Extraction, Purification and Identification of Bovine Lung Peptides and Its Antioxidant Effects on H2O2-Induced HepG2 Cells and Mice with Alcoholic Liver Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of BLP
2.2.1. Pretreatment of Bovine Lungs
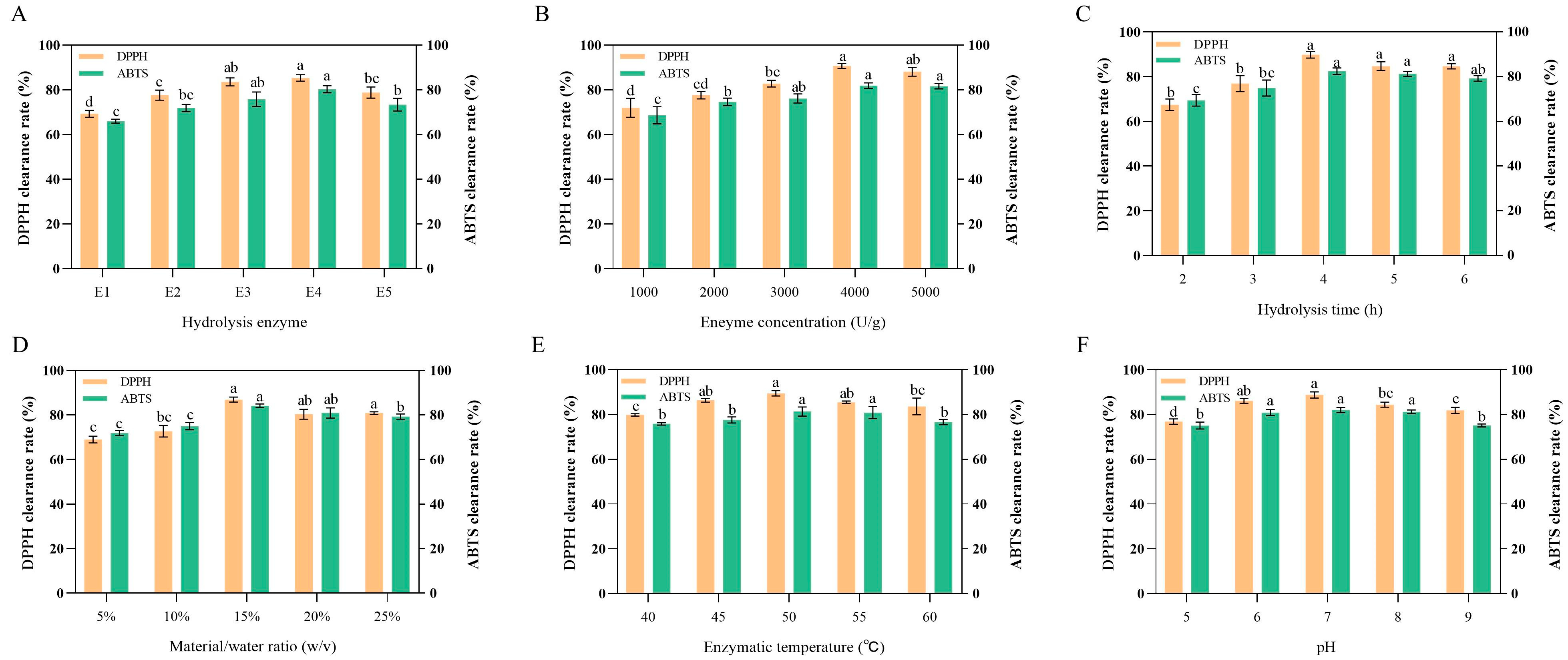
2.2.2. Screening of Proteases
2.3. Optimization of BLP Extraction Conditions
2.3.1. Single-Factor Experiment
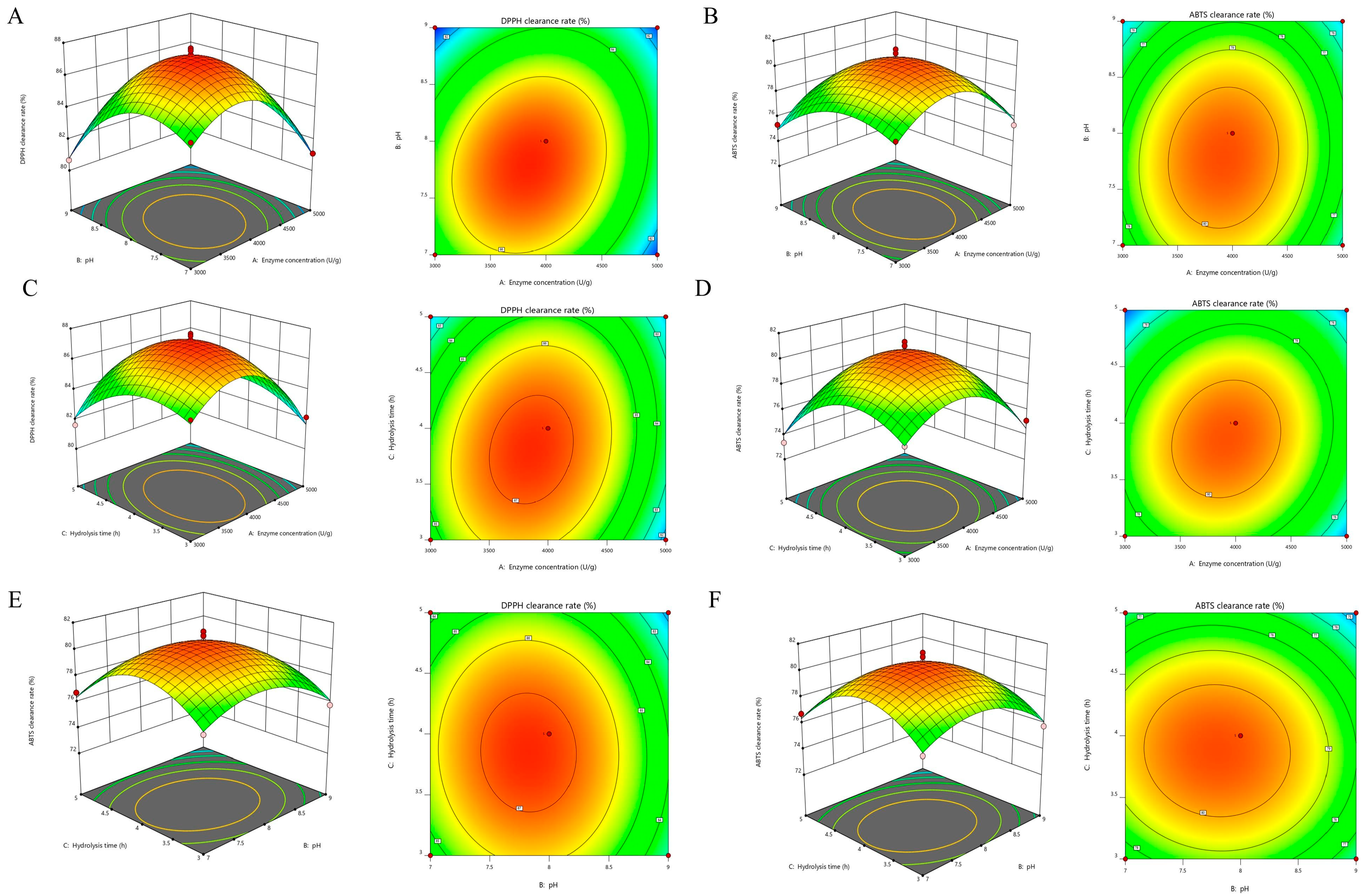
2.3.2. Design of Response Surface Methodology (RSM)
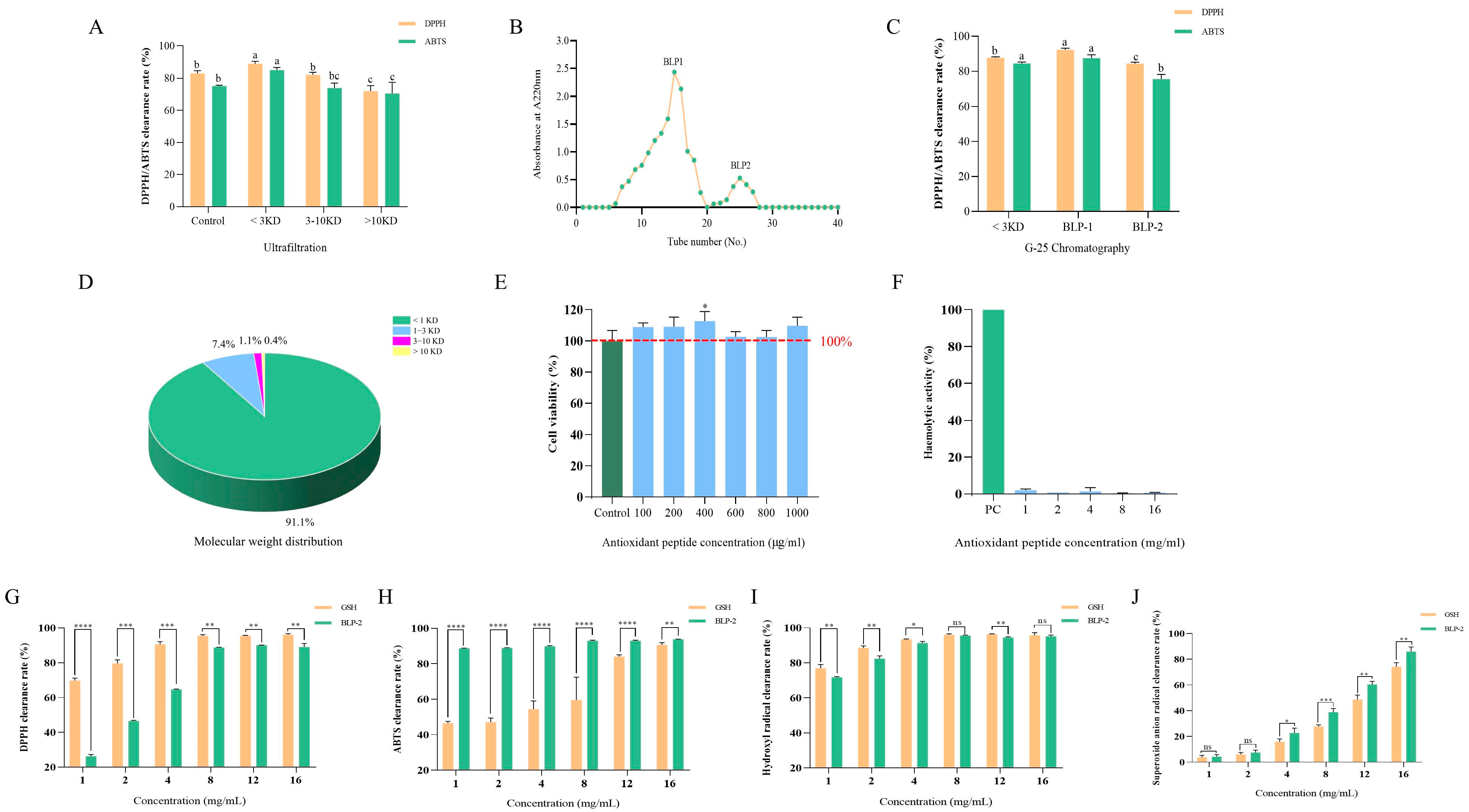
2.4. Isolation and Purification of BLP
2.4.1. Ultrafiltration
2.4.2. Gel Filtration Chromatography
2.5. Molecular Weight Determination
2.6. Biosafety Assessment
2.6.1. Cytotoxicity Assay
2.6.2. Hemolysis Test
2.7. Evaluation of Antioxidant Activity In Vitro
2.8. Protective Effect of BLP-1 on HepG2 Cells Against Oxidative Stress
2.8.1. Cell Experimental Design
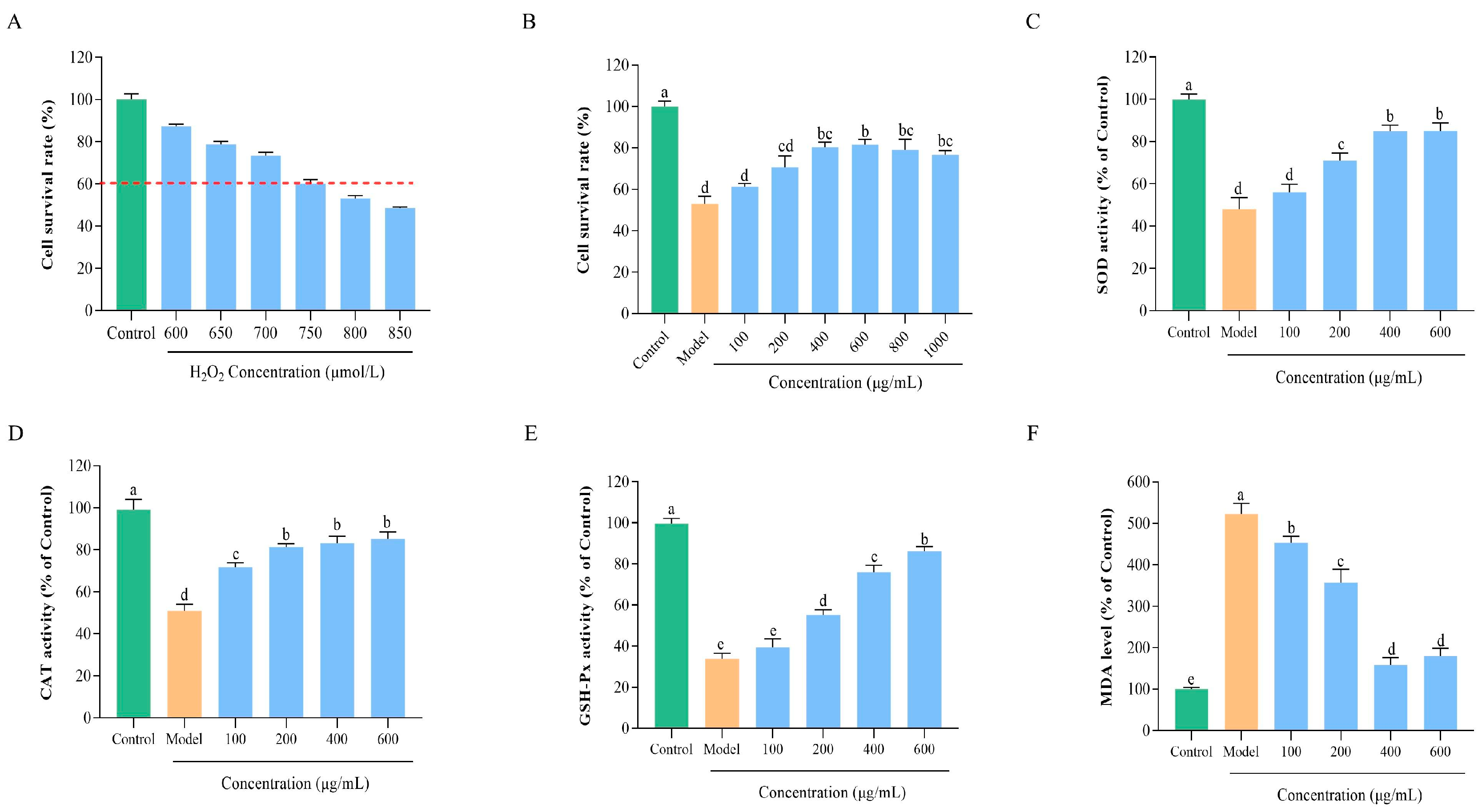
2.8.2. Selection of H2O2 Concentration
2.8.3. The Effect of BLP-1 on Cell Viability
2.8.4. Determination of Antioxidant Enzyme Activity and MDA Level
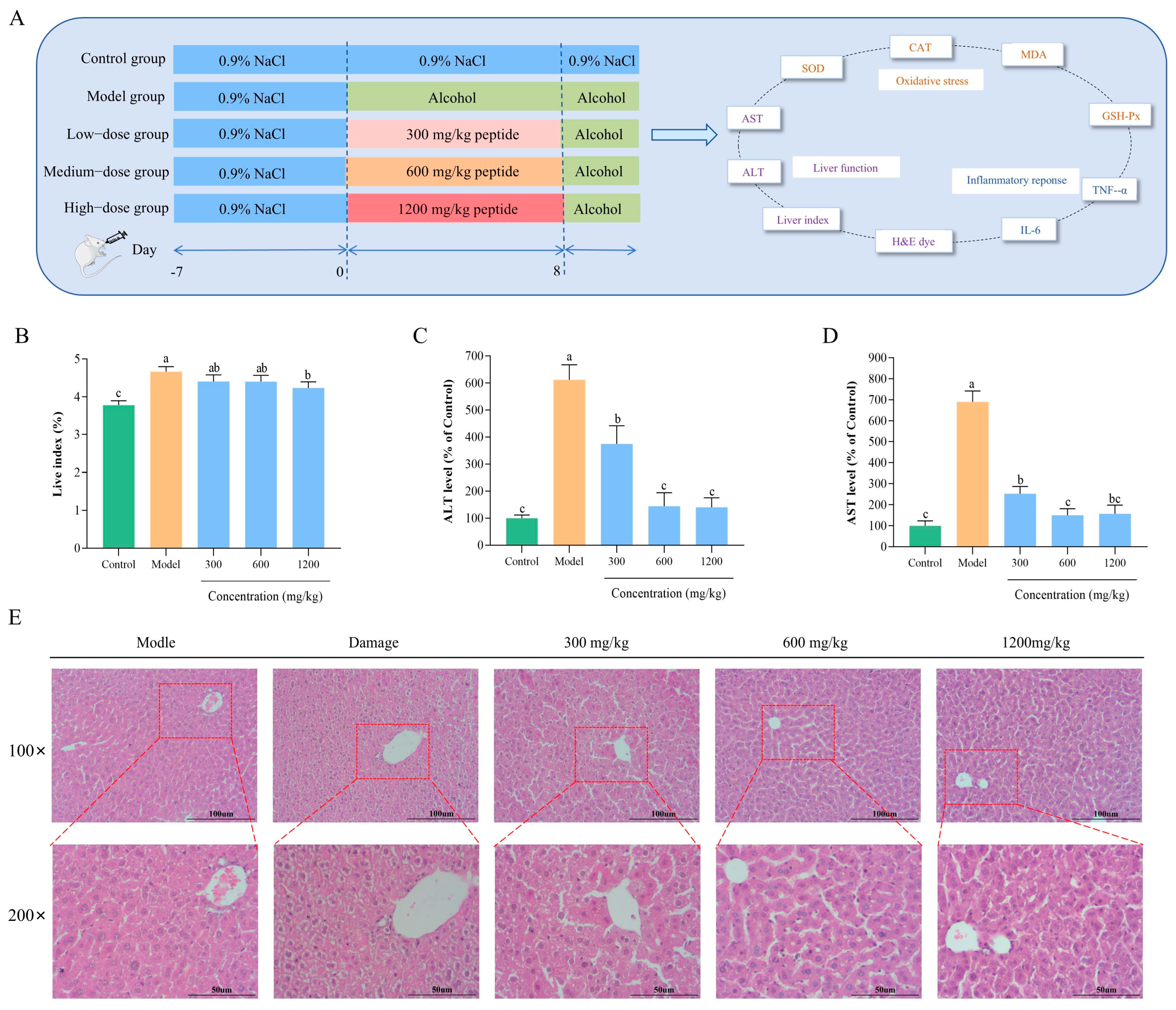
2.9. Protective Effect of BLP-1 on Mice with ALD
2.9.1. Animal Experiment
2.9.2. Biochemical Testing and ELISA Assay
2.9.3. Histopathological Analysis
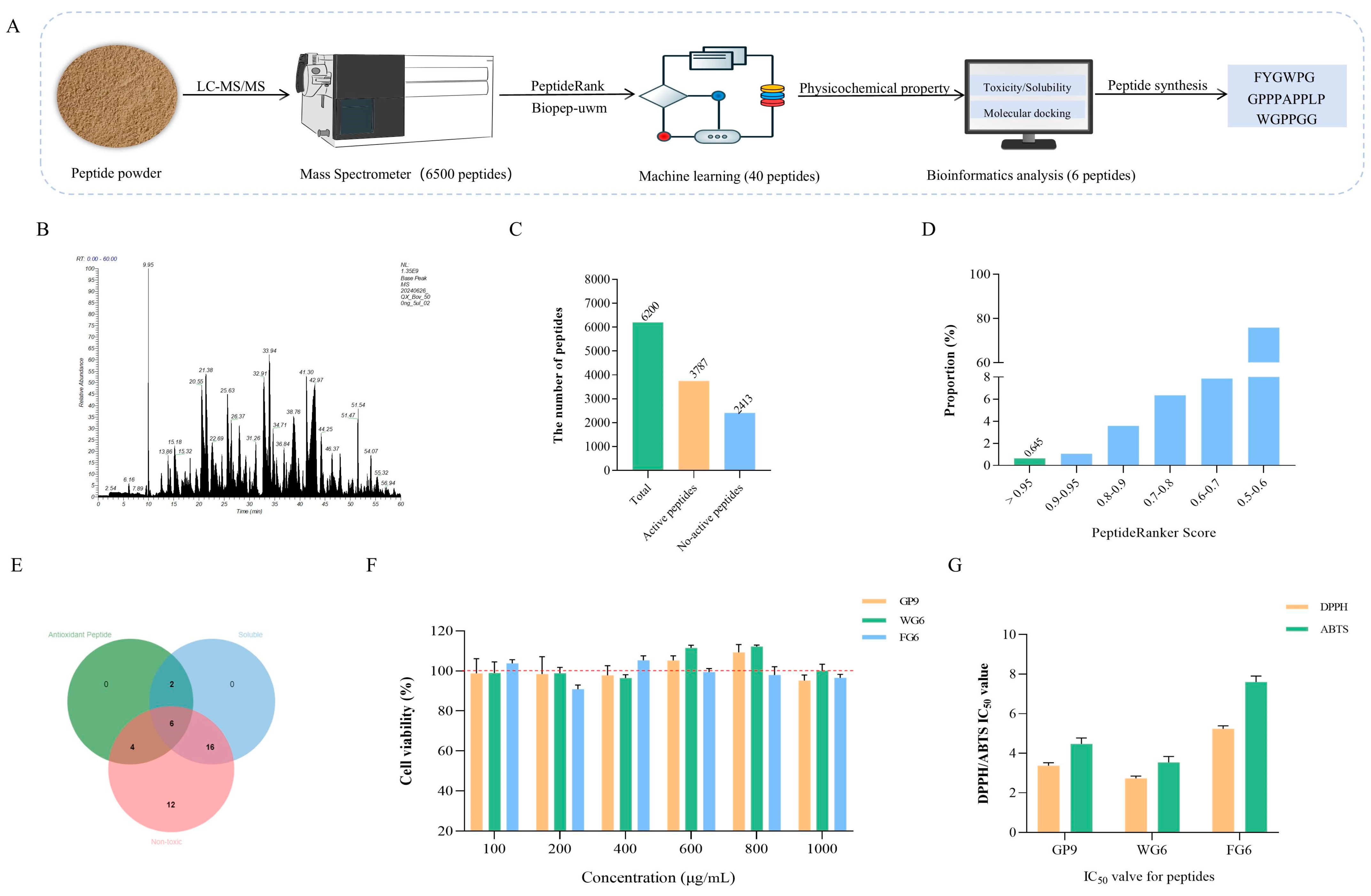
2.10. Identification of Antioxidant Peptide Sequences in BLP-1
2.10.1. LC-MS/MS Identification
2.10.2. Antioxidant Peptide Sequence Screening
2.10.3. Prediction of Antioxidant Peptide Properties
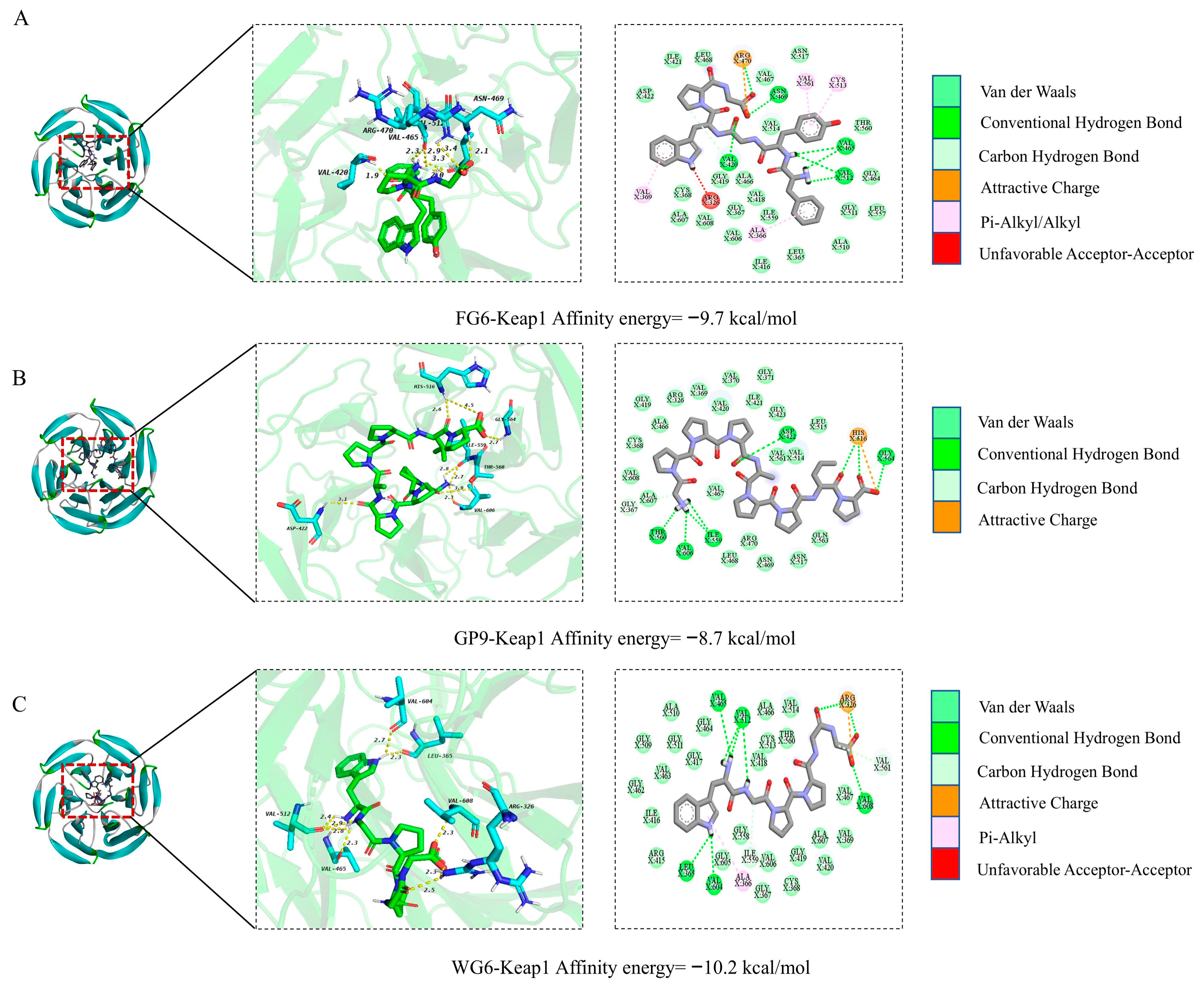
2.11. Molecular Docking
2.11.1. Docking with DPPH and ABTS
2.11.2. Docking with Keap1
2.12. Peptide Synthesis and Antioxidant Activity Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Optimal Protease Selection
3.2. Optimization of Single-Factor Experiment
3.3. Optimization of Hydrolysis Parameter by RSM
3.4. Separation and Purification of BLP
3.5. Biological Toxicity of BLP-1
3.6. Antioxidant Capacity of BLP-1 In Vitro
3.7. Cytoprotective Function of BLP-1 Against H2O2-Induced HepG2 Cells
3.7.1. Construction of an Oxidative Damage Model
3.7.2. Protective Effect of BLP-1 on Oxidatively Damaged HepG2 Cells
3.7.3. Regulation of BLP-1 on Antioxidant Enzyme Activity and MDA Levels
3.8. Protective Effect and Mechanism of BLP-1 on Mice with ALD
3.8.1. Effect of BLP-1 on Liver Index in ALD Mice
3.8.2. Changes in Transaminase Levels in ALD Mice
3.8.3. Histopathological Analysis After BLP-1 Treatment
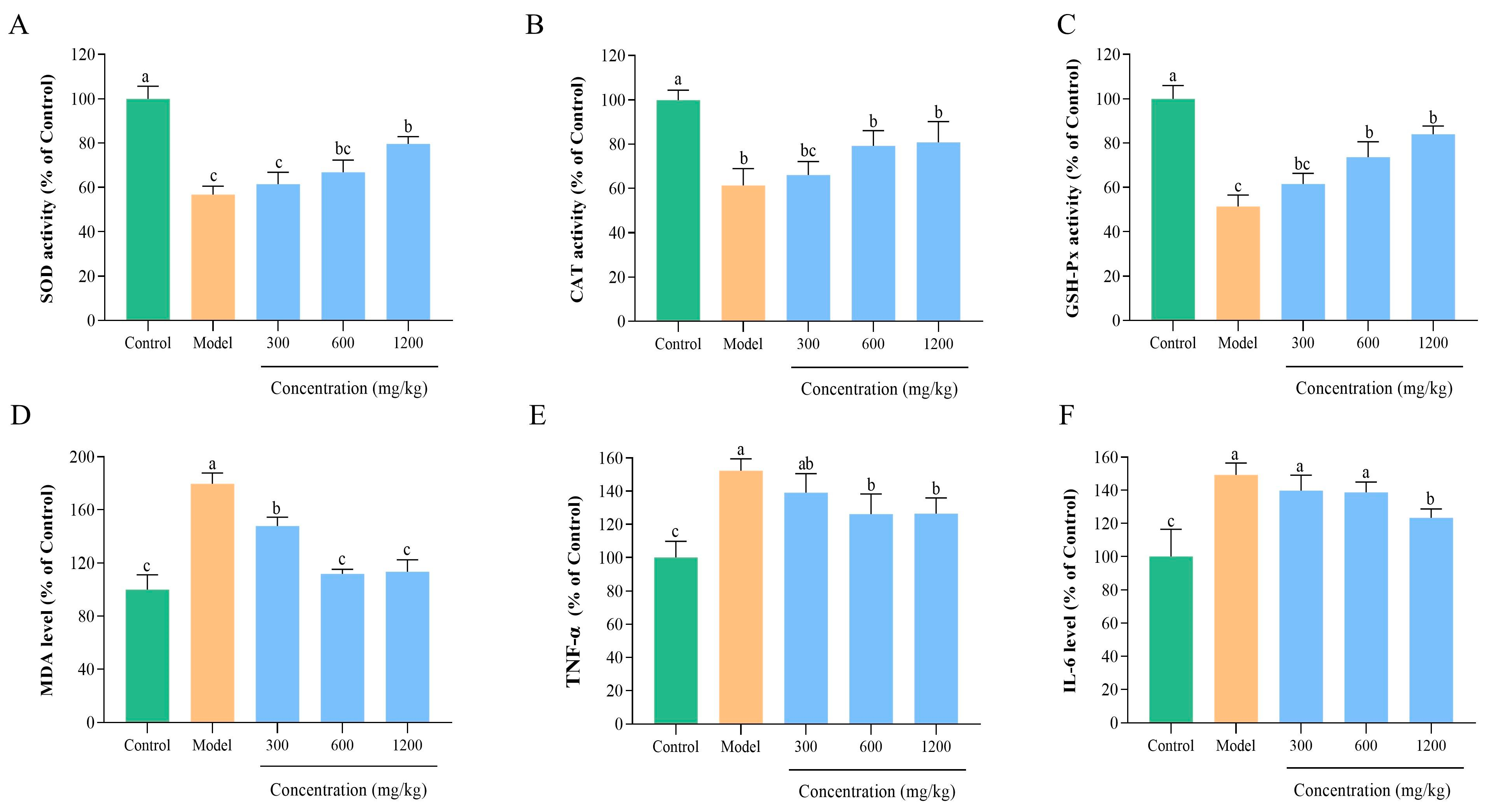
3.8.4. BLP-1 Inhibits Hepatic Oxidative Stress
3.8.5. BLP-1 Decreased Inflammatory Responses
3.9. Screening of Antioxidant Peptides in BLP-1
3.9.1. Screening Using PeptideRanker and BIOPEP Databases
3.9.2. Prediction of Peptide Physicochemical Properties
3.9.3. Molecular Docking with DPPH/ABTS
3.10. Peptide Synthesis and Validation
3.11. Molecular Docking with KeaP1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehm, J.; Guiraud, J.; Poulnais, R.; Shield, K.D. Alcohol dependence and very high risk level of alcohol consumption: A life-threatening and debilitating disease. Addict. Biol. 2018, 23, 961–968. [Google Scholar] [CrossRef]
