Resveratrol Ameliorates Chronic Stress in Kennel Dogs and Mice by Regulating Gut Microbiome and Metabolome Related to Tryptophan Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animal and Housing
2.2.1. Dogs
2.2.2. Mice
2.3. Treatment Administrations and Sample Collection
2.3.1. Experiment 1
2.3.2. Experiment 2
2.4. Behavior and Sample Analyses
2.4.1. Behavior Assessments in Dogs and Mice
2.4.2. Serum and Brain Biochemistry Analyses in Dogs and Mice
2.4.3. Gene Expression Analysis in Mice
2.4.4. Fecal SCFAs and Branched-Chain Fatty Acids (BCFAs) Analysis in Dogs
2.4.5. Feces Microbiota Analysis in Dogs
2.4.6. Untargeted Serum and Fecal Metabolome Analyses in Dogs
2.4.7. Targeted Metabolome Analyses of Serum, Feces, and Broth Fermentation in Experiment 1
2.4.8. MetOrigin Analysis for Gut Microbiota and Microbial Metabolic Pathways in Dogs
2.5. Statistical Analysis
3. Results
3.1. Experiment 1
3.1.1. Behaviors Related to Fear and Anxiety in the OFT in Dogs
3.1.2. Serum 5-HT, BDNF, and Hormones of HPA Axis in Dogs
3.1.3. Serum GABA, ACh, Glu, and Dopamine in Dogs
3.1.4. Cytokine, IgA, and Antioxidant Measures in Dogs
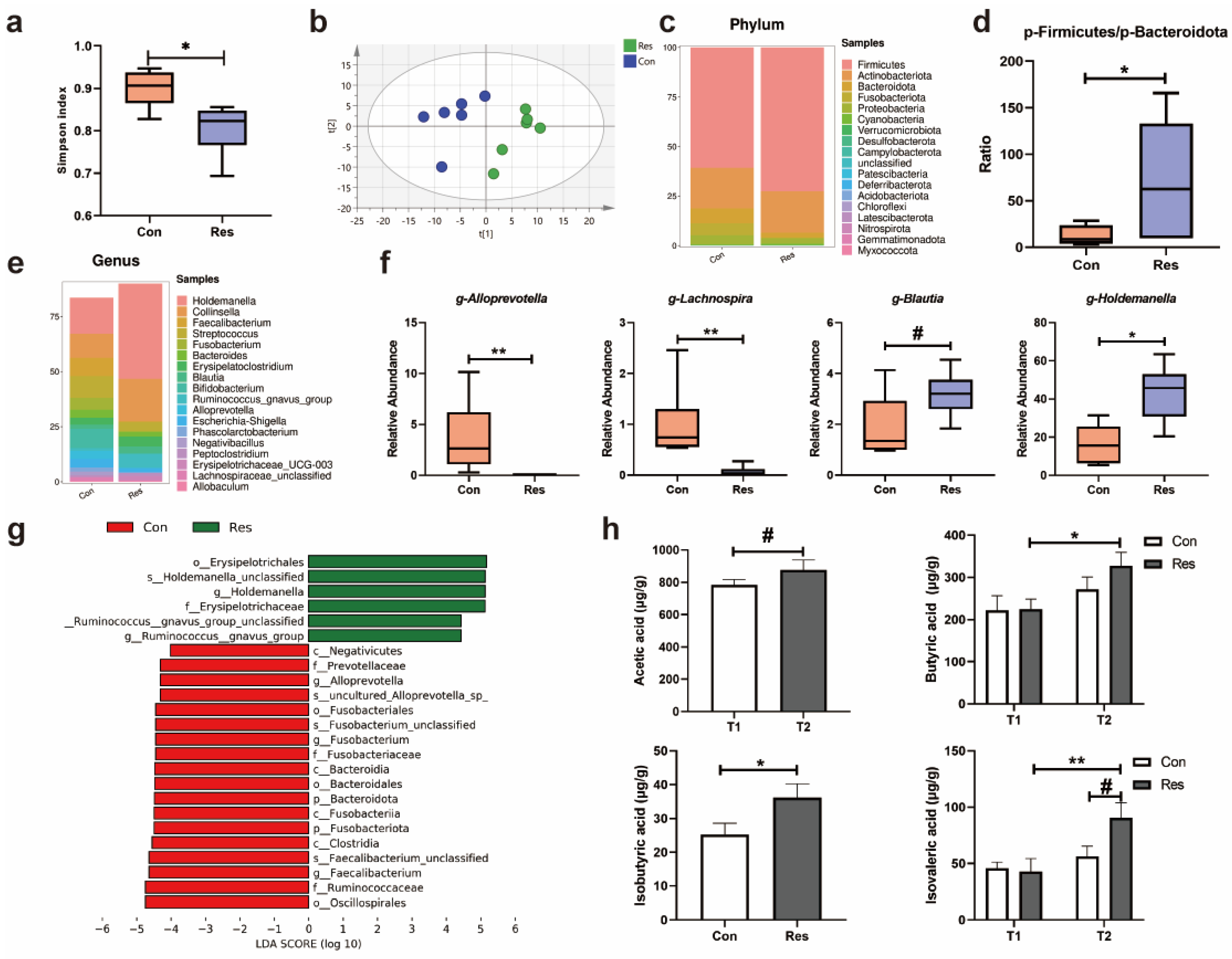
3.1.5. Gut Microbiota and Fecal SCFAs and BCFAs in Dogs
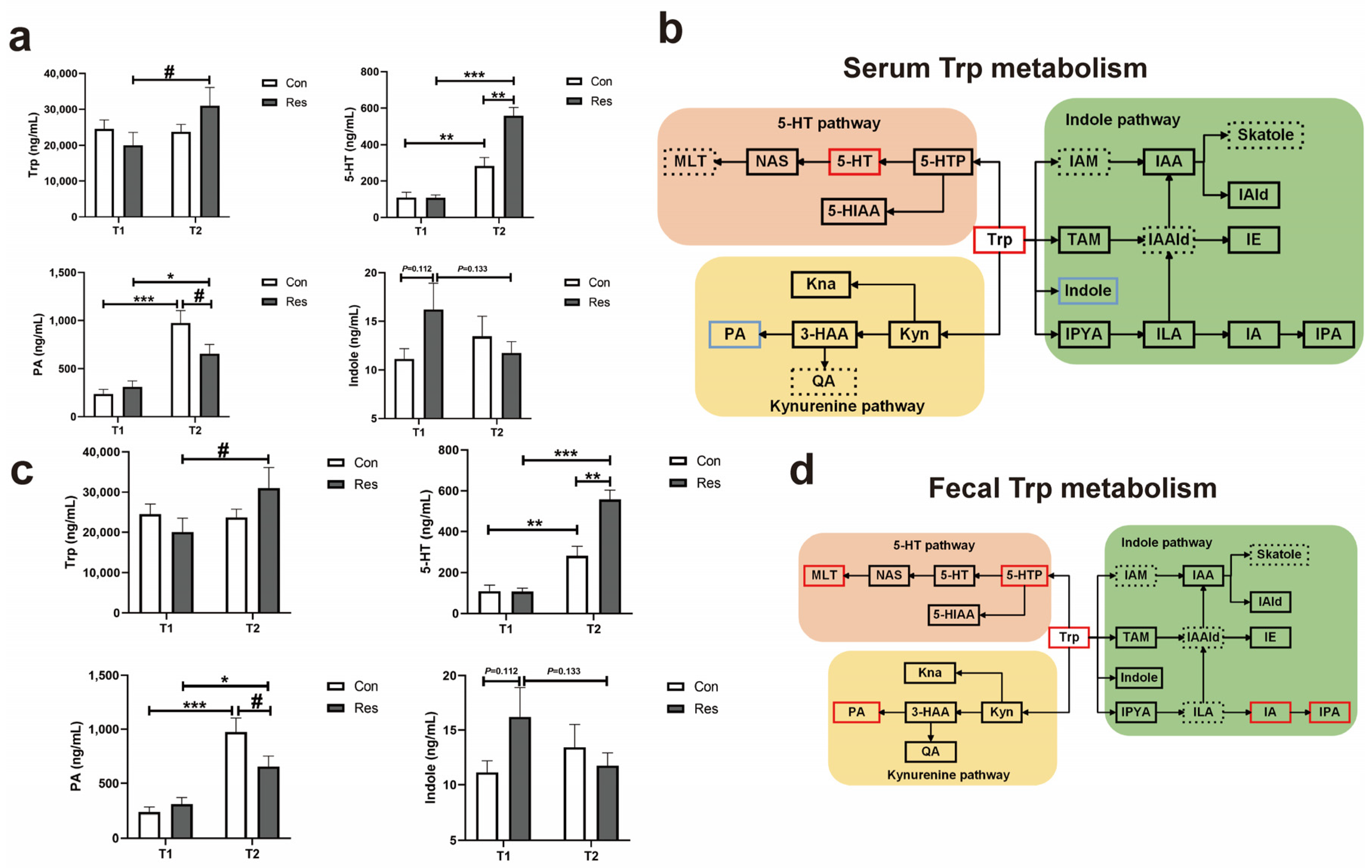
3.1.6. Metabolome in Dogs
3.1.7. Biological Relationship of Gut Trp Metabolism and Microbes in Dogs
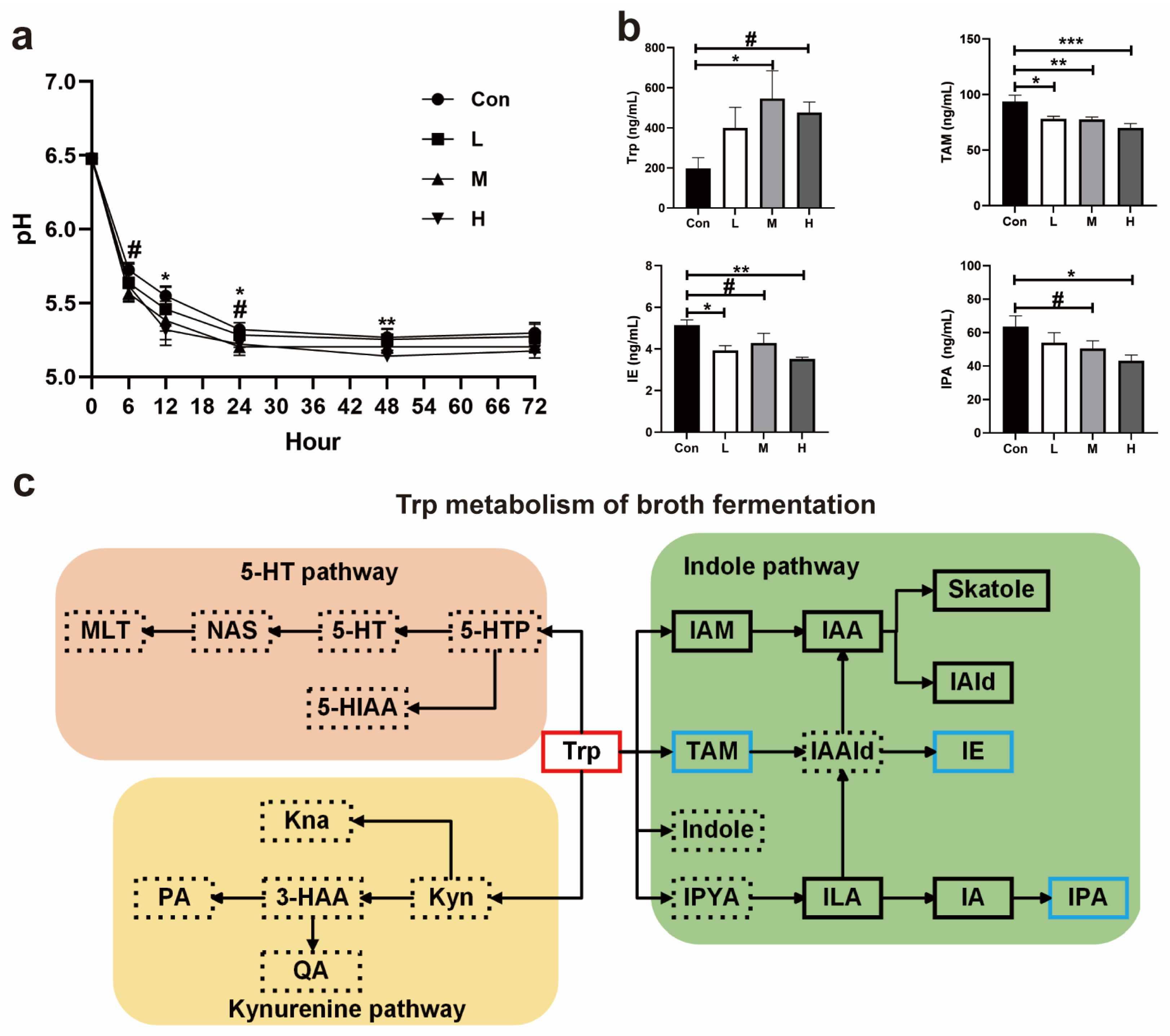
3.1.8. pH and Trp Metabolism in Fermentation Broth of Experiment 1
3.2. Experiment 2
3.2.1. Behavioral Tests in Mice
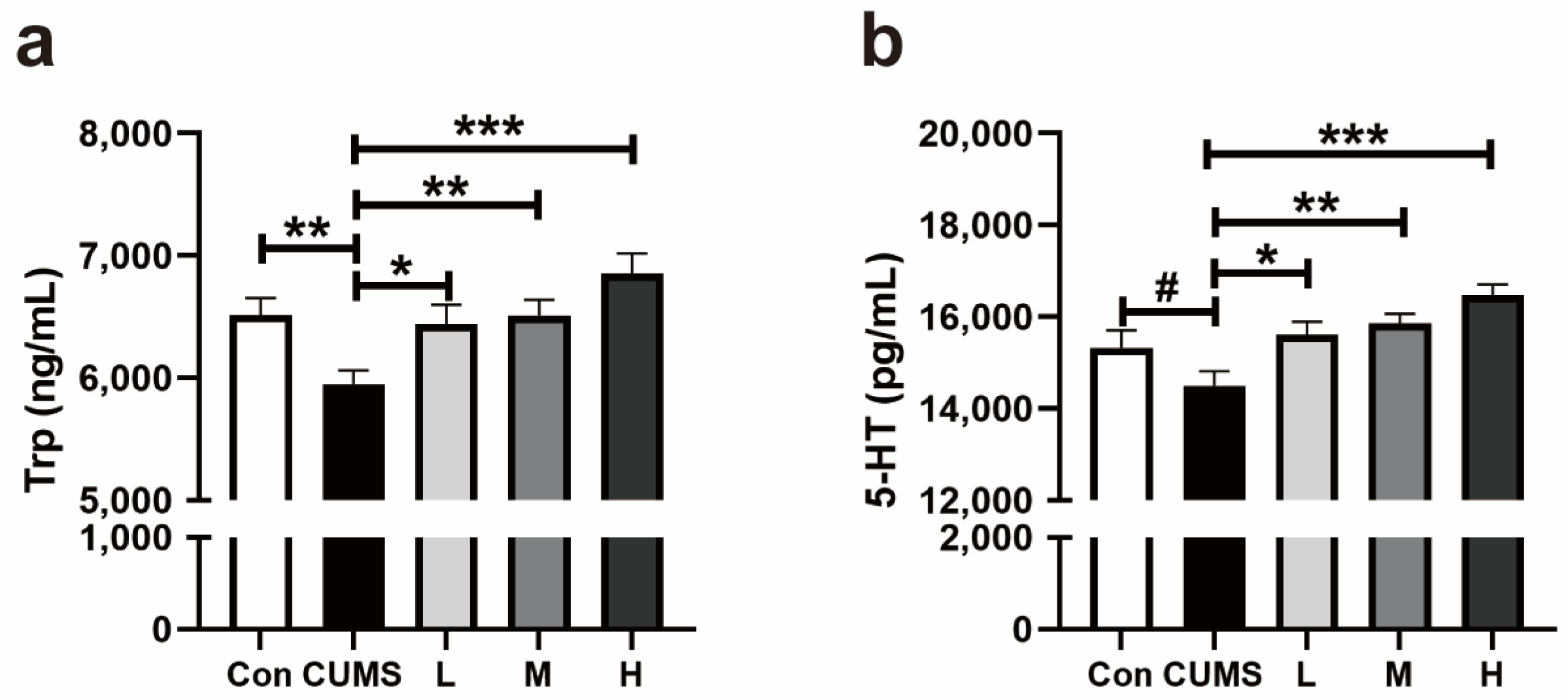
3.2.2. Trp and 5-HT in the Whole Brains of Mice
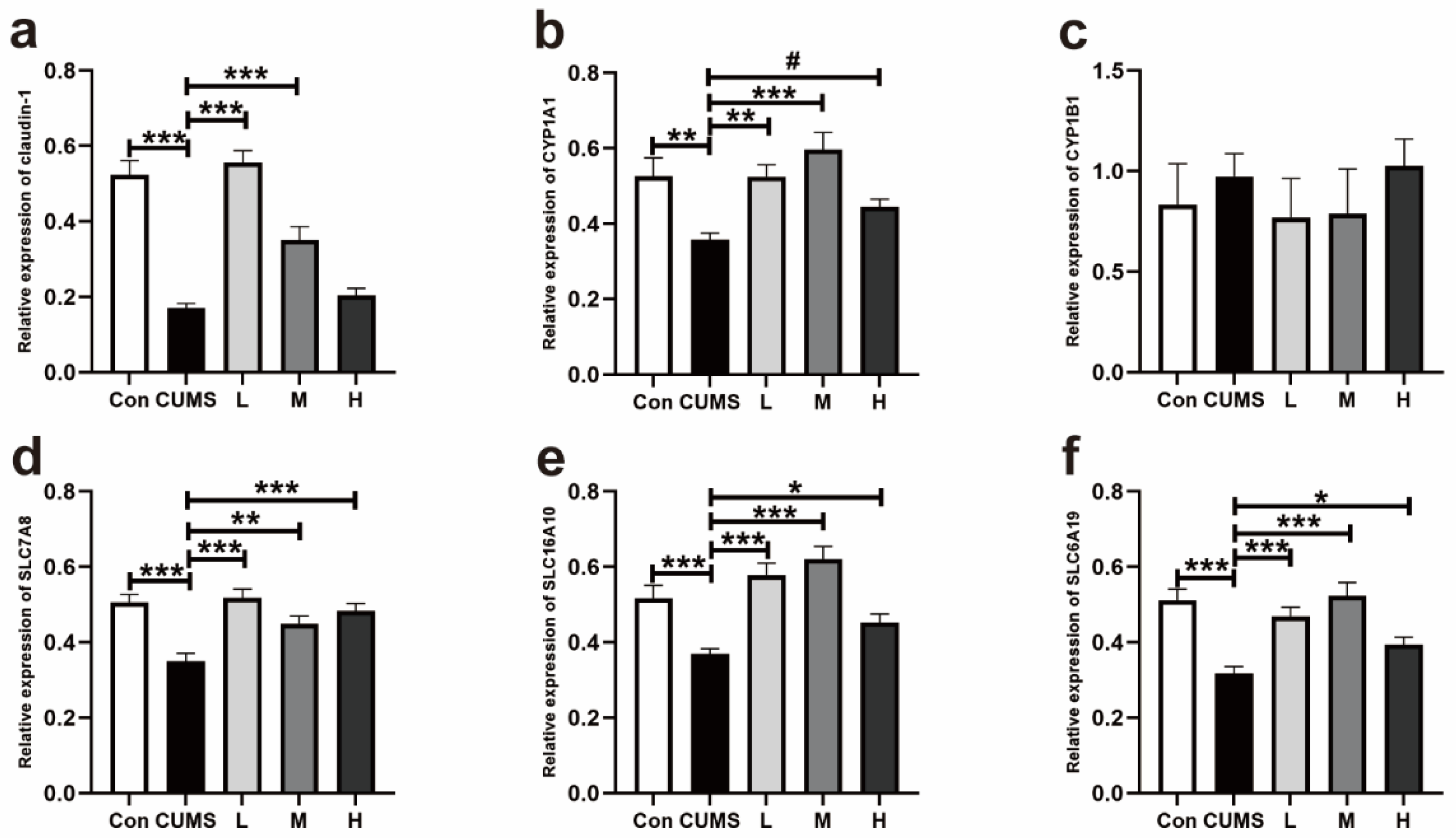
3.2.3. Expression of Genes Related to the Tight Junction Protein, aryl hydrocarbon Receptor (AhR), and Trp Transporters in the Colons of Mice
4. Discussion
4.1. Neurotransmitters and Hormones of HPA Axis
4.2. Immune Function and Oxidative Stress
4.3. Gut Microbiota, SCFAs, and BCFAs
4.4. Metabolome in Serum, Feces, and Fermentation Broth
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| 5-HT | 5-hydroxytryptamine |
| 5-HTP | 5-hydroxytryptophan |
| ACh | Acetylcholine |
| ACTH | Adreno-cortico-tropic-hormone |
| AhR | Aryl hydrocarbon receptor |
| BBB | Blood–brain barrier |
