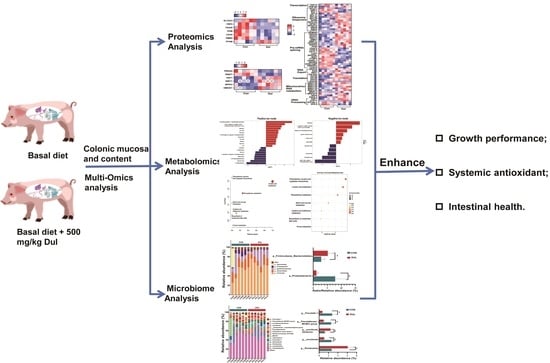
Multi-Omics Insights into the Role of Dulcitol in Weaned Piglets’ Growth Performance and Intestinal Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Serum Parameters Analysis
2.3. Colon Morphology and Goblet Cell Analysis
2.4. Western Blot Analysis
2.5. Proteomics Analysis
2.6. Untargeted Metabolism Analysis
2.7. Gut Microbiota Analysis
2.8. Data Analysis
3. Results
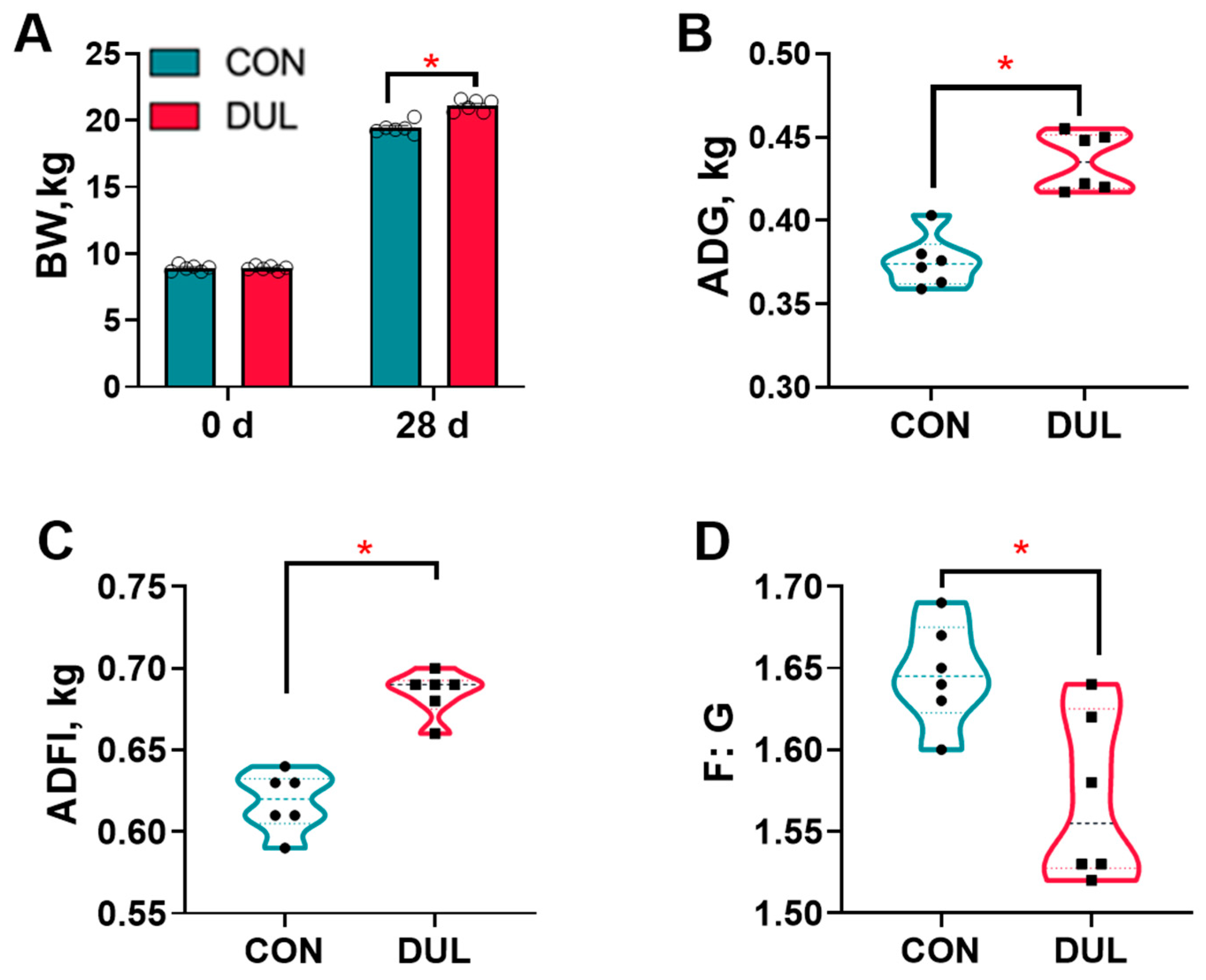
3.1. Dul Improved Growth Performance in Weaned Piglets
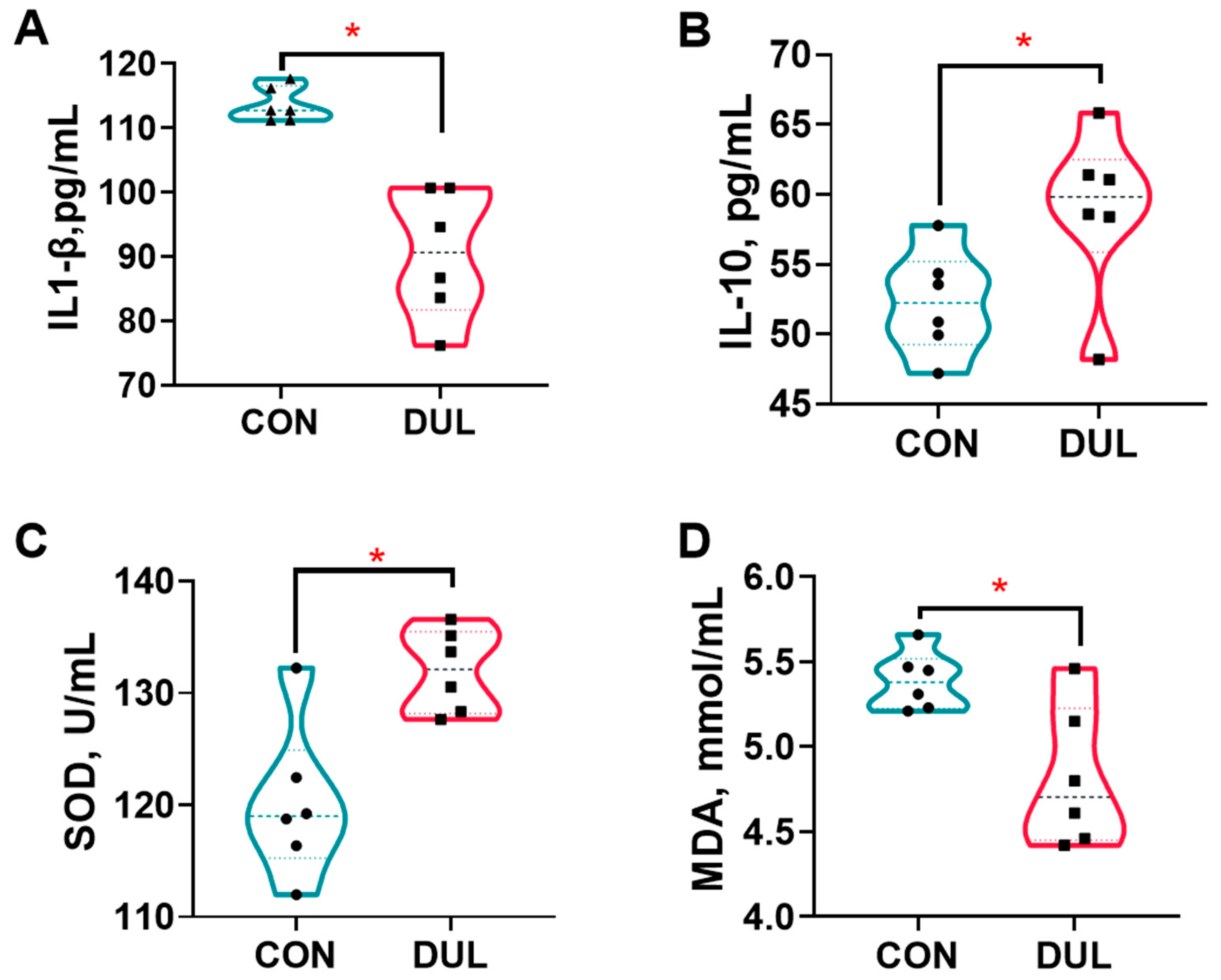
3.2. Dul Enhanced Antioxidant and Anti-Inflammatory Capacities in Weaned Piglets
3.3. Dul Decreased Intestinal Permeability but Increased the Expression of Colonic Tight Junction in Weaned Piglets
3.4. Dul Altered the Colonic Proteomics in Weaned Piglets
3.5. Dul Changed the Colonic Metabolomics in Weaned Piglets
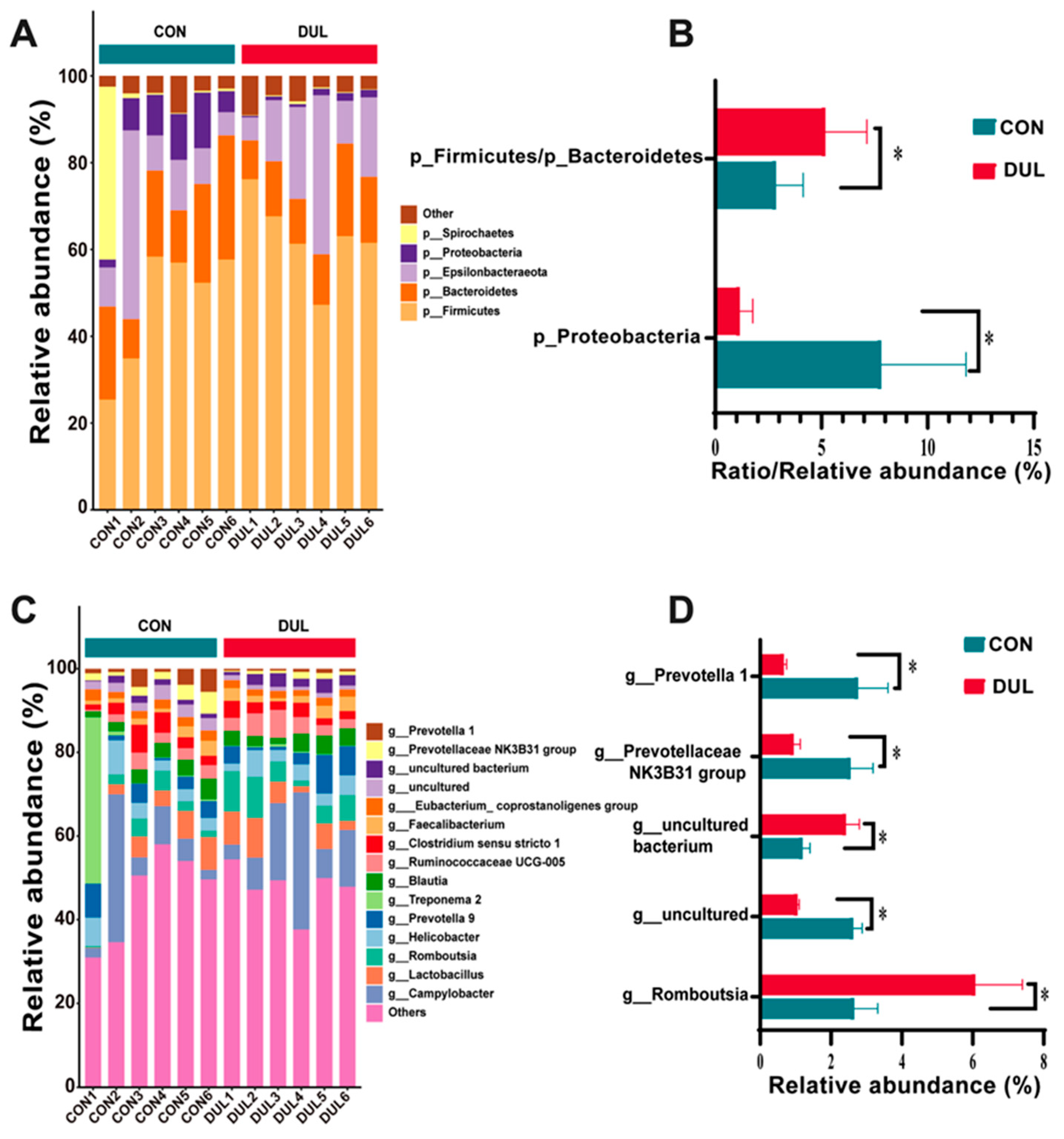
3.6. Dul Regulated the Colonic Microbial Composition in Weaned Piglets
4. Discussion
4.1. Growth Performance
4.2. Antioxidant and Anti-Inflammatory Capacities
4.3. Intestinal Barrier
4.4. Colonic Proteomics and Metabolomics
4.5. Colonic Microbiota
4.6. Study Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.K.; Deschêne, K.; Martel-Kennes, Y.; Roy, C.; Laforest, J.P.; Lessard, M.; Matte, J.J.; Lapointe, J. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS ONE 2021, 16, e0247188. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Lin, X.L.; Li, K.; Yang, Z.; Chen, B.; Zhang, T. Dulcitol suppresses proliferation and migration of hepatocellular carcinoma via regulating SIRT1/p53 pathway. Phytomedicine 2020, 66, 153112. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, F.; Xu, X.; Chen, B.; Zhang, T. Inhibitory effects of Dulcitol on rat C6 glioma by regulating autophagy pathway. Nat. Prod. Res. 2020, 34, 1437–1441. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shen, J.; Li, S.H.; Kakizoe, E.; Okunishi, H.; Chen, J.F. Suppressive effects of a plant-origin polyol, dulcitol on collagen-induced arthritis in mice. Nihon Yakurigaku Zasshi 1997, 110 (Suppl. S1), 132–137. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, S.; Wang, D.; Zheng, Z.; Li, N.; Zhang, C.; Yan, J.; Liu, R.; He, P.; Li, Q.; et al. Effect of dulcitol on growth performance, antioxidant activity, immune function, and intestinal microflora of growing-finishing pigs. Livest. Sci. 2024, 284, 105476. [Google Scholar] [CrossRef]
- Barmukh, R.; Garg, V.; Liu, H.; Chitikineni, A.; Xin, L.; Henry, R.; Varshney, R.K. Spatial omics for accelerating plant research and crop improvement. Trends Biotechnol. 2025, 43, 1904–1920. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th revised ed.; National Academies Press: Washington, WA, USA, 2012. [Google Scholar]
