Glucuronolactone Restores the Intestinal Barrier and Redox Balance Partly Through the Nrf2/Akt/FOXO1 Pathway to Alleviate Weaning Stress-Induced Intestinal Dysfunction in Piglets
Abstract
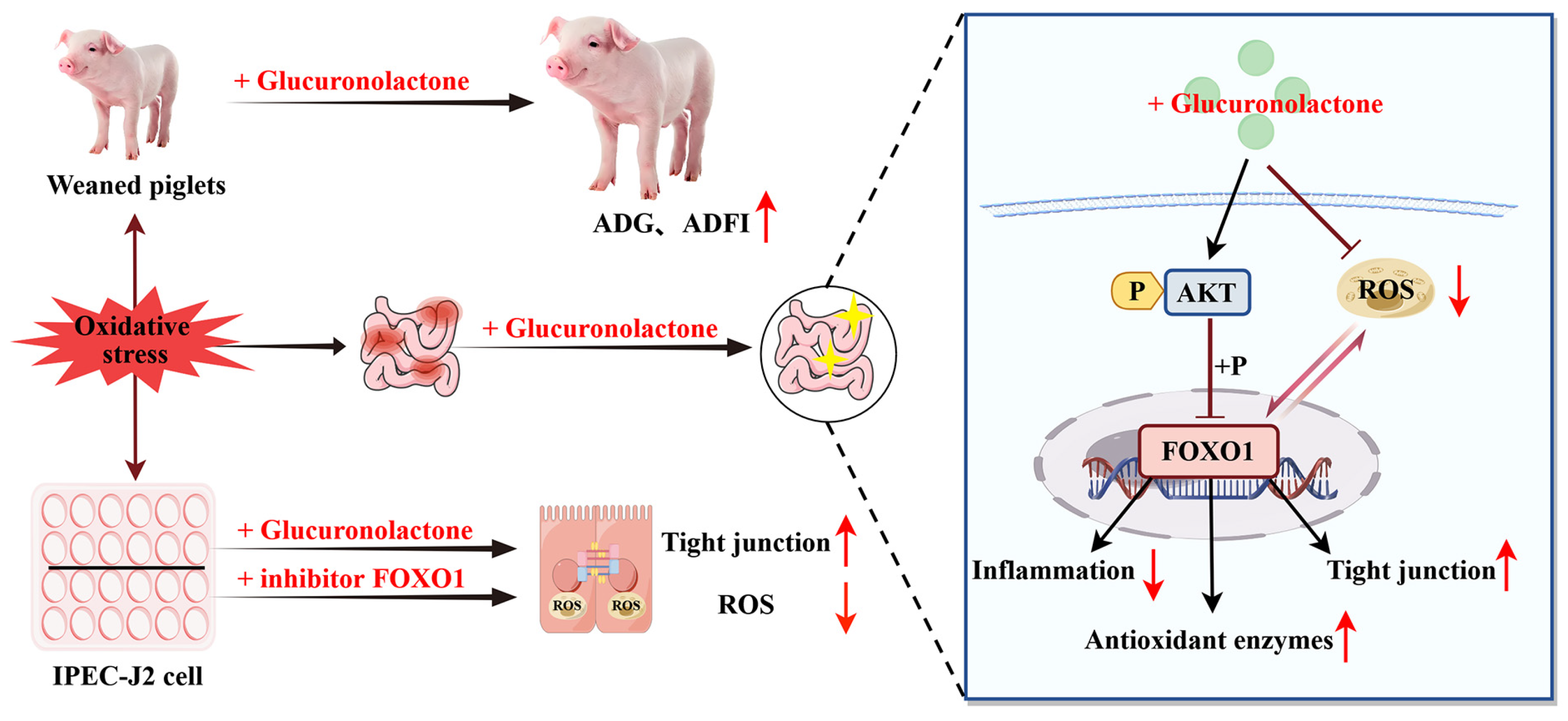
1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.1.1. Ethical Statement
2.1.2. Animals and Experimental Design
2.1.3. Data and Sample Collection
2.1.4. Serum Biochemical Indicators
2.1.5. Measurement of Intestinal Transmembrane Electrical Resistance
2.1.6. Intestinal Morphology Analysis
2.1.7. Intestinal Immunohistochemical Analysis
2.1.8. Total RNA Extraction and Real-Time Quantitative PCR
2.1.9. Chemical Analysis of Intestinal Mucosa
2.1.10. Microbiota 16S rRNA Sequencing and Analysis
2.1.11. Network Pharmacological Analysis
2.1.12. Western Blot Analysis of Intestinal
2.2. IPEC-J2 Cell Experiment
2.2.1. Cell Culture and Treatments
2.2.2. Cell Viability
2.2.3. Measurement of Intracellular ROS Levels
2.2.4. Immunofluorometric Assay
2.2.5. Western Blot Analysis of IPEC-J2 Cells
2.3. Statistical Analysis
3. Results
3.1. The Effects of GLU on the Growth Performance of Piglets
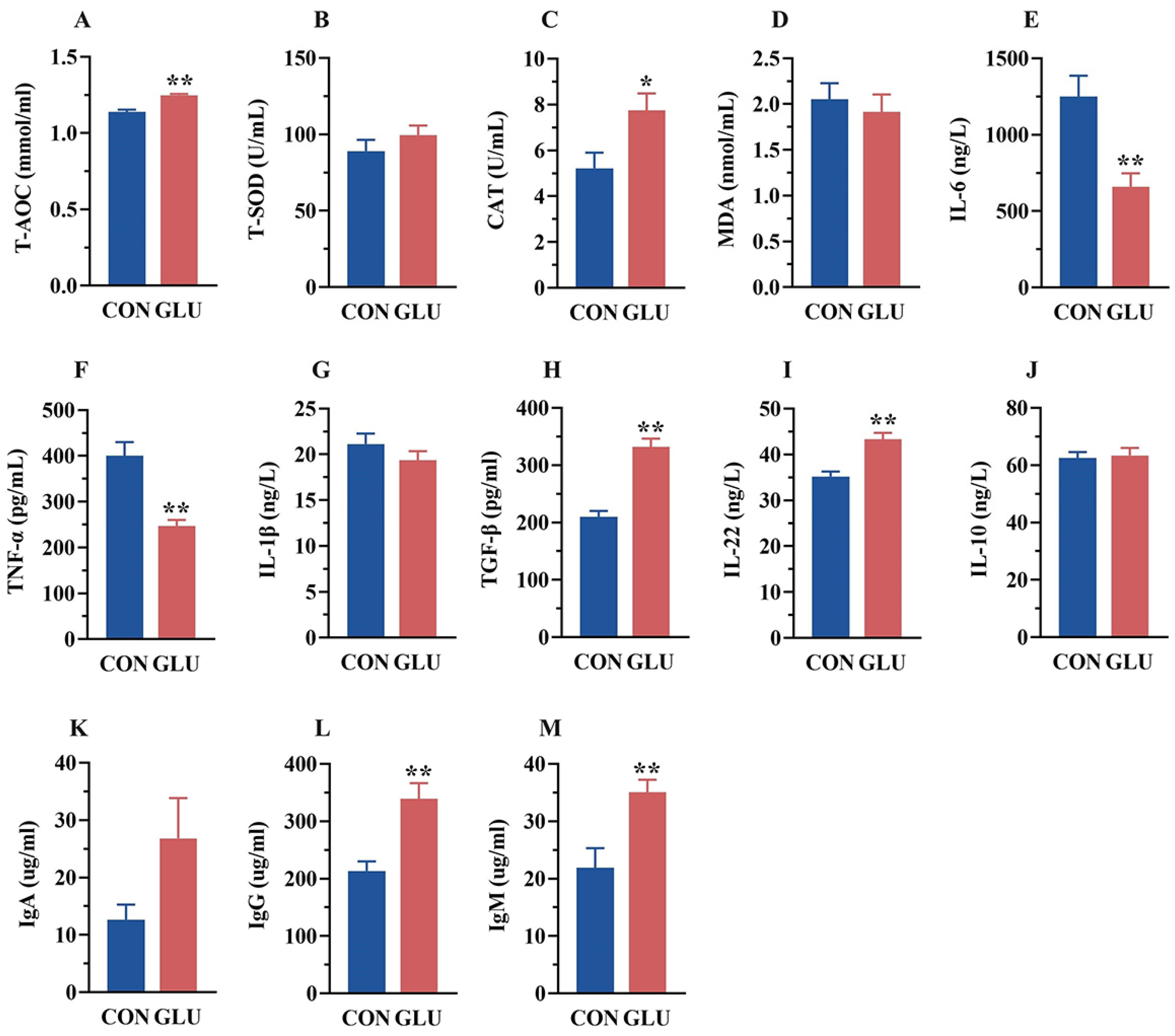
3.2. The Effects of GLU on the Serum Biochemical Parameters of Piglets
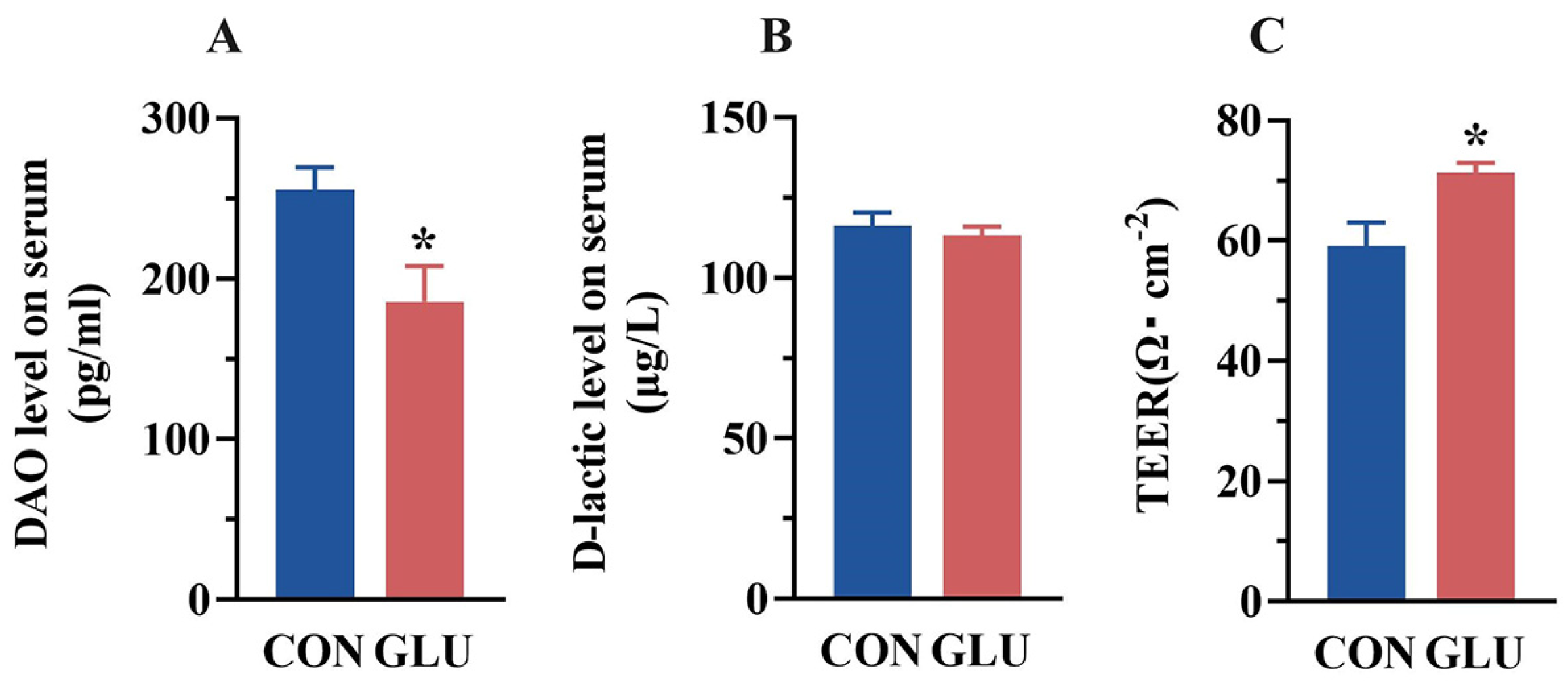
3.3. The Effects of GLU on the Intestinal Permeability of Piglets
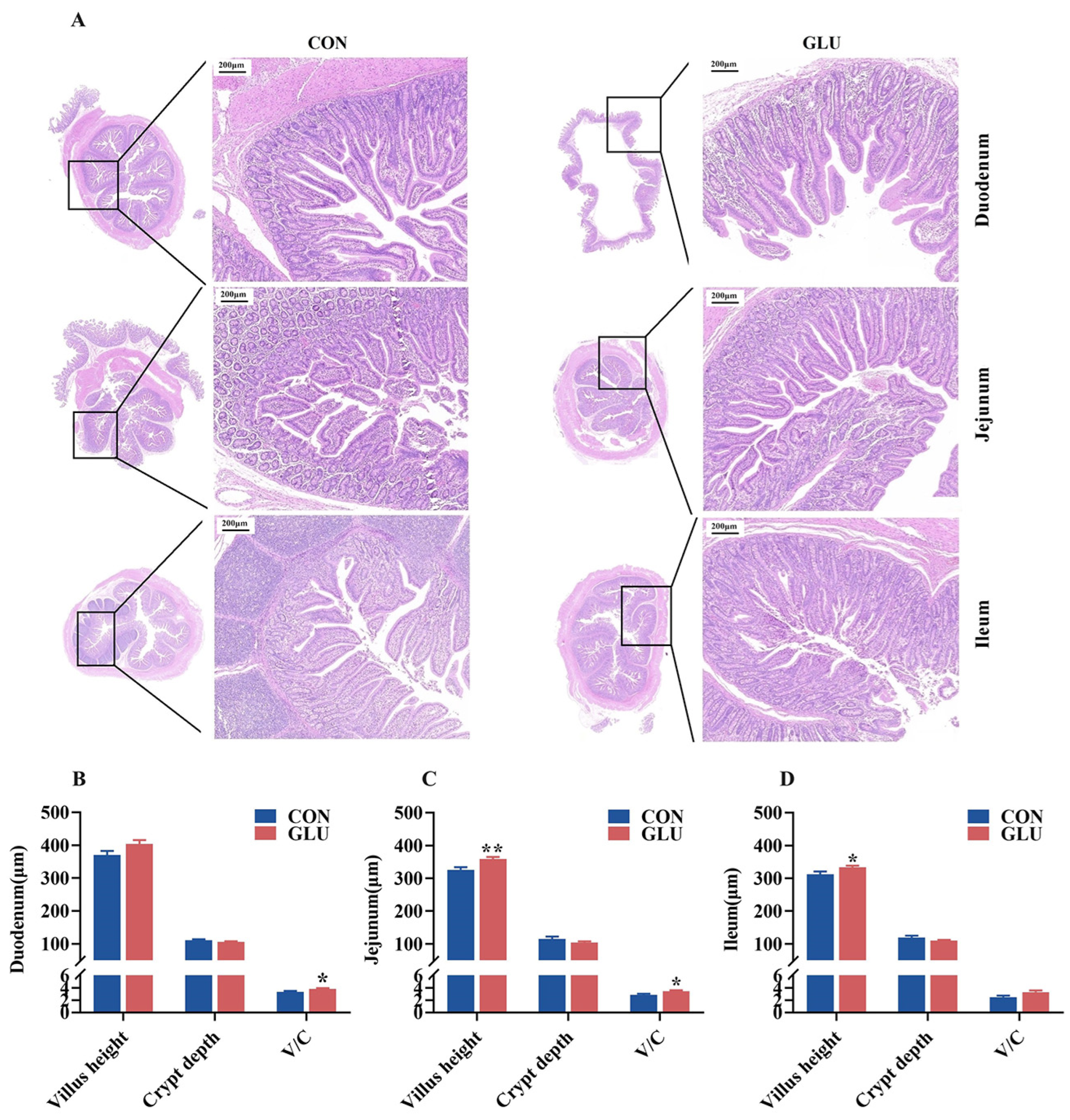
3.4. The Effects of GLU on the Intestinal Morphology in Piglets
3.5. The Effects of GLU on the Expression of Intestinal Tight Junction Proteins in Piglets
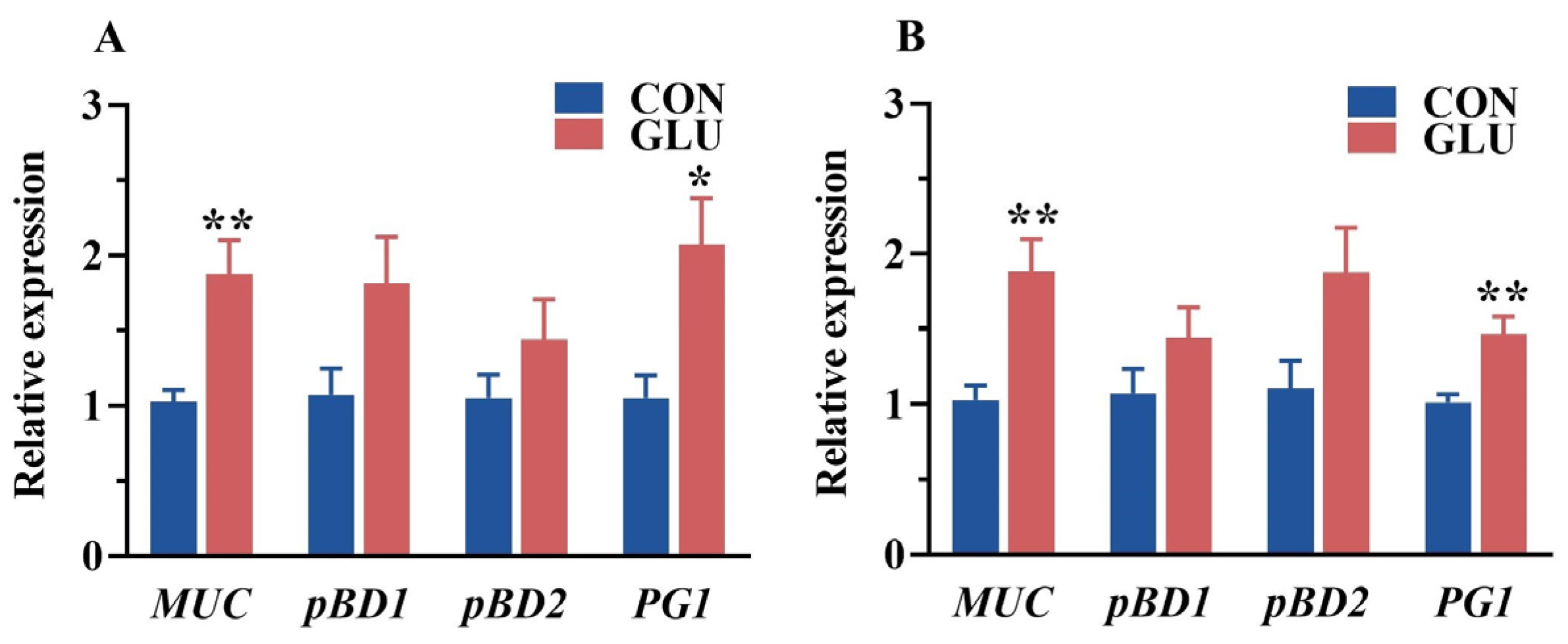
3.6. The Effects of GLU on the Relative Expression of Intestinal Mucin and Porcine β-Defensin in Piglets
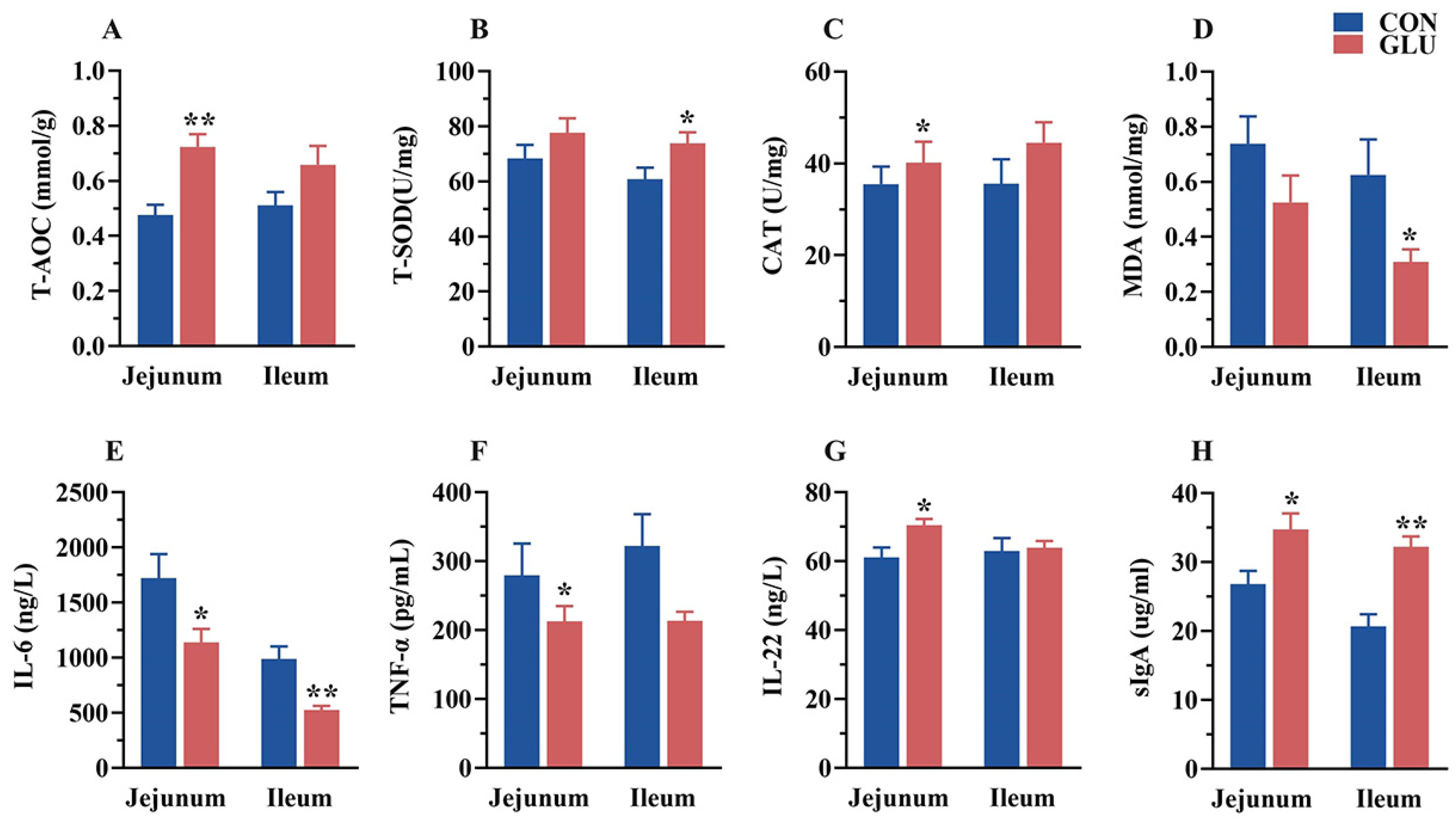
3.7. The Effects of GLU on the Antioxidant Capacity, Immunoglobulins, and Inflammatory Factors in the Intestine of Piglets
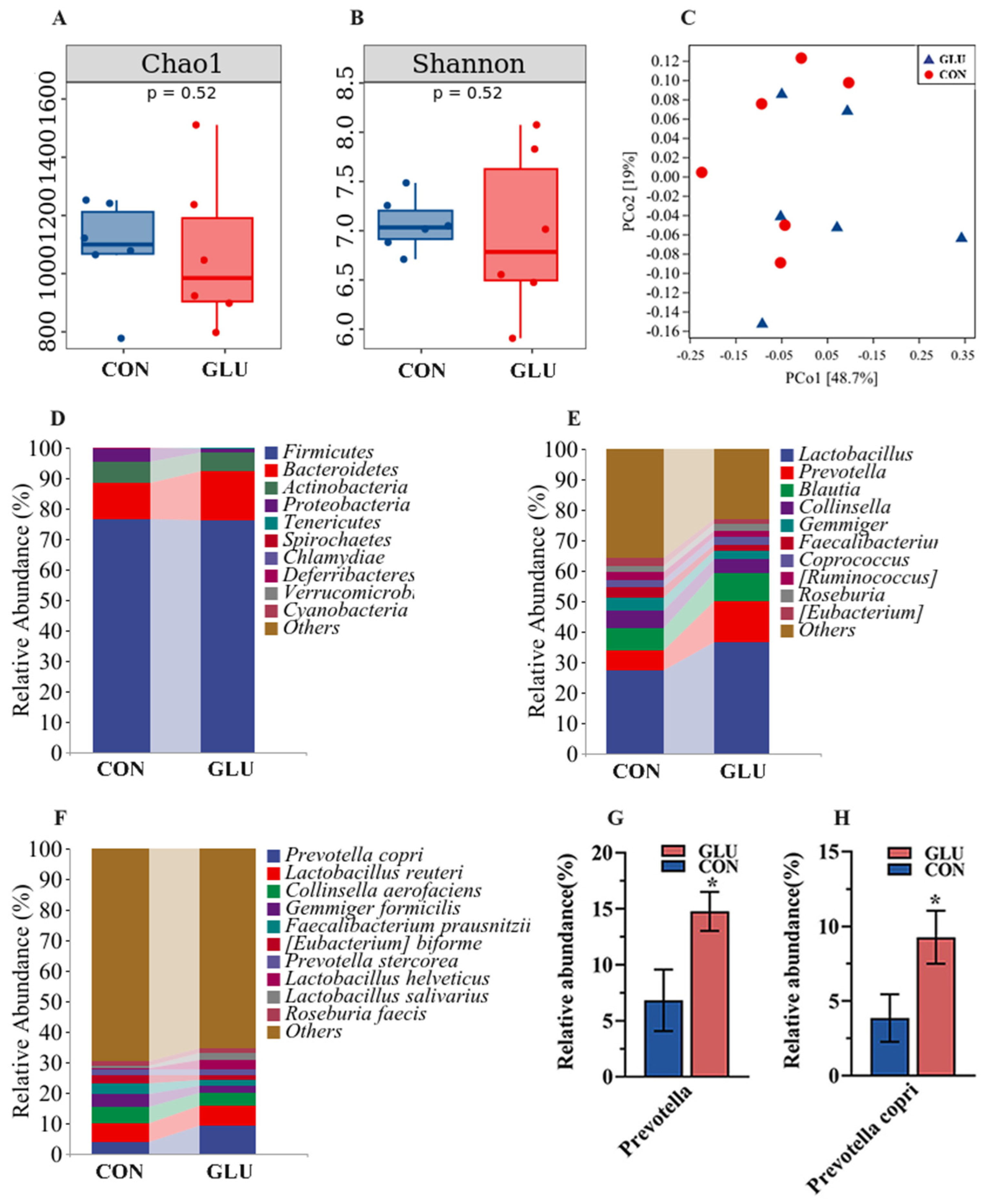
3.8. The Effects of GLU on the Microorganisms in the Contents of the Ileum and Colon in Piglets
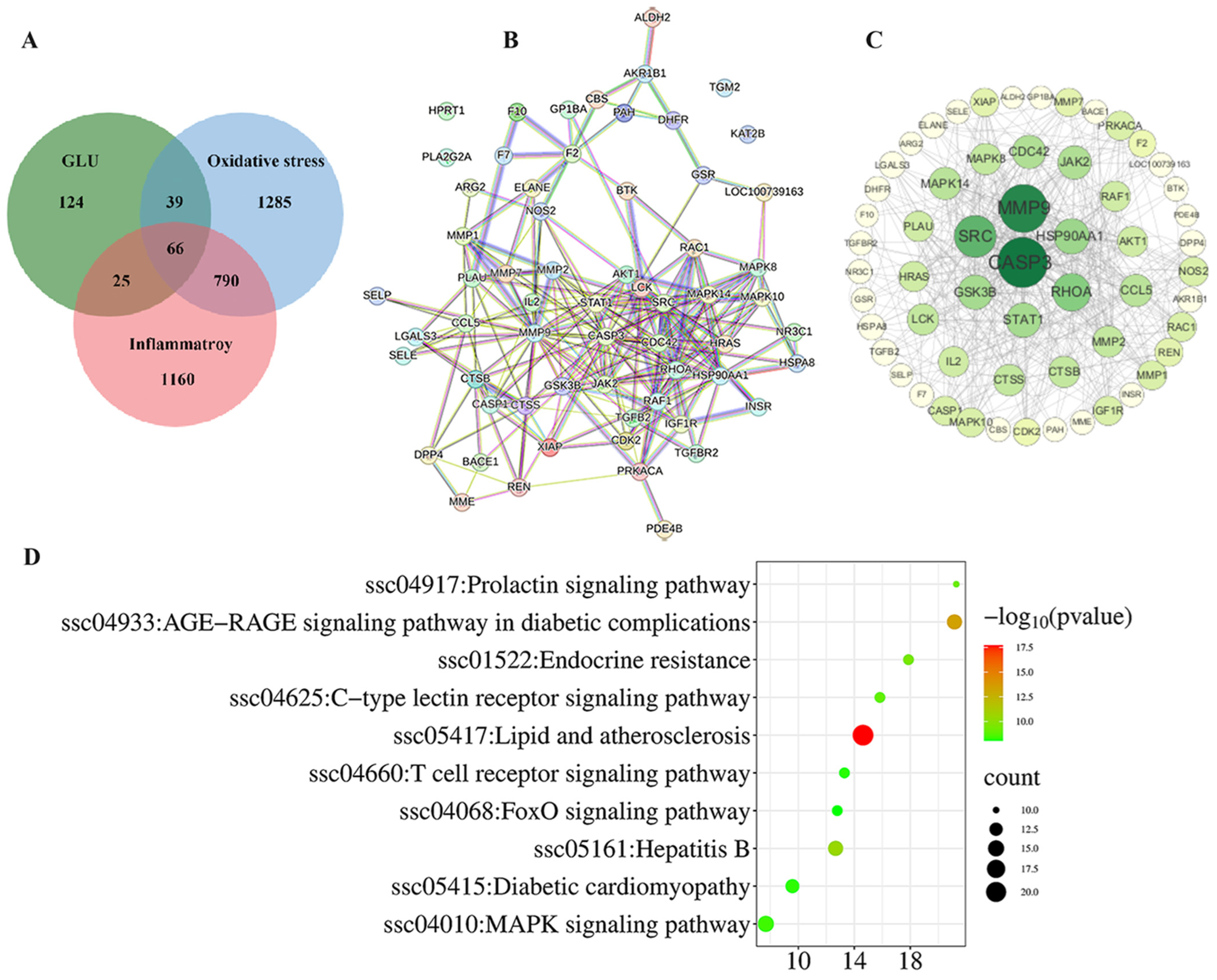
3.9. Network Pharmacological Analysis Among GLU, Oxidative Stress, and Inflammatory
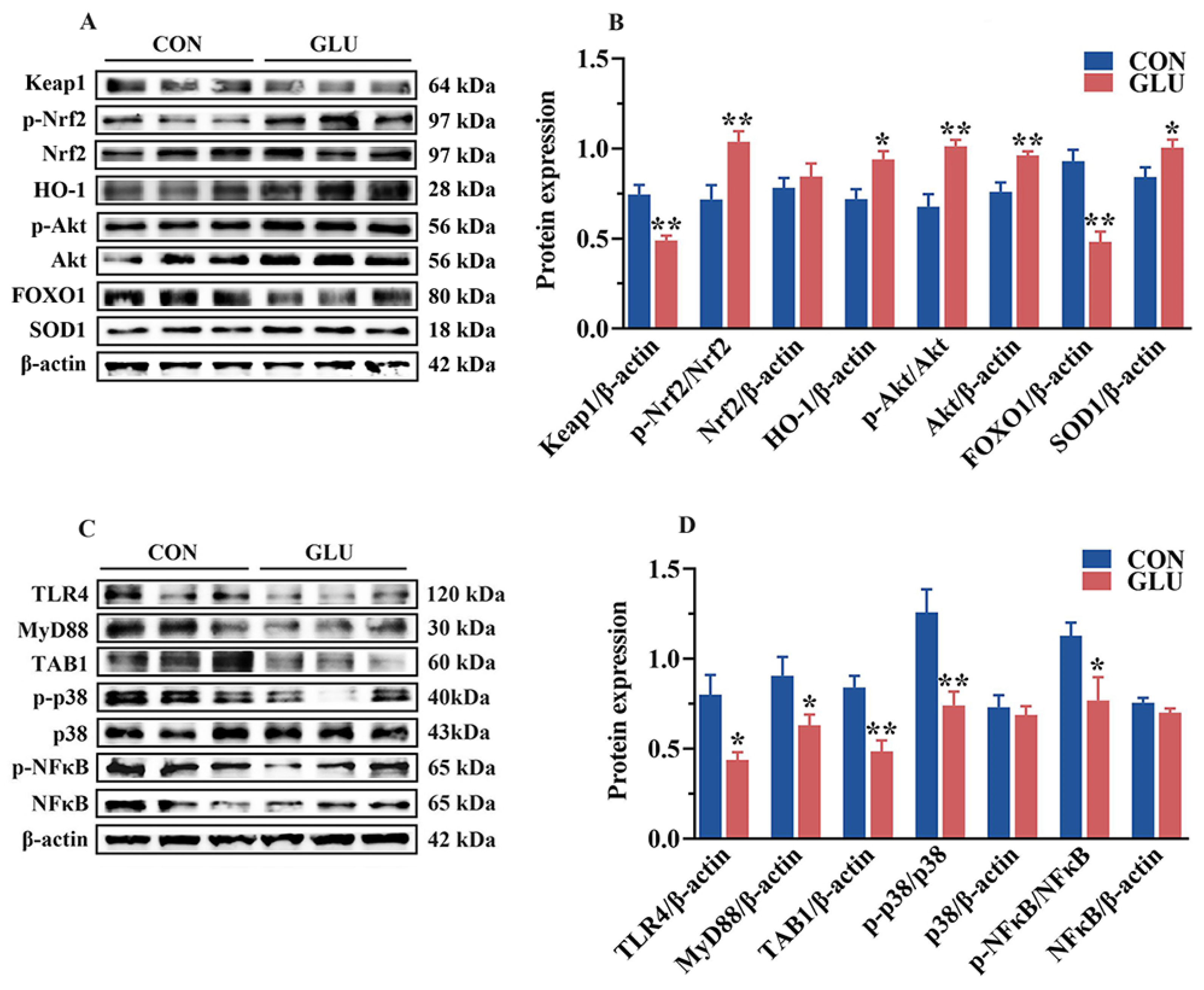
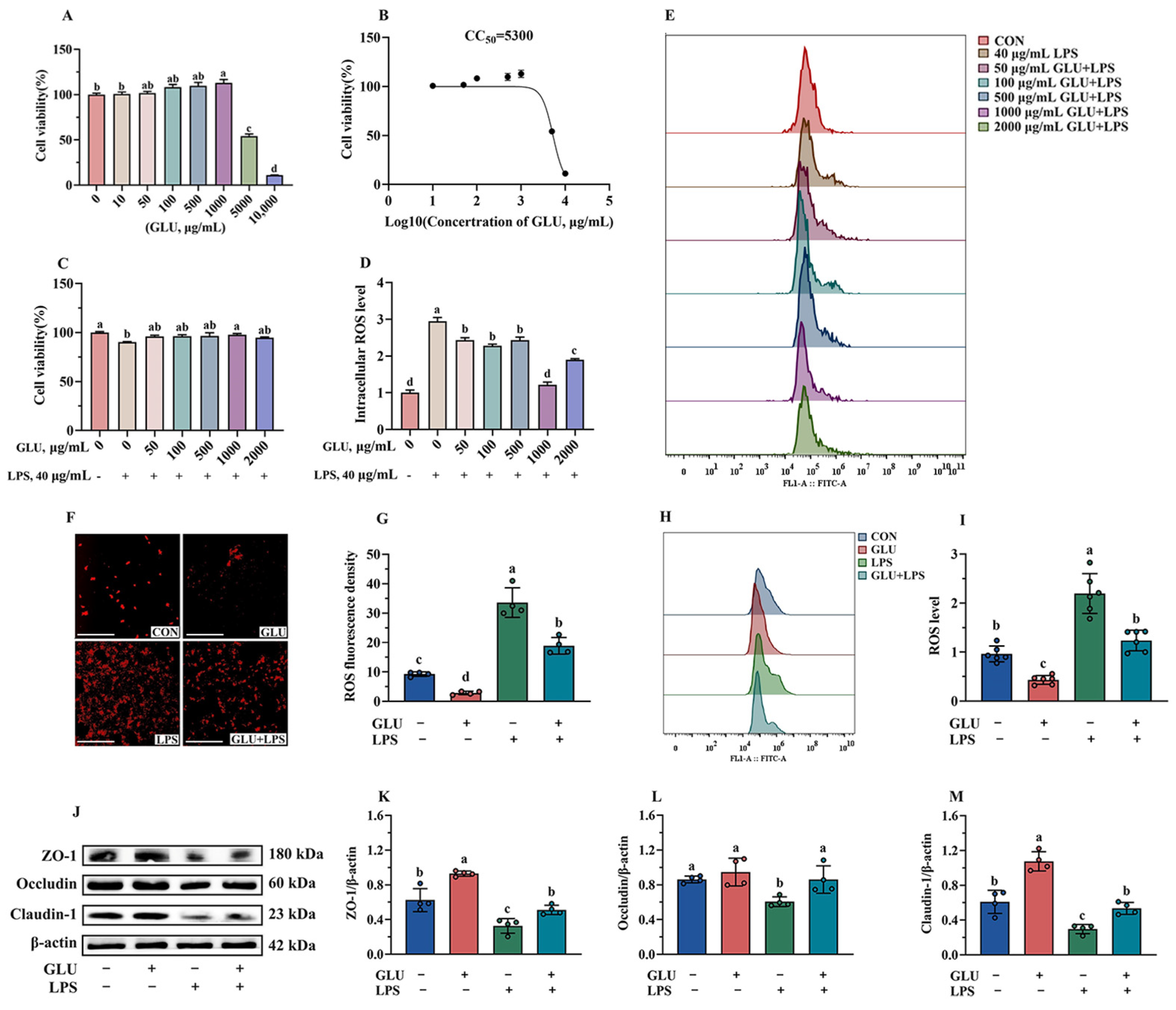
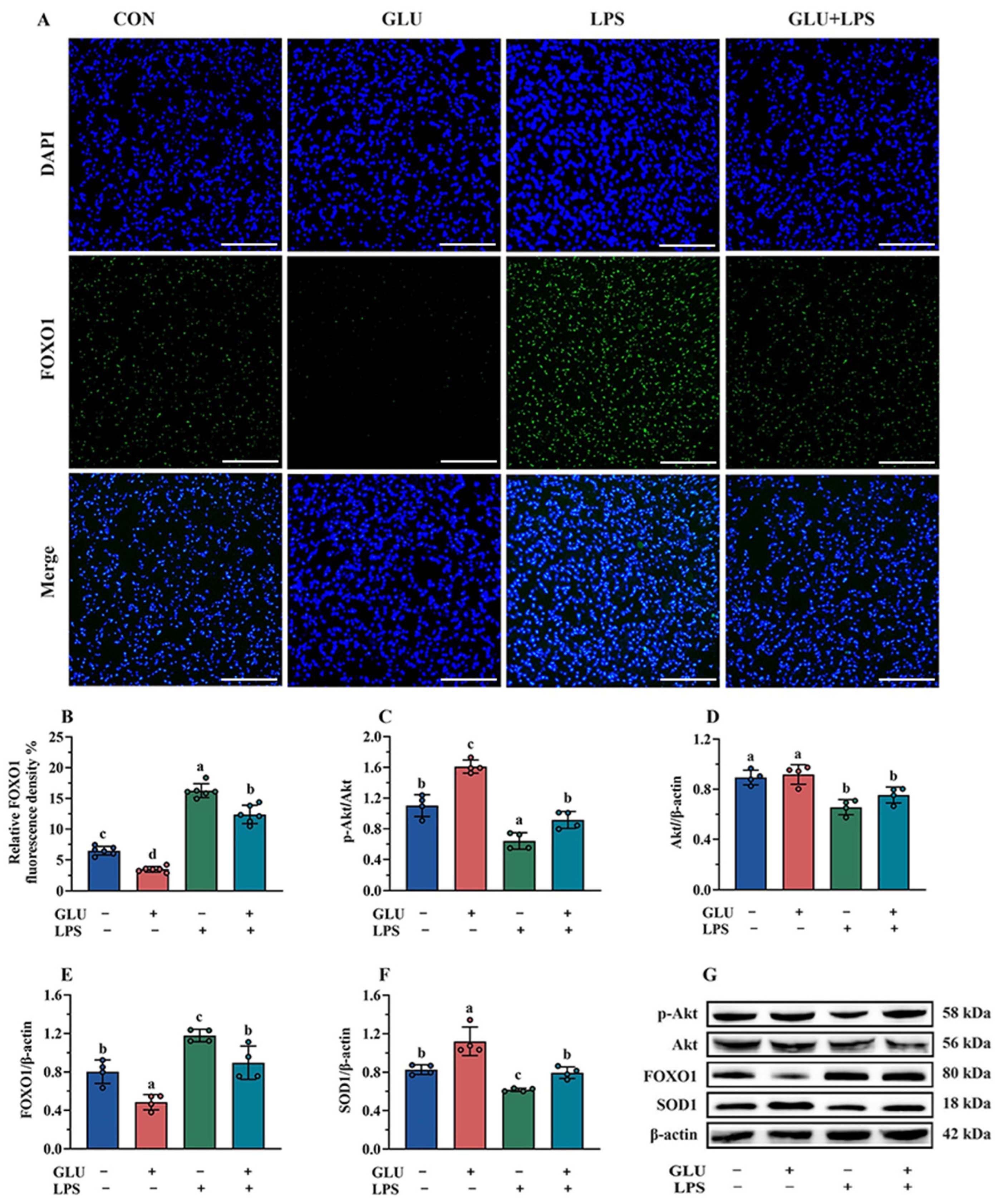
