Increased HLA-DR Expression on M2a Monocytes and Helper T Cells in Patients with COPD and Asthma–COPD Overlap Contributes to Disease Severity via Apoptosis and ROS
Abstract
1. Introduction
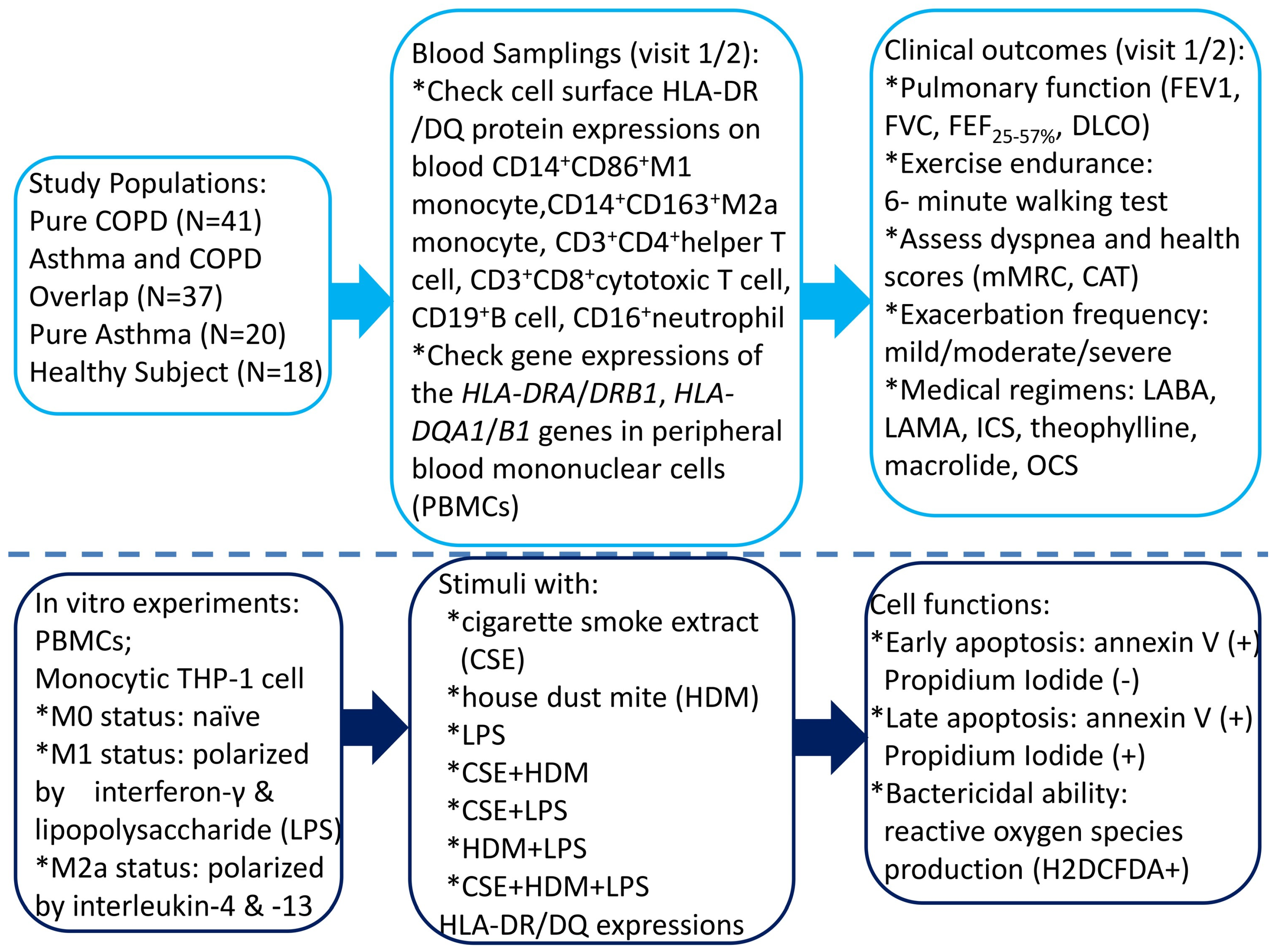
2. Materials and Methods
2.1. Study Subjects
2.2. Blood Sample Collection
2.3. Determining Cell Surface Protein Expressions of MHC Class II of Peripheral Blood CD14+ Monocyte, CD16+Neutrophil, CD125+Siglec8+ Eosinophil, CD19+ B Cell, CD3+CD4+ Helper T (Th) Cell, and CD3+CD8+Cytotoxic T (Tc) Cell by Flowcytometry
2.4. Determination of HLA-DQ/DR Gene Expressions of PBMC Samples by Quantitative Real-Time Reverse Transcriptase–Polymerase Chain Reaction (RT-PCR)
2.5. In Vitro Human Monocytic THP-1 and PBMC Culture Models Under CSE, HDM, and LPS Stimulation
2.5.1. M1/M2 Polarization Protocol
2.5.2. Measurement of Cell Apoptosis by Flow Cytometry Analysis
2.5.3. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.6. Statistical Analysis
3. Results
3.1. Demographic and Baseline Characteristics in the Study Cohort
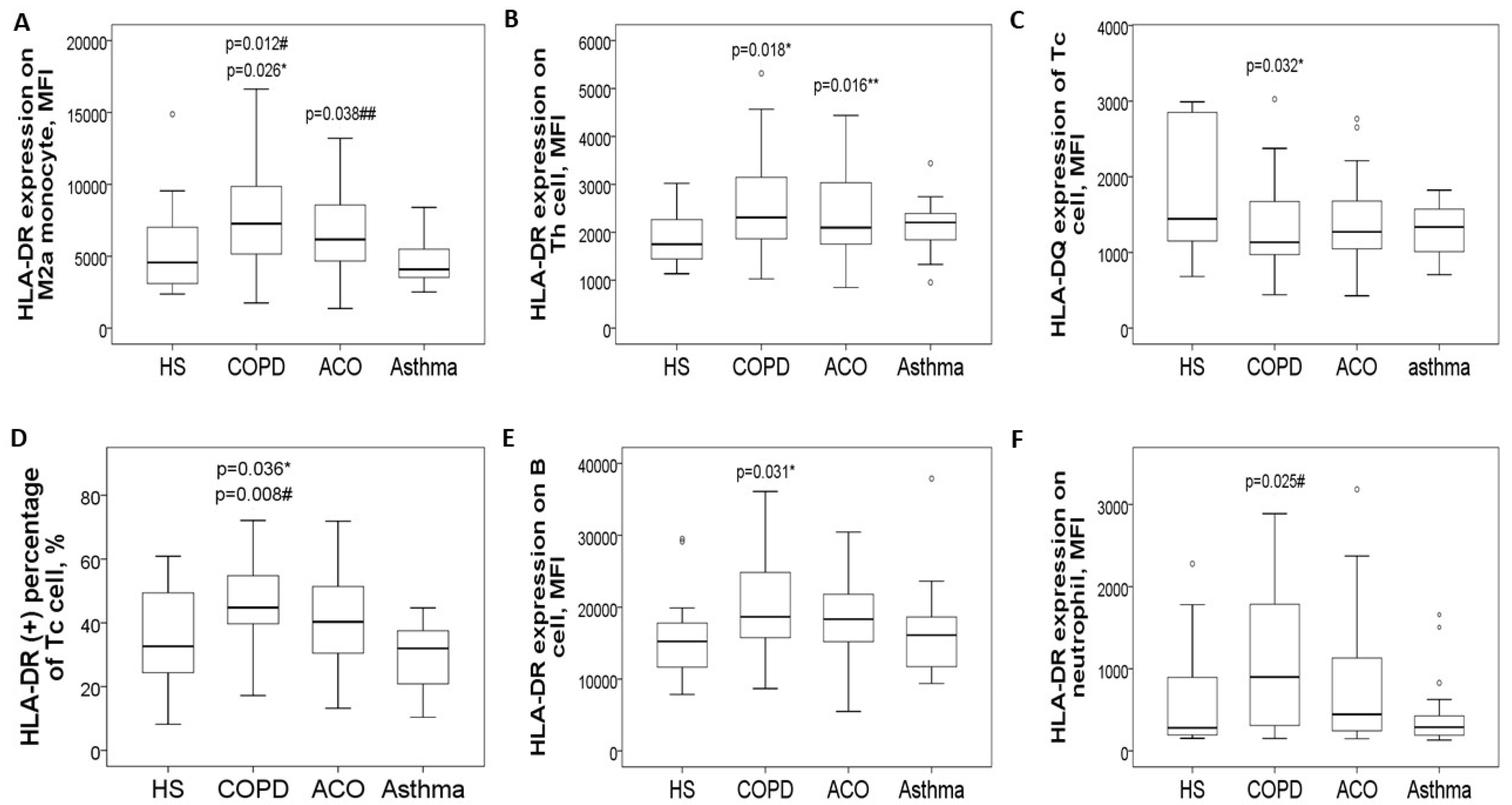
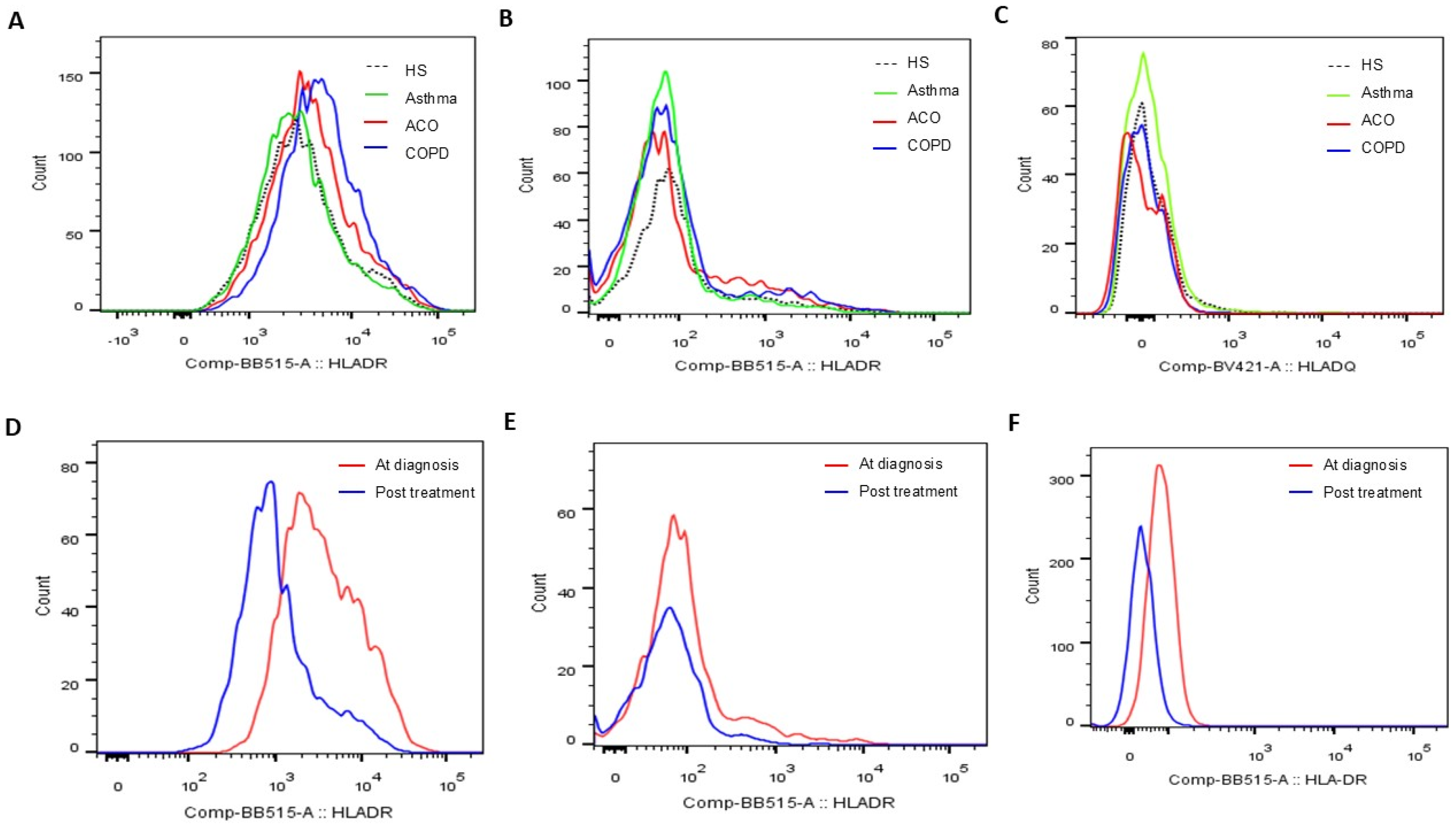
3.2. Increased HLA-DR Protein Expressions of Blood Helper T Cell and M2a Monocyte in COPD and ACO Patients
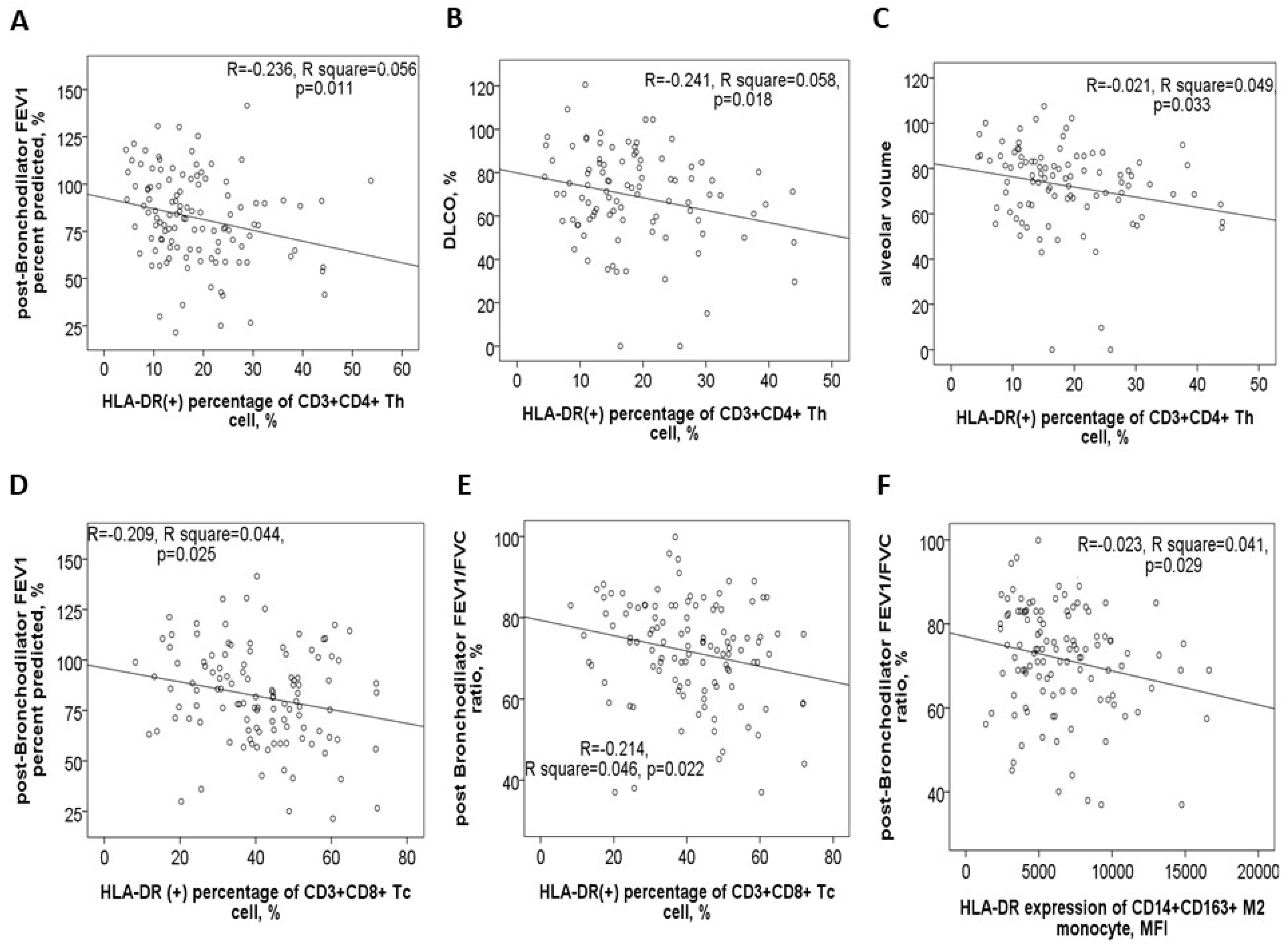
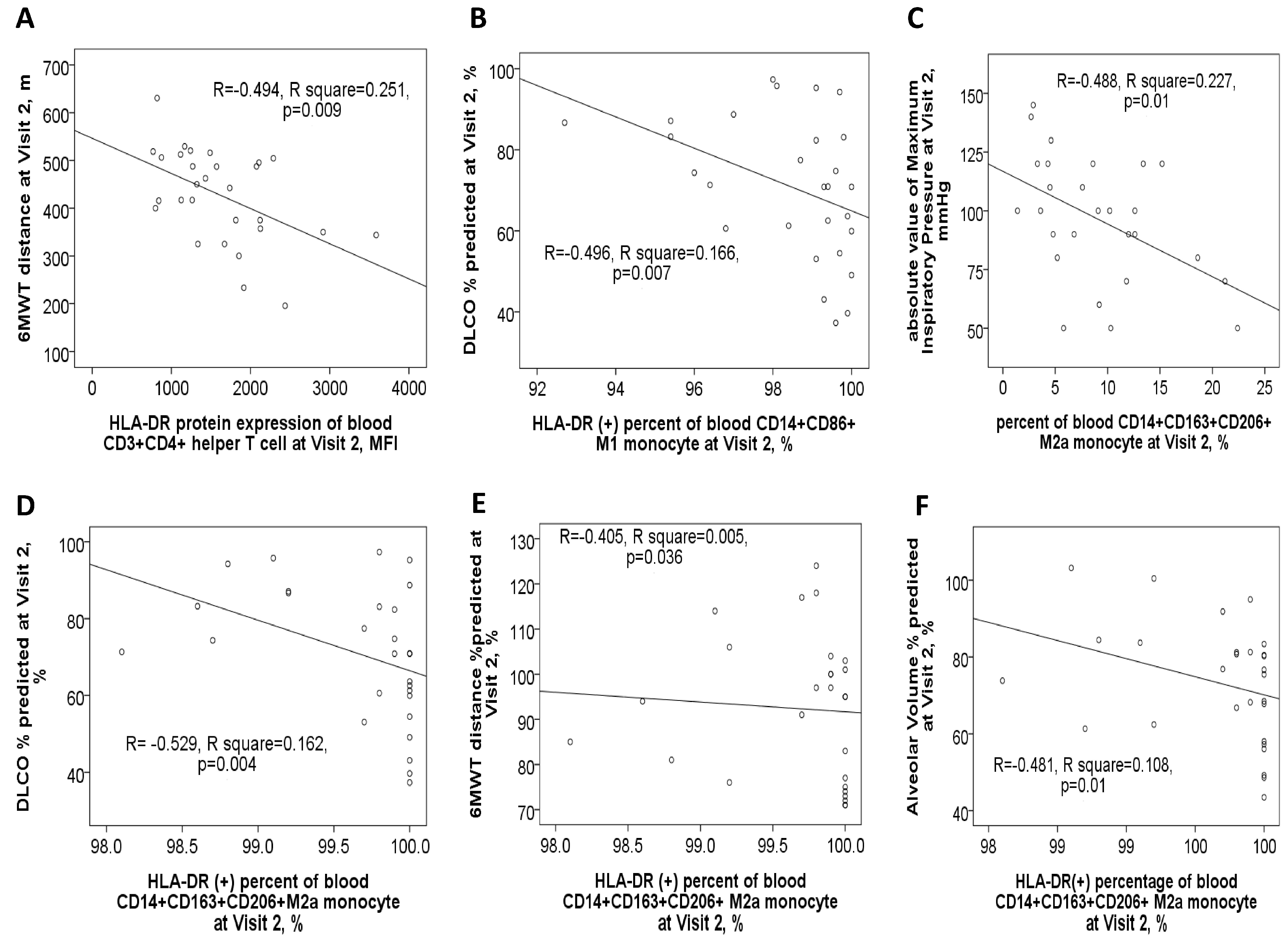
3.3. Negative Correlations Between HLA-DR Protein Expressions and Either Pulmonary Function Test Parameters or Exacerbation Frequency
3.4. Increased HLA-DRA and HLA-DRB1 Gene Expressions of PBMCs in COPD Patients
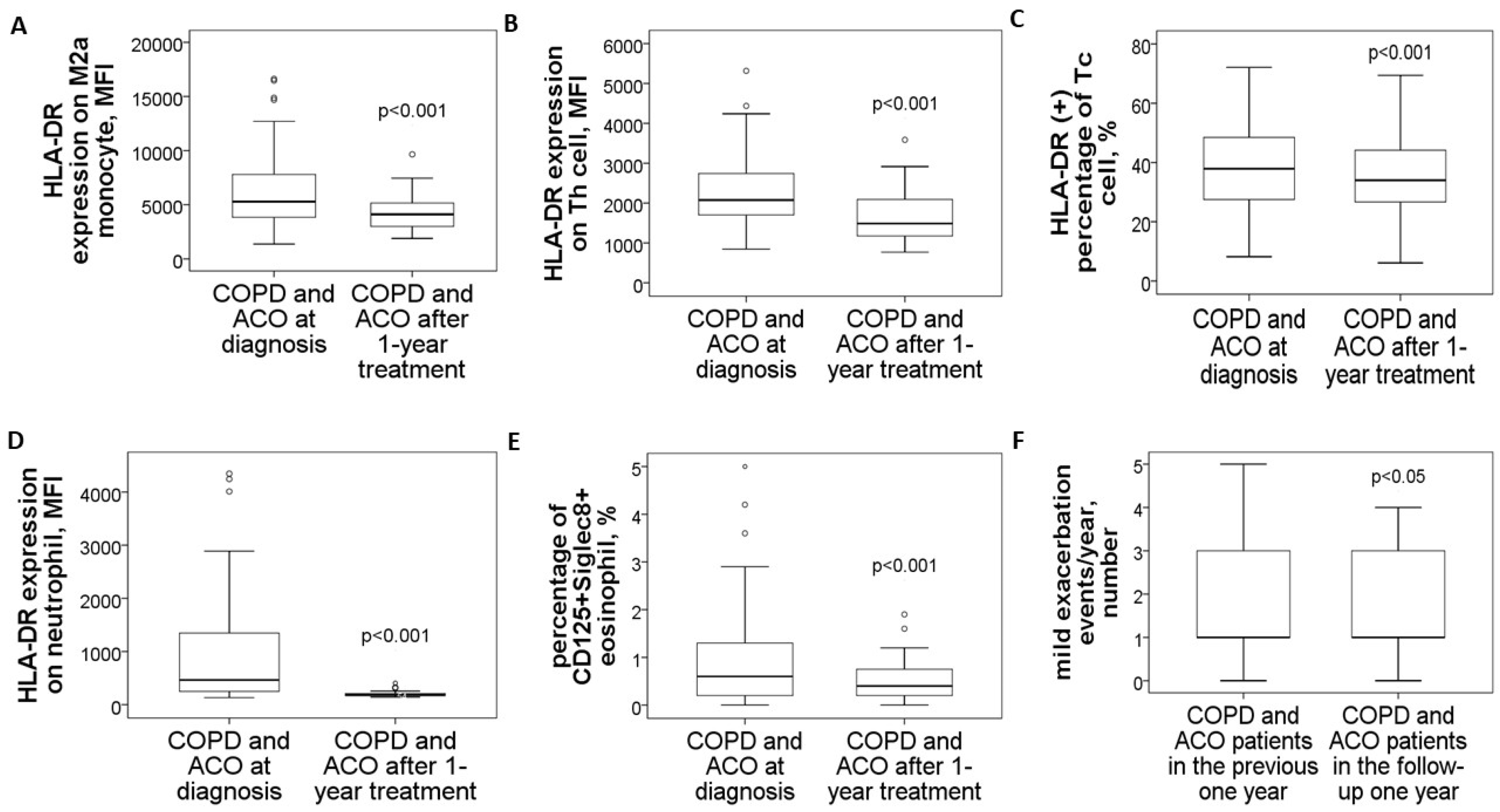
3.5. Reduced HLA-DR Protein Expressions in COPD and ACO Patients After 1-Year Treatment
3.6. Specific Medicines Prescribed for the 30 COPD or ACO Patients During the 1-Year Follow-Up Period
3.7. Negative Correlations Between HLA-DR Protein Expressions and Clinical Outcomes After 1-Year Medical Treatment
3.8. In Vitro Experiments for Stimuli with CSE, HDM, LPS, or Their Mixture
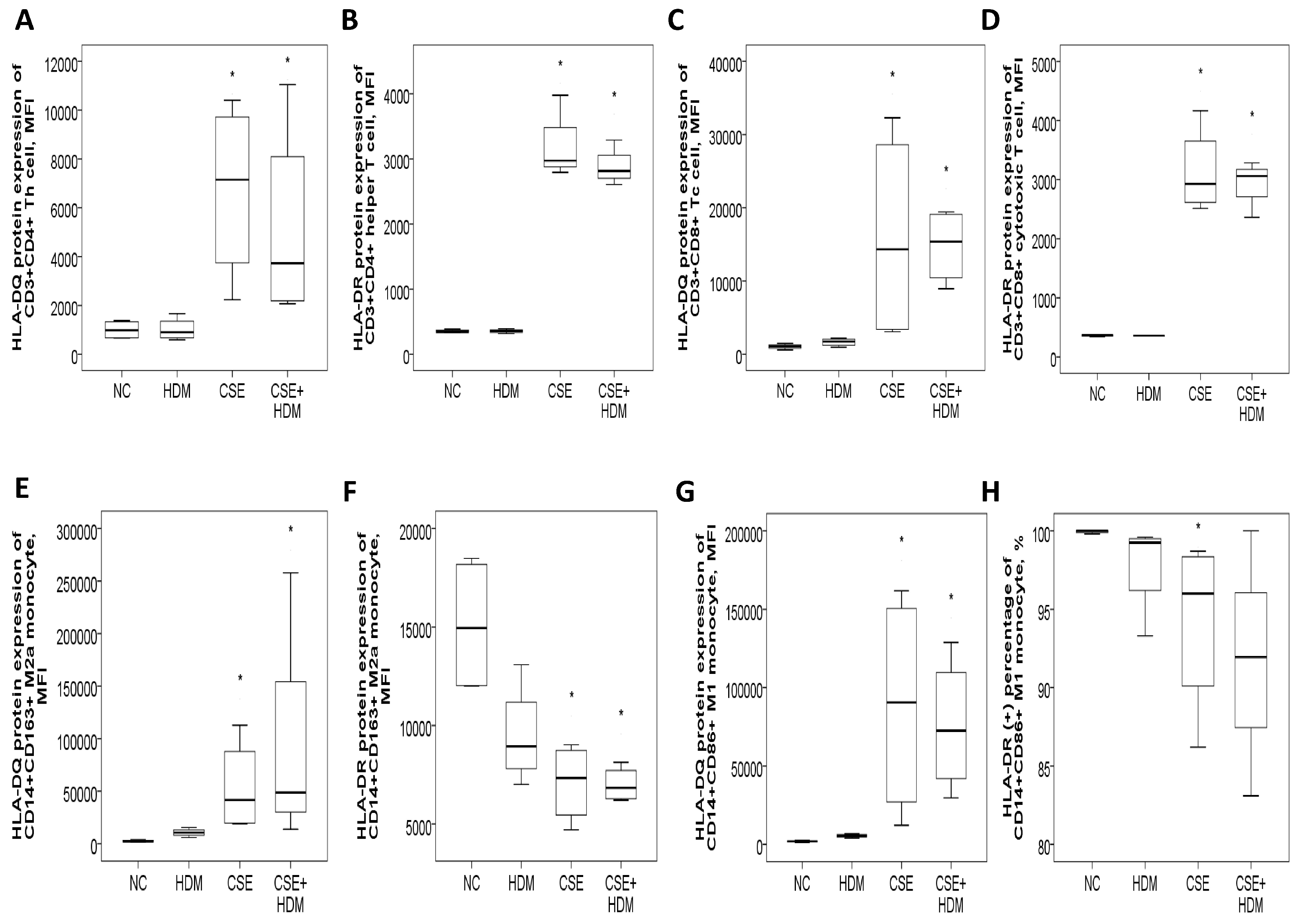
3.8.1. Increased HLA-DR/DQ Protein Expressions of Th and Tc Cells in Response to CSE Plus HDM Stimuli
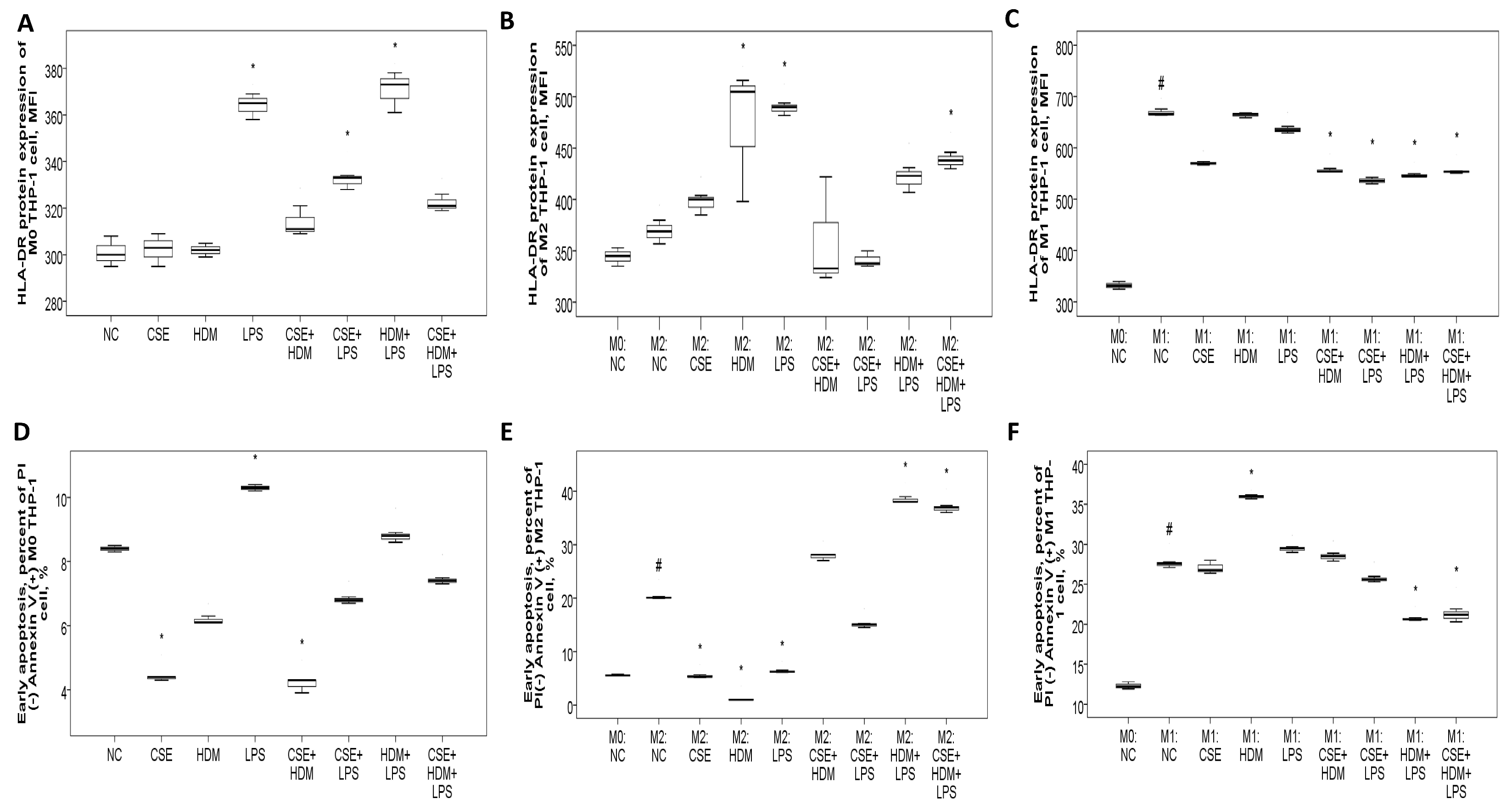
3.8.2. Increased HLA-DR Protein Expression with CSE, HDM, and LPS Mixture Stimuli in M2 Polarized THP-1 Cells or M1 Polarized THP-1 Cells with or Without Additional Stimuli
3.8.3. Apoptosis Proportions and ROS Production of THP-1 Cells Varied with Different Stimuli and at Different Polarization Statuses