- Liu, Z.; Wang, H.; Han, H.; Li, N.; Zheng, Z.; Liang, S.; Zhong, R.; Chen, L.; Yan, J.; Mu, S. The protective effect of dulcitol on lipopolysaccharide-induced intestinal injury in piglets: Mechanistic insights. J. Nutr. Biochem. 2024, 133, 109719. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. The Impact of Weaning Stress on Gut Health and the Mechanistic Aspects of Several Feed Additives Contributing to Improved Gut Health Function in Weanling Piglets-A Review. Animals 2021, 11, 2418. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Singh, Z.; Karthigesu, I.P.; Singh, P.; Kaur, R. Use of malondialdehyde as a biomarker for assessing oxidative stress in different disease pathologies: A review. Iran. J. Public Health 2014, 43, 7–16. [Google Scholar]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Li, J.Y.; Lu, Y.; Hu, S.; Sun, D.; Yao, Y.M. Preventive effect of glutamine on intestinal barrier dysfunction induced by severe trauma. World J. Gastroenterol. 2002, 8, 168–171. [Google Scholar] [CrossRef]
- Tossou, M.C.; Liu, H.; Bai, M.; Chen, S.; Cai, Y.; Duraipandiyan, V.; Liu, H.; Adebowale, T.O.; Al-Dhabi, N.A.; Long, L.; et al. Effect of High Dietary Tryptophan on Intestinal Morphology and Tight Junction Protein of Weaned Pig. Biomed. Res. Int. 2016, 2016, 2912418. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-Y.; Huang, D.-L.; Wen, B.; Wei, S.-Z.; Li, L.-Y.; Fang, W.-J.; Wu, X.-H. Effect of different minimally invasive gastric cancer surgical approaches on postoperative intestinal mucosal barrier function. J. Nutr. Oncol. 2024, 9, 98–102. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014, 48, 980–986. [Google Scholar] [CrossRef]
