Single Low-Dose Ionizing Radiation Transiently Enhances Rat RIN-m5F Cell Function via the ROS/p38 MAPK Pathway Without Inducing Cell Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Treatment
2.2. Ionizing Radiation
2.3. RNA Extraction and Reverse Transcription Quantitative PCR (RT-qPCR)
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Apoptosis Assay
2.6. Immunofluorescence
2.7. Intracellular Protein Detection by Flow Cytometry
2.8. 5-Ethynyl-2′-Deoxyuridine (EdU) Incorporation Assay
2.9. CCK-8 Assay
2.10. Reactive Oxygen Species (ROS) Detection
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
3.1. A Single Exposure to Ionizing Radiation Leads to an Increase in Insulin Synthesis by β-Cells Within a Short Period
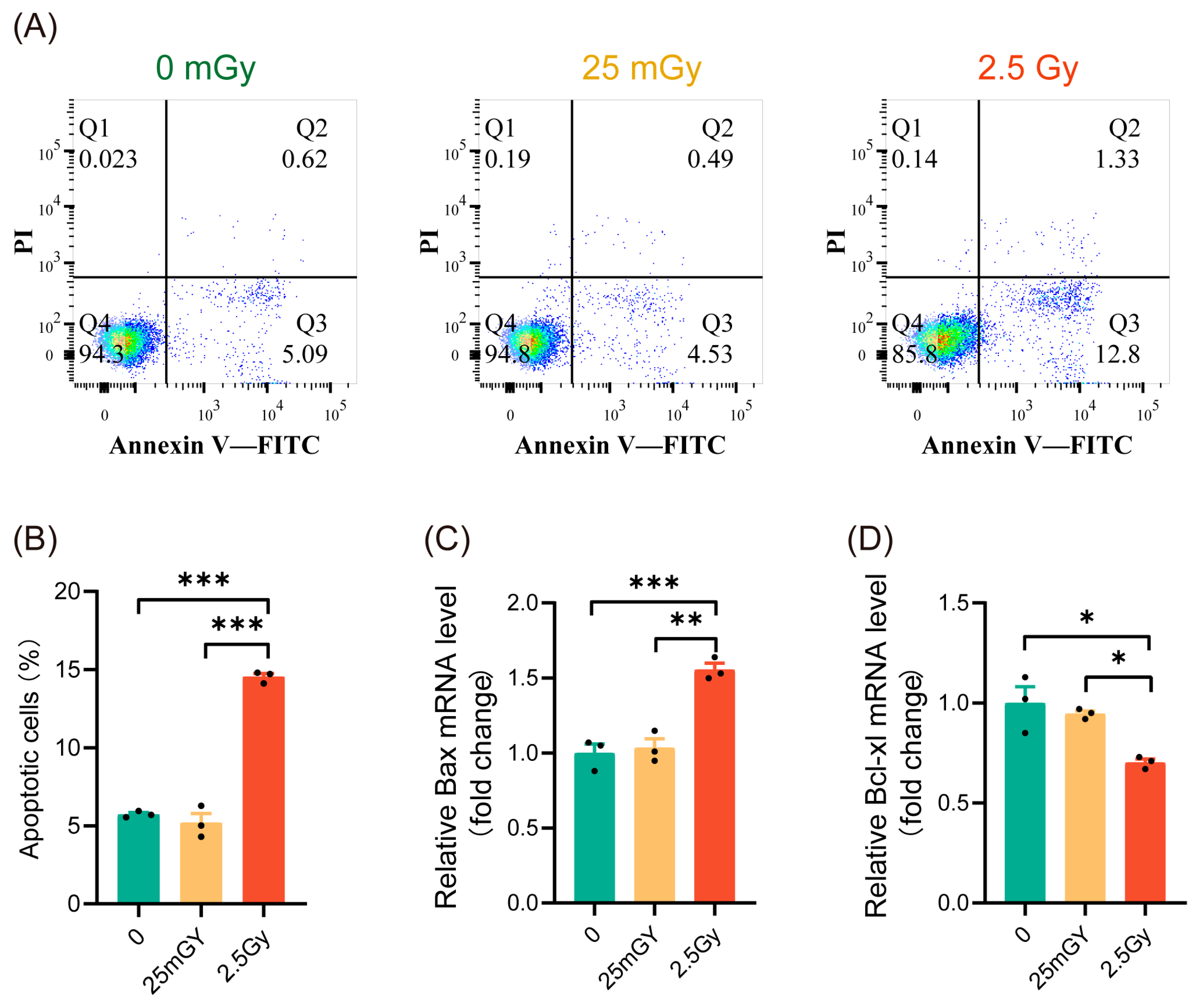
3.2. LDIR Do Not Induce Apoptosis in RIN-m5F Cells
3.3. LDIR Does Not Modify Cell Proliferation in RIN-m5F Cells
3.4. LDIR Does Not Induce DNA Double-Strand Breaks nor Activate DNA Repair Pathways in RIN-m5F Cells
3.5. LDIR Induces a Transient Increase in Reactive Oxygen Species (ROS)
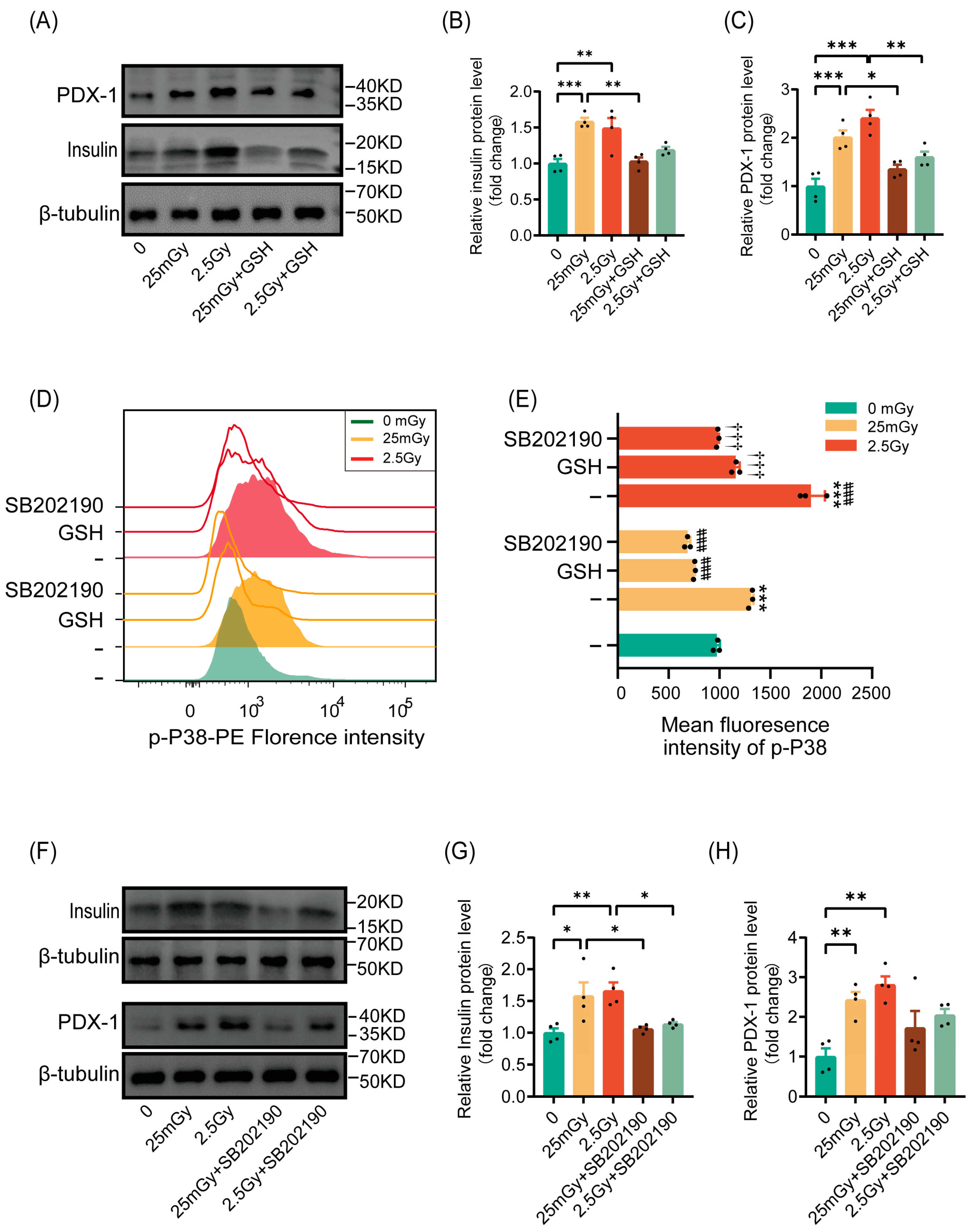
3.6. Ionizing Radiation Promotes β-Cell Function Through the ROS/p38 MAPK Pathway
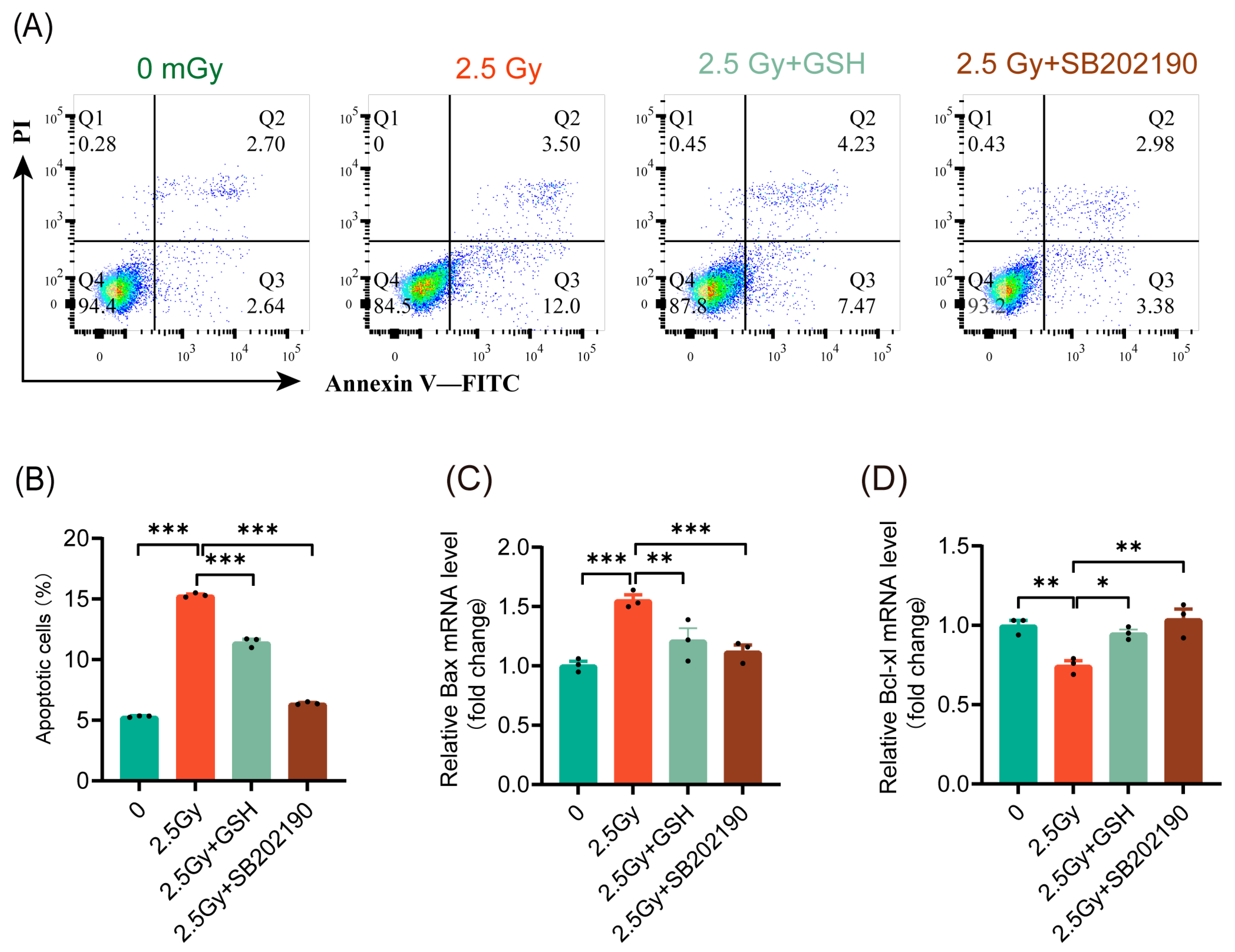
3.7. HDIR Induces Apoptosis Through Overactivation of the ROS/p38 MAPK Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanakoglou, D.S.; Michalettou, T.D.; Vasileiou, C.; Gioukakis, E.; Maneta, D.; Kyriakidis, K.V.; Georgakilas, A.G.; Michalopoulos, I. Effects of High-Dose Ionizing Radiation in Human Gene Expression: A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 1938. [Google Scholar] [CrossRef] [PubMed]
- Paithankar, J.G.; Gupta, S.C.; Sharma, A. Therapeutic potential of low dose ionizing radiation against cancer, dementia, and diabetes: Evidences from epidemiological, clinical, and preclinical studies. Mol. Biol. Rep. 2023, 50, 2823–2834. [Google Scholar] [CrossRef]
- Tang, F.R.; Loke, W.K. Molecular mechanisms of low dose ionizing radiation-induced hormesis, adaptive responses, radioresistance, bystander effects, and genomic instability. Int. J. Radiat. Biol. 2015, 91, 13–27. [Google Scholar] [CrossRef]
