Discovering Skin Anti-Aging Potentials of the Most Abundant Flavone Phytochemical Compound Reported in Siam Violet Pearl, a Medicinal Plant from Thailand by In Silico and In Vitro Assessments
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking
2.2. Prediction of Pharmacokinetic Properties and Toxicities
2.3. In Vitro Enzymatic Anti-Aging Assays
2.3.1. Chemical Reagents
2.3.2. Determination of the Anti-Collagenase Assay
2.3.3. Determination of the Anti-Elastase Assay
2.3.4. Determination of the Anti-Tyrosinase Assay
2.4. Statistical Analysis
3. Results and Discussion
3.1. Binding Interactions Predicted by Molecular Docking
3.2. Pharmacokinetic Properties and Toxicities
3.3. In Vitro Anti-Aging Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huh, J.; Ha, T.K.Q.; Kang, K.B.; Kim, K.H.; Oh, W.K.; Kim, J.; Sung, S.H. C-Methylated Flavonoid Glycosides from Pentarhizidium orientale Rhizomes and Their Inhibitory Effects on the H1N1 Influenza Virus. J. Nat. Prod. 2017, 80, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Scognamiglio, M.; D’Abrosca, B.; Piccolella, S.; Tsafantakis, N.; Gallicchio, M.; Ricci, A.; Fiorentino, A. Spectroscopic Characterization and Antiproliferative Activity on HepG2 Human Hepatoblastoma Cells of Flavonoid C-Glycosides from Petrorhagia velutina. J. Nat. Prod. 2010, 73, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Stanković, J.; Gođevac, D.; Tešević, V.; Dajić-Stevanović, Z.; Ćirić, A.; Soković, M.; Novaković, M. Antibacterial and Antibiofilm Activity of Flavonoid and Saponin Derivatives from Atriplex tatarica against Pseudomonas aeruginosa. J. Nat. Prod. 2019, 82, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jin, J.; Ruan, J.; Zhu, C.; Lin, C.; Fang, W.; Cai, Y. Antioxidant Flavonoid Glycosides from Aerial Parts of the Fern Abacopteris penangiana. J. Nat. Prod. 2007, 70, 1683–1686. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and Other Phenolic Compounds for Physiological Roles, Plant Species Delimitation, and Medical Benefits: A Promising View. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Takenaka, Y.; Chen, C.-C.; Pelletier, J. Flavonoid Glycosides from Adina racemosa and Their Inhibitory Activities on Eukaryotic Protein Synthesis. J. Nat. Prod. 2004, 67, 427–431. [Google Scholar] [CrossRef]
- Intharuksa, A.; Kuljarusnont, S.; Sasaki, Y.; Tungmunnithum, D. Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and Medical Application. Molecules 2024, 29, 5760. [Google Scholar] [CrossRef]
- Nassief, S.M.; Amer, M.E.; Shawky, E.; Sishtla, K.; Mas-Claret, E.; Muniyandi, A.; Corson, T.W.; Mulholland, D.A.; El-Masry, S. Antiangiogenic Pterocarpan and Flavonoid Constituents of Erythrina lysistemon. J. Nat. Prod. 2023, 86, 759–766. [Google Scholar] [CrossRef]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef]
- Zeng, M.; Sun, R.; Basu, S.; Ma, Y.; Ge, S.; Yin, T.; Gao, S.; Zhang, J.; Hu, M. Disposition of flavonoids via recycling: Direct biliary excretion of enterically or extrahepatically derived flavonoid glucuronides. Mol. Nutr. Food Res. 2016, 60, 1006–1019. [Google Scholar] [CrossRef]
- Hour, T.-C.; Lan Nhi, N.T.; Lai, I.J.; Chuu, C.-P.; Lin, P.-C.; Chang, H.-W.; Su, Y.-F.; Chen, C.-H.; Chen, Y.-K. Kaempferol-Enhanced Migration and Differentiation of C2C12 Myoblasts via ITG1B/FAK/Paxillin and IGF1R/AKT/mTOR Signaling Pathways. Mol. Nutr. Food Res. 2024, 68, 2300685. [Google Scholar] [CrossRef] [PubMed]
- Ozalp Unal, D.; Sel, T. Investigation of Antiproliferative Effects of Combinations of White and Black Garlic Extracts with 5-Fluorouracil (5-FU) on Caco-2 Colorectal Adenocarcinoma Cells. Mol. Nutr. Food Res. 2024, 68, 2300820. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, Y.; Zi, J.; Hu, Y.; Ma, G.; Wang, X.; Shan, S.; Cheng, G.; Xiong, J. Dietary Flavonoids Consumption and Health: An Umbrella Review. Mol. Nutr. Food Res. 2024, 68, 2300727. [Google Scholar] [CrossRef] [PubMed]
- Majma Sanaye, P.; Mojaveri, M.R.; Ahmadian, R.; Sabet Jahromi, M.; Bahramsoltani, R. Apigenin and its Dermatological Applications: A Comprehensive Review. Phytochemistry 2022, 203, 113390. [Google Scholar] [CrossRef]
- Wang, Z.; Du, X.; Ye, G.; Wang, H.; Liu, Y.; Liu, C.; Li, F.; Ågren, H.; Zhou, Y.; Li, J.; et al. Functional Characterization, Structural Basis, and Protein Engineering of a Rare Flavonoid 2′-O-glycosyltransferase from Scutellaria baicalensis. Acta Pharm. Sin. B 2024, 14, 3746–3759. [Google Scholar] [CrossRef]
- Boligon, A.A.; de Freitas, R.B.; de Brum, T.F.; Waczuk, E.P.; Klimaczewski, C.V.; de Ávila, D.S.; Athayde, M.L.; de Freitas Bauermann, L. Antiulcerogenic Activity of Scutia buxifolia on Gastric Ulcers Induced by Ethanol in Rats. Acta Pharm. Sin. B 2014, 4, 358–367. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, W.; Yang, X.; Tong, B. Kaempferol inhibits the growth and metastasis of cholangiocarcinoma in vitro and in vivo. Acta Biochim. Biophys. Sin. 2016, 48, 238–245. [Google Scholar] [CrossRef]
