Extracellular Vesicle-Mediated Delivery of Antioxidant Enzymes: Emerging Insights and Translational Opportunities
Abstract
1. Introduction
2. Antioxidant Enzymes (AOEs) in EVs
2.1. Superoxide Dismutases (SOD1/2/3)
2.2. Catalase
2.3. Peroxiredoxins (PRDXs)
2.4. Glutathione Peroxidases (GPXs)
2.5. Glutathione System Enzymes and Glutathione S-Transferase (GST)
2.6. Thioredoxin (TXN) and Related Enzymes
2.7. Additional Redox-Active Enzymes
2.8. Functional Evidence of Enzymatic Activity
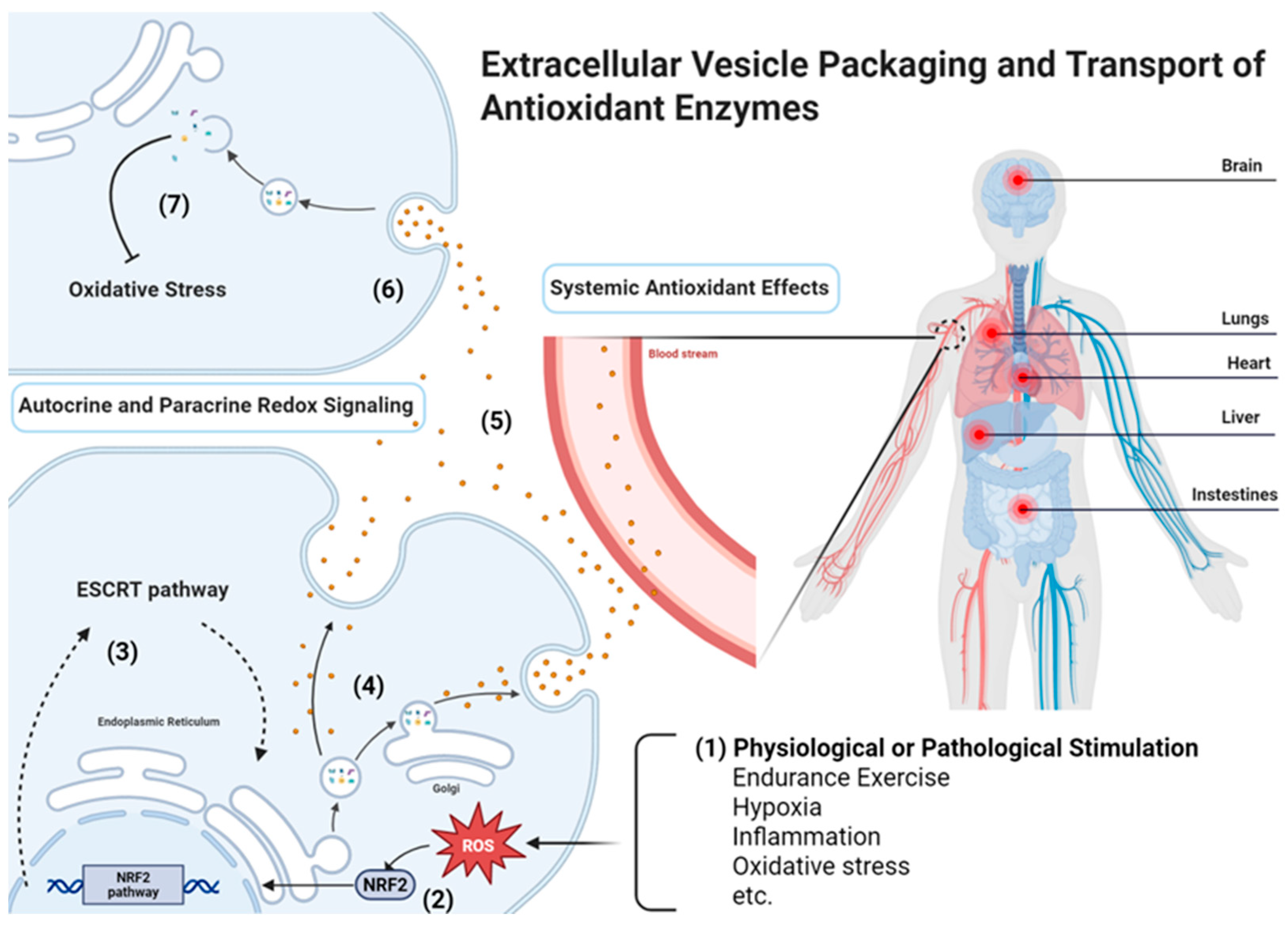
3. Mechanisms of Antioxidant Enzymes (AOEs) Packaging and Transfer
3.1. Cellular Origins of Antioxidant Enzymes (AOEs) in EVs
3.2. Passive Incorporation Versus Stress-Induced Enrichment
3.3. Sorting Pathways Influencing Antioxidant Enzymes (AOEs) Loading
3.4. Uptake Mechanisms in Recipient Cells
3.5. Functional Evidence of Enzyme Transfer
3.6. Outstanding Questions and Knowledge Gaps
4. Functional Roles of EV-Derived Antioxidant Enzymes (AOEs)
4.1. Autocrine and Paracrine Redox Signaling
4.2. Systemic Antioxidant Effects
5. Therapeutic Applications
5.1. Natural EVs
5.1.1. Stem Cell-Derived EVs
5.1.2. Immune Cell-Derived EVs
5.1.3. Plant-Derived EVs
5.1.4. Milk-Derived EVs
5.2. Engineered EVs
5.3. Nanozymes
5.4. Diagnostic Value of Antioxidant Enzymes (AOEs) in EVs
6. Challenges and Limitations
6.1. Large-Scale Production and Quality Standards
6.2. Enzyme Loading Efficiency and Activity Preservation
6.3. Stability and Storage Challenges
6.4. Targeted Delivery and Tissue Penetration
6.5. Analytical Characterization and Quality Control
6.6. Regulatory and Clinical Translation
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOE | Antioxidant enzyme |
| AML12 | Alpha mouse liver 12 cells |
| ALS | Amyotrophic lateral sclerosis |
| DFSCs | Dental follicle stem cells |
| ESCRT | Endosomal sorting complex required for transport |
| EV | Extracellular vesicle |
| ExT | Exercise training |
| EEx | Endurance exercise |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GMP | Good Manufacturing Practice |
| GPX | Glutathione peroxidase |
| GSH | Glutathione |
| GSR | Glutathione reductase |
| GST | Glutathione S-transferase |
| GSSG | Oxidized glutathione |
| H2O2 | Hydrogen peroxide |
| HMOX1 | Heme oxygenase 1 |
| hUC-MSC | Human umbilical cord–derived mesenchymal stem cell |
| hMNCs | Human mononu-clear cells |
| HDF | Human Dermal Fibroblast |
| HIIT | High-intensity interval training |
| LPS | Lipopolysaccharide |
| MASLD | Metabolic dysfunction–associated steatotic liver disease |
| MEV | Milk-derived extracellular vesicle |
| Mn-SOD | Manganese superoxide dismutase |
| MOF | Metal–organic framework |
| MSC | Mesenchymal stem cell |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NQO1 | NAD(P)H dehydrogenase [quinone] 1 |
| O2−• | Superoxide anion |
| OR | Oxidative stress |
| PRDX | Peroxiredoxin |
| PDLSCs | Periodontal ligament stem cells |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SOD1 | Cytosolic Cu/Zn-superoxide dismutase |
| SOD2 | Mitochondrial Mn-superoxide dismutase |
| SOD3 | Extracellular superoxide dismutase |
| TRXNRD1 | Thioredoxin reductase 1 |
| TXN | Thioredoxin |
| TXNRO | Thioredoxin oxidase |
References
- Lennicke, C.; Cocheme, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Zhou, N.J.; Bao, W.Q.; Zhang, C.F.; Jiang, M.L.; Liang, T.L.; Ma, G.Y.; Liu, L.; Pan, H.D.; Li, R.Z. Immunometabolism and oxidative stress: Roles and therapeutic strategies in cancer and aging. NPJ Aging 2025, 11, 59. [Google Scholar] [CrossRef]
- Stojanovic, B.; Jovanovic, I.; Dimitrijevic Stojanovic, M.; Stojanovic, B.S.; Kovacevic, V.; Radosavljevic, I.; Jovanovic, D.; Miletic Kovacevic, M.; Zornic, N.; Arsic, A.A.; et al. Oxidative Stress-Driven Cellular Senescence: Mechanistic Crosstalk and Therapeutic Horizons. Antioxidants 2025, 14, 987. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm (2020) 2025, 6, e70268. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef]
- Uti, D.E.; Atangwho, I.J.; Alum, E.U.; Ntaobeten, E.; Obeten, U.N.; Bawa, I.; Agada, S.A.; Ukam, C.I.; Egbung, G.E. Antioxidants in cancer therapy mitigating lipid peroxidation without compromising treatment through nanotechnology. Discov. Nano 2025, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xia, M.; Salas, S.S.; Trillos-Almanza, M.C.; Aguilar, M.M.; Arroyave-Ospina, J.C.; Wang, J.; Arrese, M.; Sydor, S.; Bechmann, L.P.; et al. Extracellular vesicles in metabolic dysfunction associated fatty liver disease: Mechanisms, diagnostic and therapeutic implications. Explor. Dig. Dis. 2022, 4–20. [Google Scholar] [CrossRef]
- Wang, J.; Lei, J.; Harmsen, M.C.; Moshage, H. From cooperation to collapse: Systemic failure in liver disease through a sociological lens. Explor. Dig. Dis. 2025, 4, 100580. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Sagini, K.; Selvarajah, M.; Romero, S.; Bassols-Citores, E.; Martin-Gracia, B.; Ramirez-Garrastacho, M.; Rise, F.; Endzelins, E.; Sadovska, L.; Skorinkina, D.; et al. The Redox Enzyme Thioredoxin Is Increased in Plasma Extracellular Vesicles From Endurance-Trained Females in Response to Acute Exercise. FASEB J. 2025, 39, e70927. [Google Scholar] [CrossRef]