3.8.4. Increased HLA-DRA Gene Expression in M0 THP-1 Cell and Increased HLA-DRB1 Gene Expression in M1 Polarized THP-1 Cell in Response to CSE Stimulus
4. Discussion
4.1. Overview of the Main Findings
4.2. Increased HLA-DR Expression May Play a Critical Role in Smoking, Allergy, and Microorganism-Related Airway Remodeling in the Circumference of M2 or M1 Polarization
4.3. Both External Stimuli and M1/M2 Polarization Status May Further Affect HLA-DR/-DQ Expressions and Immune Cell Maturation, Which in Turn Lead to Altered Apoptosis and Bactericidal Functions
4.4. Compatible with the Results in Previous Studies, ACO Patients Had More Frequent Exacerbations and Higher Symptom Burden than Pure COPD Patients in the Current Study
4.5. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COPD | chronic obstructive pulmonary disease |
| ACO | asthma and COPD overlap |
| HLA | human leukocyte antigen |
| MHC | major histocompatibility complex |
| FEV1 | forced expiratory volume in 1 s |
| FVC | forced vital capacity |
| BD | bronchodilator |
| 6MWT | 6-min walking test |
| RT-PCR | reverse transcriptase polymerase chain |
| IgE | immunoglobulin E |
| CSE | cigarette smoke extract |
| HDM | house dust mite |
| LPS | lipopolysaccharide |
| CAT | COPD assessment test |
| mMRC | Modified Medical Research Council |
| DLCO | diffusion capacity of carbon monoxide |
| MFI | mean fluorescence intensity |
| PI | propidium iodide |
| ROS | reactive oxygen species |
| VA | alveolar volume |
References
- Tu, X.; Donovan, C.; Kim, R.Y.; Wark, P.A.B.; Horvat, J.C.; Hansbro, P.M. Asthma-COPD overlap: Current understanding and the utility of experimental models. Eur. Respir. Rev. 2021, 30, 190185. [Google Scholar] [CrossRef]
- Mekov, E.; Nunez, A.; Sin, D.D.; Ichinose, M.; Rhee, C.K.; Maselli, D.J.; Cote, A.; Suppli Ulrik, C.; Maltais, F.; Anzueto, A.; et al. Update on Asthma-COPD Overlap (ACO): A Narrative Review. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1783–1799. [Google Scholar]
- Roman-Rodriguez, M.; Kaplan, A. GOLD 2021 Strategy Report: Implications for Asthma-COPD Overlap. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1709–1715. [Google Scholar]