- Luo, K.; Huang, W.; Qiao, L.; Zhang, X.; Yan, D.; Ning, Z.; Ma, C.; Dang, H.; Wang, D.; Guo, H.; et al. Dendrocalamus latiflorus and its Component Rutin Exhibit Glucose-lowering Activities by Inhibiting Hepatic Glucose Production via AKT Activation. Acta Pharm. Sin. B 2022, 12, 2239–2251. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically Modified Isoquercitrin Improves Endothelial Function in Volunteers at Risk of Cardiovascular Disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Validation of a High-Performance Liquid Chromatography with Photodiode Array Detection Method for the Separation and Quantification of Antioxidant and Skin Anti-Aging Flavonoids from Nelumbo nucifera Gaertn. Stamen Extract. Molecules 2022, 27, 1102. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Chen, R.; Shen, J.; Zhang, S.; Gu, Y.; Shi, J.; Meng, G. Dihydromyricetin Attenuates Diabetic Cardiomyopathy by Inhibiting Oxidative Stress, Inflammation and Necroptosis via Sirtuin 3 Activation. Antioxidants 2023, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Lorenzo, J.M.; Hano, C. Effect of Traditional Cooking and In Vitro Gastrointestinal Digestion of the Ten Most Consumed Beans from the Fabaceae Family in Thailand on Their Phytochemicals, Antioxidant and Anti-Diabetic Potentials. Plants 2022, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.D.; Lozano-Sánchez, J.; Fernández-Ochoa, Á.; Segura-Carretero, A. Enhancing the Yield of Bioactive Compounds from Sclerocarya birrea Bark by Green Extraction Approaches. Molecules 2019, 24, 966. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Garros, L.; Lorenzo, J.M.; Hano, C. Flavonoid Profiles and Antioxidant Potential of Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, a New Species from the Genus Monochoria C. Presl. Antioxidants 2022, 11, 952. [Google Scholar] [CrossRef]
- Gagandeep, M.; Biplab, S.; Ashish, K.; Rahul, S.; Awanish, M. Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top. Med. Chem. 2020, 20, 1169–1194. [Google Scholar] [CrossRef]
- Faysal, M.; Dehbia, Z.; Zehravi, M.; Sweilam, S.H.; Haque, M.A.; Kumar, K.P.; Chakole, R.D.; Shelke, S.P.; Sirikonda, S.; Nafady, M.H.; et al. Flavonoids as Potential Therapeutics Against Neurodegenerative Disorders: Unlocking the Prospects. Neurochem. Res. 2024, 49, 1926–1944. [Google Scholar] [CrossRef]
- Menichini, F.; Losi, L.; Bonesi, M.; Pugliese, A.; Loizzo, M.R.; Tundis, R. Chemical Profiling and In Vitro Biological Effects of Cardiospermum halicacabum L. (Sapindaceae) Aerial Parts and Seeds for Applications in Neurodegenerative Disorders. J. Enzyme Inhib. Med. Chem. 2014, 29, 677–685. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Tanaka, N.; Boonkerd, T. Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, the New Species of the Genus Monochoria from Thailand. Songklanakarin J. Sci. Technol. 2020, 42, 747–752. [Google Scholar]
- Tungmunnithum, D.; Boonkerd, T.; Zungsontiporn, S.; Tanaka, N. Phenetic Study of the Genus Monochoria in Thailand. Songklanakarin J. Sci. Technol. 2017, 39, 49–57. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Boonkerd, T.; Zungsontiporn, S.; Tanaka, N. Morphological Variations among Populations of Monochoria vaginalis s.l. (Pontederiaceae) in Thailand. Phytotaxa 2017, 268, 57. [Google Scholar] [CrossRef]
- Chayamarit, K. Pontederiaceae. In Flora of Thailand; Santisuk, T., Larsen, K., Eds.; Forest Herbarium, Royal Forest Department: Bangkok, Thailand, 2005; Volume 9, pp. 51–57. [Google Scholar]
- Wu, G.; Horn, C.N. Pontederiaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, pp. 40–42. [Google Scholar]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular Docking as a Tool for the Discovery of Molecular Targets of Nutraceuticals in Diseases Management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K.; Mariam, Z. Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis. Pharmaceuticals 2024, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Wołos, A.; Koszelewski, D.; Roszak, R.; Szymkuć, S.; Moskal, M.; Ostaszewski, R.; Herrera, B.T.; Maier, J.M.; Brezicki, G.; Samuel, J.; et al. Computer-designed Repurposing of Chemical Wastes into Drugs. Nature 2022, 604, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L. Grand Challenges of Computer-Aided Drug Design: The Road Ahead. Front. Drug Discov. 2021, 1, 728551. [Google Scholar] [CrossRef]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.; Oprea, T.I.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef]
- Cavasotto, C.N.; Scardino, V. Machine Learning Toxicity Prediction: Latest Advances by Toxicity End Point. ACS Omega 2022, 7, 47536–47546. [Google Scholar] [CrossRef]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L., III; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Alhayek, A.; Abdelsamie, A.S.; Schönauer, E.; Camberlein, V.; Hutterer, E.; Posselt, G.; Serwanja, J.; Blöchl, C.; Huber, C.G.; Haupenthal, J.; et al. Discovery and Characterization of Synthesized and FDA-Approved Inhibitors of Clostridial and Bacillary Collagenases. J. Med. Chem. 2022, 65, 12933–12955. [Google Scholar] [CrossRef] [PubMed]