- Lisi, V.; Senesi, G.; Balbi, C. Converging protective pathways: Exploring the linkage between physical exercise, extracellular vesicles and oxidative stress. Free Radic. Biol. Med. 2023, 208, 718–727. [Google Scholar] [CrossRef]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramirez, R.M. Microvesicles: ROS scavengers and ROS producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, X.; Chang, L.; Liu, B.; Zhang, M.; Mao, Y.; Shen, X. Exosomes Derived from Rejuvenated Stem Cells Inactivate NLRP3 Inflammasome and Pyroptosis of Nucleus Pulposus Cells via the Transfer of Antioxidants. Tissue Eng. Regen. Med. 2024, 21, 1061–1077. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Romero-Garcia, N.; Huete-Acevedo, J.; Dromant, M.; Borras, C. Small extracellular vesicles from senescent stem cells trigger adaptive mechanisms in young stem cells by increasing antioxidant enzyme expression. Redox Biol. 2023, 62, 102668. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Liu, L.; Huo, F.; Guo, S.; Tian, W. Lipopolysaccharide-Preconditioned Dental Follicle Stem Cells Derived Small Extracellular Vesicles Treating Periodontitis via Reactive Oxygen Species/Mitogen-Activated Protein Kinase Signaling-Mediated Antioxidant Effect. Int. J. Nanomed. 2022, 17, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Claridge, B.; Rai, A.; Fang, H.; Matsumoto, A.; Luo, J.; McMullen, J.R.; Greening, D.W. Proteome characterisation of extracellular vesicles isolated from heart. Proteomics 2021, 21, e2100026. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Mastrototaro, L.; Hartwig, S.; Pesta, D.; Strassburger, K.; de Filippo, E.; Jelenik, T.; Karusheva, Y.; Gancheva, S.; Markgraf, D.; et al. Metabolic responsiveness to training depends on insulin sensitivity and protein content of exosomes in insulin-resistant males. Sci. Adv. 2021, 7, eabi9551. [Google Scholar] [CrossRef] [PubMed]
- McIlvenna, L.C.; Whitham, M. Exercise, healthy ageing, and the potential role of small extracellular vesicles. J. Physiol. 2023, 601, 4937–4951. [Google Scholar] [CrossRef]
- Vasam, G.; Nadeau, R.; Cadete, V.J.J.; Lavallee-Adam, M.; Menzies, K.J.; Burelle, Y. Proteomics characterization of mitochondrial-derived vesicles under oxidative stress. FASEB J. 2021, 35, e21278. [Google Scholar] [CrossRef]
- Garaeva, L.; Tolstyko, E.; Putevich, E.; Kil, Y.; Spitsyna, A.; Emelianova, S.; Solianik, A.; Yastremsky, E.; Garmay, Y.; Komarova, E.; et al. Microalgae-Derived Vesicles: Natural Nanocarriers of Exogenous and Endogenous Proteins. Plants 2025, 14, 2354. [Google Scholar] [CrossRef]
- Lisi, V.; Moulton, C.; Fantini, C.; Grazioli, E.; Guidotti, F.; Sgro, P.; Dimauro, I.; Capranica, L.; Parisi, A.; Di Luigi, L.; et al. Steady-state redox status in circulating extracellular vesicles: A proof-of-principle study on the role of fitness level and short-term aerobic training in healthy young males. Free Radic. Biol. Med. 2023, 204, 266–275. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019, 33, 1695–1710. [Google Scholar] [CrossRef]
- Haque, S.; Kodidela, S.; Sinha, N.; Kumar, P.; Cory, T.J.; Kumar, S. Differential packaging of inflammatory cytokines/ chemokines and oxidative stress modulators in U937 and U1 macrophages-derived extracellular vesicles upon exposure to tobacco constituents. PLoS One 2020, 15, e0233054. [Google Scholar] [CrossRef]
- Yang, J.W.; Seo, Y.; Shin, T.H.; Ahn, J.S.; Oh, S.J.; Shin, Y.Y.; Kang, M.J.; Lee, B.C.; Lee, S.; Kang, K.S.; et al. Extracellular Vesicles from SOD3-Transduced Stem Cells Exhibit Improved Immunomodulatory Abilities in the Murine Dermatitis Model. Antioxidants 2020, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Xing, H.; Cao, S.; Long, X.; Liu, H.; Ma, J.; Guo, F.; Deng, Z.; Liu, X. Neutrophils restrain sepsis associated coagulopathy via extracellular vesicles carrying superoxide dismutase 2 in a murine model of lipopolysaccharide induced sepsis. Nat. Commun. 2022, 13, 4583. [Google Scholar] [CrossRef] [PubMed]
- Abdelsaid, K.; Sudhahar, V.; Harris, R.A.; Das, A.; Youn, S.W.; Liu, Y.; McMenamin, M.; Hou, Y.; Fulton, D.; Hamrick, M.W.; et al. Exercise improves angiogenic function of circulating exosomes in type 2 diabetes: Role of exosomal SOD3. FASEB J. 2022, 36, e22177. [Google Scholar] [CrossRef]
- Iversen, M.B.; Gottfredsen, R.H.; Larsen, U.G.; Enghild, J.J.; Praetorius, J.; Borregaard, N.; Petersen, S.V. Extracellular superoxide dismutase is present in secretory vesicles of human neutrophils and released upon stimulation. Free Radic. Biol. Med. 2016, 97, 478–488. [Google Scholar] [CrossRef]
- Haque, S.; Sinha, N.; Ranjit, S.; Midde, N.M.; Kashanchi, F.; Kumar, S. Monocyte-derived exosomes upon exposure to cigarette smoke condensate alter their characteristics and show protective effect against cytotoxicity and HIV-1 replication. Sci. Rep. 2017, 7, 16120. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.J.; Tian, C.; Zucker, I.H. Skeletal Muscle Nrf2 Contributes to Exercise-Evoked Systemic Antioxidant Defense Via Extracellular Vesicular Communication. Exerc. Sport. Sci. Rev. 2021, 49, 213–222. [Google Scholar] [CrossRef]