- Siegel, J.A.; Greenspan, B.S.; Maurer, A.H.; Taylor, A.T.; Phillips, W.T.; Van Nostrand, D.; Sacks, B.; Silberstein, E.B. The BEIR VII Estimates of Low-Dose Radiation Health Risks Are Based on Faulty Assumptions and Data Analyses: A Call for Reassessment. J. Nucl. Med. 2018, 59, 1017–1019. [Google Scholar] [CrossRef]
- Cioffi, D.L.; Fontana, L.; Leso, V.; Dolce, P.; Vitale, R.; Vetrani, I.; Galdi, A.; Iavicoli, I. Low dose ionizing radiation exposure and risk of thyroid functional alterations in healthcare workers. Eur. J. Radiol. 2020, 132, 109279. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wu, B.; Wang, X.; Kou, X.; Zhu, X.; Fu, K.; Zhang, Q.; Hong, S.; Wang, X. Long-term low-dose ionizing radiation induced chromosome-aberration-specific metabolic phenotype changes in radiation workers. J. Pharm. Biomed. Anal. 2022, 214, 114718. [Google Scholar] [CrossRef]
- Scott, B.R. Radiation-hormesis phenotypes, the related mechanisms and implications for disease prevention and therapy. J. Cell Commun. Signal 2014, 8, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, Y.; Nakamura, H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. Int. J. Mol. Sci. 2018, 19, 2387. [Google Scholar] [CrossRef]
- Doss, M. Evidence supporting radiation hormesis in atomic bomb survivor cancer mortality data. Dose Response 2012, 10, 584–592. [Google Scholar] [CrossRef]
- Hart, J. Cancer mortality for a single race in low versus high elevation counties in the U.S. Dose Response 2011, 9, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lin, X.; Yu, L.; Li, W.; Qian, D.; Cheng, P.; He, L.; Yang, H.; Zhang, C. Low-dose radiation prevents type 1 diabetes-induced cardiomyopathy via activation of AKT mediated anti-apoptotic and anti-oxidant effects. J. Cell Mol. Med. 2016, 20, 1352–1366. [Google Scholar] [CrossRef]
- Caratero, A.; Courtade, M.; Bonnet, L.; Planel, H.; Caratero, C. Effect of a continuous gamma irradiation at a very low dose on the life span of mice. Gerontology 1998, 44, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, G.; Hahn, D.A. Early life hormetic treatments decrease irradiation-induced oxidative damage, increase longevity, and enhance sexual performance during old age in the Caribbean fruit fly. PLoS ONE 2014, 9, e88128. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Impens, N.; Armengol, G.; Candeias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rodel, F.; Schaue, D. Low dose ionizing radiation effects on the immune system. Environ. Int. 2021, 149, 106212. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef] [PubMed]
