Peroxiredoxin 6 in Stress Orchestration and Disease Interplay
Abstract
1. Introduction
2. Dysregulation of PRDX6 Expression Induced by Various Stressors
2.1. In Vivo Dysregulation of PRDX6 Expression Induced by Various Stressors
2.2. In Vitro Dysregulation of PRDX6 Expression Induced by Various Stressors
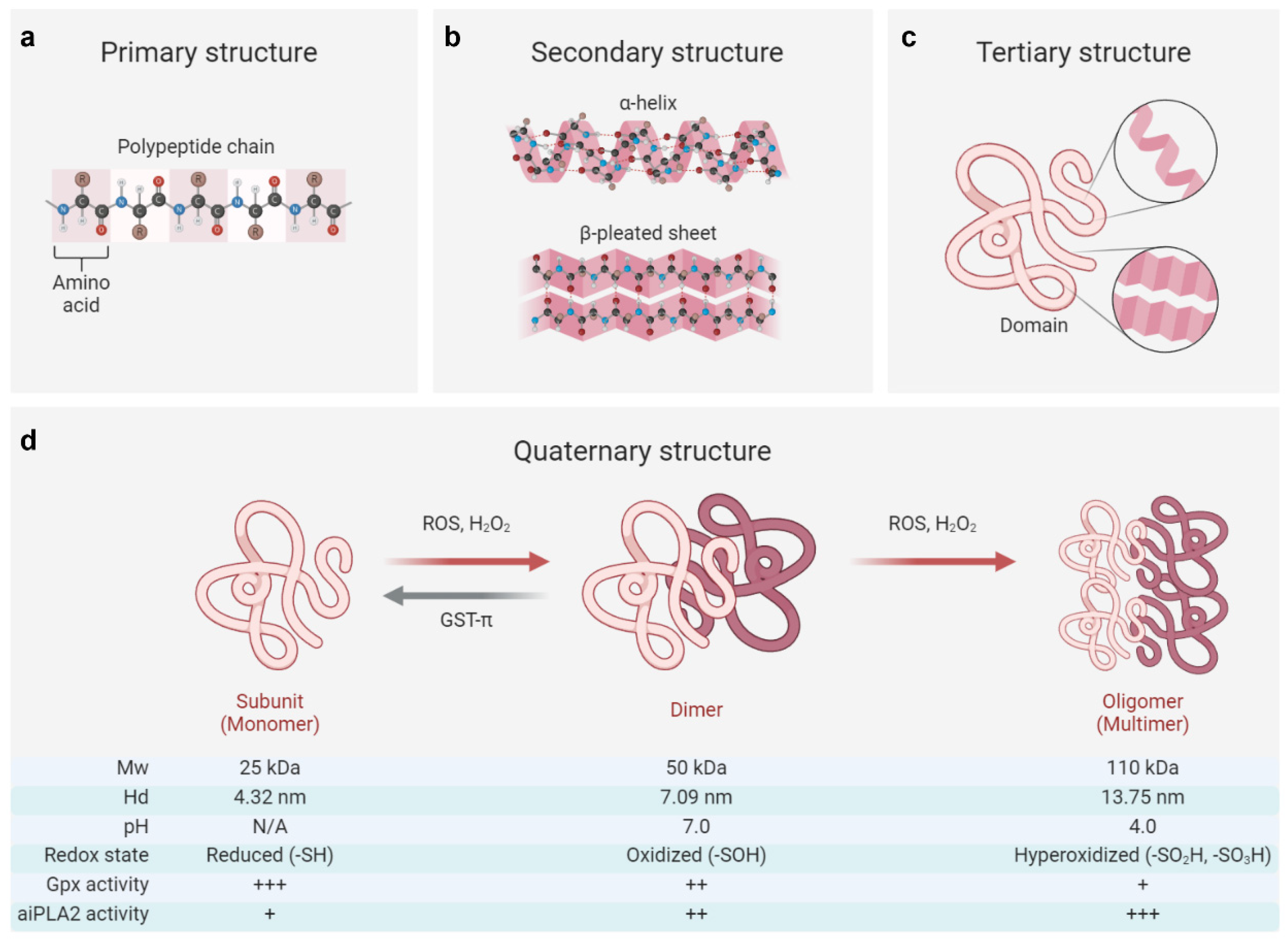
3. Conformational and Functional Switches of PRDX6 in Response to Stressors
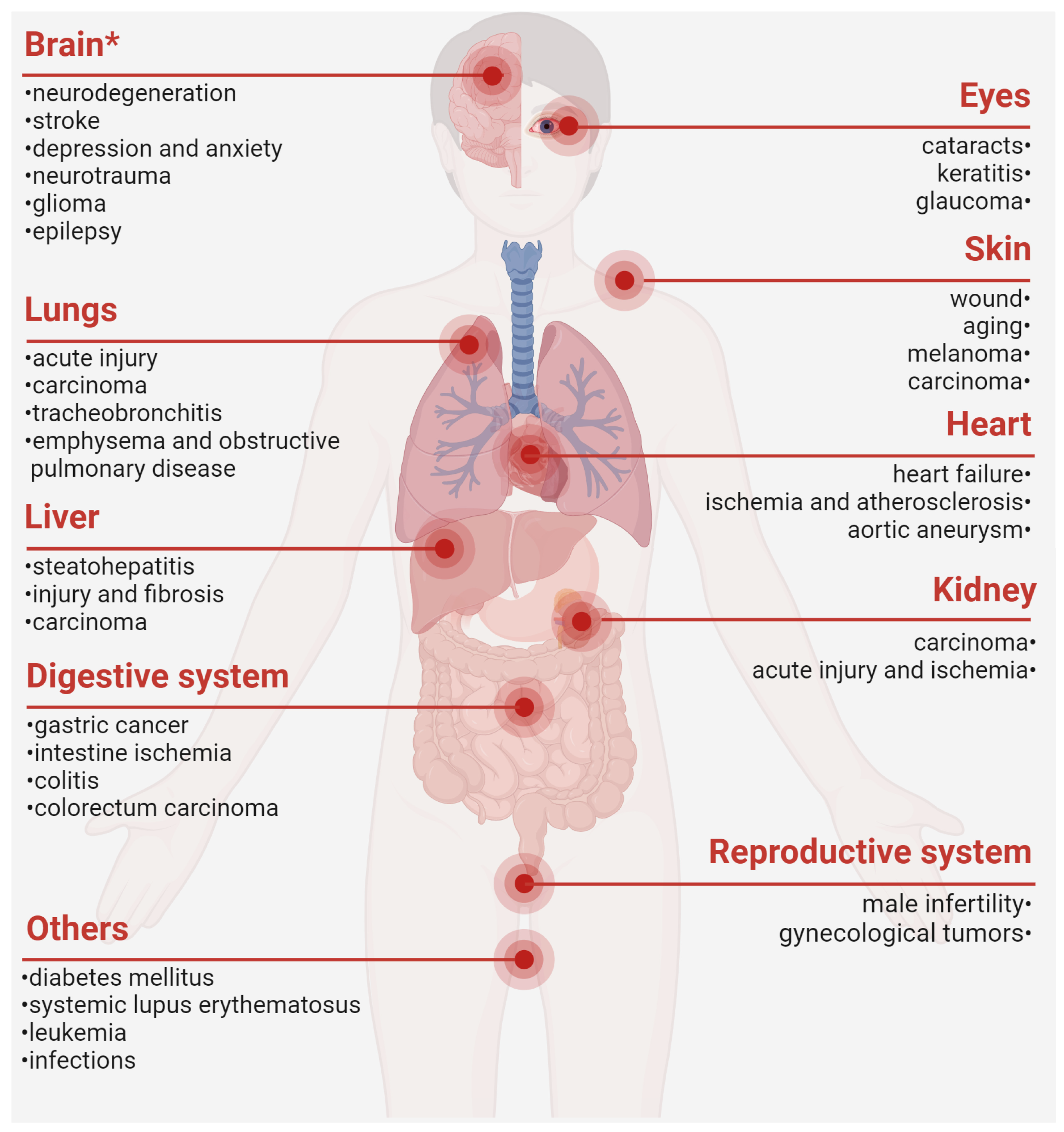
4. The Close Involvement of PRDX6 in Diverse Pathological Conditions
| Cancer Types | Expressions | Effects | Samples | Methods | References | |
|---|---|---|---|---|---|---|
| Tissue | Fluid | |||||
| Glioma | ↑ | NA | + | H | 2-DE, BA | [1,143] |
| Head and neck carcinoma | ↑ | NA | + | H | BA | [153] |
| Lung cancer | ↑ | ↑ * | + | H, M, C | BA, qPCR, WB, IHC, ELISA, IF, 2-DE | [142,144,145,146,147,148,149] |
| Esophageal carcinoma | ↑ | ↓ * | + | H, C | BA, WB, IHC, IF, 2-DE | [142,147,176,177] |
| Cervical cancer | ↑ | NA | + | H, M, C | BA, IHC, WB | [142,150] |
| Endometrial cancer | ↑ | NA | + | H, M, C | BA, WB | [142,151] |
| Ovarian cancer | ↑ | NA | + | H | BA | [142] |
| Melanoma | ↑ | NA | + | H, F, C | BA, qPCR, WB | [98,142] |
| Cholangiocarcinoma | ↑ | NA | + | H, R | IHC, IF, WB | [43] |
| Skin cancer | ↑ | NA | +/- | H, M | IHC | [152] |
| Bladder cancer | ↑ | NA | +/- | H | BA, IHC, qPCR | [142,154,155] |
| Breast carcinoma | ↑/↓ | ↓ # | + | H, M, C | 2-DE, IHC, qPCR, WB, BA | [142,156,157,158] |
| Hepatocellular carcinoma | ↑/↓ | ↑ * | +/- | H, M, C | BA, MS, qPCR, WB, IHC | [142,159,160] |
| Colorectal cancer | ↑/↓ | ↓ * | +/- | H, C | BA, IHC, ELISA | [142,162,163,164] |
| Thyroid cancer | ↑/↓ | NA | +/- | H, C | BA, qPCR, WB, IHC | [142,165] |
| Gastric cancer | ↑/↓ | NA | +/- | H, C | IHC | [166,167] |
| Prostate cancer | ↑/↓ | NA | - | H, M | BA, qPCR, IHC | [142,161] |
| Kidney cancer | ↓ | NA | + | H | BA | [142] |
5. Therapeutic Potential of Exogenous Supplementation of PRDX6
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PRDXs | peroxiredoxins |
| Cys | cysteine |
| -SH | reduced state |
| -SOH | sulfenic state |
| -SO2H | sulfinic acid |
| -SO3H | sulfonic acid |
| ATP | adenosine triphosphate |
| Gpx | glutathinone peroxidase |
| aiPLA2 | acidic calcium-independent phospholipase |
| LPCAT | lysophosphatidylcholine acyl transferase |
| SP1 | specificity protein 1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| MgSO4 | magnesium sulfate |
| CoCl2 | cobalt chloride |
| NF-κB | nuclear factor kappa B |
| MAPKs | mitogen-activated protein kinases |
| CCl4 | carbon tetrachloride |
| MANF | mesencephalic astrocyte-derived neurotrophic factor |
| PKA/C | protein kinase A/C |
| SIRT1 | sirtuin 1 |
| BMAL1 | brain and muscle ARNT-like protein 1 |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| H2O2 | hydrogen peroxide |
| IL-1β | Interleukin 1β |
| PI3K | phosphatidylinositol 3-kinase |
| Akt | protein kinase B |
| TNFα | tumor necrosis factor alpha |
| GLP-1R | glucagon-like peptide-1 receptor |
| EGFR | epidermal growth factor receptor |
| NMDAR | N-methyl-D-aspartate receptor |
| ROS | reactive oxygen species |
| PRD | PRDX6-related disorder |
| TLR | Toll-like receptor |
| TAT | HIV transactivating transduction |
| FITC | fluorescein isothiocyanate |
| I/R | ischemia/reperfusion |
| NOX1/4 | NADPH oxidase 1/4 |
References
- Liao, J.; Zhang, Y.; Chen, X.; Zhang, J. The Roles of Peroxiredoxin 6 in Brain Diseases. Mol. Neurobiol. 2021, 58, 4348–4364. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, F.; Della Morte, D.; Capuani, B.; Pastore, D.; Bellia, A.; Sbraccia, P.; Di Daniele, N.; Lauro, R.; Lauro, D. Peroxiredoxin6, a Multitask Antioxidant Enzyme Involved in the Pathophysiology of Chronic Noncommunicable Diseases. Antioxid. Redox Signal. 2019, 30, 399–414. [Google Scholar] [CrossRef]
- Chowhan, R.K.; Rahaman, H.; Singh, L.R. Structural basis of peroxidase catalytic cycle of human Prdx6. Sci. Rep. 2020, 10, 17416. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jo, H.Y.; Kim, M.H.; Cha, Y.Y.; Choi, S.W.; Shim, J.H.; Kim, T.J.; Lee, K.Y. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J. Biol. Chem. 2008, 283, 33563–33568. [Google Scholar] [CrossRef] [PubMed]
- Iakoubova, O.A.; Pacella, L.A.; Her, H.; Beier, D.R. LTW4 protein on mouse chromosome 1 is a member of a family of antioxidant proteins. Genomics 1997, 42, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Sorokina, E.M.; Li, H.; Zhou, S.; Raabe, T.; Feinstein, S.I. A novel lysophosphatidylcholine acyl transferase activity is expressed by peroxiredoxin 6. J. Lipid Res. 2016, 57, 587–596. [Google Scholar] [CrossRef]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef]
- Arevalo, J.A.; Vazquez-Medina, J.P. The Role of Peroxiredoxin 6 in Cell Signaling. Antioxidants 2018, 7, 172. [Google Scholar] [CrossRef]
- Chen, Z.; Inague, A.; Kaushal, K.; Fazeli, G.; Schilling, D.; Xavier, D.S.T.; Dos, S.A.; Cheytan, T.; Freitas, F.P.; Yildiz, U.; et al. PRDX6 contributes to selenocysteine metabolism and ferroptosis resistance. Mol. Cell 2024, 84, 4645–4659. [Google Scholar] [CrossRef]
- Wu, M.; Deng, C.; Lo, T.H.; Chan, K.Y.; Li, X.; Wong, C.M. Peroxiredoxin, Senescence, and Cancer. Cells 2022, 11, 1772. [Google Scholar] [CrossRef]
- Fatma, N.; Kubo, E.; Takamura, Y.; Ishihara, K.; Garcia, C.; Beebe, D.C.; Singh, D.P. Loss of NF-kappaB control and repression of Prdx6 gene transcription by reactive oxygen species-driven SMAD3-mediated transforming growth factor beta signaling. J. Biol. Chem. 2009, 284, 22758–22772. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Xu, L.; Huang, Y.; Chen, T.; Zhou, T.; Yang, L. Magnesium sulphate can alleviate oxidative stress and reduce inflammatory cytokines in rat placenta of intrahepatic cholestasis of pregnancy model. Arch. Gynecol. Obstet. 2018, 298, 631–638. [Google Scholar] [CrossRef]
- Pak, J.H.; Yi, J.; Ryu, S.; Kim, I.K.; Kim, J.W.; Baek, H.; Chung, J.W. Induction of Redox-Active Gene Expression by CoCl2 Ameliorates Oxidative Stress-Mediated Injury of Murine Auditory Cells. Antioxidants 2019, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Tahmasbpour, M.E.; Ghanei, M.; Panahi, Y. Oxidative stress and altered expression of peroxiredoxin genes family (PRDXS) and sulfiredoxin-1 (SRXN1) in human lung tissue following exposure to sulfur mustard. Exp. Lung Res. 2016, 42, 217–226. [Google Scholar] [CrossRef]
- Torok, S.; Almasi, N.; Veszelka, M.; Borzsei, D.; Szabo, R.; Varga, C. Protective Effects of H2S Donor Treatment in Experimental Colitis: A Focus on Antioxidants. Antioxidants 2023, 12, 1025. [Google Scholar] [CrossRef]
- Kuda, O.; Brezinova, M.; Silhavy, J.; Landa, V.; Zidek, V.; Dodia, C.; Kreuchwig, F.; Vrbacky, M.; Balas, L.; Durand, T.; et al. Nrf2-Mediated Antioxidant Defense and Peroxiredoxin 6 Are Linked to Biosynthesis of Palmitic Acid Ester of 9-Hydroxystearic Acid. Diabetes 2018, 67, 1190–1199. [Google Scholar] [CrossRef]
- Newton, B.W.; Russell, W.K.; Russell, D.H.; Ramaiah, S.K.; Jayaraman, A. Liver proteome analysis in a rodent model of alcoholic steatosis. J. Proteome Res. 2009, 8, 1663–1671. [Google Scholar] [CrossRef]
- Roede, J.R.; Stewart, B.J.; Petersen, D.R. Decreased expression of peroxiredoxin 6 in a mouse model of ethanol consumption. Free Radic. Biol. Med. 2008, 45, 1551–1558. [Google Scholar] [CrossRef]
- Tao, X.; Wang, D.; Liang, Y.; Yang, L.; He, E.; Zhou, J.; He, Y.; Liang, J.; Wang, P.; Chhetri, G.; et al. PRDX6 inhibits hepatic stellate cells activation and fibrosis via promoting MANF secretion. Biomed. Pharmacother. 2022, 156, 113931. [Google Scholar] [CrossRef]
- Luo, P.; Liu, D.; Zhang, Q.; Yang, F.; Wong, Y.K.; Xia, F.; Zhang, J.; Chen, J.; Tian, Y.; Yang, C.; et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm. Sin. B 2022, 12, 2300–2314. [Google Scholar] [CrossRef]
- Huang, S.; Cao, S.; Zhou, T.; Kong, L.; Liang, G. 4-tert-octylphenol injures motility and viability of human sperm by affecting cAMP-PKA/PKC-tyrosine phosphorylation signals. Environ. Toxicol. Pharmacol. 2018, 62, 234–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Teng, Z.; Wang, Z.; Zhu, P.; Wang, Z.; Liu, F.; Liu, X. Human umbilical cord mesenchymal stem cells (hUC-MSCs) alleviate paclitaxel-induced spermatogenesis defects and maintain male fertility. Biol. Res. 2023, 56, 47. [Google Scholar] [CrossRef]