| BW | Body weight |
| BDNF | Brain-derived neurotrophic factor |
| BCFAs | Branched-chain fatty acids |
| CMC | Carboxymethylcellulose |
| CUMS | Chronic unpredictable mild stress group |
| CRH | Corticotropin releasing hormone |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| ELISA | Enzyme-linked immune-sorbent assay |
| FST | Forced swim test |
| GC | Glucocorticoid |
| Glu | Glutamate |
| HPA | Hypothalamic-pituitary-adrenal |
| IgA | Immunoglobulin A |
| IA | Indole acrylic acid |
| IPA | Indole-3-acetic acid |
| IL-10 | Interleukin 10 |
| LDA | Linear discriminant analysis |
| LEfSe | Linear discriminant analysis Effect Size |
| MDA | Malondialdehyde |
| MLT | Melatonin |
| MGB | Microbiota–gut–brain |
| OFT | Open-field test |
| PA | Picolinic acid |
| PCA | Principal component analysis |
| PG | Prostaglandin |
| QA | Quinolinic acid |
| Res | Resveratrol |
| SCFAs | Short-chain fatty acids |
| T-AOC | Total antioxidative capacity |
| TAM | Tryptamine |
| Trp | Tryptophan |
| GABA | γ-aminobutyric acid |
References
- Grigg, E.K.; Nibblett, B.M.; Robinson, J.Q.; Smits, J.E. Evaluating Pair versus Solitary Housing in Kennelled Domestic Dogs (Canis Familiaris) Using Behaviour and Hair Cortisol: A Pilot Study. Vet. Rec. Open 2017, 4, e000193. [Google Scholar] [CrossRef] [PubMed]
- Van Haaften, K.A.; Grigg, E.K.; Kolus, C.; Hart, L.; Kogan, L.R. A Survey of Dog Owners’ Perceptions on the Use of Psychoactive Medications and Alternatives for the Treatment of Canine Behavior Problems. J. Vet. Behav. 2020, 35, 27–33. [Google Scholar] [CrossRef]
- Kartashova, I.; Ganina, K.; Karelina, E.; Tarasov, S. How to Evaluate and Manage Stress in Dogs–a Guide for Veterinary Specialist. Appl. Anim. Behav. Sci. 2021, 243, 105458. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.A.; Fahim, A.T.; Sadik, N.A.H.; Ali, B.M. Resveratrol and Dimethyl Fumarate Ameliorate Depression-like Behaviour in a Rat Model of Chronic Unpredictable Mild Stress. Brain Res. 2018, 1701, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Chu, L.; Han, Y. Therapeutic Effect of Resveratrol on Mice with Depression. Exp. Ther. Med. 2019, 17, 3061–3064. [Google Scholar] [CrossRef] [PubMed]
