Effects of Lactic Acid and Glyceryl Lactate on Growth Performance, Antioxidant Capacity, and Intestinal Health of Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Experimental Animals and Experimental Design
2.3. Growth Performance
2.4. Sampling
2.5. Digestive Enzyme Activities of Jejunum Digesta
2.6. Intestinal Morphology
2.7. Expression Level of Jejunum Gene in Piglets
2.8. Determination of Antioxidant Indexes in Jejunum
2.9. Gut Microbes
2.10. SCFAs
2.11. Statistical Analysis
3. Results
3.1. Effects of Lactic Acid and Glyceryl Lactate on Growth Performance of Piglets
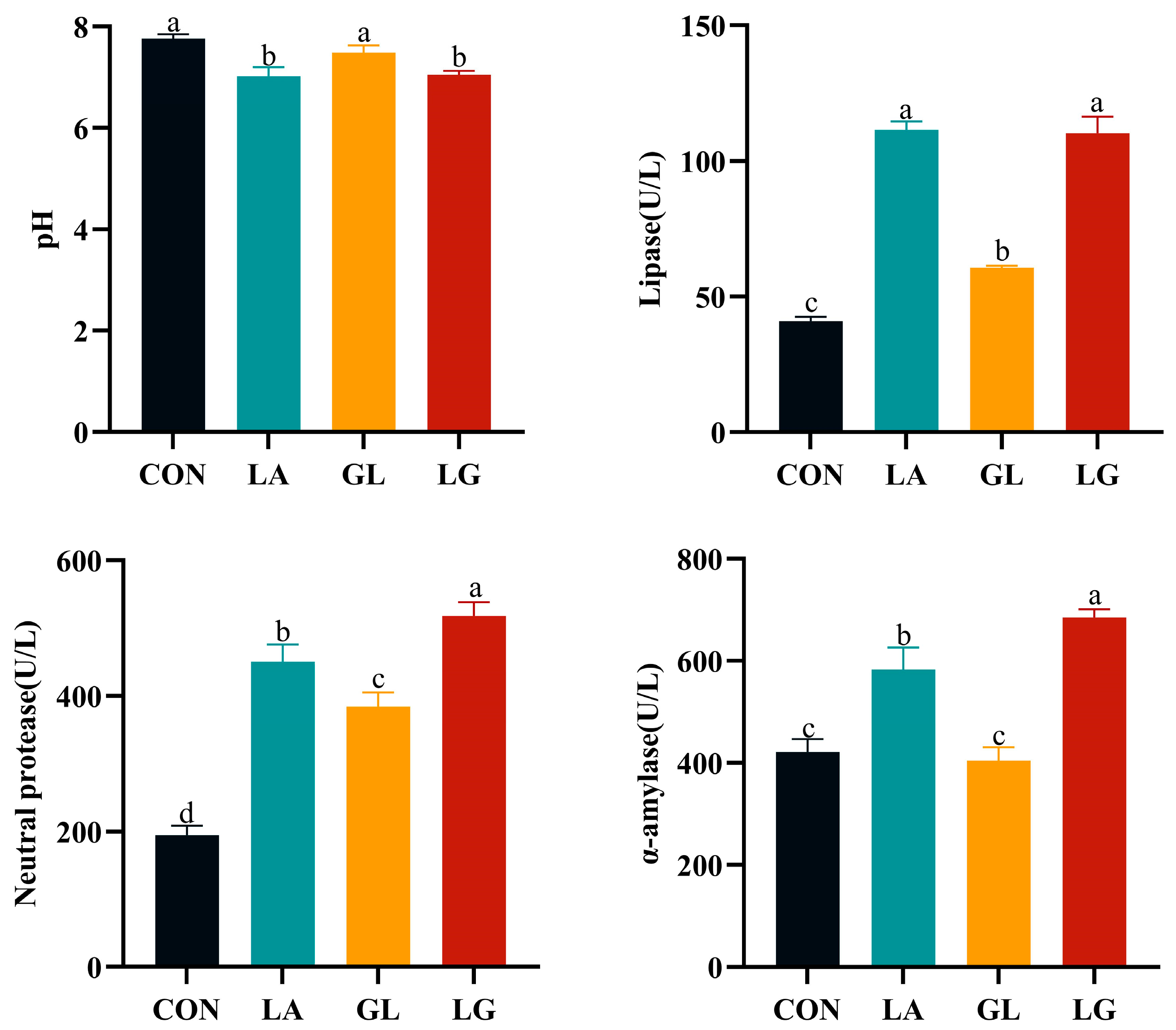
3.2. Effects of Lactic Acid and Glyceryl Lactate on pH and Digestive Enzyme Activities of Digesta of Piglets
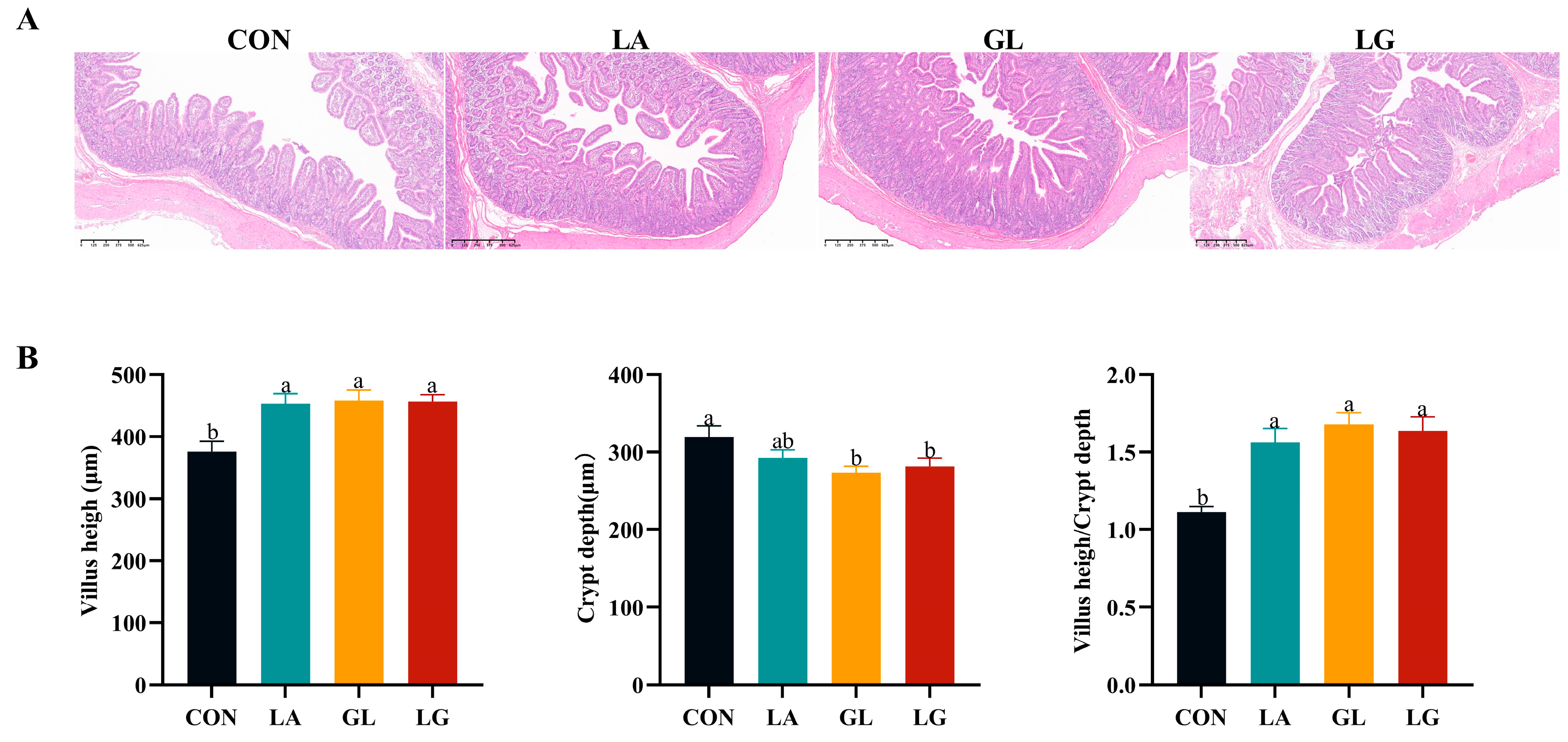
3.3. Effects of Lactic Acid and Glyceryl Lactate Jejunum Morphology of Piglets
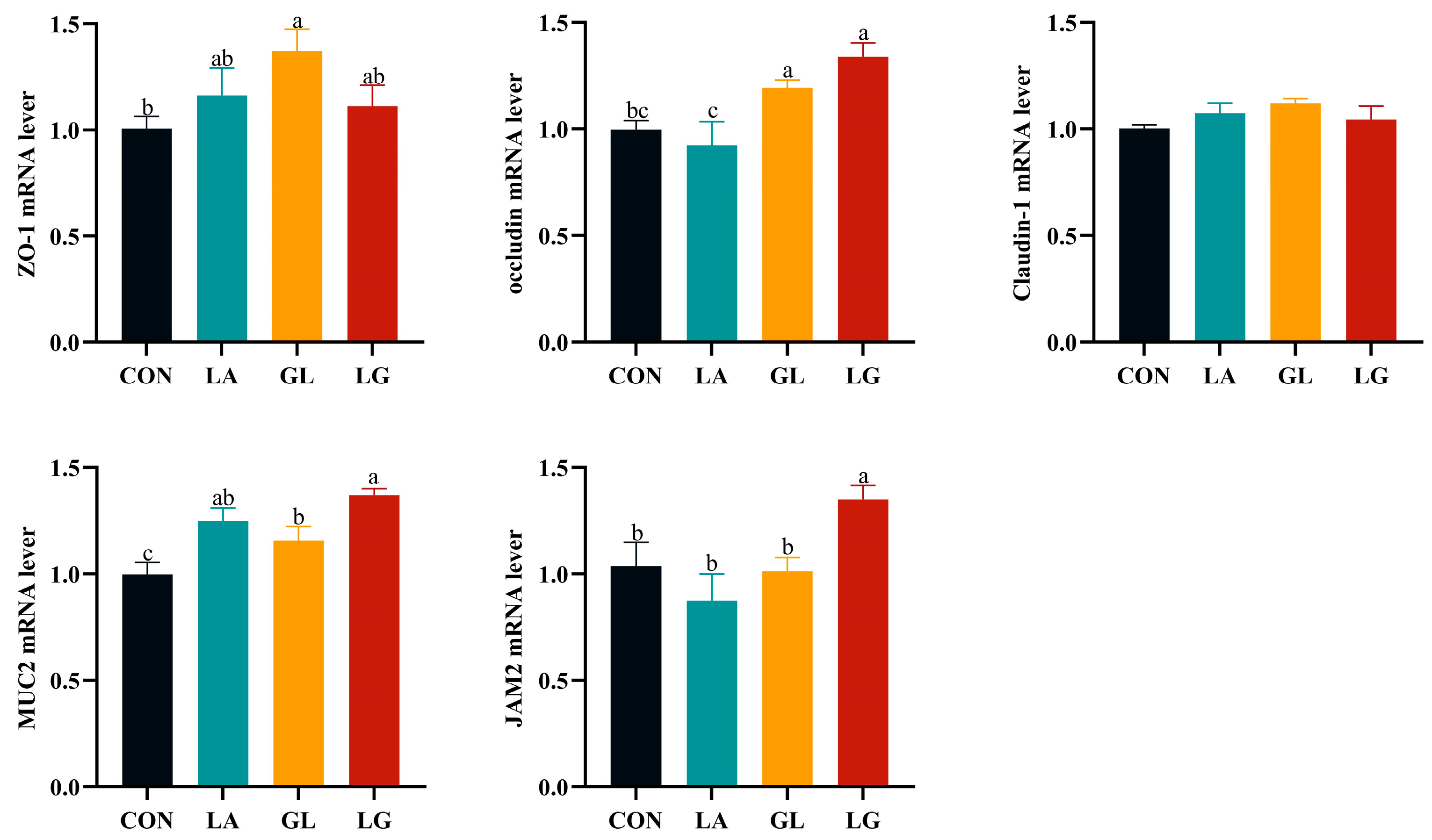
3.4. Effect of Lactic Acid and Glyceryl Lactate on Jejunal Tight Junction Proteins and Mucins in Piglets
3.5. Effects of Lactic Acid and Glyceryl Lactate Jejunum Antioxidant Markers in Piglets
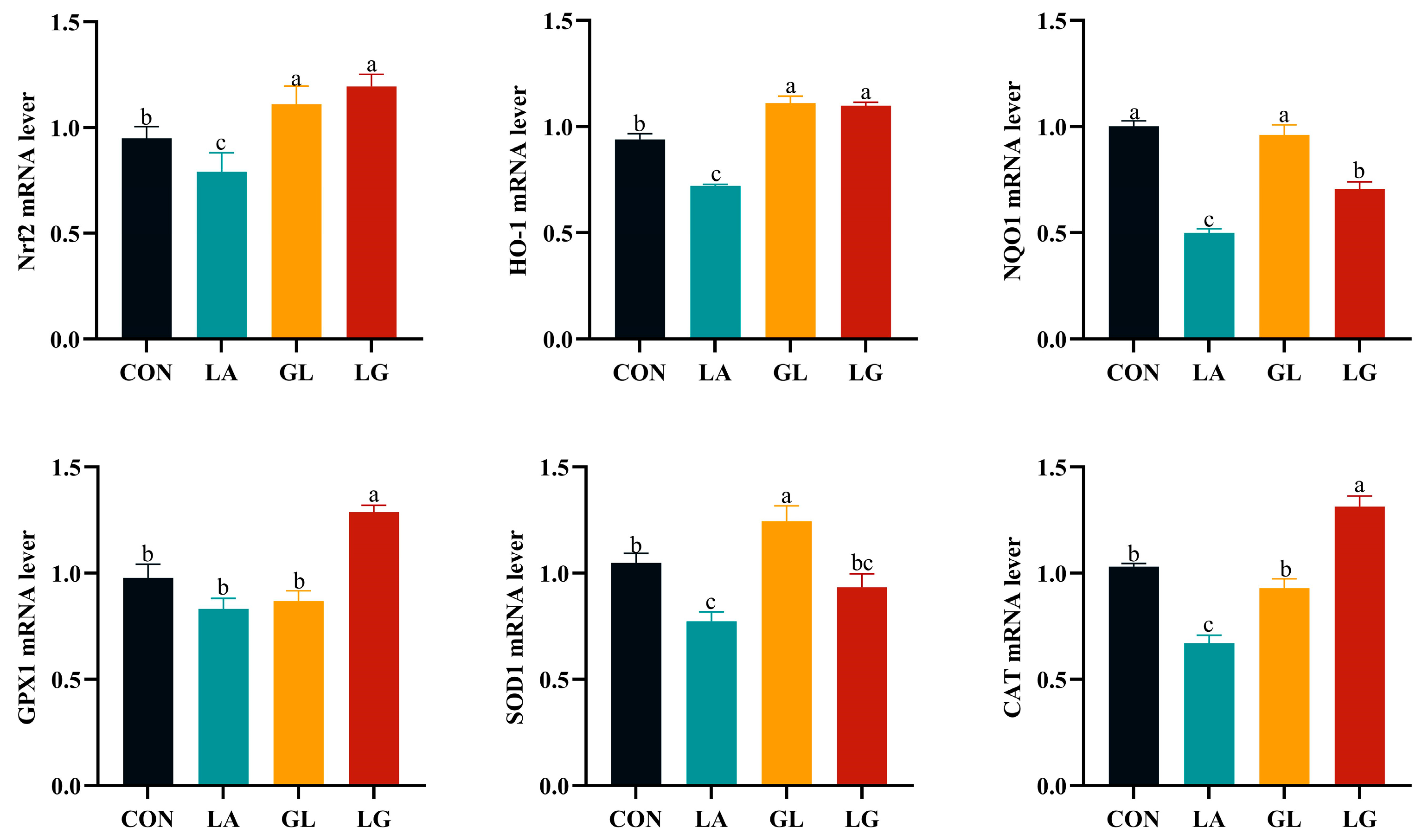
3.6. Effects of Lactic Acid and Glyceryl Lactate on mRNA Relative Expression of Nrf2 Signaling Pathway in Jejunum of Piglets
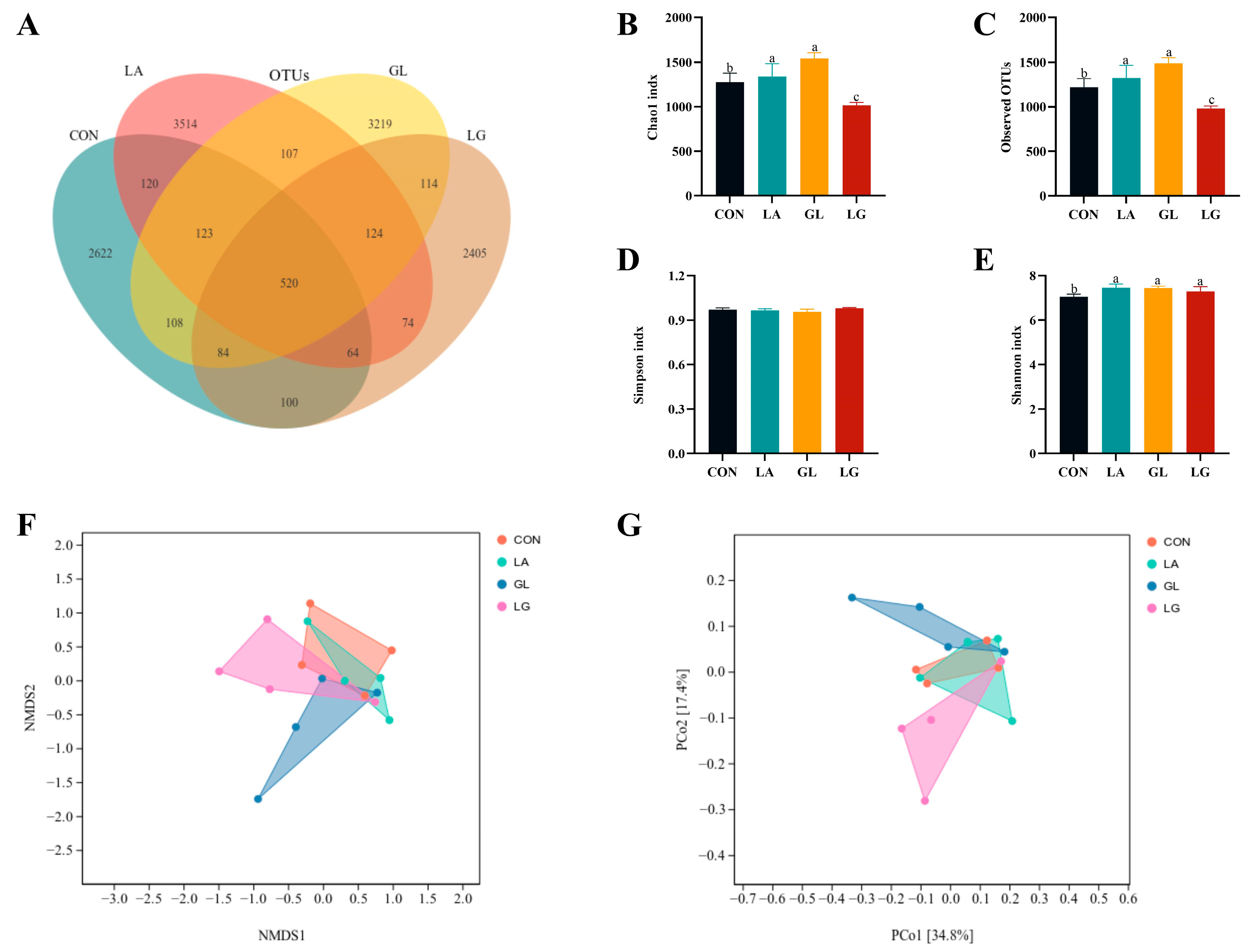
3.7. Effects of Lactic Acid and Glyceryl Lactate on α and β Diversity of Cecum Microorganisms in Piglets
3.8. Effects of Lactic Acid and Glyceryl Lactate on Phylum Level and Genus Level in Cecum of Piglets
3.9. Effects of Lactic Acid and Glyceryl Lactate on the Concentration of SCFAs in the Cecum of Piglets
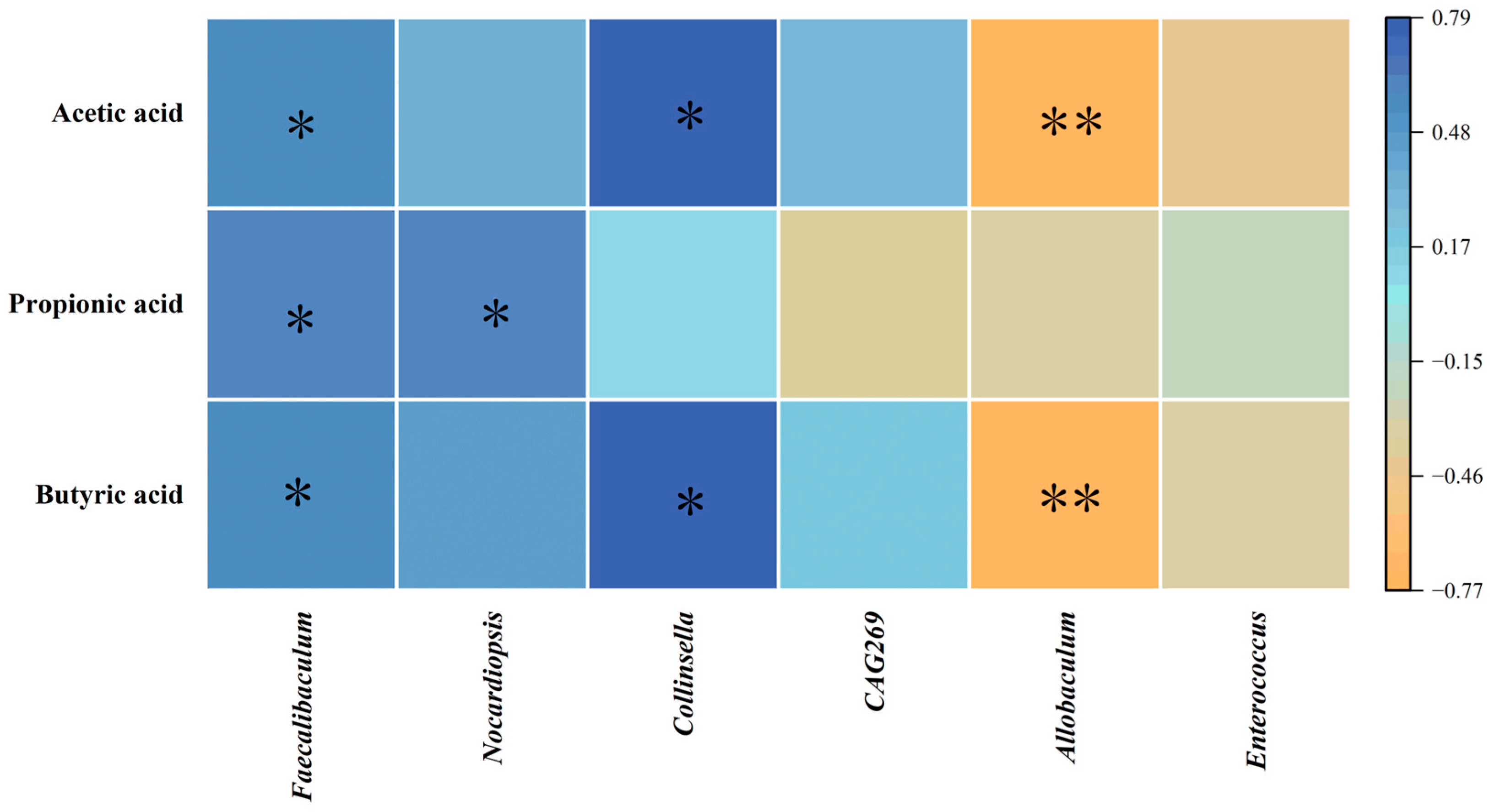
