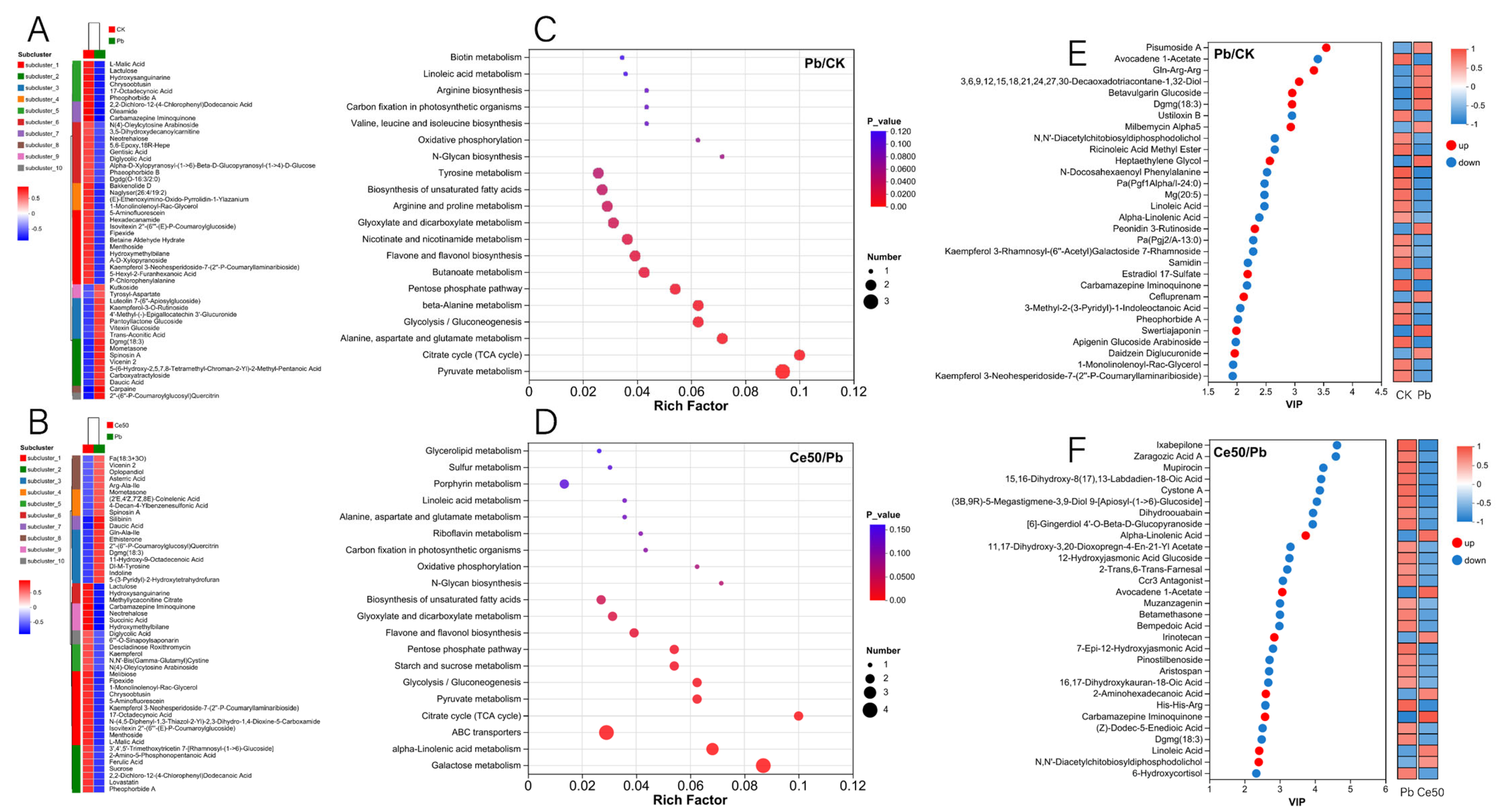
Figure 7.
Differential metabolite cluster analysis, KEGG enrichment analysis, and VIP analysis. (A,B) Differential metabolite cluster analysis; (C,D) KEGG enrichment analysis; (E,F) VIP analysis.
