Antioxidant and Photoprotective Activities of 3,4-Dihydroxybenzoic Acid and (+)-Catechin, Identified from Schima argentea Extract, in UVB-Irradiated HaCaT Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of S. argentea Using Solvents of Different Polarities
2.2. Analysis of Total Phenol and Flavonoid Contents
2.3. Analysis of ABTS and DPPH Scavenging Rates
2.4. Cell Viability Assessment
2.5. Cellular ROS Assay
2.6. UPLC-QTOF-MS Assay of the NBA Extract
2.7. Western Blot Analysis
2.8. Analysis of JNK1 and p38 α Kinase Activity
2.9. Cell Viability and Cytotoxicity Assay Using Calcein/PI
2.10. Cell Apoptosis Assay Using Hoechst Staining
2.11. Cell Apoptosis Assay Using a Flow Cytometer
2.12. Proteomic Analysis
2.13. Data Statistical Analysis
3. Results
3.1. Antioxidant Capacities and Total Phenol and Flavonoid Contents of S. argentea Extracts
3.2. Effect of NBA on ROS Production and Survival Rate of UVB-Induced Cells
3.3. Identification of NBA Components Using UPLC-QTOF-MS
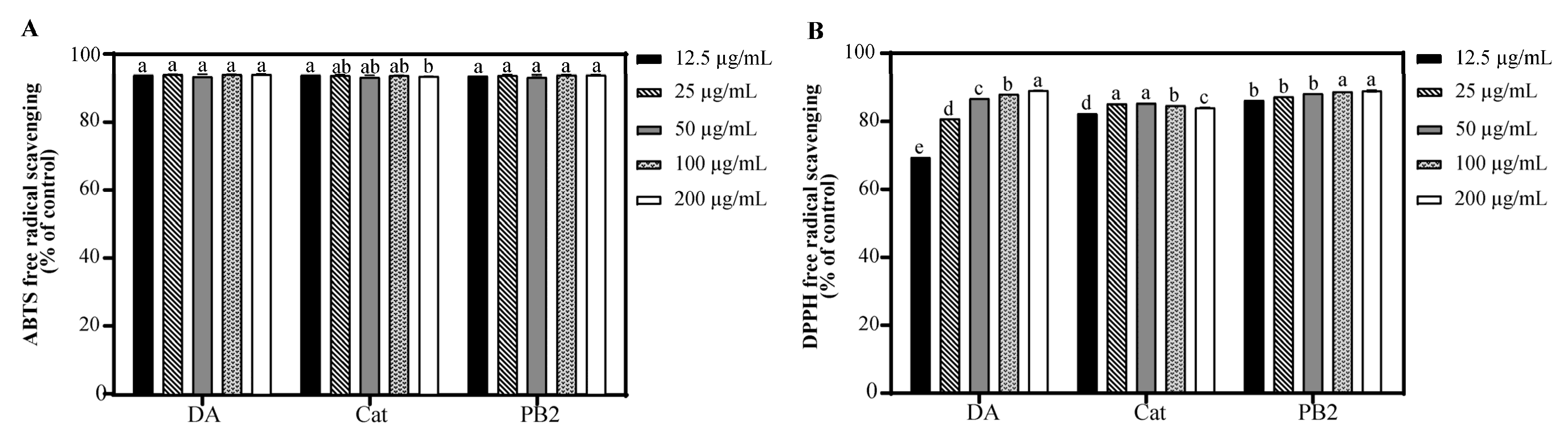
3.4. Antioxidant Capacities of Bioactive Compounds from NBA
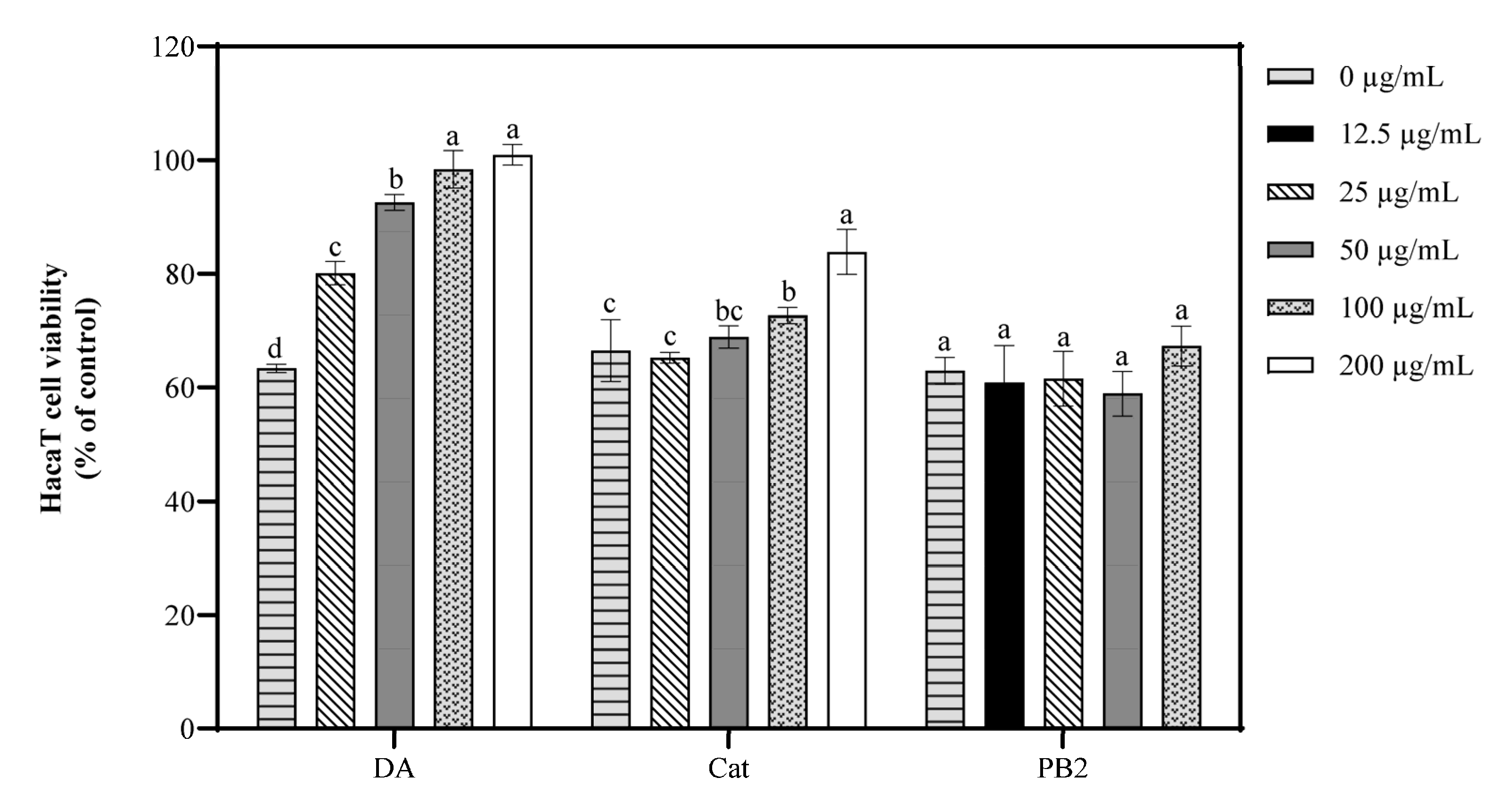
3.5. Effect of Bioactive Compounds on Cell Viability and ROS Generation of UVB-Induced Cells
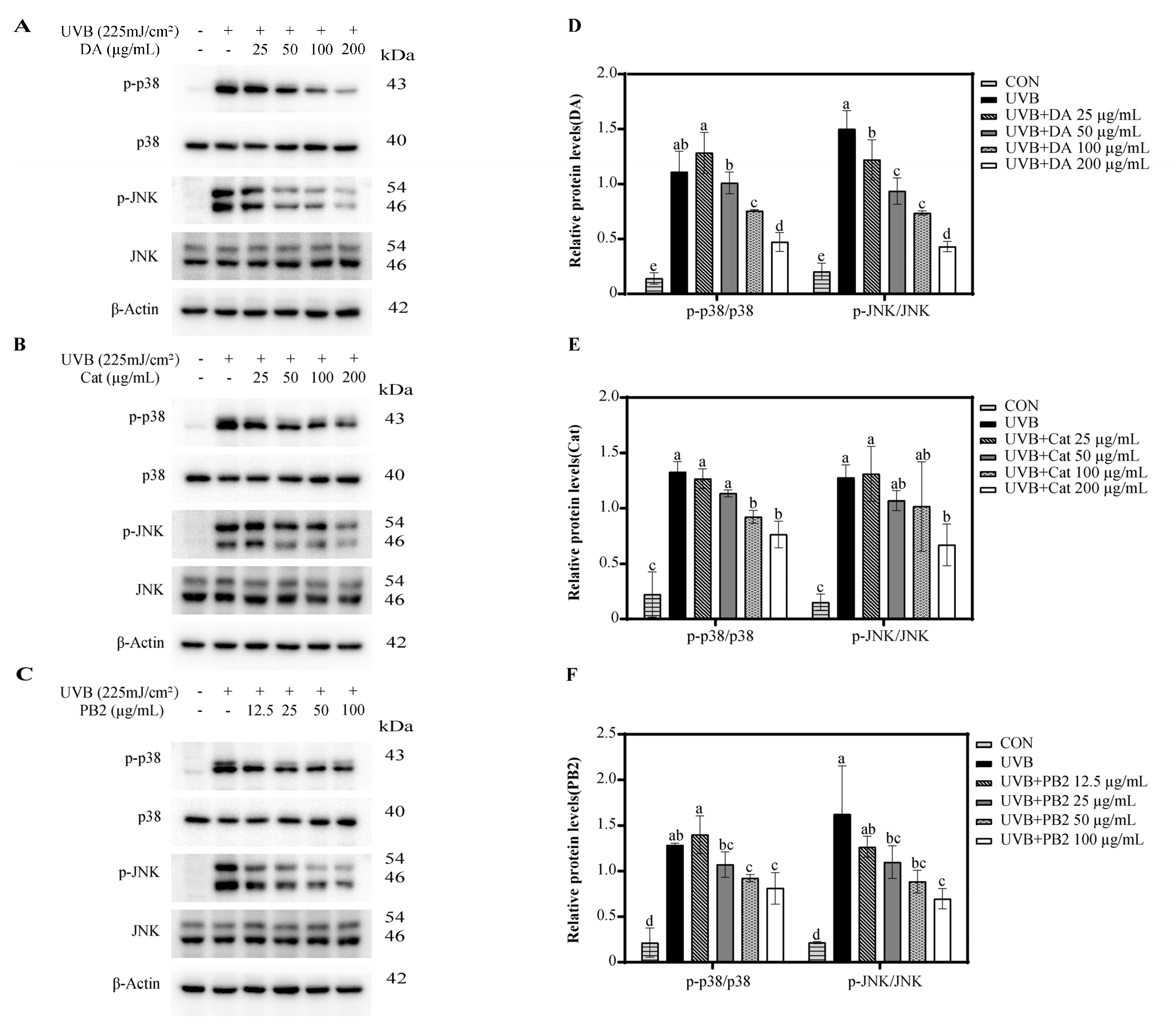
3.6. Effect of Bioactive Compounds on the Expression and Activity of p38 and JNK
3.7. Effect of Bioactive Compounds on Cellular Viability and Apoptosis
3.8. Proteomic Analysis of UVB-Induced Cells Treated with 3,4-Dihydroxybenzoic Acid and (+)-Catechin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NBA | n-Butanol Extract of Schima argentea |
| HaCaT | Human Adult Keratinocyte Cell Line |
| MAPK | Mitogen-Activated Protein Kinase |
| UPLC-QTOF-MS | Ultra-High-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| UVB | Ultraviolet B |
| JNK | c-Jun N-terminal kinase |
| c-Fos | Cellular Oncogene fos |
| PI3K-Akt | Phosphoinositide 3-Kinase/Protein Kinase B |
| Wnt | Wingless-Related Integration Site |
| ROS | Reactive Oxygen Species |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DMSO | Dimethyl Sulfoxide |
| Annexin V-FITC | Annexin V conjugated with Fluorescein Isothiocyanate |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| IC50 | Half-Maximal Inhibitory Concentration |
References
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Kim, D.J.; Iwasaki, A.; Chien, A.L.; Kang, S. UVB-mediated DNA damage induces matrix metalloproteinases to promote photoaging in an AhR- and SP1-dependent manner. JCI Insight 2022, 7, e156344. [Google Scholar] [CrossRef]
- She, Q.B.; Ma, W.Y.; Dong, Z. Role of MAP kinases in UVB-induced phosphorylation of p53 at serine 20. Oncogene 2002, 21, 1580–1589. [Google Scholar] [CrossRef]