3.10. Correlation Analysis Between Differential Bacteria and SCFAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.L.; Blackwood, A.C.; Dale, D.G. Enteritis of Early Weaned Pigs: I. Enteropathogenic Escherichia coli. Can. J. Comp. Med. Vet. Sci. 1964, 28, 239–247. [Google Scholar]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Hwang, J.A.; Hoon, J.; Mun, H.S.; Yang, C.J. Comparison of single and blend acidifiers as alternative to antibiotics on growth performance, fecal microflora, and humoral immunity in weaned piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 93–100. [Google Scholar] [CrossRef]
- Wang, H.; Long, W.; Chadwick, D.; Zhang, X.; Zhang, S.; Piao, X.; Hou, Y. Dietary acidifiers as an alternative to antibiotics for promoting pig growth performance: A systematic review and meta-analysis. Anim. Feed. Sci. Technol. 2022, 289, 115320. [Google Scholar] [CrossRef]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From Acidifiers to Intestinal Health Enhancers: How Organic Acids Can Improve Growth Efficiency of Pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef]
- Xiang, X.D.; Deng, Z.C.; Wang, Y.W.; Sun, H.; Wang, L.; Han, Y.M.; Wu, Y.Y.; Liu, J.G.; Sun, L.H. Organic Acids Improve Growth Performance with Potential Regulation of Redox Homeostasis, Immunity, and Microflora in Intestines of Weaned Piglets. Antioxidants 2021, 10, 1665. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Xie, J.; Wang, C.; Yi, D.; Wu, T.; Wang, L.; Zhao, D.; Hou, Y. Dietary Supplementation with Mono-Lactate Glyceride Enhances Intestinal Function of Weaned Piglets. Animals 2023, 13, 1303. [Google Scholar] [CrossRef]
- Wu, T.; Li, K.; Lyu, Y.; Yi, D.; Zhao, D.; Wang, L.; Ding, B.; Hou, Y.; Wu, G. Trilactic glyceride regulates lipid metabolism and improves gut function in piglets. Front. Biosci. Landmark 2020, 25, 1324–1336. [Google Scholar] [CrossRef]