3.6.2. KEGG Enrichment Analysis
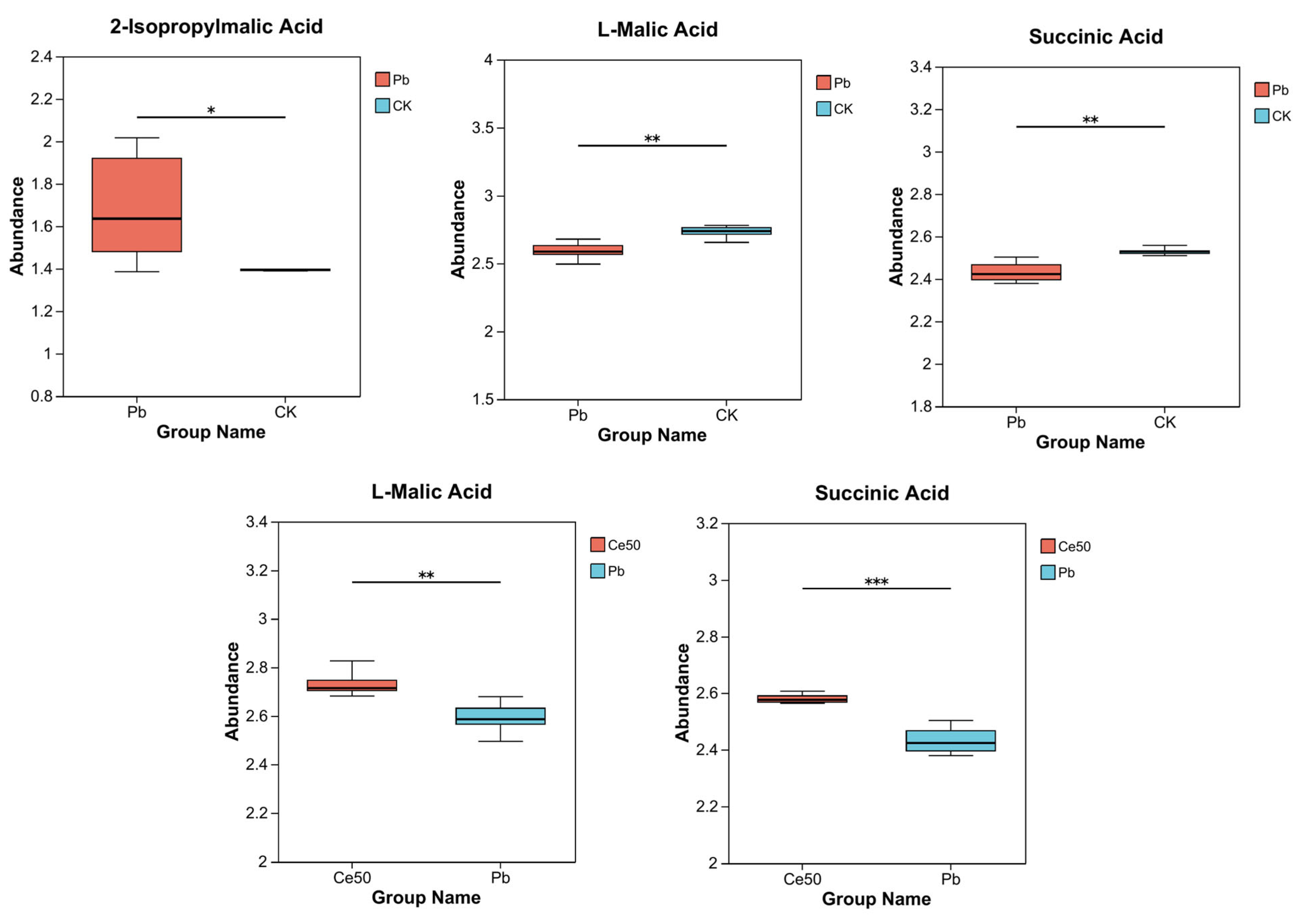
This study conducted KEGG enrichment analysis to study the functions of differential metabolites. The results showed that the P-adjust of the pyruvate metabolism pathway was the smallest in the Pb/CK comparison group. A total of three metabolites were enriched in pyruvate metabolism, namely 2-isopropylmalic acid, L-malic acid, and succinic acid, among which 2-isopropylmalic acid was upregulated, while L-malic acid and succinic acid were downregulated (Figure 7C and Figure 8).
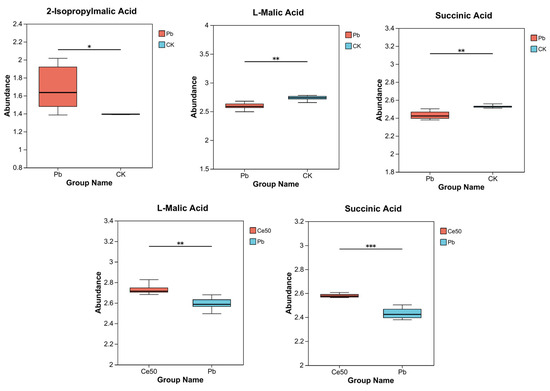
Figure 8.
Differential metabolite abundance in the Pb/CK and Ce50/Pb comparison groups. * 0.01 < p ≤ 0.05; ** 0.001 < p ≤ 0.01; *** p ≤ 0.001.
