Hydroxytyrosol as a Mitochondrial Homeostasis Regulator: Implications in Metabolic Syndrome and Related Diseases
Abstract
1. Introduction
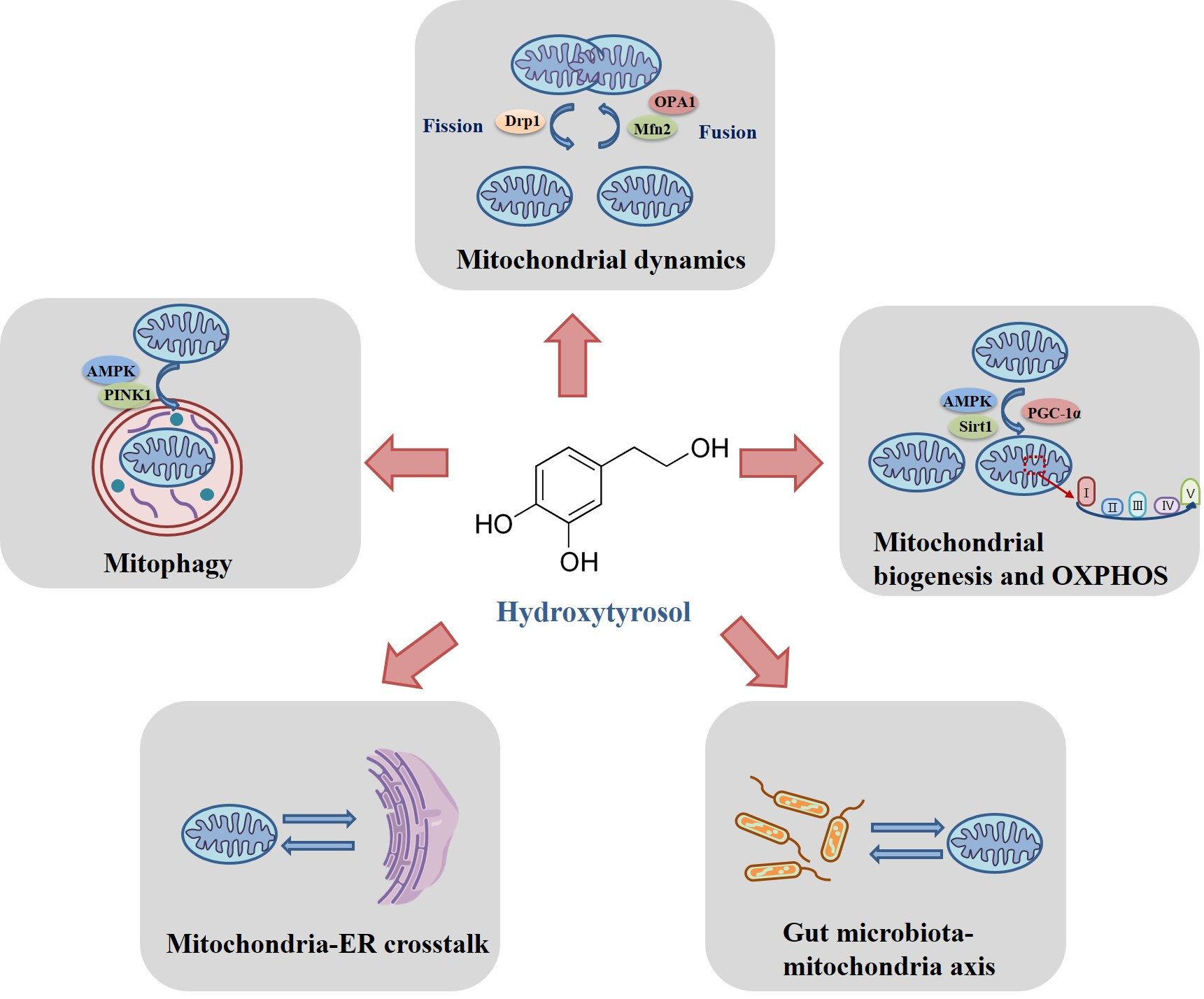
2. The Regulatory Mechanisms of Hydroxytyrosol on Mitochondrial Homeostasis
2.1. Modulation of Hydroxytyrosol on Mitochondrial Fusion and Fission
2.2. Modulation of Hydroxytyrosol on Mitochondrial Biogenesis and Oxidative Phosphorylation
2.3. Modulation of Hydroxytyrosol on Mitophagy
2.4. Modulation of Hydroxytyrosol on Mitochodnria-Endoplasmic Reticulum Crosstalk
2.5. Modulation of Hydroxytyrosol on Gut Microbiota-Mitochondria Axis
3. The Protective Role of Hydroxytyrosol in Metabolic-Related Diseases
3.1. Type 2 Diabetes and Its Complications
3.2. Obesity, Hyperlipemia, and Nonalcoholic Fatty Liver
3.3. Hypertension-Related Diseases
4. Challenges and Future Perspectives
4.1. Direct Targets of Hydroxytyrosol: Awaiting Further Elucidation
4.2. Mitochondrial Delivery System for Enhanced Targeting
4.3. Synergistic Therapies for Boosting Efficacy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HT | Hydroxytyrosol |
| MetS | Metabolic Syndrome |
| T2DM | Type 2 Diabetes Mellitus |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| OXPHOS | Oxidative Phosphorylation |
| ER | Endoplasmic Reticulum |
| OPA1 | Optic Atrophy 1 |
| Drp1 | Dynamin-Related Protein 1 |
| Mfn2 | Mitofusin 2 |
| PGC-1α | Peroxisome Proliferator-Activated Receptor γ Coactivator 1-α |
| mtDNA | Mitochondrial DNA |
| SIRT1 | Sirtuin 1 |
| ROS | Reactive Oxygen Species |
| HFD | High-Fat Diet |
| MERC | Mitochondria–ER Contact |
| UPR | Unfolded Protein Response |
| eNOS | Endothelial Nitric Oxide Synthase |
| ABPP | Activity-Based Protein Profiling |
| TPP | Thermal Proteome Profiling |
References
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, Z.; Liu, X.; He, Y. Confronting the global obesity epidemic: Investigating the role and underlying mechanisms of vitamin D in metabolic syndrome management. Front. Nutr. 2024, 11, 1416344. [Google Scholar] [CrossRef]
- Bouillon-Minois, J.B.; Dutheil, F. Biomarker of Stress, Metabolic Syndrome and Human Health. Nutrients 2022, 14, 2935. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.; Lavie, C.J.; Lippi, G.; Brzek, A.; Vollenberg, R.; Sanchis-Gomar, F.; Leischik, R. A systematic review of prevalence of metabolic syndrome in occupational groups—Does occupation matter in the global epidemic of metabolic syndrome? Prog. Cardiovasc. Dis. 2022, 75, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Despres, J.P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wang, L.; Duan, L.; Gong, F.; Zhu, H.; Pan, H.; Yang, H. Insulin resistance surrogates are associated with all-cause mortality and cardiovascular mortality in population with metabolic syndrome: A retrospective cohort study of NHANES. Sci. Rep. 2025, 15, 4706. [Google Scholar] [CrossRef]
- Xia, W.; Veeragandham, P.; Cao, Y.; Xu, Y.; Rhyne, T.E.; Qian, J.; Hung, C.W.; Zhao, P.; Jones, Y.; Gao, H.; et al. Obesity causes mitochondrial fragmentation and dysfunction in white adipocytes due to RalA activation. Nat. Metab. 2024, 6, 273–289. [Google Scholar] [CrossRef]
- Koh, J.H.; Johnson, M.L.; Dasari, S.; LeBrasseur, N.K.; Vuckovic, I.; Henderson, G.C.; Cooper, S.A.; Manjunatha, S.; Ruegsegger, G.N.; Shulman, G.I.; et al. TFAM Enhances Fat Oxidation and Attenuates High-Fat Diet-Induced Insulin Resistance in Skeletal Muscle. Diabetes 2019, 68, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.; Zhang, Q.; Hu, L.; Zhao, L.; Wang, H.; Yuan, Y.; Niu, H.; Wang, D.; Zhang, H.; et al. Metabolic regulator LKB1 controls adipose tissue ILC2 PD-1 expression and mitochondrial homeostasis to prevent insulin resistance. Immunity 2024, 57, 1289–1305. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Li, W.; Chen, H.; Du, L.; Liu, D.; Wang, X.; Xu, T.; Liu, L.; Chen, Q. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy 2019, 15, 1882–1898. [Google Scholar] [CrossRef]