- Giuliano, C.; Goodlett, C.R.; Economidou, D.; García-Pardo, M.P.; Belin, D.; Robbins, T.W.; Bullmore, E.T.; Everitt, B.J. The Novel μ-Opioid Receptor Antagonist GSK1521498 Decreases Both Alcohol Seeking and Drinking: Evidence from a New Preclinical Model of Alcohol Seeking. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 2981–2992. [Google Scholar] [CrossRef]
- Mason, B.J.; Quello, S.; Goodell, V.; Shadan, F.; Begovic, A. Gabapentin treatment for alcohol dependence: A randomized clinical trial. JAMA Intern. Med. 2014, 174, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, E.; Suzuki, H.; Bai, W.; Pazoki, R.; Elliott, P. Alcohol consumption in the general population is associated with structural changes in multiple organ systems: A population-based study in UK Biobank. eLife Sci. 2021, 10, e65325. [Google Scholar] [CrossRef]
- Addolorato, G.; Mirijello, A.; Barrio, P.; Gual, A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J. Hepatol. 2016, 65, 618–630. [Google Scholar] [CrossRef]
- Ina, B.; Craig, J.M.; Gavin, E.A. Treatment of Alcoholic Liver Disease. Dig. Dis. 2005, 23, 275–284. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2006, 29, 245–254. [Google Scholar]
- Kim, D.K.; Kim, Y.H.; Jang, H.H.; Park, J.; Kim, J.R.; Koh, M.; Jeong, W.I.; Koo, S.H.; Park, T.S.; Yun, C.H. Estrogen-related receptor γ controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut 2013, 62, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.C.; Chen, Y.L.; Yang, S.Y. The antiapoptotic effects of different doses of β-carotene in chronic ethanol-fed rats. HepatoBiliary Surg. Nutr. 2013, 2, 132–141. [Google Scholar]
- Lai, W.; Zhang, J.; Sun, J.; Min, T.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Oxidative stress in alcoholic liver disease, focusing on proteins, nucleic acids, and lipids: A review. Int. J. Biol. Macromol. 2024, 278 Pt 3, 134809. [Google Scholar] [CrossRef]
- Xu, J.; Ma, H.Y.; Liang, S.; Sun, M.; Karin, G.; Koyama, Y.; Hu, R.; Quehenberger, O.; Davidson, N.O.; Dennis, E.A.; et al. The role of human cytochrome P450 2E1 in liver inflammation and fibrosis. Hepatol. Commun. 2017, 1, 1043–1057. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Liu, J.; Li, Z.; Fan, Q.; Jiang, Z.; Yan, F.; Wang, Z.; Huang, P.; Feng, N. Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of silymarin and enhanced lipid-lowering effect in NAFLD. J. Nanobiotechnol. 2018, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. PTR 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Grădinariu, L.; Dediu, L.; Crețu, M.; Grecu, I.R.; Docan, A.; Istrati, D.I.; Dima, F.M.; Stroe, M.D.; Vizireanu, C. The Antioxidant and Hepatoprotective Potential of Berberine and Silymarin on Acetaminophen Induced Toxicity in Cyprinus carpio L. Animals 2024, 14, 373. [Google Scholar] [CrossRef]
- Xu, W.; Lu, H.; Yuan, Y.; Deng, Z.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods 2022, 11, 2439. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Scalcon, V.; Marin, O.; Bindoli, A.; Rigobello, M.P. Nrf2-Activating Bioactive Peptides Exert Anti-Inflammatory Activity through Inhibition of the NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 4382. [Google Scholar] [CrossRef]
