Abstract
As a moonlighting protein with multiple enzymatic activities, peroxiredoxin 6 (PRDX6) maintains redox homeostasis, regulates phospholipid metabolism, and mediates intra- and inter-cellular signaling transduction. Its expression and activity can be regulated by diverse stressors. However, the roles and relevant mechanisms of these regulators in various conditions have yet to be comprehensively reviewed. In this study, these stressors were systematically reviewed both in vivo and in vitro and classified into chemical, physical, and biological categories. We found that the regulatory effects of these stressors on PRDX6 expression were primarily mediated via key transcriptional factors (e.g., NRF2, HIF-1α, SP1, and NF-κB), micro-RNAs, and receptor- or kinase-dependent signaling pathways. Additionally, certain stressors, including reactive oxygen species, pH fluctuations, and post-translational modifications, induced the structure-based functional switches in the PRDX6 enzyme. We further reviewed the altered expression of PRDX6 under various disease conditions, with a particular focus on neuropsychiatric disorders and cancers, and proposed the concept of PRDX6-related disorders (PRD), which refers to a spectrum of diseases mediated by or associated with dysregulated PRDX6 expression. Finally, we found that an exogenous supplementation of PRDX6 protein provided preventive and therapeutic potentials for oxidative stress-related injuries in both in vivo and in vitro models. Taken together, this review underscores the critical role of PRDX6 as a cellular orchestrator in response to various stressors, highlighting its clinical potential for disease monitoring and the development of therapeutic strategies.
1. Introduction
Peroxiredoxins (PRDXs) are members of an evolutionarily conserved family of peroxidases capable of reducing a wide range of peroxide substrates [1,2]. These enzymes are ubiquitously expressed across all major organs of mammals and can be classified into three categories based on the number of the residues of conserved cysteines (Cys) involved in catalysis: typical 2-Cystein PRDXs (PRDX1-4), atypical 2-Cystein PRDX5, and 1-Cystein PRDX6 [2]. All PRDX isoforms share a fundamental catalytic mechanism, where a reduced Cys (-SH) at the active site is oxidized into a sulfenic form (-SOH) by peroxide substrates. However, they differ greatly in their abilities to recycle sulfenic acid back to thiol forms and in restoring hyperoxidized states, such as sulfinic acid (-SO2H) and sulfonic acid (-SO3H) [3]. It has been reported that the oxidized sulfenic form of PRDX6 can be reduced, whereas its hyperoxidized sulfinic and sulfonic forms are not reversible, contrary to the hyperoxidized sulfinic forms of other PRDXs that can be restored through the action of ATP-dependent enzyme sulfiredoxin [4]. Therefore, PRDXs play a crucial role in regulating cellular redox homeostasis and oxidative stress responses.
PRDX6 is a particularly intriguing member of the PRDXs. Ever since its initial cloning from mouse kidneys in 1997, it has received growing attention in various research fields [5]. Over the past two decades, constant efforts have been dedicated to elucidating its pathophysiological implications in living organisms. PRDX6 is widely recognized as a moonlighting protein with multiple enzymatic activities, including glutathinone peroxidase (Gpx), acidic calcium-independent phospholipase (aiPLA2), and lysophosphatidylcholine acyl transferase (LPCAT) [6], with its enzymatic functions varying under distinct conditions [1]. For instance, the optimal pH for the activities of Gpx and aiPLA2 of PRDX6 varies considerably; Gpx activity maximizes at a pH exceeding 7 in the cytosol, while aiPLA2 activity peaks at pH 4 within lysosomal lamellar bodies. Notably, PRDX6 is unique not only for its ability to utilize both glutathione and phospholipid hydroperoxides as substrates but also for its crucial downstream cellular signaling effects [1,7]. Its enzymatic activities are closely associated with lipid peroxidation, selenocysteine metabolism and ferroptosis, and the activation of NADPH oxidases and redox-dependent inflammatory pathways [8,9]. Hence, the complex interplay of these enzymatic activities suggests that PRDX6 may serve as a crucial molecular node in various pathophysiological conditions.
Emerging evidence has intriguingly indicated that the expression and functional dynamics of PRDX6 are substantially modulated by various endogenous and exogenous stressors [2,10], making this area a subject of considerable interest. Given the complex and sometimes contradictory results, a comprehensive overview of these regulators is essential to enhance our understanding of this enzyme and its potential applications. The current study systematically delineated the spectrum of in vivo and in vitro stressors that modulate PRDX6 expression and subsequently examined its structural and functional adaptations, relevant pathological implications, and therapeutic prospects in exogenous supplementation. Based on the current literature, this review highlights PRDX6 as a crucial orchestrator against various internal and external stressors, signifying its potential applications in diagnostic and therapeutic strategies for stress-related disorders.
2. Dysregulation of PRDX6 Expression Induced by Various Stressors
Previous web-based computer analyses of the 5′-flanking regions of the PRDX6 gene promoter have identified multiple putative regulatory elements, including binding sites for specificity protein 1 (SP1), nuclear factor erythroid 2-related factor 2 (NRF2), and hypoxia-inducible factor 1 alpha (HIF-1α) [11]. The available evidence suggests that a variety of stressors can modulate PRDX6 expression both in vivo and in vitro through transcriptional factor binding, rendering it an important responder to both internal disruption and external insults. These stressors may be roughly classified into chemical, physical, and biological types. In some cases, the expression of PRDX6 may exhibit a biphasic response pattern depending on both the duration and intensity of stressor exposure. Therefore, a further summary of their regulations on PRDX6 expression and the underlying mechanisms will significantly enhance our understanding of its biological functions and molecular features.
2.1. In Vivo Dysregulation of PRDX6 Expression Induced by Various Stressors
Previous studies have revealed that various chemical compounds can induce the dysregulation of PRDX6 expression. For instance, magnesium sulfate (MgSO4) has been shown to provide protection against oxidative damage and inflammatory response in the rat placenta within a model of intrahepatic cholestasis of pregnancy by enhancing the NRF2/PRDX6 signaling pathway [12]. The treatment with cobalt chloride (CoCl2) has been found to increase the expressions of two PRDX6-related redox-active transcription factors (NRF2 and HIF-1α) to ameliorate oxidative stress in auditory cells [13]. In addition, several sulfur agents, such as sulfur mustard, hydrogen sulfide, and buthionine sulfoximine, may also exert antioxidant effects by elevating the PRDX6 expression via their transcriptional effects [14,15,16]. In contrast, the expression of PRDX6 is downregulated in the livers of ethanol-fed rodents, accompanied by the activation of nuclear factor kappa B (NF-κB) and phosphorylation of mitogen-activated protein kinases (MAPKs) [17,18], implying the critical roles of PRDX6 in attenuating cellular inflammation and oxidative stress.
Notably, carbon tetrachloride (CCl4) has been reported to inhibit hepatic fibrosis but appears to modulate the PRDX6 expression bidirectionally [19,20]. Specifically, both the protein and mRNA levels of PRDX6 are elicited in hepatic fibrosis induced by CCl4 after four weeks; however, eight weeks after the treatment, only the mRNA level of PRDX6 is increased while its protein level shows a comparative decrease. This paradox may be attributed to the release of mesencephalic astrocyte-derived neurotrophic factor (MANF), which promotes the PRDX6 release in relation to the intensity and duration of CCl4 exposure or to the protein leakage from apoptotic or necrotic hepatocytes caused by CCl4 [19]. Collectively, these findings underscore the complex regulatory effects that chemical agents have on PRDX6 expression and highlight the critical roles played by various transcriptional factors (e.g., NRF2 and NF-κB) in modulating its expression.
PRDX6 has also been shown to underlie the effects of several chemical stressors on male infertility. For example, an exposure to 4-tert-octylphenol or paclitaxel can impair sperm motility and viability by downregulating the PRDX6 levels, alongside the inhibition of the activities of protein kinase A/C (PKA/C), sirtuin 1 (SIRT1), or NRF2 [21,22]. On the other hand, a controlled clinical trial reports that the expression of semen PRDX6 is significantly elevated following the consumption of date palm pollen, which positively correlates with improved parameters of sperm, including count, volume, motility, and morphology [23]. Rodent studies have also demonstrated a marked upregulation of protective PRDX6 protein in both the epididymis and spermatozoa upon exposure to Di-n-butyl phthalate or tert-butyl hydroperoxide [24,25]. Although the precise molecular mechanisms underlying these observations remain largely elusive, these findings have established a strong link between PRDX6 dysregulation and male reproductive health, indicating the critical role of this antioxidant enzyme in the pathogenesis of male infertility.
Apart from the various chemical factors mentioned above, the alteration in PRDX6 expression can be influenced by certain physical stressors. Previous proteomic analyses have identified an upregulation of PRDX6 in both the spleen and liver of mice when exposed to proton beam (2 Gy), ionizing radiation (10 Gy), and carbon nanotubes [26,27,28]; while an exposure to electron beam irradiation (45 Gy) or ultraviolet-B can downregulate the expression of PRDX6 in rodent skin by regulating miR-214 and BMAL1 (brain and muscle ARNT-like protein 1) signals [29,30]. Other physical factors, such as temperature [31,32], hyperoxia [33], or hypoxia [32], have also been documented to impact the expression of PRDX6. These findings indicate the significant associations between the alteration in PRDX6 expression and cellular antioxidant defense mechanisms.
Additionally, physiological activities, such as exercise and reproductive activities (e.g., mating or fertilization), have been shown to stimulate the expression of PRDX6 in human blood [34] and rodent oviducts or colons [35,36]. Biological infections caused by bacterial, viral, or parasitic agents can disrupt immune homeostasis in mouse pulmonary and hepatic systems by affecting PRDX6 expression possibly through several transcriptional factors, such as NF-κB, NRF2, and CCAAT/enhancer-binding protein beta (C/EBPβ) [37,38,39].
A comprehensive summary of additional external stimuli contributing to PRDX6 dysregulation in vivo is detailed in Table 1.

Table 1.
In vivo regulations of PRDX6 induced by external stimuli.
2.2. In Vitro Dysregulation of PRDX6 Expression Induced by Various Stressors
A growing body of research utilizing multiple cell lines has demonstrated the regulation of PRDX6 by various external stressors, several of which are particularly interesting and merit in-depth discussion. For instance, physical factors, such as ionizing radiation (2 or 20 Gy) and electromagnetic waves, can elevate the PRDX6 expression in both human epithelial cells and monocytes to maintain redox homeostasis and protect against oxidative stress and inflammation [70,71,72]. More intriguingly, certain biochemical agents, including curcumin, hydrogen peroxide (H2O2), and inflammatory cytokines, have been extensively investigated for their distinct regulatory effects on the PRDX6 expression [73,74] and will be overarchingly summarized below.
As a polyphenol extracted from turmeric rhizome, the mechanism underlying the protective effects of curcumin has been a research focus for decades. Treatment with curcumin, at doses of 5 µM for 48 h or 109 µM for 2 h, has been reported to mitigate inflammation and oxidative stress by increasing PRDX6 expression possibly through the enhanced transcriptional activities of specificity protein 1 (SP1) [74,75]. On the other hand, curcumin administered at a dose of 1 µM for 24 h decreases the PRDX6 level to inhibit astrocyte activation [76]. Proteomics analyses have also indicated that the administration of curcumin at doses ranging from 25 µM or 1737 µM over a period of 72 h can reverse multi-drug resistance in tumors and inhibit tumor cell proliferation by reducing the PRDX6 levels through p53 [77,78]. Therefore, it is reasonable to conclude that PRDX6 serves as a crucial downstream target in curcumin-conferred protections, although there is still no consensus regarding its optimal dosage and timing on protein expression. In addition to curcumin, an array of phytochemicals, such as baicalein, luteolin, andrographolide, and glycyrrhizin, have been extensively documented as potent modulators for PRDX6 expression [79,80,81,82,83].
Another intriguing example is H2O2, a well-known inducer of oxidative stress, which forms a much more complex relation with PRDX6 expression. Specifically, previous studies have reported that H2O2 administered at a low dose (25 µM for 9 h) may increase the expression of PRDX6 in mouse HEI-OC1 cell line [13]. Moderate doses of H2O2 (e.g., 100 µM for 30 min, 1 h, or 24 h; and 300–500 µM for 6 h or 48 h) can reduce the cellular expression of PRDX6, accompanied by increased cellular apoptosis and oxidative stress [13,50,79,84,85,86]. However, an exposure to higher concentrations of H2O2 (1000 µM) may increase the PRDX6 levels in mouse H2.35 cells after treatment for either 8 h or 24 h [87], but decrease its expression in human APRE-19 cells after a duration of 48 h [84], suggesting potential dual effects of high concentration of H2O2 on the PRDX6 expression. These reports indicate that the H2O2-mediated regulation of PRDX6 is largely contingent upon the specific dosage and exposure time. Recent, more direct evidence shows that the gradient concentration of H2O2 (ranging from 50 to 200 µM over a period of 24 h and 50 µM across time points from 2 h to 24 h) gradually upregulates the PRDX6 levels in human astrocyte cell lines (HA-sp and A172) [76]. Collectively, H2O2 may exert influences on the expression of PRDX6 in a dose- and time-dependent manner, possibly associated with the extent of cellular oxidative stress.
Inflammatory cytokines have also been demonstrated to modulate the expression of PRDX6 bidirectionally. For instance, tumor necrosis factor alpha (TNFα) treatment has been shown to upregulate the PRDX6 level in human embryonic kidney cells during short exposure (5 h, 7 h, or 9 h), while downregulating its expression in rat pancreatic beta cells (RINm5F) and retinal ganglion cells following prolonged exposure (24 h, or 10 days). These alterations in PRDX6 expression are linked to critical cellular processes, including cell migration, oxidative vulnerability, and cell death [88,89,90]. Additionally, both interferon γ (140 U/mL for 24 h) and lipopolysaccharide (1, 10, or 20 µg/mL for 8 h or 24 h) can decrease the PRDX6 expression in insulin-producing RINm5F cells [89] and tracheobronchial epithelial cells [91], thereby enhancing cellular susceptibility to oxidative stress and suppressing inflammatory responses, respectively, while combinations of them in turn stimulate its expression in mouse bone-marrow-derived macrophages, conferring protection against oxidative and nitrosative stress [92]. These differences may, of course, be attributed to variations among the cell lines used, but when considering the intricate protein post-translational modifications and enzymatic activities of PRDX6, its expression can vary under different and even identical external stressors. Despite this, these results indicate PRDX6 as a key downstream molecular target of cellular inflammation or immune cascade. It is important to concurrently assess both the protein post-translational modification states and enzymatic activities of PRDX6 when detecting its expression to obtain a comprehensive landscape.