- Kyriakoudi, S.; Theodoulou, A.; Potamiti, L.; Schumacher, F.; Zachariou, M.; Papacharalambous, R.; Kleuser, B.; Panayiotidis, M.I.; Drousiotou, A.; Petrou, P.P. Stbd1-deficient mice display insulin resistance associated with enhanced hepatic ER-mitochondria contact. Biochimie 2022, 200, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Casler, J.C.; Harper, C.S.; White, A.J.; Anderson, H.L.; Lackner, L.L. Mitochondria-ER-PM contacts regulate mitochondrial division and PI(4)P distribution. J. Cell Biol. 2024, 223, e202308144. [Google Scholar] [CrossRef]
- Juarez-Fernandez, M.; Goikoetxea-Usandizaga, N.; Porras, D.; Garcia-Mediavilla, M.V.; Bravo, M.; Serrano-Macia, M.; Simon, J.; Delgado, T.C.; Lachiondo-Ortega, S.; Martinez-Florez, S.; et al. Enhanced mitochondrial activity reshapes a gut microbiota profile that delays NASH progression. Hepatology 2023, 77, 1654–1669. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef]
- Ditano-Vazquez, P.; Torres-Pena, J.D.; Galeano-Valle, F.; Perez-Caballero, A.I.; Demelo-Rodriguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef]
- Blanco-Benitez, M.; Calderon-Fernandez, A.; Canales-Cortes, S.; Alegre-Cortes, E.; Uribe-Carretero, E.; Paredes-Barquero, M.; Gimenez-Bejarano, A.; Duque Gonzalez, G.; Gomez-Suaga, P.; Ortega-Vidal, J.; et al. Biological effects of olive oil phenolic compounds on mitochondria. Mol. Cell Oncol. 2022, 9, 2044263. [Google Scholar] [CrossRef]
- Khawula, S.; Gokul, A.; Niekerk, L.A.; Basson, G.; Keyster, M.; Badiwe, M.; Klein, A.; Nkomo, M. Insights into the Effects of Hydroxycinnamic Acid and Its Secondary Metabolites as Antioxidants for Oxidative Stress and Plant Growth under Environmental Stresses. Curr. Issues Mol. Biol. 2023, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, K. Roles of Plant-Derived Secondary Metabolites during Interactions with Pathogenic and Beneficial Microbes under Conditions of Environmental Stress. Microorganisms 2019, 7, 362. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.H.; Zhou, C.; Li, S.J.; Li, Y.H.; Shen, C.W.; Tao, Y.; Li, X. Microbial diversity across tea varieties and ecological niches: Correlating tea polyphenol contents with stress resistance. Front. Microbiol. 2024, 15, 1439630. [Google Scholar] [CrossRef]
- Lopez Sanchez, A.; Hernandez Luelmo, S.; Izquierdo, Y.; Lopez, B.; Cascon, T.; Castresana, C. Mitochondrial Stress Induces Plant Resistance Through Chromatin Changes. Front. Plant Sci. 2021, 12, 704964. [Google Scholar] [CrossRef]
- Zou, X.; Zeng, M.; Zheng, Y.; Zheng, A.; Cui, L.; Cao, W.; Wang, X.; Liu, J.; Xu, J.; Feng, Z. Comparative Study of Hydroxytyrosol Acetate and Hydroxytyrosol in Activating Phase II Enzymes. Antioxidants 2023, 12, 1834. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hou, C.; Yang, Z.; Li, C.; Jia, L.; Liu, J.; Tang, Y.; Shi, L.; Li, Y.; Long, J.; et al. Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Mol. Nutr. Food Res. 2016, 60, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Feng, Z.; Li, Y.; Wang, Y.; Wertz, K.; Weber, P.; Fu, Y.; Liu, J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: Activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012, 23, 994–1006. [Google Scholar] [CrossRef]
- Sirangelo, I.; Liccardo, M.; Iannuzzi, C. Hydroxytyrosol Prevents Doxorubicin-Induced Oxidative Stress and Apoptosis in Cardiomyocytes. Antioxidants 2022, 11, 1087. [Google Scholar] [CrossRef]
- Han, H.; Zhong, R.; Zhang, S.; Wang, M.; Wen, X.; Yi, B.; Zhao, Y.; Chen, L.; Zhang, H. Hydroxytyrosol attenuates diquat-induced oxidative stress by activating Nrf2 pathway and modulating colonic microbiota in mice. J. Nutr. Biochem. 2023, 113, 109256. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhang, L.; Yin, H.; Shen, L.; Zheng, W.; Zhang, K.; Zeng, J.; Hu, C.; Liu, Y. Hydroxytyrosol alleviates oxidative stress and neuroinflammation and enhances hippocampal neurotrophic signaling to improve stress-induced depressive behaviors in mice. Food Funct. 2021, 12, 5478–5487. [Google Scholar] [CrossRef]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, J.; Cao, W.; Zhang, J.; Feng, Z.; Cao, K.; Liu, J. Hydroxytyrosol improves strenuous exercise-associated cardiac pathological changes via modulation of mitochondrial homeostasis. Food Funct. 2022, 13, 8676–8684. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Binou, P.; Stergiou, A.; Kosta, O.; Tentolouris, N.; Karathanos, V.T. Positive contribution of hydroxytyrosol-enriched wheat bread to HbA(1)c levels, lipid profile, markers of inflammation and body weight in subjects with overweight/obesity and type 2 diabetes mellitus. Eur. J. Nutr. 2023, 62, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.; Lama, A.; Simeoli, R.; Paciello, O.; Pagano, T.B.; Mollica, M.P.; Di Guida, F.; Russo, R.; Magliocca, S.; Canani, R.B.; et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016, 30, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodriguez-Morato, J.; Varon, C.; Munoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic. Biol. Med. 2019, 143, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, A.; Nikolaou, P.E.; Symeonidi, L.; Katogiannis, K.; Pechlivani, L.; Nikou, T.; Varela, A.; Chania, C.; Zerikiotis, S.; Efentakis, P.; et al. Cardioprotective potential of oleuropein, hydroxytyrosol, oleocanthal and their combination: Unravelling complementary effects on acute myocardial infarction and metabolic syndrome. Redox Biol. 2024, 76, 103311. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Zheng, A.; Yang, L.; Liu, J.; Chen, C.; Tang, Y.; Zou, X.; Li, Y.; Long, J.; et al. Mitochondrial dysfunction-associated OPA1 cleavage contributes to muscle degeneration: Preventative effect of hydroxytyrosol acetate. Cell Death Dis. 2014, 5, e1521. [Google Scholar] [CrossRef]
- Pérez-Barrón, G.; Montes, S.; Aguirre-Vidal, Y.; Santiago, M.; Gallardo, E.; Espartero, J.L.; Ríos, C.; Monroy-Noyola, A. Antioxidant Effect of Hydroxytyrosol, Hydroxytyrosol Acetate and Nitrohydroxytyrosol in a Rat MPP (+) Model of Parkinson’s Disease. Neurochem. Res. 2021, 46, 2923–2935. [Google Scholar] [CrossRef]
- Bedoya, L.M.; Beltrán, M.; Obregón-Calderón, P.; García-Pérez, J.; de la Torre, H.E.; González, N.; Pérez-Olmeda, M.; Auñón, D.; Capa, L.; Gómez-Acebo, E.; et al. Hydroxytyrosol: A new class of microbicide displaying broad anti-HIV-1 activity. Aids 2016, 30, 2767–2776. [Google Scholar] [CrossRef]
- Ben Hassena, A.; Abidi, J.; Miled, N.; Kulinowski, Ł.; Skalicka-Woźniak, K.; Bouaziz, M. New Insights into the Antibacterial Activity of Hydroxytyrosol Extracted from Olive Leaves: Molecular Docking Simulations of its Antibacterial Mechanisms. Chem. Biodivers. 2025, 22, e202401714. [Google Scholar] [CrossRef]
- Perta, N.; Torrieri Di Tullio, L.; Cugini, E.; Fattibene, P.; Rapanotti, M.C.; Borromeo, I.; Forni, C.; Malaspina, P.; Cacciamani, T.; Di Marino, D.; et al. Hydroxytyrosol Counteracts Triple Negative Breast Cancer Cell Dissemination via Its Copper Complexing Properties. Biology 2023, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Del Saz-Lara, A.; Boughanem, H.; López de Las Hazas, M.C.; Crespo, C.; Saz-Lara, A.; Visioli, F.; Macias-González, M.; Dávalos, A. Hydroxytyrosol decreases EDNRA expression through epigenetic modification in colorectal cancer cells. Pharmacol. Res. 2023, 187, 106612. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sepulveda, M.; Fernández-Verdejo, R.; Zbinden-Foncea, H.; Rieusset, J. Mitochondria-SR interaction and mitochondrial fusion/fission in the regulation of skeletal muscle metabolism. Metab. Clin. Exp. 2023, 144, 155578. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Chen, Y.; Shi, L.X.; Cheng, H.R.; Banda, I.; Ji, Y.H.; Wang, Y.T.; Li, X.M.; Mao, Y.X.; Zhang, D.F.; et al. AKT-GSK3beta Signaling Pathway Regulates Mitochondrial Dysfunction-Associated OPA1 Cleavage Contributing to Osteoblast Apoptosis: Preventative Effects of Hydroxytyrosol. Oxid. Med. Cell Longev. 2019, 2019, 4101738. [Google Scholar] [CrossRef] [PubMed]
- Casuso, R.A.; Al-Fazazi, S.; Hidalgo-Gutierrez, A.; Lopez, L.C.; Plaza-Diaz, J.; Rueda-Robles, A.; Huertas, J.R. Hydroxytyrosol influences exercise-induced mitochondrial respiratory complex assembly into supercomplexes in rats. Free Radic. Biol. Med. 2019, 134, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Bai, L.; Yan, J.; Li, Y.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, Y.; Chen, Y.; et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: Regulatory effects of hydroxytyrosol. Free Radic. Biol. Med. 2011, 50, 1437–1446. [Google Scholar] [CrossRef]