- Falahati, A.M.; Fallahi, S.; Allamehzadeh, Z.; Izadi, R.M.; Malekzadeh, K. Effects of Date Palm Pollen Supplementations on The Expression of PRDX1 and PRDX6 Genes in Infertile Men: A Controlled Clinical Trial. Int. J. Fertil. Steril. 2023, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liao, K.; Zhang, W.; Wu, H.; Shen, B.; Xu, Z. Differential expression of peroxiredoxin 6, annexin A5 and ubiquitin carboxyl-terminal hydrolase isozyme L1 in testis of rat fetuses after maternal exposure to di-n-butyl phthalate. Reprod. Toxicol. 2013, 39, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Scarlata, E.; O’Flaherty, C. Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa. Antioxidants 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Gridley, D.S.; Freeman, T.L.; Makinde, A.Y.; Wroe, A.J.; Luo-Owen, X.; Tian, J.; Mao, X.W.; Rightnar, S.; Kennedy, A.R.; Slater, J.M.; et al. Comparison of proton and electron radiation effects on biological responses in liver, spleen and blood. Int. J. Radiat. Biol. 2011, 87, 1173–1181. [Google Scholar] [CrossRef]
- An, J.H.; Seong, J.S. Proteomics analysis of apoptosis-regulating proteins in tissues with different radiosensitivity. J. Radiat. Res. 2006, 47, 147–155. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ramezani, M.; Yazdian-Robati, R.; Behnam, B.; Razavi, A.K.; Hashem, N.A.; Mokhtarzadeh, A.; Matbou, R.M.; Razavi, B.M.; Abnous, K. Acute toxicity of functionalized single wall carbon nanotubes: A biochemical, histopathologic and proteomics approach. Chem.-Biol. Interact. 2017, 275, 196–209. [Google Scholar] [CrossRef]
- Ma, L.; Huang, M.; Sun, G.; Lin, Y.; Lu, D.; Wu, B. Puerariae lobatae radix protects against UVB-induced skin aging via antagonism of REV-ERBalpha in mice. Front. Pharmacol. 2022, 13, 1088294. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Gu, Q.; Xue, J.; Cao, H.; Tang, Y.; Xu, X.; Cao, J.; Zhou, J.; Wu, J.; et al. Protein and miRNA profiling of radiation-induced skin injury in rats: The protective role of peroxiredoxin-6 against ionizing radiation. Free Radic. Biol. Med. 2014, 69, 96–107. [Google Scholar] [CrossRef]
- Chang, C.H.; Lo, W.Y.; Lee, T.H. The Antioxidant Peroxiredoxin 6 (Prdx6) Exhibits Different Profiles in the Livers of Seawater- and Fresh Water-Acclimated Milkfish, Chanos chanos, upon Hypothermal Challenge. Front. Physiol. 2016, 7, 580. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Zanuzzo, F.S.; Xue, X.; Sandrelli, R.M.; Rise, M.L.; Gamperl, A.K. The transcriptomic responses of Atlantic salmon (Salmo salar) to high temperature stress alone, and in combination with moderate hypoxia. BMC Genom. 2021, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Wang, Z.; Chen, Y.; Li, R. Intermittent hyperbaric oxygen exposure mobilizing peroxiredoxin 6 to prevent oxygen toxicity. J. Physiol. Sci. 2019, 69, 779–790. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Moes, K.A.; Shiu, M.Y.; Hutchison, M.G.; Churchill, N.; Thomas, S.G.; Rhind, S.G. High-Intensity Interval Training Is Associated with Alterations in Blood Biomarkers Related to Brain Injury. Front. Physiol. 2018, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Popli, P.; Shukla, V.; Kaushal, J.B.; Kumar, R.; Gupta, K.; Dwivedi, A. Peroxiredoxin 6 Plays Essential Role in Mediating Fertilization and Early Embryonic Development in Rabbit Oviduct. Reprod. Sci. 2021, 29, 1560–1576. [Google Scholar] [CrossRef]
- Almasi, N.; Torok, S.; Al-Awar, A.; Veszelka, M.; Kiraly, L.; Borzsei, D.; Szabo, R.; Varga, C. Voluntary Exercise-Mediated Protection in TNBS-Induced Rat Colitis: The Involvement of NETosis and Prdx Antioxidants. Antioxidants 2023, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Limmon, G.V.; Zheng, D.; Li, N.; Li, L.; Yin, L.; Chow, V.T.; Chen, J.; Engelward, B.P. Major shifts in the spatio-temporal distribution of lung antioxidant enzymes during influenza pneumonia. PLoS ONE 2012, 7, e31494. [Google Scholar] [CrossRef]
- Sun, X.G.; Fu, X.Q.; Cai, H.B.; Liu, Q.; Li, C.H.; Liu, Y.W.; Li, Y.J.; Liu, Z.F.; Song, Y.H.; Lv, Z.P. Proteomic analysis of protective effects of polysaccharides from Salvia miltiorrhiza against immunological liver injury in mice. Phytother. Res. 2011, 25, 1087–1094. [Google Scholar] [CrossRef]
- Pak, J.H.; Son, W.C.; Seo, S.B.; Hong, S.J.; Sohn, W.M.; Na, B.K.; Kim, T.S. Peroxiredoxin 6 expression is inversely correlated with nuclear factor-kappaB activation during Clonorchis sinensis infestation. Free Radic. Biol. Med. 2016, 99, 273–285. [Google Scholar] [CrossRef]
- Liao, M.; Li, X.; Zhang, H.; Zhou, L.; Shi, L.; Li, W.; Shen, R.; Peng, G.; Zhao, H.; Shao, J.; et al. Effects and plasma proteomic analysis of GLP-1RA versus CPA/EE, in combination with metformin, on overweight PCOS women: A randomized controlled trial. Endocrine 2023, 83, 227–241. [Google Scholar] [CrossRef]
- Liu, N.; Xue, L.; Guan, Y.; Li, Q.Z.; Cao, F.Y.; Pang, S.L.; Guan, W.J. Expression of Peroxiredoxins and Pulmonary Surfactant Protein A Induced by Silica in Rat Lung Tissue. Biomed. Environ. Sci. 2016, 29, 584–588. [Google Scholar] [CrossRef]
- Bu, H.; Wang, B.; Wu, Y.; Li, P.; Cui, Y.; Jiang, X.; Yu, X.; Liu, B.; Tang, M. Curcumin strengthens a spontaneous self-protective mechanism-SP1/PRDX6 pathway, against di-n-butyl phthalate-induced testicular ferroptosis damage. Environ. Sci. Pollut. Res. 2023, 30, 122165–122181. [Google Scholar] [CrossRef]
- Li, H.; Wu, Z.; Zhong, R.; Zhang, Q.; Chen, Q.; Shen, Y. PRDX6 knockout restrains the malignant progression of intrahepatic cholangiocarcinoma. Med. Oncol. 2022, 39, 250. [Google Scholar] [CrossRef]
- Soylu, H.; Karacor, K. The effects of hydroxytyrosol on Prdx6 and insulin expression in diabetic rat pancreases. Histochem. Cell Biol. 2023, 160, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, W.; Chen, C.; Chen, X. Peroxiredoxin 6 overexpression regulates adriamycin-induced myocardial injury, oxidative stress and immune response in rats. Ann. Transl. Med. 2020, 8, 1320. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, G.; Xing, Y.; Ding, Y.; Ren, T.; Kan, J. Antioxidant activity of whole grain highland hull-less barley and its effect on liver protein expression profiles in rats fed with high-fat diets. Eur. J. Nutr. 2018, 57, 2201–2208. [Google Scholar] [CrossRef]
- Atiba, A.; Abdo, W.; Ali, E.K.; Abd-Elsalam, M.; Amer, M.; Abdel, M.A.; Taha, R.; Antar, S.; Mahmoud, A. Topical and oral applications of Aloe vera improve healing of deep second-degree burns in rats via modulation of growth factors. Biomarkers 2022, 27, 608–617. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Zhao, M.; Yu, L.; Lin, Y.; Kang, D. Ferrostatin-1 attenuates brain injury in animal model of subarachnoid hemorrhage via phospholipase A2 activity of PRDX6. Neuroreport 2023, 34, 606–616. [Google Scholar] [CrossRef]
- Ren, J.; Wu, J.; Tang, X.; Chen, S.; Wang, W.; Lv, Y.; Wu, L.; Yang, D.; Zheng, Y. Ageing- and AAA-associated differentially expressed proteins identified by proteomic analysis in mice. PeerJ 2022, 10, e13129. [Google Scholar] [CrossRef]
- Dickinson, D.; DeRossi, S.; Yu, H.; Thomas, C.; Kragor, C.; Paquin, B.; Hahn, E.; Ohno, S.; Yamamoto, T.; Hsu, S. Epigallocatechin-3-gallate modulates anti-oxidant defense enzyme expression in murine submandibular and pancreatic exocrine gland cells and human HSG cells. Autoimmunity 2014, 47, 177–184. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, J.E.; Chung, B.Y.; Lee, H.M.; Kang, H.H.; Chung, J.W.; Pak, J.H. Involvement of retinoic acid-induced peroxiredoxin 6 expression in recovery of noise-induced temporary hearing threshold shifts. Environ. Toxicol. Pharmacol. 2013, 36, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, X.Y.; Zhou, Y.Q.; Wen, X.; Zhu, L.Y. Proteomic alterations in mouse kidney induced by andrographolide sodium bisulfite. Acta Pharmacol. Sin. 2011, 32, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, S.L.; Golder, Z.J.; Johnstone, D.B.; Frankl, F. Renal peroxiredoxin 6 interacts with anion exchanger 1 and plays a novel role in pH homeostasis. Kidney Int. 2016, 89, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zi, T.; Liu, Y.; Zhang, Y.; Wang, Z.; Wang, Z.; Zhan, S.; Peng, Z.; Li, N.; Liu, X.; Liu, F. Protective effect of melatonin on alleviating early oxidative stress induced by DOX in mice spermatogenesis and sperm quality maintaining. Reprod. Biol. Endocrinol. 2022, 20, 105. [Google Scholar] [CrossRef]
