Evidence for a Functional Link Between the Nrf2 Signalling Pathway and Cytoprotective Effect of S-Petasin in Human Retinal Pigment Epithelium Cells Exposed to Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures
2.3. Cell Viability
2.4. ROS Detection
2.5. Quantification of Intracellular Nrf2 Levels
2.6. Bax and Bcl-2 Detection
2.7. Sample Processing for Metabolic Analysis
2.8. HPLC Analysis of Intracellular Antioxidants and Biomarkers of Oxidative/Nitrosative Stress
2.9. Statistical Analysis
3. Results
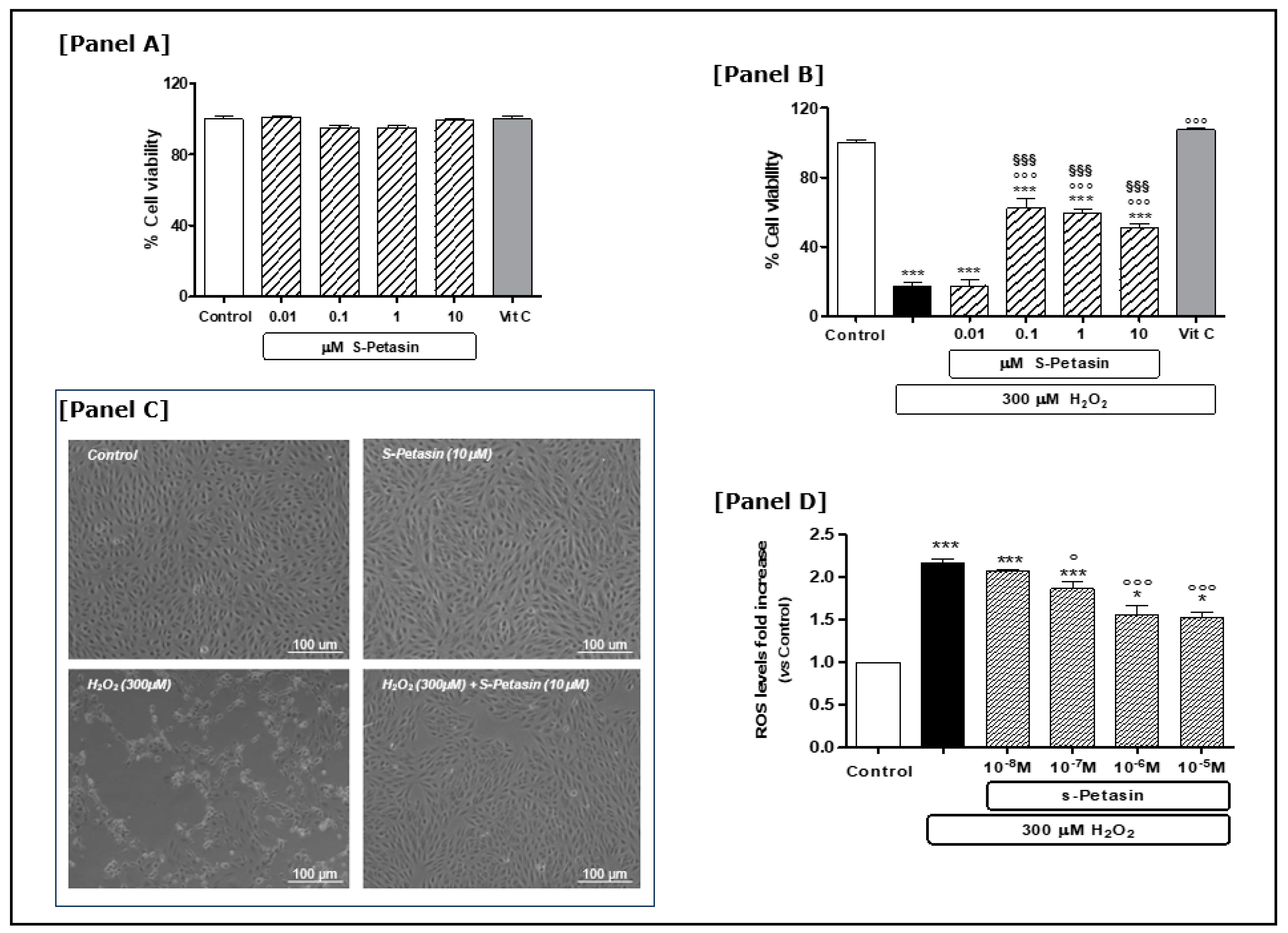
3.1. S-Petasin Cytoprotective Effects
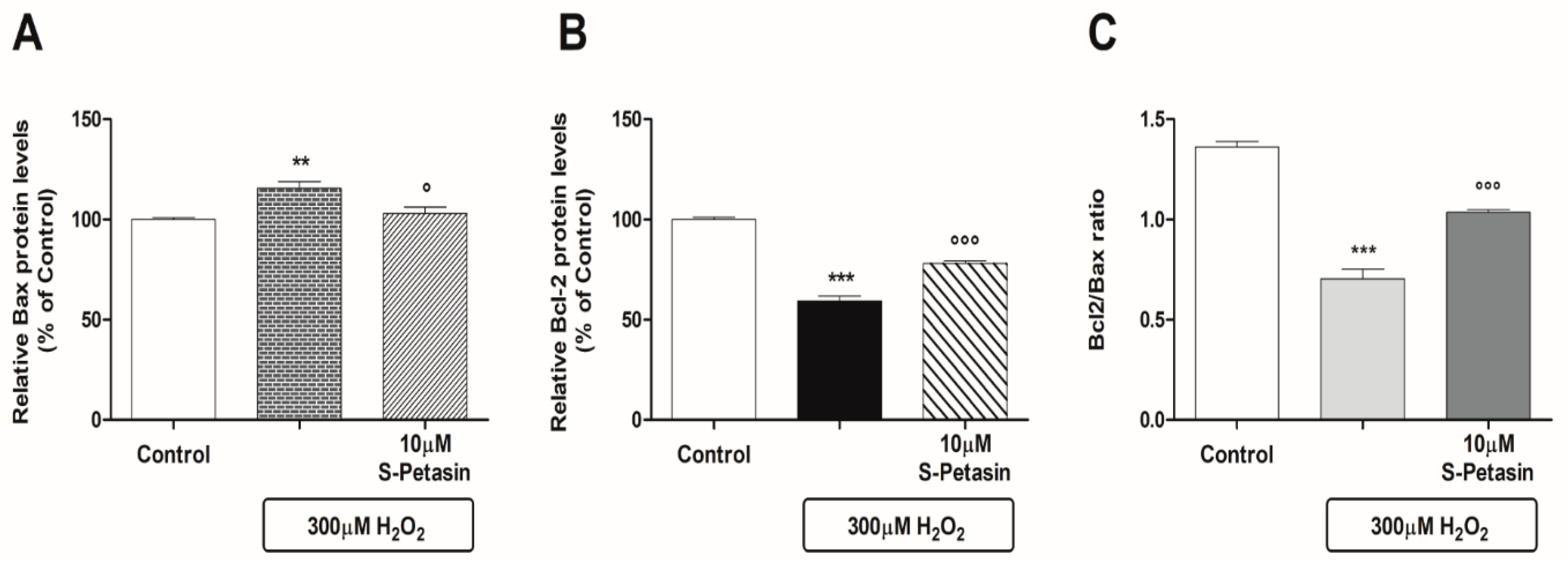
3.2. S-Petasin Increases Intracellular Nrf2 Levels and Modulates Post-Transcriptional Processes
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabre, M.; Mateo, L.; Lamaa, D.; Baillif, S.; Pagès, G.; Demange, L.; Ronco, C.; Benhida, R. Recent Advances in Age-Related Macular Degeneration Therapies. Molecules 2022, 27, 5089. [Google Scholar] [CrossRef]
- Marchesi, N.; Capierri, M.; Pascale, A.; Barbieri, A. Different Therapeutic Approaches for Dry and Wet AMD. Int. J. Mol. Sci. 2024, 25, 13053. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.; Kolekar, K.; Vishwas, S.; Shetti, P.; Kumbar, V.; Andreoli Pinto, T.J.; Paiva-Santos, A.C.; Veiga, F.; Gupta, G.; Singh, S.K.; et al. Treatment avenues for age-related macular degeneration: Breakthroughs and bottlenecks. Ageing Res. Rev. 2024, 98, 102322. [Google Scholar] [CrossRef]
- Trincão-Marques, J.; Ayton, L.N.; Hickey, D.G.; Marques-Neves, C.; Guymer, R.H.; Edwards, T.L.; Sousa, D.C. Gene and cell therapy for age-related macular degeneration: A review. Surv. Ophthalmol. 2024, 69, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.N.; Sim, D.A.; Lee, W.H.; Alfahad, N.; Dick, A.D.; Denniston, A.K.; Hill, L.J. Emerging therapies and their delivery for treating age-related macular degeneration. Br. J. Pharmacol. 2022, 179, 1908–1937. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, C.; Ayarivan, P.; Selvamuthu, P.; Natesan, S. Age-Related Macular Degeneration—Therapies and Their Delivery. Curr. Drug Deliv. 2024, 21, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, A.F.; Nguyen, V.; Barthelmes, D.; Arnold, J.J.; Cheung, C.M.G.; Murray, N.; Gillies, M.C. Smoking status and treatment outcomes of vascular endothelial growth factor inhibitors for neovascular age-related macular degeneration. Retina 2020, 40, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Banait, S. Through the Smoke: An In-Depth Review on Cigarette Smoking and Its Impact on Ocular Health. Cureus 2023, 15, e47779. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bulotta, R.M.; Oppedisano, F.; Bosco, F.; Scarano, F.; Nucera, S.; Guarnieri, L.; Ruga, S.; Macri, R.; Caminiti, R.; et al. Potential Properties of Natural Nutraceuticals and Antioxidants in Age-Related Eye Disorders. Life 2022, 13, 77. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): Design implications. AREDS report no. 1. Control Clin. Trials 1999, 20, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Strauß, O. Pharmacology of the retinal pigment epithelium, the interface between retina and body system. Eur. J. Pharmacol. 2016, 787, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, S. Not All Stressors Are Equal: Mechanism of Stressors on RPE Cell Degeneration. Front. Cell. Dev. Biol. 2020, 8, 591067. [Google Scholar] [CrossRef]
- Wong, J.H.C.; Ma, J.Y.W.; Jobling, A.I.; Brandli, A.; Greferath, U.; Fletcher, E.L.; Vessey, K.A. Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system. Front. Neurosci. 2022, 16, 1009599. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Rodella, U.; Honisch, C.; Gatto, C.; Ruzza, P.; D’Amato Tóthová, J. Antioxidant Nutraceutical Strategies in the Prevention of Oxidative Stress Related Eye Diseases. Nutrients 2023, 15, 2283. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.A.; Zerbes, V.; Parlar, H.; Letzel, T. The medical plant butterbur (Petasites): Analytical and physiological (re)view. J. Pharm. Biomed. Anal. 2013, 75, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Kang, S.; Noh, M.S.; Park, S.J.; Kim, J.M.; Chung, H.Y.; Je, N.K.; Lee, Y.G.; Choi, Y.W.; Im, D.S. Therapeutic effects of s-petasin on disease models of asthma and peritonitis. Biomol. Ther. 2015, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Borlak, J.; Diener, H.C.; Kleeberg-Hartmann, J.; Messlinger, K.; Silberstein, S. Petasites for Migraine Prevention: New Data on Mode of Action, Pharmacology and Safety. A Narrative Review. Front. Neurol. 2022, 13, 864689. [Google Scholar] [CrossRef] [PubMed]
- Sok, D.E.; Oh, S.H.; Kim, Y.B.; Kang, H.G.; Kim, M.R. Neuroprotection by extract of Petasites japonicus leaves, a traditional vegetable, against oxidative stress in brain of mice challenged with kainic acid. Eur. J. Nutr. 2006, 45, 61–69. [Google Scholar] [CrossRef]