A more comprehensive summary of various external stimuli that influence the dysregulation of PRDX6 in vitro is detailed in Table 2.

Table 2.
In vitro regulations of PRDX6 induced by external stimuli.
Taken together, extensive evidence from vertebrate models to various cell lines has revealed that the expression of PRDX6 is susceptible to change by diverse external stressors, which can be systematically classified into three major categories: chemical, physical, and biological factors. Several mechanisms may account for their regulatory effects on PRDX6 expression: (1) modulation of the activities of key transcriptional factors, particularly NRF2, HIF-1α, SP1, and NF-κB; (2) specific inductions of micro-RNAs, notably miR-214 and miR-27b; (3) activation of receptor-mediated signaling pathways, such as glucagon-like peptide-1 receptor (GLP-1R), epidermal growth factor receptor (EGFR), and N-methyl-D-aspartate receptor (NMDAR); (4) protein kinase-dependent phosphorylation cascades, primarily through PKC and MAPKs; (5) direct binding to its gene response elements; and (6) additional mechanisms currently unidentified. This intricate regulatory network elucidates the sophisticated control of PRDX6 expression in response to various stressors, offering novel insights into cellular stress orchestration.
Nevertheless, the relation between PRDX6 expression and its functional ramifications remains poorly understood, primarily due to the lack of studies that have concurrently assessed changes in enzymatic activities. Of note, an elevated PRDX6 expression may fail to inherently confer a protective effect, as such a change may also indicate an adaptive context-dependent response that can be beneficial, neutral, or potentially detrimental. Furthermore, a significant proportion of proteomic studies have merely reported the changes in PRDX6 expression without elucidating their functional implications, thereby limiting comprehensive understanding of its biological roles. Collectively, these findings highlight the pivotal role of PRDX6 in response to external stressors, enhancing our understanding of its molecular characteristics and therapeutic potentials.
3. Conformational and Functional Switches of PRDX6 in Response to Stressors
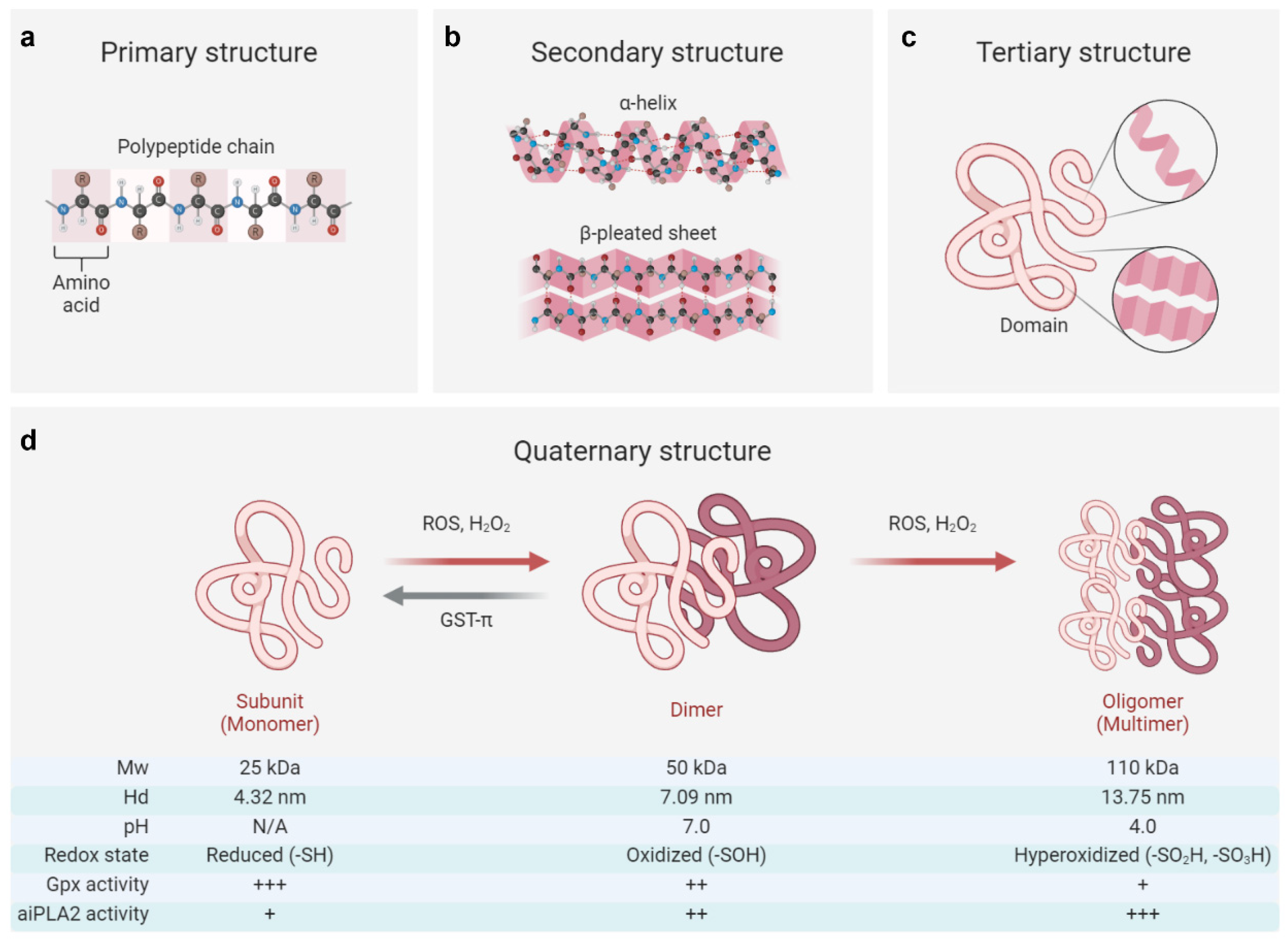
The primary structure of PRDX6 consists of a polypeptide chain comprising 224 amino acids, characterized by a single conserved redox-active C47 residue (peroxidative cysteine) at the N-terminal end of the α2 helix within βαβ motif of the thioredoxin fold. PRDX6 typically exists as a secondary or tertiary structure, forming a homodimer linked through hydrogen bonding networks between two β-strands from each monomer [126,127]. Under hyperoxidative conditions, PRDX6 may adopt an oligomeric quaternary structure [126]. Notably, both the internal and external stressors, such as reactive oxygen species, pH, and post-translational modifications, can regulate the conformation of PRDX6 [3,128,129], which is closely associated with its enzymatic properties (Figure 1).
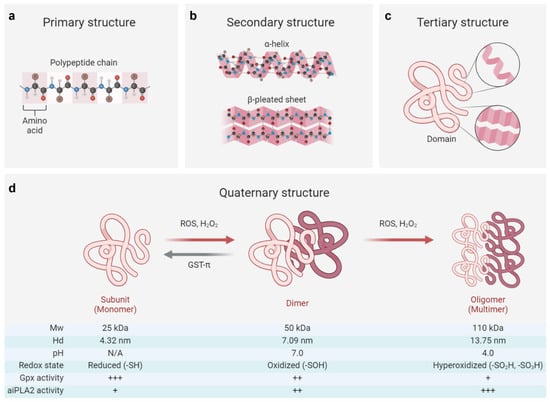
Figure 1.
Structural and functional switch of the PRDX6 protein. (a–c) The primary structure of PRDX6 consists of a polypeptide chain composed of 224 amino acids, which subsequently folds into secondary structures (including α-helix and β-pleated sheet) and a tertiary domain [1,126,127]. (d) The quaternary structure of PRDX6 exhibits dynamic regulation in response to oxidative stress and pH variations, enabling its functional switching [3,128,129]. Briefly, the reduced form (-SH) of the PRDX6 monomer (Mw: ~25 kDa, Hd: ~4.32 nm) can transition to an oxidized form (-SOH) as a dimer (Mw: ~50 kDa, Hd: ~7.09 nm) in response to oxidative stress induced by reactive oxygen species (ROS) or hydrogen peroxide (H2O2). This transformation is often associated with attenuated Gpx activity and increased aiPLA2 activity, which can be reversed by enzymes such as glutathione S-transferase π (GST-π). On the other hand, the oxidized PRDX6 dimer may undergo further hyperoxidation (-SO2H; -SO3H), resulting in a multimeric oligomer form (Mw: ~110 kDa, Hd: ~13.75 nm), achieving its maximum aiPLA2 activity within acidic organelles at approximately pH 4. Of note, the oligomeric state of its reduced form (-SH) remains controversial, with conflicting reports suggesting either a monomeric [3] or dimeric [126,127,128] configuration in solution. Mw, molecular weight; Hd, hydrodynamic diameter; +, ++, and +++, respectively, indicating the relative intensity of enzyme activity. The figure was created with Biorender.com.
Reactive oxygen species (ROS), particularly H2O2, function as critical messengers that regulate cellular activities through their interactions with the PRDX6 protein [73,130]. At low concentrations (<100 μM), H2O2 increases the PRDX6 expression via HIF-1α or NRF2 signaling pathways, while at higher concentrations (>100 μM), it can reduce the PRDX6 level by inhibiting the transcriptional activities of NRF2 and HIF-1α or activating the NF-κB signaling pathway [13,73,86]. Beyond regulating the expression of PRDX6, H2O2 also influences its structural adaptions [73]. Elevated concentrations of H2O2 (>100 μM) facilitate monomer–dimer transitions in PRDX6 and induce its transformation from dimeric to multimeric states [3,126]. Mass spectroscopy studies reveal that the monomeric form of PRDX6 (Mw: 25 kDa; Hd: 4.32 nm) exists in a reduced state while its dimeric counterpart (Mw: 50 kDa; Hd: 7.09 nm) is oxidized [3]. These oxidative modifications of PRDX6 profoundly impact its enzymatic functions and subsequent pathophysiological consequences.
Specifically, the oxidized (-SOH) form of PRDX6 can be reduced by glutathione S-transferase π (GST-π) in the presence of glutathione, providing a crucial compensatory mechanism against intracellular oxidative damage; in contrast, its hyperoxidized forms (-SO2H and -SO3H) are irreversible, exhibiting diminished Gpx activity but dramatically enhanced aiPLA2 activity [3,126]. The hyperoxidized PRDX6 has been implicated in exacerbating various pathological conditions, including acute lung injury, acute liver injury, and cerebral ischemia by triggering oxidative stress and inflammatory cascades [131,132,133]. Intriguingly, this hyperoxidized state may also confer antitumor effects by disrupting extracellular signal transduction pathways and inducing cell cycle arrest [4,126]. However, the precise relation between PRDX6 expression, the transitions of its oxidative state, and their pathophysiological consequences remains largely unknown and warrants further investigation.
Furthermore, it has been shown that the aiPLA2 activity of PRDX6 can be markedly augmented by MAPK-induced phosphorylation at the T177 residue [129]. This post-translational modification plays a crucial role in the degradation of dipalmitoyl phosphatidylcholine and the subsequent remodeling of phosphatidylcholines, thereby contributing to the pathogenesis of various diseases, such as drug addiction and acute lung injury [131,134]. Notably, the alteration in pH can also bring conformational changes in PRDX6, existing predominantly as a dimeric structure at physiological pH (7.0) while forming a tetrameric configuration (Mw: 110 kDa, Hd: 13.75 nm) under acidic conditions (pH 4.0). This structural plasticity provides a basis for its remarkable stability against lysosomal pH and thermal denaturation [128]. Additionally, pharmacological agents, such as acetaminophen and astragaloside IV, have been shown to modulate the conformational stability of PRDX6 through direct interactions with its catalytic sites, consequently impairing its antioxidant functions and cellular responses to oxidative stress [135,136].
Taken together, PRDX6 represents a structurally and functionally sophisticated enzyme whose quaternary structure is dynamically modulated by various stressors, with its multifaceted biological activities being intricately linked to its conformational states. Given the crucial role of such protein conformational and functional switches in physiological maintenance and pathological progression, further in-depth explorations are warranted to elucidate the molecular intricacies and pathophysiological implications of PRDX6 regulation.
4. The Close Involvement of PRDX6 in Diverse Pathological Conditions
PRDX6 has been implicated in the pathogenesis of various chronic noncommunicable diseases [1,2]. Our previous studies have particularly investigated its significant roles in the pathophysiology of the central nervous system, encompassing neurodegeneration, stroke, neurotrauma, gliomas, major depressive disorder, and post-traumatic stress disorder [1,137,138]. Recent advances in reproductive biology have identified PRDX6 as a key protector of spermatozoa against oxidative damage, primarily through its dual Gpx and aiPLA2 activities [8,139,140]. A deficiency in PRDX6 is associated with impaired sperm function and compromised DNA integrity in both human male infertility and rodent models [140,141], while its elevation may underlie the protective effects of curcumin and melatonin on sperm motility, viability, fertilization rates, and blastocyst development [42,54]. Notably, although the scientific community remains divided regarding its role in oncogenesis, compelling evidence suggests that PRDX6 exerts substantial influence on cancer initiation and progression across various malignancies [10], making it a focal point in the present review (Table 3).
The dysregulation of PRDX6 expression has been extensively documented across a spectrum of malignancies. Specifically, PRDX6 demonstrates tissue-specific expression patterns, with downregulation observed in renal cell carcinoma [142]; while a substantial body of pre-clinical and clinical studies suggests PRDX6 upregulation in melanoma [98] and various solid tumors, including brain tumors [1,143], lung cancer [142,144,145,146,147,148,149], cervical carcinoma [142,150], endometrial cancer [142,151], ovarian cancer [142], skin malignancies [152], head and neck squamous cell carcinoma [153], and bladder cancer [142,154,155]. A higher tissue content of PRDX6 is often correlated with lower overall survival rates and poorer outcomes [142,156,157,158], but paradox findings exist in certain cancer types. For instance, integrative bioinformatics analyses have suggested that increased expression of PRDX6 serves as a poor prognostic factor for liver cancers [142,159], while preclinical studies identify it as a suppressor for carcinogenesis in both liver and prostate tissues [160,161]. Similar results can be found in cancers from the colorectum [162,163,164], thyroid gland [142,165], and stomach [166,167]. Several plausible explanations may account for these discrepancies: (1) the complex enzymatic activities and extensive post-translational modifications of PRDX6 protein; (2) the substantial intrinsic heterogeneity across different cancer types; and (3) potential variations in experimental methodologies or differences among animal models employed in these studies.