- Jhoti, H.; Singh, O.M.P.; Wonacott, A. Structure of Porcine Pancreatic Elastase Complexed with the Elastase Inhibitor GR143783. Available online: https://www.rcsb.org/structure/1BRU (accessed on 14 September 2024).
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chao, H.; Chen, L.; Craig, P.A.; Crichlow, G.V.; Dalenberg, K.; Duarte, J.M.; et al. RCSB Protein Data Bank (RCSB.org): Delivery of Experimentally-determined PDB Structures Alongside One Million Computed Structure Models of Proteins from Artificial Intelligence/Machine Learning. Nucleic Acids Res. 2023, 51, D488–D508. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Biovia Discovery Studio® Overview. Available online: https://discover.3ds.com/sites/default/files/2023-12/biovia-discovery-studio-overview-datasheet.pdf (accessed on 9 November 2024).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory Effects of Polyphenols from Grape Pomace Extract on Collagenase and Elastase Activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Chai, W.-M.; Huang, Q.; Lin, M.-Z.; Ou-Yang, C.; Huang, W.-Y.; Wang, Y.-X.; Xu, K.-L.; Feng, H.-L. Condensed Tannins from Longan Bark as Inhibitor of Tyrosinase: Structure, Activity, and Mechanism. J. Agric. Food. Chem. 2018, 66, 908–917. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Fernie, A.R. Diversity: Current and Prospective Secondary Metabolites for Nutrition and Medicine. Curr. Opin. Biotechnol. 2022, 74, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; Kostyuk, V.; Potapovich, A.; Mayer, W.; Talib, N.; De Luca, C. Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics 2018, 5, 32. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Garros, L.; Hano, C. Differential Flavonoid and Other Phenolic Accumulations and Antioxidant Activities of Nymphaea lotus L. Populations throughout Thailand. Molecules 2022, 27, 3590. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-Assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Gohlke, H.; Hendlich, M.; Klebe, G. Knowledge-based Scoring Function to Predict Protein-ligand Interactions. J. Mol. Biol. 2000, 295, 337–356. [Google Scholar] [CrossRef]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef]
- Fischer, A.; Smieško, M.; Sellner, M.; Lill, M.A. Decision Making in Structure-Based Drug Discovery: Visual Inspection of Docking Results. J. Med. Chem. 2021, 64, 2489–2500. [Google Scholar] [CrossRef]
- Patel, N.Y.; Baria, D.M.; Pardhi, D.S.; Yagnik, S.M.; Panchal, R.R.; Rajput, K.N.; Raval, V.H. Chapter 16—Microbial enzymes in pharmaceutical industry. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 375–403. [Google Scholar]
- Fujieda, N.; Umakoshi, K.; Ochi, Y.; Nishikawa, Y.; Yanagisawa, S.; Kubo, M.; Kurisu, G.; Itoh, S. Copper–Oxygen Dynamics in the Tyrosinase Mechanism. Angew. Chem. Int. Ed. 2020, 59, 13385–13390. [Google Scholar] [CrossRef]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-based Specific Detection and Inhibition of Tyrosinase and Their Application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [CrossRef]
- Nutho, B.; Tungmunnithum, D. Anti-Aging Potential of the Two Major Flavonoids Occurring in Asian Water Lily Using In Vitro and In Silico Molecular Modeling Assessments. Antioxidants 2024, 13, 601. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Siddiquah, A.; Tungmunnithum, D.; Bose, S.; Younas, M.; Garros, L.; Drouet, S.; Giglioli-Guivarc’h, N.; Hano, C. Isodon rugosus (Wall. ex Benth.) Codd In Vitro Cultures: Establishment, Phytochemical Characterization and In Vitro Antioxidant and Anti-Aging Activities. Int. J. Mol. Sci. 2019, 20, 452. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Brás, N.F.; Gonçalves, R.; Fernandes, P.A.; Mateus, N.; Ramos, M.J.; de Freitas, V. Understanding the Binding of Procyanidins to Pancreatic Elastase by Experimental and Computational Methods. Biochemistry 2010, 49, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Cho, J.J.; Park, E.J.; Choi, J.D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int. J. Cosmet. Sci. 2001, 23, 341–346. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, X.; Jin, Z.; Cheng, H. Collagenases and their inhibitors: A review. Collagen Leather 2023, 5, 19. [Google Scholar] [CrossRef]
- Tohar, R.; Ansbacher, T.; Sher, I.; Afriat-Jurnou, L.; Weinberg, E.; Gal, M. Screening Collagenase Activity in Bacterial Lysate for Directed Enzyme Applications. Int. J. Mol. Sci. 2021, 22, 8552. [Google Scholar] [CrossRef]
- Nokinsee, D.; Shank, L.; Lee, V.S.; Nimmanpipug, P. Estimation of Inhibitory Effect against Tyrosinase Activity through Homology Modeling and Molecular Docking. Enzyme Res. 2015, 2015, 262364. [Google Scholar] [CrossRef]
- Le, J. Overview of Pharmacokinetics. Available online: https://www.msdmanuals.com/professional/clinical-pharmacology/pharmacokinetics/overview-of-pharmacokinetics (accessed on 9 November 2024).
