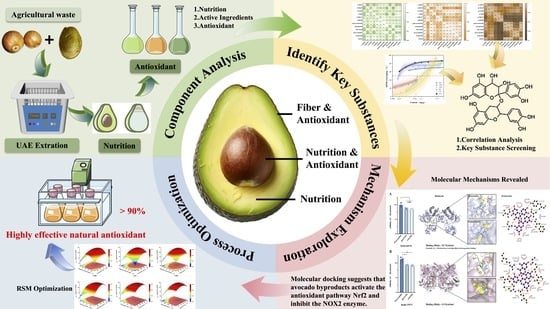
Bioactive Antioxidants from Avocado By-Products: Mechanistic Study and Laboratory-Scale Extraction Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Nutritional Analysis
2.4. Analysis of Bioactive Components
2.4.1. Total Polyphenol Content
2.4.2. Total Flavonoid Content
2.4.3. Crude Polysaccharide Content
2.4.4. Terpenoid Content
2.4.5. Proanthocyanidin Content
2.4.6. Tannic Acid Content
2.4.7. Determination of Catechin and Quercetin Content
2.5. Antioxidant Assay
2.5.1. DPPH Scavenging Activity (DPPH)
- A0 is absorbance after reacting the sample extract with the solvent;
- As is absorbance after reacting the sample extract with DPPH ethanol solution.
2.5.2. ABTS•+ Scavenging Capacity (ABTS)
2.5.3. Hydroxyl Radical Scavenging Capacity (HFR)
- A0 is absorbance after reacting with deionized water and hydrogen peroxide.
- A2 is absorbance after reacting the sample extract and deionized water.
2.5.4. Superoxide Anion Scavenging Activity (SAFR)
2.5.5. Oxygen Radical Absorbance Capacity (ORAC)
2.5.6. Iron Reducing Antioxidant Power (FRAP)
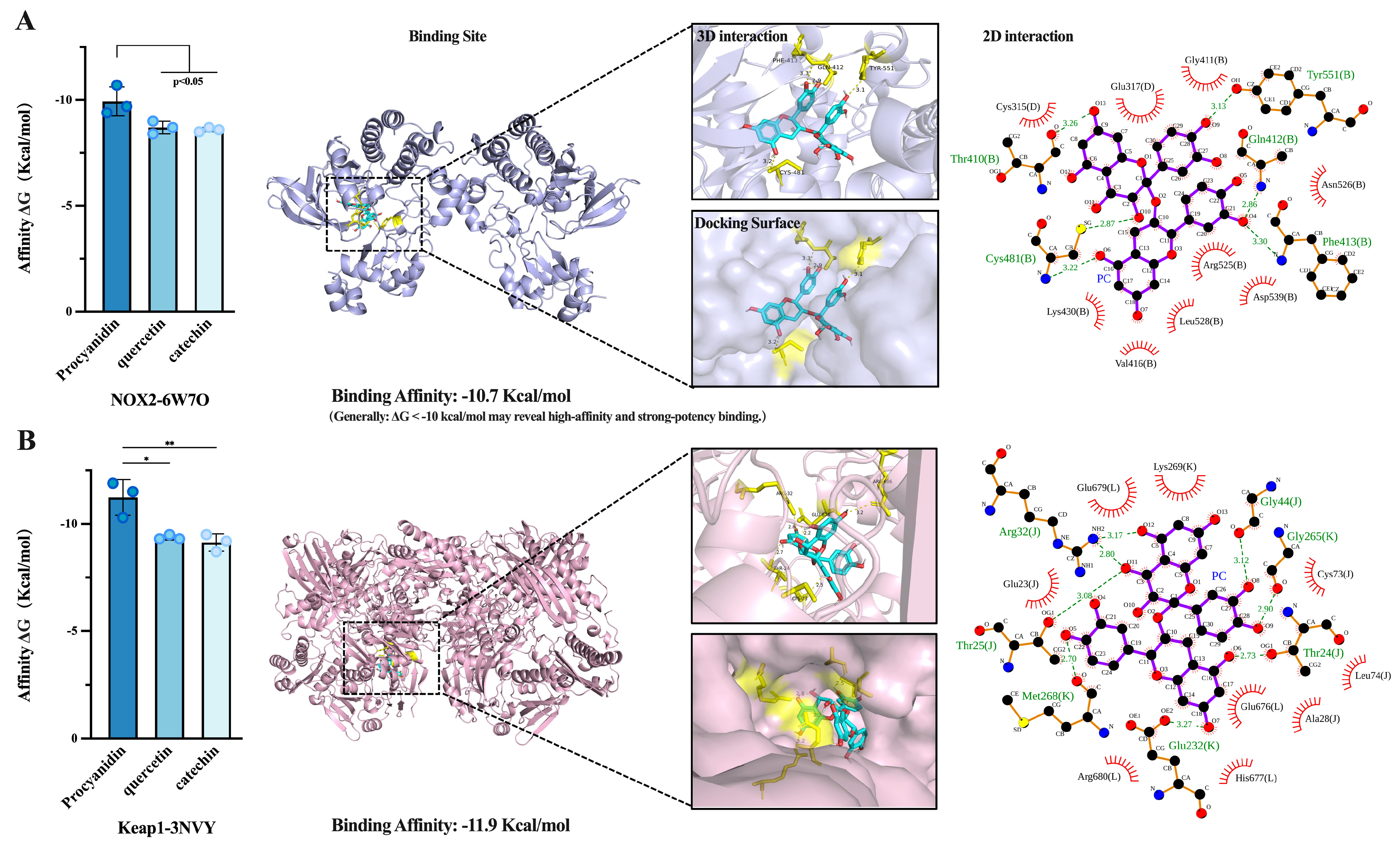
2.6. Molecular Docking Simulation of Antioxidant Mechanisms
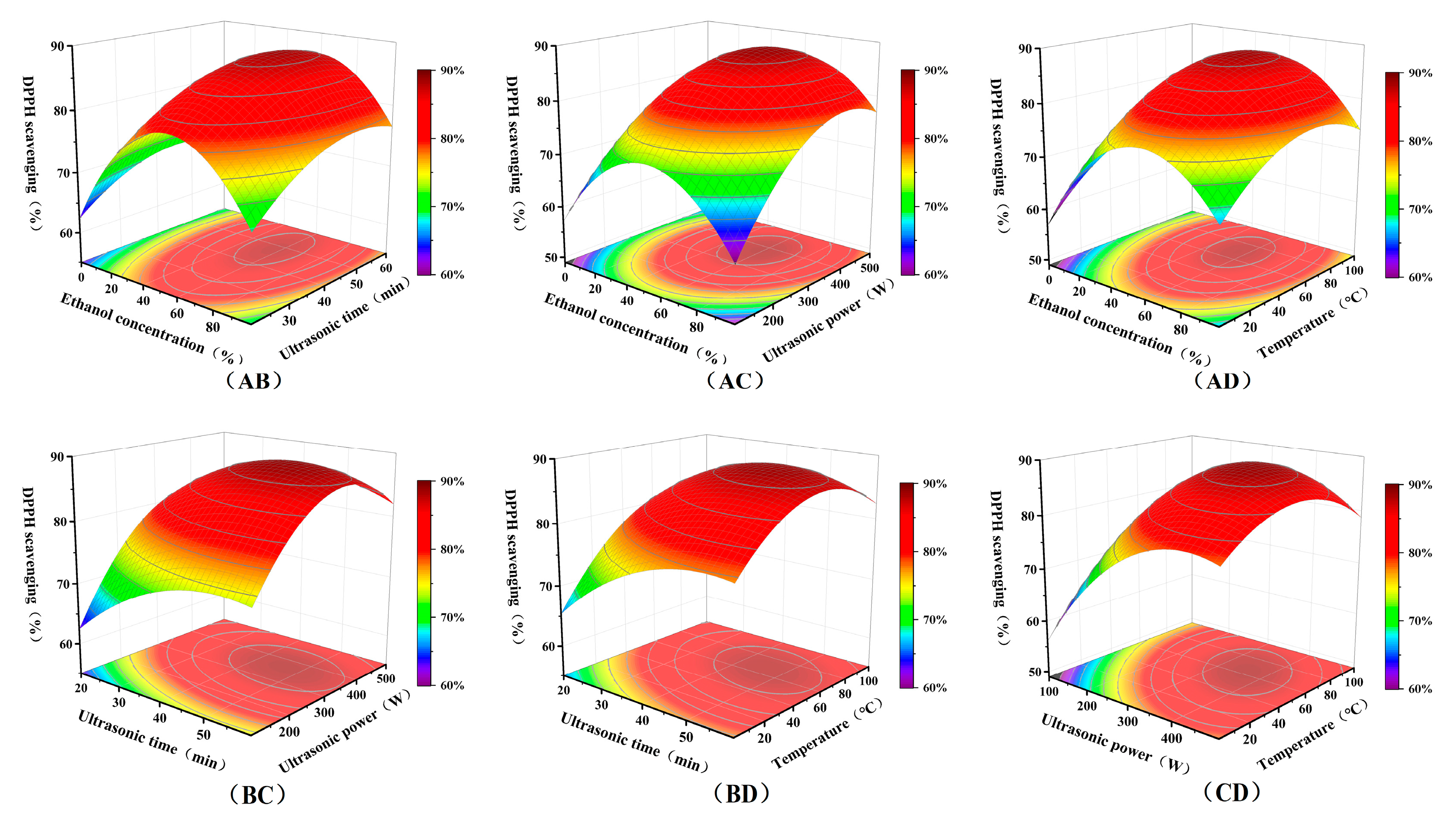
2.7. Optimization of UAE Process Parameters Using Response Surface Methodology (RSM)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Component Analysis
3.2. Analysis of Bioactive Components
3.3. Evaluation of Antioxidant Activity
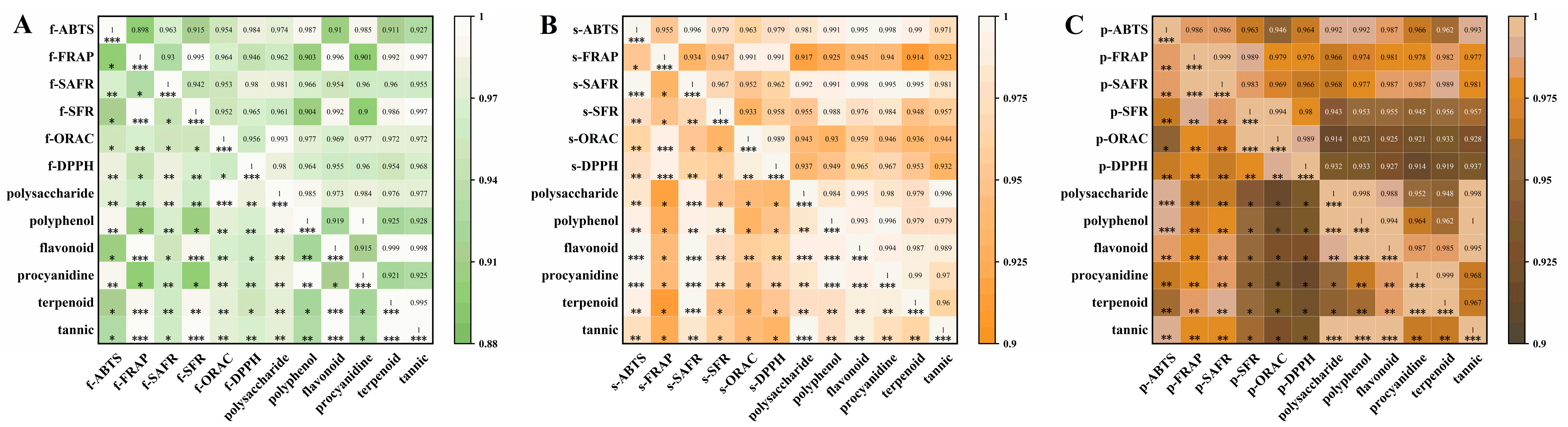
3.3.1. Correlation Analysis Between Bioactive Compound Content and Antioxidant Capacity
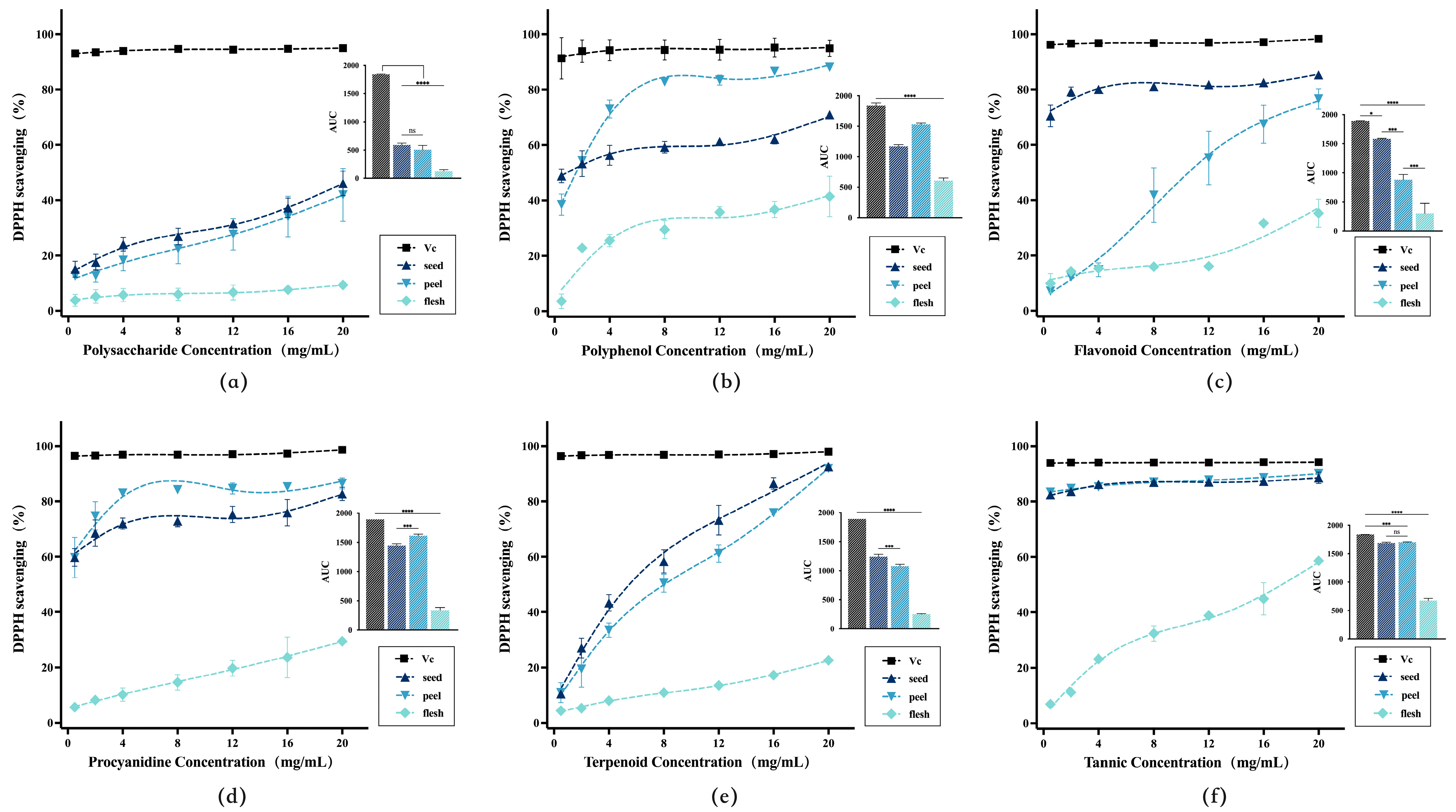
3.3.2. Preliminary Comparison of In Vitro Antioxidant Activity in Extracts of Active Compounds from Avocado Seeds
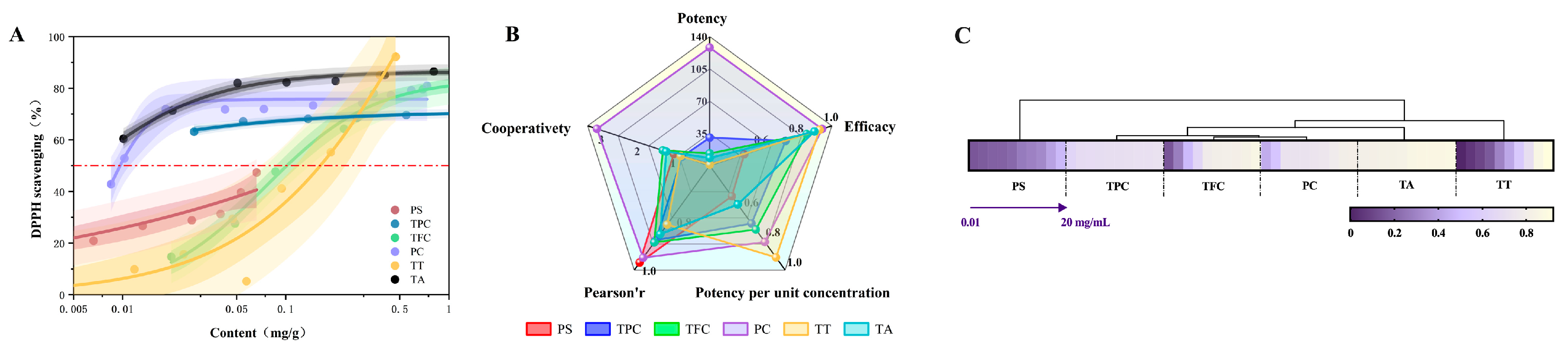
3.3.3. Analysis of Antioxidant Potency of Active Compounds in Fruit Seeds
3.4. Molecular Docking Simulation of Antioxidant Mechanisms
3.5. RSM Method for Optimizing the Extraction Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPH | DPPH Scavenging |
| ABTS | ABTS Scavenging |
| FRAP | FRAP Ion Reducing Antioxidant Power |
| HFR | Hydroxyl Radical Scavenging |