Additionally, the enzymatic activity of PRDX6 typically correlates with its expression level; however, the predominant activities of PRDX6 enzymes can vary under specific pathological conditions. For instance, in the central nervous system, PRDX6-aiPLA2 has been reported to aggravate neuroinflammation following ischemic stroke by modulating astrocyte-induced M1 microglia activation and promoting the secretion of neurotoxic inflammatory mediators, including IL-1β, IL-17, and IL-23 [133,168]. Inhibition of its aiPLA2 activity significantly reduces neurologic deficits, cerebral infarction, brain water content and inflammatory molecules [168]. It has been observed that PRDX6 is upregulated in the hippocampus of chronic epilepsy rats, accompanied by an increase in aiPLA2 activity over Gpx activity in astrocytes, which leads to prolonged seizure activity due to the autophagic degeneration of astroglial cells [169]. Although PRDX6-aiPLA2 activity has been shown to promote cancer cell death induced by TNFα in hepatocellular carcinoma [160], previous research using specific pharmacologic inhibitors and mutagenesis studies has demonstrated the critical roles played by both enzyme activities for lung tumor development [170,171]. Specifically, PRDX6-Gpx activity facilitates lung cancer growth while its aiPLA2 activity enhances invasiveness. The latter is mediated by the accumulation of arachidonic acid and subsequent activation of invasive signaling pathways involving p38 MAPK, PI3K-Akt signaling cascades, and urokinase-type plasminogen activator [170]. Therefore, these findings underscore that both enzymatic activities of PRDX6 are closely implicated in disease onset and progression.
Emerging evidence has demonstrated the presence of PRDX6 in human body fluids. Specifically, the expression of PRDX6 is documented to elevate in the sera of patients with lung squamous cell carcinoma [147] or hepatocellular carcinoma [160], but decrease in both the sera of patients with esophageal or colon carcinoma [147] and the interstitial fluid of patients with breast cancer [158]. Comparative proteomics analysis has revealed that plasma-derived small extracellular vesicles containing a signature of PRDX6 are capable of predicting the response of locally advanced rectal cancer to neoadjuvant chemoradiotherapy [172]. In addition, PRDX6 also presents in human cerebral spinal fluid and urine [173,174]. These reports merely emphasize the significance of serum PRDX6 as a biomarker for cancer diagnosis and monitoring treatment efficacy, without due attention to the underlying mechanisms. Recent research has indicated that PRDX6 may be extracellularly secreted to eliminate the ROS via its aiPLA2 activity or membrane aquaporins [73,175]. It is noteworthy to mention that the presence of PRDX6 in blood may present as a tumor antigen. Indeed, to facilitate diagnosis of early-stage malignant tumors and novel effective immunotherapies, Nakanishi et al. have detected specific antibodies against PRDX6 in peripheral blood from patients with esophageal squamous carcinoma and lung adenocarcinoma [149,176]. Despite nearly two decades of effort, it remains unknown regarding the mechanisms underlying the generation of PRDX6 antibody and its functional implications in cancer pathogenesis. With advances in tumor immunology, further studies are warranted to elucidate the role of PRDX6 and its corresponding antibodies in tumorigenesis and cancer progression.

Table 3.
The expressions and effects of PRDX6 across various solid cancers.
Table 3.
The expressions and effects of PRDX6 across various solid cancers.
| Cancer Types | Expressions | Effects | Samples | Methods | References | |
|---|---|---|---|---|---|---|
| Tissue | Fluid | |||||
| Glioma | ↑ | NA | + | H | 2-DE, BA | [1,143] |
| Head and neck carcinoma | ↑ | NA | + | H | BA | [153] |
| Lung cancer | ↑ | ↑ * | + | H, M, C | BA, qPCR, WB, IHC, ELISA, IF, 2-DE | [142,144,145,146,147,148,149] |
| Esophageal carcinoma | ↑ | ↓ * | + | H, C | BA, WB, IHC, IF, 2-DE | [142,147,176,177] |
| Cervical cancer | ↑ | NA | + | H, M, C | BA, IHC, WB | [142,150] |
| Endometrial cancer | ↑ | NA | + | H, M, C | BA, WB | [142,151] |
| Ovarian cancer | ↑ | NA | + | H | BA | [142] |
| Melanoma | ↑ | NA | + | H, F, C | BA, qPCR, WB | [98,142] |
| Cholangiocarcinoma | ↑ | NA | + | H, R | IHC, IF, WB | [43] |
| Skin cancer | ↑ | NA | +/- | H, M | IHC | [152] |
| Bladder cancer | ↑ | NA | +/- | H | BA, IHC, qPCR | [142,154,155] |
| Breast carcinoma | ↑/↓ | ↓ # | + | H, M, C | 2-DE, IHC, qPCR, WB, BA | [142,156,157,158] |
| Hepatocellular carcinoma | ↑/↓ | ↑ * | +/- | H, M, C | BA, MS, qPCR, WB, IHC | [142,159,160] |
| Colorectal cancer | ↑/↓ | ↓ * | +/- | H, C | BA, IHC, ELISA | [142,162,163,164] |
| Thyroid cancer | ↑/↓ | NA | +/- | H, C | BA, qPCR, WB, IHC | [142,165] |
| Gastric cancer | ↑/↓ | NA | +/- | H, C | IHC | [166,167] |
| Prostate cancer | ↑/↓ | NA | - | H, M | BA, qPCR, IHC | [142,161] |
| Kidney cancer | ↓ | NA | + | H | BA | [142] |
↑, upregulated expressions of PRDX6; ↓, downregulated expressions of PRDX6; +, promoting tumorigenesis; -, inhibiting tumorigenesis; NA, not applicable; H, human; R, rat; M, mouse; C, cell; F, fish (Xiphophorus); BA, bioinformatics analysis; 2-DE, two-dimensional electrophoresis; qPCR, quantitative polymerase chain reaction; WB, Western blotting; ELISA, enzyme-linked immunosorbent assay; IHC, immunohistochemistry; IF, immunofluorescence; MS, mass spectrometry; *, examined in peripheral blood serum; #, examined in interstitial fluid.
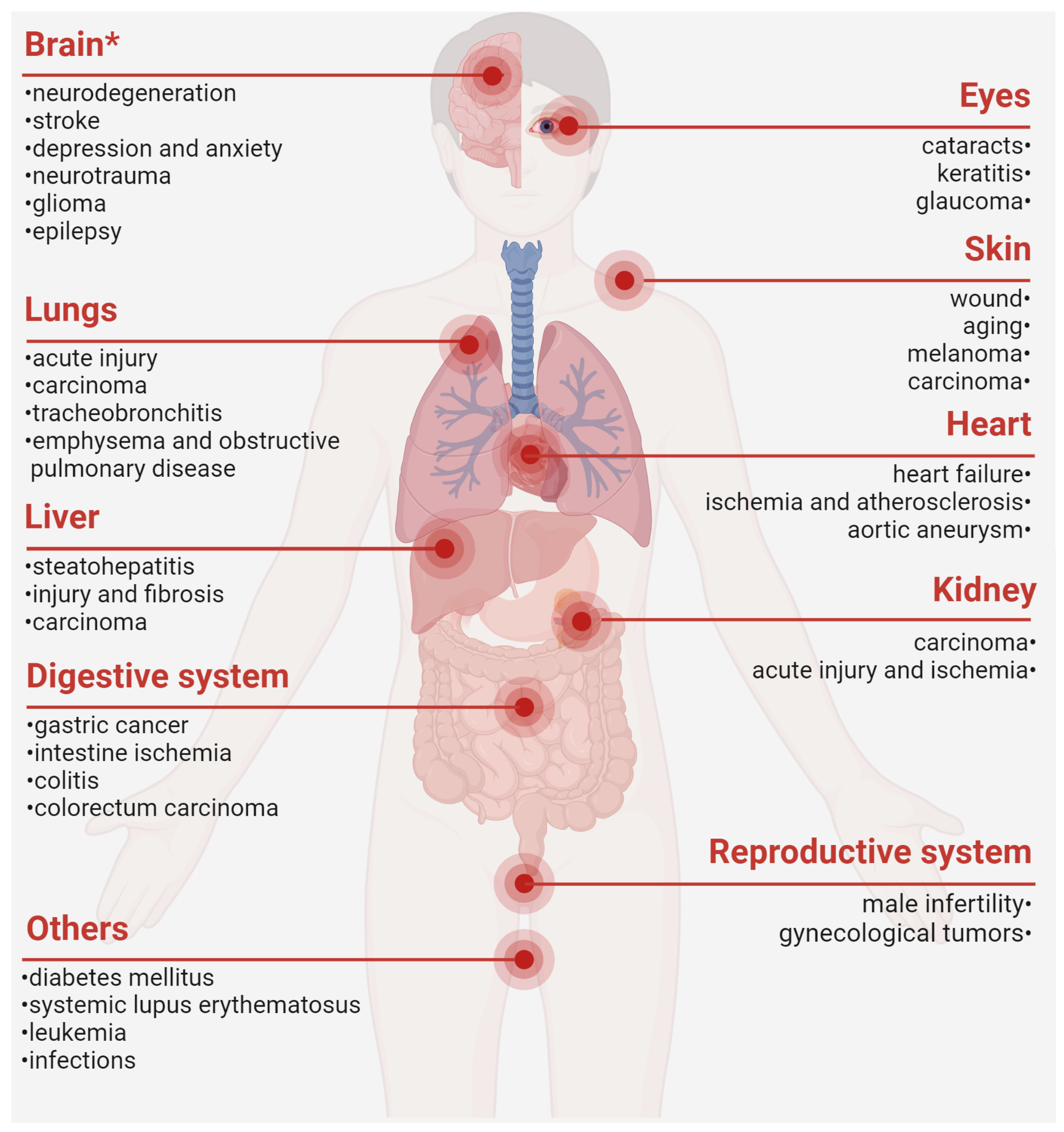
Taken together, PRDX6 is dysregulated in a cluster of diseases and closely involved in their pathogenesis, thereby substantiating the conceptualization of PRDX6-related disorders (PRD) (Figure 2). Mechanistically, PRDX6 orchestrates its effects across diverse systems via shared molecular signaling pathways, such as MAPKs, TLR (Toll-like receptors)/NF-κB, and ferroptosis [1,73,178]. For instance, PRDX6 has been implicated in neurodegeneration [1], male infertility [8,140], and senescence and cancer [10] by attenuating oxidative stress and inflammation. However, current research may still present a conundrum regarding whether the upregulation of PRDX6 is protective, neutral, or pro-pathogenic in specific diseases. Future investigations employing well-characterized mutant or transgenic animal models may better delineate the role of PRDX6 in disease progression and remission, thereby clarifying its causal relation in disease etiology.
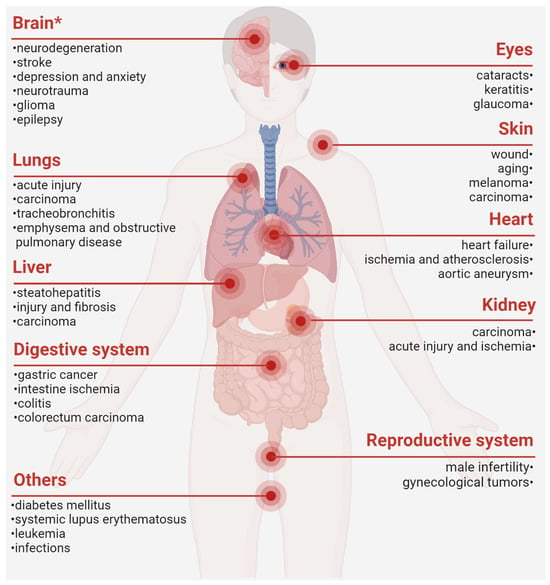
Figure 2.
PRDX6-related disorders. *, our previously published review. The figure was created with Biorender.com [1,2,8,13,15,17,19,21,22,23,24,25,30,36,39,41,45,46,47,49,75,83,84,86,100,110,111,114,125,142,144,145,146,147,148,149,150,151,152,159,160,162,163,164,166,167,175,179,180,181,182,183,184,185].
Moreover, PRDX6 holds promise as a diagnostic biomarker or therapeutic target, particularly for neurological and oncological diseases. Future comprehensive and large-scale studies are imperative to evaluate its specificity and sensitivity as a potential clinical indicator. Given recent recognitions about the critical role of peripheral immunity in disease pathogenesis, it is compelling to explore the origin of the PRDX6 antibody and its roles in disease onset and progression. Only when these uncertainties are resolved can we advance towards the development of PRDX6-targeted interventions.
5. Therapeutic Potential of Exogenous Supplementation of PRDX6
Although PRDX6 has been considered an antioxidant enzyme playing a significant role in maintaining cellular redox homeostasis, the efficacy of its exogenous administration for conferring antioxidant protection remains controversial. The challenge lies in the intracellular delivery of a mature protein because of its impermeability to the cell membrane. Previously, an HIV transactivating transduction (TAT) domain (RKKRRQRRR) has been engineered to the N-terminal region of the PRDX6, which enables 100% delivery across both the plasma membrane and blood–brain barrier. With this strategy, researchers have demonstrated that administration of PRDX6 not only restores its gene promoter activity [11] but protects against oxidative stress, apoptosis, and inflammatory insults in both the lens epithelial cells and retinal ganglion cells [90,186,187,188,189].
