Bta-Let-7d Modulation of Oxidative Stress Induced by Potassium Permanganate in Bovine Endometrial Cells via IGF1R/PI3K/AKT Signaling Pathway
Abstract
1. Introduction
2. Material and Methods
2.1. Collection of Bovine Uterine Samples and SD Rat Model
2.2. Histological Analysis
2.3. Bovine Endometrial Epithelial Cell Culture
2.4. BEEC Transfection
2.5. RNA Extraction and RT-qPCR Analysis
2.6. Western Blot Analysis
2.7. miRNA Target Prediction
2.8. Dual-Luciferase Reporter Assay
2.9. Detection of MDA, ROS, T-AOC, GPx, and SOD
2.10. Immunofluorescence Staining
2.11. Statistical Analysis
3. Result
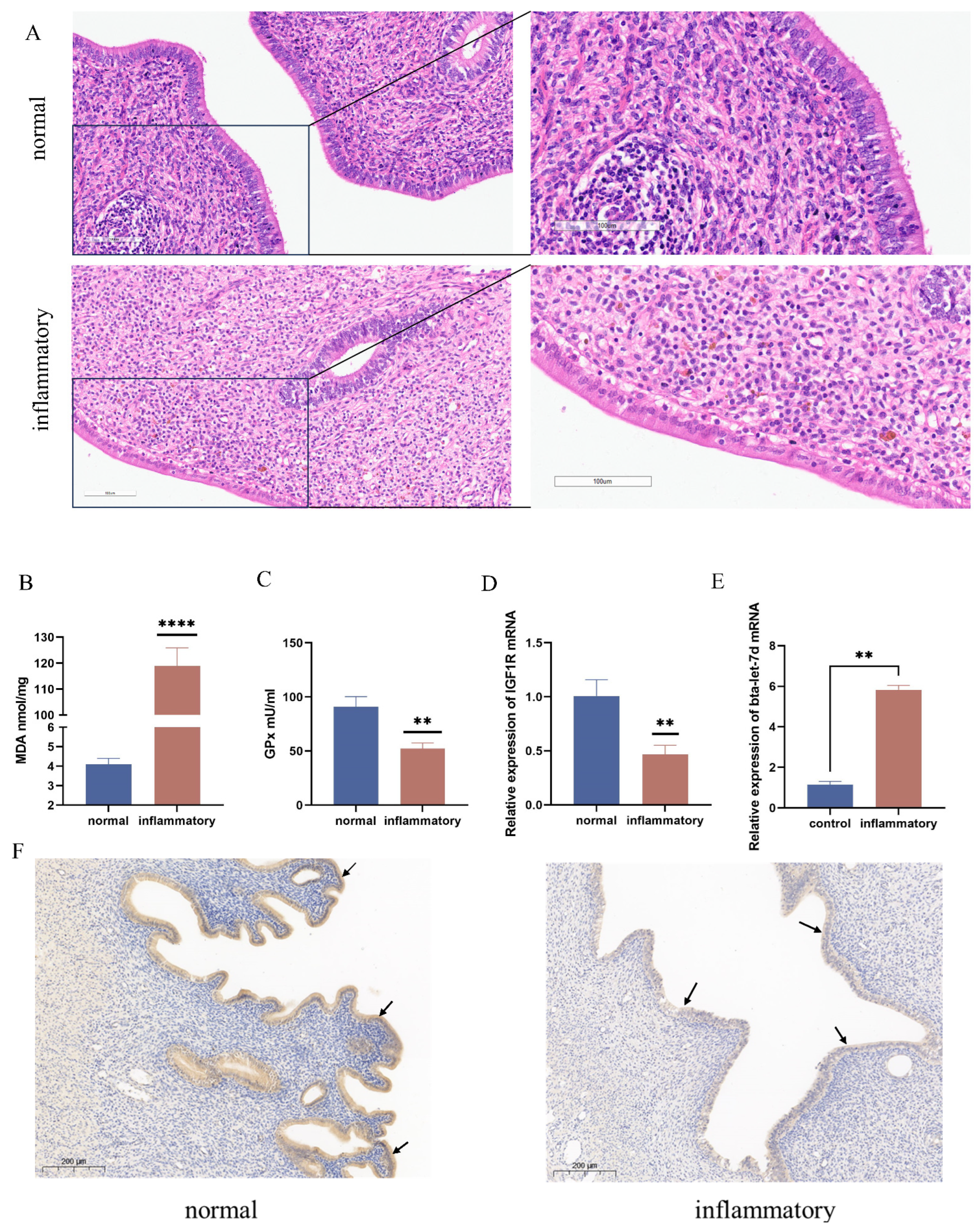
3.1. Differential Expression of IGF1R and Bta-Let-7d in Normal and Oxidative-Stress-Affected Tissues
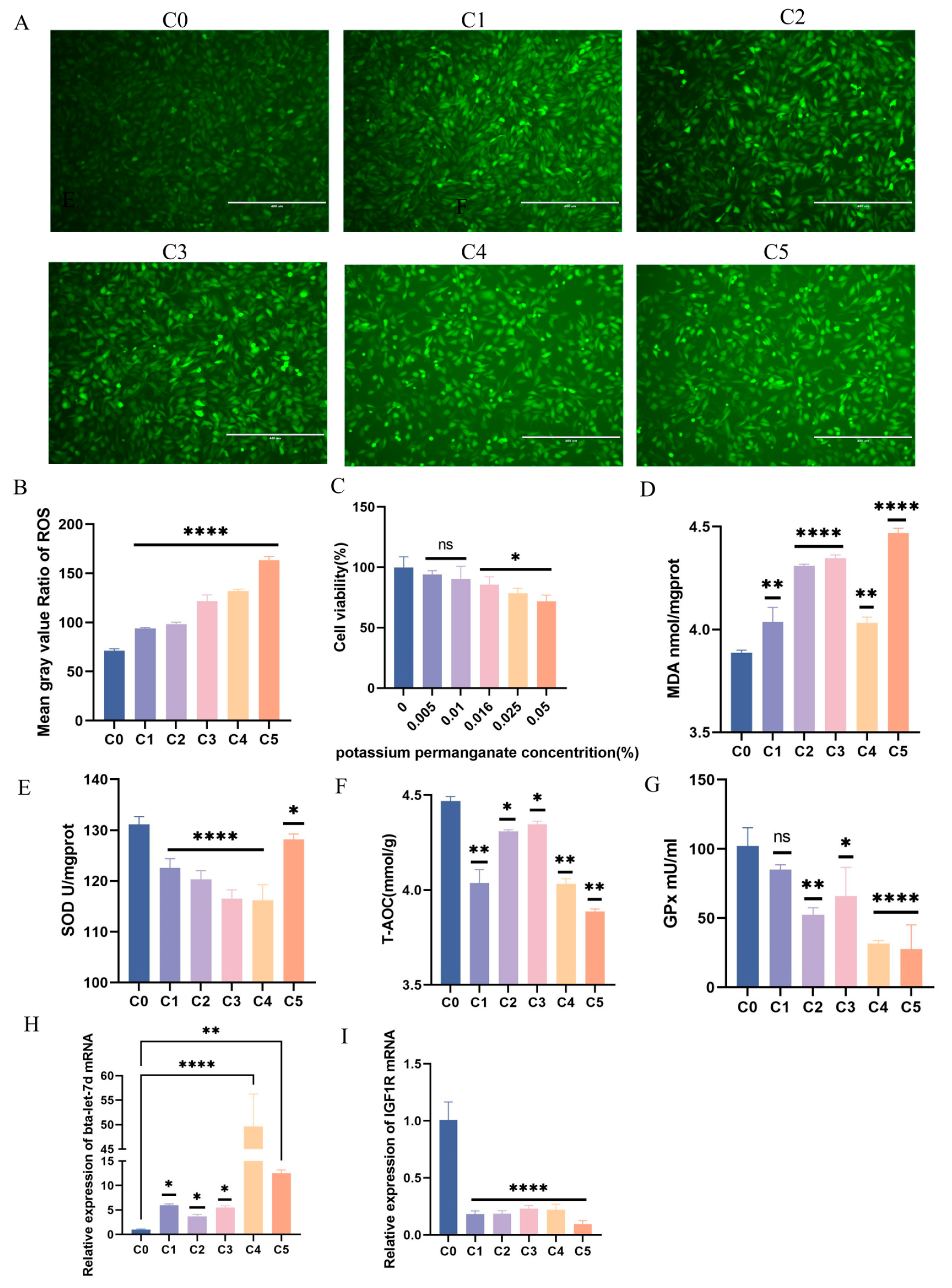
3.2. Establishing a KMnO4-Induced Oxidative Stress Model in Bovine Endometrial Epithelial Cells
3.3. Response Patterns of IGF1R and Bta-Let-7d in the Cell Model
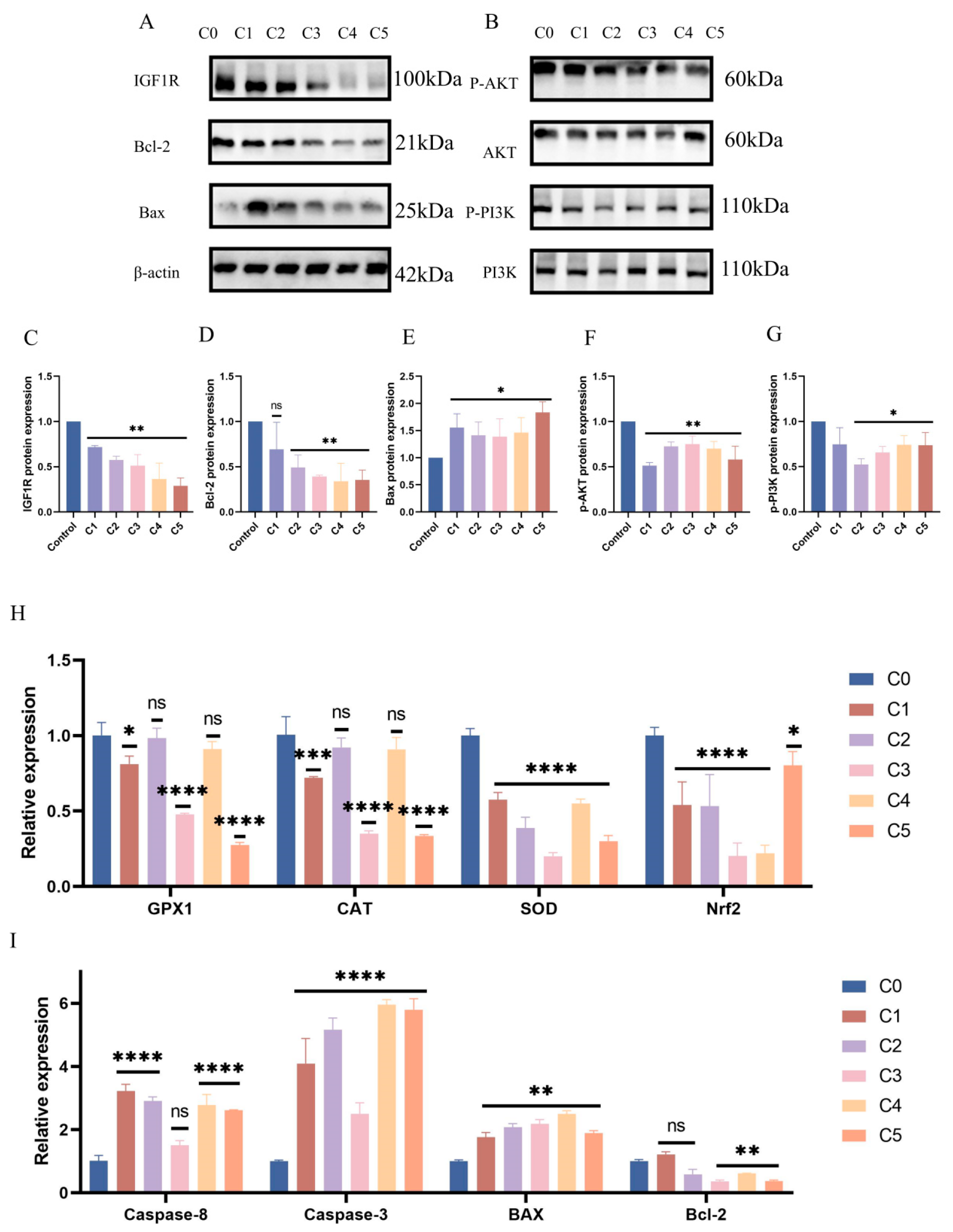
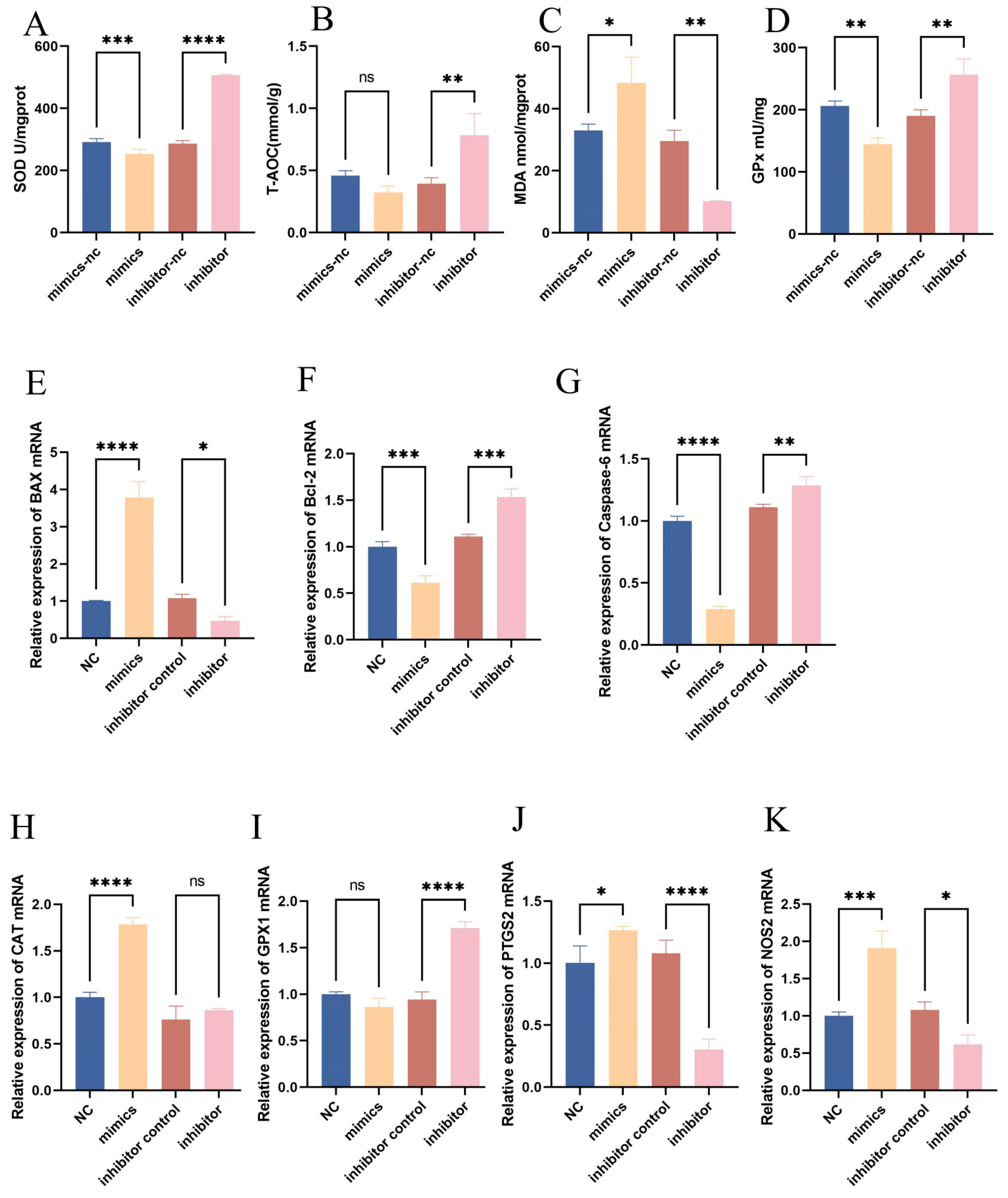
3.4. Regulatory Role of Bta-Let-7d in Oxidative Stress Marker Expression
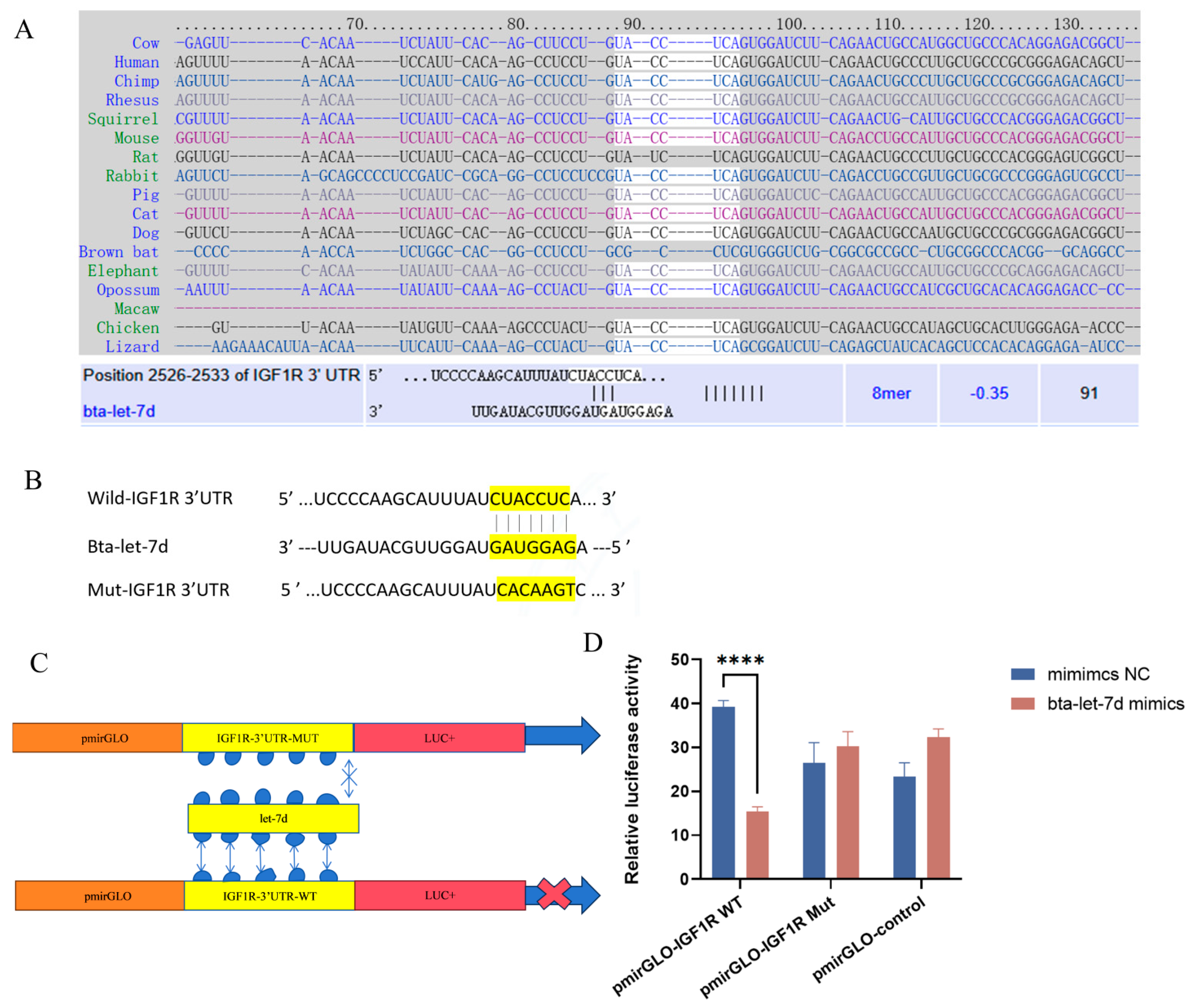
3.5. Interaction Between IGF1R and Bta-Let-7d in the Signaling Pathway
3.6. IGF1R as a Target Gene of Bta-Let-7d
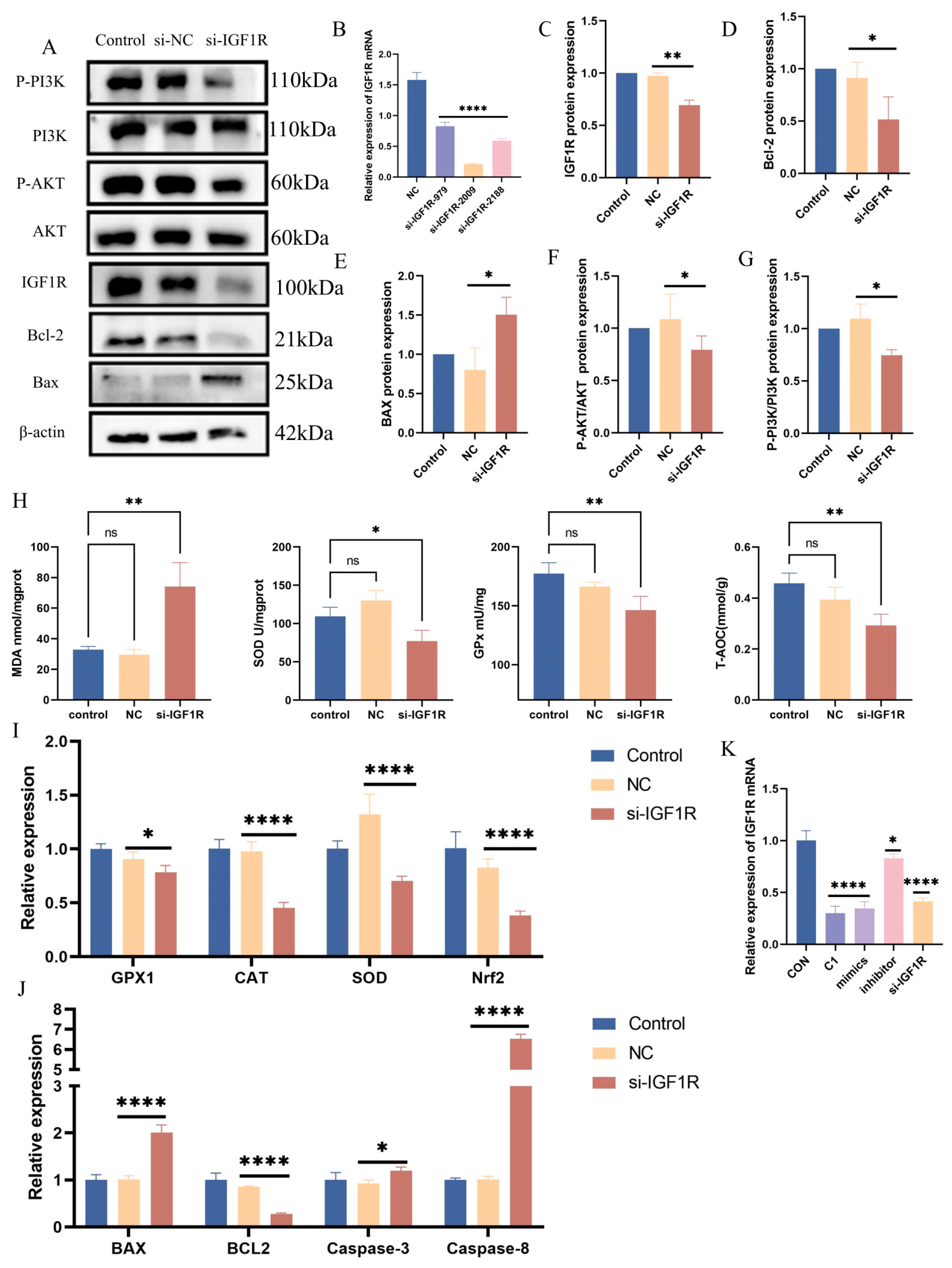
3.7. Negative Regulatory Role of IGF1R in the Oxidative Stress Response
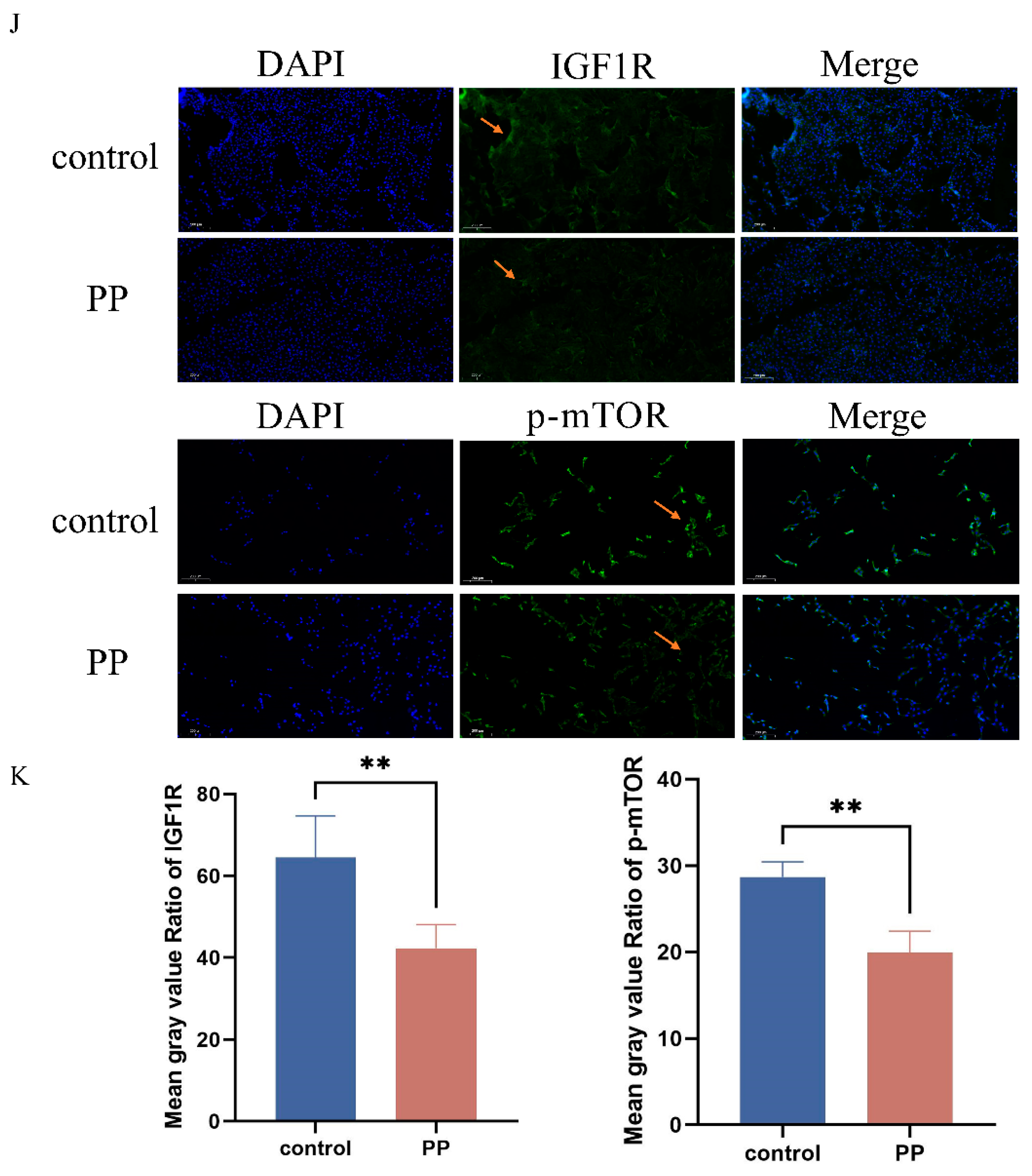
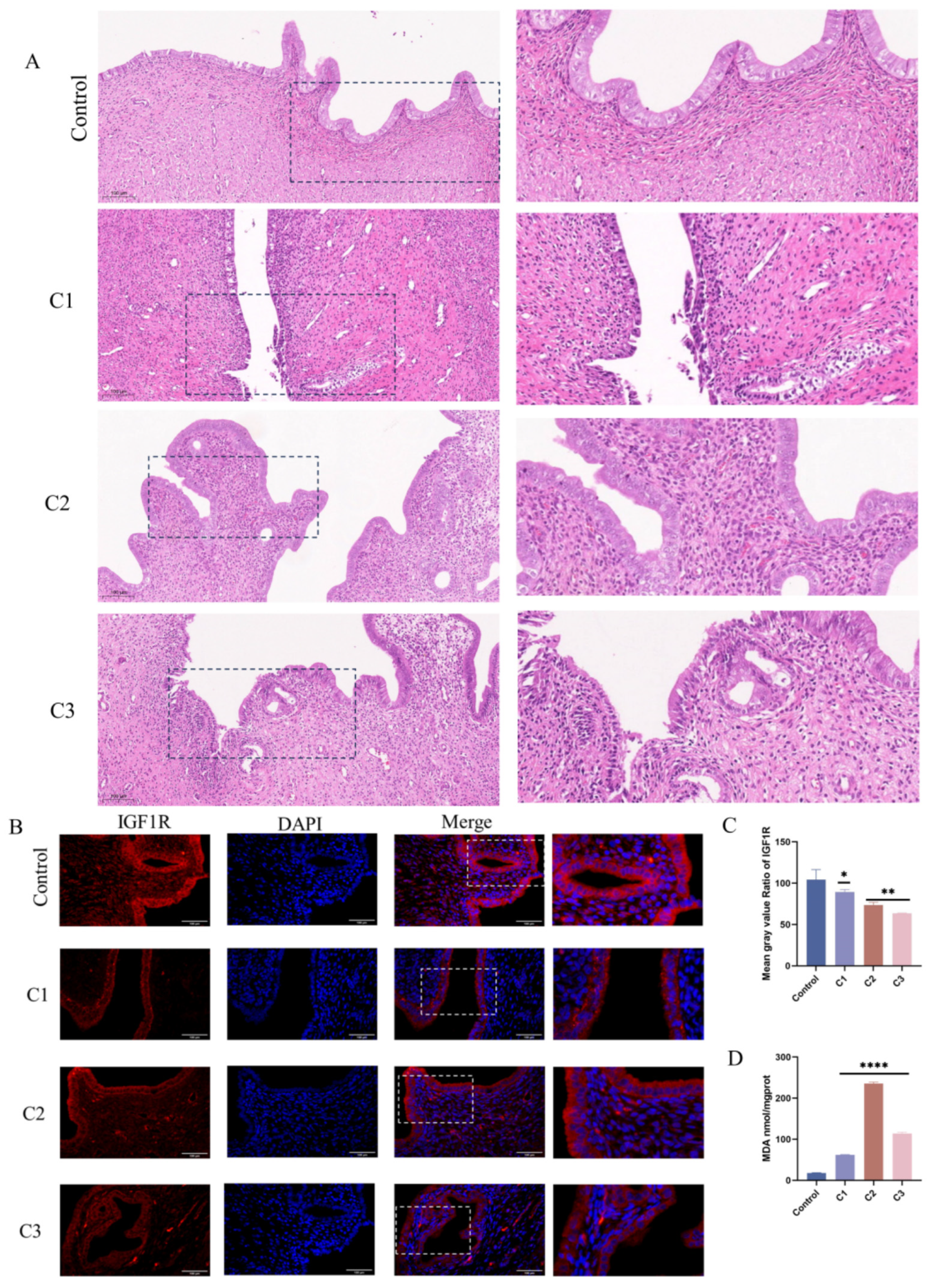
3.8. Potassium Permanganate-Induced Rat Uterine Damage and IGF1R Expression