- XueXia, L.; YaNan, L.; Zi, T.; YuSheng, Z.; ZeLin, W.; Peng, Z.; MeiNa, X.; FuJun, L. Di-2-ethylhexyl phthalate (DEHP) exposure induces sperm quality and functional defects in mice. Chemosphere 2022, 312 Pt 1, 137216. [Google Scholar] [CrossRef]
- Butt, U.J.; Shah, S.; Ahmed, T.; Zahid, S. Protective effects of Nigella sativa L. seed extract on lead induced neurotoxicity during development and early life in mouse models. Toxicol. Res. 2018, 7, 32–40. [Google Scholar] [CrossRef]
- Lee, H.L.; Park, M.H.; Son, D.J.; Song, H.S.; Kim, J.H.; Ko, S.C.; Song, M.J.; Lee, W.H.; Yoon, J.H.; Ham, Y.W.; et al. Anti-cancer effect of snake venom toxin through down regulation of AP-1 mediated PRDX6 expression. Oncotarget 2015, 6, 22139–22151. [Google Scholar] [CrossRef]
- Trudel, S.; Kelly, M.; Fritsch, J.; Nguyen-Khoa, T.; Therond, P.; Couturier, M.; Dadlez, M.; Debski, J.; Touqui, L.; Vallee, B.; et al. Peroxiredoxin 6 fails to limit phospholipid peroxidation in lung from Cftr-knockout mice subjected to oxidative challenge. PLoS ONE 2009, 4, e6075. [Google Scholar] [CrossRef]
- Guo, Z.; Han, C.; Du, J.; Zhao, S.; Fu, Y.; Zheng, G.; Sun, Y.; Zhang, Y.; Liu, W.; Wan, J.; et al. Proteomic study of differential protein expression in mouse lung tissues after aerosolized ricin poisoning. Int. J. Mol. Sci. 2014, 15, 7281–7292. [Google Scholar] [CrossRef]
- Jo, M.; Yun, H.M.; Park, K.R.; Park, M.H.; Lee, D.H.; Cho, S.H.; Yoo, H.S.; Lee, Y.M.; Jeong, H.S.; Kim, Y.; et al. Anti-cancer effect of thiacremonone through down regulation of peroxiredoxin 6. PLoS ONE 2014, 9, e91508. [Google Scholar] [CrossRef]
- Wang, J.L.; Lin, Y.W.; Chen, H.M.; Kong, X.; Xiong, H.; Shen, N.; Hong, J.; Fang, J.Y. Calcium prevents tumorigenesis in a mouse model of colorectal cancer. PLoS ONE 2011, 6, e22566. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Chen, D.; Huo, X.; Tian, X.; Li, J.; Liu, M.; Yu, Z.; Zhang, B.; Yang, Y.; et al. Prdx6-induced inhibition of ferroptosis in epithelial cells contributes to liquiritin-exerted alleviation of colitis. Food Funct. 2022, 13, 9470–9480. [Google Scholar] [CrossRef]
- Godoy-Lugo, J.A.; Thorwald, M.A.; Hui, D.Y.; Nishiyama, A.; Nakano, D.; Sonanez-Organis, J.G.; Ortiz, R.M. Chronic angiotensin receptor activation promotes hepatic triacylglycerol accumulation during an acute glucose challenge in obese-insulin-resistant OLETF rats. Endocrine 2021, 75, 92–107. [Google Scholar] [CrossRef]
- Paulino, L.; Barroso, P.; Silva, B.R.; Barroso, L.G.; Barbalho, E.C.; Bezerra, F.; Souza, A.; Monte, A.; Silva, A.; Matos, M.; et al. Immunolocalization of melatonin receptors in bovine ovarian follicles and in vitro effects of melatonin on growth, viability and gene expression in secondary follicles. Domest. Anim. Endocrinol. 2022, 81, 106750. [Google Scholar] [CrossRef]
- Azevedo, V.; De Assis, E.; Silva, A.; Costa, F.; Souza, L.F.; Silva, J. alpha-Pinene Improves Follicle Morphology and Increases the Expression of mRNA for Nuclear Factor Erythroid 2-Related Factor 2 and Peroxiredoxin 6 in Bovine Ovarian Tissues Cultured In Vitro. Animals 2024, 14, 1443. [Google Scholar] [CrossRef]
- Costa, F.; Vasconcelos, E.M.; Nunes, A.V.; Feitosa, M.P.L.; Soares, M.D.; Viana, S.J.; Barbalho, S.A.; Paz, S.A. Aloe vera increases mRNA expression of antioxidant enzymes in cryopreserved bovine ovarian tissue and promotes follicular growth and survival after in vitro culture. Cryobiology 2021, 102, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Caetano, F.F.; Paulino, L.; Bezerra, V.S.; Azevedo, V.; Barroso, P.; Costa, F.C.; Amorim, G.G.; Silva, J. Thymol increases primordial follicle activation, protects stromal cells, collagen fibers and down-regulates expression of mRNA for superoxide dismutase 1, catalase and periredoxin 6 in cultured bovine ovarian tissues. Anim. Reprod. Sci. 2024, 266, 107514. [Google Scholar] [CrossRef]
- Na-Phatthalung, P.; Teles, M.; Tort, L.; Oliveira, M. Gold nanoparticles exposure modulates antioxidant and innate immune gene expression in the gills of Sparus aurata. Genomics 2018, 110, 430–434. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Nakharuthai, C.; Schrama, D.; Duangkaew, R.; Rodrigues, P.M. Effects of dietary lipid sources on hepatic nutritive contents, fatty acid composition and proteome of Nile tilapia (Oreochromis niloticus). J. Proteom. 2019, 192, 208–222. [Google Scholar] [CrossRef]
- Hao, C.C.; Luo, J.N.; Xu, C.Y.; Zhao, X.Y.; Zhong, Z.B.; Hu, X.N.; Jin, X.M.; Ge, X. TRIAP1 knockdown sensitizes non-small cell lung cancer to ionizing radiation by disrupting redox homeostasis. Thorac. Cancer 2020, 11, 1015–1025. [Google Scholar] [CrossRef]
- Salovska, B.; Kondelova, A.; Pimkova, K.; Liblova, Z.; Pribyl, M.; Fabrik, I.; Bartek, J.; Vajrychova, M.; Hodny, Z. Peroxiredoxin 6 protects irradiated cells from oxidative stress and shapes their senescence-associated cytokine landscape. Redox Biol. 2021, 49, 102212. [Google Scholar] [CrossRef]
- Groiss, S.; Lammegger, R.; Brislinger, D. Anti-Oxidative and Immune Regulatory Responses of THP-1 and PBMC to Pulsed EMF Are Field-Strength Dependent. Int. J. Environ. Res. Public Health 2021, 18, 9519. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Novoselov, V.I.; Gudkov, S.V. Radioprotective Role of Peroxiredoxin 6. Antioxidants 2019, 8, 15. [Google Scholar] [CrossRef]
- Li, H.T.; Tan, F.; Zhang, T.H.; Cao, L.H.; Tan, H.Y.; Lin, W.Q.; Zeng, W.A.; Chi, X.J. Peroxiredoxin 6 mediates the protective function of curcumin pretreatment in acute lung injury induced by serum from patients undergoing one-lung ventilation in vitro. BMC Pulm. Med. 2022, 22, 192. [Google Scholar] [CrossRef]
- Chhunchha, B.; Fatma, N.; Bhargavan, B.; Kubo, E.; Kumar, A.; Singh, D.P. Specificity protein, Sp1-mediated increased expression of Prdx6 as a curcumin-induced antioxidant defense in lens epithelial cells against oxidative stress. Cell Death Dis. 2011, 2, e234. [Google Scholar] [CrossRef]
- Daverey, A.; Agrawal, S.K. Curcumin alleviates oxidative stress and mitochondrial dysfunction in astrocytes. Neuroscience 2016, 333, 92–103. [Google Scholar] [CrossRef]
- Li, L.; Yu, L.; Cao, X.; Zhang, C.; Liu, Q.; Chen, J. Comparative Analysis of Proteomic of Curcumin Reversing Multidrug Resistance in HCT-8/VCR Cells. J. Oncol. 2022, 2022, 3605436. [Google Scholar] [CrossRef]
- Chen, J.; Cao, X.; Qin, X.; Liu, H.; Chen, S.; Zhong, S.; Li, Y. Proteomic analysis of the molecular mechanism of curcumin/beta-cyclodextrin polymer inclusion complex inhibiting HepG2 cells growth. J. Food Biochem. 2020, 44, e13119. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, J.; Liu, X.; Li, Y.; Jiang, H.; Fang, W.; Long, X. Glycyrrhizin regulates antioxidation through Nrf2 signaling pathway in rat temporomandibular joint osteoarthritis. J. Oral Rehabil. 2023, 51, 611–622. [Google Scholar] [CrossRef]
- Huang, W.S.; Kuo, Y.H.; Chin, C.C.; Wang, J.Y.; Yu, H.R.; Sheen, J.M.; Tung, S.Y.; Shen, C.H.; Chen, T.C.; Sung, M.L.; et al. Proteomic analysis of the effects of baicalein on colorectal cancer cells. Proteomics 2012, 12, 810–819. [Google Scholar] [CrossRef]
- Yoo, D.R.; Jang, Y.H.; Jeon, Y.K.; Kim, J.Y.; Jeon, W.; Choi, Y.J.; Nam, M.J. Proteomic identification of anti-cancer proteins in luteolin-treated human hepatoma Huh-7 cells. Cancer Lett. 2009, 282, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Lian, W.; Bai, Y.; Wang, L.; Zhao, F.; Li, H.; Wang, D.; Pang, Q. TMT-based quantitative proteomics reveals the targets of andrographolide on LPS-induced liver injury. Bmc Vet. Res. 2023, 19, 199. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Yu, G.; Zhang, Y.; Xu, Z.; Wang, Y.Q.; Yan, G.R.; He, Q.Y. A novel andrographolide derivative AL-1 exerts its cytotoxicity on K562 cells through a ROS-dependent mechanism. Proteomics 2013, 13, 169–178. [Google Scholar] [CrossRef]