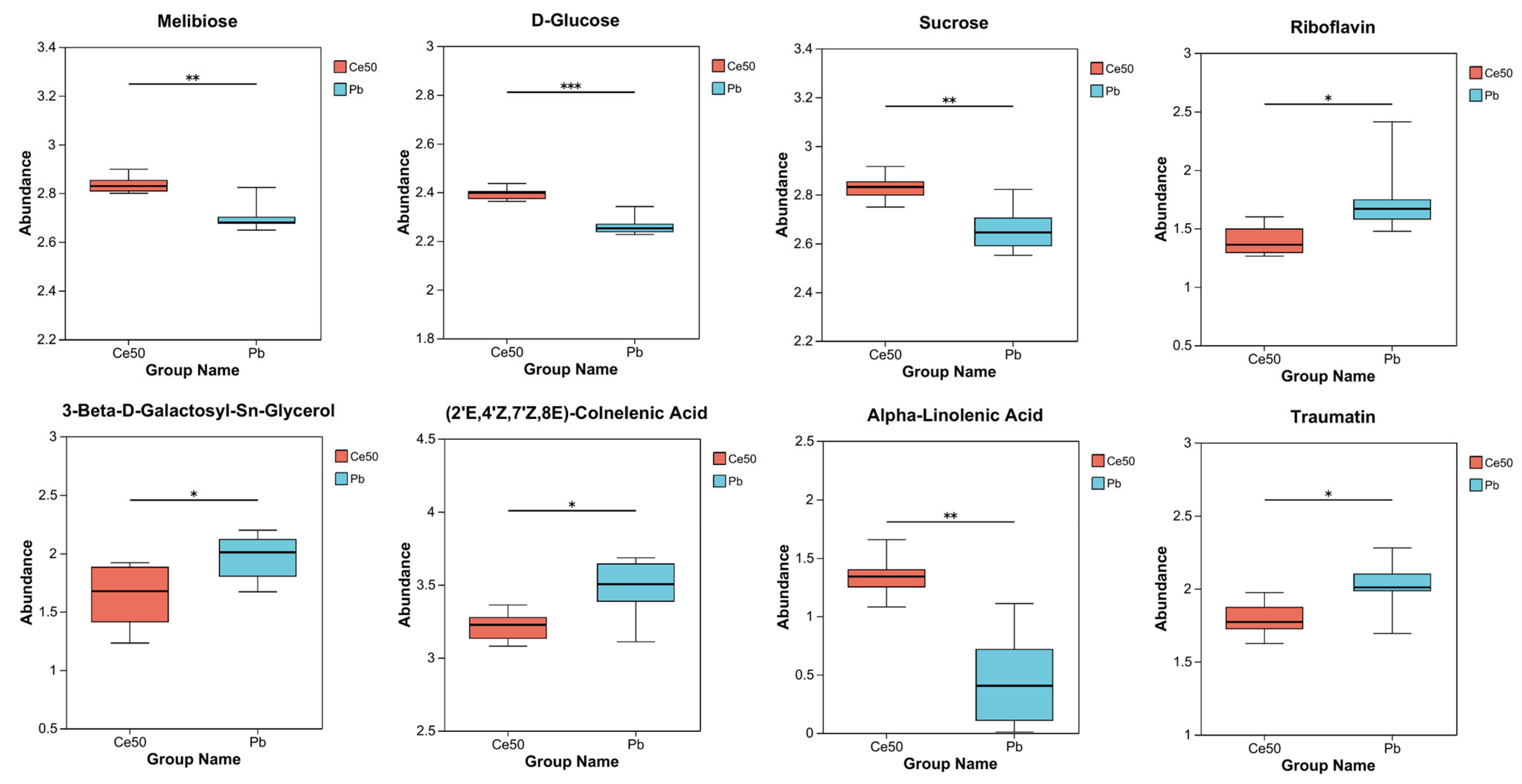
The differential metabolites in the Ce50/Pb comparison group were mainly enriched in the galactose metabolism, alpha-linolenic acid metabolism, and ABC transporters pathways. Galactose metabolism was the pathway with the smallest P-adjust in this study. The four differential metabolites enriched in the galactose metabolism pathway were melibiose, D-glucose, sucrose, and 3-beta-D-galactosyl-sn-glycerol; except for 3-beta-D-galactosyl-sn-glycerol, all the others were upregulated. The three differential metabolites enriched in the alpha-linolenic acid metabolism pathway were (2′E, 4′Z, 7′Z, 8E)-colnelenic acid, alpha-linolenic acid, and traumatin; alpha-linolenic acid was upregulated, and other metabolites were downregulated. In addition, four differential metabolites were enriched in the ABC transporters pathway, including upregulated melibiose, D-glucose, sucrose, and downregulated riboflavin (Figure 7D and Figure 9).
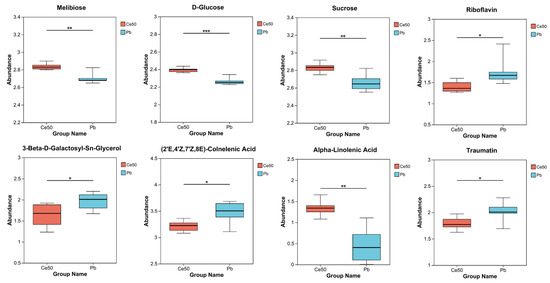
Figure 9.
Differential metabolite abundance in the Ce50/Pb comparison groups. * 0.01 < p ≤ 0.05; ** 0.001 < p ≤ 0.01; *** p ≤ 0.001.
3.6.3. VIP Analysis
In the Pb/CK comparison group, the top five differential metabolites in terms of VIP value were pisumoside a, avocadene 1-acetate, gln-arg-arg, 3,6,9,12,15,18,21,24,27,30-decaoxadotriacontane-1,32-diol, and betavulgarin glucoside; except for avocadene 1-acetate, all the others were upregulated (Figure 7E). In the Ce50/Pb comparison group, the top 5 were ixabepilone, zaragozic acid a, mupirocin, 15,16-dihydroxy-8(17),13-labdadien-18-oic acid, and cystone a; all of these metabolites were downregulated (Figure 7F).