- National Research Council; Division on Earth, Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Swine. Nutrient Requirements of Swine; The National Academic Press: Washington, DC, USA, 2012. [Google Scholar]
- ISO9831:1998. Available online: https://www.iso.org/standard/17702.html (accessed on 23 March 2025).
- GB/T6432-2018. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=9523E30B3D6AA9362B2BD5CAD8A0633F (accessed on 23 March 2025).
- GB/T13885-2017. Available online: https://openstd.samr.gov.cn/bzgk/gb/std_list?p.p1=0&p.p90=circulation_date&p.p91=desc&p.p2=GB/T%2013885-2017 (accessed on 23 March 2025).
- GB/T18246-2019. Available online: https://openstd.samr.gov.cn/bzgk/gb/std_list?p.p1=0&p.p90=circulation_date&p.p91=desc&p.p2=GB/T%2018246-2019 (accessed on 23 March 2025).
- Gong, L.; Mahmood, T.; Mercier, Y.; Xu, H.; Zhang, X.; Zhao, Y.; Luo, Y.; Guo, Y. Dietary methionine sources and levels modulate the intestinal health status of broiler chickens. Anim. Nutr. 2023, 15, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, T.; Kang, J.; Li, Y.; Li, Y.; Xi, L. Effects of Weaning Modes on the Intestinal pH, Activity of Digestive Enzymes, and Intestinal Morphology of Piglets. Animals 2022, 12, 2200. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Sun, J.; Zhao, L.; Li, Y.; Zhang, Z.; Zhu, W.; Zhang, Y.; Xu, F.; Xing, S.; Zhao, X.; et al. Effects of BBIBP-CorV vaccine on gut microbiota and short-chain fatty acids in mice exposed to bis (2-ethylhexyl) phthalate and dioctyl terephthalate. Environ. Int. 2024, 190, 108851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Li, S.; Wang, X.F.; Xing, T.; Li, J.L.; Zhu, X.D.; Zhang, L.; Gao, F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 2021, 100, 273–282. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, H.; Niu, J.; Chen, X.; Li, H.; Rao, Z.; Guo, Y.; Zhang, W.; Wang, Z. Curcumin alleviates zearalenone-induced liver injury in mice by scavenging reactive oxygen species and inhibiting mitochondrial apoptosis pathway. Ecotoxicol. Environ. Saf. 2024, 277, 116343. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, Z.; Deng, D.; Wang, B.; Zhou, X.; Zhou, B.; Wang, C.; Zeng, Y. Effects of taurine on the growth performance, diarrhea, oxidative stress and intestinal barrier function of weanling piglets. Front. Vet. Sci. 2024, 11, 1436282. [Google Scholar] [CrossRef]
- Xu, Q.-L.; Liu, C.; Mo, X.-J.; Chen, M.; Zhao, X.-L.; Liu, M.-Z.; Wang, S.-B.; Zhou, B.; Zhao, C.-X. Drinking Water Supplemented with Acidifiers Improves the Growth Performance of Weaned Pigs and Potentially Regulates Antioxidant Capacity, Immunity, and Gastrointestinal Microbiota Diversity. Antioxidants 2022, 11, 809. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Pan, L.; Zhou, P.; Yu, R.; Zhang, Z.; Lv, J.; Guo, H.; Wang, Y.; Xiao, S.; et al. Nicotinamide Efficiently Suppresses Porcine Epidemic Diarrhea Virus and Porcine Deltacoronavirus Replication. Viruses 2023, 15, 1591. [Google Scholar] [CrossRef]
- Walsh, M.C.; Sholly, D.M.; Hinson, R.B.; Saddoris, K.L.; Sutton, A.L.; Radcliffe, J.S.; Odgaard, R.; Murphy, J.; Richert, B.T. Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding. J. Anim. Sci. 2007, 85, 1799–1808. [Google Scholar] [CrossRef]
- Qiu, K.; Chen, Z.; Zheng, A.; Chang, W.; Cai, H.; Zhang, X.; Liu, G. Augmentation of Performance, Carcass Trait, Biochemical Profile and Lipid Metabolism Concerning the Use of Organic Acidifier in Broiler Chickens. Agriculture 2023, 13, 1765. [Google Scholar] [CrossRef]