| SAFR | Superoxide Anion Scavenging |
| ORAC | Oxygen Radical Absorbance Capacity |
| PS | Polysaccharide |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| PC | Procyanidin |
| TT | Terpenoid |
| TA | Tannic acid |
References
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado Seed: A Comparative Study of Antioxidant Content and Capacity in Protecting Oil Models from Oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [PubMed]
- Rosero, J.C.; Cruz, S.; Osorio, C.; Hurtado, N. Analysis of Phenolic Composition of Byproducts (Seeds and Peels) of Avocado (Persea americana Mill.) Cultivated in Colombia. Molecules 2019, 24, 3209. [Google Scholar] [CrossRef]
- Nyakang’i, C.O.; Ebere, R.; Marete, E.; Arimi, J.M. Avocado production in Kenya in relation to the world, Avocado by-products (seeds and peels) functionality and utilization in food products. Appl. Food Res. 2023, 3, 100275. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borras-Linares, I.; Lozano-Sanchez, J.; Segura-Carretero, A. Comprehensive identification of bioactive compounds of avocado peel by liquid chromatography coupled to ultra-high-definition accurate-mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Suzuki, S.; Fujisawa, S. Radical-scavenging and Pro-/anti-inflammatory Activity of Tetracycline and Related Phenolic Compounds With or Without Visible Light Irradiation. In Vivo 2020, 34, 81–94. [Google Scholar] [CrossRef]
- Karim, M.R.; Miletti-Gonzalez, K.E.; Aryee, A.N.A.; Besong, S.A. Phytochemical Analysis and Antioxidant Activities of Prunus africana Bark, Leea indica and Paullinia pinnata Leaf Extracts. Antioxidants 2025, 14, 666. [Google Scholar] [CrossRef]
- Bazdar, M.; Baghery, M.; Hosseinzadeh, N.; Sarabi-Aghdam, V. Optimization Extraction Conditions of Bioactive Compounds of Carum copticum L. Seed by Microwave Using Response Surface Methodology (RSM). Food Anal. Methods 2025, 18, 1189–1202. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, F.; Zabini, F. Industrialization of hydrodynamic cavitation in plant resource extraction. Curr. Opin. Chem. Eng. 2025, 48, 101140. [Google Scholar] [CrossRef]