- Zha, X.; Wu, G.; Zhang, H.; Yang, Y.; Zhang, Y.; Ma, L. PRDX6 regulates the H2O2 and blue light-induced APRE-19 cell apoptosis via down-regulating and interacting with RARA. Anim. Cells Syst. 2019, 23, 241–245. [Google Scholar] [CrossRef]
- Chhunchha, B.; Singh, P.; Stamer, W.D.; Singh, D.P. Prdx6 retards senescence and restores trabecular meshwork cell health by regulating reactive oxygen species. Cell Death Discov. 2017, 3, 17060. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tzekov, R.; Su, M.; Zhu, Y.; Han, A.; Li, W. Hydrogen peroxide-induced oxidative damage and protective role of peroxiredoxin 6 protein via EGFR/ERK signaling pathway in RPE cells. Front. Aging Neurosci. 2023, 15, 1169211. [Google Scholar] [CrossRef]
- Gallagher, B.M.; Phelan, S.A. Investigating transcriptional regulation of Prdx6 in mouse liver cells. Free Radic. Biol. Med. 2007, 42, 1270–1277. [Google Scholar] [CrossRef]
- Kwon, J.; Wang, A.; Burke, D.J.; Boudreau, H.E.; Lekstrom, K.J.; Korzeniowska, A.; Sugamata, R.; Kim, Y.S.; Yi, L.; Ersoy, I.; et al. Peroxiredoxin 6 (Prdx6) supports NADPH oxidase1 (Nox1)-based superoxide generation and cell migration. Free Radic. Biol. Med. 2016, 96, 99–115. [Google Scholar] [CrossRef]
- Paula, F.M.; Ferreira, S.M.; Boschero, A.C.; Souza, K.L. Modulation of the peroxiredoxin system by cytokines in insulin-producing RINm5F cells: Down-regulation of PRDX6 increases susceptibility of beta cells to oxidative stress. Mol. Cell. Endocrinol. 2013, 374, 56–64. [Google Scholar] [CrossRef]
- Fatma, N.; Kubo, E.; Sen, M.; Agarwal, N.; Thoreson, W.B.; Camras, C.B.; Singh, D.P. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res. 2008, 1233, 63–78. [Google Scholar] [CrossRef]
- Yang, D.; Jin, M.; Bai, C.; Zhou, J.; Shen, Y. Peroxiredoxin 6 suppresses Muc5ac overproduction in LPS-induced airway inflammation through H2O2-EGFR-MAPK signaling pathway. Respir. Physiol. Neurobiol. 2017, 236, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Diet, A.; Abbas, K.; Bouton, C.; Guillon, B.; Tomasello, F.; Fourquet, S.; Toledano, M.B.; Drapier, J.C. Regulation of peroxiredoxins by nitric oxide in immunostimulated macrophages. J. Biol. Chem. 2007, 282, 36199–36205. [Google Scholar] [CrossRef]
- Yazheng, L.; Kitts, D.D. Activation of antioxidant response element (ARE)-dependent genes by roasted coffee extracts. Food Funct. 2012, 3, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.M.; Lambert, P.A.; Hummon, A.B. Comparative label-free LC-MS/MS analysis of colorectal adenocarcinoma and metastatic cells treated with 5-fluorouracil. Proteomics 2012, 12, 1928–1937. [Google Scholar] [CrossRef]
- Ribeiro, T.; Lemos, F.; Preto, M.; Azevedo, J.; Sousa, M.L.; Leao, P.N.; Campos, A.; Linder, S.; Vitorino, R.; Vasconcelos, V.; et al. Cytotoxicity of portoamides in human cancer cells and analysis of the molecular mechanisms of action. PLoS ONE 2017, 12, e0188817. [Google Scholar] [CrossRef]
- Hughes, N.P.; Xu, L.; Nielsen, C.H.; Chang, E.; Hori, S.S.; Natarajan, A.; Lee, S.; Kjaer, A.; Kani, K.; Wang, S.X.; et al. A blood biomarker for monitoring response to anti-EGFR therapy. Cancer Biomark. 2018, 22, 333–344. [Google Scholar] [CrossRef]
- Chowdhury, I.; Fisher, A.B.; Christofidou-Solomidou, M.; Gao, L.; Tao, J.Q.; Sorokina, E.M.; Lien, Y.C.; Bates, S.R.; Feinstein, S.I. Keratinocyte growth factor and glucocorticoid induction of human peroxiredoxin 6 gene expression occur by independent mechanisms that are synergistic. Antioxid. Redox Signal. 2014, 20, 391–402. [Google Scholar] [CrossRef]
- Schmitt, A.; Schmitz, W.; Hufnagel, A.; Schartl, M.; Meierjohann, S. Peroxiredoxin 6 triggers melanoma cell growth by increasing arachidonic acid-dependent lipid signalling. Biochem. J. 2015, 471, 267–279. [Google Scholar] [CrossRef]
- Junkins, K.; Rodgers, M.; Phelan, S.A. Oleuropein Induces Cytotoxicity and Peroxiredoxin Over-expression in MCF-7 Human Breast Cancer Cells. Anticancer. Res. 2023, 43, 4333–4339. [Google Scholar] [CrossRef]
- Chhunchha, B.; Singh, P.; Singh, D.P.; Kubo, E. Ginkgolic Acid Rescues Lens Epithelial Cells from Injury Caused by Redox Regulated-Aberrant Sumoylation Signaling by Reviving Prdx6 and Sp1 Expression and Activities. Int. J. Mol. Sci. 2018, 19, 3520. [Google Scholar] [CrossRef]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Sulforaphane-Induced Klf9/Prdx6 Axis Acts as a Molecular Switch to Control Redox Signaling and Determines Fate of Cells. Cells 2019, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Sinha, N.; Ranjit, S.; Midde, N.M.; Kashanchi, F.; Kumar, S. Monocyte-derived exosomes upon exposure to cigarette smoke condensate alter their characteristics and show protective effect against cytotoxicity and HIV-1 replication. Sci. Rep. 2017, 7, 16120. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Kumar, S. Chronic Effects of Ethanol and/or Darunavir/Ritonavir on U937 Monocytic Cells: Regulation of Cytochrome P450 and Antioxidant Enzymes, Oxidative Stress, and Cytotoxicity. Alcohol. Clin. Exp. Res. 2016, 40, 73–82. [Google Scholar] [CrossRef]
- Karaosmanoglu, O. P38-beta/SAPK-inhibiting and apoptosis-inducing activities of (E)-4-chloro-2-((3-ethoxy-2-hydroxybenzylidene) amino)phenol. Hum. Exp. Toxicol. 2020, 39, 1374–1389. [Google Scholar] [CrossRef]
- Yumnam, S.; Venkatarame, G.S.V.; Raha, S.; Lee, H.J.; Lee, W.S.; Kim, E.K.; Lee, S.J.; Heo, J.D.; Kim, G.S. Proteomic profiling of human HepG2 cells treated with hesperidin using antibody array. Mol. Med. Rep. 2017, 16, 5386–5392. [Google Scholar] [CrossRef]
- Bibli, S.I.; Hu, J.; Leisegang, M.S.; Wittig, J.; Zukunft, S.; Kapasakalidi, A.; Fisslthaler, B.; Tsilimigras, D.; Zografos, G.; Filis, K.; et al. Shear stress regulates cystathionine gamma lyase expression to preserve endothelial redox balance and reduce membrane lipid peroxidation. Redox Biol. 2020, 28, 101379. [Google Scholar] [CrossRef]
- Li, D.X.; Chen, W.; Jiang, Y.L.; Ni, J.Q.; Lu, L. Antioxidant protein peroxiredoxin 6 suppresses the vascular inflammation, oxidative stress and endothelial dysfunction in angiotensin II-induced endotheliocyte. Gen. Physiol. Biophys. 2020, 39, 545–555. [Google Scholar] [CrossRef]
- Li, H.; Weng, Y.; Lai, L.; Lei, H.; Xu, S.; Zhang, Y.; Li, L. KLF9 regulates PRDX6 expression in hyperglycemia-aggravated bupivacaine neurotoxicity. Mol. Cell Biochem. 2021, 476, 2125–2134. [Google Scholar] [CrossRef]
- Seriani, R.; Paula, C.P.; Cunha, A.; Oliveira, M.A.; Krempel, P.G.; Frias, D.P.; Negri, E.M.; Mauad, T.; Macchione, M. Expression patterns of peroxiredoxin genes in bronchial epithelial cells exposed to diesel exhaust particles. Exp. Mol. Pathol. 2021, 120, 104641. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Wei, T.; Lang, K.; Bao, C.; Yang, D. Peroxinredoxin 6 reduction accelerates cigarette smoke extract-induced senescence by regulating autophagy in BEAS-2B cells. Exp. Ther. Med. 2023, 26, 375. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, C.; Cigna, D.; Di Sano, C.; Di Vincenzo, S.; Dino, P.; Ferraro, M.; Bini, L.; Bianchi, L.; Di Gaudio, F.; Gjomarkaj, M.; et al. Exposure to cigarette smoke extract and lipopolysaccharide modifies cytoskeleton organization in bronchial epithelial cells. Exp. Lung Res. 2017, 43, 347–358. [Google Scholar] [CrossRef]
- Leopoldino, A.M.; Squarize, C.H.; Garcia, C.B.; Almeida, L.O.; Pestana, C.R.; Sobral, L.M.; Uyemura, S.A.; Tajara, E.H.; Silvio, G.J.; Curti, C. SET protein accumulates in HNSCC and contributes to cell survival: Antioxidant defense, Akt phosphorylation and AVOs acidification. Oral. Oncol. 2012, 48, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.; Ren, K.; Hong, M.; Cui, J.; Liu, J. Cytotoxicity of alkaline serine protease (ASPNJ) on Jurkat cells and its correlation with changes in the expression of membrane-associated proteins. Biochem. Mol. Toxicol. 2023, 37, e23456. [Google Scholar] [CrossRef]
- Zhang, J.; Park, H.S.; Kim, J.A.; Hong, G.E.; Nagappan, A.; Park, K.I.; Kim, G.S. Flavonoids identified from Korean Scutellaria baicalensis induce apoptosis by ROS generation and caspase activation on human fibrosarcoma cells. Am. J. Chin. Med. 2014, 42, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, R.; Liu, T.; Ma, H.; Xue, G.; Liu, M. Peroxiredoxin 6 alleviates high glucose-induced inflammation and apoptosis in HK-2 cells by inhibiting TLR4/NF-kappaB signaling. Ann. Transl. Med. 2023, 11, 41. [Google Scholar] [CrossRef]