- Accili, D. Insulin Action Research and the Future of Diabetes Treatment: The 2017 Banting Medal for Scientific Achievement Lecture. Diabetes 2018, 67, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Mitsunobu, F.; Hanamoto, K.; Shibuya, K.; Mori, S.; Tanizaki, Y.; Sugita, K. Biochemical comparison between radon effects and thermal effects on humans in radon hot spring therapy. J. Radiat. Res. 2004, 45, 83–88. [Google Scholar] [CrossRef]
- Kojima, S.; Cuttler, J.M.; Shimura, N.; Koga, H.; Murata, A.; Kawashima, A. Radon Therapy for Autoimmune Diseases Pemphigus and Diabetes: 2 Case Reports. Dose Response 2019, 17, 1559325819850984. [Google Scholar] [CrossRef]
- Takehara, Y.; Yamaoka, K.; Hiraki, Y.; Yoshioka, T.; Utsumi, K. Protection against alloxan diabetes by low-dose 60Co gamma irradiation before alloxan administration. Physiol. Chem. Phys. Med. NMR 1995, 27, 149–159. [Google Scholar]
- Tsuruga, M.; Taki, K.; Ishii, G.; Sasaki, Y.; Furukawa, C.; Sugihara, T.; Nomura, T.; Ochiai, A.; Magae, J. Amelioration of type II diabetes in db/db mice by continuous low-dose-rate gamma irradiation. Radiat. Res. 2007, 167, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kojima, S.; Yamaoka, K.; Niki, E. Prevention of type I diabetes by low-dose gamma irradiation in NOD mice. Radiat. Res. 2000, 154, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, J.L.; Thomas, D.S. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar]
- Ellen, C.J. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar]
- Daniel, C.G.; Juan, C.A. A Simple, Reproducible Procedure for Chemiluminescent Western Blot Quantification. Bio-Protoc. 2023, 13, e4667. [Google Scholar]