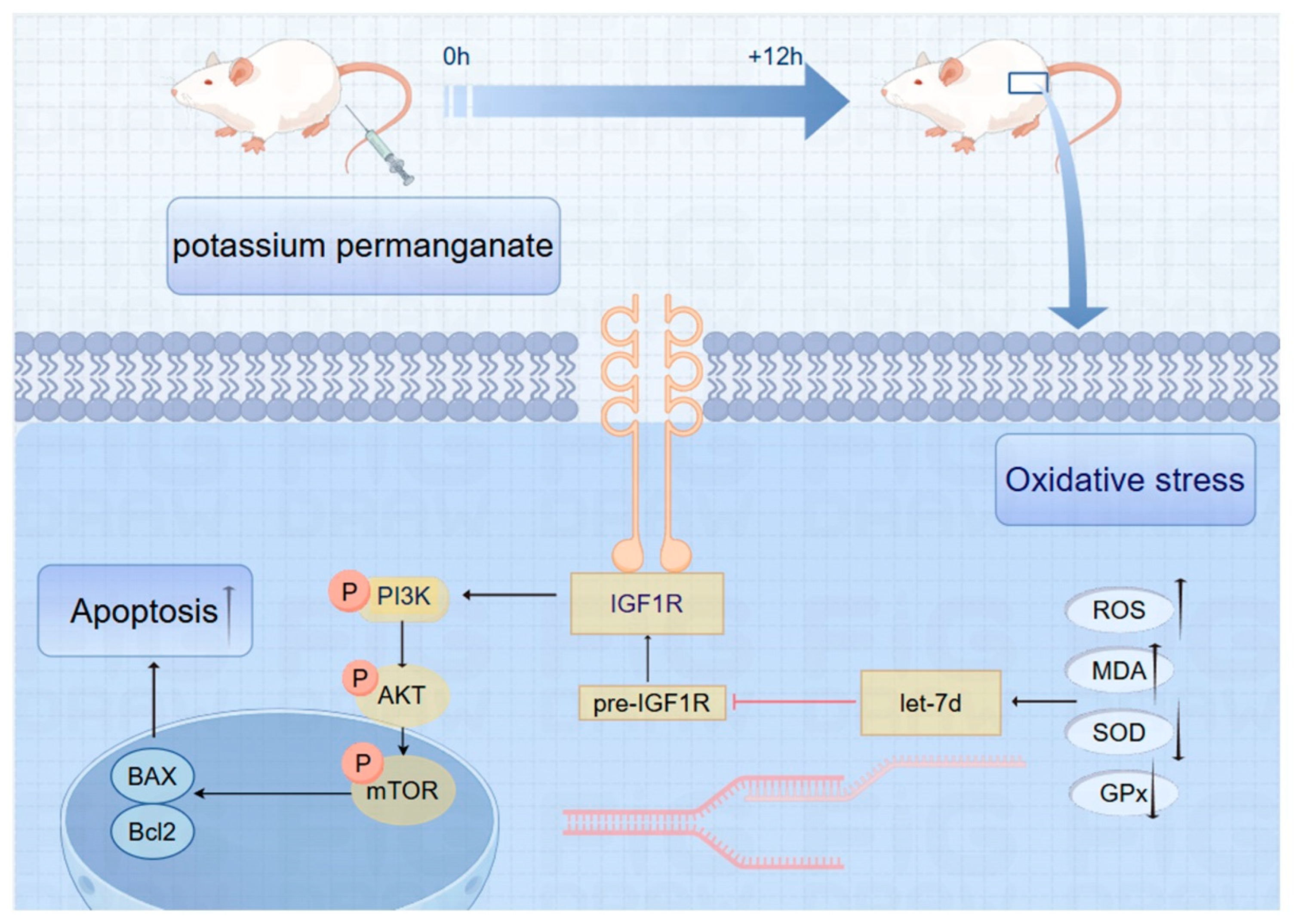
3.9. Schematic of IGF1R/PI3K/AKT Pathway Inhibition in Potassium Permanganate-Induced Endometrial Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Gad, A.; Salilew-Wondim, D.; Prastowo, S.; Held, E.; Hoelker, M.; Rings, F.; Tholen, E.; Neuhoff, C.; Looft, C.; et al. Bovine embryo survival under oxidative-stress conditions is associated with activity of the NRF2-mediated oxidative-stress-response pathway. Mol. Reprod. Dev. 2014, 81, 497–513. [Google Scholar] [PubMed]
- Ciampi, F.; Sordillo, L.M.; Gandy, J.C.; Caroprese, M.; Sevi, A.; Albenzio, M.; Santillo, A. Evaluation of natural plant extracts as antioxidants in a bovine in vitro model of oxidative stress. J. Dairy Sci. 2020, 103, 8938–8947. [Google Scholar] [PubMed]
- Azawi, O.I. Postpartum uterine infection in cattle. Anim. Reprod. Sci. 2008, 105, 187–208. [Google Scholar]
- Song, P.; Liu, C.; Sun, M.; Liu, J.; Lin, P.; Wang, A.; Jin, Y. Oxidative Stress Induces Bovine Endometrial Epithelial Cell Damage through Mitochondria-Dependent Pathways. Animals 2022, 12, 2444. [Google Scholar] [CrossRef]
- Umar, T.; Wenjing, L.; Feng, H.; Feng, W.; Bin, M.; Umar, Z.; Naeem, M.; Umar, A.S.; Asif, S.; Usman, M. A Review on the Applications of Potassium Permanganate in Veterinary Medicine: Toxicity, Efficacy and Future Considerations. Pak. Vet. J. 2024, 44, 214–221. [Google Scholar]
- Abinaya, P.; Thangarasu, S. Testing the Efficacy of Potassium Permanganate as Antiseptic Agent for the Control of Bovine Mastitis. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 609–611. [Google Scholar]