3.6.4. KEGG Compound Classification
In the Pb/CK comparison group, a total of four differential metabolites were classified into flavonoids, namely apigenin 7-O-beta-D-rutinoside, tricin, swertiajaponin, and vicenin 2; among them, tricin was downregulated, and the others were upregulated (Figures S4A and S5). In the Ce50/Pb comparison group, three differential metabolites were classified into flavonoids, including silibinin, vicenin 2, and kaempferol; kaempferol was upregulated, while the others were downregulated Figures S4B and S5).
4. Discussion
4.1. Physiological Responses of Rice to CeO2 NPs Under Pb Stress
In this study, Pb stress significantly reduced the shoot fresh weight and leaf area of rice at the first sampling; plant height, shoot fresh weight, root fresh weight, and leaf area all decreased significantly under the influence of Pb stress at the second sampling, indicating that Pb stress had an inhibitory effect on rice growth. Foliar spraying of 50 mg·L−1 of CeO2 NPs significantly increased the leaf area at the first sampling and significantly increased the shoot fresh weight, root fresh weight, and leaf area at the second sampling, indicating that 50 mg·L−1 of CeO2 NPs effectively alleviated the adverse effects of Pb toxicity on rice growth.
CeO2 NPs have been reported to exhibit antioxidant behavior and suppress nitric oxide and hydroxyl radicals in biological systems [25]. To further explore the mitigation mechanism of CeO2 NPs, this study measured the activities of different antioxidant enzymes in leaves and found that Pb stress led to a decrease in POD, CAT, and APX activities. CAT, APX, and POD activities of Ce50 were slightly higher than those of Pb. These three enzymes are mainly responsible for removing ROS. At the same time, 50 mg·L−1 CeO2 NPs upregulated many antioxidant metabolites, such as alpha-linolenic acid, linoleic acid, and ferulic acid. Alpha-linolenic acid in lipid metabolism is a strong antioxidant and a precursor for synthesizing jasmonic acid, which acts as a signaling molecule to stimulate downstream anti-stress responses [29,30,31]. Linoleic acid has been reported to enhance AsA-GSH cycle efficiency, reduce lipid peroxidation, and decrease ROS accumulation [32]. The antioxidant activity of ferulic acid has been reported to help enhance the plant’s resistance to drought stress [33,34]. These results indicated that foliar spraying of 50 mg·L−1 CeO2 NPs improved the antioxidant capacity of rice, which helped alleviate the oxidative stress caused by Pb stress.
The detection of photosynthetic pigment contents showed that Pb stress increased photosynthetic pigment-related parameters at the first sampling but reversed at the second sampling. Under Pb stress, the contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids decreased significantly compared with CK at the second sampling. We speculated that this phenomenon was because rice activated the adaptive response mechanism in the initial stress stage, resulting in a short-term increase in photosynthetic pigments. As the stress time continued to increase, the toxic effect of Pb stress began to appear, and then the content of photosynthetic pigments decreased. At the second sampling, foliar spraying of 50 mg·L−1 CeO2 NPs alleviated the decline of chlorophyll a, chlorophyll b, total chlorophyll content, and the carotenoid content under Pb stress. The changes in the content of photosynthetic pigments indicated that 50 mg·L−1 CeO2 NPs helped the photosystem to efficiently capture light energy by increasing the pigment content, alleviated the photosynthetic inhibition of rice caused by Pb stress, and enhanced the adaptability to Pb stress.
4.2. Response of Rice Metabolism to CeO2 NPs Under Pb Stress
This study performed KEGG enrichment analysis on all differential metabolites detected in the Pb/CK comparison group and found that the P-adjust of the pyruvate metabolism pathway was the smallest, indicating that Pb stress may have seriously affected the pyruvate metabolism of rice. A total of three differential metabolites were enriched in the pyruvate metabolism pathway, namely 2-isopropylmalic acid, L-malic acid, and succinic acid. Malic acid increases chlorophyll content, reduces stress damage to photosynthetic structures, and significantly increases plant biomass [35,36]. Succinic acid can be an elicitor candidate and might be used to improve plant stress tolerance [37]. According to reports, succinic acid helps increase resistance to adverse environmental effects. For example, when using a mixture of succinic and lactic acids 45:55 (10 ppm) to treat the root of Asparagus officinal, the seedlings increased their root mass by 40% [38,39]. This study showed that both L-malic acid and succinic acid were downregulated under Pb stress, indicating that Pb stress had a negative impact on the synthesis and accumulation yield of these important metabolites in rice, weakening the stress tolerance. In addition to being enriched in the pyruvate metabolism pathway, they are also essential intermediates in the tricarboxylic acid (TCA) cycle. The TCA cycle was reported to be responsible for driving ATP synthesis and providing carbon skeletons to anabolic processes [40]. Therefore, the downregulation of these metabolites may interfere with the TCA cycle, affecting energy supply and metabolic balance. Interestingly, both metabolites were upregulated in the Ce50/Pb comparison group, indicating that foliar spraying of 50 mg·L−1 CeO2 NPs under Pb stress could reverse this inhibitory effect.