- Chen, W.; Bowden, G.T. Role of p38 mitogen-activated protein kinases in ultraviolet-B irradiation-induced activator protein 1 activation in human keratinocytes. Mol. Carcinog. 2000, 28, 196–202. [Google Scholar] [CrossRef]
- Gonzales, M.; Bowden, G.T. The role of PI 3-kinase in the UVB-induced expression of c-fos. Oncogene 2002, 21, 2721–2728. [Google Scholar] [CrossRef][Green Version]
- Park, Y.K.; Jang, B.C. UVB-induced anti-survival and pro-apoptotic effects on HaCaT human keratinocytes via caspase- and PKC-dependent downregulation of PKB, HIAP-1, Mcl-1, XIAP and ER stress. Int. J. Mol. Med. 2014, 33, 695–702. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Garcia-Baeza, A.; Vidal-Limon, H.R.; Balderas-Renteria, I.; Ramírez-Cabrera, M.A.; Ramirez-Estrada, K. Plant secondary metabolites against skin photodamage: Mexican plants, a potential source of UV-radiation protectant molecules. Plants 2022, 11, 220. [Google Scholar] [CrossRef]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural products and extracts from plants as natural UV filters for sunscreens: A review. Anim. Model. Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- Charachit, N.; Sukhamwang, A.; Dejkriengkraikul, P.; Yodkeeree, S. Hyperoside and quercitrin in Houttuynia cordata extract attenuate UVB-induced human keratinocyte cell damage and oxidative stress via modulation of MAPKs and Akt signaling pathway. Antioxidants 2022, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Park, N.J.; Jo, B.G.; Lee, B.S.; Keem, M.J.; Kwon, T.H.; Kim, K.H.; Kim, S.N.; Yang, M.H. Anti-wrinkling effect of 3,4,5-tri-O-caffeoylquinic acid from the roots of Nymphoides peltata through MAPK/AP-1, NF-κB, and Nrf2 signaling in UVB-irradiated HaCaT cells. Antioxidants 2023, 12, 1899. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Zheng, S.; Fang, M.; Kim, M.; Bellere, A.D.; Jeong, J.; Yi, T.H. Anti-photoaging effect of Phaseolus angularis L. extract on UVB-exposed HaCaT keratinocytes and possibilities as cosmetic materials. Molecules 2023, 28, 1407. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.Y.; Jang, J.; Lee, H.P.; Park, S.H.; Kry, M.; Keo, O.; Lee, B.H.; Choi, W.; Lee, S.; Cho, J.Y. Anti-oxidative and anti-aging effects of ethanol extract of the officinal Breynia (Breynia vitis-idaea) in vitro. Plants 2023, 12, 1088. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, R.L.; Li, H.Y.; Hu, C.; Liu, B.L.; Li, Y.L.; Zhou, G.X. Triterpenoid saponins from the root bark of Schima superba and their cytotoxic activity on B16 melanoma cell line. Carbohydr. Res. 2015, 413, 107–114. [Google Scholar] [CrossRef]