- Tzonevaa, R.; Uzunovaa, V.; Stoyanovaa, T.; Borisovaa, B.; Momchilovaa, A.; Pankovb, R.; Maslenkova, L. Anti-cancer effect of Petasites hybridus L. (Butterbur) root extract on breast cancer cell lines. Biotechnol. Biotechnol. Equip. 2021, 35, 853–861. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hsu, H.W.; Chou, J.C.; Yu, C.H.; Bau, D.T.; Wang, G.J.; Huang, C.Y.; Wang, P.S.; Wang, S.W. Cytotoxic effect of s-petasin and iso-s-petasin on the proliferation of human prostate cancer cells. Anticancer. Res. 2015, 35, 191–199. [Google Scholar] [PubMed]
- Kulinowski, Ł.; Luca, S.V.; Minceva, M.; Skalicka-Woźniak, K. A review on the ethnobotany, phytochemistry, pharmacology and toxicology of butterbur species (Petasites L.). J. Ethnopharmacol. 2022, 293, 115263. [Google Scholar] [CrossRef]
- Shih, C.H.; Huang, T.J.; Chen, C.M.; Lin, Y.L.; Ko, W.C. S-Petasin, the Main Sesquiterpene of Petasites formosanus, Inhibits Phosphodiesterase Activity and Suppresses Ovalbumin-Induced Airway Hyperresponsiveness. Evid. -Based Complement. Altern. Med. 2011, 2011, 132374. [Google Scholar] [CrossRef]
- Guo, L.; Kang, J.S.; Park, Y.H.; Je, B.I.; Lee, Y.J.; Kang, N.J.; Park, S.Y.; Hwang, D.Y.; Choi, Y.W. S-petasin inhibits lipid accumulation in oleic acid-induced HepG2 cells through activation of the AMPK signaling pathway. Food Funct. 2020, 11, 5664–5673. [Google Scholar] [CrossRef]
- Guo, L.; Kang, J.S.; Kang, N.J.; Choi, Y.W. S-petasin induces apoptosis and inhibits cell migration through activation of p53 pathway signaling in melanoma B16F10 cells and A375 cells. Arch. Biochem. Biophys. 2020, 692, 108519. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, S.; Oreshkova, T.; Uzunova, V.; Georgieva, I.; Maslenkova, L.; Tzoneva, R.A. Standardized Extract of Petasites hybridus L., Containing the Active Ingredients Petasins, Acts as a Pro-Oxidant and Triggers Apoptosis through Elevating of NF-κB in a Highly Invasive. Human Breast Cancer Cell Line. Front. Biosci. 2023, 28, 111. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Bechir, B.; Rajhi, I. Potential Antioxidant Activity of Terpenes. In Book: Terpenes and Terpenoids—Recent Advances; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Hiemori-Kondo, M. Antioxidant compounds of Petasites japonicus and their preventive effects in chronic diseases: A review. J. Clin. Biochem. Nutr. 2020, 67, 10–18. [Google Scholar] [CrossRef]