In the Ce50/Pb comparison group, the differential metabolites were mainly enriched in the galactose metabolism, alpha-linolenic acid metabolism, and ABC transporters pathways. Specifically, sucrose and D-glucose were the two key metabolites enriched in the galactose metabolism pathway in this study. Sucrose is the transportable form of carbon predominantly utilized at the sink to supply the energy required for plant biomass production [41,42] and also to stabilize cellular membranes under stress conditions [43]. Glucose reduces the negative effects of abiotic stress by increasing antioxidant and sugar levels [44]. The upregulation of sucrose and D-glucose in the Ce50/CK comparison group in this study indicated that CeO2 NP treatment increased carbon flow and energy supply in rice, which was beneficial to improving rice’s resistance to Pb.
In addition, the KEGG compound classification of the differential metabolites in the two comparison groups revealed that the differential metabolites were mainly classified into flavonoids. In plants, flavonoids play a role in protecting against biotic and abiotic stresses [45,46]. Flavonoids improve plants’ tolerance to abiotic stress by improving antioxidant capacity [47]. In this study, foliar spraying of 50 mg·L−1 CeO2 NPs upregulated kaempferol, which functions to protect plants from oxidative stress [48], verifying that CeO2 NPs have the effect of improving antioxidant capacity.
4.3. Response of Rice Gene Expression to CeO2 NPs Under Pb Stress
Plant defense priming is a physiological process by which a plant is prepared to respond more quickly or aggressively to future biotic or abiotic stress [49,50,51]. KEGG enrichment analysis found that the diterpenoid biosynthesis in the Pb/CK and Ce50/Pb comparison groups was the most significant pathway, reflecting the core role of diterpenoid metabolism in Pb stress response and that CeO2 NPs may mediate the adaptability to Pb stress by regulating the diterpenoid metabolism pathway.
GO enrichment analysis showed that 17 DEGs were classified into antioxidant activity in the Ce50/Pb comparison group. Among these DEGs, SOD1-Fe (Os06g0115400) was significantly upregulated. This is a superoxide dismutase gene involved in the antioxidant response of rice.
Furthermore, this study found that 58 DEGs were classified into defense response in the Ce50/Pb comparison group, which meant that foliar spraying of CeO2 NPs may initiate the defense mechanism of rice against Pb stress by regulating the expression of these genes. OsOPR1 (Os06g0216300) is a cadmium stress-responsive gene [52] upregulated in the Ce50/Pb comparison group. Wu et al. reported that overexpression of OsOPR1 improved the cadmium tolerance of yeast cells by affecting the expression of antioxidant enzyme-related genes and reducing the cadmium content in yeast cells [52]. Moreover, OsOPR1 encodes 12-oxo-phytodienoic acid (OPDA) reductase, an enzyme involved in jasmonic acid biosynthesis [53]. OsPR10a; RPR10a (Os12g0555500) was another gene focused on in this study, and it was upregulated after using CeO2 NPs under Pb stress. According to the previous report, the overexpression of OsPR10a in rice can significantly enhance resistance to infection by the pathogen Xoo and Xanthomona campestris pv. campestris (Xcc), respectively [54]. Jasmonic acid has a strong induction effect on RPR10a [55]. Therefore, the simultaneous upregulation of OsOPR1 and OsPR10a indicated that foliar spraying of CeO2 NPs might activate the jasmonic acid signaling pathway, thereby improving the ability of rice to cope with Pb stress.
This study also screened some genes related to rice stress resistance, which were upregulated after CeO2 NP treatment, such as OsmiR528, OsLG3; OsERF62, OsRCI2-5, and OsHKT1; 1. OsMIR528 was reported to be a positive regulator in salt stress [56]. oshkt1;1 mutant plants show hypersensitivity to salt stress [57]. The upregulation of salt response-related genes suggested that CeO2 NPs may have the potential to provide salt tolerance to rice, which is consistent with the results of Zhou et al. [58]. Meanwhile, OsRCI2-5 was reported to be a drought-resistant gene [59]. The overexpression of OsLG3 significantly improves drought tolerance in rice [60]. These revealed that CeO2 NPs may potentially mediate drought resistance in rice.
In addition, based on the statistical results of TF families, this study found that bHLH, WRKY, MYB, and NAC families contained most TF genes in the Ce50/Pb comparison group. These TF families play a role in plant adaptive responses to abiotic stresses [61,62,63,64]. Many DEGs were classified into these families, suggesting that CeO2 NPs may mediate plant stress resistance by regulating the expression of these TF genes. However, these four families contain a large number of DEGs, and these DEGs may be involved in a complex gene regulatory network. Further studies on these transcription factors’ interactions and downstream target genes will help reveal more details of the regulation of CeO2 NPs on rice under Pb stress.