- Sampadi, B.; Vermeulen, S.; Misovic, B.; Boei, J.J.; Batth, T.S.; Chang, J.G.; Paulsen, M.T.; Magnuson, B.; Schimmel, J.; Kool, H.; et al. Divergent Molecular and Cellular Responses to Low and High-Dose Ionizing Radiation. Cells 2022, 11, 3794. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Hauer-Jensen, M. Ionizing radiation-induced responses: Where free radical chemistry meets redox biology and medicine. Antioxid. Redox Signal 2014, 20, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Saitoh, I.; Watanabe, M. Novel cell-permeable p38-MAPK inhibitor efficiently prevents porcine islet apoptosis and improves islet graft function. Am. J. Transplant. 2020, 20, 1296–1308. [Google Scholar] [CrossRef]
- Bacarella, N.; Ruggiero, A.; Davis, A.T.; Uberseder, B.; Davis, M.A.; Bracy, D.P.; Wasserman, D.H.; Cline, J.M.; Sherrill, C.; Kavanagh, K. Whole Body Irradiation Induces Diabetes and Adipose Insulin Resistance in Nonhuman Primates. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 878–886. [Google Scholar] [CrossRef]
- Yahyapour, R.; Amini, P.; Rezapour, S.; Cheki, M.; Rezaeyan, A.; Farhood, B.; Shabeeb, D.; Musa, A.E.; Fallah, H.; Najafi, M. Radiation-induced inflammation and autoimmune diseases. Mil. Med. Res. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Middleton, R.J.; Banati, R.B.; Liu, G.J. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016, 9, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Woodbine, L.; Haines, J.; Coster, M.; Ricket, N.; Barazzuol, L.; Ainsbury, E.; Sienkiewicz, Z.; Jeggo, P. Increased apoptosis and DNA double-strand breaks in the embryonic mouse brain in response to very low-dose X-rays but not 50 Hz magnetic fields. J. R. Soc. Interface 2014, 11, 20140783. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.; Souissi-Sahraoui, I.; Deynoux, M.; Lefevre, A.; Barroca, V.; Campalans, A.; Menard, V.; Calvo, J.; Pflumio, F.; Arcangeli, M.L. Human hematopoietic stem/progenitor cells display reactive oxygen species-dependent long-term hematopoietic defects after exposure to low doses of ionizing radiations. Haematologica 2020, 105, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- Ojima, M.; Eto, H.; Ban, N.; Kai, M. Radiation-induced bystander effects induce radioadaptive response by low-dose radiation. Radiat. Prot. Dosim. 2011, 146, 276–279. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zeng, Z.; Li, J.; Luo, Y.; Sun, W.; Gong, Y.; Zhang, J.; Wu, Q.; Xie, C. Immunomodulation of NK Cells by Ionizing Radiation. Front. Oncol. 2020, 10, 874. [Google Scholar] [CrossRef]