- Ana, P.C.C.; Francisco, E.S.L.; Francisca, F.N.A.; Yago, O.P.; Claudia, R.A.; Felipe, P.M.; Gabrielly, O.S.; Cleverson, D.T.F.; Pedro, F.N.S. Antioxidant peptides from plants: A review. Phytochem. Rev. 2023, 23, 95–104. [Google Scholar] [CrossRef]
- Mao, Z.; Jiang, H.; Sun, J.; Zhao, Y.; Gao, X.; Mao, X. Research progress in the preparation and structure-activity relationship of bioactive peptides derived from aquatic foods. Trends Food Sci. Technol. 2024, 147, 104443. [Google Scholar] [CrossRef]
- Lu, W.C.; Chiu, C.S.; Chan, Y.J.; Guo, T.P.; Lin, C.C.; Wang, P.C.; Lin, P.Y.; Mulio, A.T.; Li, P.H. An In Vivo Study to Evaluate the Efficacy of Blue Shark (Prionace glauca) Cartilage Collagen as a Cosmetic. Mar. Drugs 2022, 20, 633. [Google Scholar] [CrossRef] [PubMed]
- Leticia, M.; Milagro, R.; Fidel, T. Bioactive peptides generated from meat industry by-products. Food Res. Int. 2014, 65, 344–349. [Google Scholar] [CrossRef]
- Jayawardena, S.R.; Morton, J.D.; Brennan, C.S.; Bekhit, A.E.-D.A. Utilisation of beef lung protein powder as a functional ingredient to enhance protein and iron content of fresh pasta. Int. J. Food Sci. Technol. 2019, 54, 610–618. [Google Scholar] [CrossRef]
- Jayawardena, S.R.; Morton, J.D.; Bekhit, A.E.-D.A.; Bhat, Z.F.; Brennan, C.S. Effect of drying temperature on nutritional, functional and pasting properties and storage stability of beef lung powder, a prospective protein ingredient for food supplements. LWT 2022, 161, 113315. [Google Scholar] [CrossRef]
- O’SUllivan, S.M.; Lafarga, T.; Hayes, M.; O’BRien, N.M. Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and Alcalase. J. Food Biochem. 2017, 41, e12406. [Google Scholar] [CrossRef]
- Wei, G.; Zhao, Q.; Wang, D.; Fan, Y.; Shi, Y.; Huang, A. Novel ACE inhibitory, antioxidant and α-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking. LWT 2022, 159, 113196. [Google Scholar] [CrossRef]
- Fan, X.; Han, Y.; Sun, Y.; Zhang, T.; Tu, M.; Du, L.; Pan, D. Preparation and characterization of duck liver-derived antioxidant peptides based on LC-MS/MS, molecular docking, and machine learning. LWT 2023, 175, 114479. [Google Scholar] [CrossRef]
- Xiao, X.; Meng, J.; Chen, X.; Li, Y.; Zheng, S.; Zhang, X.; Zhang, L.; Fang, J.; Su, T.; Ma, Z.; et al. Preparation, Identification, and Cellular Antioxidant Activity Evaluation of Antioxidant Peptides from the Antler Tips of Sika Deer (Cervus nippon). J. Food Biochem. 2025, 2025, 9974213. [Google Scholar] [CrossRef]
- Chen, X.; Xia, P.; Zheng, S.; Li, Y.; Fang, J.; Ma, Z.; Zhang, L.; Zhang, X.; Hao, L.; Zhang, H. Antioxidant Peptides from the Collagen of Antler Ossified Tissue and Their Protective Effects against H2O2-Induced Oxidative Damage toward HaCaT Cells. Molecules 2023, 28, 6887. [Google Scholar] [CrossRef]
- Su, T.; Liu, L.; Lv, Q.; Zhang, X.; Hao, L.; Lv, Y.; Song, Q. Preparation of Bovine Bone Collagen Peptide Wine and Its Protective Effect on Alcoholic Liver Injury in Mice. J. Jilin Agric. Univ. 2024, 46, 1074–1080. [Google Scholar]
- Liu, H.; Fan, H.; Teng, X.; Sun, T.; Zhang, S.; Wang, N.; Zhang, X.; Liu, T.; Zhang, Y.; Wang, D. Exploring novel antioxidant cyclic peptides in corn protein hydrolysate: Preparation, identification and molecular docking analysis. Food Chem. 2025, 464, 141747. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Li, J.; Song, Y.; Chen, S.; Zhou, S.; Yang, X. Preparation, purification, and identification of novel antioxidant peptides derived from Gracilariopsis lemaneiformis protein hydrolysates. Front. Nutr. 2022, 9, 971419. [Google Scholar] [CrossRef]