5. Conclusions
This study preliminarily explored the positive regulatory role of CeO2 NPs in alleviating Pb stress in rice, especially in improving antioxidant capacity, which is beneficial to alleviate rice oxidative stress under Pb stress and is one of the characteristics of the regulatory mechanism of CeO2 NPs in this study. In addition, CeO2 NP treatment increased carbon flow and energy supply. KEGG enrichment analysis of DEGs revealed the core role of diterpenoid metabolism in CeO2 NPs regulating Pb stress in rice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14050552/s1. Figure S1. KEGG annotation and TF gene statistics. AC, in the Pb/CK comparison group; BD, in the Ce50/Pb comparison group. Figure S2. Diterpenoid biosynthesis pathway in the Pb/CK comparison group. In the pathway diagram, red represents upregulated genes; blue represents downregulated genes. The copyright of the KEGG map has been obtained. Figure S3. Cluster analysis of DEGs in antioxidant activity and defense response in the Ce50/Pb comparison group. A, defense response; B, antioxidant activity. Figure S4. Differential metabolite classification. A, in the Pb/CK comparison group; B, in the Ce50/Pb comparison group. Figure S5. Differential metabolite abundance in the Pb/CK and Ce50/Pb comparison groups. Table S1. Primer sequence. Table S2. Sequencing data statistics. Table S3. Alignment result statistics. Table S4. Expression levels of stress-related genes
Author Contributions
H.Z.: Software, writing—original draft, formal analysis, writing—review and editing, methodology. Z.C. and J.L.: Visualization. L.Z., X.S., Y.X., G.R., J.H., N.F., D.Z., Q.B. and T.S.: Resources. J.A. and R.Z.: Funding acquisition, project administration, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support in this study was from the Program for Scientific Research Start-Up Funds of Guangdong Ocean University (060302052316), the Project of Science and Technology of Zhanjiang City (2023B01028), and the Project of Guangzhou Science and Technology Plan (2023A04J1451).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to confidentiality.
Acknowledgments
Thanks to all the participants for their contributions to this study. The authors thank Kanehisa Laboratories for the copyright of KEGG map (Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. and Ishiguro-Watanabe, M.; KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672-D677 (2025). https://doi.org/10.1093/nar/gkae909).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Obaje, S.O.; Ogunyele, A.C.; Adeola, A.O.; Akingboye, A.S. Assessment of stream sediments pollution by potentially toxic elements in the active mining area of Okpella, Edo State, Nigeria. Rud.-Geološko-Naft. Zb. 2019, 34, 43–50. [Google Scholar] [CrossRef]
- Gupta, S.; Jena, V.; Matić, N.; Kapralova, V.; Solanki, J. Assessment of geo-accumulation indeks of heavy metal and source of contamination by multivariate factor analysis. Int. J. Hazard. Mater. 2014, 2, 18–22. [Google Scholar]
- Ale, T.O.; Ogunribido, T.H.; Ademila, O.; Akingboye, A.S. Soil pollution status due to potentially toxic elements in active open dumpsites: Insights from different Nigerian geological environments. Environ. Earth Sci. 2024, 83, 535. [Google Scholar] [CrossRef]
- Yang, H.; Wang, F.; Yu, J.; Huang, K.; Zhang, H.; Fu, Z. An improved weighted index for the assessment of heavy metal pollution in soils in Zhejiang, China. Environ. Res. 2021, 192, 110246. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y.; Lin, S.; Liu, Y.; Xie, Y. Soil Pollution Management in China: A Brief Introduction. Sustainability 2019, 11, 556. [Google Scholar] [CrossRef]
- Yang, H.; Huang, X.; Thompson, J.R.; Flower, R.J. Soil pollution: Urban brownfields. Science 2014, 344, 691–692. [Google Scholar] [CrossRef]
- Chen, R.; De Sherbinin, A.; Ye, C.; Shi, G. China’s soil pollution: Farms on the frontline. Science 2014, 344, 691. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Peng, J.-Y.; Zhang, S.; Han, Y.; Bate, B.; Ke, H.; Chen, Y. Soil heavy metal pollution of industrial legacies in China and health risk assessment. Sci. Total Environ. 2022, 816, 151632. [Google Scholar] [CrossRef]
- Kumar, S.; Rahman, M.A.; Islam, M.R.; Hashem, M.A.; Rahman, M.M. Lead and other elements-based pollution in soil, crops and water near a lead-acid battery recycling factory in Bangladesh. Chemosphere 2022, 290, 133288. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Tripathi, R.D.; Sinha, S.; Maheshwari, R.; Srivastava, H. Response of higher plants to lead contaminated environment. Chemosphere 1997, 34, 2467–2493. [Google Scholar] [CrossRef]
- Qing, Z. Effect of La on Glycine max seedling under Pb stress. Chin. J. Appl. Environ. Biol. 1999, 5, 22–25. [Google Scholar]