- Pan, R.; Jones, A.D.; Hu, J. Cardiolipin-mediated mitochondrial dynamics and stress response in Arabidopsis. Plant Cell 2014, 26, 391–409. [Google Scholar] [CrossRef]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality control of the mitochondrion. Dev. Cell 2021, 56, 881–905. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, L.; Zhu, L.; Jia, X.; Li, X.; Jia, H.; Wang, Y.; Weber, P.; Long, J.; Liu, J. Hydroxytyrosol protects retinal pigment epithelial cells from acrolein-induced oxidative stress and mitochondrial dysfunction. J. Neurochem. 2007, 103, 2690–2700. [Google Scholar] [CrossRef]
- Dong, Y.; Kang, H.; Peng, R.; Liu, Z.; Liao, F.; Hu, S.-a.; Ding, W.; Wang, P.; Yang, P.; Zhu, M.; et al. A clinical-stage Nrf2 activator suppresses osteoclast differentiation via the iron-ornithine axis. Cell Metab. 2024, 36, 1679–1695. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, Y.F.; Liu, X.T.; Li, Y.C.; Zhu, H.M.; Sun, M.R.; Li, P.; Liu, B.; Yang, H. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021, 38, 101771. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Liu, J.; Feng, Z. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem. 2015, 26, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Li, L.; Espe, M.; Lu, K.L.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef] [PubMed]
- Signorile, A.; Micelli, L.; De Rasmo, D.; Santeramo, A.; Papa, F.; Ficarella, R.; Gattoni, G.; Scacco, S.; Papa, S. Regulation of the biogenesis of OXPHOS complexes in cell transition from replicating to quiescent state: Involvement of PKA and effect of hydroxytyrosol. Biochim. Biophys. Acta 2014, 1843, 675–684. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, Y.; Li, C.; Xie, Y.; Klionsky, D.J.; Kang, R.; Tang, D. TMEM164 is a new determinant of autophagy-dependent ferroptosis. Autophagy 2023, 19, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15, 1774. [Google Scholar] [CrossRef]
- Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms. Biochim. Biophys. Acta 2016, 1860, 1181–1191. [Google Scholar] [CrossRef]
- Sun, T.; Chen, Q.; Zhu, S.Y.; Wu, Q.; Liao, C.R.; Wang, Z.; Wu, X.H.; Wu, H.T.; Chen, J.T. Hydroxytyrosol promotes autophagy by regulating SIRT1 against advanced oxidation protein product-induced NADPH oxidase and inflammatory response. Int. J. Mol. Med. 2019, 44, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; et al. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, M.; Gao, Y.; Komianou, A.C.; Georgiou, E.A.; Wang, Y.; Zheng, Y.; Liu, J.; Kostakis, I.K.; Zhao, L. A Novel Synthesized Cyclohexane-Hydroxytyrosol Derivative Suppresses Ovarian Cancer Cell Growth Through Inducing Reactive Oxidative Species and Blocking Autophagic Flux. Antioxid. Redox Signal. 2024, 41, 430–461. [Google Scholar] [CrossRef]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in human health, ageing and disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef]
- Wen, X.; Tang, S.; Wan, F.; Zhong, R.; Chen, L.; Zhang, H. The PI3K/Akt-Nrf2 Signaling Pathway and Mitophagy Synergistically Mediate Hydroxytyrosol to Alleviate Intestinal Oxidative Damage. Int. J. Biol. Sci. 2024, 20, 4258–4276. [Google Scholar] [CrossRef]
- Jiang, Y.; Krantz, S.; Qin, X.; Li, S.; Gunasekara, H.; Kim, Y.M.; Zimnicka, A.; Bae, M.; Ma, K.; Toth, P.T.; et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission-fusion dynamics and mitophagy. Redox Biol. 2022, 52, 102304. [Google Scholar] [CrossRef]
- Raffin, J.; de Souto Barreto, P.; Le Traon, A.P.; Vellas, B.; Aubertin-Leheudre, M.; Rolland, Y. Sedentary behavior and the biological hallmarks of aging. Ageing Res. Rev. 2023, 83, 101807. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Pan, K.; Guo, J.; Liu, Z.; Zhang, C.; Zhang, J.; Qian, X.; Shen, H.; Zhao, J. Exercise ameliorates fine particulate matter-induced metabolic damage through the SIRT1/AMPKalpha/PGC1-alpha/NRF1 signaling pathway. Environ. Res. 2024, 245, 117973. [Google Scholar] [CrossRef]
- Chen, C.C.W.; Erlich, A.T.; Crilly, M.J.; Hood, D.A. Parkin is required for exercise-induced mitophagy in muscle: Impact of aging. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E404–E415. [Google Scholar] [CrossRef]
- Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012, 13, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Ruhmkorf, A.; Harbauer, A.B. Role of Mitochondria-ER Contact Sites in Mitophagy. Biomolecules 2023, 13, 1198. [Google Scholar] [CrossRef]
- Yepuri, G.; Ramirez, L.M.; Theophall, G.G.; Reverdatto, S.V.; Quadri, N.; Hasan, S.N.; Bu, L.; Thiagarajan, D.; Wilson, R.; Diez, R.L.; et al. DIAPH1-MFN2 interaction regulates mitochondria-SR/ER contact and modulates ischemic/hypoxic stress. Nat. Commun. 2023, 14, 6900. [Google Scholar] [CrossRef]
- Li, Y.; Huang, D.; Jia, L.; Shangguan, F.; Gong, S.; Lan, L.; Song, Z.; Xu, J.; Yan, C.; Chen, T.; et al. LonP1 Links Mitochondria-ER Interaction to Regulate Heart Function. Research 2023, 6, 175. [Google Scholar] [CrossRef]
- Rieusset, J. Mitochondria and endoplasmic reticulum: Mitochondria-endoplasmic reticulum interplay in type 2 diabetes pathophysiology. Int. J. Biochem. Cell Biol. 2011, 43, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Katti, P.; Glancy, B. Rebalancing Cardiac Structure and Function with Synthetic Mitochondria-Endoplasmic Reticulum Tethers. Circ. Res. 2023, 132, 1465–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, F.; Jiang, Y.; Lu, C. Renal Denervation Ameliorates Cardiomyocyte Apoptosis in Myocardial Ischemia-Reperfusion Injury Through Regulating Mitochondria-Endoplasmic Reticulum Contact. Anatol. J. Cardiol. 2024, 28, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Davalos, A.; Nicod, N.; Visioli, F. Hydroxytyrosol attenuates tunicamycin-induced endoplasmic reticulum stress in human hepatocarcinoma cells. Mol. Nutr. Food Res. 2014, 58, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Liao, W.C.; Lin, R.A.; Chen, I.S.; Wang, J.L.; Chien, J.M.; Kuo, C.C.; Hao, L.J.; Chou, C.T.; Jan, C.R. Hydroxytyrosol [2-(3,4-dihydroxyphenyl)-ethanol], a natural phenolic compound found in the olive, alters Ca(2+) signaling and viability in human HepG2 hepatoma cells. Chin. J. Physiol. 2022, 65, 30–36. [Google Scholar] [CrossRef]
- Hsu, S.S.; Lin, Y.S.; Liang, W.Z. Inhibition of the pesticide rotenone-induced Ca(2+) signaling, cytotoxicity and oxidative stress in HCN-2 neuronal cells by the phenolic compound hydroxytyrosol. Pestic. Biochem. Physiol. 2021, 179, 104979. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, L.; Chen, A.; Xu, C.; Feng, Q. Protective Effects of Olive Leaf Extract on Acrolein-Exacerbated Myocardial Infarction via an Endoplasmic Reticulum Stress Pathway. Int. J. Mol. Sci. 2018, 19, 493. [Google Scholar] [CrossRef]
- Wu, L.X.; Xu, Y.Y.; Yang, Z.J.; Feng, Q. Hydroxytyrosol and olive leaf extract exert cardioprotective effects by inhibiting GRP78 and CHOP expression. J. Biomed. Res. 2018, 32, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, Y.; Ma, Y.; Wen, D. Hydroxytyrosol ameliorates insulin resistance by modulating endoplasmic reticulum stress and prevents hepatic steatosis in diet-induced obesity mice. J. Nutr. Biochem. 2018, 57, 180–188. [Google Scholar] [CrossRef]
- Vezza, T.; Abad-Jimenez, Z.; Marti-Cabrera, M.; Rocha, M.; Victor, V.M. Microbiota-Mitochondria Inter-Talk: A Potential Therapeutic Strategy in Obesity and Type 2 Diabetes. Antioxidants 2020, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Huang, D.; Wang, J.; Li, H.; Gao, J.; Zhong, Y.; Xia, L.; Zhang, A.; Lin, Z.; Ke, X. The role of the "gut microbiota-mitochondria" crosstalk in the pathogenesis of multiple sclerosis. Front. Microbiol. 2024, 15, 1404995. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.; Zhuge, A.; Li, L.; Ni, S. Gut microbiota modulates osteoclast glutathione synthesis and mitochondrial biogenesis in mice subjected to ovariectomy. Cell Prolif. 2022, 55, e13194. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [PubMed]