- Jeong, I.; Lee, J.; Park, S.J.; Kim, O.K. High fat diet enhances catalase loading into adipose tissue derived extracellular vesicles with limited effect on oxidative stress. Sci. Rep. 2025, 15, 31010. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Nanovesicles from Organic Agriculture-Derived Fruits and Vegetables: Characterization and Functional Antioxidant Content. Int. J. Mol. Sci. 2021, 22, 8170. [Google Scholar] [CrossRef]
- Di Raimo, R.; Mizzoni, D.; Aloi, A.; Pietrangelo, G.; Dolo, V.; Poppa, G.; Fais, S.; Logozzi, M. Antioxidant Effect of a Plant-Derived Extracellular Vesicles’ Mix on Human Skin Fibroblasts: Induction of a Reparative Process. Antioxidants 2024, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Shiri, E.; Azadian, Z.; Piryaei, F.; Jalali, A.; Nouri, F.; Dalirfardouei, R. Preparation of Allium cepa-derived exosome-like nanovesicles and their anti-inflammatory potential in a skin wound healing mouse model. Mol. Biol. Rep. 2025, 52, 769. [Google Scholar] [CrossRef] [PubMed]
- Lisi, V.; Senesi, G.; Bertola, N.; Pecoraro, M.; Bolis, S.; Gualerzi, A.; Picciolini, S.; Raimondi, A.; Fantini, C.; Moretti, E.; et al. Plasma-derived extracellular vesicles released after endurance exercise exert cardioprotective activity through the activation of antioxidant pathways. Redox Biol. 2023, 63, 102737. [Google Scholar] [CrossRef]
- Deng, J.; Li, W.; Wang, Z.; Zeng, J.; Cai, Q. KatB, a bacterial extracellular vesicles (EVs)-secreted catalase, detoxifies reactive oxygen species (ROS) and promotes pathogen proliferation in plants. J. Integr. Plant Biol. 2025, 67, 1928–1946. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Eguchi, A.; Tamai, Y.; Fukuda, S.; Tempaku, M.; Izuoka, K.; Iwasa, M.; Takei, Y.; Togashi, K. Protein Composition of Circulating Extracellular Vesicles Immediately Changed by Particular Short Time of High-Intensity Interval Training Exercise. Front. Physiol. 2021, 12, 693007. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mahairaki, V.; Bai, H.; Ding, Z.; Li, J.; Witwer, K.W.; Cheng, L. Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. Stem Cells 2019, 37, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Warnier, G.; van Doorslaer de Ten Ryen, S.; Lannoy, C.; Mahy, T.; Antoine, N.; Boyer, E.; Kienlen-Campard, P.; Verboven, K.; Copine, S.; Francaux, M.; et al. Effect of a 12-Week Endurance Training Program on Circulating Extracellular Vesicle Proteome in Sedentary Adults With Obesity. J. Extracell. Biol. 2025, 4, e70087. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Li, F.; Huang, Q.; Huang, X.; Maj, T. Macrophages and macrophage extracellular vesicles confer cancer ferroptosis resistance via PRDX6-mediated mitophagy inhibition. Redox Biol. 2025, 86, 103826. [Google Scholar] [CrossRef]
- Huang, J.; Li, S.; Yang, Y.; Li, C.; Zuo, Z.; Zheng, R.; Chai, J.; Jiang, S. GPX5-Enriched Exosomes Improve Sperm Quality and Fertilization Ability. Int. J. Mol. Sci. 2024, 25, 569. [Google Scholar] [CrossRef]
- Lei, F.J.; Chiang, J.Y.; Chang, H.J.; Chen, D.C.; Wang, H.L.; Yang, H.A.; Wei, K.Y.; Huang, Y.C.; Wang, C.C.; Wei, S.T.; et al. Cellular and exosomal GPx1 are essential for controlling hydrogen peroxide balance and alleviating oxidative stress in hypoxic glioblastoma. Redox Biol. 2023, 65, 102831. [Google Scholar] [CrossRef]
- Chong, M.C.; Silva, A.; James, P.F.; Wu, S.S.X.; Howitt, J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD(+) activity in recipient cells. Aging Cell 2022, 21, e13647. [Google Scholar] [CrossRef]
- Bryl-Gorecka, P.; Sathanoori, R.; Al-Mashat, M.; Olde, B.; Jogi, J.; Evander, M.; Laurell, T.; Erlinge, D. Effect of exercise on the plasma vesicular proteome: A methodological study comparing acoustic trapping and centrifugation. Lab. Chip 2018, 18, 3101–3111. [Google Scholar] [CrossRef]
- Glorieux, C.; Buc Calderon, P. Targeting catalase in cancer. Redox Biol. 2024, 77, 103404. [Google Scholar] [CrossRef]
- Hayes, S.H.; Liu, Q.; Selvakumaran, S.; Haney, M.J.; Batrakova, E.V.; Allman, B.L.; Walton, P.A.; Kiser, P.; Whitehead, S.N. Brain Targeting and Toxicological Assessment of the Extracellular Vesicle-Packaged Antioxidant Catalase-SKL Following Intranasal Administration in Mice. Neurotox. Res. 2021, 39, 1418–1429. [Google Scholar] [CrossRef]
- Wang, W.; Qiao, S.; Kong, X.; Zhang, G.; Cai, Z. The role of exosomes in immunopathology and potential therapeutic implications. Cell Mol. Immunol. 2025, 22, 975–995. [Google Scholar] [CrossRef]
- Yu, J.; Sane, S.; Kim, J.E.; Yun, S.; Kim, H.J.; Jo, K.B.; Wright, J.P.; Khoshdoozmasouleh, N.; Lee, K.; Oh, H.T.; et al. Biogenesis and delivery of extracellular vesicles: Harnessing the power of EVs for diagnostics and therapeutics. Front. Mol. Biosci. 2023, 10, 1330400. [Google Scholar] [CrossRef]
- Pust, S.; Brech, A.; Wegner, C.S.; Stenmark, H.; Haglund, K. Vesicle-mediated transport of ALIX and ESCRT-III to the intercellular bridge during cytokinesis. Cell Mol. Life Sci. 2023, 80, s00018–s00023. [Google Scholar] [CrossRef]
