Photoreceptors Are Involved in Antioxidant Effects of Melatonin Under High Light in Arabidopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Treatments
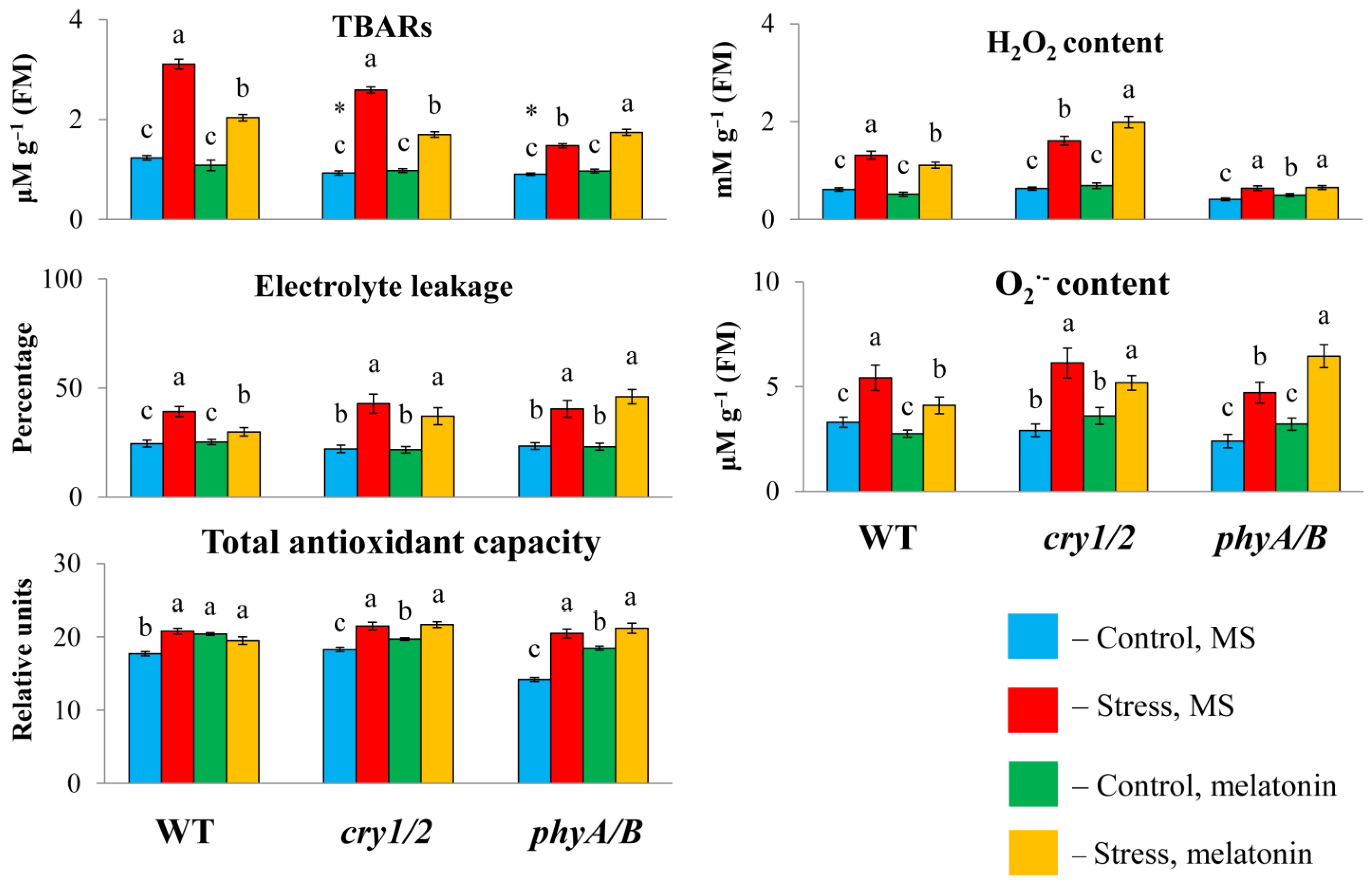
2.2. Determination of Chlorophyll and TBARs Contents and Electrolyte Leakage
2.3. Quantification of Antioxidant Capacity, H2O2 and O2−
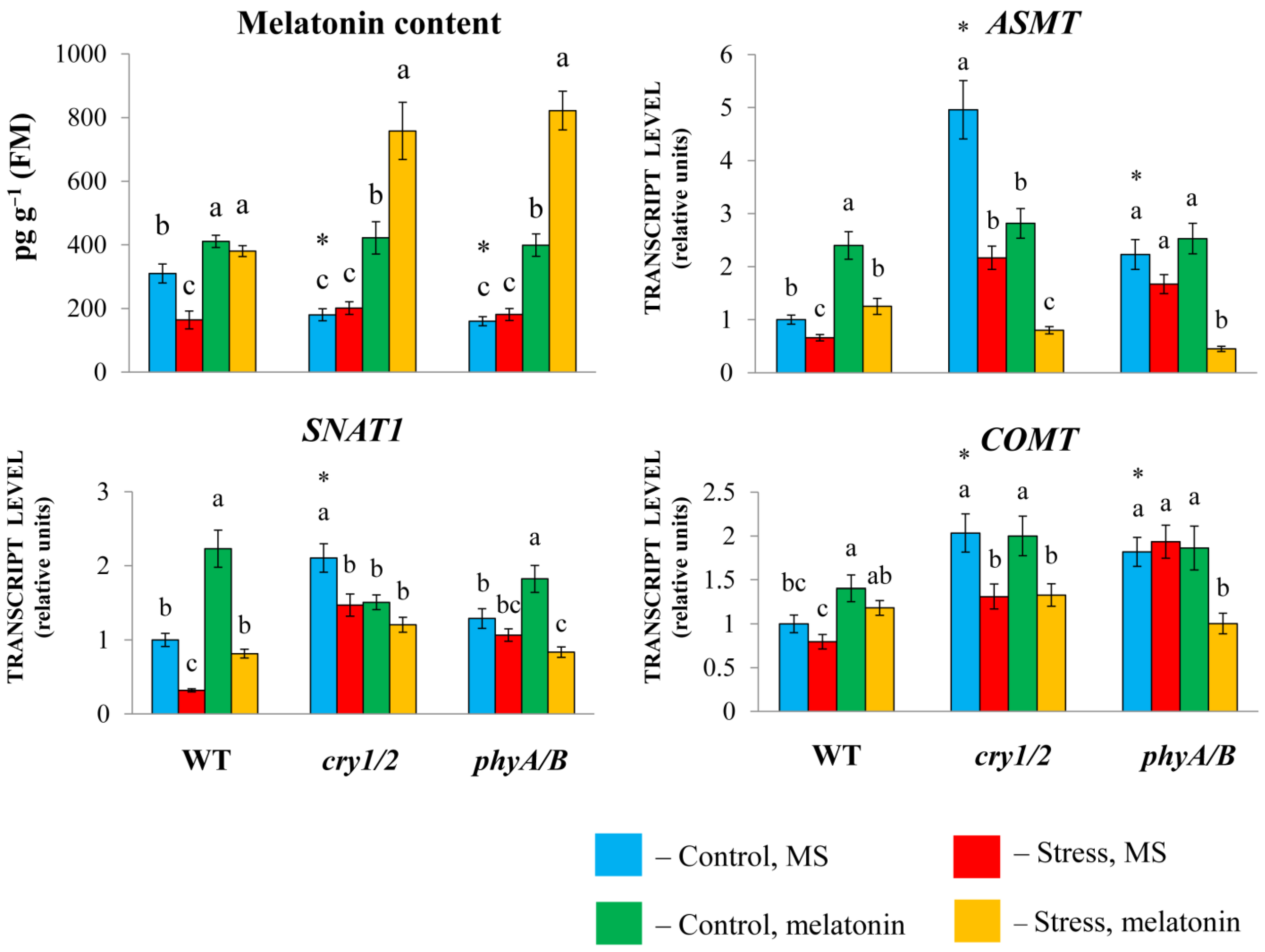
2.4. Determination of Melatonin Content
2.5. Fluorescence Measurements
2.6. qRT–PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Increased Melatonin Levels in Photoreceptor Mutants Alter Their Response to Melatonin Treatment Under HL Stress
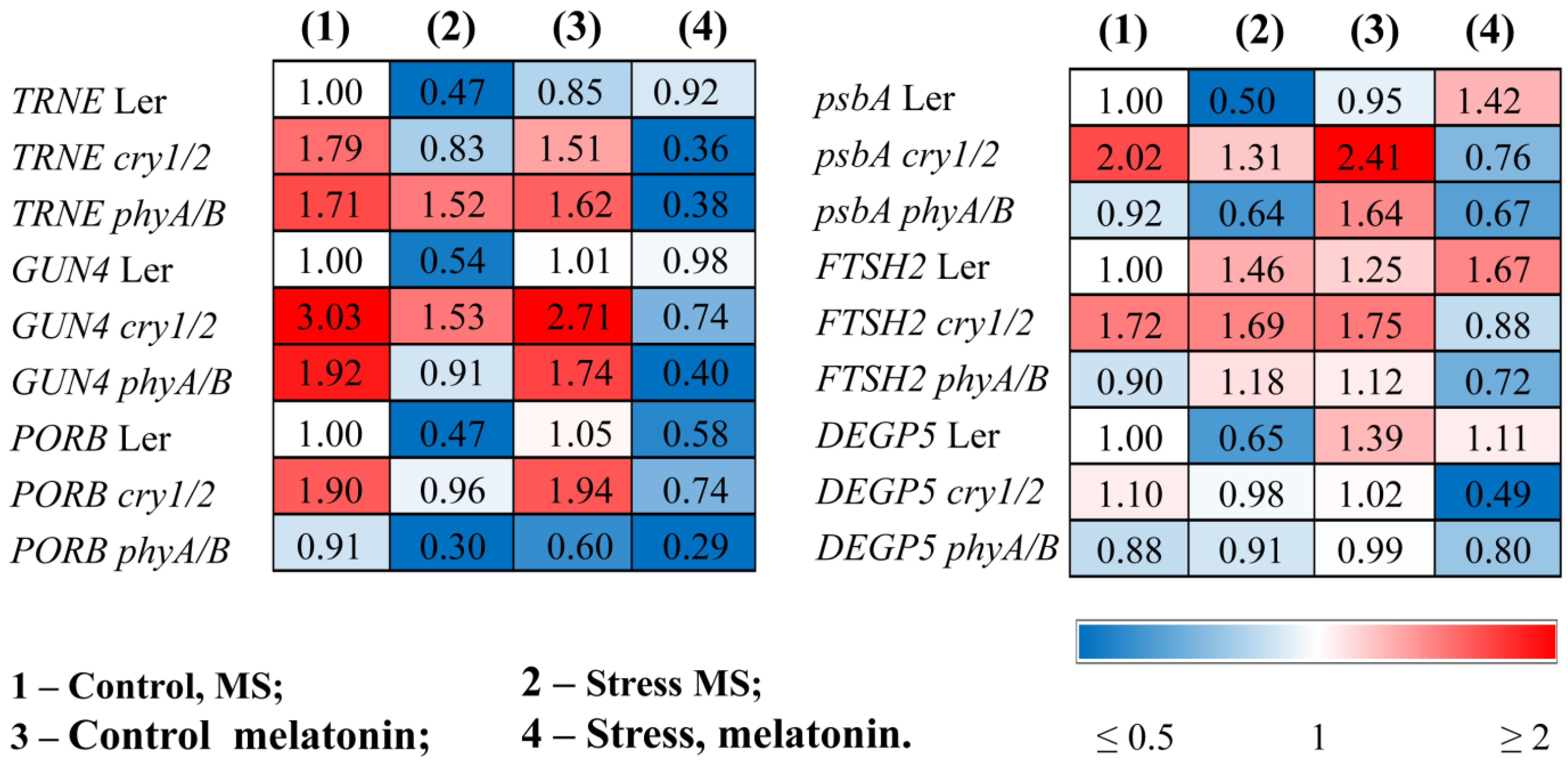
3.2. Increased Susceptibility to HL Stress in the Presence of MT in Photoreceptor Mutants Is Accompanied by Perturbed Physiological Indicators and Molecular Markers
3.3. Exogenous MT Reduced the Photosynthetic Activity and Expression of Photosynthesis-Related Genes in cry1/2 and phyA/B Under HL Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossel, J.B.; Wilson, I.W.; Pogson, B.J. Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol. 2002, 130, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ming, Y.; Wang, H.B.; Jin, H.L. Strategies for adaptation to high light in plants. aBIOTECH 2024, 5, 381–393. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Moyá, M.L.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Comparative evaluation of the antioxidant activity of melatonin and related indoles. J. Food Compost. Anal. 2012, 12, 16–22. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, P.; Wang, R.; Sun, L.; Ju, Q.; Xu, J. Comparative physiological responses and transcriptome analysis reveal the 541 roles of melatonin and serotonin in regulating growth and metabolism in Arabidopsis. BMC Plant Biol. 2018, 18, 362. [Google Scholar] [CrossRef]
- Hernández, I.G.; Gomez, F.J.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana acts as plant growth 544 regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiol. Biochem. 2015, 94, 191–196. [Google Scholar] [CrossRef]
- Kusnetsov, V.V.; Bychkov, I.A.; Kudryakova, N.V. Phytomelatonin As an Element of the Plant Hormonal System. Russ. J. Plant Physiol. 2024, 71, 134. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in plants—Diversity of levels and multiplicity of functions. Front Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. Melatonin induction and its role in high light stress tolerance in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12504. [Google Scholar] [CrossRef]
- Yang, S.J.; Huang, B.; Zhao, Y.Q.; Hu, D.; Chen, T.; Ding, C.B.; Chen, Y.E.; Yuan, S.; Yuan, M. Melatonin Enhanced the Tolerance of Arabidopsis thaliana to High Light Through Improving Anti-oxidative System and Photosynthesis. Front. Plant Sci. 2021, 12, 752584. [Google Scholar] [CrossRef]
- Bychkov, I.A.; Kudryakova, N.V.; Pojidaeva, E.S.; Kusnetsov, V.V. The melatonin receptor CAND2 is involved in the regulation of photosynthesis and chloroplast gene expression in Arabidopsis thaliana under photooxidative stress. Photosynthetica 2021, 9, 683–692. [Google Scholar] [CrossRef]
- Georgieva, M.; Vassileva, V. Stress Management in Plants: Examining Provisional and Unique Dose-Dependent Responses. Int. J. Mol. Sci. 2023, 24, 5105. [Google Scholar] [CrossRef] [PubMed]
- Kleine, T.; Kindgren, P.; Benedict, C.; Hendrickson, L.; Strand, A. Genome-wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol. 2007, 144, 1391–1406. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]
- Shaikhali, J.; de Dios Barajas-Lopéz, J.; Ötvös, K.; Kremnev, D.; Garcia, A.S.; Srivastava, V.; Wingsle, G.; Bako, L.; Strand, Å. The cryptochrome1-dependent response to excess light is mediated through the transcriptional activators zinc finger protein expressed in inflorescence meristem LIKE1 and ZML2 in Arabidopsis. Plant Cell 2012, 24, 3009–3025. [Google Scholar] [CrossRef] [PubMed]