- Pang, X.; Wang, D.; Xing, X.; Peng, A.; Zhang, F.; Li, C. Effect of La3+ on the activities of antioxidant enzymes in wheat seedlings under lead stress in solution culture. Chemosphere 2002, 47, 1033–1039. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Ashraf, M.; Tabassam, Q.; Hussain, M.; Firdous, H. Lead (Pb)-induced regulation of growth, photosynthesis, and mineral nutrition in maize (Zea mays L.) plants at early growth stages. Biol. Trace Elem. Res. 2011, 144, 1229–1239. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Z.; Ma, J.; Yang, L.; Wei, Y. The effects of lead stress on photosynthetic function and chloroplast ultrastructure of Robinia pseudoacacia seedlings. Environ. Sci. Pollut. Res. 2017, 24, 10718–10726. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The Combined Effects of Arbuscular Mycorrhizal Fungi (AMF) and Lead (Pb) Stress on Pb Accumulation, Plant Growth Parameters, Photosynthesis, and Antioxidant Enzymes in Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef]
- Rahman, S.U.; Qin, A.; Zain, M.; Mushtaq, Z.; Mehmood, F.; Riaz, L.; Naveed, S.; Ansari, M.J.; Saeed, M.; Ahmad, I. Pb uptake, accumulation, and translocation in plants: Plant physiological, biochemical, and molecular response: A review. Heliyon 2024, 10, e27724. [Google Scholar] [CrossRef]
- Hu, J.; Shi, G.; Xu, Q.; Wang, X.; Yuan, Q.; Du, K. Effects of Pb2+ on the active oxygen-scavenging enzyme activities and ultrastructure in Potamogeton crispus leaves. Russ. J. Plant Physiol. 2007, 54, 414–419. [Google Scholar] [CrossRef]
- Maleki, M.; Ghorbanpour, M.; Kariman, K. Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene 2017, 11, 247–254. [Google Scholar] [CrossRef]
- Sarraf, M.; Vishwakarma, K.; Kumar, V.; Arif, N.; Das, S.; Johnson, R.; Janeeshma, E.; Puthur, J.T.; Aliniaeifard, S.; Chauhan, D.K. Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: An overview of the mechanisms. Plants 2022, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Preetha, J.S.Y.; Sriram, D.; Premasudha, P.; Pudake, R.N.; Arun, M. Cerium oxide as a nanozyme for plant abiotic stress tolerance: An overview of the mechanisms. Plant Nano Biol. 2023, 6, 100049. [Google Scholar] [CrossRef]
- Fallah Imani, A.; Gomarian, M.; Ghorbanpour, M.; Ramak, P.; Chavoshi, S. Foliar-applied nano-cerium dioxide differentially affect morpho-physiological traits and essential oil profile of Salvia mirzayanii Rech. f. & Esfand under drought stress and post-stress recovery conditions. Plant Physiol. Biochem. 2023, 203, 108046. [Google Scholar] [CrossRef]
- Ayub, M.A.; Ahmad, H.R.; Zia ur Rehman, M.; Waraich, E.A. Cerium oxide nanoparticles alleviates stress in wheat grown on Cd contaminated alkaline soil. Chemosphere 2023, 338, 139561. [Google Scholar] [CrossRef]
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.C.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging investigator series: Molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, D.; Feng, N.; Qiu, Q.-S.; Zhou, H.; Liu, M.; Li, Y.; Meng, F.; Huang, X.; Huang, A. Prohexadione calcium enhances rice growth and tillering under NaCl stress. PeerJ 2023, 11, e14804. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The oxylipin pathways: Biochemistry and function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef]
- Zi, X.; Zhou, S.; Wu, B. Alpha-linolenic acid mediates diverse drought responses in maize (Zea mays L.) at seedling and flowering stages. Molecules 2022, 27, 771. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Teng, Z.; Shu, Y.; Wang, Y.; Wang, D.; Sun, C.; Lin, X. Linoleic acid alleviates aluminum toxicity by modulating fatty acid composition and redox homeostasis in wheat (Triticum aestivum) seedlings. J. Hazard. Mater. 2025, 487, 137156. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.T.; Xuan, T.D. Foliar application of vanillic and p-hydroxybenzoic acids enhanced drought tolerance and formation of phytoalexin momilactones in rice. Arch. Agron. Soil Sci. 2018, 64, 1831–1846. [Google Scholar] [CrossRef]
- Khan, K.A.; Saleem, M.H.; Afzal, S.; Hussain, I.; Ameen, F.; Fahad, S. Ferulic acid: Therapeutic potential due to its antioxidant properties, role in plant growth, and stress tolerance. Plant Growth Regul. 2024, 104, 1329–1353. [Google Scholar] [CrossRef]
- Chen, H.-C.; Zhang, S.-L.; Wu, K.-J.; Li, R.; He, X.-R.; He, D.-N.; Huang, C.; Wei, H. The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. Under Cd stress. Ecotoxicol. Environ. Saf. 2020, 187, 109790. [Google Scholar] [CrossRef]
- Si, P.; Shao, W.; Yu, H.; Xu, G.; Du, G. Differences in microbial communities stimulated by malic acid have the potential to improve nutrient absorption and fruit quality of grapes. Front. Microbiol. 2022, 13, 850807. [Google Scholar] [CrossRef]
- KiliÇ, T. Seed treatments with salicylic and succinic acid to mitigate drought stress in flowering kale cv. ‘Red Pigeon F1’. Sci. Hortic. 2023, 313, 111939. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hirai, N.; Wakabayashi, K.; Sugizaki, H.; Iwamura, H. Succinic and lactic acids as plant growth promoting compounds produced by rhizospheric Pseudomonas putida. Can. J. Microbiol. 1993, 39, 1150–1154. [Google Scholar] [CrossRef]