- Shayganfard, M. Molecular and Biological Functions of Resveratrol in Psychiatric Disorders: A Review of Recent Evidence. Cell Biosci. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Wu, J.; Zhang, H.; Perry, S.W.; Yin, B.; Tan, X.; Chai, T.; Liang, W.; Huang, Y.; Li, Y.; et al. The Gut Microbiome Modulates Gut–Brain Axis Glycerophospholipid Metabolism in a Region-Specific Manner in a Nonhuman Primate Model of Depression. Mol. Psychiatry 2021, 26, 2380–2392. [Google Scholar] [CrossRef]
- Yu, M.; Mu, C.; Yang, Y.; Zhang, C.; Su, Y.; Huang, Z.; Yu, K.; Zhu, W. Increases in Circulating Amino Acids with In-Feed Antibiotics Correlated with Gene Expression of Intestinal Amino Acid Transporters in Piglets. Amino Acids 2017, 49, 1587–1599. [Google Scholar] [CrossRef]
- Gao, K.; Pi, Y.; Mu, C.; Farzi, A.; Liu, Z.; Zhu, W. Increasing Carbohydrate Availability in the Hindgut Promotes Hypothalamic Neurotransmitter Synthesis: Aromatic Amino Acids Linking the Microbiota–Brain Axis. J. Neurochem. 2019, 149, 641–659. [Google Scholar] [CrossRef]
- Magaji, M.G.; Iniaghe, L.O.; Abolarin, M.; Abdullahi, O.I.; Magaji, R.A. Neurobehavioural Evaluation of Resveratrol in Murine Models of Anxiety and Schizophrenia. Metab. Brain Dis. 2017, 32, 437–442. [Google Scholar] [CrossRef]
- Ibáñez, M.; Anzola, B. Use of Fluoxetine, Diazepam, and Behavior Modification as Therapy for Treatment of Anxiety-Related Disorders in Dogs. J. Vet. Behav. 2009, 4, 223–229. [Google Scholar] [CrossRef]
- Van Den Abbeele, P.; Moens, F.; Pignataro, G.; Schnurr, J.; Ribecco, C.; Gramenzi, A.; Marzorati, M. Yeast-Derived Formulations Are Differentially Fermented by the Canine and Feline Microbiome As Assessed in a Novel In Vitro Colonic Fermentation Model. J. Agric. Food Chem. 2020, 68, 13102–13110. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, J.; Zhu, P.; Xie, H.; Lu, L.; Bai, W.; Pan, W.; Shi, R.; Ye, J.; Xia, B.; et al. Tryptophan-Rich Diet Ameliorates Chronic Unpredictable Mild Stress Induced Depression- and Anxiety-like Behavior in Mice: The Potential Involvement of Gut-Brain Axis. Food Res. Int. 2022, 157, 111289. [Google Scholar] [CrossRef] [PubMed]
- Gruen, M.E.; Case, B.C.; Foster, M.L.; Lazarowski, L.; Fish, R.E.; Landsberg, G.; DePuy, V.; Dorman, D.C.; Sherman, B.L. The Use of an Open-Field Model to Assess Sound-Induced Fear and Anxiety-Associated Behaviors in Labrador Retrievers. J. Vet. Behav. 2015, 10, 338–345. [Google Scholar] [CrossRef]