Under normal conditions, studies using recombinant PRDX6 labeled with fluorescein isothiocyanate (FITC) and molecular docking techniques have observed an approach of PRDX6 to the cell surface, serving as a close engagement with the membrane TLR4 receptor rather than a simple interaction [123]. Moreover, a part of the PRDX6 protein can intracellularly penetrate via its aiPLA2 activity, presumably accumulating in the transport vesicles from endoplasmic reticulum rather than in the nucleus [73,123,190]. More intriguingly, in vivo studies indicate that intravenous pretreatments with PRDX6 not only mitigate ischemia/reperfusion (I/R)-induced injuries in both intestinal and renal tissues [179,180] but also reduce mortality rates, glycemia- and cytokine-induced cytotoxicity, and radiation-induced injuries [175,191]. An intraperitoneal injection of exogenous PRDX6 protein has been shown to enhance blood–brain barrier integrity and improve overall health status in mice suffering from relapsing/remitting experimental autoimmune encephalomyelitis, which may be mediated by a reduced expression of NADPH oxidase 1/4 (NOX1/4) in the brain [192]. Other researchers have also demonstrated that topical administration or subcutaneous injection of PRDX6 can ameliorate wound-induced scar formation, ultraviolet-induced corneal injury, and myocardial ischemic damage in rat models [181,182,183].
Mechanistically, the administration of the recombinant PRDX6 protein restores cellular gene signatures associated with oxidative stress response (e.g., NRF2 and MAPKs), cell cycle regulation and apoptosis (e.g., p21, p53, and caspase-3), glucose metabolism (e.g., glucose transporter 2), cellular senescence (e.g., SA-β-Gal), and inflammatory pathways (e.g., TLR4, NF-κB, and IL-1β) [175,190,193,194]. Although the specific mechanisms by which PRDX6 triggers these gene cascades remain obscure, these findings strongly support the immense therapeutic potential of PRDX6 in attenuating oxidative stress and relevant pathological conditions, particularly in ocular disorders such as cataract and neurological diseases including encephalomyelitis.
The dual enzymatic properties of PRDX6 are essential for conferring its cytoprotective effects. Studies of site-directed mutagenesis have demonstrated that the C47 mutation, which results in a loss of Gpx activity of PRDX6, significantly attenuates its protective capacity against I/R-induced renal and intestinal injuries [179,180] and irradiation-induced lens epithelial cell damage [186,187]. However, the C47S mutant PRDX6 retains partial radioprotection against X-ray-induced oxidative stress, possibly owing to its extracellular form as a signaling molecule [123,191]. On the other hand, the S32 mutation in PRDX6, while abolishing its aiPLA2 enzymatic activity, displays greater cardioprotective effects against myocardial injury through its augmented antioxidant capacity [183]. These findings collectively reveal that the biological functions of PRDX6 are mediated through a complex interplay between its Gpx and aiPLA2 enzymatic activities, along with its extracellular penetration capability.
It is an intriguing question to inquire about the distribution of PRDX6 protein in the body upon intravenous administration. Pre-clinical studies have reported that PRDX6 is specifically localized within the blood vessel lumen after intravenous injection but can penetrate through the vascular wall [179]. Recent investigations have shown that PRDX6 exhibits a diffuse distribution in intestinal epithelium 15 min later, with approximately 50% remaining in circulation one hour after intravenous administration [179,191]. Pharmacokinetic analyses reveal that PRDX6 retains its highest concentration in the bloodstream for 10 min post-intravenous delivery but gradually decreases over time, by around 80% within the first hour and about 30% after six hours [180]. These data suggest that intravenous administration of PRDX6 attains peak concentrations in the blood and enables rapid distribution to multiple organs within 10–15 min, suggesting potential applications particularly under emergency or critical conditions.
Several critical issues remain to be addressed regarding the therapeutic application of recombinant PRDX6 protein. First, significant gaps remain in our understanding of its safety profile, therapeutic efficacy, and potential adverse effects, particularly the immunogenicity risk associated with repeated administration. Further investigations are awaited to elucidate the pharmacokinetic properties, immune responses, post-translational modifications (e.g., hyperoxidation), and comparative effectiveness in relation to established antioxidant therapies. Second, balancing the Gpx and aiPLA2 enzymatic activities within recombinant PRDX6 protein remains a significant challenge, as excessive PRDX6 activity in certain contexts may promote inflammation through arachidonic acid release. Additionally, the therapeutic window, including optimal dosage, administration timing, and delivery methods, may need to be tailored to specific disease pathophysiology. Lastly, repeated administration of recombinant PRDX6 protein may pose clinical challenges for the management of chronic diseases, including potential economic burdens. Alternative delivery strategies, such as viral vectors, mRNA-based therapies, and nanoparticle-mediated delivery systems, may overcome the limitations associated with repeated protein administration.
Despite these challenges, accumulated pre-clinical studies have demonstrated that exogenous supplementation of recombinant PRDX6 protein, regardless of the dosage or administration strategies, effectively mitigates oxidative stress-related pathological alterations (Table 4). This therapeutic approach offers distinct advantages, particularly its capacity to rapidly induce both local and systemic antioxidant and immunomodulatory effects. These findings also provide a valuable foundation for the development of novel pharmaceutical interventions targeting oxidative stress-related disorders. Nevertheless, the path to clinical translation remains complex and protracted, requiring sustained effort and extensive exploration to address these barriers.

Table 4.
Protective effects of exogenous PRDX6 supplementation.
6. Conclusions and Future Perspectives
PRDX6, a moonlighting protein endowed with diverse enzymatic activities, has garnered significant attention in recent years. Although it is predominantly localized within the cellular compartment, this enzyme demonstrates the capacity for extracellular secretion under specific pathophysiological conditions. The expression profile and subcellular distribution of PRDX6 are dynamically regulated by a spectrum of external stimuli, which can be systematically classified into chemical, physical, and biological categories. Furthermore, both internal and external stressors, such as ROS, pH fluctuations, and post-translational modifications, can modulate the structural dynamics and functional switches of this enzyme. Given these findings, it is interesting to speculate that PRDX6 may play a pivotal role not only in intercellular signaling transduction, but also in mediating gene-environmental interactions. The inherent susceptibility of PRDX6 to various stressors qualifies it as a promising biomarker for disease recognition and treatment monitoring.
Accumulating evidence has indicated the dysregulation and pathophysiological involvement of PRDX6 in various disease states, particularly in neuropsychiatric disorders and oncological conditions. Emerging genetic studies have revealed significant associations between the PRDX6 gene polymorphisms and individual susceptibility to systemic lupus erythematosus [184] and chronic obstructive pulmonary disease [185], emphasizing its critical roles in disease-related genetic variability. Whilst the precise mechanisms need further elaboration, the dysregulation of PRDX6 appears to indicate a common molecular pathway or pathological event in multiple diseases. Based on these scientific observations, we propose the concept of PRDX6-related disorders (PRD), which refers to a spectrum of diseases mediated by or associated with dysregulation of PRDX6 expression or enzymatic activity.
The remarkable antioxidant capacity of PRDX6 has prompted investigations into its preventive and therapeutic potential through exogenous supplementation, either as the native protein or in compound formulations, particularly for conditions involving oxidative stress, a ubiquitous pathological process across numerous diseases. More intriguingly, emerging evidence suggests that PRDX6 may play a critical role in immune modulation, given its presence in various immune cells (e.g., neutrophils, monocytes, and macrophages) and its detection in bodily fluids alongside a specific antibody. These findings suggest new avenues for the development of PRDX6-targeted immunotherapeutic strategies for PRD management.
The present comprehensive review systemically examined the various stressors regulating the PRDX6 expression and its structure-based functional switches, introduced the novel concept of PRDX6-related disorders, and evaluated the therapeutic potential of exogenous PRDX6 administration in oxidative stress-related pathologies. Our primary objective was to provide a conceptual framework for and offer a macroscopic perspective into the response of PRDX6 to diverse stressors, rather than delving into intricate mechanistical investigations. While we acknowledge certain limitations in this approach, we have also incorporated discussions of several key stressor-induced regulatory mechanisms for the PRDX6 expression and activity. Collectively, this review positions PRDX6 as a cellular orchestrator in response to both internal and external stressors, underscoring its clinical potential in disease diagnosis and therapeutic intervention.
Author Contributions
J.L.: Data curation, investigation, methodology, project administration, software, funding acquisition, and writing—original draft. Y.Z. and J.Y.: Data curation, investigation, methodology, and software. L.C.: Resources, supervision, and data curation; J.Z. and X.C.: Conceptualization, project administration, resources, supervision, data curation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (82301707), Fujian Provincial Health Technology Project (2022GGA027), and the First Affiliated Hospital of Fujian Medical University (YJRC3975; PT-82301707).
Acknowledgments
We thank Hongzhi Huang from Fujian Medical University for his language help and writing assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PRDXs | peroxiredoxins |
| Cys | cysteine |
| -SH | reduced state |
| -SOH | sulfenic state |
| -SO2H | sulfinic acid |
| -SO3H | sulfonic acid |
| ATP | adenosine triphosphate |
| Gpx | glutathinone peroxidase |
| aiPLA2 | acidic calcium-independent phospholipase |
| LPCAT | lysophosphatidylcholine acyl transferase |
| SP1 | specificity protein 1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| MgSO4 | magnesium sulfate |
| CoCl2 | cobalt chloride |
| NF-κB | nuclear factor kappa B |
| MAPKs | mitogen-activated protein kinases |
| CCl4 | carbon tetrachloride |
| MANF | mesencephalic astrocyte-derived neurotrophic factor |
| PKA/C | protein kinase A/C |
| SIRT1 | sirtuin 1 |
| BMAL1 | brain and muscle ARNT-like protein 1 |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| H2O2 | hydrogen peroxide |
| IL-1β | Interleukin 1β |
| PI3K | phosphatidylinositol 3-kinase |
| Akt | protein kinase B |
| TNFα | tumor necrosis factor alpha |
| GLP-1R | glucagon-like peptide-1 receptor |
| EGFR | epidermal growth factor receptor |
| NMDAR | N-methyl-D-aspartate receptor |
| ROS | reactive oxygen species |
| PRD | PRDX6-related disorder |
| TLR | Toll-like receptor |
| TAT | HIV transactivating transduction |
| FITC | fluorescein isothiocyanate |
| I/R | ischemia/reperfusion |
| NOX1/4 | NADPH oxidase 1/4 |
References
- Liao, J.; Zhang, Y.; Chen, X.; Zhang, J. The Roles of Peroxiredoxin 6 in Brain Diseases. Mol. Neurobiol. 2021, 58, 4348–4364. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, F.; Della Morte, D.; Capuani, B.; Pastore, D.; Bellia, A.; Sbraccia, P.; Di Daniele, N.; Lauro, R.; Lauro, D. Peroxiredoxin6, a Multitask Antioxidant Enzyme Involved in the Pathophysiology of Chronic Noncommunicable Diseases. Antioxid. Redox Signal. 2019, 30, 399–414. [Google Scholar] [CrossRef]