- Aliverdi-Nasab, K.; Zhandi, M.; Yousefi, A.R.; Zahedi, V.; Rafieian-Naeini, H.R. The effect of acidifier supplementation on egg production performance and intestinal histology of Japanese quail (Coturnix japonica). Vet. Med. Sci. 2023, 9, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Huting, A.M.S.; Middelkoop, A.; Guan, X.; Molist, F. Using Nutritional Strategies to Shape the Gastro-Intestinal Tracts of Suckling and Weaned Piglets. Animals 2021, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Yousefi, M.; Afzali-Kordmahalleh, A.; Pagheh, E.; Taheri Mirghaed, A. Effects of Dietary Lactic Acid Supplementation on the Activity of Digestive and Antioxidant Enzymes, Gene Expressions, and Bacterial Communities in the Intestine of Common Carp, Cyprinus carpio. Animals 2023, 13, 1934. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, M.M.O.; Ali, A.M.; Blanch, A.; Kehlet, A.B.; Madekurozwa, M.C. Morphological Responses of the Small Intestine of Broiler Chicks to Dietary Supplementation with a Probiotic, Acidifiers, and Their Combination. J. Appl. Poult. Res. 2019, 28, 108–117. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Tan, X.; Wang, L.; Xiong, X.; Wang, Y.; Wang, Q.; Yang, H.; Yin, Y. The developmental changes in intestinal epithelial cell proliferation, differentiation, and shedding in weaning piglets. Anim. Nutr. 2022, 9, 214–222. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Applications of Organic Acids in Poultry Production: An Updated and Comprehensive Review. Agriculture 2024, 14, 1756. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- de Groot, N.; Fariñas, F.; Cabrera-Gómez, C.G.; Pallares, F.J.; Ramis, G. Blend of organic acids improves gut morphology and affects inflammation response in piglets after weaning. Front. Anim. Sci. 2024, 5, 1308514. [Google Scholar] [CrossRef]