- Delgado-Enciso, I.; Madrigal-Perez, V.M.; Lara-Esqueda, A.; Diaz-Sanchez, M.G.; Guzman-Esquivel, J.; Rosas-Vizcaino, L.E.; Virgen-Jimenez, O.O.; Kleiman-Trujillo, J.; Lagarda-Canales, M.R.; Ceja-Espiritu, G.; et al. Topical 5% potassium permanganate solution accelerates the healing process in chronic diabetic foot ulcers. Biomed. Rep. 2018, 8, 156–159. [Google Scholar]
- Barik, C.; Jindal, A. Potassium permanganate toxicity: A rare case with difficult airway management and hepatic damage. Indian J. Crit. Care Med. 2015, 19, 129. [Google Scholar]
- Chin, G.; Nicholson, H.; Demirel, S.; Affleck, A. Topical potassium permanganate solution use in dermatology: Comparison of guidelines and clinical practice. Clin. Exp. Dermatol. 2022, 47, 966–967. [Google Scholar]
- Subramanya, S.H.; Pai, V.; Bairy, I.; Nayak, N.; Gokhale, S.; Sathian, B. Potassium permanganate cleansing is an effective sanitary method for the reduction of bacterial bioload on raw Coriandrum sativum. BMC Res. Notes 2018, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Rai, V. What is the evidence for the use of potassium permanganate for wound care? Drug Ther. Bull. 2020, 58, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Veerendranath, H.P.; Wali, S.; Mohan, M.N.; Kumar, P.A.; Trimurty, G. Suicidal ingestion of potassium permanganate crystals: A rare encounter. Toxicol. Int. 2014, 21, 331–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Zeng, J.; Yu, X. Potassium permanganate as a promising pre-oxidant to treat low-viability cyanobacteria and associated removal of cyanotoxins and extracellular organic matters. Water Res. 2021, 202, 117353. [Google Scholar] [CrossRef]