- Wu, C.; Wu, H.T.; Wang, Q.; Wang, G.H.; Yi, X.; Chen, Y.P.; Zhou, G.X. Anticandidal potential of stem bark extract from Schima superba and the identification of its major anticandidal compound. Molecules 2019, 24, 1587. [Google Scholar] [CrossRef]
- Lin, T.K.; Hung, C.F.; Weng, J.R.; Hsieh, T.Y.; Wang, S.J. Kaempferol 3-rhamnoside on glutamate release from rat cerebrocortical nerve terminals involves P/Q-type Ca2+ channel and Ca2+/calmodulin-dependent protein kinase II-dependent pathway suppression. Molecules 2022, 27, 1342. [Google Scholar] [CrossRef]
- Chen, W.-H.; Wu, D.-G. A structural study of sapogenin of Schima argentea Pritz. Acta Chim. Sin. 1978, 3, 229–232. (In Chinese) [Google Scholar]
- Zhang, P.C.; Hong, Y.; Zong, S.Q.; Chen, L.; Zhang, C.; Tian, D.Z.; Ke, D.; Tian, L.M. Variation of ferroptosis-related markers in HaCaT cell photoaging models Induced by UVB. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3147–3155. [Google Scholar] [CrossRef]
- Bianchini Silva, L.S.; Perasoli, F.B.; Carvalho, K.V.; Vieira, K.M.; Paz Lopes, M.T.; Bianco de Souza, G.H.; Henrique Dos Santos, O.D.; Freitas, K.M. Melaleuca leucadendron (L.) L. flower extract exhibits antioxidant and photoprotective activities in human keratinocytes exposed to ultraviolet B radiation. Free Radic. Biol. Med. 2020, 159, 54–65. [Google Scholar] [CrossRef]
- Fang, M.; Lee, H.M.; Oh, S.; Zheng, S.; Bellere, A.D.; Kim, M.; Choi, J.; Kim, M.; Yu, D.; Yi, T.H. Rosa davurica inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and Nrf2/HO-1 signaling in UVB-irradiated HaCaTs. Photochem. Photobiol. Sci. 2022, 21, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Azmi, W.A.; Santhanam, R.; Abd Rahman, N.E.; Ismail, W.I.W. Photoprotective properties of four structure propolis from Heterotrigona itama stingless beehive: Fractionation, bioactivity analysis, and chemical profiling. Heliyon 2024, 10, e39164. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Bansal, A.; Aggarwal, K.; Nagpal, K. Development and validation of UV spectrophotometric method for the quantitative estimation of quercetin in bulk followed by its solubility studies. J. Appl. Spectrosc. 2024, 91, 700–708. [Google Scholar] [CrossRef]
- Agrawal, O.D.; Kulkarni, Y.A. Mini-review of analytical methods used in quantification of ellagic acid. Rev. Anal. Chem. 2020, 39, 31–44. [Google Scholar] [CrossRef]
- Malikov, V.M.; Bruskov, N.P.; Yuldashev, M.P.; Khushbaktova, Z.A. Quercetin-3-O-arabinoside (Gvajaverin). In Natural Compounds: Flavonoids; Azimova, S.S., Vinogradova, V.I., Eds.; Springer: New York, NY, USA, 2013; p. 204. [Google Scholar] [CrossRef]
- Nagaraju, M.; Ramulla, S.; Murthy, N.Y.S. Extraction and preliminary analysis of aloin obtained from Aloe barbadensis miller. Asian J. Chem. 2011, 23, 2421–2423. [Google Scholar]