- Long, S.F.; Xu, Y.T.; Pan, L.; Wang, Q.Q.; Wang, C.L.; Wu, J.Y.; Wu, Y.Y.; Han, Y.M.; Yun, C.H.; Piao, X.S. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim. Feed. Sci. Technol. 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Ma, J.; Long, S.; Wang, J.; Gao, J.; Piao, X. Microencapsulated essential oils combined with organic acids improves immune antioxidant capacity and intestinal barrier function as well as modulates the hindgut microbial community in piglets. J. Anim. Sci. Biotechnol. 2022, 13, 16. [Google Scholar] [CrossRef]
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Orengo, J.; Hernández, F.; Martínez-Miró, S.; Sánchez, C.J.; Peres Rubio, C.; Madrid, J. Effects of Commercial Antioxidants in Feed on Growth Performance and Oxidative Stress Status of Weaned Piglets. Animals 2021, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; He, X.M.; Zhu, G.F.; Li, D.D.; Shi, J.S.; Zhang, F. Ellagic acid supports neuron by regulating astroglia Nrf2. Biotechnol. Appl. Biochem. 2019, 66, 738–743. [Google Scholar] [CrossRef]
- Wei, Y.Z.; Zhu, G.F.; Zheng, C.Q.; Li, J.J.; Sheng, S.; Li, D.D.; Wang, G.Q.; Zhang, F. Ellagic acid protects dopamine neurons from rotenone-induced neurotoxicity via activation of Nrf2 signalling. J. Cell. Mol. Med. 2020, 24, 9446–9456. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, R.; Wang, N.; Deng, Y.; Tan, B.; Yin, Y.; Qi, M.; Wang, J. Ellagic Acid Alleviates Oxidative Stress by Mediating Nrf2 Signaling Pathways and Protects against Paraquat-Induced Intestinal Injury in Piglets. Antioxidants 2022, 11, 252. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Y.; Su, Y.; Gao, Z.; Diao, X.; Li, J.; Zhu, X.; Li, D.; Li, Z.; Wang, C.; et al. Dietary Fiber Ameliorates Lipopolysaccharide-Induced Intestinal Barrier Function Damage in Piglets by Modulation of Intestinal Microbiome. mSystems 2021, 6, e01374-20. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- Hirayama, M.; Nishiwaki, H.; Hamaguchi, T.; Ito, M.; Ueyama, J.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ohno, K. Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS ONE 2021, 16, e0260451. [Google Scholar] [CrossRef]