- Pak, J.H.; Choi, W.H.; Lee, H.M.; Joo, W.D.; Kim, J.H.; Kim, Y.T.; Kim, Y.M.; Nam, J.H. Peroxiredoxin 6 overexpression attenuates cisplatin-induced apoptosis in human ovarian cancer cells. Cancer Investig. 2011, 29, 21–28. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Wang, N.; Wang, D.M.; Li, Y.W.; Han, F.; Shen, J.G.; Yang, D.P.; Guan, X.Y.; Chen, J.P. Dioscin induces cancer cell apoptosis through elevated oxidative stress mediated by downregulation of peroxiredoxins. Cancer Biol. Ther. 2012, 13, 138–147. [Google Scholar] [CrossRef]
- Berntsen, H.F.; Duale, N.; Bjorklund, C.G.; Rangel-Huerta, O.D.; Dyrberg, K.; Hofer, T.; Rakkestad, K.E.; Ostby, G.; Halsne, R.; Boge, G.; et al. Effects of a human-based mixture of persistent organic pollutants on the in vivo exposed cerebellum and cerebellar neuronal cultures exposed in vitro. Environ. Int. 2021, 146, 106240. [Google Scholar] [CrossRef]
- Wu, J.; Wang, F.; Gong, Y.; Li, D.; Sha, J.; Huang, X.; Han, X. Proteomic analysis of changes induced by nonylphenol in Sprague-Dawley rat Sertoli cells. Chem. Res. Toxicol. 2009, 22, 668–675. [Google Scholar] [CrossRef]
- Walsh, B.; Pearl, A.; Suchy, S.; Tartaglio, J.; Visco, K.; Phelan, S.A. Overexpression of Prdx6 and resistance to peroxide-induced death in Hepa1-6 cells: Prdx suppression increases apoptosis. Redox Rep. 2009, 14, 275–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Hu, J.E.; Ding, Y.; Shen, Y.; Xu, H.; Chen, H.; Wu, N. Sp1-mediated upregulation of Prdx6 expression prevents podocyte injury in diabetic nephropathy via mitigation of oxidative stress and ferroptosis. Life Sci. 2021, 278, 119529. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Glushkova, O.V.; Parfenyuk, S.B.; Gudkov, S.V.; Lunin, S.M.; Novoselova, E.G. The role of TLR4/NF-kappaB signaling in the radioprotective effects of exogenous Prdx6. Arch. Biochem. Biophys. 2021, 702, 108830. [Google Scholar] [CrossRef]
- Hong, M.W.; Kim, H.; Choi, S.Y.; Sharma, N.; Lee, S.J. Effect of Gossypol on Gene Expression in Swine Granulosa Cells. Toxins 2024, 16, 436. [Google Scholar] [CrossRef]
- Wang, C.; Feng, H.; Zhang, X.; Li, K.; Yang, F.; Cao, W.; Liu, H.; Gao, L.; Xue, Z.; Liu, X.; et al. Porcine Picornavirus 3C Protease Degrades PRDX6 to Impair PRDX6-mediated Antiviral Function. Virol. Sin. 2021, 36, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Shahnaj, S.; Chowhan, R.K.; Meetei, P.A.; Kakchingtabam, P.; Herojit, S.K.; Rajendrakumar, S.L.; Nongdam, P.; Fisher, A.B.; Rahaman, H. Hyperoxidation of Peroxiredoxin 6 Induces Alteration from Dimeric to Oligomeric State. Antioxidants 2019, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, W.; Kim, E.E. Crystal structures of human peroxiredoxin 6 in different oxidation states. Biochem. Biophys. Res. Commun. 2016, 477, 717–722. [Google Scholar] [CrossRef]
- Chowhan, R.K.; Hotumalani, S.; Rahaman, H.; Singh, L.R. pH induced conformational alteration in human peroxiredoxin 6 might be responsible for its resistance against lysosomal pH or high temperature. Sci. Rep. 2021, 11, 9657. [Google Scholar] [CrossRef]
- Wu, Y.; Feinstein, S.I.; Manevich, Y.; Chowdhury, I.; Pak, J.H.; Kazi, A.; Dodia, C.; Speicher, D.W.; Fisher, A.B. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. Biochem. J. 2009, 419, 669–679. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Switching of Redox Signaling by Prdx6 Expression Decides Cellular Fate by Hormetic Phenomena Involving Nrf2 and Reactive Oxygen Species. Cells 2022, 11, 1266. [Google Scholar] [CrossRef]
- Vazquez-Medina, J.P.; Tao, J.Q.; Patel, P.; Bannitz-Fernandes, R.; Dodia, C.; Sorokina, E.M.; Feinstein, S.I.; Chatterjee, S.; Fisher, A.B. Genetic inactivation of the phospholipase A2 activity of peroxiredoxin 6 in mice protects against LPS-induced acute lung injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L656–L668. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jung, Y.S.; Yun, J.; Han, S.B.; Roh, Y.S.; Song, M.J.; Hong, J.T. Peroxiredoxin 6 mediates acetaminophen-induced hepatocyte death through JNK activation. Redox Biol. 2020, 32, 101496. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Ji, Y.; Li, Y.; You, Y.; Zhou, Y. PRDX6-iPLA2 aggravates neuroinflammation after ischemic stroke via regulating astrocytes-induced M1 microglia. Cell Commun. Signal. 2024, 22, 76. [Google Scholar] [CrossRef]
- Schattauer, S.S.; Land, B.B.; Reichard, K.L.; Abraham, A.D.; Burgeno, L.M.; Kuhar, J.R.; Phillips, P.E.M.; Ong, S.E.; Chavkin, C. Prdx6 mediates Gαi protein-coupled receptor inactivation by cJun kinase. Nat. Commun. 2017, 8, 743. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, K.; Shen, F.; Zhang, J.; Xie, Y.; Li, S.; Hou, Y.; Bai, G. Astragaloside IV targets PRDX6, inhibits the activation of RAC subunit in NADPH oxidase 2 for oxidative damage. Phytomedicine 2023, 114, 154795. [Google Scholar] [CrossRef]
- Mohar, I.; Stamper, B.D.; Rademacher, P.M.; White, C.C.; Nelson, S.D.; Kavanagh, T.J. Acetaminophen-induced liver damage in mice is associated with gender-specific adduction of peroxiredoxin-6. Redox Biol. 2014, 2, 377–387. [Google Scholar] [CrossRef]
- Liao, J.; Hu, W.; Chen, S.; Huang, C.; Dong, S.; Chen, W.; Chen, X.; Chen, L. Multidimensional features of sporadic Creutzfeldt-Jakob disease in the elderly: A case report and systematic review. Front. Aging Neurosci. 2024, 16, 1379011. [Google Scholar] [CrossRef]
- Liao, J.; Mi, X.; Zeng, G.; Wei, Y.; Dai, X.; Ye, Q.; Chen, X.; Zhang, J. Circuit-wide proteomics profiling reveals brain region-specific protein signatures in the male WKY rats with endogenous depression. J. Affect. Disord. 2023, 320, 98–107. [Google Scholar] [CrossRef]
- Bumanlag, E.; Scarlata, E.; O’Flaherty, C. Peroxiredoxin 6 Peroxidase and Ca2+-Independent Phospholipase A2 Activities Are Essential to Support Male-Mouse Fertility. Antioxidants 2022, 11, 226. [Google Scholar] [CrossRef]
- O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015, 5, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Meng, J.; Yue, C.; Wu, X.; Su, Q.; Wu, H.; Zhang, Z.; Yu, Q.; Gao, S.; Fan, S.; et al. Integrative analysis the characterization of peroxiredoxins in pan-cancer. Cancer Cell Int. 2021, 21, 366. [Google Scholar] [CrossRef]
- Szeliga, M. Comprehensive analysis of the expression levels and prognostic values of PRDX family genes in glioma. Neurochem. Int. 2021, 153, 105256. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, Q.; Gao, M.; Liang, Q.; Li, J.; Chen, X. Differential Expression and Effects of Peroxiredoxin-6 on Drug Resistance and Cancer Stem Cell-like Properties in Non-Small Cell Lung Cancer. Oncotargets Ther. 2019, 12, 10477–10486. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Li, B.; Zhen, H.; Chen, W.; Men, Q. PRDX6 Overexpression Promotes Proliferation, Invasion, and Migration of A549 Cells in vitro and in vivo. Cancer Manag. Res. 2021, 13, 1245–1255. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, K.R.; Park, M.H.; Kim, D.H.; Jo, M.R.; Kim, J.Y.; Kim, E.C.; Yoon, D.Y.; Han, S.B.; Hong, J.T. PRDX6 promotes tumor development via the JAK2/STAT3 pathway in a urethane-induced lung tumor model. Free Radic. Biol. Med. 2015, 80, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Xiao, Z.F.; Li, C.; Xiao, Z.Q.; Yang, F.; Li, D.J.; Li, M.Y.; Li, F.; Chen, Z.C. Triosephosphate isomerase and peroxiredoxin 6, two novel serum markers for human lung squamous cell carcinoma. Cancer Sci. 2009, 100, 2396–2401. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Zhou, C.; Wang, Z.; Cui, Z.; Wu, X.; Xu, Z.; Yang, J.; Zhang, X. Construction and validation of a novel ferroptosis-related prognostic signature for lung adenocarcinoma. Transl. Lung Cancer R. 2023, 12, 1766–1781. [Google Scholar] [CrossRef]
- Nakanishi, T.; Takeuchi, T.; Ueda, K.; Murao, H.; Shimizu, A. Detection of eight antibodies in cancer patients’ sera against proteins derived from the adenocarcinoma A549 cell line using proteomics-based analysis. J. Chromatogr. B 2006, 838, 15–20. [Google Scholar] [CrossRef]