- Grogan, S.; Preuss, C.V. Pharmacokinetics. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557744/ (accessed on 9 November 2024).
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Chen, C.P.; Chen, C.C.; Huang, C.W.; Chang, Y.C. Evaluating Molecular Properties Involved in Transport of Small Molecules in Stratum Corneum: A Quantitative Structure-Activity Relationship for Skin Permeability. Molecules 2018, 23, 911. [Google Scholar] [CrossRef]
- Alves, V.M.; Muratov, E.; Fourches, D.; Strickland, J.; Kleinstreuer, N.; Andrade, C.H.; Tropsha, A. Predicting Chemically-induced Skin Reactions. Part II: QSAR Models of Skin Permeability and the Relationships Between Skin Permeability and Skin Sensitization. Toxicol. Appl. Pharmacol. 2015, 284, 273–280. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 10th ed.; United Nations Publications: New York, NY, USA, 2023. [Google Scholar]
- Sezen Karaoğlan, E.; Hancı, H.; Koca, M.; Kazaz, C. Some Bioactivities of Isolated Apigenin-7-O-glucoside and Luteolin-7-O-glucoside. Appl. Sci. 2023, 13, 1503. [Google Scholar] [CrossRef]
- Nasr Bouzaiene, N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of Apigenin-7-glucoside, Genkwanin and Naringenin on Tyrosinase Activity and Melanin Synthesis in B16F10 Melanoma Cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Tahtaci, H.; Ozcan, I.; Mirghani, A.H.; Erdogan, T.; Kisa, D.; Yıldırım, B. 1,2,4-Triazole-derived oxime ether derivatives: Synthesis, characterization, in vitro tyrosinase inhibition properties and in silico studies. J. Mol. Struct. 2025, 1323, 140722. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, H.J.; Park, H.S.; Park, H.S.; Ko, J.; Yoon, D.; Park, Y.; Chun, P.; Chung, H.Y.; Moon, H.R. Design, Synthesis, and Antioxidant and Anti-Tyrosinase Activities of (Z)-5-Benzylidene-2-(naphthalen-1-ylamino)thiazol-4(5H)-one Analogs: In Vitro and In Vivo Insights. Molecules 2025, 30, 289. [Google Scholar] [CrossRef]
- Vasilev, H.; Šmejkal, K.; Jusková, S.; Vaclavik, J.; Treml, J. Five New Tamarixetin Glycosides from Astragalus thracicus Griseb. Including Some Substituted with the Rare 3-Hydroxy-3-methylglutaric Acid and Their Collagenase Inhibitory Effects In Vitro. ACS Omega 2024, 9, 18023–18031. [Google Scholar] [CrossRef]
- Choi, S.; Youn, J.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.-S.; Kwon, S.; Youn, H.J.; et al. Apigenin Inhibits UVA-Induced Cytotoxicity In Vitro and Prevents Signs of Skin Aging In Vivo. Int. J. Mol. Med. 2016, 38, 627–634. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism. Cells 2022, 11, 599. [Google Scholar] [CrossRef]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Marjanovic Vicentic, J.; Popovic, J.; Golic Grdadolnik, S.; Markovic, D.; Sankovic-Babice, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside Versus Apigenin: Insight into the Modes of Anticandidal and Cytotoxic Actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [CrossRef]
- Wang, W.; Yue, R.-F.; Jin, Z.; He, L.-M.; Shen, R.; Du, D.; Tang, Y.-Z. Efficiency Comparison of Apigenin-7-O-glucoside and Trolox in Antioxidative Stress and Anti-inflammatory Properties. J. Pharm. Pharmacol. 2020, 72, 1645–1656. [Google Scholar] [CrossRef]