- Enns, L.; Rasouli-Nia, A.; Hendzel, M.; Marples, B.; Weinfeld, M. Association of ATM activation and DNA repair with induced radioresistance after low-dose irradiation. Radiat. Prot. Dosim. 2015, 166, 131–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seth, I.; Joiner, M.C.; Tucker, J.D. Cytogenetic Low-Dose Hyperradiosensitivity Is Observed in Human Peripheral Blood Lymphocytes. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, V.A.; Ershova, E.S.; Veiko, N.N.; Malinovskaya, E.M.; Kalyanov, A.A.; Kameneva, L.V.; Stukalov, S.V.; Dolgikh, O.A.; Konkova, M.S.; Ermakov, A.V.; et al. Low-Dose Ionizing Radiation Affects Mesenchymal Stem Cells via Extracellular Oxidized Cell-Free DNA: A Possible Mediator of Bystander Effect and Adaptive Response. Oxidative Med. Cell. Longev. 2017, 2017, 9515809. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, J.A.; Sharma, N.; Allen, C.P.; Taylor, L.; Allen, S.J.; Jaiswal, A.S.; Hromas, R. Roles of homologous recombination in response to ionizing radiation-induced DNA damage. Int. J. Radiat. Biol. 2023, 99, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules--mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Socol, Y.; Welsh, J.S. Changing Attitude Toward Radiation Carcinogenesis and Prospects for Novel Low-Dose Radiation Treatments. Technol. Cancer Res. Treat. 2016, 15, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Einor, D.; Bonisoli-Alquati, A.; Costantini, D.; Mousseau, T.A.; Moller, A.P. Ionizing radiation, antioxidant response and oxidative damage: A meta-analysis. Sci. Total Environ. 2016, 548–549, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Montoro, A. Ionizing Radiation, Antioxidant Response and Oxidative Damage: Radiomodulators. Antioxidants 2023, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.L.; Ktorza, A.; Casteilla, L.; Penicaud, L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 2009, 58, 673–681. [Google Scholar] [CrossRef]