- Hofer, S.; Stonig, M.; Wally, V.; Hartmann, A.; Fuchs, D.; Hermann, M.; Paparella, M.; Ganzera, M.; Gostner, J.M. Contradictory effects of chemical filters in UV/ROS-stressed human keratinocyte and fibroblast cells. ALTEX—Altern. Anim. Exp. 2019, 36, 231–244. [Google Scholar] [CrossRef]
- Park, J.M.; Cho, J.-K.; Mok, J.Y.; Jeon, I.H.; Kim, H.S.; Kang, H.J.; Jang, S.I. Protective effect of astragalin and quercetin on ultraviolet (UV)-irradiated damage in HaCaT cells and Balb/c mice. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 443–446. [Google Scholar] [CrossRef]
- Ding, M.; Zhao, J.; Bowman, L.; Lu, Y.; Shi, X. Inhibition of AP-1 and MAPK signaling and activation of Nrf2/ARE pathway by quercitrin. Int. J. Oncol. 2010, 36, 59–67. [Google Scholar] [CrossRef]
- Daré, R.G.; Oliveira, M.M.; Truiti, M.C.T.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Abilities of protocatechuic acid and its alkyl esters, ethyl and heptyl protocatechuates, to counteract UVB-induced oxidative injuries and photoaging in fibroblasts L929 cell line. J. Photochem. Photobiol. B 2020, 203, 111771. [Google Scholar] [CrossRef]
- Wu, W.B.; Chiang, H.S.; Fang, J.Y.; Chen, S.K.; Huang, C.C.; Hung, C.F. (+)-Catechin prevents ultraviolet B-induced human keratinocyte death via inhibition of JNK phosphorylation. Life Sci. 2006, 79, 801–807. [Google Scholar] [CrossRef]
- Wang, H.; Hao, W.; Yang, L.; Li, T.; Zhao, C.; Yan, P.; Wei, S. Procyanidin B2 alleviates heat-induced oxidative stress through the Nrf2 pathway in bovine mammary epithelial cells. Int. J. Mol. Sci. 2022, 23, 7769. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003, 2003, Re2. [Google Scholar] [CrossRef]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Hyun, C.L.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; et al. Protective effect of 3,4-dihydroxybenzoic acid isolated from Cladophora wrightiana Harvey against ultraviolet B radiation-induced cell damage in human HaCaT keratinocytes. Appl. Biochem. Biotechnol. 2014, 172, 2582–2592. [Google Scholar] [CrossRef]
- Mittraphab, Y.; Amen, Y.; Nagata, M.; Matsumoto, M.; Wang, D.; Shimizu, K. Anti-phototoxicity effect of phenolic compounds from acetone extract of Entada phaseoloides leaves via activation of COX-2 and iNOS in human epidermal keratinocytes. Molecules 2022, 27, 440. [Google Scholar] [CrossRef]
- Tanigawa, T.; Kanazawa, S.; Ichibori, R.; Fujiwara, T.; Magome, T.; Shingaki, K.; Miyata, S.; Hata, Y.; Tomita, K.; Matsuda, K.; et al. (+)-Catechin protects dermal fibroblasts against oxidative stress-induced apoptosis. BMC Complement. Altern. Med. 2014, 14, 133. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Sun, T.; Yao, X.; Sun, X. Supplementation of procyanidins B2 attenuates photooxidation-induced apoptosis in ARPE-19 cells. Int. J. Food Sci. Nutr. 2016, 67, 650–659. [Google Scholar] [CrossRef]