- Hu, X.; Lu, E.; Pan, C.; Xu, Y.; Zhu, X. Overexpression and biological function of PRDX6 in human cervical cancer. J. Cancer 2020, 11, 2390–2400. [Google Scholar] [CrossRef]
- Roh, J.W.; Choi, J.E.; Han, H.D.; Hu, W.; Matsuo, K.; Nishimura, M.; Lee, J.S.; Kwon, S.Y.; Cho, C.H.; Kim, J.; et al. Clinical and biological significance of EZH2 expression in endometrial cancer. Cancer Biol. Ther. 2020, 21, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rolfs, F.; Huber, M.; Gruber, F.; Bohm, F.; Pfister, H.J.; Bochkov, V.N.; Tschachler, E.; Dummer, R.; Hohl, D.; Schafer, M.; et al. Dual role of the antioxidant enzyme peroxiredoxin 6 in skin carcinogenesis. Cancer Res. 2013, 73, 3460–3469. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.Y.; Zhang, Y.C.; Wu, Y.X.; Guo, D.D.; Long, D.; Liu, Z.H. A Novel Ferroptosis-Related Gene Signature to Predict Prognosis in Patients with Head and Neck Squamous Cell Carcinoma. Dis. Markers 2021, 2021, 5759927. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yue, W.; You, J.; Wei, X.; Huang, Y.; Ling, Z.; Hou, J. Identification of a Novel Ferroptosis-Related Gene Prognostic Signature in Bladder Cancer. Front. Oncol. 2021, 11, 730716. [Google Scholar] [CrossRef]
- Quan, C.; Cha, E.J.; Lee, H.L.; Han, K.H.; Lee, K.M.; Kim, W.J. Enhanced expression of peroxiredoxin I and VI correlates with development, recurrence and progression of human bladder cancer. J. Urol. 2006, 175, 1512–1516. [Google Scholar] [CrossRef]
- Karihtala, P.; Mantyniemi, A.; Kang, S.W.; Kinnula, V.L.; Soini, Y. Peroxiredoxins in breast carcinoma. Clin. Cancer Res. 2003, 9, 3418–3424. [Google Scholar]
- Chang, X.Z.; Li, D.Q.; Hou, Y.F.; Wu, J.; Lu, J.S.; Di, G.H.; Jin, W.; Ou, Z.L.; Shen, Z.Z.; Shao, Z.M. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007, 9, R76. [Google Scholar] [CrossRef]
- Cortesi, L.; Barchetti, A.; De Matteis, E.; Rossi, E.; Della, C.L.; Marcheselli, L.; Tazzioli, G.; Lazzaretti, M.G.; Ficarra, G.; Federico, M.; et al. Identification of protein clusters predictive of response to chemotherapy in breast cancer patients. J. Proteome Res. 2009, 8, 4916–4933. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Qiu, G. Bioinformatics Analysis Using ATAC-seq and RNA-seq for the Identification of 15 Gene Signatures Associated With the Prediction of Prognosis in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 726551. [Google Scholar] [CrossRef]
- Xu, X.; Lu, D.; Zhuang, R.; Wei, X.; Xie, H.; Wang, C.; Zhu, Y.; Wang, J.; Zhong, C.; Zhang, X.; et al. The phospholipase A2 activity of peroxiredoxin 6 promotes cancer cell death induced by tumor necrosis factor alpha in hepatocellular carcinoma. Mol. Carcinog. 2016, 55, 1299–1308. [Google Scholar] [CrossRef]
- Ouyang, X.; DeWeese, T.L.; Nelson, W.G.; Abate-Shen, C. Loss-of-function of Nkx3.1 promotes increased oxidative damage in prostate carcinogenesis. Cancer Res. 2005, 65, 6773–6779. [Google Scholar] [CrossRef]
- Huang, W.S.; Huang, C.Y.; Hsieh, M.C.; Kuo, Y.H.; Tung, S.Y.; Shen, C.H.; Hsieh, Y.Y.; Teng, C.C.; Lee, K.C.; Lee, K.F.; et al. Expression of PRDX6 Correlates with Migration and Invasiveness of Colorectal Cancer Cells. Cell Physiol. Biochem. 2018, 51, 2616–2630. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F.; Li, N.; Zhang, J.; Dai, L.; Yang, H. Peroxiredoxins and Immune Infiltrations in Colon Adenocarcinoma: Their Negative Correlations and Clinical Significances, an In Silico Analysis. J. Cancer 2020, 11, 3124–3143. [Google Scholar] [CrossRef] [PubMed]
- Falidas, E.; Kitsiouli, E.; Tsounis, D.; Kalogirou, A.; Tsiambas, E.; Tsouvelas, G.; Papadopoulos, S.; Mitsis, M.; Lekka, M.; Vlachos, K. Impact of peroxiredoxin-6 expression on colon adenocarcinoma. J. Buon 2021, 26, 1893–1897. [Google Scholar] [PubMed]
- Nicolussi, A.; D’Inzeo, S.; Mincione, G.; Buffone, A.; Di Marcantonio, M.C.; Cotellese, R.; Cichella, A.; Capalbo, C.; Di Gioia, C.; Nardi, F.; et al. PRDX1 and PRDX6 are repressed in papillary thyroid carcinomas via BRAF V600E-dependent and -independent mechanisms. Int. J. Oncol. 2014, 44, 548–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mu, R.; Li, Y.; Xing, J.; Li, Y.; Lin, R.; Ye, S.; Zhang, Y.; Mu, H.; Guo, X.; An, L. Effect of lentivirus-mediated peroxiredoxins 6 gene silencing on the phenotype of human gastric cancer BGC-823 cells. J. Cancer Res. Ther. 2022, 18, 411–417. [Google Scholar] [CrossRef]
- Li, Q.; Wang, N.; Wei, H.; Li, C.; Wu, J.; Yang, G. miR-24-3p Regulates Progression of Gastric Mucosal Lesions and Suppresses Proliferation and Invasiveness of N87 Via Peroxiredoxin 6. Dig. Dis. Sci. 2016, 61, 3486–3497. [Google Scholar] [CrossRef]
- Shanshan, Y.; Beibei, J.; Li, T.; Minna, G.; Shipeng, L.; Li, P.; Yong, Z. Phospholipase A2 of Peroxiredoxin 6 Plays a Critical Role in Cerebral Ischemia/Reperfusion Inflammatory Injury. Front. Cell. Neurosci. 2017, 11, 99. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Kang, T.C. Sp1-Mediated Prdx6 Upregulation Leads to Clasmatodendrosis by Increasing Its aiPLA2 Activity in the CA1 Astrocytes in Chronic Epilepsy Rats. Antioxidants 2022, 11, 1883. [Google Scholar] [CrossRef]
- Ho, J.N.; Lee, S.B.; Lee, S.S.; Yoon, S.H.; Kang, G.Y.; Hwang, S.G.; Um, H.D. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol. Cancer Ther. 2010, 9, 825–832. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, K.R.; Lee, H.P.; Lee, D.H.; Jo, M.; Shin, D.H.; Yoon, D.Y.; Han, S.B.; Hong, J.T. PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities. Free Radic. Biol. Med. 2014, 69, 367–376. [Google Scholar] [CrossRef]
- Chen, H.; Fang, Y.; Dai, S.; Jiang, K.; Shen, L.; Zhao, J.; Huang, K.; Zhou, X.; Ding, K. Characterization and proteomic analysis of plasma-derived small extracellular vesicles in locally advanced rectal cancer patients. Cell Oncol. 2024, 47, 1995–2009. [Google Scholar] [CrossRef]
- Kuang, X.; Wang, L.F.; Yu, L.; Li, Y.J.; Wang, Y.N.; He, Q.; Chen, C.; Du, J.R. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: Involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic. Biol. Med. 2014, 71, 165–175. [Google Scholar] [CrossRef]
- Huang, Y.; Driedonks, T.; Cheng, L.; Rajapaksha, H.; Routenberg, D.A.; Nagaraj, R.; Redding, J.; Arab, T.; Powell, B.H.; Pletnikova, O.; et al. Brain Tissue-Derived Extracellular Vesicles in Alzheimer’s Disease Display Altered Key Protein Levels Including Cell Type-Specific Markers. J. Alzheimers Dis. 2022, 90, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Novoselova eg Glushkova, O.V.; Lunin, S.M.; Khrenov, M.O.; Parfenyuk, S.B.; Novoselova, T.V.; Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E. Peroxiredoxin 6 Attenuates Alloxan-Induced Type 1 Diabetes Mellitus in Mice and Cytokine-Induced Cytotoxicity in RIN-m5F Beta Cells. J. Diabetes Res. 2020, 2020, 7523892. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Nakanishi, T.; Hiramatsu, M.; Mabuchi, H.; Miyamoto, Y.; Miyamoto, A.; Shimizu, A.; Tanigawa, N. Proteomics-based approach identifying autoantibody against peroxiredoxin VI as a novel serum marker in esophageal squamous cell carcinoma. Clin. Cancer Res. 2006, 12, 6415–6420. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, W.; Xiao, Y.; Pan, L.; Chen, G.; Tang, Y.; Zhou, J.; Wu, J.; Zhu, W.; Zhang, S.; et al. Overexpression of Peroxiredoxin 6 (PRDX6) Promotes the Aggressive Phenotypes of Esophageal Squamous Cell Carcinoma. J. Cancer 2018, 9, 3939–3949. [Google Scholar] [CrossRef]
- Lu, B.; Chen, X.B.; Hong, Y.C.; Zhu, H.; He, Q.J.; Yang, B.; Ying, M.D.; Cao, J. Identification of PRDX6 as a regulator of ferroptosis. Acta Pharmacol. Sin. 2019, 40, 1334–1342. [Google Scholar] [CrossRef]
- Gordeeva, A.E.; Temnov, A.A.; Charnagalov, A.A.; Sharapov, M.G.; Fesenko, E.E.; Novoselov, V.I. Protective Effect of Peroxiredoxin 6 in Ischemia/Reperfusion-Induced Damage of Small Intestine. Dig. Dis. Sci. 2015, 60, 3610–3619. [Google Scholar] [CrossRef]
- Goncharov, R.G.; Rogov, K.A.; Temnov, A.A.; Novoselov, V.I.; Sharapov, M.G. Protective role of exogenous recombinant peroxiredoxin 6 under ischemia-reperfusion injury of kidney. Cell Tissue Res. 2019, 378, 319–332. [Google Scholar] [CrossRef]