- Stoten, C.L.; Carlton, J.G. ESCRT-dependent control of membrane remodelling during cell division. Semin. Cell Dev. Biol. 2018, 74, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Karkossa, I.; Furst, S.; Grosskopf, H.; von Bergen, M.; Schubert, K. Oxidation is an underappreciated post-translational modification in the regulation of immune responses associated with changes in phosphorylation. Front. Immunol. 2023, 14, 1244431. [Google Scholar] [CrossRef] [PubMed]
- Vrettou, S.; Wirth, B. S-Glutathionylation and S-Nitrosylation in Mitochondria: Focus on Homeostasis and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 15849. [Google Scholar] [CrossRef]
- Kwok, Z.H.; Wang, C.; Jin, Y. Extracellular Vesicle Transportation and Uptake by Recipient Cells: A Critical Process to Regulate Human Diseases. Processes 2021, 9, 273. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Rubinstein, E.; Thery, C.; Zimmermann, P. Tetraspanins affect membrane structures and the trafficking of molecular partners: What impact on extracellular vesicles? Biochem. Soc. Trans. 2025, 0, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Czosseck, A.; Chen, M.M.; Hsu, C.C.; Shamrin, G.; Meeson, A.; Oldershaw, R.; Nguyen, H.; Livkisa, D.; Lundy, D.J. Extracellular vesicles from human cardiac stromal cells up-regulate cardiomyocyte protective responses to hypoxia. Stem Cell Res. Ther. 2024, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, J.; Zhang, H.; Zheng, W.; Yang, Y. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging 2021, 13, 21642–21658. [Google Scholar] [CrossRef]
- Pistono, C.; Bister, N.; Stanova, I.; Malm, T. Glia-Derived Extracellular Vesicles: Role in Central Nervous System Communication in Health and Disease. Front. Cell Dev. Biol. 2020, 8, 623771. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, B.; Charoenthanakitkul, D.; Suntornnont, N.; Ariyachet, C.; Tangkijvanich, P. Extracellular vesicles in metabolic dysfunction-associated steatotic liver disease: From intercellular signaling to clinical translation. World J. Hepatol. 2025, 17, 108259. [Google Scholar] [CrossRef]
- Liu, G.; Yin, X.M. The Role of Extracellular Vesicles in Liver Pathogenesis. Am. J. Pathol. 2022, 192, 1358–1367. [Google Scholar] [CrossRef]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; van den Berg, E.H.; Gansevoort, R.T.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; et al. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Buist-Homan, M.; Harmsen, M.C.; Moshage, H. Extracellular vesicle-dependent crosstalk between hepatic stellate cells and Kupffer cells promotes their mutual activation. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167914. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, J.; Serna-Salas, S.A.; Villanueva, A.H.; Buist-Homan, M.; Arrese, M.; Olinga, P.; Blokzijl, H.; Moshage, H. Hepatic stellate cells induce an inflammatory phenotype in Kupffer cells via the release of extracellular vesicles. J. Cell Physiol. 2023, 238, 2293–2303. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Xia, M.; Salas, S.S.; Ospina, J.A.; Buist-Homan, M.; Harmsen, M.C.; Moshage, H. Extracellular vesicles derived from liver sinusoidal endothelial cells inhibit the activation of hepatic stellate cells and Kupffer cells in vitro. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167020. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, M.; Wang, J.; Aguilar, M.M.; Buist-Homan, M.; Moshage, H. Extracellular vesicles originating from steatotic hepatocytes promote hepatic stellate cell senescence via AKT/mTOR signaling. Cell Biochem. Funct. 2024, 42, e4077. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, T.; Lu, Y.; Zhao, W.; Zhang, J.; Chen, Q.; Ge, G.; Hua, Y.; Chen, K.; Ullah, I.; et al. Exosomal thioredoxin-1 from hypoxic human umbilical cord mesenchymal stem cells inhibits ferroptosis in doxorubicin-induced cardiotoxicity via mTORC1 signaling. Free Radic. Biol. Med. 2022, 193, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.a.; Garin, J.m.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles1. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Li, W.; Chang, C.J. Anti-inflammatory and antioxidative effects of Perilla frutescens-derived extracellular vesicles: Insights from Zebrafish models. Mol. Immunol. 2025, 182, 126–138. [Google Scholar] [CrossRef]
- Xu, R.; Lu, Y.; Cai, L.; Zhang, L. Utilizing Extracellular Vesicles from Phaeodactylum tricornutum as a Novel Approach for Protecting the Skin from Oxidative Damage. ACS Biomater. Sci. Eng. 2025, 11, 3400–3415. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, X.; Xie, Y.; Yang, Z.; Spanos, M.; Guo, Z.; Jin, Y.; Li, G.; Lei, Z.; Schiffelers, R.M.; et al. GEV (Sod2) Powder: A Modified Product Based on Biovesicles Functioned in Air Pollution PM2.5-Induced Cardiopulmonary Injury. Research 2025, 8, 0609. [Google Scholar] [CrossRef]
- Gaweł, P.; Karcz, K.; Zaręba-Wdowiak, N.; Królak-Olejnik, B. Antioxidant Capacity of Colostrum of Mothers with Gestational Diabetes Mellitus-A Cross-Sectional Study. Nutrients 2025, 17, 3324. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, S.; Liu, Q.; Huang, C.; Hao, H.; Tan, M.S.; Yu, X.; Lou, C.K.L.; Huang, R.; Zhang, Z.; et al. Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis. Sci. Adv. 2023, 9, eade5041. [Google Scholar] [CrossRef]