- Lai, W.W.; Hsiao, Y.P.; Chung, J.G.; Wei, Y.H.; Cheng, Y.W.; Yang, J.H. Synergistic phototoxic effects of glycolic acid in a human keratinocyte cell line (HaCaT). J. Dermatol. Sci. 2011, 64, 191–198. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.Y.; Cheon, Y.H.; Baek, J.M.; Ahn, S.J.; Yoon, K.H.; Lee, M.S.; Oh, J. Protocatechuic acid attenuates osteoclastogenesis by downregulating JNK/c-Fos/NFATc1 signaling and prevents inflammatory bone loss in mice. Phytother. Res. 2016, 30, 604–612. [Google Scholar] [CrossRef]
- Kanellou, A.; Giakoumakis, N.N.; Panagopoulos, A.; Tsaniras, S.C.; Lygerou, Z. The licensing factor Cdt1 links cell cycle progression to the DNA damage response. Anticancer Res. 2020, 40, 2449–2456. [Google Scholar] [CrossRef]
- Gan, Y.; Ye, F.; He, X.X. The role of YWHAZ in cancer: A maze of opportunities and challenges. J. Cancer 2020, 11, 2252–2264. [Google Scholar] [CrossRef]
- Drigeard Desgarnier, M.C.; Rochette, P.J. Enhancement of UVB-induced DNA damage repair after a chronic low-dose UVB pre-stimulation. DNA Repair 2018, 63, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Bergiers, I.; Bridoux, L.; Nguyen, N.; Twizere, J.C.; Rezsöhazy, R. The homeodomain transcription factor Hoxa2 interacts with and promotes the proteasomal degradation of the E3 ubiquitin protein ligase RCHY1. PLoS ONE 2013, 8, e80387. [Google Scholar] [CrossRef]
- Guo, F.; Jiao, D.; Sui, G.Q.; Sun, L.N.; Gao, Y.J.; Fu, Q.F.; Jin, C.X. Anticancer effect of YWHAZ silencing via inducing apoptosis and autophagy in gastric cancer cells. Neoplasma 2018, 65, 693–700. [Google Scholar] [CrossRef]
- Leenders, F.; Möpert, K.; Schmiedeknecht, A.; Santel, A.; Czauderna, F.; Aleku, M.; Penschuck, S.; Dames, S.; Sternberger, M.; Röhl, T.; et al. PKN3 is required for malignant prostate cell growth downstream of activated PI 3-kinase. EMBO J. 2004, 23, 3303–3313. [Google Scholar] [CrossRef]
- Möpert, K.; Löffler, K.; Röder, N.; Kaufmann, J.; Santel, A. Depletion of protein kinase N3 (PKN3) impairs actin and adherens junctions dynamics and attenuates endothelial cell activation. Eur. J. Cell Biol. 2012, 91, 694–705. [Google Scholar] [CrossRef]
- Cui, D.; Dai, X.; Shu, J.; Ma, Y.; Wei, D.; Xiong, X.; Zhao, Y. The cross talk of two family members of β-TrCP in the regulation of cell autophagy and growth. Cell Death Differ. 2020, 27, 1119–1133. [Google Scholar] [CrossRef]