- Ramirez-Brewer, D.; Quintana, S.E.; Garcia-Zapateiro, L.A. Modeling and optimization of microwave-assisted extraction of total phenolics content from mango (Mangifera indica) peel using response surface methodology (RSM) and artificial neural networks (ANN). Food Chem. X 2024, 22, 101420. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lu, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef]
- Perez, M.; Dominguez-Lopez, I.; Lamuela-Raventos, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Zulkifli, S.A.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E. Optimization of Total Phenolic and Flavonoid Contents of Defatted Pitaya (Hylocereus polyrhizus) Seed Extract and Its Antioxidant Properties. Molecules 2020, 25, 787. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Z.; Liu, Y.; Dong, B.; Yang, C.; Li, H. Ultrasound-assisted extraction, optimization, and purification of total flavonoids from Daphnegenkwa and analysis of their antioxidant, anti-inflammatory, and analgesic activities. Ultrason. Sonochem. 2024, 111, 107079. [Google Scholar] [CrossRef]
- Wang, F.; Ye, S.; Ding, Y.; Ma, Z.; Zhao, Q.; Zang, M.; Li, Y. Research on structure and antioxidant activity of polysaccharides from Ginkgo biloba leaves. J. Mol. Struct. 2022, 1252, 132185. [Google Scholar] [CrossRef]
- Poirier, B.C.; Buchanan, D.A.; Rudell, D.R.; Mattheis, J.P. Differential Partitioning of Triterpenes and Triterpene Esters in Apple Peel. J. Agric. Food Chem. 2018, 66, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.M.; de Castro, W.V.; Castro, A.H.F.; Duarte-Almeida, J.M. Validated spectrophotometric method for quantification of total triterpenes in plant matrices. Daru 2020, 28, 281–286. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Li, D.; Liu, X. Anthocyanin and proanthocyanidin from Aronia melanocarpa (Michx.) Ell.: Purification, fractionation, and enzyme inhibition. Food Sci. Nutr. 2023, 11, 3911–3922. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.S. Valorization of faba bean peels for fungal tannase production and its application in coffee tannin removal. Food Chem. X 2024, 23, 101678. [Google Scholar] [CrossRef] [PubMed]