- Fujino, N.; Sugiura, H. ACO (Asthma-COPD Overlap) Is Independent from COPD, a Case in Favor: A Systematic Review. Diagnostics 2021, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Abu Khweek, A.; Kim, E.; Joldrichsen, M.R.; Amer, A.O.; Boyaka, P.N. Insights Into Mucosal Innate Immune Responses in House Dust Mite-Mediated Allergic Asthma. Front. Immunol. 2020, 11, 534501. [Google Scholar] [CrossRef] [PubMed]
- Guo-Parke, H.; Linden, D.; Weldon, S.; Kidney, J.C.; Taggart, C.C. Mechanisms of Virus-Induced Airway Immunity Dysfunction in the Pathogenesis of COPD Disease, Progression, and Exacerbation. Front. Immunol. 2020, 11, 1205. [Google Scholar] [CrossRef]
- De Cunto, G.; Cavarra, E.; Bartalesi, B.; Lucattelli, M.; Lungarella, G. Innate Immunity and Cell Surface Receptors in the Pathogenesis of COPD: Insights from Mouse Smoking Models. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1143–1154. [Google Scholar] [CrossRef]
- Hikichi, M.; Hashimoto, S.; Gon, Y. Asthma and COPD overlap pathophysiology of ACO. Allergol. Int. 2018, 67, 179–186. [Google Scholar] [CrossRef]
- Liu, B.; Shao, Y.; Fu, R. Current research status of HLA in immune-related diseases. Immun. Inflamm. Dis. 2021, 9, 340–350. [Google Scholar] [PubMed]
- Carey, B.S.; Poulton, K.V.; Poles, A. Factors affecting HLA expression: A review. Int. J. Immunogenet. 2019, 46, 307–320. [Google Scholar]
- Plusa, T. Azithromycin in the treatment of patients with exacerbation of chronic obstructive pulmonary disease. Pol. Merkur. Lek. 2020, 48, 65–68. [Google Scholar]
- Diaz-Pena, R.; Silva, R.S.; Hosgood, H.D.; Jaime, S., 3rd; Miravitlles, M.; Olloquequi, J. HLA-DRB1 Alleles are Associated with COPD in a Latin American Admixed Population. Arch. Bronconeumol. (Engl. Ed.) 2021, 57, 291–297. [Google Scholar]
- Lee, Y.J.; Choi, S.; Kwon, S.Y.; Lee, Y.; Lee, J.K.; Heo, E.Y.; Chung, H.S.; Kim, D.K. A Genome-Wide Association Study in Early COPD: Identification of One Major Susceptibility Loci. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2967–2975. [Google Scholar] [CrossRef]
- Faner, R.; Nunez, B.; Sauleda, J.; Garcia-Aymerich, J.; Pons, J.; Crespi, C.; Mila, J.; Gonzalez, J.R.; Maria Anto, J.; Agusti, A. Group P-CS: HLA distribution in COPD patients. COPD 2013, 10, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nie, X.; Luo, Z.; Wei, B.; Teng, G. The Role of Human Leukocyte Antigen-DR in Regulatory T Cells in Patients with Virus-Induced Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Med. Sci. Monit. 2021, 27, e928051. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Ye, R.; Wang, C.; Bai, S.; Zhao, L. Identification of proteomic signatures associated with COPD frequent exacerbators. Life Sci. 2019, 230, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kubysheva, N.; Soodaeva, S.; Novikov, V.; Eliseeva, T.; Li, T.; Klimanov, I.; Kuzmina, E.; Baez-Medina, H.; Solovyev, V.; Ovsyannikov, D.Y.; et al. Soluble HLA-I and HLA-II Molecules Are Potential Prognostic Markers of Progression of Systemic and Local Inflammation in Patients with COPD. Dis. Markers 2018, 2018, 3614341. [Google Scholar] [CrossRef]
- Singh, D.; Fox, S.M.; Tal-Singer, R.; Bates, S.; Riley, J.H.; Celli, B. Altered gene expression in blood and sputum in COPD frequent exacerbators in the ECLIPSE cohort. PLoS ONE 2014, 9, e107381. [Google Scholar]
- Mahdi, B.M.; Al-Hadithi, A.T.R.; Raouf, H.; Zalzala, H.H.; Abid, L.A.; Nehad, Z. Effect of HLA on development of asthma. Ann. Med. Surg. 2018, 36, 118–121. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, L.; Li, J.; Jin, Y.; He, L. Association of HLA-DRB1 Gene Polymorphism with Risk of Asthma: A Meta-Analysis. Med. Sci. Monit. Basic Res. 2016, 22, 80–86. [Google Scholar]
- Pino-Yanes, M.; Corrales, A.; Acosta-Herrera, M.; Perez-Rodriguez, E.; Cumplido, J.; Campo, P.; Barreto-Luis, A.; Sanchez-Garcia, F.; Felipe, T.; Sanchez-Machin, I.; et al. HLA-DRB1*15:01 allele protects from asthma susceptibility. J. Allergy Clin. Immunol. 2014, 134, 1201–1203. [Google Scholar] [CrossRef]
- Kostara, M.; Chondrou, V.; Sgourou, A.; Douros, K.; Tsabouri, S. HLA Polymorphisms and Food Allergy Predisposition. J. Pediatr. Genet. 2020, 9, 77–86. [Google Scholar] [CrossRef]
- Kawasaki, A.; Hasebe, N.; Hidaka, M.; Hirano, F.; Sada, K.E.; Kobayashi, S.; Yamada, H.; Furukawa, H.; Yamagata, K.; Sumida, T.; et al. Protective Role of HLA-DRB1*13:02 against Microscopic Polyangiitis and MPO-ANCA-Positive Vasculitides in a Japanese Population: A Case-Control Study. PLoS ONE 2016, 11, e0154393. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.; et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef]
- Ma, P.; Li, S.; Yang, H.; Yuan, J.; Zhang, Z.; Li, X.; Fang, N.; Lin, M.; Hou, Q. Comparative RNA-Seq Transcriptome Analysis on Pulmonary Inflammation in a Mouse Model of Asthma-COPD Overlap Syndrome. Front. Cell Dev. Biol. 2021, 9, 628957. [Google Scholar] [CrossRef]