- Neha, S.; Gily, N.; Tenzin, T.; Daniel, N.A.B. Sequence dependencies and biophysical features both govern cleavage of diverse cut-sites by HIV protease. Protein Sci. 2022, 31, e4366. [Google Scholar]
- Sun, K.-L.; Gao, M.; Wang, Y.-Z.; Li, X.-R.; Wang, P.; Wang, B. Antioxidant Peptides From Protein Hydrolysate of Marine Red Algae Eucheuma cottonii: Preparation, Identification, and Cytoprotective Mechanisms on H2O2 Oxidative Damaged HUVECs. Front. Microbiol. 2022, 13, 791248. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K.; Lee, S.-J.; Jeon, B.-T.; Moon, S.-H.; Kim, B.; Park, T.-K.; Han, J.-S.; Park, P.-J. Purification and characterisation of antioxidative peptides from enzymatic hydrolysates of venison protein. Food Chem. 2009, 114, 1365–1370. [Google Scholar] [CrossRef]
- Lee, W.-S.; Jeon, J.-K.; Byun, H.-G. Characterization of a novel antioxidative peptide from the sand eel Hypoptychus dybowskii. Process Biochem. 2011, 46, 1207–1211. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, G.; Li, X.; Zhao, B.; Zhou, S. Enzymatic hydrolysis of protein from small yellow croaker (Psendosciaena polyactis) and evaluation of its antioxidant activity. J. Food Biochem. 2013, 37, 278–285. [Google Scholar] [CrossRef]
- Esfandi, R.; Willmore, W.G.; Tsopmo, A. Peptidomic analysis of hydrolyzed oat bran proteins, and their in vitro antioxidant and metal chelating properties. Food Chem. 2019, 279, 49–57. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Y.; Wan, Y.; Liang, Y.; Liu, Q.; Wei, M.; Hou, T. Preparation, Structural Identification, and Screening of Egg-Derived Peptides with Facilitating Alcohol Metabolism Activity. Foods 2024, 13, 745. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, P.; Zhu, C.; Liu, Y.; Zhao, Z. Preparation of Antioxidant Peptides from Chicken Bone Proteins and the Influence of Their Compositional Characteristics on Antioxidant Activity. Foods 2024, 13, 4171. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Yu, S.; He, M.; Yu, S.; Xiao, H.; Song, Y. Antioxidant and functional properties of cowhide collagen peptides. J. Food Sci. 2021, 86, 1802–1818. [Google Scholar] [CrossRef]
- Chen, B.; Xia, Z.; Ye, H.; Wang, Q.; Huang, W.; Zhang, L.; Chen, W.; Liang, D.; Liang, X.; Yin, Y. Response surface optimization of selenium-enriched Moringa oleifera seed peptides with antioxidant, ACEI and XOI activities. J. Food Meas. Charact. 2023, 17, 1289–1299. [Google Scholar] [CrossRef]
- Liu, Q.; Xun, G.; Feng, Y. The state-of-the-art strategies of protein engineering for enzyme stabilization. Biotechnol. Adv. 2019, 37, 530–537. [Google Scholar] [CrossRef]
- Gao, D.; Chen, H.; Liu, H.; Yang, X.; Guo, P.; Cao, X.; Cai, Y.; Xu, H.; Yang, J. Structure characterization and antioxidant activity analysis of polysaccharides from Lanzhou Lily. Front. Nutr. 2022, 9, 976607. [Google Scholar] [CrossRef]
- Yuan, E.; Nie, S.; Liu, L.; Ren, J. Study on the interaction of Hericium erinaceus mycelium polysaccharides and its degradation products with food additive silica nanoparticles. Food Chem. X 2021, 12, 100172. [Google Scholar] [CrossRef]