- Döner, D.; Icier, F. Exergoeconomic analysis of ultrasound-assisted extraction of tannins from acorn fruit. J. Food Eng. 2024, 367, 111851. [Google Scholar] [CrossRef]
- Moore, G.; Brooks, P.; Pappalardo, L.; Boufridi, A. Phenolic profiles of Australian monofloral Eucalyptus, Corymbia, Macadamia and Lophostemon honeys via HPLC-DAD analysis. Food Chem. 2025, 462, 140900. [Google Scholar] [CrossRef] [PubMed]
- Anticona, M.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J.; Blesa, J. Comprehensive analysis of polyphenols from hybrid Mandarin peels by SPE and HPLC-UV. LWT 2022, 165, 113770. [Google Scholar] [CrossRef]
- Diment, D.; Musl, O.; Balakshin, M.; Rigo, D. Guidelines for Evaluating the Antioxidant Activity of Lignin via the 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay. ChemSusChem 2025, 18, e202402383. [Google Scholar] [CrossRef]
- Srivastava, R.; Mishra, N.; Arshi; Tripathi, S.; Smriti; Fatima, N.T.; Mishra, N. Influence of fruit stages on chemical compositions, phytochemicals, and antioxidant activity of wood apple (Feronia limonia (L.) Swingle). Heliyon 2025, 11, e42223. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, J.; Lee, S.H.; Kim, J.H.; Kim, D.G.; Ha, T.H.; Kim, S.H. Evaluation of Anthocyanin Profiling, Total Phenolic and Flavonoid Content, and Antioxidant Activity of Korean Rubus Accessions for Functional Food Applications and Breeding. Antioxidants 2025, 14, 12. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Wen, F.; Yan, X.; Zhang, Y.; Zhong, Z. Preparation, characterization, antioxidant and antifungal activities of benzoic acid compounds grafted onto chitosan. Int. J. Biol. Macromol. 2024, 259, 129096. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Z.; Liu, Y.; Wang, X.; Zhang, H.; Shang, R.; Laba, C.; Wujin, C.; Hao, B.; Wang, S. Structural characteristic of polysaccharide isolated from Nostoc commune, and their potential as radical scavenging and antidiabetic activities. Sci. Rep. 2022, 12, 22155. [Google Scholar] [CrossRef]