- Armitage, M.N.; Spittle, D.A.; Turner, A.M. A Systematic Review and Meta-Analysis of the Prevalence and Impact of Pulmonary Bacterial Colonisation in Stable State Chronic Obstructive Pulmonary Disease (COPD). Biomedicines 2021, 10, 81. [Google Scholar] [CrossRef]
- Joshi, I.; Carney, W.P.; Rock, E.P. Utility of monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis immunoparalysis. Front. Immunol. 2023, 14, 1130214. [Google Scholar] [CrossRef]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Boulet, L.P.; Reddel, H.K.; Bateman, E.; Pedersen, S.; FitzGerald, J.M.; O’Byrne, P.M. The Global Initiative for Asthma (GINA): 25 years later. Eur. Respir. J. 2019, 54, 1900598. [Google Scholar] [CrossRef]
- Sin, D.D.; Miravitlles, M.; Mannino, D.M.; Soriano, J.B.; Price, D.; Celli, B.R.; Leung, J.M.; Nakano, Y.; Park, H.Y.; Wark, P.A.; et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur. Respir. J. 2016, 48, 664–673. [Google Scholar] [CrossRef]
- Roos-Engstrand, E.; Ekstrand-Hammarstrom, B.; Pourazar, J.; Behndig, A.F.; Bucht, A.; Blomberg, A. Influence of smoking cessation on airway T lymphocyte subsets in COPD. COPD 2009, 6, 112–120. [Google Scholar]
- Peres, A.; Dorneles, G.P.; Dias, A.S.; Vianna, P.; Chies, J.A.B.; Monteiro, M.B. T-cell profile and systemic cytokine levels in overweight-obese patients with moderate to very-severe COPD. Respir. Physiol. Neurobiol. 2018, 247, 74–79. [Google Scholar] [CrossRef]
- Burchiel, S.W.; Lauer, F.T.; Factor-Litvak, P.; Liu, X.; Santella, R.M.; Islam, T.; Eunus, M.; Alam, N.; Islam, T.; Rahman, M.; et al. An increase in circulating B cells and B cell activation markers in peripheral blood is associated with cigarette smoking in a male cohort in Bangladesh. Toxicol. Appl. Pharmacol. 2019, 384, 114783. [Google Scholar] [CrossRef]
- Holownia, A.; Wielgat, P.; Stasiak-Barmuta, A.; Kwolek, A.; Jakubow, P.; Szepiel, P.; Chyczewska, E.; Braszko, J.J.; Mroz, R.M. Tregs and HLA-DR expression in sputum cells of COPD patients treated with tiotropium and formoterol. Adv. Exp. Med. Biol. 2015, 839, 7–12. [Google Scholar]
- Li, H.; Yang, T.; Ning, Q.; Li, F.; Chen, T.; Yao, Y.; Sun, Z. Cigarette smoke extract-treated mast cells promote alveolar macrophage infiltration and polarization in experimental chronic obstructive pulmonary disease. Inhal. Toxicol. 2015, 27, 822–831. [Google Scholar]