- Leonardelli, M.; Tissot, N.; Podolec, R.; Ares-Orpel, F.; Glauser, G.; Ulm, R.; Demarsy, E. Photoreceptor-induced sinapate synthesis contributes to photoprotection in Arabidopsis. Plant Physiol. 2024, 196, 1518–1533. [Google Scholar] [CrossRef] [PubMed]
- Fichman, Y.; Xiong, H.; Sengupta, S.; Morrow, J.; Loog, H.; Azad, R.K.; Hibberd, J.M.; Liscum, E.; Mittler, R. Phytochrome B regulates reactive oxygen signaling during abiotic and biotic stress in plants. New Phytol. 2023, 237, 1711–1727. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Heath, L.R.; Packer, L. Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Campos, P.S.; Quartin, V.; Ramalho, J.C.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. Plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kumar, C.N.; Knowles, N.R. Changes in lipid peroxidation and lipolytic and freeradical scavenging enzyme during aging and sprouting of potato (Solanum tuberosum L.) seed-tubers. Plant Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Glutathione-induced drought stress tolerance in mung bean: Coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants. 2015, 7, plv069. [Google Scholar] [CrossRef] [PubMed]
- Kozuleva, M.A.; Lysenko, E.A.; Klaus, A.A.; Kuznetsov, V.V. Long-term hyperthermia impairs activity of both photosystems. Dokl. Biochem. Biophys. 2017, 472, 71–73. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Akmakjiana, G.Z.; Riaza, N.; Guerinota, M.L. Photoprotection during iron deficiency is mediated by the bHLH transcription factors PYE and ILR3. Proc. Natl. Acad. Sci. USA 2021, 118, e202491811. [Google Scholar] [CrossRef]
- Tzvetkova-Chevolleau, T.; Franck, F.; Alawady, A.E.; Dall’Osto, L.; Carrière, F.; Bassi, R.; Grimm, B.; Nussaume, L.; Havaux, M. The light stress-induced protein ELIP2 is a regulator of chlorophyll synthesis in Arabidopsis thaliana. Plant J. 2007, 50, 795–809. [Google Scholar] [CrossRef]
- Ruban, A.V. Quantifying the efficiency of photoprotection. Phil. Trans. R. Soc. B 2017, 372, 20160393. [Google Scholar] [CrossRef]
- Kato, Y.; Sakamoto, W. Protein quality control in chloroplasts: A current model of D1 protein degradation in the photosystem II repair cycle. J. Biochem. 2009, 146, 463–469. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Wang, F.; Liu, A.; Chen, S.; Li, X. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant Physiol. Biochem. 2024, 207, 108398. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Xiao, Q.; Chen, Z.; Han, Y. Crosstalk between Melatonin and Reactive Oxygen Species in Plant Abiotic Stress Responses: An Update. Int. J. Mol. Sci. 2022, 23, 5666. [Google Scholar] [CrossRef] [PubMed]
- Ponnu, J.; Hoecker, U. Signaling Mechanisms by Arabidopsis Cryptochromes. Front. Plant Sci. 2022, 13, 844714. [Google Scholar] [CrossRef]
- Khudyakova, A.Y.; Kosobryukhov, A.A.; Pashkovskiy, P.P.; Kreslavski, V.D. Cryptochromes and Their Role in the Process of Plant Adaptation. Russ J. Plant Physiol. 2024, 71, 42. [Google Scholar] [CrossRef]
- Hwang, O.J.; Back, K. Suppression of Rice Cryptochrome 1b Decreases Both Melatonin and Expression of Brassinosteroid Biosynthetic Genes Resulting in Salt Tolerance. Molecules 2021, 26, 1075. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Xu, P.; Lian, H.; Wang, W.; Xu, F.; Mao, Z.; Zhang, T.; Yang, H. CRY1 interacts directly with HBI1 to regulate its transcriptional activity and photomorphogenesis in Arabidopsis. J. Exp. Bot. 2018, 69, 3867–3881. [Google Scholar] [CrossRef]
- D’Amico-Damião, V.; Carvalho, R.F. Cryptochrome-Related Abiotic Stress Responses in Plants. Front. Plant Sci. 2018, 9, 1897. [Google Scholar] [CrossRef]
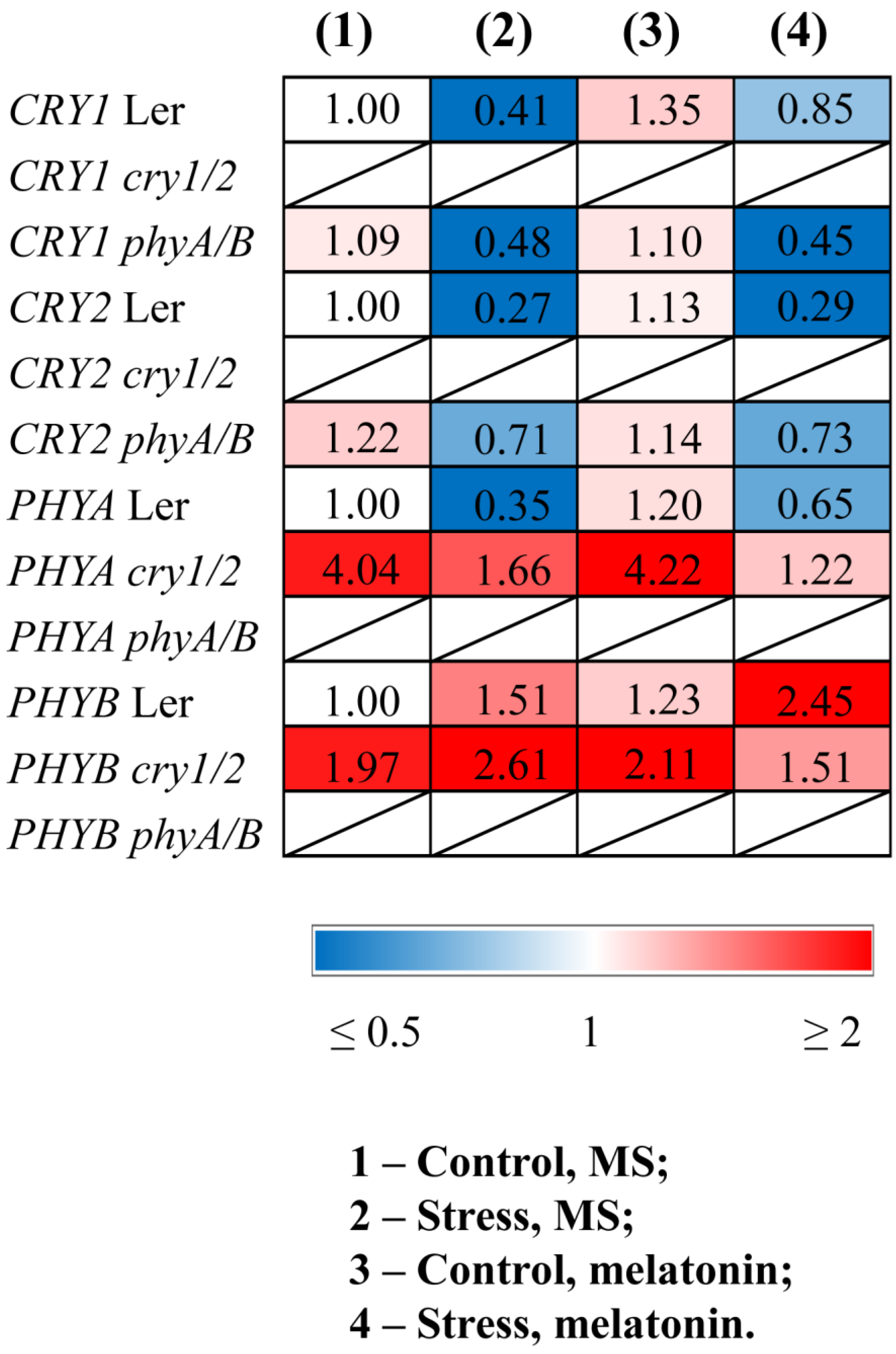