- Saganuwan, S.; Ahur, V.; Yohanna, C. Acute toxicity studies of potassium permanganate in Swiss albino mice. Niger. J. Physiol. Sci. 2008, 23, 31–35. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Zhou, Y.; Zhou, Y.; Wang, J.; Ji, R. Mechanism of miR-98 inhibiting tumor proliferation and invasion by targeting IGF1R in diabetic patients combined with colon cancer. Oncol. Lett. 2020, 20, 1719–1726. [Google Scholar] [CrossRef]
- Griffiths, C.D.; Bilawchuk, L.M.; McDonough, J.E.; Jamieson, K.C.; Elawar, F.; Cen, Y.; Duan, W.; Lin, C.; Song, H.; Casanova, J.L.; et al. IGF1R is an entry receptor for respiratory syncytial virus. Nature 2020, 583, 615–619. [Google Scholar] [CrossRef]
- Li, R.L.; Zhang, Q.; Liu, J.; Sun, J.Y.; He, L.Y.; Duan, H.X.; Peng, W.; Wu, C.J. Hydroxy-alpha-sanshool Possesses Protective Potentials on H2O2-Stimulated PC12 Cells by Suppression of Oxidative Stress-Induced Apoptosis through Regulation of PI3K/Akt Signal Pathway. Oxid. Med. Cell Longev. 2020, 2020, 3481758. [Google Scholar]
- Hong, F.; Kwon, S.J.; Jhun, B.S.; Kim, S.S.; Ha, J.; Kim, S.J.; Sohn, N.W.; Kang, C.; Kang, I. Insulin-like growth factor-1 protects H9c2 cardiac myoblasts from oxidative stress-induced apoptosis via phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Life Sci. 2001, 68, 1095–1105. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Yao, Y. Hydrogen peroxide preconditioning is of dual role in cardiac ischemia/reperfusion. Eur. J. Pharmacol. 2023, 947, 175684. [Google Scholar] [CrossRef]