- Jiang, Y.; Zhao, Y.; Wang, Q.; Chen, H.; Zhou, X. Fine particulate matter exposure promotes M2 macrophage polarization through inhibiting histone deacetylase 2 in the pathogenesis of chronic obstructive pulmonary disease. Ann. Transl. Med. 2020, 8, 1303. [Google Scholar] [CrossRef]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. 2018, 191, 1–14. [Google Scholar] [CrossRef]
- Jiang, P.; Li, X. Regulatory Mechanism of lncRNAs in M1/M2 Macrophages Polarization in the Diseases of Different Etiology. Front. Immunol. 2022, 13, 835932. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, Y.; Li, F.; Hao, Y.; Kong, Y.; Chen, C.; Hao, X.; Han, D.; Li, G.; Wang, Z.; et al. Phenotypic and functional alterations of monocyte subsets with aging. Immun. Ageing 2022, 19, 63. [Google Scholar] [CrossRef]
- Deshane, J.S.; Redden, D.T.; Zeng, M.; Spell, M.L.; Zmijewski, J.W.; Anderson, J.T.; Deshane, R.J.; Gaggar, A.; Siegal, G.P.; Abraham, E.; et al. Subsets of airway myeloid-derived regulatory cells distinguish mild asthma from chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015, 135, 413–424.e5. [Google Scholar] [CrossRef][Green Version]
- Zou, L.; Chen, K.; Hong, X.; Ye, B. Single-cell RNA sequencing reveals immunological link between house dust mite allergy and childhood asthma. Sci. Rep. 2025, 15, 16812. [Google Scholar] [CrossRef]
- Hasan, A.; Al-Ozairi, E.; Hassan, N.Y.M.; Ali, S.; Ahmad, R.; Al-Shatti, N.; Alshemmari, S.; Al-Mulla, F. Fatal COVID-19 is Associated with Reduced HLA-DR, CD123 or CD11c Expression on Circulating Dendritic Cells. J. Inflamm. Res. 2022, 15, 5665–5675. [Google Scholar] [CrossRef]
- Bourdin, A.; Ahmed, E.; Vachier, I.; Roche, N.; Pissarra, J.; Malafaye, N.; Molinari, N. Hospitalizations for Chronic Obstructive Pulmonary Disease Exacerbation During COVID-19. JAMA Netw. Open 2024, 7, e2412383. [Google Scholar] [CrossRef]
- Casasola-LaMacchia, A.; Ritorto, M.S.; Seward, R.J.; Ahyi-Amendah, N.; Ciarla, A.; Hickling, T.P.; Neubert, H. Human leukocyte antigen class II quantification by targeted mass spectrometry in dendritic-like cell lines and monocyte-derived dendritic cells. Sci. Rep. 2021, 11, 1028. [Google Scholar] [CrossRef]
- Leung, C.; Sin, D.D. Asthma-COPD Overlap: What Are the Important Questions? Chest 2022, 161, 330–344. [Google Scholar] [CrossRef]
- Peng, J.; Wang, M.; Wu, Y.; Shen, Y.; Chen, L. Clinical Indicators for Asthma-COPD Overlap: A Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2567–2575. [Google Scholar] [CrossRef]