- Cui, H.S.; Kim, M.R.; Sok, D.E. Protection by petaslignolide A, a major neuroprotective compound in the butanol extract of Petasites japonicus leaves, against oxidative damage in the brains of mice challenged with kainic acid. J. Agric. Food Chem. 2005, 53, 8526–8532. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, M.H.; Li, M.; Li, K.; Park, G.; Choi, Y.W. Petatewalide B alleviates oxygen-glucose deprivation/reoxygenation-induced neuronal injury via activation of the AMPK/Nrf2 signaling pathway. Mol. Med. Rep. 2020, 22, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Tringali, G.; Pizzoferrato, M.; Lisi, L.; Marinelli, S.; Buccarello, L.; Falsini, B.; Cattaneo, A.; Navarra, P.A. Vicious NGF-p75NTR Positive Feedback Loop Exacerbates the Toxic Effects of Oxidative Damage in the Human Retinal Epithelial Cell Line ARPE-19. Int. J. Mol. Sci. 2023, 24, 16237. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Pizzoferrato, M.; Bianchetti, G.; Brancato, A.; Sampaolese, B.; Maulucci, G.; Tringali, G. Cytoprotective Effect of Idebenone through Modulation of the Intrinsic Mitochondrial Pathway of Apoptosis in Human Retinal Pigment Epithelial Cells Exposed to Oxidative Stress Induced by Hydrogen Peroxide. Biomedicines 2022, 10, 503. [Google Scholar] [CrossRef]
- Clementi, M.E.; Maulucci, G.; Bianchetti, G.; Pizzoferrato, M.; Sampaolese, B.; Tringali, G. Cytoprotective Effects of Punicalagin on Hydrogen-Peroxide-Mediated Oxidative Stress and Mitochondrial Dysfunction in Retinal Pigment Epithelium Cells. Antioxidants 2021, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Sampaolese, B.; Sciandra, F.; Tringali, G. Punicalagin Protects Human Retinal Pigment Epithelium Cells from Ultraviolet Radiation-Induced Oxidative Damage by Activating Nrf2/HO-1 Signaling Pathway and Reducing Apoptosis. Antioxidants 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, C.; Lastres-Becker, I.; Demirdöğen, B.C.; Costa, V.M.; Daiber, A.; Foresti, R.; Motterlini, R.; Kalyoncu, S.; Arioz, B.I.; Genc, S.; et al. Biomarkers of NRF2 signalling: Current status and future challenges. Redox Biol. 2024, 72, 103134. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Lazzarino, G.; Sampaolese, B.; Brancato, A.; Tringali, G. DHA protects PC12 cells against oxidative stress and apoptotic signals through the activation of the NFE2L2/HO-1 axis. Int. J. Mol. Med. 2019, 43, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Amorini, A.M.; Fazzina, G.; Vagnozzi, R.; Signoretti, S.; Donzelli, S.; Di Stasio, E.; Giardina, B.; Tavazzi, B. Single-sample preparation for simultaneous cellular redox and energy state determination. Anal. Biochem. 