- Llanos, P.; Contreras-Ferrat, A.; Barrientos, G.; Valencia, M.; Mears, D.; Hidalgo, C. Glucose-Dependent Insulin Secretion in Pancreatic β-Cell Islets from Male Rats Requires Ca2+ Release via ROS-Stimulated Ryanodine Receptors. PLoS ONE 2015, 10, e0129238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, T.; Zhang, D.; Leung, P.S. GPR120 protects lipotoxicity-induced pancreatic β-cell dysfunction through regulation of PDX1 expression and inhibition of islet inflammation. Clin. Sci. 2019, 133, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Kassouf, T.; Sumara, G. Impact of Conventional and Atypical MAPKs on the Development of Metabolic Diseases. Biomolecules 2020, 10, 1256. [Google Scholar] [CrossRef]
- Song, H.; Wohltmann, M.; Tan, M.; Bao, S.; Ladenson, J.H.; Turk, J. Group VIA PLA2 (iPLA2β) is activated upstream of p38 mitogen-activated protein kinase (MAPK) in pancreatic islet β-cell signaling. J. Biol. Chem. 2012, 287, 5528–5541. [Google Scholar] [CrossRef]
- MacFarlane, W.M.; Read, M.L.; Gilligan, M.; Bujalska, I.; Docherty, K. Glucose modulates the binding activity of the β-cell transcription factor IUF1 in a phosphorylation-dependent manner. Biochem. J. 1994, 303 Pt 2, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, W.M.; Smith, S.B.; James, R.F.; Clifton, A.D.; Doza, Y.N.; Cohen, P.; Docherty, K. The p38/reactivating kinase mitogen-activated protein kinase cascade mediates the activation of the transcription factor insulin upstream factor 1 and insulin gene transcription by high glucose in pancreatic β-cells. J. Biol. Chem. 1997, 272, 20936–20944. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, H.; Liu, S.H.; Shahi, K.M.; Lin, X.; Wu, J.; Feng, X.H.; Qin, J.; Tan, T.H.; Brunicardi, F.C. p38 MAP kinase interacts with and stabilizes pancreatic and duodenal homeobox-1. Curr. Mol. Med. 2013, 13, 377–386. [Google Scholar]





| Gene | Accession Number | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| Ins1 | NM_019129 | ACAACTGGAGCTGGGTGGAGG | GTTGCAGTAGTTCTCCAGTTGGTAGAG |
| Ins2 | NM_019130 | CAGCACCTTTGTGGTTCTCA | AGAGCAGATGCTGGTGCAG |
| Bax | NM_017059 | CTGGACAACAACATGGAG | AAGTAGAAAAGGGCAACC |
| Bcl-xl | NM_001033670 | TAGGTGGTCATTCAGGTAGG | GTGGAAAGCGTAGACAAGG |
| Pcna | NM_022381 | AAGTTTTCTGCGAGTGGGGA | ACAGTGGAGTGGCTTTTGTGA |
| β-actin | NM_031144 | GTCGTACCACTGGCATTGTG | CTCTCAGCTGTGGTGGTGAA |