- Muttiah, B.; Law, J.X. Milk-derived extracellular vesicles and gut health. NPJ Sci. Food 2025, 9, 12. [Google Scholar] [CrossRef]
- Kong, C.; Huang, L.B.; Yang, M.F.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Wang, Y.H.; Wei, D.R.; Shi, R.Y.; Liang, Y.J.; et al. Milk-derived extracellular vesicles: Nature’s nanocarriers for drug delivery and therapeutics. Front. Pharmacol. 2025, 16, 1595891. [Google Scholar] [CrossRef]
- Prasadani, M.; Kodithuwakku, S.; Pennarossa, G.; Fazeli, A.; Brevini, T.A.L. Therapeutic Potential of Bovine Milk-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 5543. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Parra, A.; Martínez-Díaz, P.; Rubio, C.P.; Lucas, X.; Yeste, M.; Roca, J.; Barranco, I. Protective role of extracellular vesicles against oxidative DNA damage. Biol. Res. 2025, 58, 14. [Google Scholar] [CrossRef]
- Francesco, D.D.; Mantovani, D.; Hussey, G.; Boccafoschi, F. Matrix bound nanovesicles: A great promise for TERM in less than a decade of research. Matrix Biol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Chen, Y.; He, J.; Zhao, L.; Huang, Y.; Zhao, F.; Jiang, Y.; Fu, S.; Hong, Z. Improving therapeutic effects of exosomes encapsulated gelatin methacryloyl/hyaluronic acid blended and oxygen releasing injectable hydrogel by cardiomyocytes induction and vascularization in rat myocardial infarction model. Int. J. Biol. Macromol. 2024, 271, 132412. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Z.; Li, D.; Ruan, X.; Yang, J.; Chen, S.; Li, X.; Ling, W. Integrating oxygen-boosted sonodynamic therapy and ferroptosis via engineered exosomes for effective cancer treatment. Theranostics 2025, 15, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, Y.; Cao, Y.; Liu, Z. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma. Adv. Mater. 2022, 34, e2110364. [Google Scholar] [CrossRef]
- Cheng, Q.; Dai, Z.; Shi, X.; Duan, X.; Wang, Y.; Hou, T.; Zhang, Y. Expanding the toolbox of exosome-based modulators of cell functions. Biomaterials 2021, 277, 121129. [Google Scholar] [CrossRef]
- Cao, H.; Li, W.; Zhang, H.; Hong, L.; Feng, X.; Gao, X.; Li, H.; Lv, N.; Liu, M. Bio-nanoparticles loaded with synovial-derived exosomes ameliorate osteoarthritis progression by modifying the oxidative microenvironment. J. Nanobiotechnology 2024, 22, 271. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, M.; Chen, Y.; Sun, S.; Yang, S.; Li, Q. Exosome-mediated delivery of superoxide dismutase for anti-aging studies in Caenorhabditis elegans. Int. J. Pharm. 2023, 641, 123090. [Google Scholar] [CrossRef]
- Shao, X.; Feng, X.; Feng, J.; Qiu, T.; Yang, S.; Li, Q. Exosome-mediated co-delivery of superoxide dismutase and chondroitinase ABC for multiple sclerosis therapy. Int. J. Biol. Macromol. 2025, 327, 147438. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Li, Z.; Yang, Y.; Li, L.; Zhao, Y.; Zhao, J. Enzyme-Loaded Catalytic Macrophage Vesicles with Cascade Amplification of Tumor-Targeting for Oxygenated Photodynamic Therapy. Int. J. Nanomed. 2021, 16, 7801–7812. [Google Scholar] [CrossRef]
- Xu, Z.; Hong, W.; Mo, Y.; Shu, F.; Liu, Y.; Cheng, Y.; Tan, N.; Jiang, L. Stem cells derived exosome laden oxygen generating hydrogel composites with good electrical conductivity for the tissue-repairing process of post-myocardial infarction. J. Nanobiotechnology 2025, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yan, X.; Gao, L.; Fan, K. Nanozymes expanding the boundaries of biocatalysis. Nat. Commun. 2025, 16, 6817. [Google Scholar] [CrossRef]
- Zamanian Dastmalchi, H.; Dashtestani, F.; Ghourchian, H. Superoxide dismutase-mimetic nanozymes: A promising alternative to natural superoxide dismutases for biomedical and industrial applications. Colloids Surf. B Biointerfaces 2025, 257, 115138. [Google Scholar] [CrossRef]
- Shen, J.; Pan, Y.; Han, L.; Luo, L.; Sun, T.; Yu, Y. Nanozymes as next-generation ROS scavengers: Design strategies, catalytic mechanisms, and therapeutic frontiers. J. Mater. Chem. B 2025, 13, 8286–8297. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Y.; Jiang, Y.; Li, T.; Qiu, X. Advances in total antioxidant capacity detection based on nanozyme. Talanta 2025, 292, 127941. [Google Scholar] [CrossRef]
- Tagaras, N.; Song, H.; Sahar, S.; Tong, W.; Mao, Z.; Buerki-Thurnherr, T. Safety Landscape of Therapeutic Nanozymes and Future Research Directions. Adv. Sci. 2024, 11, e2407816. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhong, H.; Liao, J.; Huo, Q.; Miao, B.; Zeng, L.; Zhang, B.; Nie, G. Antioxidant activities of metal single-atom nanozymes in biomedicine. Biomater. Sci. 2024, 12, 5150–5163. [Google Scholar] [CrossRef]
- Zhao, H.; Serre, C.; Steunou, N. Metal-Organic Frameworks for the Therapy of Inflammatory Diseases. Adv. Healthc. Mater. 2025, 14, e2404334. [Google Scholar] [CrossRef]
- Ameen, S.S.M.; Omer, K.M. Metal-organic framework-based nanozymes for water-soluble antioxidants and Total antioxidant capacity detection: Principles and applications. Food Chem. 2025, 479, 143876. [Google Scholar] [CrossRef]