2003, 322, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, B.; Lazzarino, G.; Leone, P.; Amorini, A.M.; Bellia, F.; Janson, C.G.; Di Pietro, V.; Ceccarelli, L.; Donzelli, S.; Francis, J.S.; et al. Simultaneous high performance liquid chromatographic separation of purines, pyrimidines, N-acetylated amino acids, and dicarboxylic acids for the chemical diagnosis of inborn errors of metabolism. Clin. Biochem. 2005, 38, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.J.; Lee, E.K.; Park, S.W.; Yu, H.G. Antioxidative Effects of Ascorbic Acid and Astaxanthin on ARPE-19 Cells in an Oxidative Stress Model. Antioxidants 2020, 9, 833. [Google Scholar] [CrossRef]
- Yin, J.; Thomas, F.; Lang, J.C.; Chaum, E. Modulation of oxidative stress responses in the human retinal pigment epithelium following treatment with vitamin C. J. Cell Physiol. 2011, 226, 2025–2032. [Google Scholar] [CrossRef]
- Chen, X.; Tzekov, R.; Su, M.; Zhu, Y.; Han, A.; Li, W. Hydrogen peroxide-induced oxidative damage and protective role of peroxiredoxin 6 protein via EGFR/ERK signaling pathway in RPE cells. Front. Aging Neurosci. 2023, 15, 1169211. [Google Scholar] [CrossRef] [PubMed]
- Hassel, C.; Couchet, M.; Jacquemot, N.; Blavignac, C.; Loï, C.; Moinard, C.; Cia, D. Citrulline protects human retinal pigment epithelium from hydrogen peroxide and iron/ascorbate induced damages. J. Cell Mol. Med. 2022, 26, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Phumsuay, R.; Jongjitphisut, N.; Waikasikorn, P.; Sangsawat, M.; Rashatasakhon, P.; Paraoan, L.; Rojsitthisak, P. Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 4722. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, Z.; Zhang, R.; Quan, Z.; Xu, Y.; He, B.; Ren, Y. Mitochondrial-Targeted Antioxidant Peptide SS31 Prevents RPE Cell Death under Oxidative Stress. Biomed. Res. Int. 2022, 2022, 6180349. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Markitantova, Y.; Simirskii, V. Endogenous and Exogenous Regulation of Redox Homeostasis in Retinal Pigment Epithelium Cells: An Updated Antioxidant Perspective. Int. J. Mol. Sci. 2023, 24, 10776. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.; Usategui-Martin, R.; Sanabria, M.R.; Fernandez-Perez, E.; Telleria, J.J.; Coco-Martin, R.M. Pathophysiology of Age-Related Macular Degeneration: Implications for Treatment. Ophthalmic Res. 2022, 65, 615–636. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Albon, J.; Boulton, M. The contribution of DNA repair and antioxidants in determining cell type-specific resistance to oxidative stress. Free Radic. Res. 2006, 40, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.J.; Krebs, M.P.; Mao, H.; Jones, K.; Conners, M.; Lewin, A.S. Pathological consequences of long-term mitochondrial oxidative stress in the mouse retinal pigment epithelium. Exp. Eye Res. 2012, 101, 60–71. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.Y.; Lee, H.M.; Seo, D.I.; Kim, Y.M. Antiproliferative effect of the methanol extract from the roots of Petasites japonicus on Hep3B hepatocellular carcinoma cells in vitro and in vivo. Exp. Ther. Med. 2015, 9, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, L.; Zhang, Y.; Geriletu; Yang, J.; Zhang, Y.; Xing, Y. Vitamin C protected human retinal pigmented epithelium from oxidant injury depending on regulating SIRT1. Sci. World J. 2014, 2014, 750634. [Google Scholar] [CrossRef]
- Wang, R.; Liang, L.; Matsumoto, M.; Iwata, K.; Umemura, A.; He, F. Reactive Oxygen Species and NRF2 Signaling, Friends or Foes in Cancer? Biomolecules 2023, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Torrente, L.; DeNicola, G.M. Targeting NRF2 and Its Downstream Processes: Opportunities and Challenges. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 279–300. [Google Scholar] [CrossRef]
- Grassmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Jaiswal, A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, S.; Toothman, B.; Li, B.; Engel, A.L.; Lim, R.R.; Niernberger, S.; Lu, J.; Ratliff, C.; Xiang, Y.; Eminhizer, M.; et al. Metabolic Phenotyping of Healthy and Diseased Human RPE Cells. Investig. Ophthalmol. Vis. Sci. 2024, 65, 5. [Google Scholar] [CrossRef]
- Bharti, K.; den Hollander, A.I.; Lakkaraju, A.; Sinha, D.; Williams, D.S.; Finnemann, S.C.; Bowes-Rickman, C.; Malek, G.; D’Amore, P.A. Cell culture models to study retinal pigment epithelium-related pathogenesis in age-related macular degeneration. Exp. Eye Res. 2022, 222, 109170. [Google Scholar] [CrossRef]
- Ablonczy, Z.; Dahrouj, M.; Tang, P.H.; Liu, Y.; Sambamurti, K.; Marmorstein, A.D.; Crosson, C.E. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8614–8620. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, B.A.; Fliesler, S.J. Reassessing the suitability of ARPE-19 cells as a valid model of native RPE biology. Exp. Eye Res. 2022, 219, 109046. [Google Scholar] [CrossRef] [PubMed]

| Basal Conditions | |||||
| Biomarker | Control Mean ± SEM (n) | S-Petasin 10−6 Mean ± SEM (n) | S-Petasin 10−5 Mean ± SEM (n) | ||
| GSH | 1.171 ± 0.101 (6) | 1.022 ± 0.180 (6) | 0.918 ± 0.029 (6) | ||
| MDA | 0.010 ± 0.001 (6) | 0.009 ± 0.001 (6) | 0.009 ± 0.002 (6) | ||