- Vijay, A.; Kouraki, A.; Gohir, S.; Turnbull, J.; Kelly, A.; Chapman, V.; Barrett, D.A.; Bulsiewicz, W.J.; Valdes, A.M. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes 2021, 13, 1997559. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, W.F.; Deane, S.M.; Dicks, L.M.T. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes 2020, 12, 1831339. [Google Scholar] [CrossRef] [PubMed]
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species: Incidence, ecological roles and adaptations. Microbiol. Res. 2015, 174, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wang, L.; Le, S.; Yang, Y.; Zhao, C.; Zhang, X.; Yang, X.; Xu, T.; Xu, L.; Wiklund, P.; et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 2022, 13, 2555. [Google Scholar] [CrossRef]

| Contents | CON | LA | GL | LG | SEM | p-Value |
|---|---|---|---|---|---|---|
| Initial weight, kg/piglet | 7.99 | 7.89 | 7.92 | 7.89 | 0.03 | 0.794 |
| Final weight, kg/piglet | 19.41 c | 19.93 bc | 21.30 ab | 21.89 a | 0.32 | 0.010 |
| ADG, kg/d | 0.41 c | 0.43 bc | 0.48 ab | 0.50 a | 0.01 | 0.023 |
| ADFI, kg/d | 0.69 | 0.68 | 0.74 | 0.75 | 0.01 | 0.113 |
| F/G, kg/kg | 1.70 a | 1.59 ab | 1.57 b | 1.49 b | 0.02 | 0.020 |
| Diarrhea rate, % | 7.96 a | 3.56 b | 3.27 b | 3.29 b | 0.67 | 0.020 |
| Contents | CON | LA | GL | LG | SEM | p-Value |
|---|---|---|---|---|---|---|
| GPX, U/mL | 55.40 c | 94.48 b | 119.65 a | 136.58 a | 7.02 | 0.013 |
| CAT, U/mL | 36.39 c | 45.13 b | 54.30 a | 55.04 a | 2.03 | 0.016 |
| SOD, U/mL | 90.27 bc | 81.28 c | 95.73 b | 109.93 a | 2.87 | 0.014 |