- Ye, H.; Lai, Y.; Wu, Z.; Li, G.; Hua, Q.; Zhu, W. Carbon-based nanozymes: Catalytic mechanisms, performance tuning, and environmental and biomedical applications. Anal. Methods 2025, 17, 6264–6281. [Google Scholar] [CrossRef] [PubMed]
- Darabi, S.; Ariaei, A.; Rustamzadeh, A.; Afshari, D.; Charkhat Gorgich, E.A.; Darabi, L. Cerebrospinal fluid and blood exosomes as biomarkers for amyotrophic lateral sclerosis; a systematic review. Diagn. Pathol. 2024, 19(47), s13000–s13024. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, S.; Zhang, J.; Li, R.; Zhang, Y.; Wang, Z.; Kong, Q.; Cho, W.C.; Ju, X.; Shen, Y.; et al. Proteomic profiling of serum extracellular vesicles identifies diagnostic markers for echinococcosis. PLoS Negl. Trop. Dis. 2022, 16, e0010814. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- de Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef]
- Nelson, B.C.; Maragh, S.; Ghiran, I.C.; Jones, J.C.; DeRose, P.C.; Elsheikh, E.; Vreeland, W.N.; Wang, L. Measurement and standardization challenges for extracellular vesicle therapeutic delivery vectors. Nanomedicine 2020, 15, 2149–2170. [Google Scholar] [CrossRef]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef]
- Cheng, G.; Li, W.; Ha, L.; Han, X.; Hao, S.; Wan, Y.; Wang, Z.; Dong, F.; Zou, X.; Mao, Y.; et al. Self-Assembly of Extracellular Vesicle-like Metal-Organic Framework Nanoparticles for Protection and Intracellular Delivery of Biofunctional Proteins. J. Am. Chem. Soc. 2018, 140, 7282–7291. [Google Scholar] [CrossRef]
- Sharma, S.; Masud, M.K.; Kaneti, Y.V.; Rewatkar, P.; Koradia, A.; Hossain, M.S.A.; Yamauchi, Y.; Popat, A.; Salomon, C. Extracellular Vesicle Nanoarchitectonics for Novel Drug Delivery Applications. Small 2021, 17, e2102220. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, B.; Zhang, S.; Cao, C.; Qi, L. Fabrication of Hierarchically Mesoporous Multishelled Metal-Organic Frameworks via Template-Induced Assembly/Grinding Strategy for Immobilization of Enzyme and Enhancing Catalytic Performance. ACS Appl. Mater. Interfaces 2025. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Kang, J.Y.; Mun, D.; Chun, Y.; Park, D.S.; Kim, H.; Yun, N.; Joung, B. Engineered small extracellular vesicle-mediated NOX4 siRNA delivery for targeted therapy of cardiac hypertrophy. J. Extracell. Vesicles 2023, 12, e12371. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2020, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef]

| Antioxidant Enzymes (AOEs) in EVs | EV Source | EV Targeting | Alterations in AOEs-Loading EVs | Detection Methods | Reference |
|---|---|---|---|---|---|
| Superoxide Dismutase (SOD) | The media of human dental pulp stem cells (senescent) | Human dental pulp stem cells (young) | EVs ↑ SOD2 in EVs ↓ under oxidative stress (OR) | qPCR | Mas-Bargues et al., 2023 [20] |
| Human dental follicle stem cells (DFSCs) | Periodontal ligament stem cells (PDLSCs) | EVs ↑ SOD1 in EVs √ under LPS | Proteomics analysis | Huang et al., 2022 [21] | |
| The cardiac EVs from the mouse heart | SOD1/2/3 in EVs √ | Claridge et al., 2021 [22] | |||
| Human serum samples and skeletal muscle cells | SOD2 in EVs ↑ after high-intensity interval training (HIIT) | Lisi et al., 2023; Apostolopoulou et al., 2021 [17,23] | |||
| Human blood plasma | SOD1/2 in EVs √ | McIlvenna et al., 2023 [24] | |||
| Mitochondrial-derived vesicles from rat heart | SOD2 in EVs √ | Vasam et al., 2021 [25] | |||
| Chlamydomonas reinhardtii | SOD in EVs √ | Garaeva et al. [26] | |||
| Human blood plasma | SOD1 in EVs ─ SOD2 in EVs ↑ after endurance exercise (EEx) | Western blot analysis | Lisi et al., 2023 [17,27] | ||
| Human umbilical cord mesenchymal stem cells (hUC-MSCs) | Hepatocytes; neutrophils | SOD2 in EVs ↓ after knocking down SOD2 in hUC-MSCs | Yao et al., 2019 [28] | ||
| Monocyte-derived macrophages | SOD1 in EVs ↓ after HIV infects cells | Haque et al., 2020 [29] | |||
| Human umbilical cord blood-derived MSCs | Human mononuclear cells (hMNCs); HaCaT Cell; Human Dermal Fibroblast (HDF); | SOD3 in EVs √ after SOD3 transduction in MSCs | Yang et al., 2020 [30] | ||
| Mouse neutrophils | Mouse liver endothelial cell | EVs ↑ SOD2 in EVs ↑ under LPS | Bao et al., 2022 [31] | ||
| Mouse/human blood plasma | Human umbilical vein endothelial cells (HUVECs) | EVs ↑ SOD3 in EVs ↑ after exercise training (ExT) | Abdelsaid et al., 2022 [32] | ||
| The media of human bone marrow MSCs | Nucleus pulposus cells | SOD1 in EVs ↑, SOD2 in EVs ─ after MSCs were treated with quercetin | Peng et al., 2024 [19] | ||
| Human neutrophils | SOD in EVs √ | Western blot analysis; Enzymatic assays | Iversen et al., 2016 [33] | ||
| Supernatant from cell culture media: human peripheral blood mononuclear cells | Monocytic cell lines (U937 cells) | SOD1/2 in EVs √ SOD 1 ↑ after U937 cells are exposed to cigarette smoke condensate (CSC) | qPCR; Western blot analysis | Haque et al., 2017 [34] | |
| Plasma of exercise training wild-type mice | The central nervous system and myocardium | EVs ↑ SOD2 in EVs ↑ after exercise training (ExT) | Proteomics analysis; Western blot analysis | Gao et al., 2021 [35] | |
| Skeletal muscle-derived EVs | |||||
| The adipose tissue from mice | AML12 (alpha mouse liver 12) cells | SOD1 in EVs ⅹ SOD activity in EVs ─ under a high-fat diet (HFD) | Enzymatic assays | Jeong et al., 2025 [36] | |