- Acosta-Quiroga, K.; Rocha-Valderrama, E.; Zuniga-Bustos, M.; Mera-Adasme, R.; Cabrera-Barjas, G.; Olea-Azar, C.; Moncada-Basualto, M. Gross Antioxidant Capacity and Anti-Inflammatory Potential of Flavonol Oxidation Products: A Combined Experimental and Theoretical Study. Antioxidants 2025, 14, 479. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, S.; Wu, X.; He, Y.; Shen, H.; Tang, S.; Zhu, F.; Luo, Y. Phytochemical Profile and Antioxidant Activity of the Tuber and Peel of Pachyrhizus erosus. Antioxidants 2025, 14, 416. [Google Scholar] [CrossRef]
- Lima, T.P.B.; Coimbra, P.P.S.; da Silva Antonio, A.; Pereira, H.M.G.; da Silva, G.R.P.; da Veiga-Junior, V.F.; Silva, O.F.; Felzenszwalb, I.; Araujo-Lima, C.F.; Teodoro, A.J. Antioxidant activity, phytochemical composition, and antitumor capacity of Amazonian fruits tapereba (Spondias mombin) and murici (Byrsonima crassifolia). Arch. Pharm. 2025, 358, e2400758. [Google Scholar] [CrossRef]
- Puscas, A.; Tanislav, A.E.; Marc, R.A.; Muresan, V.; Muresan, A.E.; Pall, E.; Cerbu, C. Cytotoxicity Evaluation and Antioxidant Activity of a Novel Drink Based on Roasted Avocado Seed Powder. Plants 2022, 11, 83. [Google Scholar] [CrossRef]
- Sanchez-Quezada, V.; Campos-Vega, R.; Loarca-Pina, G. Prediction of the Physicochemical and Nutraceutical Characteristics of ‘Hass’ Avocado Seeds by Correlating the Physicochemical Avocado Fruit Properties According to Their Ripening State. Plant Foods Hum. Nutr. 2021, 76, 311–318. [Google Scholar] [CrossRef]
- Salazar-Irrazabal, M.D.; Ramirez-Tixe, E.E.; Velasquez-Barreto, F.F.; Bello-Perez, L.A. Avocado seed starch: Effect of the variety on molecular, physicochemical, and digestibility characteristics. Int. J. Biol. Macromol. 2023, 247, 125746. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.G.; Spricigo, P.C.; Purgatto, E.; Alencar, S.M.; Sartori, S.F.; Jacomino, A.P. Chemical composition, nutritional value and bioactive compounds in six uvaia accessions. Food Chem. 2019, 294, 547–556. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, F. Characterization of physicochemical properties, fatty acids, flavor volatiles and phenolic compounds of avocado varieties. Food Chem. 2025, 482, 143533. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104 (Suppl. 3), S28–S39. [Google Scholar] [CrossRef]