| NO2− | 1.190 ± 0.139 (6) | 1.086 ± 0.074 (6) | 1.092 ± 0.016(6) | ||
| NO3− | 30.29 ± 0.484 (6) | 24.01 ± 2.413 (6) * | 25.16 ± 0.207 (6) * | ||
| NADP+/NADPH | 0.667 ± 0.105 (6) | 0.814 ± 0.077 (6) | 0.970 ± 0.026 (6) * | ||
| Stress Conditions | |||||
| Biomarker | Control Mean ± SEM (n) | H2O2 Mean ± SEM (n) | H2O2 + S-Petasin 10−7 Mean ± SEM (n) | H2O2 + S-Petasin 10−6 Mean ± SEM (n) | H2O2 + S-Petasin 10−5 Mean ± SEM (n) |
| GSH | 1.171 ± 0.101 (6) | 0.235 ± 0.038 (6) *** | 0.813 ± 0.052 (6) °°° | 0.708 ± 0.068 (6) °°° | 0.705 ± 0.061 (6) °°° |
| MDA | 0.010 ± 0.001 (6) | 0.027 ± 0.004 (6) *** | 0.017 ± 0.003 (6) °°° | 0.019 ± 0.001 (6) °° | 0.020 ± 0.002 (6) °° |
| NO2− | 1.190 ± 0.139 (6) | 2.014 ± 0.221 (6) *** | 0.979 ± 0.112 (6) °°° | 0.856 ± 0.074 (6) °°° | 1.231 ± 0.096 (6) °°° |
| NO3− | 30.29 ± 0.484 (6) | 43.22 ± 5.334 (6) *** | 23.60 ± 1.880 (6) °°° | 30.40 ± 1.900 (6) °° | 32.77 ± 1.943 (6) ° |
| NADP+/NADPH | 0.667 ± 0.105 (6) | 5.551 ± 0.743 (6) *** | 2.088 ± 0.240 (6) °°° | 1.533 ± 0.052 (6) °°° | 2.350 ± 0.197 (6) °°° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzoferrato, M.; Lazzarino, G.; Brancato, A.; Tabolacci, E.; Clementi, M.E.; Tringali, G. Evidence for a Functional Link Between the Nrf2 Signalling Pathway and Cytoprotective Effect of S-Petasin in Human Retinal Pigment Epithelium Cells Exposed to Oxidative Stress. Antioxidants 2025, 14, 180. https://doi.org/10.3390/antiox14020180
Pizzoferrato M, Lazzarino G, Brancato A, Tabolacci E, Clementi ME, Tringali G. Evidence for a Functional Link Between the Nrf2 Signalling Pathway and Cytoprotective Effect of S-Petasin in Human Retinal Pigment Epithelium Cells Exposed to Oxidative Stress. Antioxidants. 2025; 14(2):180. https://doi.org/10.3390/antiox14020180
Chicago/Turabian StylePizzoferrato, Michela, Giacomo Lazzarino, Anna Brancato, Elisabetta Tabolacci, Maria Elisabetta Clementi, and Giuseppe Tringali. 2025. "Evidence for a Functional Link Between the Nrf2 Signalling Pathway and Cytoprotective Effect of S-Petasin in Human Retinal Pigment Epithelium Cells Exposed to Oxidative Stress" Antioxidants 14, no. 2: 180. https://doi.org/10.3390/antiox14020180
APA StylePizzoferrato, M., Lazzarino, G., Brancato, A., Tabolacci, E., Clementi, M. E., & Tringali, G. (2025). Evidence for a Functional Link Between the Nrf2 Signalling Pathway and Cytoprotective Effect of S-Petasin in Human Retinal Pigment Epithelium Cells Exposed to Oxidative Stress. Antioxidants, 14(2), 180. https://doi.org/10.3390/antiox14020180