- Maria, I.; Elisavet, B.; Stamatia, C.; Adriana, S.; Paschalina, C. Modeling and Optimization of Phenolic Compounds from Sage (Salvia fruticosa L.) Post-Distillation Residues: Ultrasound- versus Microwave-Assisted Extraction. Antioxidants 2023, 12, 549. [Google Scholar]
- Wang, L.; Li, Z.; Fan, X.; Zhang, T.; Wang, H.; Ye, K. Novel antioxidant peptides from bovine blood: Purification, identification and mechanism of action. LWT 2024, 205, 116499. [Google Scholar] [CrossRef]
- Yang, W. Evaluation of the antioxidant activity and identification of potential antioxidant peptides in commercially available probiotic Cheddar cheese. LWT 2024, 205, 116486. [Google Scholar] [CrossRef]
- Jin, H.-X.; Xu, H.-P.; Li, Y.; Zhang, Q.-W.; Xie, H. Preparation and Evaluation of Peptides with Potential Antioxidant Activity by Microwave Assisted Enzymatic Hydrolysis of Collagen from Sea Cucumber Acaudina Molpadioides Obtained from Zhejiang Province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-M.; Lu, S.-Z.; Li, Y.-S.; Wang, H.; Shi, Y.; Zhang, L.; Tu, Z.-C. Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem. 2022, 373, 131539. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, H.; Zhang, H.; Wang, X.; Fu, Y.; Wang, Q.; Liu, H.; Yong, Y.; Guo, J.; Liu, J. Biosafety consideration of nanocellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2024, 265, 130900. [Google Scholar] [CrossRef] [PubMed]
- Asadian, S.; Piryaei, A.; Gheibi, N.; Kalantari, B.A.; Davarpanah, M.R.; Azad, M.; Kapustina, V.; Alikhani, M.; Nejad, S.M.; Alikhani, H.K.; et al. Rhenium Perrhenate ((188)ReO(4)) Induced Apoptosis and Reduced Cancerous Phenotype in Liver Cancer Cells. Cells 2022, 11, 305. [Google Scholar] [CrossRef]
- He, Y.; Peng, L.; Xiong, H.; Liu, W.; Zhang, H.; Peng, X.; Zhu, X.; Guo, F.; Sun, Y. The profiles of durian (Durio zibethinus Murr.) shell phenolics and their antioxidant effects on H2O2-treated HepG2 cells as well as the metabolites and organ distribution in rats. Food Res. Int. 2023, 163, 112122. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, M.; Chao, X.; Zhang, C.; Yang, H.; Chen, J.; Zhao, C.; Zhou, B. Acidifiers Attenuate Diquat-Induced Oxidative Stress and Inflammatory Responses by Regulating NF-κB/MAPK/COX-2 Pathways in IPEC-J2 Cells. Antioxidants 2022, 11, 2002. [Google Scholar] [CrossRef]
- Kong, Y.; Feng, M.; Sun, J. Novel antioxidant peptides in fermented pork sausage: Purification, characterization, and cytoprotective functions on Caco-2 cells. Food Chem. 2023, 426, 136566. [Google Scholar] [CrossRef]
- Taghikhani, E.; Fromm, M.F.; König, J. Assays for Analyzing the Role of Transport Proteins in the Uptake and the Vectorial Transport of Substances Affecting Cell Viability. Methods Mol. Biol. 2017, 1601, 123–135. [Google Scholar]