3.10. Effect of GLU Treatment on LPS-Challenged IPEC-J2 Cells
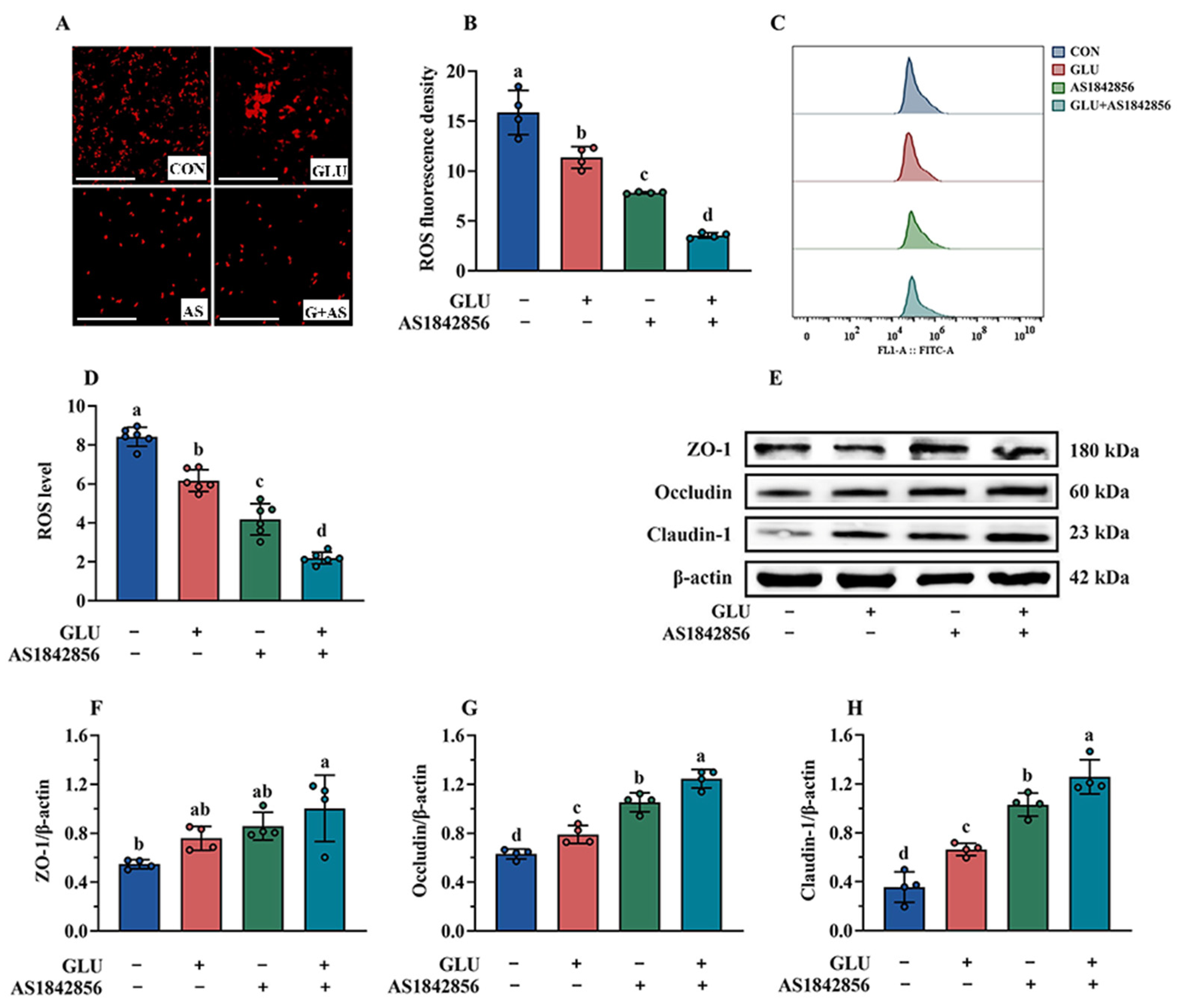
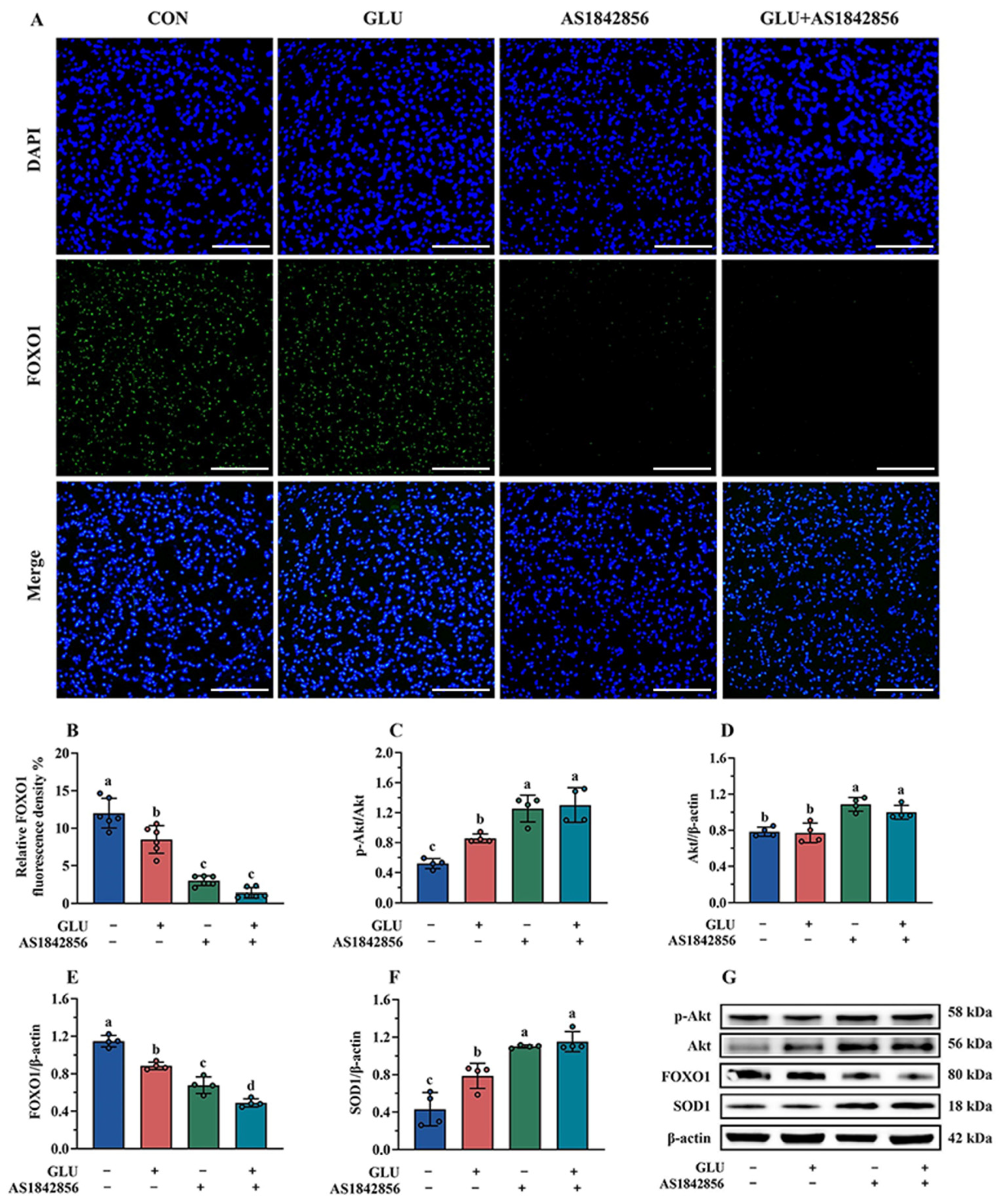
3.11. Effect of GLU Treatment and FOXO1 Pathway Inhibition on ROS Accumulation in IPEC-J2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xiong, X.; Wang, K.; Cheng, P.; Zou, L.; Zhou, J.; Qi, M.; Yin, Y. Early weaning leads to the remodeling of lipid profile in piglet jejunal crypt cells during post-weaning days. Anim. Nutr. 2022, 11, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Tang, Y.; Huang, Y. Gut health: The results of microbial and mucosal immune interactions in pigs. Anim. Nutr. 2021, 7, 282–294. [Google Scholar] [CrossRef]
- Tian, J.; Jiang, Q.; Bao, X.; Yang, F.; Li, Y.; Sun, H.; Yao, K.; Yin, Y. Plant-derived squalene supplementation improves growth performance and alleviates acute oxidative stress-induced growth retardation and intestinal damage in piglets. Anim. Nutr. 2023, 15, 386–398. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.A.M.; Zeng, W.; Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Cao, S.; Xiao, H.; Li, X.; Zhu, J.; Gao, J.; Wang, L.; Hu, C. AMPK-PINK1/Parkin Mediated Mitophagy Is Necessary for Alleviating Oxidative Stress-Induced Intestinal Epithelial Barrier Damage and Mitochondrial Energy Metabolism Dysfunction in IPEC-J2. Antioxidants 2021, 10, 2010. [Google Scholar] [CrossRef]
- Rajput, S.A.; Liang, S.J.; Wang, X.Q.; Yan, H.C. Lycopene Protects Intestinal Epithelium from Deoxynivalenol-Induced Oxidative Damage via Regulating Keap1/Nrf2 Signaling. Antioxidants 2021, 10, 1493. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, Q.; Qin, J.; Zhao, Q.; Shi, B. Resveratrol alleviates oxidative stress induced by oxidized soybean oil and improves gut function via changing gut microbiota in weaned piglets. J. Anim. Sci. Biotechnol. 2023, 14, 54. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Y.; Cheng, X.; Chen, J.; Cao, H.; Guo, X.; Zhang, C.; Zhuang, Y.; Hu, G. Baicalin Attenuates H2O2-Induced Oxidative Stress by Regulating the AMPK/Nrf2 Signaling Pathway in IPEC-J2 Cells. Int. J. Mol. Sci. 2023, 24, 9435. [Google Scholar] [CrossRef] [PubMed]