- Mejia, J.; Giovagnoli-Vicuna, C.; Jacob, C.; Montenegro, G.; Moreno-Switt, A.I.; Giordano, A. Antioxidant, Antibacterial, and Bioaccessibility Properties of Ultrasound-Extracted Chilean Propolis. Antioxidants 2025, 14, 651. [Google Scholar] [CrossRef] [PubMed]
- Madeswaran, A.; Mohan, S. Neuroprotective effects of terpenoids against streptozotocin-nicotinamide-induced diabetic rats: An in silico, in vitro and in vivo study. Int. J. Biol. Macromol. 2023, 247, 125817. [Google Scholar] [CrossRef]
- Mei, X.; Yang, W.; Huang, G.; Huang, H. The antioxidant activities of balsam pear polysaccharide. Int. J. Biol. Macromol. 2020, 142, 232–236. [Google Scholar] [CrossRef]
- Pozzo, L.; Raffaelli, A.; Ciccone, L.; Zabini, F.; Vornoli, A.; Calderone, V.; Testai, L.; Meneguzzo, F. Conifer By-Products Extracted Using Hydrodynamic Cavitation as a Convenient Source of Phenolic Compounds and Free Amino Acids with Antioxidant and Antimicrobial Properties. Molecules 2025, 30, 2722. [Google Scholar] [CrossRef] [PubMed]
| Name | Moisture (%) | Ash (%) | Crude Liquid (%) | Crude Fiber (%) | Total Acid (%) | Protein (%) | Total Sugar (g/100 g) |
|---|---|---|---|---|---|---|---|
| flesh | 75.63 ± 0.81 a | 1.64 ± 0.38 a | 18.95 ± 0.22 a | 2.53 ± 0.19 c | 1.14 ± 0.09 a | 2.28 ± 0.07 b | 3.31 ± 0.26 b |
| seed | 52.37 ± 1.21 c | 1.24 ± 0.32 ab | 3.36 ± 0.06 b | 5.58 ± 0.90 b | 1.18 ± 0.06 a | 2.77 ± 0.15 a | 8.11 ± 0.76 a |
| peel | 64.45 ± 1.63 b | 0.85 ± 0.02 b | 2.68 ± 0.03 c | 16.33 ± 0.24 a | 0.73 ± 0.04 b | 1.60 ± 0.14 c | 3.13 ± 0.72 b |
| Total Polyphenol (mg/100 g) | Flavonoid (mg/100 g) | Polysaccharose (g/100 g) | Procyanidin (mg/100 g) | Terpenoid (mg/100 g) | Tannic (mg/100 g) | Catechin (mg/g) | Quercetin (ug/g) | |
|---|---|---|---|---|---|---|---|---|
| flesh | 240.70 ± 23.98 c | 255.37 ± 22.01 c | 0.47 ± 0.00 a | 34.08 ± 1.08 c | 1717.37 ± 157.19 a | 356.63 ± 3.61 b | 0.70 ± 0.03 b | - |
| seed | 2343.61 ± 31.44 a | 2645.59 ± 84.68 a | 0.03 ± 0.01 c | 485.76 ± 15.84 a | 241.20 ± 2.41 b | 1043.33 ± 24.13 a | 4.45 ± 0.61 a | 259.49 ± 44.35 a |
| peel | 1055.23 ± 30.97 b | 521.49 ± 14.57 b | 0.16 ± 0.01 b | 281.39 ± 11.23 b | 182.28 ± 9.98 b | 1040.51 ± 7.77 a | 4.12 ± 1.77 a | 178.19 ± 15.85 b |