- Tod, E.; Brander, D.; Waran, N. Efficacy of Dog Appeasing Pheromone in Reducing Stress and Fear Related Behaviour in Shelter Dogs. Appl. Anim. Behav. Sci. 2005, 93, 295–308. [Google Scholar] [CrossRef]
- Seo, M.K.; Jeong, S.; Seog, D.-H.; Lee, J.A.; Lee, J.-H.; Lee, Y.; McIntyre, R.S.; Park, S.W.; Lee, J.G. Effects of Liraglutide on Depressive Behavior in a Mouse Depression Model and Cognition in the Probe Trial of Morris Water Maze Test. J. Affect. Disord. 2023, 324, 8–15. [Google Scholar] [CrossRef]
- Bian, Z.; Jian, X.; Liu, G.; Jian, S.; Wen, J.; Zhang, H.; Lin, X.; Huang, H.; Deng, J.; Deng, B.; et al. Wet-Food Diet Promotes the Recovery from Surgery of Castration and Control of Body Weight in Adult Young Cats. J. Anim. Sci. 2023, 101, skad039. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Ma, S.; Ren, D.; Liu, W.; Han, B.; Zhang, Y.; Xiao, J.; Yi, L.; Deng, B. UPLC–Orbitrap–MS/MS Combined with Chemometrics Establishes Variations in Chemical Components in Green Tea from Yunnan and Hunan Origins. Food Chem. 2018, 266, 534–544. [Google Scholar] [CrossRef]
- Liu, B.; Yu, D.; Sun, J.; Wu, X.; Xin, Z.; Deng, B.; Fan, L.; Fu, J.; Ge, L.; Ren, W. Characterizing the Influence of Gut Microbiota on Host Tryptophan Metabolism with Germ-Free Pigs. Anim. Nutr. 2022, 11, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Xu, C.; Zhang, D.; Ju, F.; Ni, Y. MetOrigin: Discriminating the Origins of Microbial Metabolites for Integrative Analysis of the Gut Microbiome and Metabolome. IMeta 2022, 1, e10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhong, Z.; Wang, B.; Wang, Y. Xiaoyao San Ameliorates High-Fat Diet-Induced Anxiety and Depression via Regulating Gut Microbiota in Mice. Biomed. Pharmacother. 2022, 156, 113902. [Google Scholar] [CrossRef]
- Baghaei Naeini, F.; Hassanpour, S.; Asghari, A. Resveratrol Exerts Anxiolytic-like Effects through Anti-Inflammatory and Antioxidant Activities in Rats Exposed to Chronic Social Isolation. Behav. Brain Res. 2023, 438, 114201. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Le Brech, S.; Camps, T.; Torrente, C.; Mariotti, V.M.; Ruiz, J.L.; Manteca, X. Differences in Serotonin Serum Concentration between Aggressive English Cocker Spaniels and Aggressive Dogs of Other Breeds. J. Vet. Behav. 2013, 8, 19–25. [Google Scholar] [CrossRef]