- Hamada, K.; Shitara, Y.; Sekine, S.; Horie, T. Zonula Occludens-1 alterations and enhanced intestinal permeability in methotrexate-treated rats. Cancer Chemother. Pharmacol. 2010, 66, 1031–1038. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zhai, P.; Del Re, D.P.; Sciarretta, S.; Yabuta, N.; Nojima, H.; Lim, D.S.; Pan, D.; Sadoshima, J. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat. Commun. 2014, 5, 3315. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, S.; Li, C.; Cui, L.; Ma, J.; Hui, Y. The non-canonical effects of heme oxygenase-1, a classical fighter against oxidative stress. Redox Biol. 2021, 47, 102170. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Wu, J.; Gu, X.; Zhang, J.; Mi, Z.; He, Z.; Dong, Y.; Ge, W.; Ghimire, K.; Rong, P.; Wang, W.; et al. 4-OI Protects MIN6 Cells from Oxidative Stress Injury by Reducing LDHA-Mediated ROS Generation. Biomolecules 2022, 12, 1236. [Google Scholar] [CrossRef]
- Kapoor, R.; Huang, Y.S. Gamma linolenic acid: An antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, Y.; Wu, L.; Fu, J.; Du, J.; Luo, Z.; Guo, L.; Xu, J.; Liu, Y. Porphyromonas gingivalis aggravates colitis via a gut microbiota-linoleic acid metabolism-Th17/Treg cell balance axis. Nat. Commun. 2024, 15, 1617. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jiang, G.; Wang, Y.; Yan, E.; He, L.; Guo, J.; Yin, J.; Zhang, X. Maternal consumption of l-malic acid enriched diets improves antioxidant capacity and glucose metabolism in offspring by regulating the gut microbiota. Redox Biol. 2023, 67, 102889. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, N.; Wang, H.; Zhong, R.; Cao, S.; Zheng, Z.; Liu, J.; Chen, L.; Yan, J.; Mu, S. Multi-Omics Insights into the Role of Dulcitol in Weaned Piglets’ Growth Performance and Intestinal Health. Antioxidants 2025, 14, 1346. https://doi.org/10.3390/antiox14111346
Liu Z, Li N, Wang H, Zhong R, Cao S, Zheng Z, Liu J, Chen L, Yan J, Mu S. Multi-Omics Insights into the Role of Dulcitol in Weaned Piglets’ Growth Performance and Intestinal Health. Antioxidants. 2025; 14(11):1346. https://doi.org/10.3390/antiox14111346
Chicago/Turabian StyleLiu, Zhengqun, Ning Li, Han Wang, Ruqing Zhong, Shanchuan Cao, Zi Zheng, Jingbo Liu, Liang Chen, Jun Yan, and Shuqin Mu. 2025. "Multi-Omics Insights into the Role of Dulcitol in Weaned Piglets’ Growth Performance and Intestinal Health" Antioxidants 14, no. 11: 1346. https://doi.org/10.3390/antiox14111346
APA StyleLiu, Z., Li, N., Wang, H., Zhong, R., Cao, S., Zheng, Z., Liu, J., Chen, L., Yan, J., & Mu, S. (2025). Multi-Omics Insights into the Role of Dulcitol in Weaned Piglets’ Growth Performance and Intestinal Health. Antioxidants, 14(11), 1346. https://doi.org/10.3390/antiox14111346