- Zorea, J.; Prasad, M.; Cohen, L.; Li, N.; Schefzik, R.; Ghosh, S.; Rotblat, B.; Brors, B.; Elkabets, M. IGF1R upregulation confers resistance to isoform-specific inhibitors of PI3K in PIK3CA-driven ovarian cancer. Cell Death Dis. 2018, 9, 944. [Google Scholar] [PubMed]
- Shu, S.; Liu, X.; Xu, M.; Gao, X.; Fan, J.; Liu, H.; Li, R. MicroRNA-424 regulates epithelial-mesenchymal transition of endometrial carcinoma by directly targeting insulin-like growth factor 1 receptor. J. Cell Biochem. 2019, 120, 2171–2179. [Google Scholar] [PubMed]
- Esen, N.; Vejalla, A.; Sharma, R.; Treuttner, J.S.; Dore-Duffy, P. Hypoxia-Induced Let-7d Has a Role in Pericyte Differentiation. Adv. Exp. Med. Biol. 2016, 923, 37–42. [Google Scholar] [PubMed]
- Hou, W.; Tian, Q.; Steuerwald, N.M.; Schrum, L.W.; Bonkovsky, H.L. The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes. Biochim. Biophys. Acta 2012, 1819, 1113–1122. [Google Scholar]
- Song, L.; Li, D.; Gu, Y.; Li, X.; Peng, L. Let-7a modulates particulate matter (≤2.5 μm)-induced oxidative stress and injury in human airway epithelial cells by targeting arginase 2. J. Appl. Toxicol. 2016, 36, 1302–1310. [Google Scholar] [CrossRef]
- Balzeau, J.; Menezes, M.R.; Cao, S.; Hagan, J.P. The LIN28/let-7 Pathway in Cancer. Front. Genet. 2017, 8, 31. [Google Scholar]