- Shen, J.-D.; Zhang, Y.-W.; Wang, B.-Y.; Bai, L.; Lu, S.-F.; Zhu, L.-L.; Bai, M.; Li, Y.-C.; Xu, E.-P. Effects of Resveratrol on the Levels of ATP, 5-HT and GAP-43 in the Hippocampus of Mice Exposed to Chronic Unpredictable Mild Stress. Neurosci. Lett. 2020, 735, 135232. [Google Scholar] [CrossRef]
- Niu, J.; Cao, Y.; Ji, Y. Resveratrol, a SIRT1 Activator, Ameliorates MK-801-Induced Cognitive and Motor Impairments in a Neonatal Rat Model of Schizophrenia. Front. Psychiatry 2020, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, S.-Q.; Xu, Y. Resveratrol Ameliorates Chronic Unpredictable Mild Stress-Induced Depression-like Behavior: Involvement of the HPA Axis, Inflammatory Markers, BDNF, and Wnt/β-Catenin Pathway in Rats. Neuropsychiatr. Dis. Treat. 2017, 13, 2727–2736. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The Master Regulator of Immunity to Infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Tian, J.; Wang, J.; Khan, M.A.; Wang, Y.; Zhang, L.; Wang, T. Effects of Dietary Sodium Selenite and Selenium Yeast on Antioxidant Enzyme Activities and Oxidative Stability of Chicken Breast Meat. J. Agric. Food Chem. 2012, 60, 7111–7120. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lv, X.; Ze, X.; Ma, Z.; Zhang, X.; He, R.; Fan, J.; Zhang, M.; Sun, B.; Wang, F.; et al. Combined Probiotics Attenuate Chronic Unpredictable Mild Stress-Induced Depressive-like and Anxiety-like Behaviors in Rats. Front. Psychiatry 2022, 13, 990465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants Fluoxetine and Amitriptyline Induce Alterations in Intestinal Microbiota and Gut Microbiome Function in Rats Exposed to Chronic Unpredictable Mild Stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hansen, C.; Mullan, B.; Pluske, J. Nutrition and Pathology of Weaner Pigs: Nutritional Strategies to Support Barrier Function in the Gastrointestinal Tract. Anim. Feed. Sci. Technol. 2012, 173, 3–16. [Google Scholar] [CrossRef]