- Chowhan, R.K.; Rahaman, H.; Singh, L.R. Structural basis of peroxidase catalytic cycle of human Prdx6. Sci. Rep. 2020, 10, 17416. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jo, H.Y.; Kim, M.H.; Cha, Y.Y.; Choi, S.W.; Shim, J.H.; Kim, T.J.; Lee, K.Y. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J. Biol. Chem. 2008, 283, 33563–33568. [Google Scholar] [CrossRef] [PubMed]
- Iakoubova, O.A.; Pacella, L.A.; Her, H.; Beier, D.R. LTW4 protein on mouse chromosome 1 is a member of a family of antioxidant proteins. Genomics 1997, 42, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Sorokina, E.M.; Li, H.; Zhou, S.; Raabe, T.; Feinstein, S.I. A novel lysophosphatidylcholine acyl transferase activity is expressed by peroxiredoxin 6. J. Lipid Res. 2016, 57, 587–596. [Google Scholar] [CrossRef]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef]
- Arevalo, J.A.; Vazquez-Medina, J.P. The Role of Peroxiredoxin 6 in Cell Signaling. Antioxidants 2018, 7, 172. [Google Scholar] [CrossRef]
- Chen, Z.; Inague, A.; Kaushal, K.; Fazeli, G.; Schilling, D.; Xavier, D.S.T.; Dos, S.A.; Cheytan, T.; Freitas, F.P.; Yildiz, U.; et al. PRDX6 contributes to selenocysteine metabolism and ferroptosis resistance. Mol. Cell 2024, 84, 4645–4659. [Google Scholar] [CrossRef]
- Wu, M.; Deng, C.; Lo, T.H.; Chan, K.Y.; Li, X.; Wong, C.M. Peroxiredoxin, Senescence, and Cancer. Cells 2022, 11, 1772. [Google Scholar] [CrossRef]
- Fatma, N.; Kubo, E.; Takamura, Y.; Ishihara, K.; Garcia, C.; Beebe, D.C.; Singh, D.P. Loss of NF-kappaB control and repression of Prdx6 gene transcription by reactive oxygen species-driven SMAD3-mediated transforming growth factor beta signaling. J. Biol. Chem. 2009, 284, 22758–22772. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Xu, L.; Huang, Y.; Chen, T.; Zhou, T.; Yang, L. Magnesium sulphate can alleviate oxidative stress and reduce inflammatory cytokines in rat placenta of intrahepatic cholestasis of pregnancy model. Arch. Gynecol. Obstet. 2018, 298, 631–638. [Google Scholar] [CrossRef]
- Pak, J.H.; Yi, J.; Ryu, S.; Kim, I.K.; Kim, J.W.; Baek, H.; Chung, J.W. Induction of Redox-Active Gene Expression by CoCl2 Ameliorates Oxidative Stress-Mediated Injury of Murine Auditory Cells. Antioxidants 2019, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Tahmasbpour, M.E.; Ghanei, M.; Panahi, Y. Oxidative stress and altered expression of peroxiredoxin genes family (PRDXS) and sulfiredoxin-1 (SRXN1) in human lung tissue following exposure to sulfur mustard. Exp. Lung Res. 2016, 42, 217–226. [Google Scholar] [CrossRef]
- Torok, S.; Almasi, N.; Veszelka, M.; Borzsei, D.; Szabo, R.; Varga, C. Protective Effects of H2S Donor Treatment in Experimental Colitis: A Focus on Antioxidants. Antioxidants 2023, 12, 1025. [Google Scholar] [CrossRef]
- Kuda, O.; Brezinova, M.; Silhavy, J.; Landa, V.; Zidek, V.; Dodia, C.; Kreuchwig, F.; Vrbacky, M.; Balas, L.; Durand, T.; et al. Nrf2-Mediated Antioxidant Defense and Peroxiredoxin 6 Are Linked to Biosynthesis of Palmitic Acid Ester of 9-Hydroxystearic Acid. Diabetes 2018, 67, 1190–1199. [Google Scholar] [CrossRef]
- Newton, B.W.; Russell, W.K.; Russell, D.H.; Ramaiah, S.K.; Jayaraman, A. Liver proteome analysis in a rodent model of alcoholic steatosis. J. Proteome Res. 2009, 8, 1663–1671. [Google Scholar] [CrossRef]
- Roede, J.R.; Stewart, B.J.; Petersen, D.R. Decreased expression of peroxiredoxin 6 in a mouse model of ethanol consumption. Free Radic. Biol. Med. 2008, 45, 1551–1558. [Google Scholar] [CrossRef]
- Tao, X.; Wang, D.; Liang, Y.; Yang, L.; He, E.; Zhou, J.; He, Y.; Liang, J.; Wang, P.; Chhetri, G.; et al. PRDX6 inhibits hepatic stellate cells activation and fibrosis via promoting MANF secretion. Biomed. Pharmacother. 2022, 156, 113931. [Google Scholar] [CrossRef]
- Luo, P.; Liu, D.; Zhang, Q.; Yang, F.; Wong, Y.K.; Xia, F.; Zhang, J.; Chen, J.; Tian, Y.; Yang, C.; et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm. Sin. B 2022, 12, 2300–2314. [Google Scholar] [CrossRef]
- Huang, S.; Cao, S.; Zhou, T.; Kong, L.; Liang, G. 4-tert-octylphenol injures motility and viability of human sperm by affecting cAMP-PKA/PKC-tyrosine phosphorylation signals. Environ. Toxicol. Pharmacol. 2018, 62, 234–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Teng, Z.; Wang, Z.; Zhu, P.; Wang, Z.; Liu, F.; Liu, X. Human umbilical cord mesenchymal stem cells (hUC-MSCs) alleviate paclitaxel-induced spermatogenesis defects and maintain male fertility. Biol. Res. 2023, 56, 47. [Google Scholar] [CrossRef]
- Falahati, A.M.; Fallahi, S.; Allamehzadeh, Z.; Izadi, R.M.; Malekzadeh, K. Effects of Date Palm Pollen Supplementations on The Expression of PRDX1 and PRDX6 Genes in Infertile Men: A Controlled Clinical Trial. Int. J. Fertil. Steril. 2023, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liao, K.; Zhang, W.; Wu, H.; Shen, B.; Xu, Z. Differential expression of peroxiredoxin 6, annexin A5 and ubiquitin carboxyl-terminal hydrolase isozyme L1 in testis of rat fetuses after maternal exposure to di-n-butyl phthalate. Reprod. Toxicol. 2013, 39, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Scarlata, E.; O’Flaherty, C. Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa. Antioxidants 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Gridley, D.S.; Freeman, T.L.; Makinde, A.Y.; Wroe, A.J.; Luo-Owen, X.; Tian, J.; Mao, X.W.; Rightnar, S.; Kennedy, A.R.; Slater, J.M.; et al. Comparison of proton and electron radiation effects on biological responses in liver, spleen and blood. Int. J. Radiat. Biol. 2011, 87, 1173–1181. [Google Scholar] [CrossRef]
- An, J.H.; Seong, J.S. Proteomics analysis of apoptosis-regulating proteins in tissues with different radiosensitivity. J. Radiat. Res. 2006, 47, 147–155. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ramezani, M.; Yazdian-Robati, R.; Behnam, B.; Razavi, A.K.; Hashem, N.A.; Mokhtarzadeh, A.; Matbou, R.M.; Razavi, B.M.; Abnous, K. Acute toxicity of functionalized single wall carbon nanotubes: A biochemical, histopathologic and proteomics approach. Chem.-Biol. Interact. 2017, 275, 196–209. [Google Scholar] [CrossRef]
- Ma, L.; Huang, M.; Sun, G.; Lin, Y.; Lu, D.; Wu, B. Puerariae lobatae radix protects against UVB-induced skin aging via antagonism of REV-ERBalpha in mice. Front. Pharmacol. 2022, 13, 1088294. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Gu, Q.; Xue, J.; Cao, H.; Tang, Y.; Xu, X.; Cao, J.; Zhou, J.; Wu, J.; et al. Protein and miRNA profiling of radiation-induced skin injury in rats: The protective role of peroxiredoxin-6 against ionizing radiation. Free Radic. Biol. Med. 2014, 69, 96–107. [Google Scholar] [CrossRef]
- Chang, C.H.; Lo, W.Y.; Lee, T.H. The Antioxidant Peroxiredoxin 6 (Prdx6) Exhibits Different Profiles in the Livers of Seawater- and Fresh Water-Acclimated Milkfish, Chanos chanos, upon Hypothermal Challenge. Front. Physiol. 2016, 7, 580. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Zanuzzo, F.S.; Xue, X.; Sandrelli, R.M.; Rise, M.L.; Gamperl, A.K. The transcriptomic responses of Atlantic salmon (Salmo salar) to high temperature stress alone, and in combination with moderate hypoxia. BMC Genom. 2021, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Wang, Z.; Chen, Y.; Li, R. Intermittent hyperbaric oxygen exposure mobilizing peroxiredoxin 6 to prevent oxygen toxicity. J. Physiol. Sci. 2019, 69, 779–790. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Moes, K.A.; Shiu, M.Y.; Hutchison, M.G.; Churchill, N.; Thomas, S.G.; Rhind, S.G. High-Intensity Interval Training Is Associated with Alterations in Blood Biomarkers Related to Brain Injury. Front. Physiol. 2018, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Popli, P.; Shukla, V.; Kaushal, J.B.; Kumar, R.; Gupta, K.; Dwivedi, A. Peroxiredoxin 6 Plays Essential Role in Mediating Fertilization and Early Embryonic Development in Rabbit Oviduct. Reprod. Sci. 2021, 29, 1560–1576. [Google Scholar] [CrossRef]
- Almasi, N.; Torok, S.; Al-Awar, A.; Veszelka, M.; Kiraly, L.; Borzsei, D.; Szabo, R.; Varga, C. Voluntary Exercise-Mediated Protection in TNBS-Induced Rat Colitis: The Involvement of NETosis and Prdx Antioxidants. Antioxidants 2023, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Limmon, G.V.; Zheng, D.; Li, N.; Li, L.; Yin, L.; Chow, V.T.; Chen, J.; Engelward, B.P. Major shifts in the spatio-temporal distribution of lung antioxidant enzymes during influenza pneumonia. PLoS ONE 2012, 7, e31494. [Google Scholar] [CrossRef]
- Sun, X.G.; Fu, X.Q.; Cai, H.B.; Liu, Q.; Li, C.H.; Liu, Y.W.; Li, Y.J.; Liu, Z.F.; Song, Y.H.; Lv, Z.P. Proteomic analysis of protective effects of polysaccharides from Salvia miltiorrhiza against immunological liver injury in mice. Phytother. Res. 2011, 25, 1087–1094. [Google Scholar] [CrossRef]
- Pak, J.H.; Son, W.C.; Seo, S.B.; Hong, S.J.; Sohn, W.M.; Na, B.K.; Kim, T.S. Peroxiredoxin 6 expression is inversely correlated with nuclear factor-kappaB activation during Clonorchis sinensis infestation. Free Radic. Biol. Med. 2016, 99, 273–285. [Google Scholar] [CrossRef]
- Liao, M.; Li, X.; Zhang, H.; Zhou, L.; Shi, L.; Li, W.; Shen, R.; Peng, G.; Zhao, H.; Shao, J.; et al. Effects and plasma proteomic analysis of GLP-1RA versus CPA/EE, in combination with metformin, on overweight PCOS women: A randomized controlled trial. Endocrine 2023, 83, 227–241. [Google Scholar] [CrossRef]
- Liu, N.; Xue, L.; Guan, Y.; Li, Q.Z.; Cao, F.Y.; Pang, S.L.; Guan, W.J. Expression of Peroxiredoxins and Pulmonary Surfactant Protein A Induced by Silica in Rat Lung Tissue. Biomed. Environ. Sci. 2016, 29, 584–588. [Google Scholar] [CrossRef]
- Bu, H.; Wang, B.; Wu, Y.; Li, P.; Cui, Y.; Jiang, X.; Yu, X.; Liu, B.; Tang, M. Curcumin strengthens a spontaneous self-protective mechanism-SP1/PRDX6 pathway, against di-n-butyl phthalate-induced testicular ferroptosis damage. Environ. Sci. Pollut. Res. 2023, 30, 122165–122181. [Google Scholar] [CrossRef]
- Li, H.; Wu, Z.; Zhong, R.; Zhang, Q.; Chen, Q.; Shen, Y. PRDX6 knockout restrains the malignant progression of intrahepatic cholangiocarcinoma. Med. Oncol. 2022, 39, 250. [Google Scholar] [CrossRef]
- Soylu, H.; Karacor, K. The effects of hydroxytyrosol on Prdx6 and insulin expression in diabetic rat pancreases. Histochem. Cell Biol. 2023, 160, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, W.; Chen, C.; Chen, X. Peroxiredoxin 6 overexpression regulates adriamycin-induced myocardial injury, oxidative stress and immune response in rats. Ann. Transl. Med. 2020, 8, 1320. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, G.; Xing, Y.; Ding, Y.; Ren, T.; Kan, J. Antioxidant activity of whole grain highland hull-less barley and its effect on liver protein expression profiles in rats fed with high-fat diets. Eur. J. Nutr. 2018, 57, 2201–2208. [Google Scholar] [CrossRef]
- Atiba, A.; Abdo, W.; Ali, E.K.; Abd-Elsalam, M.; Amer, M.; Abdel, M.A.; Taha, R.; Antar, S.; Mahmoud, A. Topical and oral applications of Aloe vera improve healing of deep second-degree burns in rats via modulation of growth factors. Biomarkers 2022, 27, 608–617. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Zhao, M.; Yu, L.; Lin, Y.; Kang, D. Ferrostatin-1 attenuates brain injury in animal model of subarachnoid hemorrhage via phospholipase A2 activity of PRDX6. Neuroreport 2023, 34, 606–616. [Google Scholar] [CrossRef]
- Ren, J.; Wu, J.; Tang, X.; Chen, S.; Wang, W.; Lv, Y.; Wu, L.; Yang, D.; Zheng, Y. Ageing- and AAA-associated differentially expressed proteins identified by proteomic analysis in mice. PeerJ 2022, 10, e13129. [Google Scholar] [CrossRef]