| Run | A | B | C | D | Actual | Predicted | Residual |
|---|---|---|---|---|---|---|---|
| 1 | 0 (−1) | 20 (−1) | 100 (−1) | 20 (−1) | 0.25868 | 0.2789 | −2.02% |
| 2 | 100 (1) | 20 (−1) | 100 (−1) | 20 (−1) | 0.315794 | 0.3192 | −0.35% |
| 3 | 0 (−1) | 60 (1) | 100 (−1) | 20 (−1) | 0.42033 | 0.4267 | −0.64% |
| 4 | 100 (1) | 60 (1) | 100 (−1) | 20 (−1) | 0.501974 | 0.4901 | 1.18% |
| 5 | 0 (−1) | 20 (−1) | 500 (1) | 20 (−1) | 0.47531 | 0.4917 | −1.64% |
| 6 | 100 (1) | 20 (−1) | 500 (1) | 20 (−1) | 0.625494 | 0.6318 | −0.63% |
| 7 | 0 (−1) | 60 (1) | 500 (1) | 20 (−1) | 0.5933 | 0.5554 | 3.79% |
| 8 | 100 (1) | 60 (1) | 500 (1) | 20 (−1) | 0.688426 | 0.7184 | −3.00% |
| 9 | 0 (−1) | 20 (−1) | 100 (−1) | 100 (1) | 0.590798 | 0.5461 | 4.47% |
| 10 | 100 (1) | 20 (−1) | 100 (−1) | 100 (1) | 0.506254 | 0.5373 | −3.11% |
| 11 | 0 (−1) | 60 (1) | 100 (−1) | 100 (1) | 0.605521 | 0.5924 | 1.31% |
| 12 | 100 (1) | 60 (1) | 100 (−1) | 100 (1) | 0.637822 | 0.6067 | 3.11% |
| 13 | 0 (−1) | 20 (−1) | 500 (1) | 100 (1) | 0.565849 | 0.5709 | −0.50% |
| 14 | 100 (1) | 20 (−1) | 500 (1) | 100 (1) | 0.682848 | 0.6618 | 2.11% |
| 15 | 0 (−1) | 60 (1) | 500 (1) | 100 (1) | 0.551169 | 0.533 | 1.81% |
| 16 | 100 (1) | 60 (1) | 500 (1) | 100 (1) | 0.673935 | 0.6469 | 2.70% |
| 17 | 0 (−1) | 40 (0) | 300 (0) | 60 (0) | 0.21579 | 0.2379 | −2.22% |
| 18 | 100 (1) | 40 (0) | 300 (0) | 60 (0) | 0.392891 | 0.3922 | 0.06% |
| 19 | 50 (0) | 20 (−1) | 300 (0) | 60 (0) | 0.652859 | 0.6338 | 1.91% |
| 20 | 50 (0) | 60 (1) | 300 (0) | 60 (0) | 0.726293 | 0.7669 | −4.06% |
| 21 | 50 (0) | 40 (0) | 100 (−1) | 60 (0) | 0.391613 | 0.4007 | −0.90% |
| 22 | 50 (0) | 40 (0) | 500 (1) | 60 (0) | 0.641289 | 0.6537 | −1.25% |
| 23 | 50 (0) | 40 (0) | 300 (0) | 20 (−1) | 0.538643 | 0.5114 | 2.73% |
| 24 | 50 (0) | 40 (0) | 300 (0) | 100 (1) | 0.658328 | 0.7071 | −4.88% |
| 25 | 50 (0) | 40 (0) | 300 (0) | 60 (0) | 0.858378 | 0.8707 | −1.23% |
| 26 | 50 (0) | 40 (0) | 300 (0) | 60 (0) | 0.884378 | 0.8707 | 1.37% |
| 27 | 50 (0) | 40 (0) | 300 (0) | 60 (0) | 0.878805 | 0.8707 | 0.81% |
| 28 | 50 (0) | 40 (0) | 300 (0) | 60 (0) | 0.884853 | 0.8707 | 1.41% |