- Pray, B.A.; Youssef, Y.; Alinari, L. TBL1X: At the crossroads of transcriptional and posttranscriptional regulation. Exp. Hematol. 2022, 116, 18–25. [Google Scholar] [CrossRef]
- Anaga, N.; Lekshmy, K.; Purushothaman, J. (+)-Catechin mitigates impairment in insulin secretion and beta cell damage in methylglyoxal-induced pancreatic beta cells. Mol. Biol. Rep. 2024, 51, 434. [Google Scholar] [CrossRef]
- Chencen, L.; Shuo, Z.; Zhiyu, C.; Xiaoyu, F.; Min, Z.; Pengjiao, W.; Xiuli, G. (+)-catechin protects PC12 cells against CORT-induced oxidative stress and pyroptosis through the pathways of PI3K/AKT and Nrf2/HO-1/NF-κB. Front. Pharmacol. 2024, 15, 1450211. [Google Scholar] [CrossRef]







| Ion Mode | No. | Compounds | Retention Time (min) | m/z | Relative Abundance |
|---|---|---|---|---|---|
| Negative | 1 | PG(16:0/0:0) [U] | 9.074 | 483.272 | 1.779 × 106 ± 1.401 × 105 |
| 2 | 3-O-Mycarosylerythronolide B | 2.250 | 1151.765 | 7.988 × 105 ± 3.729 × 105 | |
| 3 | Thr Asp Phe Glu | 3.496 | 509.220 | 6.163 × 105 ± 4.755 × 104 | |
| 4 | Ellagic acid | 3.340 | 300.999 | 5.059 × 105 ± 2.010 × 104 | |
| 5 | Quercetin 3-arabinoside | 3.560 | 433.078 | 3.158 × 105 ± 1.658 × 104 | |
| 6 | Nodularin | 8.732 | 823.469 | 3.263 × 105 ± 3.273 × 104 | |
| 7 | Hieracin | 4.522 | 301.036 | 1.894 × 105 ± 1.438 × 104 | |
| 8 | 3,3-Dimethylglutaric acid | 2.477 | 205.072 | 1.635 × 105 ± 5.904 × 103 | |
| 9 | Procyanidin B2 | 2.670 | 577.135 | 1.183 × 105 ± 1.417 × 104 | |
| 10 | Leukotriene F4 | 8.925 | 567.285 | 9.387 × 104 ± 5.607 × 103 | |
| 11 | Aloin A | 3.852 | 439.107 | 8.512 × 104 ± 1.232 × 104 | |
| 12 | Sapindoside A | 8.497 | 809.455 | 8.456 × 104 ± 6.602 × 103 | |
| 13 | TyrMe-Met-OH | 2.157 | 447.115 | 7.200 × 104 ± 9.69 × 102 | |
| 14 | (+)-Catechin | 2.855 | 289.072 | 5.214 × 104 ± 3.94 × 102 | |
| 15 | 3,4-Dihydroxybenzoic acid | 2.228 | 153.019 | 5.750 × 104 ± 5.411 × 103 | |
| Positive | 1 | PC(16:0/20:4(5E,8E,11E,14E)) | 10.007 | 782.569 | 2.897 × 106 ± 2.466 × 105 |
| 2 | Lucidal | 5.825 | 437.342 | 1.032 × 106 ± 3.346 × 104 | |
| 3 | Harderoporphyrin | 10.164 | 609.271 | 1.033 × 106 ± 1.541 × 104 | |
| 4 | 1-Linoleoylglycerophosphocholine | 7.621 | 520.340 | 8.125 × 105 ± 6.997 × 104 | |
| 5 | LysoPC(18:3(6Z,9Z,12Z)) | 7.129 | 518.325 | 7.264 × 105 ± 5.692 × 104 | |
| 6 | Docosa-4,7,10,13,16-pentaenoyl carnitine | 8.447 | 474.379 | 7.351 × 105 ± 3.464 × 104 | |
| 7 | PG(16:1(9Z)/0:0) | 8.490 | 447.251 | 6.402 × 105 ± 1.171 × 104 | |
| 8 | Ricinoleic acid methyl ester | 9.159 | 313.274 | 4.714 × 105 ± 1.046 × 104 | |
| 9 | PC(0:0/18:0) | 8.967 | 524.372 | 4.662 × 105 ± 1.622 × 104 | |
| 10 | Leukotriene C4 | 10.022 | 625.267 | 4.749 × 105 ± 7.798 × 103 | |
| 11 | Thr Trp Met Arg | 10.598 | 593.277 | 3.980 × 105 ± 4.239 × 103 | |