- Zhu, H.; Shyh-Chang, N.; Segrè, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011, 147, 81–94. [Google Scholar]
- De Santis, C.; Gotte, M. The Role of microRNA Let-7d in Female Malignancies and Diseases of the Female Reproductive Tract. Int. J. Mol. Sci. 2021, 22, 7359. [Google Scholar] [CrossRef]
- Barrey, E.; Saint-Auret, G.; Bonnamy, B.; Damas, D.; Boyer, O.; Gidrol, X. Pre-microRNA and mature microRNA in human mitochondria. PLoS ONE 2011, 6, e20220. [Google Scholar] [CrossRef]
- Umar, T.; Feng, H.; Feng, W.; Zhou, H.; Chen, N.; Zhang, J.; Liu, W.; Wang, X.; Umer, S.; Umar, Z.; et al. Comparative Transcriptional Analysis of Long Noncoding RNAs in Oxidative Stress and Inflammation Induced by Potassium Permanganate and Lipopolysaccharide in Rat Uterine Tissues. Antioxidants 2025, 14, 251. [Google Scholar] [CrossRef]
- Stryhn, J.K.G.; Larsen, J.; Pedersen, P.L.; Gæde, P.H. Expressions of mitochondria-related genes in pregnant women with subclinical hypothyroidism, and expressions of miRNAs in maternal and cord blood. Thyroid. Res. 2023, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Radajewska, A.; Krzywonos-Zawadzka, A.; Bil-Lula, I. Klotho inhibits IGF1R/PI3K/AKT signalling pathway and protects the heart from oxidative stress during ischemia/reperfusion injury. Sci. Rep. 2023, 13, 20312. [Google Scholar]