- Shi, Q.-Q.; Dang, J.; Wen, H.-X.; Yuan, X.; Tao, Y.-D.; Wang, Q.-L. Anti-hepatitis, Antioxidant Activities and Bioactive Compounds of Dracocephalum heterophyllum Extracts. Bot. Stud. 2016, 57, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, H.; Li, J.; Fang, X.; Wu, H.; Hu, W. Apigetrin Alleviates Intervertebral Disk Degeneration by Regulating Nucleus Pulposus Cell Autophagy. JOR SPINE 2024, 7, e1325. [Google Scholar] [CrossRef] [PubMed]
- Cetiz, M.; Isah, M.; Ak, G.; Bakar, K.; Ahamada-Himidi, A.; Mohamed, A.; Glamoclija, J.; Nikolić, F.; Gašić, U.; Cespedes-Acuña, C.L.; et al. Exploring of Chemical Profile and Biological Activities of Three Ocimum Species From Comoros Islands: A Combination of In Vitro and In Silico Insights. Cell Biochem. Funct. 2024, 42, e70000. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, U.; Schönauer, E.; Nüss, D.; Brandstetter, H. Structure of Collagenase G Reveals a Chew-and-digest Mechanism of Bacterial Collagenolysis. Nat. Struct. Mol. Biol. 2011, 18, 1109–1114. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Li, J.; Xiong, J.; Xiao, B.-L. Inhibitory Mechanism on Tyrosinase Activity of Flavonoids from Flower Buds of Sophora japonica L. Heliyon 2024, 10, e38252. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef]
- Boran, R. Investigations of anti-aging potential of Hypericum origanifolium Willd. for skincare formulations. Ind. Crop Prod. 2018, 118, 290–295. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crop Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Coricovac, D.; Soica, C.; Muntean, D.; Popovici, R.; Dehelean, C.; Hogea, E. Assessment of the effects induced by two triterpenoids on liver mitochondria respiratory function isolated from aged rats. Rev. Chim. 2015, 66, 1707–1710. [Google Scholar]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and Instrumental Approaches to Treat Hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef]