- Zha, A.; Yuan, D.; Cui, Z.; Qi, M.; Liao, S.; Liao, P.; Tan, B. The Evaluation of the Antioxidant and Intestinal Protective Effects of Baicalin-Copper in Deoxynivalenol-Challenged Piglets. Oxid. Med. Cell. Longev. 2020, 2020, 5363546. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Liu, Z.; Lv, H.; Cai, X.; Zhong, R.; Chen, L.; Zhang, H. Baicalin restore intestinal damage after early-life antibiotic therapy: The role of the MAPK signaling pathway. Pharmacol. Res. 2024, 204, 107194. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, F.; Tian, J.; Guo, X.; An, R. Protective effects of compound ammonium glycyrrhizin, L-arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes. Mol. Med. Rep. 2018, 18, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Chiu, C.-H.; Tseng, J.-K.; Yang, K.-T.; Chen, Y.-C. Ameliorative effects of D-glucuronolactone on oxidative stress and inflammatory/fibrogenic responses in livers of thioacetamide-treated rats. J. Funct. Foods 2015, 14, 154–162. [Google Scholar] [CrossRef]
- Link, K.A.; Spurzem, G.N.; Tuladhar, A.; Chase, Z.; Wang, Z.; Wang, H.; Walker, R.A. Organic Enrichment at Aqueous Interfaces: Cooperative Adsorption of Glucuronic Acid to DPPC Monolayers Studied with Vibrational Sum Frequency Generation. J. Phys. Chem. A 2019, 123, 5621–5632. [Google Scholar] [CrossRef]
- Stachulski, A.V. Convenient syntheses of deoxypyranose sugars from glucuronolactone. Tetrahedron Lett. 2007, 48, 2361–2364. [Google Scholar] [CrossRef]
- Tukey, R.H.; Strassburg, C.P. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616. [Google Scholar] [CrossRef]
- Peng, X.Y.; Luo, X.H.; Yang, Q.; Cheng, M.L.; Han, B.; Xie, R.J. Interventional effect of bicyclol on isoniazid-induced liver injury in rats and the expression of glucose-regulated protein 78, and growth arrest and DNA-damage-inducible gene 153. Zhonghua Gan Zang Bing Za Zhi 2019, 27, 133–139. [Google Scholar] [CrossRef]
- Nutrient Requirements of Swine; The National Academies Press: Washington, DC, USA, 2012.
- Lu, D.; Feng, C.; Pi, Y.; Ye, H.; Wu, Y.; Huang, B.; Zhao, J.; Han, D.; Soede, N.; Wang, J. Maternal dietary inulin intake during late gestation and lactation ameliorates intestinal oxidative stress in piglets with the involvements of gut microbiota and bile acids metabolism. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2025, 20, 318–331. [Google Scholar] [CrossRef]