- Jimenez Calvente, C.; Del Pilar, H.; Tameda, M.; Johnson, C.D.; Feldstein, A.E. MicroRNA 223 3p Negatively Regulates the NLRP3 Inflammasome in Acute and Chronic Liver Injury. Mol. Ther. 2020, 28, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Yang, J.; Yang, C.; Zhang, T.; Shaukat, A.; Yang, X.; Dai, A.; Wu, H.; Deng, G. miR-148a suppresses inflammation in lipopolysaccharide-induced endometritis. J. Cell Mol. Med. 2020, 24, 405–417. [Google Scholar]

| Species | Gene Name | Primer Sequence (5′–3′) | GenBank Accession Number | Product Size (bp) |
|---|---|---|---|---|
| Bos taurus | IGF1R | Forward: CCAAAACCGAAGCTGAGAAG | XM_054377836.1 | 199 |
| Reverse: TCCGGGTCTGTGATGTTGTA | ||||
| GPx1 | Forward: CTTGCTGCTTGGCGGTCA | NM_174076.3 | 139 | |
| Reverse: AGGGGAGGCTGGGATGGAT | ||||
| SOD3 | Forward: CTTCTTCCACCTTGAGGGCTTC | NM_001428313.1 | 125 | |
| Reverse: CGGACATCGGGTTGTAGTGC | ||||
| CAT | Forward: CTGGGACCCAACTATCTCCA | NM_001035386.2 | 148 | |
| Reverse: GATGCTCGGGAGCACTAAAG | ||||
| Nrf2 | Forward: GGTTGCCCACATTCCCAAATC | XM_005202314.5 | 119 | |
| Reverse: CAAGTGACTGAAACGTAGCCG | ||||
| NOS2 | Forward: ACCTACCAGCTGACGGGAGAT | XM_024979646.2 | 316 | |
| Reverse: TGGCAGGGTCCCCTCTGATG | ||||
| Ptgs2 | Forward: TCCTGAAACCCACTCCCAACA | NM_174445.2 | 242 | |
| Reverse: TGGGCAGTCAGGCACAG | ||||
| Bax | Forward: CAGATCATGAAGACAGGGGC | NM_173894.1 | 389 | |
| Reverse: CGCTCTCGAAGGAAGTCCAA | ||||
| Bcl-2 | Forward: ATGTGTGTGGAGAGCGTCAA | NM_001166486.1 | 143 | |
| Reverse: GGGCCATACAGCTCCACAAA | ||||
| Caspase-3 | Forward: AAGCCATGGTGAAGAAGGAA | XM_010820245.4 | 134 | |
| Reverse: GGCAGGCCTGAATAATGAAA | ||||
| Caspase-9 | Forward: CGCCACCATCTTCTCCCTG | XM_024999238.2 | 83 | |
| Reverse: CCAACGTCTCCTTCTCCTCC | ||||