| Antibody Name | Catalog No. | Vendor | Application and Dilution |
|---|---|---|---|
| Primary antibody | |||
| Rabbit anti-insulin antibody | A19066 | ABclonal (Wuhan, China) | 1:1000 (WB) |
| Rabbit anti-pdx-1 antibody | 20989-1-AP | Proteintech (Wuhan, China) | 1:1000 (WB) |
| Rabbit anti-phospho-Histone H2A.X (ser139) (γH2A.X) antibody | 381558 | Zenbio (Chengdu, China) | 1:200 (IF), 1:1000 (WB) |
| Mouse anti-phospho-ATM antibody | AA866 | Biotime (Shanghai, China) | 1:200 (IF), 1:100 (Flow) |
| Rabbit anti-phospho-p38 MAPK (Thr180, Tyr182) antibody, PE | MA5-36912 | Invitrogen (Waltham, MA, USA) | 5 µL/1 × 106 cells (Flow) |
| Mouse anti-β-tubulin antibody | 66240-1-Ig | Proteintech | 1:20,000 (WB) |
| Secondary antibody | |||
| Alexa Fluor 594 goat anti-rabbit IgG (H + L) antibody | A11012 | ThermoFisher (Waltham, MA, USA) | 1:1000 (IF), 1:500 (Flow) |
| Alexa Fluor 594 goat anti-mouse IgG (H+ L) antibody | A11005 | ThermoFisher | 1:1000 (IF), 1:500 (Flow) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Dai, K.; An, R.; Wang, C.; Zhou, X.; Tian, Z.; Liao, Z. Single Low-Dose Ionizing Radiation Transiently Enhances Rat RIN-m5F Cell Function via the ROS/p38 MAPK Pathway Without Inducing Cell Damage. Antioxidants 2025, 14, 120. https://doi.org/10.3390/antiox14020120
Zhang J, Dai K, An R, Wang C, Zhou X, Tian Z, Liao Z. Single Low-Dose Ionizing Radiation Transiently Enhances Rat RIN-m5F Cell Function via the ROS/p38 MAPK Pathway Without Inducing Cell Damage. Antioxidants. 2025; 14(2):120. https://doi.org/10.3390/antiox14020120
Chicago/Turabian StyleZhang, Jitai, Kaicen Dai, Ruike An, Chengying Wang, Xuanting Zhou, Zhujun Tian, and Zhonglu Liao. 2025. "Single Low-Dose Ionizing Radiation Transiently Enhances Rat RIN-m5F Cell Function via the ROS/p38 MAPK Pathway Without Inducing Cell Damage" Antioxidants 14, no. 2: 120. https://doi.org/10.3390/antiox14020120
APA StyleZhang, J., Dai, K., An, R., Wang, C., Zhou, X., Tian, Z., & Liao, Z. (2025). Single Low-Dose Ionizing Radiation Transiently Enhances Rat RIN-m5F Cell Function via the ROS/p38 MAPK Pathway Without Inducing Cell Damage. Antioxidants, 14(2), 120. https://doi.org/10.3390/antiox14020120