| Aloe vera peels | Human keratinocytes and fibroblasts | SOD activity in EVs √ | Kim et al. [37] | ||
| Fruits-derived EVs | Logozzi et al. [38] | ||||
| Fruits-derived EVs | HDF | Di Raimo et al. [39] | |||
| Allium cepa | Mouse skin | Azizi et al. [40] | |||
| Catalase (CAT) | The media of human dental pulp stem cells (senescent) | Human dental pulp stem cells (young) | EVs ↑ CAT in EVs ─ under OR | qPCR | Mas-Bargues et al., 2023 [20] |
| Human blood plasma | Human iPSC-derived cardiomyocytes | CAT activity in EVs ↑ after EEx | Proteomics analysis; Enzymatic assays | Lisi et al., 2023 [41] | |
| The adipose tissue from mice | AML12 | CAT in EVs ↑ CAT activity in EVs ↑ under HFD | Western blot analysis; Enzymatic assays | Jeong et al., 2025 [36] | |
| Cardiac EVs from the mouse heart | CAT in EVs √ | Proteomics analysis | Claridge et al., 2021 [22] | ||
| Human serum samples and skeletal muscle cells | CAT in EVs ↑ after HIIT | Lisi et al., 2023; Apostolopoulou et al., 2021 [17,23] | |||
| Human blood plasma | CAT in EVs √ | McIlvenna et al., 2023 [24] | |||
| Pseudomonas syringae pv. tomato DC3000 (Pto DC3000) | Deng et al., 2025 [42] | ||||
| Chlamydomonas reinhardtii | Garaeva et al. [26] | ||||
| Human blood plasma | CAT in EVs ↑ after EEx | Western blot analysis | Lisi et al., 2023 [17,27] | ||
| Monocyte-derived macrophages | CAT in EVs ↓ after cells are infected by HIV | Haque et al., 2020 [29] | |||
| Supernatant from cell culture media: human peripheral blood mononuclear cells | Monocytic cell lines (U937 cells) | CAT ↑ after U937 cells are exposed to CSC | qPCR; Western blot analysis | Haque et al., 2017 [34] | |
| Human serum samples | CAT in EVs ↑ after HIIT | Proteomics analysis; Western blot analysis | Kobayashi et al., 2021 [43] | ||
| Fruits-derived EVs | CAT in EVs √ | Enzymatic assays | Logozzi et al. [38] | ||
| Fruits-derived EVs | HDF | Di Raimo et al. [39] | |||
| Peroxiredoxin (PRDX) | Human serum samples and skeletal muscle cells | PRDX1/2 in EVs ↑ after HIIT | Proteomics analysis | Lisi et al., 2023; Apostolopoulou et al., 2021 [17,23] | |
| Human blood plasma | PRDX1/2/6 in EVs √ | McIlvenna et al., 2023 [24] | |||
| Mitochondrial-derived vesicles from rat heart | PRDX3/5/6 in EVs √ | Vasam et al., 2021 [25] | |||
| Induced pluripotent stem cell (iPSC); mesenchymal stem cell (MSC) | PRDX1/2 in EVs ↓ under progerin-induced senescence | Proteomics analysis; Western blot analysis | Liu et al., 2019 [44] | ||
| Human blood plasma | PRDX1 in EVs ↑ after EEx | Warnier et al., 2025 [45] | |||
| Human serum samples | PRDX2 in EVs ↑ after HIIT | Kobayashi et al., 2021 [43] | |||
| Supernatant from cell culture media: human peripheral blood mononuclear cells | Monocytic cell lines (U937 cells) | PRDX 6 ↓ after U937 cells are exposed to CSC | qPCR; Western blot analysis | Haque et al., 2017 [34] | |
| The media of human bone marrow MSCs | Nucleus pulposus cells | PRDX1/2 in EVs ─ after MSCs were treated with quercetin | Western blot analysis | Peng et al., 2024 [19] | |
| Macrophages | Cancer cells | PRDX 6 in EVs √ | Zheng et al., 2025 [46] | ||
| Glutathione Peroxidase (GPX) | The media of human dental pulp stem cells (senescent) | Human dental pulp stem cells (young) | EVs ↑ GPX in EVs ↓ under OR | qPCR | Mas-Bargues et al., 2023 [20] |
| The adipose tissue from mice | AML12 | GPX1 in EVs ⅹ GPx in EVs activity ─ under HFD | Enzymatic assays | Jeong et al., 2025 [36] | |
| Cardiac EVs from the mouse heart | GPX1/3/4/7/8 in EVs √ | Proteomics analysis | Claridge et al., 2021 [22] | ||
| Human blood plasma | GPX in EVs √ | McIlvenna et al., 2023 [24] | |||
| Pig seminal plasma exosomes | Sperm | GPX5 in EVs √ | Proteomics analysis; Western blot analysis | Huang et al., 2024 [47] | |
| The media of human glioblastoma cell line, GBM8401, and primary glioblastoma cells, GBM04T | Glioblastoma; HUVECs; U251 cells | GPX1 in EVs ↑ under hypoxia | Western blot analysis | Lei et al., 2023 [48] | |
| Glutathione Reductase (GSR) | Human blood plasma | Human iPSC-derived cardiomyocytes | GSR activity in EVs ↑ after EEx | Proteomics analysis; Enzymatic assays; | Lisi et al., 2023 [41] |
| DFSCs | PDLSCs | EVs ↑ GSR in EVs ↑ under LPS | Proteomics analysis | Huang et al., 2022 [21] | |
| Human blood plasma | GSR in EVs √ | McIlvenna et al., 2023 [24] | |||
| Thioredoxin Reductase (TXNRD) | Human blood plasma | TXNRD1 in EVs ─ after EEx | Western blot analysis | Lisi et al., 2023 [17,27] | |
| Mitochondrial-derived vesicles from rat heart | TXNRD2 in EVs √ | Proteomics analysis | Vasam et al., 2021 [25] | ||
| Thioredoxin Oxidase (TXNRO) | Human blood plasma | TXNRO in EVs √ | Proteomics analysis | McIlvenna et al., 2023 [24] | |
| Human blood plasma | The breast cancer cell line MDA-MB-231 | TXNRO in EVs ↑ after EEx | Proteomics analysis; Western blot analysis | Sagini et al., 2025 [16] | |
| NAD(P)H dehydrogenase [quinone] 1 (NQO1) | Human blood plasma | The central nervous system and myocardium | EVs ↑ NQO1 in EVs ↑ after ExT | Proteomics analysis; Western blot analysis | Gao et al., 2021 [35] |