| β-actin | Forward: CATCACCATCGGCAATGAGC | NM_173979.3 | 156 | |
| Reverse: AGCACCGTGTTGGCGTAGAG | ||||
| RT-U6 | Forward: ACGUGACACGUUCGGAGAATT | |||
| U6 | Forward: CTCGCTTCGGCAGCACA | |||
| Reverse: AACGCTTCACGAATTTGCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Umar, T.; Feng, W.; Zhang, B.; Zhang, J.; Zhou, H.; Chen, N.; Deng, G.; Xiao, S. Bta-Let-7d Modulation of Oxidative Stress Induced by Potassium Permanganate in Bovine Endometrial Cells via IGF1R/PI3K/AKT Signaling Pathway. Antioxidants 2025, 14, 444. https://doi.org/10.3390/antiox14040444
Liu W, Umar T, Feng W, Zhang B, Zhang J, Zhou H, Chen N, Deng G, Xiao S. Bta-Let-7d Modulation of Oxidative Stress Induced by Potassium Permanganate in Bovine Endometrial Cells via IGF1R/PI3K/AKT Signaling Pathway. Antioxidants. 2025; 14(4):444. https://doi.org/10.3390/antiox14040444
Chicago/Turabian StyleLiu, Wenjing, Talha Umar, Wen Feng, Bohan Zhang, Jinxin Zhang, Han Zhou, Nuoer Chen, Ganzhen Deng, and Siyu Xiao. 2025. "Bta-Let-7d Modulation of Oxidative Stress Induced by Potassium Permanganate in Bovine Endometrial Cells via IGF1R/PI3K/AKT Signaling Pathway" Antioxidants 14, no. 4: 444. https://doi.org/10.3390/antiox14040444
APA StyleLiu, W., Umar, T., Feng, W., Zhang, B., Zhang, J., Zhou, H., Chen, N., Deng, G., & Xiao, S. (2025). Bta-Let-7d Modulation of Oxidative Stress Induced by Potassium Permanganate in Bovine Endometrial Cells via IGF1R/PI3K/AKT Signaling Pathway. Antioxidants, 14(4), 444. https://doi.org/10.3390/antiox14040444