| 29 | 50 (0) | 40 (0) | 300 (0) | 60 (0) | 0.885875 | 0.8707 | 1.52% |
| 30 | 50 (0) | 40 (0) | 300 (0) | 60 (0) | 0.881995 | 0.8707 | 1.13% |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.9754 | 14 | 0.0697 | 53.78 | <0.0001 *** |
| A-Ethanol concentration | 0.0357 | 1 | 0.0357 | 27.57 | <0.0001 *** |
| B-Ultrasonic time | 0.0266 | 1 | 0.0266 | 20.50 | 0.0004 ** |
| C-Ultrasonic power | 0.0961 | 1 | 0.0961 | 74.16 | <0.0001 *** |
| D-Temperature | 0.0575 | 1 | 0.0575 | 44.35 | <0.0001 *** |
| AB | 0.0005 | 1 | 0.0005 | 0.4091 | 0.5321 |
| AC | 0.0099 | 1 | 0.0099 | 7.66 | 0.0143 * |
| AD | 0.0024 | 1 | 0.0024 | 1.86 | 0.1923 |
| BC | 0.0071 | 1 | 0.0071 | 5.47 | 0.0336 * |
| BD | 0.0103 | 1 | 0.0103 | 7.95 | 0.0129 * |
| CD | 0.0354 | 1 | 0.0354 | 27.31 | 0.0001 ** |
| A2 | 0.5292 | 1 | 0.5292 | 408.52 | <0.0001 *** |
| B2 | 0.0498 | 1 | 0.0498 | 38.42 | <0.0001 *** |
| C2 | 0.2023 | 1 | 0.2023 | 156.15 | <0.0001 *** |
| D2 | 0.1172 | 1 | 0.1172 | 90.47 | <0.0001 *** |
| Residual | 0.0194 | 15 | 0.0013 | ||
| Lack of Fit | 0.0147 | 10 | 0.0015 | 1.57 | 0.3231 |
| Pure Error | 0.0047 | 5 | 0.0009 | ||
| Cor Total | 0.9948 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Z.; Gao, Y.; He, L.; Xiu, Z.; Sun, L. Bioactive Antioxidants from Avocado By-Products: Mechanistic Study and Laboratory-Scale Extraction Optimization. Antioxidants 2025, 14, 1225. https://doi.org/10.3390/antiox14101225
Xin Z, Gao Y, He L, Xiu Z, Sun L. Bioactive Antioxidants from Avocado By-Products: Mechanistic Study and Laboratory-Scale Extraction Optimization. Antioxidants. 2025; 14(10):1225. https://doi.org/10.3390/antiox14101225
Chicago/Turabian StyleXin, Ziyao, Yicheng Gao, Leiyu He, Zhilong Xiu, and Lihui Sun. 2025. "Bioactive Antioxidants from Avocado By-Products: Mechanistic Study and Laboratory-Scale Extraction Optimization" Antioxidants 14, no. 10: 1225. https://doi.org/10.3390/antiox14101225
APA StyleXin, Z., Gao, Y., He, L., Xiu, Z., & Sun, L. (2025). Bioactive Antioxidants from Avocado By-Products: Mechanistic Study and Laboratory-Scale Extraction Optimization. Antioxidants, 14(10), 1225. https://doi.org/10.3390/antiox14101225