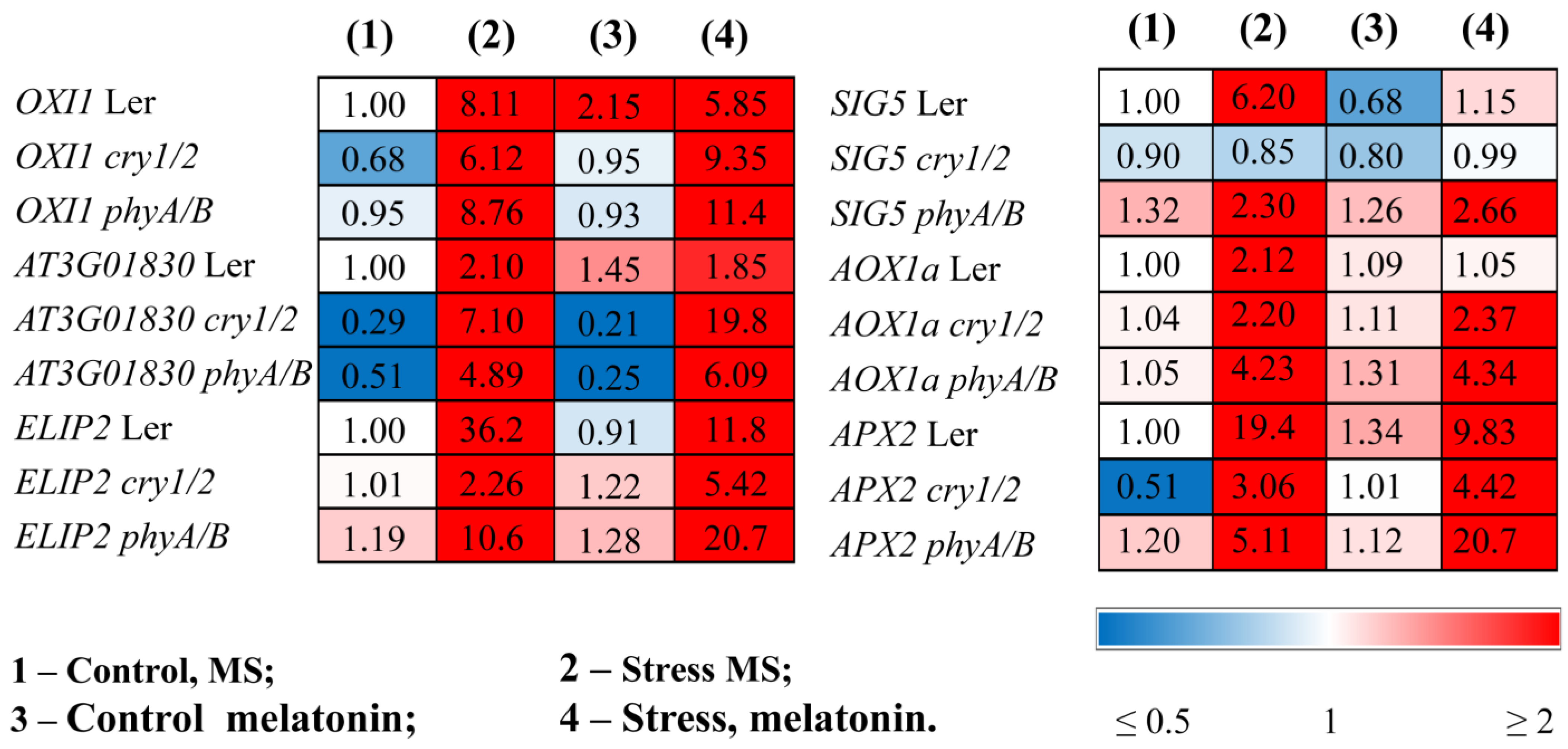
| Genotype | Control/MS | Stress/MS | Control/Melatonin | Stress/Melatonin |
|---|---|---|---|---|
| Fv/Fm | ||||
| Ler | 0.821 ± 0.011 a | 0.550 ± 0.027 c | 0.826 ± 0.014 a | 0.661 ± 0.019 b |
| cry1/2 | 0.819 ± 0.009 a | 0.541 ± 0.030 c | 0.806 ± 0.016 a | 0.645 ± 0.025 b |
| phyA/B | 0.814 ± 0.013 a | 0.599 ± 0.011 b | 0.808 ± 0.021 a | 0.593 ± 0.029 b |
| ΦPSII | ||||
| Ler | 0.732 ± 0.019 a | 0.488 ± 0.016 c | 0.709 ± 0.010 a | 0.579 ± 0.010 b |
| cry1/2 | 0.704 ± 0.015 a | 0.473 ± 0.018 c | 0.692 ± 0.017 a | 0.572 ± 0.022 b |
| phyA/B | 0.678 ± 0.019 a | 0.435 ± 0.024 b | 0.671 ± 0.018 a | 0.456 ± 0.029 b |
| NPQ | ||||
| Ler | 0.187 ± 0.006 c | 0.337 ± 0.015 a | 0.186 ± 0.011 c | 0.228 ± 0.018 b |
| cry1/2 | 0.480 ± 0.021 a* | 0.395 ± 0.032 a | 0.391 ± 0.027 a | 0.360 ± 0.022 a |
| phyA/B | 0.326 ± 0.016 c | 0.484 ± 0.019 b | 0.313 ± 0.013 c | 0.691 ± 0.020 a |
| Chlorophyll content (a + b), mg g−1 (FM) | ||||
| Ler | 1.107 ± 0.051 a | 0.369 ± 0.019 b | 1.052 ± 0.039 a | 0.733 ± 0.026 c |
| cry1/2 | 0.931 ± 0.034 a* | 0.632 ± 0.037 c | 0.821 ± 0.029 b | 0.643 ± 0.046 c |
| phyA/B | 0.816 ± 0.043 a* | 0.452 ± 0.027 b | 0.746 ± 0.039 a | 0.437 ± 0.022 b |
| Carotenoid content, mg g−1 (FM) | ||||