- Zhong, Z.; Zhang, Y.; Wei, Y.; Li, X.; Ren, L.; Li, Y.; Zhang, X.; Chen, C.; Yin, X.; Liu, R.; et al. Fucoidan Improves Early Stage Diabetic Nephropathy via the Gut Microbiota-Mitochondria Axis in High-Fat Diet-Induced Diabetic Mice. J. Agric. Food Chem. 2024, 72, 9755–9767. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, L.; Escobar Marcillo, D.I.; Simonelli, V.; Iorio, E.; Rinaldi, T.; Sbraccia, P.; Fortini, P.; Guglielmi, V. The Crosstalk between Gut Microbiota and White Adipose Tissue Mitochondria in Obesity. Nutrients 2023, 15, 1723. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tapia, M.; Tobon-Cornejo, S.; Noriega, L.G.; Vazquez-Manjarrez, N.; Coutino-Hernandez, D.; Granados-Portillo, O.; Roman-Calleja, B.M.; Ruiz-Margain, A.; Macias-Rodriguez, R.U.; Tovar, A.R.; et al. Hepatic Steatosis Can Be Partly Generated by the Gut Microbiota-Mitochondria Axis via 2-Oleoyl Glycerol and Reversed by a Combination of Soy Protein, Chia Oil, Curcumin and Nopal. Nutrients 2024, 16, 2594. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.N.; Theiss, A.L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 2020, 11, 285–304. [Google Scholar] [CrossRef]
- Kramer, P. Mitochondria-Microbiota Interaction in Neurodegeneration. Front. Aging Neurosci. 2021, 13, 776936. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, M.; Liu, J.; Wang, J.; Zhou, A.; Cao, Y.; Duan, S.; Wang, Q. Screening study of hydroxytyrosol metabolites from in vitro fecal fermentation and their interaction with intestinal barrier repair receptor AhR. J. Food Sci. 2024, 89, 10134–10151. [Google Scholar] [CrossRef]
- Han, H.; Zhong, R.; Zhou, Y.; Xiong, B.; Chen, L.; Jiang, Y.; Liu, L.; Sun, H.; Tan, J.; Tao, F.; et al. Hydroxytyrosol Benefits Boar Semen Quality via Improving Gut Microbiota and Blood Metabolome. Front. Nutr. 2021, 8, 815922. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Abdullah; Tian, W.; Qiu, Z.; Song, M.; Cao, Y.; Xiao, J. Hydroxytyrosol Alleviates Dextran Sulfate Sodium-Induced Colitis by Modulating Inflammatory Responses, Intestinal Barrier, and Microbiome. J. Agric. Food Chem. 2022, 70, 2241–2252. [Google Scholar] [CrossRef]
- Miao, F. Hydroxytyrosol alleviates dextran sodium sulfate-induced colitis by inhibiting NLRP3 inflammasome activation and modulating gut microbiota in vivo. Nutrition 2022, 97, 111579. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Liu, Z.; Liu, L.; Yang, K.; Wei, Y.; Liu, Y.; Chen, X.; Sun, X.; Wen, D. Hydroxytyrosol prevents PM (2.5)-induced adiposity and insulin resistance by restraining oxidative stress related NF-kappaB pathway and modulation of gut microbiota in a murine model. Free Radic. Biol. Med. 2019, 141, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, V.; Vaag, A. Genetics of diabetes-associated microvascular complications. Diabetologia 2023, 66, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Villodres, J.A.; Abdel-Karim, M.; De La Cruz, J.P.; Rodriguez-Perez, M.D.; Reyes, J.J.; Guzman-Moscoso, R.; Rodriguez-Gutierrez, G.; Fernandez-Bolanos, J.; Gonzalez-Correa, J.A. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016, 37, 94–100. [Google Scholar] [CrossRef]
- Reyes, J.J.; Villanueva, B.; Lopez-Villodres, J.A.; De La Cruz, J.P.; Romero, L.; Rodriguez-Perez, M.D.; Rodriguez-Gutierrez, G.; Fernandez-Bolanos, J.; Gonzalez-Correa, J.A. Neuroprotective Effect of Hydroxytyrosol in Experimental Diabetes Mellitus. J. Agric. Food Chem. 2017, 65, 4378–4383. [Google Scholar] [CrossRef]
- Achour, O.; Haffani, Y.Z.; Mbarek, S.; Hammami, O.; Feki, M.; Zemmel, A.; Picaud, S.; Boudhrioua, N.; Chaouacha-Chekir, R.B. Hydroxytyrosol-Rich Olive Mill Wastewater, a Potential Protector Against Dyslipidemia, Diabetes, and Diabetic Retinopathy in Psammomys obesus. Chem. Biodivers. 2025, e202401351. [Google Scholar] [CrossRef]
- Zeljkovic, A.; Vekic, J.; Stefanovic, A. Obesity and dyslipidemia in early life: Impact on cardiometabolic risk. Metabolism 2024, 156, 155919. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Hadrich, F.; Feki, I.; Ghorbel, H.; Bouallagui, Z.; Marrekchi, R.; Fourati, H.; Sayadi, S. Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018, 9, 3220–3234. [Google Scholar] [CrossRef]
- Liu, X.; Gao, J.; Yan, Y.; Georgiou, E.A.; Lou, J.; Feng, M.; Zhang, X.; Gao, F.; Liu, J.; Kostakis, I.K.; et al. Mitochondria-Targeted Triphenylphosphonium-Hydroxytyrosol Prevents Lipotoxicity-Induced Endothelial Injury by Enhancing Mitochondrial Function and Redox Balance via Promoting FoxO1 and Nrf2 Nuclear Translocation and Suppressing Inflammation via Inhibiting p38/NF-small ka, CyrillicB Pathway. Antioxidants 2023, 12, 175. [Google Scholar] [CrossRef]