- Elzayat, H.E.; Taha, N.M.; Shakweer, N.H. Spraying biostimulants on Le Conte pear trees reduces fruit drop and enhances yield, improves fruit quality, and storability. Egypt. J. Agric. Res. 2024, 102, 251–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. The role of TCA cycle enzymes in plants. Adv. Biol. 2023, 7, 2200238. [Google Scholar] [CrossRef]
- Lemoine, R. Sucrose transporters in plants: Update on function and structure. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2000, 1465, 246–262. [Google Scholar] [CrossRef]
- Aluko, O.O.; Li, C.; Wang, Q.; Liu, H. Sucrose utilization for improved crop yields: A review article. Int. J. Mol. Sci. 2021, 22, 4704. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Beena, R. Sucrose Metabolism in Plants under Drought Stress Condition: A Review. Indian J. Agric. Res. 2024, 58, 943. [Google Scholar] [CrossRef]
- Siddiqui, H.; Sami, F.; Hayat, S. Glucose: Sweet or bitter effects in plants-a review on current and future perspective. Carbohydr. Res. 2020, 487, 107884. [Google Scholar] [CrossRef]
- De Luna, S.R.; Ramirez-Garza, R.; Saldivar, S.S. Review Article–Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Ramzan, M.; Haider, S.T.A.; Hussain, M.B.; Ehsan, A.; Datta, R.; Alahmadi, T.A.; Ansari, M.J.; Alharbi, S.A. Potential of kaempferol and caffeic acid to mitigate salinity stress and improving potato growth. Sci. Rep. 2024, 14, 21657. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.; Poinssot, B.; Pozo, M.J. Priming: Getting ready for battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef]
- Krokene, P. Conifer defense and resistance to bark beetles. In Bark Beetles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 177–207. [Google Scholar]
- Wu, L.; Wang, R.; Li, M.; Du, Z.; Jin, Y.; Shi, Y.; Jiang, W.; Chen, J.; Jiao, Y.; Hu, B. Functional analysis of a rice 12-oxo-phytodienoic acid reductase gene (OsOPR1) involved in Cd stress tolerance. Mol. Biol. Rep. 2024, 51, 198. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Jwa, N.-S.; Shibato, J.; Han, O.; Iwahashi, H.; Rakwal, R. Diverse environmental cues transiently regulate OsOPR1 of the “octadecanoid pathway” revealing its importance in rice defense/stress and development. Biochem. Biophys. Res. Commun. 2003, 310, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-F.; Lin, K.-H.; He, S.-L.; Chen, J.-L.; Jiang, J.-Z.; Chen, B.-H.; Hou, Y.-S.; Chen, R.-S.; Hong, C.-Y.; Ho, S.-L. Multiple patterns of regulation and overexpression of a ribonuclease-like pathogenesis-related protein gene, OsPR10a, conferring disease resistance in rice and Arabidopsis. PLoS ONE 2016, 11, e0156414. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.D.; Hamer, J.E.; Hodges, T.K. Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea. Mol. Plant-Microbe Interact. 2001, 14, 877–886. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, K.; Cheng, Y.; Zhong, Z.; Tian, L.; Tang, X.; Tang, A.; Zheng, X.; Zhang, T.; Qi, Y. CRISPR-Cas9 based genome editing reveals new insights into microRNA function and regulation in rice. Front. Plant Sci. 2017, 8, 1598. [Google Scholar] [CrossRef]
- Wang, R.; Jing, W.; Xiao, L.; Jin, Y.; Shen, L.; Zhang, W. The rice high-affinity potassium transporter1; 1 is involved in salt tolerance and regulated by an MYB-type transcription factor. Plant Physiol. 2015, 168, 1076–1090. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, H.; Zhang, F.; Su, Y.; Guan, W.; Xie, Y.; Giraldo, J.P.; Shen, W. Molecular basis of cerium oxide nanoparticle enhancement of rice salt tolerance and yield. Environ. Sci. Nano 2021, 8, 3294–3311. [Google Scholar] [CrossRef]
- Li, L.; Li, N.; Song, S.; Li, Y.; Xia, X.; Fu, X.; Chen, G.; Deng, H. Cloning and characterization of the drought-resistance OsRCI2-5 gene in rice (Oryza sativa L.). Genet. Mol. Res. 2014, 13, 4022–4035. [Google Scholar] [CrossRef]
- Xiong, H.; Yu, J.; Miao, J.; Li, J.; Zhang, H.; Wang, X.; Liu, P.; Zhao, Y.; Jiang, C.; Yin, Z. Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol. 2018, 178, 451–467. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, T.; Yu, Y.; Gou, L.; Yang, J.; Xu, J.; Pi, E. Regulatory mechanisms of bHLH transcription factors in plant adaptive responses to various abiotic stresses. Front. Plant Sci. 2021, 12, 677611. [Google Scholar] [CrossRef]
- Wagan, S.; Ali, M.; Khoso, M.A.; Alam, I.; Dinislam, K.; Hussain, A.; Brohi, N.A.; Manghwar, H.; Liu, F. Deciphering the role of WRKY transcription factors in plant resilience to alkaline salt stress. Plant Stress 2024, 13, 100526. [Google Scholar] [CrossRef]
- Mariyam, S.; Kumar, V.; Roychoudhury, A.; Ghodake, G.S.; Muneer, S.; Duhan, J.S.; Ahmad, F.; Sharma, R.K.; Singh, J.; Seth, C.S. Functional Diversification and Mechanistic Insights of MYB Transcription Factors in Mediating Plant Growth and Development, Secondary Metabolism, and Stress Responses. J. Plant Growth Regul. 2025, 44, 1465–1484. [Google Scholar] [CrossRef]
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC transcription factors in plant abiotic stress responses. Biochim. Et Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 97–103. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).