- Zhang, L.; Yang, Z.; Zeng, W.; Liang, B.; Huang, Z.; Wang, Q.; Li, Z.; Chen, T.; Yan, B. Hierarchical Vanadium Detoxification Mechanisms in Gram-Positive Enterococcus faecalis: Extracellular Chelation, Antioxidant Defense, and Metabolic Reprogramming. ACS EST Eng. 2025. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Feng, B.; Xu, S.; et al. Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model. Int. J. Mol. Sci. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Xenofontos, R.; Chatzimichail, G.; Christou, A.; Kashfi, K.; Fotopoulos, V. Exploring the Potential of Nitric Oxide and Hydrogen Sulfide (NOSH)-Releasing Synthetic Compounds as Novel Priming Agents against Drought Stress in Medicago sativa Plants. Biomolecules 2020, 10, 120. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, C.I. Potentials of Raspberry Ketone as a Natural Antioxidant. Antioxidants 2021, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zheng, Y.; Cheng, Z.; Ji, N.; Niu, C.; Wang, Y.; Huang, T.; Li, R.; Huang, M.; Chen, X.; et al. One-year follow-up study after patients with severe COVID-19 received human umbilical cord mesenchymal stem cells treatment. Stem Cell Res. Ther. 2022, 13, 321. [Google Scholar] [CrossRef]
- Chen, J.; Shu, Y.; Chen, Y.; Ge, Z.; Zhang, C.; Cao, J.; Li, X.; Wang, Y.; Sun, C. Evaluation of Antioxidant Capacity and Gut Microbiota Modulatory Effects of Different Kinds of Berries. Antioxidants 2022, 11, 1020. [Google Scholar] [CrossRef]
- Xie, R.-H.; Xiao, S.; Ma, D.; Wang, B.; Chen, G.-C.; Xiang, J.-H.; Wang, J.-H. Protective mechanism of antioxidant peptides derived from dry-cured ham against ultraviolet A-induced oxidative damage in HaCat cells. Food Biosci. 2024, 62, 105394. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, L.; Yin, Y.; Gong, S.; Yang, Y.; Chen, S.; Han, M.; Duan, Y. Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice. Antioxidants 2022, 11, 1391. [Google Scholar] [CrossRef]
- de Stoppelaar, S.F.; van ‘t Veer, C.; Claushuis, T.A.; Albersen, B.J.; Roelofs, J.J.; van der Poll, T. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 2014, 124, 3781–3790. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, Q.; Chen, H.; Hu, E.; Zhao, C.; Gong, X. Characterizations and hepatoprotective effect of polysaccharides from Mori Fructus in rats with alcoholic-induced liver injury. Int. J. Biol. Macromol. 2017, 102, 60–67. [Google Scholar] [CrossRef]
- Govindan, S.; Jayabal, A.; Shanmugam, J.; Ramani, P. Antioxidant and hepatoprotective effects of Hypsizygus ulmarius polysaccharide on alcoholic liver injury in rats. Food Sci. Hum. Wellness 2021, 10, 523–535. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Hartmut, J. Oxidant Stress, Antioxidant Defense, and Liver Injury. In Drug-Induced Liver Disease; Academic Press: Cambridge, MA, USA, 2013; pp. 71–84. [Google Scholar]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R.; Kaneider, N.C. Pathways of liver injury in alcoholic liver disease. J. Hepatol. 2011, 55, 1159–1161. [Google Scholar] [CrossRef]
- Cerrato, A.; Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Montone, A.M.I.; Montone, C.M.; Laganà, A. Investigating the Short Peptidome Profile of Italian Dry-Cured Ham at Different Processing Times by High-Resolution Mass Spectrometry and Chemometrics. Int. J. Mol. Sci. 2022, 23, 3193. [Google Scholar] [CrossRef] [PubMed]
- Chiozzi, R.Z.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Samperi, R.; Laganà, A. Purification and identification of endogenous antioxidant and ACE-inhibitory peptides from donkey milk by multidimensional liquid chromatography and nanoHPLC-high resolution mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 5657–5666. [Google Scholar] [CrossRef]