| Skeletal muscle-derived EVs | |||||
| Nicotinamide Phosphoribosyltransferase (NAMPT) | Human blood plasma | NAMPT in EVs ↑ after ExT | Western blot analysis; Enzymatic assays | Lisi et al., 2023; Chong et al., 2022 [17,49] | |
| Heme oxygenase 1 (HMOX1) | Human blood plasma | HMOX1 in EVs ↑ after ExT | Proteomics analysis | Lisi et al., 2023; Bryl-Gorecka., 2018 [17,41,50] | |
| Glucose-6-phosphate dehydrogenase (G6PD) | Human serum samples and skeletal muscle cells | EVs ↑ G6PD in EVs ↑ after HIIT | Proteomics analysis | Lisi et al., 2023; Apostolopoulou et al., 2021 [17,23] | |
| Human blood plasma | G6PD in EVs √ | McIlvenna et al., 2023 [24] |
| Enzyme Type | EV Source | Assembly Method | Therapeutic Effect | Reference |
|---|---|---|---|---|
| SOD3 | Synovial fibroblast | SOD3 plasmid overexpressing fibroblasts → isolate S-EXOs → load onto polydopamine-coated GelMA microspheres (GM@PDA@S-EXO) for sustained release | Enhance chondrocyte antioxidant capacity, reduce ROS and mitoROS, preserve cartilage extracellular matrix. | Cao et al., 2024 [90] |
| SOD | Human embryonic kidney cells (HEK293T) | Mechanical extrusion + saponin permeabilization | Reduce ROS level and delay aging in the C. elegans model | Shao et al., 2023 [91] |
| SOD + Chondroitinase ABC | HEK293T | Mechanical extrusion + saponin permeabilization | Reduce ROS, inhibit apoptosis, and promote remyelination in the experimental autoimmune encephalomyelitis mouse model. | Shao et al., 2025 [92] |
| SOD2 | Healthy young human plasma, skimmed milk, and grapes | Sod2-overexpressing plasmid | Grape-derived EVSod2 was the most effective carrier, significantly reducing PM2.5-induced cardiopulmonary injury. | Zhang et al., 2025 [78] |
| Catalase-SKL | RAW 264.7 macrophages | Sonication | Intranasal administration provides broad brain distribution and no off-target toxicity. | Hayes et al., 2021 [52] |
| Catalase | J774.A.1 cells | CAT@SiO2-ICG (CSI) was transfected into AS1411 aptamer-modified mononuclear macrophage exosomes. | Efficient blood−brain barrier penetration, good cancer-cell-targeting capability, and enhanced sonodynamic therapy of glioblastoma | Wu et al., 2022 [88] |
| Catalase-Ce6 | RAW 264.7 cells (M1 macrophages) | Catalase-Ce6 nanocomplex co-extruded with glucose oxidase-modified M1 macrophage EVs | Cascade oxygenation to enhance photodynamic therapy, efficient tumor targeting, and significant tumor suppression with minimal systemic toxicity | Liu et al., 2021 [93] |
| Catalase | Mesenchymal stem cells (MSCs) | Encapsulated catalase and MSC-derived exosomes into a blended GelMA/hyaluronic acid hydrogel with thioketal-PEG, forming an O2-generating injectable Exo–O2 (+) hydrogel | Sustained release of oxygen, which resulted in continuous oxygenation of the metabolically demanding heart cells | Wang et al., 2024 [86] |
| Catalase & ACSL4 (Acyl-CoA synthetase long-chain family member 4) | 4T1 breast cancer cells (engineered via lentiviral overexpression of catalase and ACSL4) | Extraction of exosomes from lentivirus-transfected 4T1 cells and loading of sonosensitizer tetrakis via electroporation to obtain engineered exosomes | Combine oxygen-enhanced sonodynamic therapy and ferroptosis induction | Wu M. et al., 2025 [87] |
| Catalase | MSCs | Exo/hydrogel loaded with Au nanoparticles and catalase | Oxygen generation to reduce cell apoptosis and necrosis, improve heart cell survival, and promote repair of infarcted cardiac tissue post-myocardial infarction. | Xu et al., 2025, [94] |
| Catalase | Expi293F cells transfected with the expression constructs | Genetic fusion of proteins (CD9, PhoCl, mCherry, apoptin, catalase) | Light-controlled release of catalase, efficient intracellular delivery for antioxidant and apoptosis-inducing therapy, targeted delivery of proteins to treat liver injury, and induce tumor cell apoptosis | Cheng et al. 2021, [89] |
| GPX5 | HEK293T | Transfect cells with the GPX5 overexpression vector | Enhanced sperm motility, acrosome integrity, reduced oxidative damage, and improved fertilization ability. | Huang et al., 2024 [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, Y.; Dullaart, R.P.F.; Olinga, P.; Moshage, H. Extracellular Vesicle-Mediated Delivery of Antioxidant Enzymes: Emerging Insights and Translational Opportunities. Antioxidants 2025, 14, 1504. https://doi.org/10.3390/antiox14121504
Wang J, Li Y, Dullaart RPF, Olinga P, Moshage H. Extracellular Vesicle-Mediated Delivery of Antioxidant Enzymes: Emerging Insights and Translational Opportunities. Antioxidants. 2025; 14(12):1504. https://doi.org/10.3390/antiox14121504
Chicago/Turabian StyleWang, Junyu, Yakun Li, Robin P. F. Dullaart, Peter Olinga, and Han Moshage. 2025. "Extracellular Vesicle-Mediated Delivery of Antioxidant Enzymes: Emerging Insights and Translational Opportunities" Antioxidants 14, no. 12: 1504. https://doi.org/10.3390/antiox14121504
APA StyleWang, J., Li, Y., Dullaart, R. P. F., Olinga, P., & Moshage, H. (2025). Extracellular Vesicle-Mediated Delivery of Antioxidant Enzymes: Emerging Insights and Translational Opportunities. Antioxidants, 14(12), 1504. https://doi.org/10.3390/antiox14121504