| 12 | Dehydro(11,12)ursolic acid lactone | 6.125 | 437.342 | 3.726 × 105 ± 2.402 × 103 | |
| 13 | Quercetin | 3.660 | 303.051 | 3.505 × 105 ± 6.453 × 103 | |
| 14 | Phaeophorbide B | 9.537 | 607.256 | 3.154 × 105 ± 1.193 × 104 | |
| 15 | MG(0:0/16:1(9Z)/0:0) | 8.511 | 311.259 | 3.153 × 105 ± 1.042 × 104 |
| KEGG Description | Gene | Protein Name | FC | Log2FC | p-Value |
|---|---|---|---|---|---|
| Cell cycle | CDT1 | DNA replication factor Cdt1 | 32.00 | 5.00 | 1.02 × 10−7 |
| CCND3 | G1/S-specific cyclin-D3 | 32.00 | 5.00 | 5.38 × 10−6 | |
| CCNB1 | G2/mitotic-specific cyclin-B1 | 2.40 | 1.24 | 5.74 × 10−5 | |
| - | Cell division control protein | 32.00 | 5.00 | 1.84 × 10−10 | |
| PTTG1 | Pituitary tumor-transforming protein 1 | 3.61 | 1.85 | 2.78 × 10−4 | |
| CDK4 | Cyclin-dependent kinase 4 | 2.30 | 1.20 | 6.82 × 10−5 | |
| CCNB2 | G2/mitotic-specific cyclin-B2 | 2.14 | 1.10 | 1.43 × 10−4 | |
| WEE1 | Wee1-like protein kinase | 3.48 | 1.80 | 4.24 × 10−5 | |
| - | Aurora kinase | 2.11 | 1.08 | 1.19 × 10−4 | |
| AURKB | Aurora kinase B | 2.05 | 1.04 | 1.64 × 10−4 | |
| ESCO2 | N-acetyltransferase ESCO2 | 3.17 | 1.67 | 4.97 × 10−5 | |
| MCM4 | DNA replication licensing factor MCM4 | 32.00 | 5.00 | 2.26 × 10−6 | |
| ORC6 | Origin recognition complex subunit 6 | 2.08 | 1.06 | 1.23 × 10−3 | |
| CCNA2 | Cyclin-A2 | 2.75 | 1.46 | 2.15 × 10−6 | |
| CDC20 | Cell division cycle protein 20 homolog | 2.23 | 1.16 | 2.29 × 10−6 | |
| p53 signaling pathway | RCHY1 | RING finger and CHY zinc finger domain-containing protein 1 | 32.00 | 5.00 | 1.01 × 10−7 |
| CCND3 | G1/S-specific cyclin-D3 | 32.00 | 5.00 | 5.38 × 10−6 | |
| CCNB1 | G2/mitotic-specific cyclin-B1 | 2.36 | 1.24 | 5.74 × 10−5 | |
| CDK4 | Cyclin-dependent kinase 4 | 2.30 | 1.20 | 6.82 × 10−5 | |
| CCNB2 | G2/mitotic-specific cyclin-B2 | 2.14 | 1.10 | 1.43 × 10−4 | |
| DDB2 | DNA damage-binding protein 2 | 6.12 | 2.61 | 3.10 × 10−4 | |
| ZNF385A | Zinc finger protein 385A | 3.20 | 1.68 | 8.34 × 10−4 | |
| Pathways in cancer | CCND3 | G1/S-specific cyclin-D3 | 32.00 | 5.00 | 5.38 × 10−6 |
| CDK4 | Cyclin-dependent kinase 4 | 2.30 | 1.20 | 6.82 × 10−5 | |
| GSTM4 | Glutathione S-transferase Mu 4 | 2.23 | 1.16 | 1.70 × 10−2 | |
| DDB2 | DNA damage-binding protein 2 | 6.12 | 2.61 | 3.10 × 10−4 | |
| STAT3 | Signal transducer and activator of transcription | 2.58 | 1.37 | 1.09 × 10−6 | |
| STAT3 | Signal transducer and activator of transcription | 3.72 | 1.90 | 7.51 × 10−3 | |
| LAMB3 | Laminin subunit beta-3 | 2.02 | 1.01 | 3.81 × 10−6 | |
| FOS | Protein c-Fos | 0.17 | −2.59 | 6.53 × 10−4 | |
| NOTCH2 | Neurogenic locus notch homolog protein 2 | 2.16 | 1.11 | 5.25 × 10−3 | |
| - | JUN | 0.45 | −1.15 | 3.00 × 10−4 | |