- Cabrera Lopez, C.; Sanchez Santos, A.; Lemes Castellano, A.; Cazorla Rivero, S.; Brena Atienza, J.; Gonzalez Davila, E.; Celli, B.; Casanova Macario, C. Eosinophil Subtypes in Adults with Asthma and Adults with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2023, 208, 155–162. [Google Scholar] [CrossRef]

| Healthy Subjects N = 18 | COPD Only N = 41 | Asthma and COPD Overlap N = 37 | Asthma Only N = 20 | p Value | |
|---|---|---|---|---|---|
| Age, years | 65 ± 11.4 | 70.7 ± 11.2 | 68.8 ± 9.8 | 60 ± 14.4 | 0.006 |
| Male Gender, n (%) | 12 (66.7) | 35 (85.4) | 36 (97.3) | 8 (40) | <0.001 |
| Cigarette smoke, pack-years | 5.1 ± 8.4 | 38.4 ± 25.1 | 40.7 ± 24.5 | 1.8 ± 5.6 | <0.001 |
| Smoke history, n (%) | <0.001 | ||||
| Current heavy smoker | 0 (0) | 21 (51.2) | 25 (67.6) | 0 (0) | |
| Remote heavy smoker | 6 (33.3) | 20 (48.8) | 12 (32.4) | 0 (0) | |
| Charlson Comorbidity Index | 3.9 ± 1.2 | 5.6 ± 2.5 | 5.2 ± 1.8 | 4.0 ± 2.1 | 0.005 |
| COPD assessment test (CAT) | 7.5 ± 6.7 | 13.1 ± 7.2 | 17.1 ± 7.4 | 16.3 ± 9.9 | <0.001 |
| mMRC, point | 0.6 ± 0.9 | 1.3 ± 0.8 | 1.3 ± 0.9 | 1.1 ± 0.7 | 0.036 |
| Absolute eosinophil count, cells/ml | 113.4 ± 57.5 | 213.2 ± 118 | 295.5 ± 214.9 | 311.1 ± 232.1 | 0.001 |
| Post-BD FEV1/FVC, % predicted | 83.1 ± 5.1 | 67.8 ± 12.5 | 66.3 ± 11.5 | 78.7 ± 11.2 | <0.001 |
| Post-BD FEV1, % predicted | 104.8 ± 11.1 | 75.2 ± 23.5 | 73.5 ± 21.3 | 90.4 ± 22.4 | <0.001 |
| Post-BD FEF25–75%, % predicted | 94.2 ± 25.3 | 44.5 ± 24.1 | 42.8 ± 21.5 | 71.2 ± 37.4 | <0.001 |
| DLCO, % predicted | 82.4 ± 18.2 | 63.6 ± 23.1 | 66.5 ± 19.7 | 74 ± 20.3 | 0.032 |
| Alveolar Volume (VA), % predicted | 82.4 ± 10.9 | 64 ± 22.1 | 73.2 ± 14 | 80.3 ± 17.6 | 0.003 |
| Exacerbation events/year, n | 0.5 ± 0.8 | 2.3 ± 1.5 | 3.8 ± 1.8 | 2.6 ± 1.9 | <0.001 |
| Mild exacerbation, n | 0.5 ± 0.8 | 1.9 ± 1.1 | 2.4 ± 1.1 | 1.8 ± 1.2 | <0.001 |
| Moderate, n | 0 ± 0 | 0.3 ± 0.6 | 1.2 ± 1.1 | 0.8 ± 1 | <0.001 |
| Severe, n | 0 ± 0 | 0 ± 0.2 | 0.2 ± 0.5 | 0 ± 0 | 0.044 |
| HLA-DR on Th Cell, MFI | HLA-DR on Tc Cell, MFI | HLA-DR on M2a Monocyte, MFI | HLA-DR on M1 Monocyte, MFI | HLA-DR on B Cell, MFI | HLA-DR on Neutrophil, MFI | |
|---|---|---|---|---|---|---|
| Age, years | 12.6 (−2.76 to 27.96) | 4.8 (−4.04 to 13.64) | 61.37 * (11.56 to 111.18) | 113.73 (−0.29 to 227.76) | −39.82 (−186.7 to 107.05) | 0.54 (−21.89 to 22.98) |