- Liu, S.; Lu, Y.; Tian, D.; Zhang, T.; Zhang, C.; Hu, C.Y.; Chen, P.; Meng, Y. Hydroxytyrosol Alleviates Obesity-Induced Cognitive Decline by Modulating the Expression Levels of Brain-Derived Neurotrophic Factors and Inflammatory Factors in Mice. J. Agric. Food Chem. 2024, 72, 6250–6264. [Google Scholar] [CrossRef]
- Dhuli, K.; Ceccarini, M.R.; Precone, V.; Maltese, P.E.; Bonetti, G.; Paolacci, S.; Dautaj, A.; Guerri, G.; Marceddu, G.; Beccari, T.; et al. Improvement of quality of life by intake of hydroxytyrosol in patients with lymphedema and association of lymphedema genes with obesity. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 33–42. [Google Scholar] [CrossRef]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol- and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. Biomed. Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Soto-Alarcon, S.A.; Orellana, P.; Espinosa, A.; Campos, C.; Lopez-Arana, S.; Rincon, M.A.; Illesca, P.; Valenzuela, R.; Videla, L.A. Suppression of high-fat diet-induced obesity-associated liver mitochondrial dysfunction by docosahexaenoic acid and hydroxytyrosol co-administration. Dig. Liver Dis. 2020, 52, 895–904. [Google Scholar] [CrossRef]
- Gori, M.; Giannitelli, S.M.; Zancla, A.; Mozetic, P.; Trombetta, M.; Merendino, N.; Rainer, A. Quercetin and hydroxytyrosol as modulators of hepatic steatosis: A NAFLD-on-a-chip study. Biotechnol. Bioeng. 2021, 118, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, F.; Valenzuela, R.; Bustamante, A.; Alvarez, D.; Ortiz, M.; Espinosa, A.; Illesca, P.; Gonzalez-Manan, D.; Videla, L.A. High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism: Attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration. Food Funct. 2019, 10, 6170–6183. [Google Scholar] [CrossRef]
- Lopez de Las Hazas, M.C.; Del Saz-Lara, A.; Cedo, L.; Crespo, M.C.; Tome-Carneiro, J.; Chapado, L.A.; Macia, A.; Visioli, F.; Escola-Gil, J.C.; Davalos, A. Hydroxytyrosol Induces Dyslipidemia in an ApoB100 Humanized Mouse Model. Mol. Nutr. Food Res. 2024, 68, e2300508. [Google Scholar] [CrossRef]
- Menichini, D.; Alrais, M.; Liu, C.; Xia, Y.; Blackwell, S.C.; Facchinetti, F.; Sibai, B.M.; Longo, M. Maternal Supplementation of Inositols, Fucoxanthin, and Hydroxytyrosol in Pregnant Murine Models of Hypertension. Am. J. Hypertens. 2020, 33, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudi, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.M.; Farras, M.; Suarez, M.; Fernandez-Castillejo, S.; Fito, M.; Konstantinidou, V.; Fuentes, F.; Lopez-Miranda, J.; Giralt, M.; Covas, M.I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef]
- Iakovis, N.; Ikonomidis, I.; Andreadou, I.; Xanthopoulos, A.; Chamaidi, A.; Chrysakis, N.; Giamouzis, G.; Skoularigis, J.; Tseti, I.; Triposkiadis, F. The Short-Term Effect of Olive Oil Extract Enriched with Hydroxytyrosol on Cardiovascular Function. J. Med. Food 2023, 26, 939–942. [Google Scholar] [CrossRef]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.B.L.; Heilig, R.; Kessler, B.M.; Pinto-Fernandez, A. Activity-Based Protein Profiling (ABPP) for Cellular Deubiquitinase (DUB) and Inhibitor Profiling at Deep and High-Throughput Levels. Methods Mol. Biol. 2023, 2591, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Li, L.; Liao, M.; Liu, D.; Rehman, A.; Liu, Y.; Liu, Z.P.; Tu, P.F.; Zeng, K.W. Thermal Proteome Profiling Strategy Identifies CNPY3 as a Cellular Target of Gambogic Acid for Inducing Prostate Cancer Pyroptosis. J. Med. Chem. 2024, 67, 10005–10011. [Google Scholar] [CrossRef]
- Tollervey, F.; Zhang, X.; Bose, M.; Sachweh, J.; Woodruff, J.B.; Franzmann, T.M.; Mahamid, J. Cryo-Electron Tomography of Reconstituted Biomolecular Condensates. Methods Mol. Biol. 2023, 2563, 297–324. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xing, W.; Shi, X.; Zhang, T.; Lou, H.; Fan, P. Antitumor and toxicity study of mitochondria-targeted triptolide derivatives using triphenylphosphine (TPP(+)) as a carrier. Bioorg. Med. Chem. 2021, 50, 116466. [Google Scholar] [CrossRef]