| CCNA2 | Cyclin-A2 | 2.75 | 1.46 | 2.15 × 10−6 | |
| LAMA5 | Laminin subunit alpha-5 | 2.04 | 1.03 | 1.15 × 10−4 | |
| GNA12 | G protein subunit alpha 12 | 32.00 | 5.00 | 5.94 × 10−7 |
| KEGG Description | Gene | Protein Name | FC | Log2FC | p-Value |
|---|---|---|---|---|---|
| Cell cycle | CDT1 | DNA replication factor Cdt1 | 32.00 | 5.00 | 1.59 × 10−8 |
| YWHAZ | Tyrosine 3-monooxygenase | 2.03 | 1.02 | 1.58 × 10−2 | |
| - | Cell division control protein | 32.00 | 5.00 | 9.33 × 10−7 | |
| p53 signaling pathway | RCHY1 | RING finger and CHY zinc finger domain-containing protein 1 | 32.00 | 5.00 | 4.52 × 10−8 |
| DDB2 | DNA damage-binding protein 2 | 2.02 | 1.01 | 1.22 × 10−2 | |
| - | E3 ubiquitin-protein ligase | 2.43 | 1.28 | 3.11 × 10−2 | |
| PI3K-Akt signaling pathway | YWHAZ | Tyrosine 3-monooxygenase | 2.03 | 1.02 | 1.58 × 10−2 |
| COL9A3 | Collagen alpha-3(IX) chain | 2.09 | 1.06 | 4.77 × 10−3 | |
| PKN3 | Serine/threonine-protein kinase N3 | 32.00 | 5.00 | 1.19 × 10−3 | |
| Pathways in cancer | DDB2 | DNA damage-binding protein 2 | 2.02 | 1.01 | 1.22 × 10−2 |
| GNA12 | G protein subunit alpha 12 | 32.00 | 5.00 | 8.86 × 10−9 | |
| Wnt signaling pathway | BTRC | Beta-transducin repeat containing isoform 3 | 32.00 | 5.00 | 2.58 × 10−4 |
| TBL1X | F-box-like/WD repeat-containing protein TBL1X | 32.00 | 5.00 | 6.95 × 10−8 | |
| - | E3 ubiquitin-protein ligase | 32.00 | 5.00 | 5.85 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Chen, Y.-P.; Li, J.; Wu, H.; Chen, F.; Li, M.; Wu, C. Antioxidant and Photoprotective Activities of 3,4-Dihydroxybenzoic Acid and (+)-Catechin, Identified from Schima argentea Extract, in UVB-Irradiated HaCaT Cells. Antioxidants 2025, 14, 241. https://doi.org/10.3390/antiox14020241
He Q, Chen Y-P, Li J, Wu H, Chen F, Li M, Wu C. Antioxidant and Photoprotective Activities of 3,4-Dihydroxybenzoic Acid and (+)-Catechin, Identified from Schima argentea Extract, in UVB-Irradiated HaCaT Cells. Antioxidants. 2025; 14(2):241. https://doi.org/10.3390/antiox14020241
Chicago/Turabian StyleHe, Qi, Yu-Pei Chen, Junhao Li, Hongtan Wu, Fangfang Chen, Mingyu Li, and Chun Wu. 2025. "Antioxidant and Photoprotective Activities of 3,4-Dihydroxybenzoic Acid and (+)-Catechin, Identified from Schima argentea Extract, in UVB-Irradiated HaCaT Cells" Antioxidants 14, no. 2: 241. https://doi.org/10.3390/antiox14020241
APA StyleHe, Q., Chen, Y.-P., Li, J., Wu, H., Chen, F., Li, M., & Wu, C. (2025). Antioxidant and Photoprotective Activities of 3,4-Dihydroxybenzoic Acid and (+)-Catechin, Identified from Schima argentea Extract, in UVB-Irradiated HaCaT Cells. Antioxidants, 14(2), 241. https://doi.org/10.3390/antiox14020241