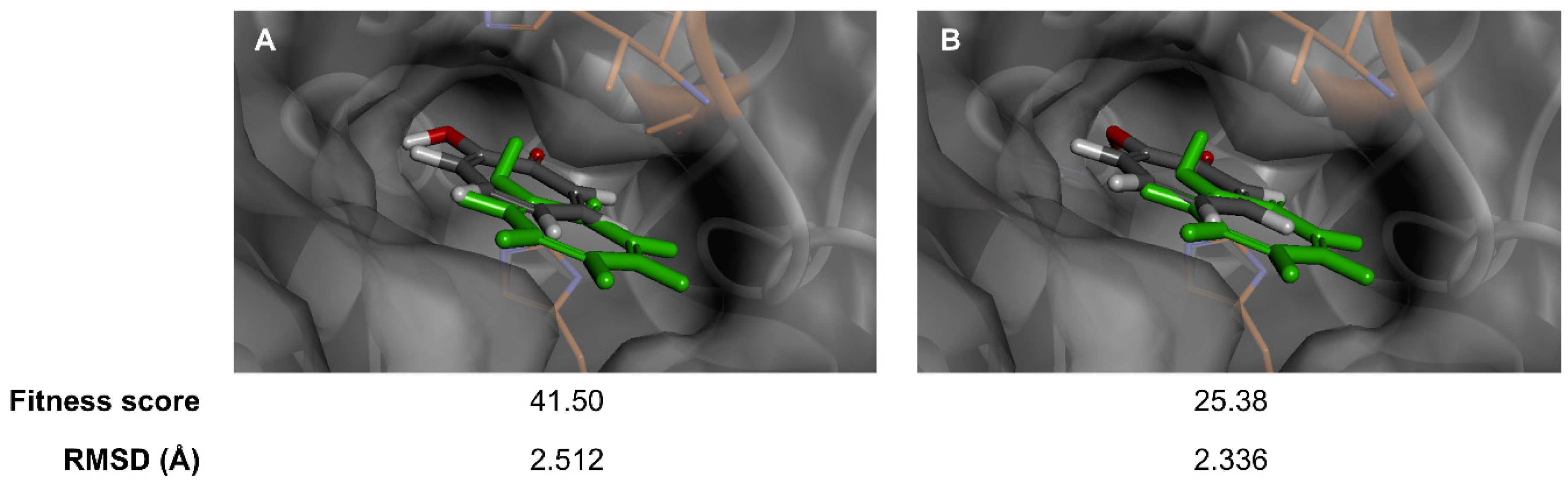
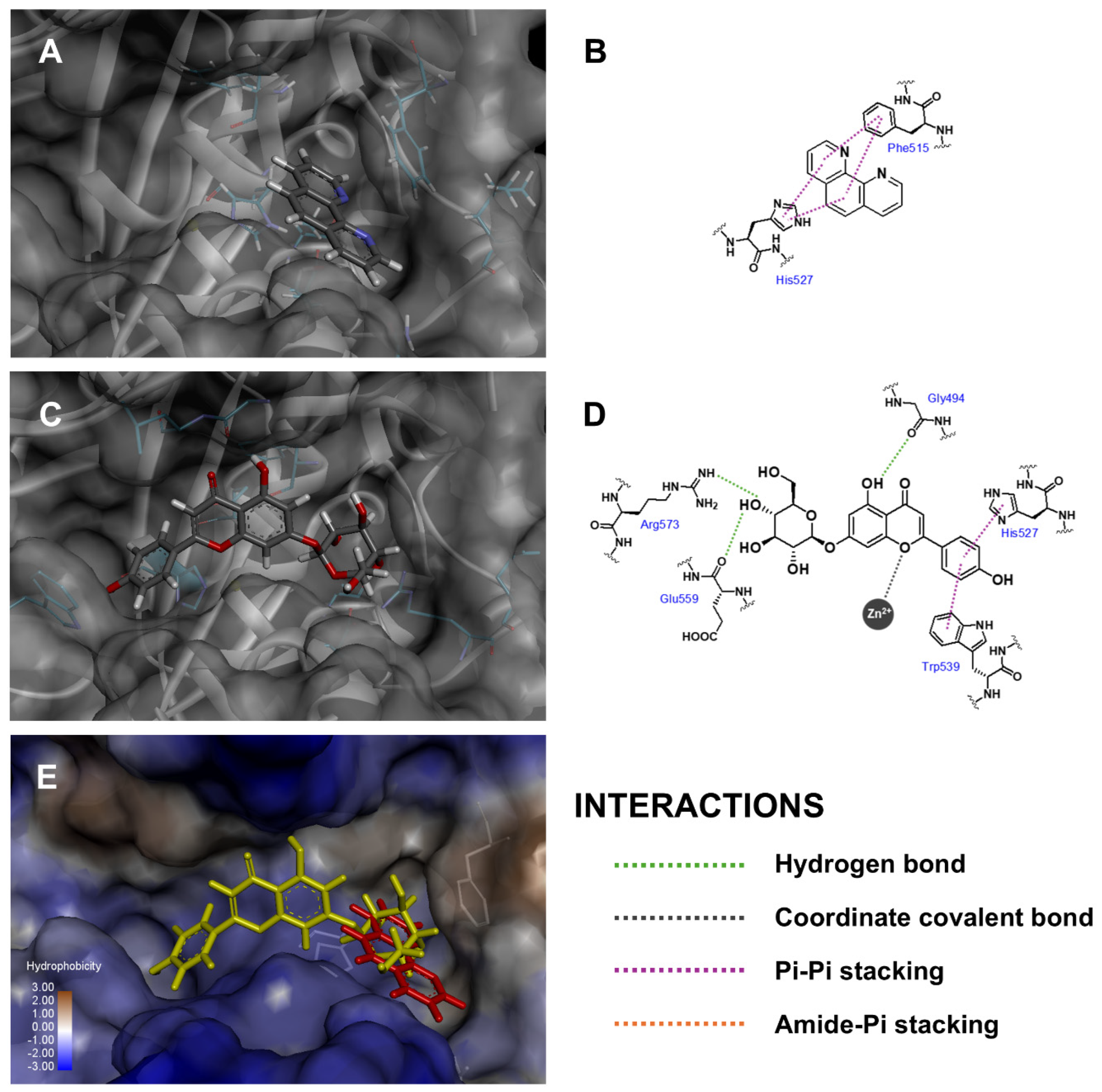
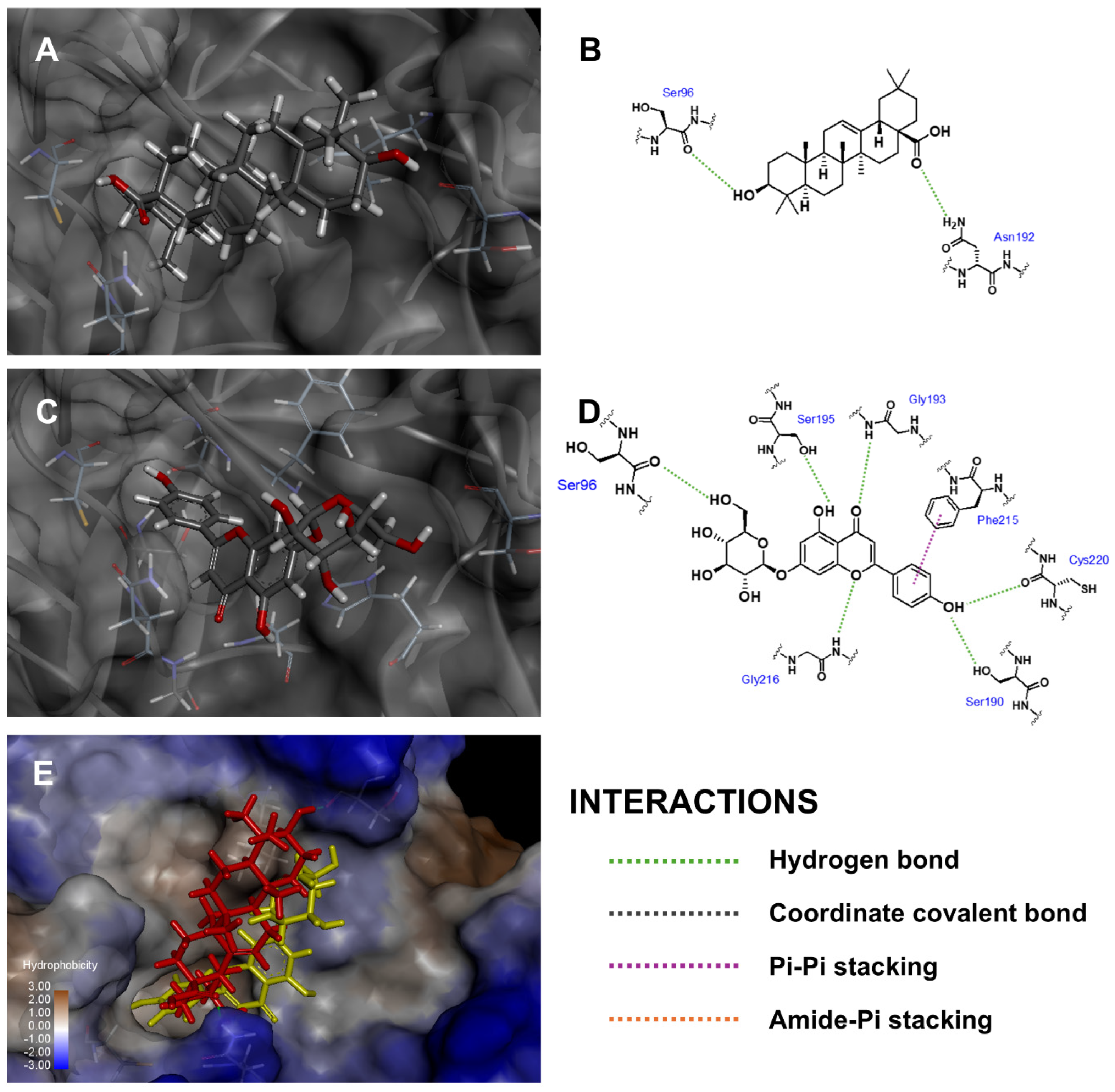
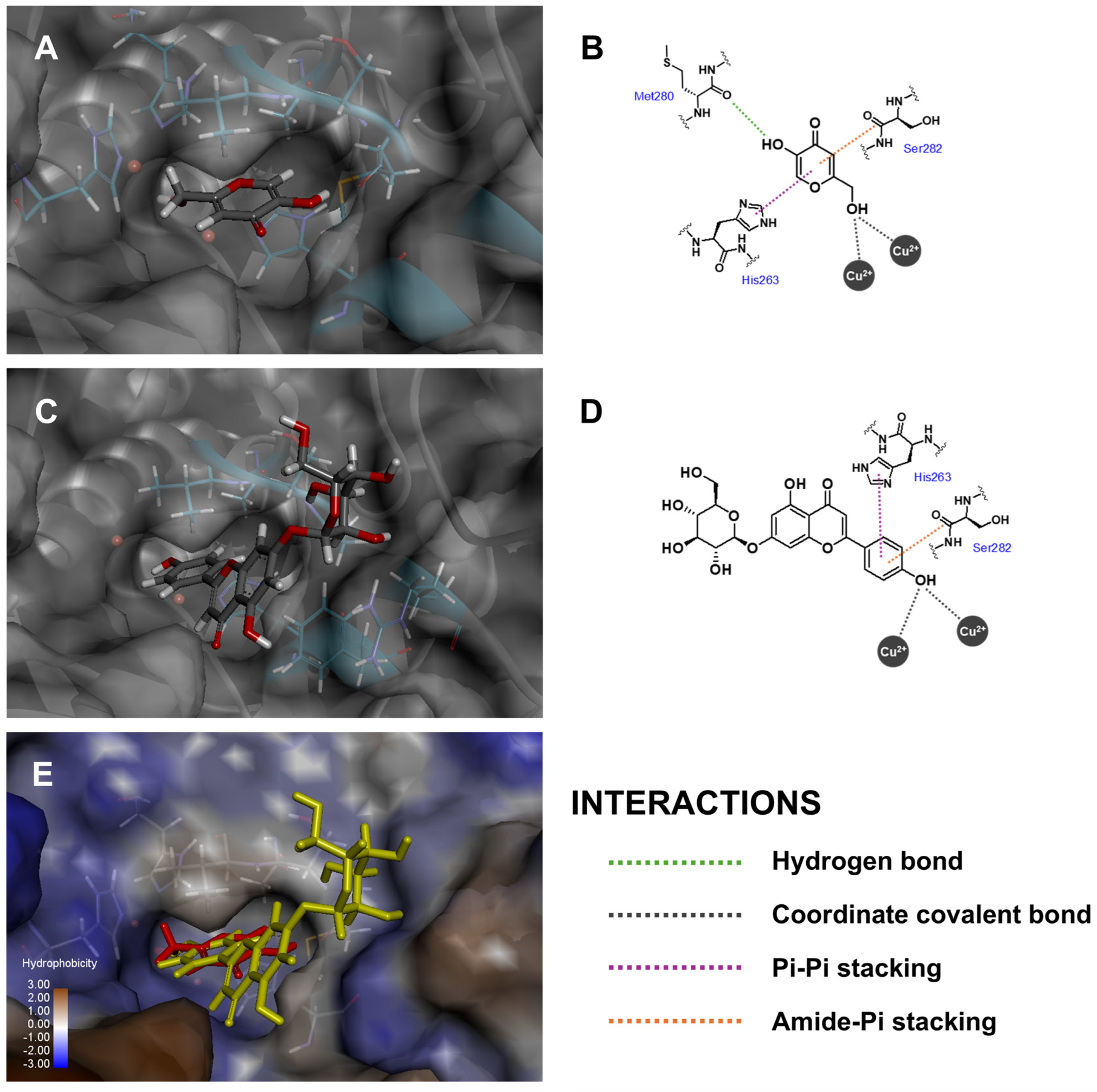
| Compound 1 | Fitness Score | ||
|---|---|---|---|
| Collagenase | Elastase | Tyrosinase | |
| Api-7-O-Glc | 67.30 | 56.57 | 55.40 |
| 1,10-Phenanthroline | 41.54 | - | - |
| Oleanolic acid | - | 37.04 | - |
| Kojic acid | - | - | 48.26 |
| Compound | Intermolecular Interaction | ||||
|---|---|---|---|---|---|
| H-Bond | Coordinate Covalent Bond | Pi–Pi Stacking | Amide–Pi Stacking | Van der Waal’s Force | |
| Collagenase | |||||
| Api-7-O-Glc | Gly494, Glu559, Arg573 | Zn (II) | His527, Trp539 | ||
| 1,10-Phenanthroline | Phe515, His523 | Glu524, Arg573 | |||
| Elastase | |||||
| Api-7-O-Glc | Ser96, Ser190, Gly193, Ser195, Gly216, Cys220 | Phe215 | Leu99 | ||
| Oleanolic acid | Ser96, Asn192 | ||||
| Tyrosinase | |||||
| Api-7-O-Glc | Cu (II) 400, 401 | His263 | Ser282 | His61, His85 | |
| Kojic acid | Met280 | Cu (II) 400, 401 | His263 | Ser282 | |
| ADMET Property | Api-7-O-Glc |
|---|---|
| MW (g/mol) | 432.38 |
| Rotatable bonds | 4 |
| Hydrogen bond donors | 6 |
| Hydrogen bond acceptors | 10 |
| Topological polar surface area (Å2) | 170.05 |
| Partition coefficient (Log P) | 1.98 |
| Solubility (Log S) | −3.78 |
| Water solubility (mg/mL) | 0.0719 |
| GI absorption | Low |
| Skin permeability (Log Kp, cm/h) | −2.735 |
| Skin sensitization | No |
| Hepatotoxicity | No |
| Carcinogenicity | Inactive |
| LD50 (mg/kg) | 5000 |