- Han, R.; Maycock, J.; Murray, B.S.; Boesch, C. Identification of angiotensin converting enzyme and dipeptidyl peptidase-IV inhibitory peptides derived from oilseed proteins using two integrated bioinformatic approaches. Food Res. Int. 2019, 115, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lu, J.; Zhang, Y.; Wang, J.; Wang, S.; Fan, H.; Zhang, J.; Dai, W.; Gao, J.; Yu, H. Structural properties, antioxidant and immune activities of low molecular weight peptides from soybean dregs (Okara). Food Chem. X 2021, 12, 100175. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Pliszka, M.; Mogut, D.; Darewicz, M. Characteristics of Biopeptides Released In Silico from Collagens Using Quantitative Parameters. Foods 2020, 9, 965. [Google Scholar] [CrossRef]
- Li, X.-X.; Han, L.-J.; Chen, L.-J. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. J. Sci. Food Agric. 2008, 88, 1660–1666. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Wang, G.; Sun, S.; Liu, R.; Hong, B.; Gao, R.; Bai, K. Processing Optimization and Characterization of Angiotensin-Ι-Converting Enzyme Inhibitory Peptides from Lizardfish (Synodus macrops) Scale Gelatin. Mar. Drugs 2018, 16, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, Y.; Tao, S.; Liang, W.; Zhang, J.; Zhang, Y.; Xuan, Z.; Xu, J.; Peng, C.; Wu, H.; et al. The Integrated Study on the Chemical Profiling to Explore the Constituents and Mechanism of Traditional Chinese Medicine Preparation Huatuo Jiuxin Pills Based on UPLC-Q-TOF/MS(E) and Network Pharmacology. Front. Mol. Biosci. 2022, 9, 818285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Abubaker, M.A.; Li, Z.; He, Y.; Shu, Q.; Li, L.; Liu, Y. Bioactive peptides with antioxidant and ACE inhibitory properties in goat milk protein hydrolysates: Peptidomics and molecular docking study. Int. J. Biol. Macromol. 2025, 299, 140286. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wen, H.; Zhou, S.; Yan, X.; Li, H. Patchouli Alcohol Inhibits D-Gal Induced Oxidative Stress and Ameliorates the Quality of Aging Cartilage via Activating the Nrf2/HO-1 Pathway in Mice. Oxidative Med. Cell. Longev. 2022, 2022, 6821170. [Google Scholar] [CrossRef]
| Source | DPPH Clearance Rate (%) | ABTS Clearance Rate (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F-Value | p-Value | Significance | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance | |
| Model | 100.71 | 9 | 11.19 | 39.87 | <0.0001 | Yes | 104.28 | 9 | 11.59 | 28.45 | 0.0001 | Yes |
| A | 4.31 | 1 | 4.31 | 15.35 | 0.0058 | 1.19 | 1 | 1.19 | 2.91 | 0.1317 | ||
| B | 5.99 | 1 | 5.99 | 21.33 | 0.0024 | 4.99 | 1 | 4.99 | 12.26 | 0.0100 | ||
| C | 2.13 | 1 | 2.13 | 7.60 | 0.0283 | 3.54 | 1 | 3.54 | 8.69 | 0.0215 | ||
| AB | 3.48 | 1 | 3.48 | 12.39 | 0.0097 | 0.6806 | 1 | 0.6806 | 1.67 | 0.2371 | ||
| AC | 2.04 | 1 | 2.04 | 7.29 | 0.0307 | 2.42 | 1 | 2.42 | 5.94 | 0.0450 | ||
| BC | 0.06 | 1 | 0.06 | 0.2139 | 0.6578 | 0.1332 | 1 | 0.1332 | 0.3271 | 0.5852 | ||
| A2 | 38.84 | 1 | 38.84 | 138.37 | <0.0001 | 37.00 | 1 | 37.00 | 90.85 | <0.0001 | ||
| B2 | 24.65 | 1 | 24.65 | 87.85 | <0.0001 | 15.43 | 1 | 15.43 | 37.89 | 0.0005 | ||