- Dickinson, D.; DeRossi, S.; Yu, H.; Thomas, C.; Kragor, C.; Paquin, B.; Hahn, E.; Ohno, S.; Yamamoto, T.; Hsu, S. Epigallocatechin-3-gallate modulates anti-oxidant defense enzyme expression in murine submandibular and pancreatic exocrine gland cells and human HSG cells. Autoimmunity 2014, 47, 177–184. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, J.E.; Chung, B.Y.; Lee, H.M.; Kang, H.H.; Chung, J.W.; Pak, J.H. Involvement of retinoic acid-induced peroxiredoxin 6 expression in recovery of noise-induced temporary hearing threshold shifts. Environ. Toxicol. Pharmacol. 2013, 36, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, X.Y.; Zhou, Y.Q.; Wen, X.; Zhu, L.Y. Proteomic alterations in mouse kidney induced by andrographolide sodium bisulfite. Acta Pharmacol. Sin. 2011, 32, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, S.L.; Golder, Z.J.; Johnstone, D.B.; Frankl, F. Renal peroxiredoxin 6 interacts with anion exchanger 1 and plays a novel role in pH homeostasis. Kidney Int. 2016, 89, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zi, T.; Liu, Y.; Zhang, Y.; Wang, Z.; Wang, Z.; Zhan, S.; Peng, Z.; Li, N.; Liu, X.; Liu, F. Protective effect of melatonin on alleviating early oxidative stress induced by DOX in mice spermatogenesis and sperm quality maintaining. Reprod. Biol. Endocrinol. 2022, 20, 105. [Google Scholar] [CrossRef]
- XueXia, L.; YaNan, L.; Zi, T.; YuSheng, Z.; ZeLin, W.; Peng, Z.; MeiNa, X.; FuJun, L. Di-2-ethylhexyl phthalate (DEHP) exposure induces sperm quality and functional defects in mice. Chemosphere 2022, 312 Pt 1, 137216. [Google Scholar] [CrossRef]
- Butt, U.J.; Shah, S.; Ahmed, T.; Zahid, S. Protective effects of Nigella sativa L. seed extract on lead induced neurotoxicity during development and early life in mouse models. Toxicol. Res. 2018, 7, 32–40. [Google Scholar] [CrossRef]
- Lee, H.L.; Park, M.H.; Son, D.J.; Song, H.S.; Kim, J.H.; Ko, S.C.; Song, M.J.; Lee, W.H.; Yoon, J.H.; Ham, Y.W.; et al. Anti-cancer effect of snake venom toxin through down regulation of AP-1 mediated PRDX6 expression. Oncotarget 2015, 6, 22139–22151. [Google Scholar] [CrossRef]
- Trudel, S.; Kelly, M.; Fritsch, J.; Nguyen-Khoa, T.; Therond, P.; Couturier, M.; Dadlez, M.; Debski, J.; Touqui, L.; Vallee, B.; et al. Peroxiredoxin 6 fails to limit phospholipid peroxidation in lung from Cftr-knockout mice subjected to oxidative challenge. PLoS ONE 2009, 4, e6075. [Google Scholar] [CrossRef]
- Guo, Z.; Han, C.; Du, J.; Zhao, S.; Fu, Y.; Zheng, G.; Sun, Y.; Zhang, Y.; Liu, W.; Wan, J.; et al. Proteomic study of differential protein expression in mouse lung tissues after aerosolized ricin poisoning. Int. J. Mol. Sci. 2014, 15, 7281–7292. [Google Scholar] [CrossRef]
- Jo, M.; Yun, H.M.; Park, K.R.; Park, M.H.; Lee, D.H.; Cho, S.H.; Yoo, H.S.; Lee, Y.M.; Jeong, H.S.; Kim, Y.; et al. Anti-cancer effect of thiacremonone through down regulation of peroxiredoxin 6. PLoS ONE 2014, 9, e91508. [Google Scholar] [CrossRef]
- Wang, J.L.; Lin, Y.W.; Chen, H.M.; Kong, X.; Xiong, H.; Shen, N.; Hong, J.; Fang, J.Y. Calcium prevents tumorigenesis in a mouse model of colorectal cancer. PLoS ONE 2011, 6, e22566. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Chen, D.; Huo, X.; Tian, X.; Li, J.; Liu, M.; Yu, Z.; Zhang, B.; Yang, Y.; et al. Prdx6-induced inhibition of ferroptosis in epithelial cells contributes to liquiritin-exerted alleviation of colitis. Food Funct. 2022, 13, 9470–9480. [Google Scholar] [CrossRef]
- Godoy-Lugo, J.A.; Thorwald, M.A.; Hui, D.Y.; Nishiyama, A.; Nakano, D.; Sonanez-Organis, J.G.; Ortiz, R.M. Chronic angiotensin receptor activation promotes hepatic triacylglycerol accumulation during an acute glucose challenge in obese-insulin-resistant OLETF rats. Endocrine 2021, 75, 92–107. [Google Scholar] [CrossRef]
- Paulino, L.; Barroso, P.; Silva, B.R.; Barroso, L.G.; Barbalho, E.C.; Bezerra, F.; Souza, A.; Monte, A.; Silva, A.; Matos, M.; et al. Immunolocalization of melatonin receptors in bovine ovarian follicles and in vitro effects of melatonin on growth, viability and gene expression in secondary follicles. Domest. Anim. Endocrinol. 2022, 81, 106750. [Google Scholar] [CrossRef]
- Azevedo, V.; De Assis, E.; Silva, A.; Costa, F.; Souza, L.F.; Silva, J. alpha-Pinene Improves Follicle Morphology and Increases the Expression of mRNA for Nuclear Factor Erythroid 2-Related Factor 2 and Peroxiredoxin 6 in Bovine Ovarian Tissues Cultured In Vitro. Animals 2024, 14, 1443. [Google Scholar] [CrossRef]
- Costa, F.; Vasconcelos, E.M.; Nunes, A.V.; Feitosa, M.P.L.; Soares, M.D.; Viana, S.J.; Barbalho, S.A.; Paz, S.A. Aloe vera increases mRNA expression of antioxidant enzymes in cryopreserved bovine ovarian tissue and promotes follicular growth and survival after in vitro culture. Cryobiology 2021, 102, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Caetano, F.F.; Paulino, L.; Bezerra, V.S.; Azevedo, V.; Barroso, P.; Costa, F.C.; Amorim, G.G.; Silva, J. Thymol increases primordial follicle activation, protects stromal cells, collagen fibers and down-regulates expression of mRNA for superoxide dismutase 1, catalase and periredoxin 6 in cultured bovine ovarian tissues. Anim. Reprod. Sci. 2024, 266, 107514. [Google Scholar] [CrossRef]
- Na-Phatthalung, P.; Teles, M.; Tort, L.; Oliveira, M. Gold nanoparticles exposure modulates antioxidant and innate immune gene expression in the gills of Sparus aurata. Genomics 2018, 110, 430–434. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Nakharuthai, C.; Schrama, D.; Duangkaew, R.; Rodrigues, P.M. Effects of dietary lipid sources on hepatic nutritive contents, fatty acid composition and proteome of Nile tilapia (Oreochromis niloticus). J. Proteom. 2019, 192, 208–222. [Google Scholar] [CrossRef]
- Hao, C.C.; Luo, J.N.; Xu, C.Y.; Zhao, X.Y.; Zhong, Z.B.; Hu, X.N.; Jin, X.M.; Ge, X. TRIAP1 knockdown sensitizes non-small cell lung cancer to ionizing radiation by disrupting redox homeostasis. Thorac. Cancer 2020, 11, 1015–1025. [Google Scholar] [CrossRef]
- Salovska, B.; Kondelova, A.; Pimkova, K.; Liblova, Z.; Pribyl, M.; Fabrik, I.; Bartek, J.; Vajrychova, M.; Hodny, Z. Peroxiredoxin 6 protects irradiated cells from oxidative stress and shapes their senescence-associated cytokine landscape. Redox Biol. 2021, 49, 102212. [Google Scholar] [CrossRef]
- Groiss, S.; Lammegger, R.; Brislinger, D. Anti-Oxidative and Immune Regulatory Responses of THP-1 and PBMC to Pulsed EMF Are Field-Strength Dependent. Int. J. Environ. Res. Public Health 2021, 18, 9519. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Novoselov, V.I.; Gudkov, S.V. Radioprotective Role of Peroxiredoxin 6. Antioxidants 2019, 8, 15. [Google Scholar] [CrossRef]
- Li, H.T.; Tan, F.; Zhang, T.H.; Cao, L.H.; Tan, H.Y.; Lin, W.Q.; Zeng, W.A.; Chi, X.J. Peroxiredoxin 6 mediates the protective function of curcumin pretreatment in acute lung injury induced by serum from patients undergoing one-lung ventilation in vitro. BMC Pulm. Med. 2022, 22, 192. [Google Scholar] [CrossRef]
- Chhunchha, B.; Fatma, N.; Bhargavan, B.; Kubo, E.; Kumar, A.; Singh, D.P. Specificity protein, Sp1-mediated increased expression of Prdx6 as a curcumin-induced antioxidant defense in lens epithelial cells against oxidative stress. Cell Death Dis. 2011, 2, e234. [Google Scholar] [CrossRef]
- Daverey, A.; Agrawal, S.K. Curcumin alleviates oxidative stress and mitochondrial dysfunction in astrocytes. Neuroscience 2016, 333, 92–103. [Google Scholar] [CrossRef]
- Li, L.; Yu, L.; Cao, X.; Zhang, C.; Liu, Q.; Chen, J. Comparative Analysis of Proteomic of Curcumin Reversing Multidrug Resistance in HCT-8/VCR Cells. J. Oncol. 2022, 2022, 3605436. [Google Scholar] [CrossRef]
- Chen, J.; Cao, X.; Qin, X.; Liu, H.; Chen, S.; Zhong, S.; Li, Y. Proteomic analysis of the molecular mechanism of curcumin/beta-cyclodextrin polymer inclusion complex inhibiting HepG2 cells growth. J. Food Biochem. 2020, 44, e13119. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, J.; Liu, X.; Li, Y.; Jiang, H.; Fang, W.; Long, X. Glycyrrhizin regulates antioxidation through Nrf2 signaling pathway in rat temporomandibular joint osteoarthritis. J. Oral Rehabil. 2023, 51, 611–622. [Google Scholar] [CrossRef]
- Huang, W.S.; Kuo, Y.H.; Chin, C.C.; Wang, J.Y.; Yu, H.R.; Sheen, J.M.; Tung, S.Y.; Shen, C.H.; Chen, T.C.; Sung, M.L.; et al. Proteomic analysis of the effects of baicalein on colorectal cancer cells. Proteomics 2012, 12, 810–819. [Google Scholar] [CrossRef]
- Yoo, D.R.; Jang, Y.H.; Jeon, Y.K.; Kim, J.Y.; Jeon, W.; Choi, Y.J.; Nam, M.J. Proteomic identification of anti-cancer proteins in luteolin-treated human hepatoma Huh-7 cells. Cancer Lett. 2009, 282, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Lian, W.; Bai, Y.; Wang, L.; Zhao, F.; Li, H.; Wang, D.; Pang, Q. TMT-based quantitative proteomics reveals the targets of andrographolide on LPS-induced liver injury. Bmc Vet. Res. 2023, 19, 199. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Yu, G.; Zhang, Y.; Xu, Z.; Wang, Y.Q.; Yan, G.R.; He, Q.Y. A novel andrographolide derivative AL-1 exerts its cytotoxicity on K562 cells through a ROS-dependent mechanism. Proteomics 2013, 13, 169–178. [Google Scholar] [CrossRef]
- Zha, X.; Wu, G.; Zhang, H.; Yang, Y.; Zhang, Y.; Ma, L. PRDX6 regulates the H2O2 and blue light-induced APRE-19 cell apoptosis via down-regulating and interacting with RARA. Anim. Cells Syst. 2019, 23, 241–245. [Google Scholar] [CrossRef]
- Chhunchha, B.; Singh, P.; Stamer, W.D.; Singh, D.P. Prdx6 retards senescence and restores trabecular meshwork cell health by regulating reactive oxygen species. Cell Death Discov. 2017, 3, 17060. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tzekov, R.; Su, M.; Zhu, Y.; Han, A.; Li, W. Hydrogen peroxide-induced oxidative damage and protective role of peroxiredoxin 6 protein via EGFR/ERK signaling pathway in RPE cells. Front. Aging Neurosci. 2023, 15, 1169211. [Google Scholar] [CrossRef]
- Gallagher, B.M.; Phelan, S.A. Investigating transcriptional regulation of Prdx6 in mouse liver cells. Free Radic. Biol. Med. 2007, 42, 1270–1277. [Google Scholar] [CrossRef]
- Kwon, J.; Wang, A.; Burke, D.J.; Boudreau, H.E.; Lekstrom, K.J.; Korzeniowska, A.; Sugamata, R.; Kim, Y.S.; Yi, L.; Ersoy, I.; et al. Peroxiredoxin 6 (Prdx6) supports NADPH oxidase1 (Nox1)-based superoxide generation and cell migration. Free Radic. Biol. Med. 2016, 96, 99–115. [Google Scholar] [CrossRef]
- Paula, F.M.; Ferreira, S.M.; Boschero, A.C.; Souza, K.L. Modulation of the peroxiredoxin system by cytokines in insulin-producing RINm5F cells: Down-regulation of PRDX6 increases susceptibility of beta cells to oxidative stress. Mol. Cell. Endocrinol. 2013, 374, 56–64. [Google Scholar] [CrossRef]
- Fatma, N.; Kubo, E.; Sen, M.; Agarwal, N.; Thoreson, W.B.; Camras, C.B.; Singh, D.P. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res. 2008, 1233, 63–78. [Google Scholar] [CrossRef]
- Yang, D.; Jin, M.; Bai, C.; Zhou, J.; Shen, Y. Peroxiredoxin 6 suppresses Muc5ac overproduction in LPS-induced airway inflammation through H2O2-EGFR-MAPK signaling pathway. Respir. Physiol. Neurobiol. 2017, 236, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Diet, A.; Abbas, K.; Bouton, C.; Guillon, B.; Tomasello, F.; Fourquet, S.; Toledano, M.B.; Drapier, J.C. Regulation of peroxiredoxins by nitric oxide in immunostimulated macrophages. J. Biol. Chem. 2007, 282, 36199–36205. [Google Scholar] [CrossRef]
- Yazheng, L.; Kitts, D.D. Activation of antioxidant response element (ARE)-dependent genes by roasted coffee extracts. Food Funct. 2012, 3, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.M.; Lambert, P.A.; Hummon, A.B. Comparative label-free LC-MS/MS analysis of colorectal adenocarcinoma and metastatic cells treated with 5-fluorouracil. Proteomics 2012, 12, 1928–1937. [Google Scholar] [CrossRef]
- Ribeiro, T.; Lemos, F.; Preto, M.; Azevedo, J.; Sousa, M.L.; Leao, P.N.; Campos, A.; Linder, S.; Vitorino, R.; Vasconcelos, V.; et al. Cytotoxicity of portoamides in human cancer cells and analysis of the molecular mechanisms of action. PLoS ONE 2017, 12, e0188817. [Google Scholar] [CrossRef]
- Hughes, N.P.; Xu, L.; Nielsen, C.H.; Chang, E.; Hori, S.S.; Natarajan, A.; Lee, S.; Kjaer, A.; Kani, K.; Wang, S.X.; et al. A blood biomarker for monitoring response to anti-EGFR therapy. Cancer Biomark. 2018, 22, 333–344. [Google Scholar] [CrossRef]
- Chowdhury, I.; Fisher, A.B.; Christofidou-Solomidou, M.; Gao, L.; Tao, J.Q.; Sorokina, E.M.; Lien, Y.C.; Bates, S.R.; Feinstein, S.I. Keratinocyte growth factor and glucocorticoid induction of human peroxiredoxin 6 gene expression occur by independent mechanisms that are synergistic. Antioxid. Redox Signal. 2014, 20, 391–402. [Google Scholar] [CrossRef]
- Schmitt, A.; Schmitz, W.; Hufnagel, A.; Schartl, M.; Meierjohann, S. Peroxiredoxin 6 triggers melanoma cell growth by increasing arachidonic acid-dependent lipid signalling. Biochem. J. 2015, 471, 267–279. [Google Scholar] [CrossRef]