- Novoselov, V.I.; Baryshnikova, L.M.; Yanin, V.A.; Amelina, S.E.; Fesenko, E.E. The influence of peroxyredoxin VI on incised-wound healing in rats. Dokl. Biochem. Biophys. 2003, 393, 326–327. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, H.J.; Wang, H.Y.; Wang, W.T.; Jin, S.H.; Zhu, P.; Li, S.J.; Rong, C.T.; Li, J.Y. Topical administration of peroxiredoxin-6 on the cornea suppresses inflammation and neovascularization induced by ultraviolet radiation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8016–8028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mu, R.; Ye, S.; Lin, R.; Li, Y.; Guo, X.; An, L. Effects of Peroxiredoxin 6 and Its Mutants on the Isoproterenol Induced Myocardial Injury in H9C2 Cells and Rats. Oxidative Med. Cell. Longev. 2022, 2022, 2576310. [Google Scholar] [CrossRef]
- Zhang, X.X.; You, J.P.; Liu, X.R.; Zhao, Y.F.; Cui, Y.; Zhao, Z.Z.; Qi, Y.Y. PRDX6 AS1 gene polymorphisms and SLE susceptibility in Chinese populations. Front. Immunol. 2022, 13, 987385. [Google Scholar] [CrossRef]
- Xiong, M.; Guo, M.; Huang, D.; Li, J.; Zhou, Y. Effect of PRDX6 gene polymorphism on susceptibility to chronic obstructive pulmonary disease in the Chinese Han population. Clin. Respir. J. 2023, 17, 638–646. [Google Scholar] [CrossRef]
- Kubo, E.; Fatma, N.; Akagi, Y.; Beier, D.R.; Singh, S.P.; Singh, D.P. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am. J. Physiol.-Cell Physiol. 2008, 294, C842–C855. [Google Scholar] [CrossRef]
- Shibata, S.; Shibata, N.; Shibata, T.; Sasaki, H.; Singh, D.P.; Kubo, E. The role of Prdx6 in the protection of cells of the crystalline lens from oxidative stress induced by UV exposure. Jpn. J. Ophthalmol. 2016, 60, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Kubo, E.; Hasanova, N.; Tanaka, Y.; Fatma, N.; Takamura, Y.; Singh, D.P.; Akagi, Y. Protein expression profiling of lens epithelial cells from Prdx6-depleted mice and their vulnerability to UV radiation exposure. Am. J. Physiol.-Cell Physiol. 2010, 298, C342–C354. [Google Scholar] [CrossRef]
- Fatma, N.; Singh, P.; Chhunchha, B.; Kubo, E.; Shinohara, T.; Bhargavan, B.; Singh, D.P. Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am. J. Physiol.-Cell Physiol. 2011, 301, C954–C967. [Google Scholar] [CrossRef]
- Novoselova eg Sharapov, M.G.; Lunin, S.M.; Parfenyuk, S.B.; Khrenov, M.O.; Mubarakshina, E.K.; Kuzekova, A.A.; Novoselova, T.V.; Goncharov, R.G.; Glushkova, O.V. Peroxiredoxin 6 Applied after Exposure Attenuates Damaging Effects of X-ray Radiation in 3T3 Mouse Fibroblasts. Antioxidants 2021, 10, 1951. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The role of peroxiredoxin 6 in neutralization of X-ray mediated oxidative stress: Effects on gene expression, preservation of radiosensitive tissues and postradiation survival of animals. Free Radical Res. 2017, 51, 148–166. [Google Scholar] [CrossRef]
- Lunin, S.M.; Novoselova eg Glushkova, O.V.; Parfenyuk, S.B.; Kuzekova, A.A.; Novoselova, T.V.; Sharapov, M.G.; Mubarakshina, E.K.; Goncharov, R.G.; Khrenov, M.O. Protective effect of exogenous peroxiredoxin 6 and thymic peptide thymulin on BBB conditions in an experimental model of multiple sclerosis. Arch. Biochem. Biophys. 2023, 746, 109729. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Khater, S.M.; Ashamallah, S.A.; Rashed, S.A.; Fouad, A.M.; Ismail, A.M.; Ghoneim, M.A. PRDX6 Promotes the Differentiation of Human Mesenchymal Stem (Stromal) Cells to Insulin-Producing Cells. Biomed. Res. Int. 2020, 2020, 7103053. [Google Scholar] [CrossRef]
- Parfenyuk, S.B.; Glushkova, O.V.; Sharapov, M.G.; Khrenov, M.O.; Lunin, S.M.; Kuzekova, A.A.; Mubarakshina, E.K.; Novoselova, T.V.; Cherenkov, D.A.; Novoselova, E.G. Protective Effects of Peroxiredoxin 6 in Pro-Inflammatory Response Model Using Raw 264.7 Macrophages. Biochemistry 2023, 88, 1156–1164. [Google Scholar] [CrossRef]
| Species | Samples | External Stimuli | Alteration of PRDX6 | Upstream Molecules | References |
|---|---|---|---|---|---|
| Human | Lung | Sulfur mustard | ↑ | N/A | [14] |
| Blood | High-intensity interval training | ↑ | N/A | [34] | |
| Liraglutide and metformin | ↓ | GLP-1R | [40] | ||
| Sperm | Date palm pollen | ↑ | N/A | [23] | |
| 4-tert-octylphenol | ↓ | cAMP-PKA/C | [21] | ||
| Rat | Lung | SiO2 | ↑ | SP-A | [41] |
| Testis | Di-n-butyl phthalate; Curcumin | ↑ | SP1 | [24,42] | |
| Epididymis | Tert-butyl hydroperoxide | ↑ | N/A | [25] | |
| Placenta | MgSO4 | ↑ | NRF2 | [12] | |
| Bile duct | Thioacetamide | ↑ | Wnt7a/b | [43] | |
| Pancreas | Hydroxytyrosol | ↑ | N/A | [44] | |
| Heart | Adriamycin | ↓ | N/A | [45] | |
| Colon | Voluntary exercise; Hydrogen sulfide | ↑ | N/A | [15,36] | |
| 2,4,6-trinitrobenzenesulfonic acid | ↓ | N/A | [15] | ||
| Liver | High-fat diet; whole grain | ↑ | N/A | [46] | |
| CCl4 | ↑ | N/A | [19] | ||
| Chronic ethanol exposure | ↓ | N/A | [17] | ||
| Skin | Aloe vera | ↑ | N/A | [47] | |
| Electron beam (45 Gy) | ↓ | miR-214 | [30] | ||
| Brain | Ferrostatin-1 | ↑ | Fer1 | [48] | |
| Mouse | Blood | Angiotensin II | ↑ | N/A | [49] |
| Brain; lung | Hyperbaric oxygen exposure | ↑ | N/A | [33] | |
| Pancreas; Salivary glands | Epigallocatechin-3-gallate | ↑ | p38 and JNK | [50] | |
| Cochleae | All-trans retinoic acid | ↑ | RARα | [51] | |
| CoCl2 | ↑ | HIF-1α and NRF2 | [13] | ||
| Spleen | Ionizing radiation (10 Gy) | ↑ | N/A | [27] | |
| Kidney | Andrographolide sodium bisulfite | ↑ | Mitochondrial complex I | [52] | |
| NH4Cl | ↑ | AE1 | [53] | ||
| Testis | Melatonin | ↑ | N/A | [54] | |
| Di-2-ethylhexyl phthalate | ↓ | N/A | [55] | ||
| Paclitaxel | ↓ | SIRT1 and NRF2 | [22] | ||
| White adipose | Buthionine sulfoximine | ↑ | NRF2 | [16] | |
| Skin | Puerariae lobatae radix | ↑ | REV-ERBα/BMAL1/NRF2 | [29] | |
| Ultraviolet-B | ↓ | ||||
| Brain | Lead | ↓ | N/A | [56] | |
| Lung | Snake venom toxin | ↓ | AP-1 | [57] | |
| Paraquat; Lipopolysaccharide; Ricin; Thiacremonone; H1N1 influenza virus | ↓ | N/A | [37,58,59,60] | ||
| Liver | Salvia miltiorrhiza polysaccharide | ↑ | N/A | [38] | |
| Clonorchis sinensis | ↑ | NF-κB; KEAP1/NRF2, HIF-1α, and C/EBPβ | [39] | ||
| Single-walled carbon nanotubes; Proton irradiation (2 Gy); Ionizing radiation (10 Gy) | ↑ | N/A | [26,27,28] | ||
| Chronic ethanol treatment | ↓ | NF-κB; MEK1/2 | [18] | ||
| CCl4 | ↓ | N/A | [20] | ||
| Colon | CaCO3 | ↑ | FoxM1 and NF-κB | [61] | |
| Liquiritin | ↑ | Direct binding | [62] | ||
| Dextran sulfate sodium | ↓ | N/A | [62] | ||
| Rabbit | Oviduct | Mating and fertilization | ↑ | N/A | [35] |
| Liver | Olmesartan | ↓ | AT1 | [63] | |
| Bovine | Ovary | Melatonin | ↑ | MTNR1A/B | [64] |
| α-pinene | ↑ | NRF2 | [65] | ||
| Aloe vera | ↑ | N/A | [66] | ||
| Thymol | ↓ | N/A | [67] | ||
| Fish | Gills | Citrate | ↑ | N/A | [68] |
| Liver | Soybean oil; low temperature | ↑ | N/A | [31,69] | |
| High temperature and hypoxia | ↓ | N/A | [32] |
| Species | Various Cell Lines | External Stimuli | Alterations of PRDX6 | Upstream Molecule | References | |
|---|---|---|---|---|---|---|
| Human | Colorectal epithelial cell | Caco-2 | Roasted coffee extracts | ↑ | ARE | [93] |
| SW-480 SW-620 | 5-fluorouracil | ↑ | N/A | [94] | ||
| DLD-1 | Baicalein | ↑ | N/A | [80] | ||
| HT-29 | Portoamides | ↑ | N/A | [95] | ||
| HCT-8 | Curcumin (25 µM, for 72 h) | ↓ | N/A | [77] | ||
| Alveolar epithelial cell | H460 | Ionizing radiation (2 Gy) | ↑ | TRIAP1 | [70] | |
| HCC-827 | Gefitinib | ↑ | EGFR | [96] | ||
| A549 | Ionizing radiation (2 Gy) | ↑ | TRIAP1 | [70] | ||
| Keratinocyte growth factor | ↑ | NRF2/ARE | [97] | |||
| Dexamethasone (1 µM) | ↑ | GRE | [97] | |||
| Endothelial growth factor | ↑ | EGFR/PI3K | [98] | |||
| Curcumin (109 µM, for 2 h) * | ↑ | N/A | [74] | |||
| Snake venom toxin; | ↓ | AP-1 | [57] | |||
| Thiacremonone | ↓ | Direct binding | [60] | |||
| Breast epithelial cell | MCF-7; MCF-10A | Oleuropein | ↑ | N/A | [99] | |
| Lens epithelial cell | hLEC | Curcumin (5 µM, for 48 h) Ginkgolic acid | ↑ | SP1 | [75,100] | |
| Sulforaphane | ↑ (6 µM) | NRF2/ARE | [101] | |||
| ↓ (>6 µM) | NRF2/KLF9 | [102] | ||||
| Betulinic acid | ↓ | SP1 | [100] | |||
| Monocyte | THP-1 | Electromagnetic fields | ↑ | N/A | [72] | |