- Tian, M.; Heng, J.; Song, H.; Zhang, Y.; Chen, F.; Guan, W.; Zhang, S. Branched chain amino acids stimulate gut satiety hormone cholecystokinin secretion through activation of the umami taste receptor T1R1/T1R3 using an in vitro porcine jejunum model. Food Funct. 2019, 10, 3356–3367. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, J.; Wang, L.; Yang, X.; Gao, K.; Zhu, C.; Jiang, Z. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Lixia, W.; Shanling, Y.; Jianzhong, L.; Yali, L.; Xueqin, D.; Jia, Y.; Xia, X.; Yulong, Y.; Huansheng, Y. Rapid Communication: The relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J. Anim. Sci. 2018, 1, 353–358. [Google Scholar]
- Li, X.; Gou, F.; Xiao, K.; Zhu, J.; Lin, Q.; Yu, M.; Hong, Q.; Hu, C. Effects of DON on Mitochondrial Function, Endoplasmic Reticulum Stress, and Endoplasmic Reticulum Mitochondria Contact Sites in the Jejunum of Piglets. J. Agric. Food Chem. 2023, 71, 13234–13243. [Google Scholar] [CrossRef]
- Bei-Bei, Z.; Xu, S.; Xiu-Jin, L.; Yun-Bo, T.; Hong-Jia, O.; Yun-Mao, H. Reference gene selection for expression studies in the reproductive axis tissues of Magang geese at different reproductive stages under light treatment. Sci. Rep. 2021, 11, 7573. [Google Scholar] [CrossRef]
- Villarino, N.F.; Lecleir, G.R.; Denny, J.E.; Dearth, S.P.; Schmidt, N.W. Composition of the gut microbiota modulates the severity of malaria. Proc. Natl. Acad. Sci. USA 2016, 113, 2235–2240. [Google Scholar] [CrossRef]
- Xing, J.-H.; Niu, T.-M.; Zou, B.-S.; Yang, G.-L.; Shi, C.-W.; Yan, Q.-S.; Sun, M.-J.; Yu, T.; Zhang, S.-M.; Feng, X.-Z.; et al. Gut microbiota-derived LCA mediates the protective effect of PEDV infection in piglets. Microbiome 2024, 12, 20. [Google Scholar] [CrossRef]
- Xie, W.; Song, L.; Wang, X.; Xu, Y.; Liu, Z.; Zhao, D.; Wang, S.; Fan, X.; Wang, Z.; Gao, C.; et al. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes 2021, 13, 1956281. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Verdile, N.; Mirmahmoudi, R.; Brevini, T.A.L.; Gandolfi, F. Evolution of pig intestinal stem cells from birth to weaning. Animal 2019, 13, 2830–2839. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Sun, S.; Luo, Z.; Shi, B.; Shan, A.; Cheng, B. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, M.; Wu, J.; Qiu, Y.; Xu, X.; Tian, C.; Hou, J.; Wang, L.; Gao, K.; Yang, X.; et al. Chlorogenic Acid Enhances the Intestinal Health of Weaned Piglets by Inhibiting the TLR4/NF-κB Pathway and Activating the Nrf2 Pathway. Int. J. Mol. Sci. 2024, 25, 9954. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Huang, Z.; Yan, H.; et al. Chlorogenic Acid Attenuates Oxidative Stress-Induced Intestinal Mucosa Disruption in Weaned Pigs. Front. Vet. Sci. 2022, 9, 806253. [Google Scholar] [CrossRef]
- Ji, F.; Yang, H.; Wang, Q.; Li, J.; Zhou, H.; Liu, S. Porcine intestinal antimicrobial peptide as an in-feed antibiotic alternative improves intestinal digestion and immunity by shaping the gut microbiota in weaned piglets. Anim. Nutr. 2023, 14, 43–55. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Y.; Yu, B.; Zheng, P.; Yu, J.; Huang, Z.; Mao, X.; Luo, J.; Yan, H.; He, J. Agrobacterium sp. ZX09 β-Glucan Attenuates Enterotoxigenic Escherichia coli-Induced Disruption of Intestinal Epithelium in Weaned Pigs. Int. J. Mol. Sci. 2022, 23, 10290. [Google Scholar] [CrossRef]
- Chen, J.L.; Zheng, P.; Zhang, C.; Yu, B.; He, J.; Yu, J.; Luo, J.Q.; Mao, X.B.; Huang, Z.Q.; Chen, D.W. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1137–1146. [Google Scholar] [CrossRef]
- Thompson, J.S.; Vaughan, W.P.; Forst, C.F.; Jacobs, D.L.; Weekly, J.S.; Rikkers, L.F. The effect of the route of nutrient delivery on gut structure and diamine oxidase levels. JPEN J. Parenter. Enter. Nutr. 1987, 11, 28–32. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Wang, F. Roles of Transepithelial Electrical Resistance in Mechanisms of Retinal Pigment Epithelial Barrier and Retinal Disorders. Discov. Med. 2022, 34, 19–24. [Google Scholar]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Blasig, I.E.; Haseloff, R.F. Tight junctions and tissue barriers. Antioxid. Redox Signal. 2011, 15, 1163–1166. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Liu, X.; Antonetti, D.A. Tight Junctions in Cell Proliferation. Int. J. Mol. Sci. 2019, 20, 5972. [Google Scholar] [CrossRef]