- Junkins, K.; Rodgers, M.; Phelan, S.A. Oleuropein Induces Cytotoxicity and Peroxiredoxin Over-expression in MCF-7 Human Breast Cancer Cells. Anticancer. Res. 2023, 43, 4333–4339. [Google Scholar] [CrossRef]
- Chhunchha, B.; Singh, P.; Singh, D.P.; Kubo, E. Ginkgolic Acid Rescues Lens Epithelial Cells from Injury Caused by Redox Regulated-Aberrant Sumoylation Signaling by Reviving Prdx6 and Sp1 Expression and Activities. Int. J. Mol. Sci. 2018, 19, 3520. [Google Scholar] [CrossRef]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Sulforaphane-Induced Klf9/Prdx6 Axis Acts as a Molecular Switch to Control Redox Signaling and Determines Fate of Cells. Cells 2019, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Sinha, N.; Ranjit, S.; Midde, N.M.; Kashanchi, F.; Kumar, S. Monocyte-derived exosomes upon exposure to cigarette smoke condensate alter their characteristics and show protective effect against cytotoxicity and HIV-1 replication. Sci. Rep. 2017, 7, 16120. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Kumar, S. Chronic Effects of Ethanol and/or Darunavir/Ritonavir on U937 Monocytic Cells: Regulation of Cytochrome P450 and Antioxidant Enzymes, Oxidative Stress, and Cytotoxicity. Alcohol. Clin. Exp. Res. 2016, 40, 73–82. [Google Scholar] [CrossRef]
- Karaosmanoglu, O. P38-beta/SAPK-inhibiting and apoptosis-inducing activities of (E)-4-chloro-2-((3-ethoxy-2-hydroxybenzylidene) amino)phenol. Hum. Exp. Toxicol. 2020, 39, 1374–1389. [Google Scholar] [CrossRef]
- Yumnam, S.; Venkatarame, G.S.V.; Raha, S.; Lee, H.J.; Lee, W.S.; Kim, E.K.; Lee, S.J.; Heo, J.D.; Kim, G.S. Proteomic profiling of human HepG2 cells treated with hesperidin using antibody array. Mol. Med. Rep. 2017, 16, 5386–5392. [Google Scholar] [CrossRef]
- Bibli, S.I.; Hu, J.; Leisegang, M.S.; Wittig, J.; Zukunft, S.; Kapasakalidi, A.; Fisslthaler, B.; Tsilimigras, D.; Zografos, G.; Filis, K.; et al. Shear stress regulates cystathionine gamma lyase expression to preserve endothelial redox balance and reduce membrane lipid peroxidation. Redox Biol. 2020, 28, 101379. [Google Scholar] [CrossRef]
- Li, D.X.; Chen, W.; Jiang, Y.L.; Ni, J.Q.; Lu, L. Antioxidant protein peroxiredoxin 6 suppresses the vascular inflammation, oxidative stress and endothelial dysfunction in angiotensin II-induced endotheliocyte. Gen. Physiol. Biophys. 2020, 39, 545–555. [Google Scholar] [CrossRef]
- Li, H.; Weng, Y.; Lai, L.; Lei, H.; Xu, S.; Zhang, Y.; Li, L. KLF9 regulates PRDX6 expression in hyperglycemia-aggravated bupivacaine neurotoxicity. Mol. Cell Biochem. 2021, 476, 2125–2134. [Google Scholar] [CrossRef]
- Seriani, R.; Paula, C.P.; Cunha, A.; Oliveira, M.A.; Krempel, P.G.; Frias, D.P.; Negri, E.M.; Mauad, T.; Macchione, M. Expression patterns of peroxiredoxin genes in bronchial epithelial cells exposed to diesel exhaust particles. Exp. Mol. Pathol. 2021, 120, 104641. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Wei, T.; Lang, K.; Bao, C.; Yang, D. Peroxinredoxin 6 reduction accelerates cigarette smoke extract-induced senescence by regulating autophagy in BEAS-2B cells. Exp. Ther. Med. 2023, 26, 375. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, C.; Cigna, D.; Di Sano, C.; Di Vincenzo, S.; Dino, P.; Ferraro, M.; Bini, L.; Bianchi, L.; Di Gaudio, F.; Gjomarkaj, M.; et al. Exposure to cigarette smoke extract and lipopolysaccharide modifies cytoskeleton organization in bronchial epithelial cells. Exp. Lung Res. 2017, 43, 347–358. [Google Scholar] [CrossRef]
- Leopoldino, A.M.; Squarize, C.H.; Garcia, C.B.; Almeida, L.O.; Pestana, C.R.; Sobral, L.M.; Uyemura, S.A.; Tajara, E.H.; Silvio, G.J.; Curti, C. SET protein accumulates in HNSCC and contributes to cell survival: Antioxidant defense, Akt phosphorylation and AVOs acidification. Oral. Oncol. 2012, 48, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.; Ren, K.; Hong, M.; Cui, J.; Liu, J. Cytotoxicity of alkaline serine protease (ASPNJ) on Jurkat cells and its correlation with changes in the expression of membrane-associated proteins. Biochem. Mol. Toxicol. 2023, 37, e23456. [Google Scholar] [CrossRef]
- Zhang, J.; Park, H.S.; Kim, J.A.; Hong, G.E.; Nagappan, A.; Park, K.I.; Kim, G.S. Flavonoids identified from Korean Scutellaria baicalensis induce apoptosis by ROS generation and caspase activation on human fibrosarcoma cells. Am. J. Chin. Med. 2014, 42, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, R.; Liu, T.; Ma, H.; Xue, G.; Liu, M. Peroxiredoxin 6 alleviates high glucose-induced inflammation and apoptosis in HK-2 cells by inhibiting TLR4/NF-kappaB signaling. Ann. Transl. Med. 2023, 11, 41. [Google Scholar] [CrossRef]
- Pak, J.H.; Choi, W.H.; Lee, H.M.; Joo, W.D.; Kim, J.H.; Kim, Y.T.; Kim, Y.M.; Nam, J.H. Peroxiredoxin 6 overexpression attenuates cisplatin-induced apoptosis in human ovarian cancer cells. Cancer Investig. 2011, 29, 21–28. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Wang, N.; Wang, D.M.; Li, Y.W.; Han, F.; Shen, J.G.; Yang, D.P.; Guan, X.Y.; Chen, J.P. Dioscin induces cancer cell apoptosis through elevated oxidative stress mediated by downregulation of peroxiredoxins. Cancer Biol. Ther. 2012, 13, 138–147. [Google Scholar] [CrossRef]
- Berntsen, H.F.; Duale, N.; Bjorklund, C.G.; Rangel-Huerta, O.D.; Dyrberg, K.; Hofer, T.; Rakkestad, K.E.; Ostby, G.; Halsne, R.; Boge, G.; et al. Effects of a human-based mixture of persistent organic pollutants on the in vivo exposed cerebellum and cerebellar neuronal cultures exposed in vitro. Environ. Int. 2021, 146, 106240. [Google Scholar] [CrossRef]
- Wu, J.; Wang, F.; Gong, Y.; Li, D.; Sha, J.; Huang, X.; Han, X. Proteomic analysis of changes induced by nonylphenol in Sprague-Dawley rat Sertoli cells. Chem. Res. Toxicol. 2009, 22, 668–675. [Google Scholar] [CrossRef]
- Walsh, B.; Pearl, A.; Suchy, S.; Tartaglio, J.; Visco, K.; Phelan, S.A. Overexpression of Prdx6 and resistance to peroxide-induced death in Hepa1-6 cells: Prdx suppression increases apoptosis. Redox Rep. 2009, 14, 275–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Hu, J.E.; Ding, Y.; Shen, Y.; Xu, H.; Chen, H.; Wu, N. Sp1-mediated upregulation of Prdx6 expression prevents podocyte injury in diabetic nephropathy via mitigation of oxidative stress and ferroptosis. Life Sci. 2021, 278, 119529. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Glushkova, O.V.; Parfenyuk, S.B.; Gudkov, S.V.; Lunin, S.M.; Novoselova, E.G. The role of TLR4/NF-kappaB signaling in the radioprotective effects of exogenous Prdx6. Arch. Biochem. Biophys. 2021, 702, 108830. [Google Scholar] [CrossRef]
- Hong, M.W.; Kim, H.; Choi, S.Y.; Sharma, N.; Lee, S.J. Effect of Gossypol on Gene Expression in Swine Granulosa Cells. Toxins 2024, 16, 436. [Google Scholar] [CrossRef]
- Wang, C.; Feng, H.; Zhang, X.; Li, K.; Yang, F.; Cao, W.; Liu, H.; Gao, L.; Xue, Z.; Liu, X.; et al. Porcine Picornavirus 3C Protease Degrades PRDX6 to Impair PRDX6-mediated Antiviral Function. Virol. Sin. 2021, 36, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Shahnaj, S.; Chowhan, R.K.; Meetei, P.A.; Kakchingtabam, P.; Herojit, S.K.; Rajendrakumar, S.L.; Nongdam, P.; Fisher, A.B.; Rahaman, H. Hyperoxidation of Peroxiredoxin 6 Induces Alteration from Dimeric to Oligomeric State. Antioxidants 2019, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, W.; Kim, E.E. Crystal structures of human peroxiredoxin 6 in different oxidation states. Biochem. Biophys. Res. Commun. 2016, 477, 717–722. [Google Scholar] [CrossRef]
- Chowhan, R.K.; Hotumalani, S.; Rahaman, H.; Singh, L.R. pH induced conformational alteration in human peroxiredoxin 6 might be responsible for its resistance against lysosomal pH or high temperature. Sci. Rep. 2021, 11, 9657. [Google Scholar] [CrossRef]
- Wu, Y.; Feinstein, S.I.; Manevich, Y.; Chowdhury, I.; Pak, J.H.; Kazi, A.; Dodia, C.; Speicher, D.W.; Fisher, A.B. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. Biochem. J. 2009, 419, 669–679. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Switching of Redox Signaling by Prdx6 Expression Decides Cellular Fate by Hormetic Phenomena Involving Nrf2 and Reactive Oxygen Species. Cells 2022, 11, 1266. [Google Scholar] [CrossRef]
- Vazquez-Medina, J.P.; Tao, J.Q.; Patel, P.; Bannitz-Fernandes, R.; Dodia, C.; Sorokina, E.M.; Feinstein, S.I.; Chatterjee, S.; Fisher, A.B. Genetic inactivation of the phospholipase A2 activity of peroxiredoxin 6 in mice protects against LPS-induced acute lung injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L656–L668. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jung, Y.S.; Yun, J.; Han, S.B.; Roh, Y.S.; Song, M.J.; Hong, J.T. Peroxiredoxin 6 mediates acetaminophen-induced hepatocyte death through JNK activation. Redox Biol. 2020, 32, 101496. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Ji, Y.; Li, Y.; You, Y.; Zhou, Y. PRDX6-iPLA2 aggravates neuroinflammation after ischemic stroke via regulating astrocytes-induced M1 microglia. Cell Commun. Signal. 2024, 22, 76. [Google Scholar] [CrossRef]
- Schattauer, S.S.; Land, B.B.; Reichard, K.L.; Abraham, A.D.; Burgeno, L.M.; Kuhar, J.R.; Phillips, P.E.M.; Ong, S.E.; Chavkin, C. Prdx6 mediates Gαi protein-coupled receptor inactivation by cJun kinase. Nat. Commun. 2017, 8, 743. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, K.; Shen, F.; Zhang, J.; Xie, Y.; Li, S.; Hou, Y.; Bai, G. Astragaloside IV targets PRDX6, inhibits the activation of RAC subunit in NADPH oxidase 2 for oxidative damage. Phytomedicine 2023, 114, 154795. [Google Scholar] [CrossRef]
- Mohar, I.; Stamper, B.D.; Rademacher, P.M.; White, C.C.; Nelson, S.D.; Kavanagh, T.J. Acetaminophen-induced liver damage in mice is associated with gender-specific adduction of peroxiredoxin-6. Redox Biol. 2014, 2, 377–387. [Google Scholar] [CrossRef]
- Liao, J.; Hu, W.; Chen, S.; Huang, C.; Dong, S.; Chen, W.; Chen, X.; Chen, L. Multidimensional features of sporadic Creutzfeldt-Jakob disease in the elderly: A case report and systematic review. Front. Aging Neurosci. 2024, 16, 1379011. [Google Scholar] [CrossRef]
- Liao, J.; Mi, X.; Zeng, G.; Wei, Y.; Dai, X.; Ye, Q.; Chen, X.; Zhang, J. Circuit-wide proteomics profiling reveals brain region-specific protein signatures in the male WKY rats with endogenous depression. J. Affect. Disord. 2023, 320, 98–107. [Google Scholar] [CrossRef]
- Bumanlag, E.; Scarlata, E.; O’Flaherty, C. Peroxiredoxin 6 Peroxidase and Ca2+-Independent Phospholipase A2 Activities Are Essential to Support Male-Mouse Fertility. Antioxidants 2022, 11, 226. [Google Scholar] [CrossRef]
- O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015, 5, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Meng, J.; Yue, C.; Wu, X.; Su, Q.; Wu, H.; Zhang, Z.; Yu, Q.; Gao, S.; Fan, S.; et al. Integrative analysis the characterization of peroxiredoxins in pan-cancer. Cancer Cell Int. 2021, 21, 366. [Google Scholar] [CrossRef]
- Szeliga, M. Comprehensive analysis of the expression levels and prognostic values of PRDX family genes in glioma. Neurochem. Int. 2021, 153, 105256. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, Q.; Gao, M.; Liang, Q.; Li, J.; Chen, X. Differential Expression and Effects of Peroxiredoxin-6 on Drug Resistance and Cancer Stem Cell-like Properties in Non-Small Cell Lung Cancer. Oncotargets Ther. 2019, 12, 10477–10486. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Li, B.; Zhen, H.; Chen, W.; Men, Q. PRDX6 Overexpression Promotes Proliferation, Invasion, and Migration of A549 Cells in vitro and in vivo. Cancer Manag. Res. 2021, 13, 1245–1255. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, K.R.; Park, M.H.; Kim, D.H.; Jo, M.R.; Kim, J.Y.; Kim, E.C.; Yoon, D.Y.; Han, S.B.; Hong, J.T. PRDX6 promotes tumor development via the JAK2/STAT3 pathway in a urethane-induced lung tumor model. Free Radic. Biol. Med. 2015, 80, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Xiao, Z.F.; Li, C.; Xiao, Z.Q.; Yang, F.; Li, D.J.; Li, M.Y.; Li, F.; Chen, Z.C. Triosephosphate isomerase and peroxiredoxin 6, two novel serum markers for human lung squamous cell carcinoma. Cancer Sci. 2009, 100, 2396–2401. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Zhou, C.; Wang, Z.; Cui, Z.; Wu, X.; Xu, Z.; Yang, J.; Zhang, X. Construction and validation of a novel ferroptosis-related prognostic signature for lung adenocarcinoma. Transl. Lung Cancer R. 2023, 12, 1766–1781. [Google Scholar] [CrossRef]