| Ler | 0.186 ± 0.009 a | 0.149 ± 0.007 b | 0.178 ± 0.011 a | 0.180 ± 0.013 a |
| cry1/2 | 0.189 ± 0.014 a | 0.151 ± 0.009 bc | 0.169 ± 0.011 ab | 0.132 ± 0.009 c |
| phyA/B | 0.158 ± 0.012 a | 0.108 ± 0.009 b | 0.130 ± 0.010 a | 0.109 ± 0.011 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bychkov, I.; Doroshenko, A.; Kudryakova, N.; Kusnetsov, V. Photoreceptors Are Involved in Antioxidant Effects of Melatonin Under High Light in Arabidopsis. Antioxidants 2025, 14, 458. https://doi.org/10.3390/antiox14040458
Bychkov I, Doroshenko A, Kudryakova N, Kusnetsov V. Photoreceptors Are Involved in Antioxidant Effects of Melatonin Under High Light in Arabidopsis. Antioxidants. 2025; 14(4):458. https://doi.org/10.3390/antiox14040458
Chicago/Turabian StyleBychkov, Ivan, Anastasia Doroshenko, Natalia Kudryakova, and Victor Kusnetsov. 2025. "Photoreceptors Are Involved in Antioxidant Effects of Melatonin Under High Light in Arabidopsis" Antioxidants 14, no. 4: 458. https://doi.org/10.3390/antiox14040458
APA StyleBychkov, I., Doroshenko, A., Kudryakova, N., & Kusnetsov, V. (2025). Photoreceptors Are Involved in Antioxidant Effects of Melatonin Under High Light in Arabidopsis. Antioxidants, 14(4), 458. https://doi.org/10.3390/antiox14040458