| C2 | 11.15 | 1 | 11.15 | 39.71 | 0.0004 | 29.66 | 1 | 29.66 | 75.84 | <0.0001 | ||
| Residual | 1.96 | 7 | 0.2807 | 2.85 | 7 | 0.4072 | ||||||
| Lack of Fit | 1.50 | 3 | 0.4998 | 4.30 | 0.0965 | No | 1.60 | 3 | 0.5342 | 1.71 | 0.3017 | No |
| Pure Error | 0.4653 | 4 | 0.1163 | 1.25 | 4 | 0.3120 | ||||||
| Cor Total | 102.68 | 16 | 107.13 | 16 | ||||||||
| R2 = 0.9809 | Radj2 = 0.9563 | R2 = 0.9734 | Radj2 = 0.9392 | |||||||||
| CV% | 0.6311 | 0.8276 | ||||||||||
| Name | Sequence | Pepitidebank Score | Sequence | Charge | Mw | Toxin | GRAVY |
|---|---|---|---|---|---|---|---|
| KF6 | KPFPFF | 0.992914 | 6 | 1 | 781.95 | Non-Toxin | 0.217 |
| MP6 | MWPPLP | 0.980232 | 6 | 0 | 739.94 | Non-Toxin | −2.96 |
| FG6 | FYGWPG | 0.978696 | 6 | 0 | 725.80 | Non-Toxin | −0.300 |
| DW6 | DGGGWW | 0.970555 | 6 | −1 | 676.69 | Non-Toxin | −1.083 |
| FP7 | FGPPPPP | 0.969438 | 7 | 0 | 707.83 | Toxin | −0.800 |
| WG6 | WGPPGG | 0.959932 | 6 | 0 | 569.62 | Non-Toxin | −0.883 |
| FW7 | FFSPGVW | 0.95771 | 7 | 0 | 838.96 | Non-Toxin | 0.871 |
| PG16 | PPPGPPPPPGPPPPPG | 0.955487 | 16 | 0 | 1451.69 | Toxin | −1.375 |
| DGG6 | DGGAWW | 0.954871 | 6 | −1 | 690.71 | Non-Toxin | −0.717 |
| GL8 | GWNIPMGL | 0.953627 | 8 | 0 | 887.07 | Non-Toxin | 0.425 |
| GP9 | GPPPAPPLP | 0.953422 | 9 | 0 | 842.00 | Non-Toxin | −0.489 |
| MF6 | MIKPFF | 0.95295 | 6 | 1 | 782.02 | Non-Toxin | 1.083 |
| Name | DPPH | ABTS | ||||
|---|---|---|---|---|---|---|
| Binding Energy (kcal/mol) | Conventional Hydrogen Bond | Binding Site | Binding Energy (kcal/mol) | Conventional Hydrogen Bond | Binding Site | |
| MP6 | −3.4 | 0 | / | −3.4 | 1 | Met1 |
| FG6 | −4.0 | 0 | / | −4.3 | 2 | Trp4 |
| DW6 | −3.6 | 2 | Trp5, Trp6 | −3.6 | / | / |
| WG6 | −3.9 | 2 | Gly2, Gly6 | −3.6 | 2 | Pro3, Gly5 |
| DGG6 | −3.4 | 0 | / | −3.9 | 3 | Ala4, Trp5, Trp6 |
| GP9 | −4.3 | 2 | Ala5, Leu8 | −4.3 | 2 | Leu8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Zhang, X.; Li, Y.; Su, T.; Zheng, S.; Fang, J.; Lv, Q.; Wang, D.; Hao, L. Extraction, Purification and Identification of Bovine Lung Peptides and Its Antioxidant Effects on H2O2-Induced HepG2 Cells and Mice with Alcoholic Liver Injury. Antioxidants 2025, 14, 1314. https://doi.org/10.3390/antiox14111314
Xiao X, Zhang X, Li Y, Su T, Zheng S, Fang J, Lv Q, Wang D, Hao L. Extraction, Purification and Identification of Bovine Lung Peptides and Its Antioxidant Effects on H2O2-Induced HepG2 Cells and Mice with Alcoholic Liver Injury. Antioxidants. 2025; 14(11):1314. https://doi.org/10.3390/antiox14111314
Chicago/Turabian StyleXiao, Xingyu, Xunming Zhang, Yi Li, Tong Su, Shuo Zheng, Jiayuan Fang, Qinchuan Lv, Dacheng Wang, and Linlin Hao. 2025. "Extraction, Purification and Identification of Bovine Lung Peptides and Its Antioxidant Effects on H2O2-Induced HepG2 Cells and Mice with Alcoholic Liver Injury" Antioxidants 14, no. 11: 1314. https://doi.org/10.3390/antiox14111314
APA StyleXiao, X., Zhang, X., Li, Y., Su, T., Zheng, S., Fang, J., Lv, Q., Wang, D., & Hao, L. (2025). Extraction, Purification and Identification of Bovine Lung Peptides and Its Antioxidant Effects on H2O2-Induced HepG2 Cells and Mice with Alcoholic Liver Injury. Antioxidants, 14(11), 1314. https://doi.org/10.3390/antiox14111314