- Nakanishi, T.; Takeuchi, T.; Ueda, K.; Murao, H.; Shimizu, A. Detection of eight antibodies in cancer patients’ sera against proteins derived from the adenocarcinoma A549 cell line using proteomics-based analysis. J. Chromatogr. B 2006, 838, 15–20. [Google Scholar] [CrossRef]
- Hu, X.; Lu, E.; Pan, C.; Xu, Y.; Zhu, X. Overexpression and biological function of PRDX6 in human cervical cancer. J. Cancer 2020, 11, 2390–2400. [Google Scholar] [CrossRef]
- Roh, J.W.; Choi, J.E.; Han, H.D.; Hu, W.; Matsuo, K.; Nishimura, M.; Lee, J.S.; Kwon, S.Y.; Cho, C.H.; Kim, J.; et al. Clinical and biological significance of EZH2 expression in endometrial cancer. Cancer Biol. Ther. 2020, 21, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rolfs, F.; Huber, M.; Gruber, F.; Bohm, F.; Pfister, H.J.; Bochkov, V.N.; Tschachler, E.; Dummer, R.; Hohl, D.; Schafer, M.; et al. Dual role of the antioxidant enzyme peroxiredoxin 6 in skin carcinogenesis. Cancer Res. 2013, 73, 3460–3469. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.Y.; Zhang, Y.C.; Wu, Y.X.; Guo, D.D.; Long, D.; Liu, Z.H. A Novel Ferroptosis-Related Gene Signature to Predict Prognosis in Patients with Head and Neck Squamous Cell Carcinoma. Dis. Markers 2021, 2021, 5759927. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yue, W.; You, J.; Wei, X.; Huang, Y.; Ling, Z.; Hou, J. Identification of a Novel Ferroptosis-Related Gene Prognostic Signature in Bladder Cancer. Front. Oncol. 2021, 11, 730716. [Google Scholar] [CrossRef]
- Quan, C.; Cha, E.J.; Lee, H.L.; Han, K.H.; Lee, K.M.; Kim, W.J. Enhanced expression of peroxiredoxin I and VI correlates with development, recurrence and progression of human bladder cancer. J. Urol. 2006, 175, 1512–1516. [Google Scholar] [CrossRef]
- Karihtala, P.; Mantyniemi, A.; Kang, S.W.; Kinnula, V.L.; Soini, Y. Peroxiredoxins in breast carcinoma. Clin. Cancer Res. 2003, 9, 3418–3424. [Google Scholar]
- Chang, X.Z.; Li, D.Q.; Hou, Y.F.; Wu, J.; Lu, J.S.; Di, G.H.; Jin, W.; Ou, Z.L.; Shen, Z.Z.; Shao, Z.M. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007, 9, R76. [Google Scholar] [CrossRef]
- Cortesi, L.; Barchetti, A.; De Matteis, E.; Rossi, E.; Della, C.L.; Marcheselli, L.; Tazzioli, G.; Lazzaretti, M.G.; Ficarra, G.; Federico, M.; et al. Identification of protein clusters predictive of response to chemotherapy in breast cancer patients. J. Proteome Res. 2009, 8, 4916–4933. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Qiu, G. Bioinformatics Analysis Using ATAC-seq and RNA-seq for the Identification of 15 Gene Signatures Associated With the Prediction of Prognosis in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 726551. [Google Scholar] [CrossRef]
- Xu, X.; Lu, D.; Zhuang, R.; Wei, X.; Xie, H.; Wang, C.; Zhu, Y.; Wang, J.; Zhong, C.; Zhang, X.; et al. The phospholipase A2 activity of peroxiredoxin 6 promotes cancer cell death induced by tumor necrosis factor alpha in hepatocellular carcinoma. Mol. Carcinog. 2016, 55, 1299–1308. [Google Scholar] [CrossRef]
- Ouyang, X.; DeWeese, T.L.; Nelson, W.G.; Abate-Shen, C. Loss-of-function of Nkx3.1 promotes increased oxidative damage in prostate carcinogenesis. Cancer Res. 2005, 65, 6773–6779. [Google Scholar] [CrossRef]
- Huang, W.S.; Huang, C.Y.; Hsieh, M.C.; Kuo, Y.H.; Tung, S.Y.; Shen, C.H.; Hsieh, Y.Y.; Teng, C.C.; Lee, K.C.; Lee, K.F.; et al. Expression of PRDX6 Correlates with Migration and Invasiveness of Colorectal Cancer Cells. Cell Physiol. Biochem. 2018, 51, 2616–2630. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F.; Li, N.; Zhang, J.; Dai, L.; Yang, H. Peroxiredoxins and Immune Infiltrations in Colon Adenocarcinoma: Their Negative Correlations and Clinical Significances, an In Silico Analysis. J. Cancer 2020, 11, 3124–3143. [Google Scholar] [CrossRef] [PubMed]
- Falidas, E.; Kitsiouli, E.; Tsounis, D.; Kalogirou, A.; Tsiambas, E.; Tsouvelas, G.; Papadopoulos, S.; Mitsis, M.; Lekka, M.; Vlachos, K. Impact of peroxiredoxin-6 expression on colon adenocarcinoma. J. Buon 2021, 26, 1893–1897. [Google Scholar] [PubMed]
- Nicolussi, A.; D’Inzeo, S.; Mincione, G.; Buffone, A.; Di Marcantonio, M.C.; Cotellese, R.; Cichella, A.; Capalbo, C.; Di Gioia, C.; Nardi, F.; et al. PRDX1 and PRDX6 are repressed in papillary thyroid carcinomas via BRAF V600E-dependent and -independent mechanisms. Int. J. Oncol. 2014, 44, 548–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mu, R.; Li, Y.; Xing, J.; Li, Y.; Lin, R.; Ye, S.; Zhang, Y.; Mu, H.; Guo, X.; An, L. Effect of lentivirus-mediated peroxiredoxins 6 gene silencing on the phenotype of human gastric cancer BGC-823 cells. J. Cancer Res. Ther. 2022, 18, 411–417. [Google Scholar] [CrossRef]
- Li, Q.; Wang, N.; Wei, H.; Li, C.; Wu, J.; Yang, G. miR-24-3p Regulates Progression of Gastric Mucosal Lesions and Suppresses Proliferation and Invasiveness of N87 Via Peroxiredoxin 6. Dig. Dis. Sci. 2016, 61, 3486–3497. [Google Scholar] [CrossRef]
- Shanshan, Y.; Beibei, J.; Li, T.; Minna, G.; Shipeng, L.; Li, P.; Yong, Z. Phospholipase A2 of Peroxiredoxin 6 Plays a Critical Role in Cerebral Ischemia/Reperfusion Inflammatory Injury. Front. Cell. Neurosci. 2017, 11, 99. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Kang, T.C. Sp1-Mediated Prdx6 Upregulation Leads to Clasmatodendrosis by Increasing Its aiPLA2 Activity in the CA1 Astrocytes in Chronic Epilepsy Rats. Antioxidants 2022, 11, 1883. [Google Scholar] [CrossRef]
- Ho, J.N.; Lee, S.B.; Lee, S.S.; Yoon, S.H.; Kang, G.Y.; Hwang, S.G.; Um, H.D. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol. Cancer Ther. 2010, 9, 825–832. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, K.R.; Lee, H.P.; Lee, D.H.; Jo, M.; Shin, D.H.; Yoon, D.Y.; Han, S.B.; Hong, J.T. PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities. Free Radic. Biol. Med. 2014, 69, 367–376. [Google Scholar] [CrossRef]
- Chen, H.; Fang, Y.; Dai, S.; Jiang, K.; Shen, L.; Zhao, J.; Huang, K.; Zhou, X.; Ding, K. Characterization and proteomic analysis of plasma-derived small extracellular vesicles in locally advanced rectal cancer patients. Cell Oncol. 2024, 47, 1995–2009. [Google Scholar] [CrossRef]
- Kuang, X.; Wang, L.F.; Yu, L.; Li, Y.J.; Wang, Y.N.; He, Q.; Chen, C.; Du, J.R. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: Involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic. Biol. Med. 2014, 71, 165–175. [Google Scholar] [CrossRef]
- Huang, Y.; Driedonks, T.; Cheng, L.; Rajapaksha, H.; Routenberg, D.A.; Nagaraj, R.; Redding, J.; Arab, T.; Powell, B.H.; Pletnikova, O.; et al. Brain Tissue-Derived Extracellular Vesicles in Alzheimer’s Disease Display Altered Key Protein Levels Including Cell Type-Specific Markers. J. Alzheimers Dis. 2022, 90, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Novoselova eg Glushkova, O.V.; Lunin, S.M.; Khrenov, M.O.; Parfenyuk, S.B.; Novoselova, T.V.; Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E. Peroxiredoxin 6 Attenuates Alloxan-Induced Type 1 Diabetes Mellitus in Mice and Cytokine-Induced Cytotoxicity in RIN-m5F Beta Cells. J. Diabetes Res. 2020, 2020, 7523892. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Nakanishi, T.; Hiramatsu, M.; Mabuchi, H.; Miyamoto, Y.; Miyamoto, A.; Shimizu, A.; Tanigawa, N. Proteomics-based approach identifying autoantibody against peroxiredoxin VI as a novel serum marker in esophageal squamous cell carcinoma. Clin. Cancer Res. 2006, 12, 6415–6420. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, W.; Xiao, Y.; Pan, L.; Chen, G.; Tang, Y.; Zhou, J.; Wu, J.; Zhu, W.; Zhang, S.; et al. Overexpression of Peroxiredoxin 6 (PRDX6) Promotes the Aggressive Phenotypes of Esophageal Squamous Cell Carcinoma. J. Cancer 2018, 9, 3939–3949. [Google Scholar] [CrossRef]
- Lu, B.; Chen, X.B.; Hong, Y.C.; Zhu, H.; He, Q.J.; Yang, B.; Ying, M.D.; Cao, J. Identification of PRDX6 as a regulator of ferroptosis. Acta Pharmacol. Sin. 2019, 40, 1334–1342. [Google Scholar] [CrossRef]
- Gordeeva, A.E.; Temnov, A.A.; Charnagalov, A.A.; Sharapov, M.G.; Fesenko, E.E.; Novoselov, V.I. Protective Effect of Peroxiredoxin 6 in Ischemia/Reperfusion-Induced Damage of Small Intestine. Dig. Dis. Sci. 2015, 60, 3610–3619. [Google Scholar] [CrossRef]
- Goncharov, R.G.; Rogov, K.A.; Temnov, A.A.; Novoselov, V.I.; Sharapov, M.G. Protective role of exogenous recombinant peroxiredoxin 6 under ischemia-reperfusion injury of kidney. Cell Tissue Res. 2019, 378, 319–332. [Google Scholar] [CrossRef]
- Novoselov, V.I.; Baryshnikova, L.M.; Yanin, V.A.; Amelina, S.E.; Fesenko, E.E. The influence of peroxyredoxin VI on incised-wound healing in rats. Dokl. Biochem. Biophys. 2003, 393, 326–327. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, H.J.; Wang, H.Y.; Wang, W.T.; Jin, S.H.; Zhu, P.; Li, S.J.; Rong, C.T.; Li, J.Y. Topical administration of peroxiredoxin-6 on the cornea suppresses inflammation and neovascularization induced by ultraviolet radiation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8016–8028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mu, R.; Ye, S.; Lin, R.; Li, Y.; Guo, X.; An, L. Effects of Peroxiredoxin 6 and Its Mutants on the Isoproterenol Induced Myocardial Injury in H9C2 Cells and Rats. Oxidative Med. Cell. Longev. 2022, 2022, 2576310. [Google Scholar] [CrossRef]
- Zhang, X.X.; You, J.P.; Liu, X.R.; Zhao, Y.F.; Cui, Y.; Zhao, Z.Z.; Qi, Y.Y. PRDX6 AS1 gene polymorphisms and SLE susceptibility in Chinese populations. Front. Immunol. 2022, 13, 987385. [Google Scholar] [CrossRef]
- Xiong, M.; Guo, M.; Huang, D.; Li, J.; Zhou, Y. Effect of PRDX6 gene polymorphism on susceptibility to chronic obstructive pulmonary disease in the Chinese Han population. Clin. Respir. J. 2023, 17, 638–646. [Google Scholar] [CrossRef]
- Kubo, E.; Fatma, N.; Akagi, Y.; Beier, D.R.; Singh, S.P.; Singh, D.P. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am. J. Physiol.-Cell Physiol. 2008, 294, C842–C855. [Google Scholar] [CrossRef]
- Shibata, S.; Shibata, N.; Shibata, T.; Sasaki, H.; Singh, D.P.; Kubo, E. The role of Prdx6 in the protection of cells of the crystalline lens from oxidative stress induced by UV exposure. Jpn. J. Ophthalmol. 2016, 60, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Kubo, E.; Hasanova, N.; Tanaka, Y.; Fatma, N.; Takamura, Y.; Singh, D.P.; Akagi, Y. Protein expression profiling of lens epithelial cells from Prdx6-depleted mice and their vulnerability to UV radiation exposure. Am. J. Physiol.-Cell Physiol. 2010, 298, C342–C354. [Google Scholar] [CrossRef]
- Fatma, N.; Singh, P.; Chhunchha, B.; Kubo, E.; Shinohara, T.; Bhargavan, B.; Singh, D.P. Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am. J. Physiol.-Cell Physiol. 2011, 301, C954–C967. [Google Scholar] [CrossRef]
- Novoselova eg Sharapov, M.G.; Lunin, S.M.; Parfenyuk, S.B.; Khrenov, M.O.; Mubarakshina, E.K.; Kuzekova, A.A.; Novoselova, T.V.; Goncharov, R.G.; Glushkova, O.V. Peroxiredoxin 6 Applied after Exposure Attenuates Damaging Effects of X-ray Radiation in 3T3 Mouse Fibroblasts. Antioxidants 2021, 10, 1951. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The role of peroxiredoxin 6 in neutralization of X-ray mediated oxidative stress: Effects on gene expression, preservation of radiosensitive tissues and postradiation survival of animals. Free Radical Res. 2017, 51, 148–166. [Google Scholar] [CrossRef]
- Lunin, S.M.; Novoselova eg Glushkova, O.V.; Parfenyuk, S.B.; Kuzekova, A.A.; Novoselova, T.V.; Sharapov, M.G.; Mubarakshina, E.K.; Goncharov, R.G.; Khrenov, M.O. Protective effect of exogenous peroxiredoxin 6 and thymic peptide thymulin on BBB conditions in an experimental model of multiple sclerosis. Arch. Biochem. Biophys. 2023, 746, 109729. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Khater, S.M.; Ashamallah, S.A.; Rashed, S.A.; Fouad, A.M.; Ismail, A.M.; Ghoneim, M.A. PRDX6 Promotes the Differentiation of Human Mesenchymal Stem (Stromal) Cells to Insulin-Producing Cells. Biomed. Res. Int. 2020, 2020, 7103053. [Google Scholar] [CrossRef]
- Parfenyuk, S.B.; Glushkova, O.V.; Sharapov, M.G.; Khrenov, M.O.; Lunin, S.M.; Kuzekova, A.A.; Mubarakshina, E.K.; Novoselova, T.V.; Cherenkov, D.A.; Novoselova, E.G. Protective Effects of Peroxiredoxin 6 in Pro-Inflammatory Response Model Using Raw 264.7 Macrophages. Biochemistry 2023, 88, 1156–1164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).