- Monaco, A.; Ovryn, B.; Axis, J.; Amsler, K. The Epithelial Cell Leak Pathway. Int. J. Mol. Sci. 2021, 22, 7677. [Google Scholar] [CrossRef]
- Balda, M.S.; Matter, K. Tight junctions. Curr. Biol. 2023, 33, R1135–R1140. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, Z.; Deng, D.; Wang, B.; Zhou, X.; Zhou, B.; Wang, C.; Zeng, Y. Effects of taurine on the growth performance, diarrhea, oxidative stress and intestinal barrier function of weanling piglets. Front. Vet. Sci. 2024, 11, 1436282. [Google Scholar] [CrossRef]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef]
- Gan, Z.; Guo, Y.; Zhao, M.; Ye, Y.; Liao, Y.; Liu, B.; Yin, J.; Zhou, X.; Yan, Y.; Yin, Y. Excitatory amino acid transporter supports inflammatory macrophage responses. Sci. Bull. 2024, 69, 15. [Google Scholar] [CrossRef]
- Accili, D.; Arden, K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Colman, M.J.; Dansen, T.B.; Burgering, B.M.T. FOXO transcription factors as mediators of stress adaptation. Nat. Rev. Mol. Cell Biol. 2024, 25, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Huang, J.; Yue, S.; Ke, B.; Zhu, J.; Shen, X.D.; Zhai, Y.; Yamamoto, M.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Nuclear factor erythroid 2-related factor 2 regulates toll-like receptor 4 innate responses in mouse liver ischemia-reperfusion injury through Akt-forkhead box protein O1 signaling network. Transplantation 2014, 98, 721–728. [Google Scholar] [CrossRef]
- Evans-Anderson, H.J.; Alfieri, C.M.; Yutzey, K.E. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ. Res. 2008, 102, 686–694. [Google Scholar] [CrossRef]
- Layden, H.M.; Ellis, J.D.; Bomber, M.L.; Bartlett, L.N.; Hiebert, S.W.; Stengel, K.R. Mutant FOXO1 controls an oncogenic network via enhancer accessibility. Cell Genom. 2024, 4, 100537. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction. Antioxidants 2022, 11, 2379. [Google Scholar] [CrossRef]