| Toxicity class | 5 |
| Anti-Aging Activities | % of Enzyme Inhibition # | Statistical Significance | |
|---|---|---|---|
| Api-7-O-Glc | Controls | ||
| Collagenase | 59.18 ± 6.24 | 33.14 ± 1.84 | ** |
| Elastase | 56.13 ± 7.49 | 49.90 ± 1.70 | * |
| Tyrosinase | 54.18 ± 6.54 | 67.90 ± 1.90 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aonsri, C.; Kuljarusnont, S.; Tungmunnithum, D. Discovering Skin Anti-Aging Potentials of the Most Abundant Flavone Phytochemical Compound Reported in Siam Violet Pearl, a Medicinal Plant from Thailand by In Silico and In Vitro Assessments. Antioxidants 2025, 14, 272. https://doi.org/10.3390/antiox14030272
Aonsri C, Kuljarusnont S, Tungmunnithum D. Discovering Skin Anti-Aging Potentials of the Most Abundant Flavone Phytochemical Compound Reported in Siam Violet Pearl, a Medicinal Plant from Thailand by In Silico and In Vitro Assessments. Antioxidants. 2025; 14(3):272. https://doi.org/10.3390/antiox14030272
Chicago/Turabian StyleAonsri, Chaiyawat, Sompop Kuljarusnont, and Duangjai Tungmunnithum. 2025. "Discovering Skin Anti-Aging Potentials of the Most Abundant Flavone Phytochemical Compound Reported in Siam Violet Pearl, a Medicinal Plant from Thailand by In Silico and In Vitro Assessments" Antioxidants 14, no. 3: 272. https://doi.org/10.3390/antiox14030272
APA StyleAonsri, C., Kuljarusnont, S., & Tungmunnithum, D. (2025). Discovering Skin Anti-Aging Potentials of the Most Abundant Flavone Phytochemical Compound Reported in Siam Violet Pearl, a Medicinal Plant from Thailand by In Silico and In Vitro Assessments. Antioxidants, 14(3), 272. https://doi.org/10.3390/antiox14030272