| Sex | 328.15 (−75.66 to 731.98) | −8.58 (−240.99 to 223.81) | 1178.54 (−300 to 2657.1) | −157.23 (−3153.5 to 2839.0) | 1233.59 (−2625.9 to 5093.1) | 363.28 (−226.38 to 952.84) |
| Smoking exposure, pack-years | 3.0 (−3.41 to 9.42) | 2.04 (−1.65 to 5.73) | 29.3 * (7.57 to 51.03) | 22.7 (−24.91 to 70.32) | 68.29 * (6.95 to 129.63) | 11.18 * (2.78 to 19.58) |
| Charlson comorbidity index | 27.33 (−54.36 to 109.02) | 23.24 (−23.77 to 70.26) | −73.61 (−372.7 to 225.5) | −104.79 (−710.9 to 501.3) | −111.88 (−892.68 to 668.91) | 3.48 (−115.81 to 122.77) |
| Medicine | COPD Patients, n = 14 | ACO Patients, n = 16 | p Value |
|---|---|---|---|
| Dual bronchodilator | 9 (64.3%) | 4 (25%) | 0.03 |
| Triple therapy | 5 (35.7%) | 12 (75%) | 0.03 |
| Oral corticosteroid | 2 (14.3%) | 3 (18.8%) | 0.743 |
| theophylline | 7 (50%) | 10 (62.5%) | 0.491 |
| macrolide | 2 (14.3%) | 3 (18.8%) | 0.743 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Huang, K.-T.; Lee, C.-P.; Hsu, P.-Y.; Chang, Y.-P.; Wu, C.-C.; Leung, S.-Y.; Hsiao, C.-C.; Lin, M.-C. Increased HLA-DR Expression on M2a Monocytes and Helper T Cells in Patients with COPD and Asthma–COPD Overlap Contributes to Disease Severity via Apoptosis and ROS. Antioxidants 2025, 14, 1507. https://doi.org/10.3390/antiox14121507
Chen Y-C, Huang K-T, Lee C-P, Hsu P-Y, Chang Y-P, Wu C-C, Leung S-Y, Hsiao C-C, Lin M-C. Increased HLA-DR Expression on M2a Monocytes and Helper T Cells in Patients with COPD and Asthma–COPD Overlap Contributes to Disease Severity via Apoptosis and ROS. Antioxidants. 2025; 14(12):1507. https://doi.org/10.3390/antiox14121507
Chicago/Turabian StyleChen, Yung-Che, Kuo-Tung Huang, Chiu-Ping Lee, Po-Yuan Hsu, Yu-Ping Chang, Chao-Chien Wu, Sum-Yee Leung, Chang-Chun Hsiao, and Meng-Chih Lin. 2025. "Increased HLA-DR Expression on M2a Monocytes and Helper T Cells in Patients with COPD and Asthma–COPD Overlap Contributes to Disease Severity via Apoptosis and ROS" Antioxidants 14, no. 12: 1507. https://doi.org/10.3390/antiox14121507
APA StyleChen, Y.-C., Huang, K.-T., Lee, C.-P., Hsu, P.-Y., Chang, Y.-P., Wu, C.-C., Leung, S.-Y., Hsiao, C.-C., & Lin, M.-C. (2025). Increased HLA-DR Expression on M2a Monocytes and Helper T Cells in Patients with COPD and Asthma–COPD Overlap Contributes to Disease Severity via Apoptosis and ROS. Antioxidants, 14(12), 1507. https://doi.org/10.3390/antiox14121507