- Zhuang, Y.; Huang, H.; Liu, S.; Liu, F.; Tu, Q.; Yin, Y.; He, S. Resveratrol Improves Growth Performance, Intestinal Morphology, and Microbiota Composition and Metabolism in Mice. Front. Microbiol. 2021, 12, 726878. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut Microbial Metabolites in Depression: Understanding the Biochemical Mechanisms. Microbial Cell 2019, 6, 454. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Yu, Z.; Zhang, Z.; Deng, M.; Zhao, J.; Ruan, B. Altered Gut Microbiota Profile in Patients with Generalized Anxiety Disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; He, Y.; Tian, L.; Yu, L.; Cheng, Q.; Li, Z.; Gao, L.; Gao, S.; Yu, C. Gut Microbiota-SCFAs-Brain Axis Associated with the Antidepressant Activity of Berberine in CUMS Rats. J. Affect. Disord. 2023, 325, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Capra, J.C.; Cunha, M.P.; Machado, D.G.; Zomkowski, A.D.; Mendes, B.G.; Santos, A.R.S.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like Effect of Scopoletin, a Coumarin Isolated from Polygala Sabulosa (Polygalaceae) in Mice: Evidence for the Involvement of Monoaminergic Systems. Eur. J. Pharmacol. 2010, 643, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Kamobayashi, H.; Sato, D.; Komori, M.; Yoshimura, M.; Hamada, A.; Kohda, Y.; Tomita, K.; Saito, H. Alleviation of Cisplatin-Induced Acute Kidney Injury Using Phytochemical Polyphenols Is Accompanied by Reduced Accumulation of Indoxyl Sulfate in Rats. Clin. Exp. Nephrol. 2011, 15, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Watanabe, T.; Endo, S.; Hata, S.; Watanabe, T.; Osada, K.; Takenaka, A. Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Anxiety-like Behavior in Socially Isolated Rats. Biosci. Biotechnol. Biochem. 2018, 82, 716–723. [Google Scholar] [CrossRef]
- Fan, L.; Yang, L.; Li, X.; Teng, T.; Xiang, Y.; Liu, X.; Jiang, Y.; Zhu, Y.; Zhou, X.; Xie, P. Proteomic and Metabolomic Characterization of Amygdala in Chronic Social Defeat Stress Rats. Behav. Brain Res. 2021, 412, 113407. [Google Scholar] [CrossRef] [PubMed]