| U937 | Cigarette smoke condensate; Ethanol; Darunavir/ritonavir | ↓ | N/A | [103,104] | ||
| U1 | Cigarette smoke condensate | ↓ | N/A | [103] | ||
| Hepatocyte | Huh-7 | Luteolin | ↑ | N/A | [81] | |
| (E)-4-chloro-2-((3-ethoxy-2-hydroxybenzylidene)amino)phenol | ↓ | p38β/SAPK | [105] | |||
| LMH | Andrographolide | ↑ | N/A | [82] | ||
| HepG2 | Curcumin (1737 µM, for 72 h) * | ↓ | p53 | [78] | ||
| Hesperidin | ↓ | N/A | [106] | |||
| Vascular cell | UVEC | Sheer stress | ↑ | KLF2/miR-27b/CSE | [107] | |
| Angiotensin II | ↓ | AT1R | [108] | |||
| Retinal epithelial cell | RPE-1 | Ionizing radiation (20 Gy); BrdU | ↑ | N/A | [71] | |
| APRE-19 | H2O2 (300, 400, or 500 µM, for 6 h; 500 or 1000 µM, for 48 h); Blue light | ↓ | N/A | [84,86] | ||
| Salivary gland epithelial cell | HSG | Epigallocatechin-3-gallate | ↑ | p38 and JNK | [50] | |
| H2O2 (100 µM, for 30 min) | ↓ | N/A | [50] | |||
| Astrocyte | A172 HA-sp | H2O2 (50, 100, or 200 µM, for 24 h) | ↑ | N/A | [76] | |
| Curcumin (1 µM, for 24 h) | ↓ | N/A | [76] | |||
| Neuron | SH-SY5Y | Hyperglycemia + bupivacaine | ↓ | KLF9 | [109] | |
| Trabecular meshwork cell | TM | H2O2 (100 µM, for 8 h) | ↓ | N/A | [85] | |
| Bronchial epithelial cell | BEAS-2B | Diesel exhaust particles; Cigarette smoke extract | ↓ | N/A | [110,111] | |
| 16-HBE | Cigarette + LPS (1 µg/mL, for 8 h) | ↓ | N/A | [112] | ||
| Oral epithelial cell | HN13 | Tert-butyl hydroperoxide | ↑ (50 µM), ↓ (250 µM) | N/A | [113] | |
| Leukemia cell | K562 | Andrographolide-lipoic acid | ↑ | N/A | [83] | |
| Jurkat | Alkaline serine protease | ↓ | N/A | [114] | ||
| Fibrosarcoma cell | HT-1080 | Korean Scutellaria baicalensis flavonoids | ↓ | N/A | [115] | |
| Embryonic kidney cell | HEK-293 | TNFα (500–2500 U/mL, for 5, 7, or 9 h) * | ↑ | TNFα/NOX1 | [88] | |
| Renal tubular epithelial cell | HK-2 | High glucose | ↓ | N/A | [116] | |
| Embryonic lung fibroblast | MRC-5 | Ionizing radiation (20 Gy); BrdU | ↑ | N/A | [71] | |
| Ovarian cell | SKOV-3 | Cisplatin (40 µM) | ↑ (3–9 h), ↓ (15–24 h) | N/A | [117] | |
| Esophageal epithelial cell | Kyse510 | Dioscin | ↓ | N/A | [118] | |
| Melanocytes | A375 | Endothelial growth factor | ↑ | EGFR | [98] | |
| Rat | Cerebellar granule neuron | CGN | Persistent organic pollutants | ↑ | N/A | [119] |
| Sertoli cell | TSC | Nonylphenol | ↑ | N/A | [120] | |
| Alveolar epithelial cell | AT-II | Keratinocyte growth factor | ↑ | NRF2/ARE | [97] | |
| Condylar chondrocyte | RCC | Glycyrrhizin | ↑ | NRF2 | [79] | |
| H2O2 (100 µM, for 24 h) | ↓ | N/A | [79] | |||
| Lens epithelial cell | rLEC | Sulforaphane | ↑ (6 µM) | NRF2/ARE | [101] | |
| ↓ (>6 µM) | NRF2/KLF9 | [102] | ||||
| Pancreatic beta cell | RINm5F | TNFα (1850 U/mL, for 24 h); IFNγ (140 U/mL, for 24 h); IL-4 | ↓ | TNFα; IFNγ; IL-4 | [89] | |
| Retinal ganglion cell | RGC-5 | TNFα (50ng/mL, for 10 days; 100 ng/mL, for 72 h) | ↓ | TNFα | [90] | |
| Glutamate (5 mM) | ↓ | NMDAR | [90] | |||
| Mouse | Lens epithelial cell | mLEC | Ginkgolic acid | ↑ | SP1 | [100] |
| Betulinic acid | ↓ | SP1 | [100] | |||
| Hepatocyte | Hepa1-6 | Mithramycin A | ↓ | SP1 | [121] | |
| H2.35 | Dexamethasone (0.1 µM) | ↑ | N/A | [87] | ||
| Keratinocyte growth factor | ↑ | PKC/MAPK | [87] | |||
| TNFα (10 ng/mL, for 8 or 24 h) | ↑ | TNFα/PKC/MAPK | [87] | |||
| BAY117082 | ↑ | NF-κB | [87] | |||
| H2O2 (1000 µM, for 8 or 24 h) | ↑ | N/A | [87] | |||
| Auditory cell | HEI-OC1 | All-trans retinoic acid | ↑ | RARα | [51] | |
| CoCl2 | ↑ | HIF-1α and NRF2 | [13] | |||
| H2O2 (25 µM, for 9 h) | ↑ | N/A | [13] | |||
| H2O2 (100 µM, for 24 h) | ↓ | N/A | [13] | |||
| Glomerular podocyte | MPC5 | High glucose | ↓ | SP1 | [122] | |
| Tracheobronchial epithelial cell | TBE | LPS (1, 10, or 20 µg/mL, for 8 or 24 h) | ↓ | N/A | [91] | |
| Embryonic fibroblast cell | 3T3 | X-ray (16 Gy) | ↑ | N/A | [123] | |
| Macrophage | BMM | NO | ↑ | NO/Srx | [92] | |
| IFNγ (100 U/mL) + LPS (0.5 µg/mL), for 18 h | ↑ | N/A | [92] | |||
| Porcine | Granulosa cell | GCs | Gossypol | ↑ | N/A | [124] |
| Kidney cell | PK-15 | Foot-and-mouth virus | ↓ | N/A | [125] | |
| Study | Model | Dose | Strategies | Significance | References |
|---|---|---|---|---|---|
| In vivo | SCR rat | 20 μg | Every 72 h, for 2 weeks (TAT, s.c.i.) | Delay the progression of cataracts | [186] |
| UCI rat | 30 μg | 4 times per day, for 14 days after injury (t.a.) | Suppress ultraviolet radiation-induced inflammation and neovascularization | [182] | |
| IW rat | 0.5 mg/mL | 1 and 3 h after incision, 2 times per day (t.a.) | Accelerate incised wound healing and decrease the size of the scar formation | [181] | |
| MII rat | 2 μg/kg | for 14 days (s.i.) | A certain repair effect on myocardial injury | [183] | |
| ASMAO rat | 10 μg/g | 15 min before I/R injury (i.v.) | Protect against I/R-induced damage of small intestine | [179] | |
| T1DM mouse | 20 μg/g | The first day and repeatedly on the eighth day (i.v.) | Decrease the mortality rate and glycemia, lower splenocytic apoptosis and plasma cytokines, and increase the pancreatic β cell mass | [175] | |
| RIRI mouse | 20 μg/g | 15 min before I/R injury (i.v.) | Reduce the degree of kidney I/R injury | [180] | |
| Irradiated mouse | 20 μg/g | 15 min before X-ray irradiation (i.v. or i.p.) | Reduce the radiation-induced organism injuries (e.g., leuko- and thrombopenia, small intestine, and red bone marrow) | [191] | |
| EAE mouse | 6 μg/g | Days 2, 7, and 10 after EAE induction (i.p.) | Improve the EAE-induced symptoms and blood–brain barrier dysfunction | [192] | |
| In vitro | hLECs | 10 and 20 μg/mL | Pretreated before ultraviolet-B irradiation (TAT) | Protect the crystalline lens cells from oxidative stress induced by ultraviolet-B irradiation | [187] |
| hMSCs | 10 ng/mL | Incubation for 7 days (d.a.) | Promote cell differentiation into insulin-producing cells | [193] | |
| RIN-m5F | 150 μg/mL | 30 min before additions of cytokines (d.a.) | Reduce the inflammation cascades, cytokine-induced cytotoxicity and apoptosis, and oxidative stress | [175] | |
| rRGCs | 4 μg/mL | 3 h before additions of glutamate and/or TNFα (TAT) | Attenuate TNFα- and glutamate-induced cell death by limiting ROS levels and maintaining Ca2+ homeostasis | [90] | |
| H9C2 | 0.1 μg/mL | Pretreatment for 2 h (d.a.) | Attenuate isoprenaline-induced cell death, apoptosis and oxidative stress | [183] | |
| r/mLECs | 5–10 μg/mL | 24 h before additions of H2O2 and/or TGF1β (TAT) | Protect against H2O2- and TGF1β-induced cell death and oxidative stress, and inhibit ROS-mediated adverse signaling | [186] | |
| mLECs | 4 μg/mL | Incubation for 72 h (TAT) | Restore the Prdx6 gene promoter activity by attenuating SMAD3/TGF1β signaling | [11] | |
| 10 μg/mL | Incubation for 96 h (TAT) | Attenuate adverse signaling in cells and maintain cellular homeostasis | [188] | ||
| N/A | Pretreated before hypoxia stress (TAT) | Optimize hypoxia-induced overstimulation of endoplasmic reticulum stress | [189] | ||
| Raw 264.7 | 150 μg/mL | Within the first hour after LPS (d.a.) | Decrease lipolyaccharide-induced ROS, apoptosis, and pro-inflammation | [194] | |
| 3T3 | 300 μg/mL | Incubation for 3 h; 4 h after irradiation (d.a.) | Increase survival, suppress oxidative stress, senescence, apoptosis, and necrosis under X-ray exposure | [123,190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Zhang, Y.; Yang, J.; Chen, L.; Zhang, J.; Chen, X. Peroxiredoxin 6 in Stress Orchestration and Disease Interplay. Antioxidants 2025, 14, 379. https://doi.org/10.3390/antiox14040379
Liao J, Zhang Y, Yang J, Chen L, Zhang J, Chen X. Peroxiredoxin 6 in Stress Orchestration and Disease Interplay. Antioxidants. 2025; 14(4):379. https://doi.org/10.3390/antiox14040379
Chicago/Turabian StyleLiao, Jiangfeng, Yusi Zhang, Jianwei Yang, Longfei Chen, Jing Zhang, and Xiaochun Chen. 2025. "Peroxiredoxin 6 in Stress Orchestration and Disease Interplay" Antioxidants 14, no. 4: 379. https://doi.org/10.3390/antiox14040379
APA StyleLiao, J., Zhang, Y., Yang, J., Chen, L., Zhang, J., & Chen, X. (2025). Peroxiredoxin 6 in Stress Orchestration and Disease Interplay. Antioxidants, 14(4), 379. https://doi.org/10.3390/antiox14040379