- Yue, C.; Yang, Y.; Song, J.; Alfranca, G.; Zhang, C.; Zhang, Q.; Yin, T.; Pan, F.; de la Fuente, J.M.; Cui, D. Mitochondria-targeting near-infrared light-triggered thermosensitive liposomes for localized photothermal and photodynamic ablation of tumors combined with chemotherapy. Nanoscale 2017, 9, 11103–11118. [Google Scholar] [CrossRef]
- Qin, C.; Hu, S.; Zhang, S.; Zhao, D.; Wang, Y.; Li, H.; Peng, Y.; Shi, L.; Xu, X.; Wang, C.; et al. Hydroxytyrosol Acetate Improves the Cognitive Function of APP/PS1 Transgenic Mice in ERbeta-dependent Manner. Mol. Nutr. Food Res. 2021, 65, e2000797. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Wang, Y.; Zhang, Y.; Wang, Z.; Xu, X.; Zhang, T.; Zhang, T.; Zhang, S.; Hu, R.; et al. Sleep Deprivation Triggers Mitochondrial DNA Release in Microglia to Induce Neural Inflammation: Preventative Effect of Hydroxytyrosol Butyrate. Antioxidants 2024, 13, 833. [Google Scholar] [CrossRef]
- Shi, L.; Gao, P.; Zhang, Y.; Liu, Q.; Hu, R.; Zhao, Z.; Hu, Y.; Xu, X.; Shen, Y.; Liu, J.; et al. 2-(3,4-Dihydroxyphenyl)ethyl 3-hydroxybutanoate Ameliorates Cognitive Dysfunction and Inflammation Via Modulating Gut Microbiota in Aged Senescence-Accelerated Mouse Prone8 Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae220. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Feng, Z.; Shi, W.; Qu, L.; Li, Y.; Liu, J.; Long, J. Reloading functionally ameliorates disuse-induced muscle atrophy by reversing mitochondrial dysfunction, and similar benefits are gained by administering a combination of mitochondrial nutrients. Free Radic. Biol. Med. 2014, 69, 116–128. [Google Scholar] [CrossRef]
- Laviano, H.D.; Gomez, G.; Escudero, R.; Nunez, Y.; Garcia-Casco, J.M.; Munoz, M.; Heras-Molina, A.; Lopez-Bote, C.; Gonzalez-Bulnes, A.; Ovilo, C.; et al. Maternal Supplementation of Vitamin E or Its Combination with Hydroxytyrosol Increases the Gut Health and Short Chain Fatty Acids of Piglets at Weaning. Antioxidants 2023, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Panera, N.; Braghini, M.R.; Crudele, A.; Smeriglio, A.; Bianchi, M.; Condorelli, A.G.; Nobili, R.; Conti, L.A.; De Stefanis, C.; Lioci, G.; et al. Combination Treatment with Hydroxytyrosol and Vitamin E Improves NAFLD-Related Fibrosis. Nutrients 2022, 14, 3791. [Google Scholar] [CrossRef] [PubMed]

| Model | Experimental Outcome | Ref. | |
|---|---|---|---|
| Antioxidant | MPP+-Induced Striatal Lipid Peroxidation in Rats model | HT increased GSH activity and GSH/GSSG ratio | [37] |
| Anti-viral | Peripheral blood mononuclear cells isolated from healthy blood donors | Hydroxytyrosol inhibited HIV-1 infections in cell | [38] |
| Antibacterial | Spectrum Beta-Lactamases | HT disrupted bacterial enzymes crucial for maintaining cell integrity and DNA replication | [39] |
| Anti-cancer | breast cancer cell lines (MDA-MB-231, MDA-MB-468, and SUM159) colorectal cancer cell line (Caco-2) | HT modulated intracellular copper levels inhibited tumor progression HT increased Caco-2 cell DNA methylation, decreased EDNRA expression | [40] [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wei, H.; Sun, Z.; Li, W.; Long, J.; Liu, J.; Feng, Z.; Cao, K. Hydroxytyrosol as a Mitochondrial Homeostasis Regulator: Implications in Metabolic Syndrome and Related Diseases. Antioxidants 2025, 14, 398. https://doi.org/10.3390/antiox14040398
Xu J, Wei H, Sun Z, Li W, Long J, Liu J, Feng Z, Cao K. Hydroxytyrosol as a Mitochondrial Homeostasis Regulator: Implications in Metabolic Syndrome and Related Diseases. Antioxidants. 2025; 14(4):398. https://doi.org/10.3390/antiox14040398
Chicago/Turabian StyleXu, Jie, Huanglong Wei, Zhenyu Sun, Wankang Li, Jiangang Long, Jiankang Liu, Zhihui Feng, and Ke Cao. 2025. "Hydroxytyrosol as a Mitochondrial Homeostasis Regulator: Implications in Metabolic Syndrome and Related Diseases" Antioxidants 14, no. 4: 398. https://doi.org/10.3390/antiox14040398
APA StyleXu, J., Wei, H., Sun, Z., Li, W., Long, J., Liu, J., Feng, Z., & Cao, K. (2025). Hydroxytyrosol as a Mitochondrial Homeostasis Regulator: Implications in Metabolic Syndrome and Related Diseases. Antioxidants, 14(4), 398. https://doi.org/10.3390/antiox14040398