- Latruffe, N.; Lançon, A.; Frazzi, R.; Aires, V.; Delmas, D.; Michaille, J.-J.; Djouadi, F.; Bastin, J.; Cherkaoui-Malki, M. Exploring New Ways of Regulation by Resveratrol Involving miRNAs, with Emphasis on Inflammation: MicroRNA-Dependent Anti-Inflammatory Effect of Resveratrol. Ann. N. Y. Acad. Sci. 2015, 1348, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Hou, W.; Bi, D.; Yin, F.; Gao, Y.; Huang, D.; Li, Y.; Cao, Z.; Yan, Y.; et al. Fecal Microbiota Transplantation Improves VPA-Induced ASD Mice by Modulating the Serotonergic and Glutamatergic Synapse Signaling Pathways. Transl. Psychiatry 2023, 13, 17. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wu, J.; Zhu, Q.; Chen, C.; Li, Y. Implications of Gut Microbiota Dysbiosis and Fecal Metabolite Changes in Psychologically Stressed Mice. Front. Microbiol. 2023, 14, 1124454. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, J.; Tiira, K.; Lehtonen, M.; Hanhineva, K.; Lohi, H. Non-Targeted Metabolite Profiling Reveals Changes in Oxidative Stress, Tryptophan and Lipid Metabolisms in Fearful Dogs. Behav. Brain Funct. 2016, 12, 7. [Google Scholar] [CrossRef]
- Guo, X.; Lin, F.; Yang, F.; Chen, J.; Cai, W.; Zou, T. Gut Microbiome Characteristics of Comorbid Generalized Anxiety Disorder and Functional Gastrointestinal Disease: Correlation with Alexithymia and Personality Traits. Front. Psychiatry 2022, 13, 946808. [Google Scholar] [CrossRef]
- Borisova, M.A.; Snytnikova, O.A.; Litvinova, E.A.; Achasova, K.M.; Babochkina, T.I.; Pindyurin, A.V.; Tsentalovich, Y.P.; Kozhevnikova, E.N. Fucose Ameliorates Tryptophan Metabolism and Behavioral Abnormalities in a Mouse Model of Chronic Colitis. Nutrients 2020, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Seow, H.F.; Bröer, S.; Bröer, A.; Bailey, C.G.; Potter, S.J.; Cavanaugh, J.A.; Rasko, J.E.J. Hartnup Disorder Is Caused by Mutations in the Gene Encoding the Neutral Amino Acid Transporter SLC6A19. Nat. Genet. 2004, 36, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, T.; Camargo, S.M.R.; Summa, V.; Hunziker, P.; Chesnov, S.; Pos, K.M.; Verrey, F. Basolateral Aromatic Amino Acid Transporter TAT1 (Slc16a10) Functions as an Efflux Pathway. J. Cell. Physiol. 2006, 206, 771–779. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Y.; Zhao, Z.; Chu, Y.; Yang, W. The Role of Amino Acid Metabolism in Inflammatory Bowel Disease and Other Inflammatory Diseases. Front. Immunol. 2023, 14, 1284133. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Modulation of Immunity by Tryptophan Microbial Metabolites. Front. Nutr. 2023, 10, 1209613. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Ramachandran, S.; Ganapathy, V. Cell-Surface and Nuclear Receptors in the Colon as Targets for Bacterial Metabolites and Its Relevance to Colon Health. Nutrients 2017, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37. [Google Scholar] [CrossRef]
- Jennis, M.; Cavanaugh, C.; Leo, G.; Mabus, J.; Lenhard, J.; Hornby, P. Microbiota-Derived Tryptophan Indoles Increase after Gastric Bypass Surgery and Reduce Intestinal Permeability in Vitro and in Vivo. Neurogastroenterol. Motil. 2018, 30, e13178. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.Z.; Yue, Q.; Fei, W.; Yao, P.Z.; Han, R.Q.; Tang, J. PE (0:0/14:0), an Endogenous Metabolite of the Gut Microbiota, Exerts Protective Effects against Sepsis-Induced Intestinal Injury by Modulating the AHR/CYP1A1 Pathway. Clin. Sci. 2023, 137, 1753–1769. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Z.; Li, Z.; Chang, H.; Luo, J.; Jian, S.; Zhang, J.; Lin, P.; Deng, B.; Deng, J.; Zhang, L. Resveratrol Ameliorates Chronic Stress in Kennel Dogs and Mice by Regulating Gut Microbiome and Metabolome Related to Tryptophan Metabolism. Antioxidants 2025, 14, 195. https://doi.org/10.3390/antiox14020195
Bian Z, Li Z, Chang H, Luo J, Jian S, Zhang J, Lin P, Deng B, Deng J, Zhang L. Resveratrol Ameliorates Chronic Stress in Kennel Dogs and Mice by Regulating Gut Microbiome and Metabolome Related to Tryptophan Metabolism. Antioxidants. 2025; 14(2):195. https://doi.org/10.3390/antiox14020195
Chicago/Turabian StyleBian, Zhaowei, Ziyang Li, Hao Chang, Jun Luo, Shiyan Jian, Jie Zhang, Peixin Lin, Baichuan Deng, Jinping Deng, and Lingna Zhang. 2025. "Resveratrol Ameliorates Chronic Stress in Kennel Dogs and Mice by Regulating Gut Microbiome and Metabolome Related to Tryptophan Metabolism" Antioxidants 14, no. 2: 195. https://doi.org/10.3390/antiox14020195
APA StyleBian, Z., Li, Z., Chang, H., Luo, J., Jian, S., Zhang, J., Lin, P., Deng, B., Deng, J., & Zhang, L. (2025). Resveratrol Ameliorates Chronic Stress in Kennel Dogs and Mice by Regulating Gut Microbiome and Metabolome Related to Tryptophan Metabolism. Antioxidants, 14(2), 195. https://doi.org/10.3390/antiox14020195