- Jin, S.; Wang, Y.S.; Huang, J.C.; Wang, T.T.; Li, B.Y.; Guo, B.; Yue, Z.P. Osthole exhibits the remedial potential for polycystic ovary syndrome mice through Nrf2-Foxo1-GSH-NF-κB pathway. Cell Biol. Int. 2024, 48, 1111–1123. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Fan, L.; Xia, Y.; Wang, Y.; Han, D.; Liu, Y.; Li, J.; Fu, J.; Wang, L.; Gan, Z.; Liu, B.; et al. Gut microbiota bridges dietary nutrients and host immunity. Sci. China Life Sci. 2023, 66, 2466–2514. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.N.; Cai, C.J.; Zeng, X.F.; Zhang, F.R.; Zhang, G.L.; Thacker, P.A.; Wang, J.J.; Qiao, S.Y. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J. Appl. Microbiol. 2013, 114, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Liu, H.; Zhang, J.; Zhang, S.; Yang, F.; Zeng, X.; Thacker, P.A.; Zhang, G.; Qiao, S. Intestinal microbiota succession and immunomodulatory consequences after introduction of Lactobacillus reuteri I5007 in neonatal piglets. PLoS ONE 2015, 10, e0119505. [Google Scholar] [CrossRef]
- Liu, H.; Hou, C.; Wang, G.; Jia, H.; Yu, H.; Zeng, X.; Thacker, P.A.; Zhang, G.; Qiao, S. Lactobacillus reuteri I5007 Modulates Intestinal Host Defense Peptide Expression in the Model of IPEC-J2 Cells and Neonatal Piglets. Nutrients 2017, 9, 559. [Google Scholar] [CrossRef]
- Tian, M.; Li, L.; Tian, Z.; Zhao, H.; Chen, F.; Guan, W.; Zhang, S. Glyceryl butyrate attenuates enterotoxigenic Escherichia coli-induced intestinal inflammation in piglets by inhibiting the NF-kappaB/MAPK pathways and modulating the gut microbiota. Food Funct. 2022, 13, 6282–6292. [Google Scholar] [CrossRef]



| Ingredient | % | Nutrient Levels 2 | |
|---|---|---|---|
| Corn | 41.21 | NE, kcal/kg | 2600.00 |
| Enzyme-treated soybean meal | 19.46 | CP, % | 22.73 |
| Expanded soybean | 11.00 | SID CP, % | 18.43 |
| Low protein whey powder | 10.00 | SID Lys, % | 1.43 |
| Whey protein concentrate | 4.00 | SID Met + Cys, % | 0.78 |
| Fishmeal | 3.00 | SID Thr, % | 0.84 |
| Soybean oil | 2.82 | SID Trp, % | 0.26 |
| Sucrose | 2.00 | SID Ile, % | 0.85 |
| Soybean hulls | 2.00 | SID Val, % | 0.91 |
| Lysine HCl | 0.33 | SID Leu, % | 1.64 |
| DL-Methionine | 0.14 | SID Lys/ME (g/MJ) | 5.49 |
| L-Threonine | 0.09 | Ca, % | 0.79 |
| Calcium hydrophosphate | 0.80 | STTD P, % | 0.62 |
| NaCl | 0.35 | Na, % | 0.31 |
| Limestone powder | 1.80 | ||
| Premix 1 | 1.00 | ||
| Total | 100.00 |
| Genes | Primer Sequence (5′-3′) | GenBank | Product Size (bp) |
|---|---|---|---|
| β-actin | F: TGCGGGACATCAAGGAGAAGC | XM_021086047 | 273 |