| T-AOC, U/L | 1.15 | 1.26 | 1.38 | 1.40 | 0.03 | 0.060 |
| MDA, nmol/mL | 1.83 a | 1.65 b | 1.56 b | 1.48 b | 0.04 | 0.020 |
| GPX, U/mL | 55.40 c | 94.48 b | 119.65 a | 136.58 a | 7.02 | 0.013 |
| Contents | CON | LA | GL | LG | SEM | p-Value |
|---|---|---|---|---|---|---|
| Acetic acid, mg/g | 2.14 b | 3.04 a | 2.87 a | 2.81 a | 0.11 | 0.005 |
| Propionic acid, mg/g | 0.92 b | 1.23 a | 1.19 a | 1.08 ab | 0.04 | 0.013 |
| Butyric acid, mg/g | 0.31 c | 0.38 c | 0.53 b | 0.62 a | 0.03 | 0.001 |
| Isovaleric acid, mg/g | 0.02 | 0.04 | 0.06 | 0.1 | 0.01 | 0.098 |
| Valeric acid, mg/g | 0.12 | 0.21 | 0.29 | 0.21 | 0.03 | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Chu, H.; Peng, B.; Niu, J.; Yang, X.; Guo, Y.; Wang, Z.; Zhang, W. Effects of Lactic Acid and Glyceryl Lactate on Growth Performance, Antioxidant Capacity, and Intestinal Health of Piglets. Antioxidants 2025, 14, 391. https://doi.org/10.3390/antiox14040391
Guo S, Chu H, Peng B, Niu J, Yang X, Guo Y, Wang Z, Zhang W. Effects of Lactic Acid and Glyceryl Lactate on Growth Performance, Antioxidant Capacity, and Intestinal Health of Piglets. Antioxidants. 2025; 14(4):391. https://doi.org/10.3390/antiox14040391
Chicago/Turabian StyleGuo, Shuaiju, Huiling Chu, Bangwang Peng, Junlong Niu, Xiaopeng Yang, Yongpeng Guo, Zhixiang Wang, and Wei Zhang. 2025. "Effects of Lactic Acid and Glyceryl Lactate on Growth Performance, Antioxidant Capacity, and Intestinal Health of Piglets" Antioxidants 14, no. 4: 391. https://doi.org/10.3390/antiox14040391
APA StyleGuo, S., Chu, H., Peng, B., Niu, J., Yang, X., Guo, Y., Wang, Z., & Zhang, W. (2025). Effects of Lactic Acid and Glyceryl Lactate on Growth Performance, Antioxidant Capacity, and Intestinal Health of Piglets. Antioxidants, 14(4), 391. https://doi.org/10.3390/antiox14040391