| R: ACAGCACCGTGTTGGCGTAGAG | |||
| MUC | F: CTGCTCCGGGTCCTGTGGGA | XM_021082584.1 | 101 |
| R: CCCGCTGGCTGGTGCGATAC | |||
| pBD-1 | F: ACCGCCTCCTCCTTGTATTC | NM_213838.1 | 150 |
| R: CACAGGTGCCGATCTGTTTC | |||
| pBD-2 | F: CCAGAGGTCCGACCACTACA | NM_214442.2 | 88 |
| R: GGTCCCTTCAATCCTGTTGAA | |||
| PG1 | F: GTAGGTTCTGCGTCTGTGTCG | NM_001123149.2 | 166 |
| R: CAAATCCTTCACCGTCTACCA |
| Item | CON | GLU | SEM | p-Value |
|---|---|---|---|---|
| Initial BW, kg | 6.33 | 6.32 | 0.13 | 0.979 |
| Final BW, kg | 12.97 a | 14.25 b | 0.36 | 0.02 |
| ADG, g/d | 315.95 a | 374.0 b | 19.63 | 0.038 |
| ADFI, g/d | 414.12 ab | 490.74 ab | 20.28 | 0.026 |
| G/F | 0.77 | 0.76 | 0.03 | 0.973 |
| Diarrhea rate, % | 24.45 a | 15.00 b | 2.58 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Tian, M.; Qiu, Y.; Wu, J.; Cui, C.; Liu, S.; Hou, J.; Tian, C.; Wang, L.; Gao, K.; et al. Glucuronolactone Restores the Intestinal Barrier and Redox Balance Partly Through the Nrf2/Akt/FOXO1 Pathway to Alleviate Weaning Stress-Induced Intestinal Dysfunction in Piglets. Antioxidants 2025, 14, 352. https://doi.org/10.3390/antiox14030352
Zhang B, Tian M, Qiu Y, Wu J, Cui C, Liu S, Hou J, Tian C, Wang L, Gao K, et al. Glucuronolactone Restores the Intestinal Barrier and Redox Balance Partly Through the Nrf2/Akt/FOXO1 Pathway to Alleviate Weaning Stress-Induced Intestinal Dysfunction in Piglets. Antioxidants. 2025; 14(3):352. https://doi.org/10.3390/antiox14030352
Chicago/Turabian StyleZhang, Beibei, Min Tian, Yueqin Qiu, Jing Wu, Chenbin Cui, Shilong Liu, Jing Hou, Chaoyang Tian, Li Wang, Kaiguo Gao, and et al. 2025. "Glucuronolactone Restores the Intestinal Barrier and Redox Balance Partly Through the Nrf2/Akt/FOXO1 Pathway to Alleviate Weaning Stress-Induced Intestinal Dysfunction in Piglets" Antioxidants 14, no. 3: 352. https://doi.org/10.3390/antiox14030352
APA StyleZhang, B., Tian, M., Qiu, Y., Wu, J., Cui, C., Liu, S., Hou, J., Tian, C., Wang, L., Gao, K., Jiang, Z., & Yang, X. (2025). Glucuronolactone Restores the Intestinal Barrier and Redox Balance Partly Through the Nrf2/Akt/FOXO1 Pathway to Alleviate Weaning Stress-Induced Intestinal Dysfunction in Piglets. Antioxidants, 14(3), 352. https://doi.org/10.3390/antiox14030352







