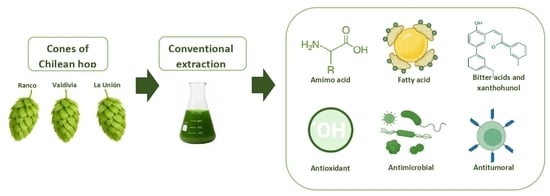
Comprehensive Characterization of Bioactive Properties in Extracts from Different Chilean Hop Ecotypes (Humulus lupulus L.): Antioxidant, Antimicrobial and Antitumor Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Hop Cones Preparation
2.2. Extraction Procedure
2.3. Proximate Analysis
2.4. Determination of the Amino Acid Profile
2.5. Determination of the Fatty Acid (FA) Profile
2.6. Determination of Bitter Acids and Xanthohumol
2.7. Antioxidant Capacity
2.7.1. DPPH Assay
2.7.2. FRAP Assay
2.7.3. ABTS Assay
2.8. Antimicrobial Activity
2.8.1. Strains and Characterization
2.8.2. Minimal Inhibitory Concentration (MIC)
2.9. Anti-Tumoral Activity
2.9.1. Cell Culture
2.9.2. Cell Viability
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Characterization
3.2. Amino Acid Profile
3.3. Fatty Acid (FA) Profile
3.4. Alpha and Beta Acids Content
3.5. Xanthohumol Content
3.6. Antioxidant Capacity
3.7. Antimicrobial Activity
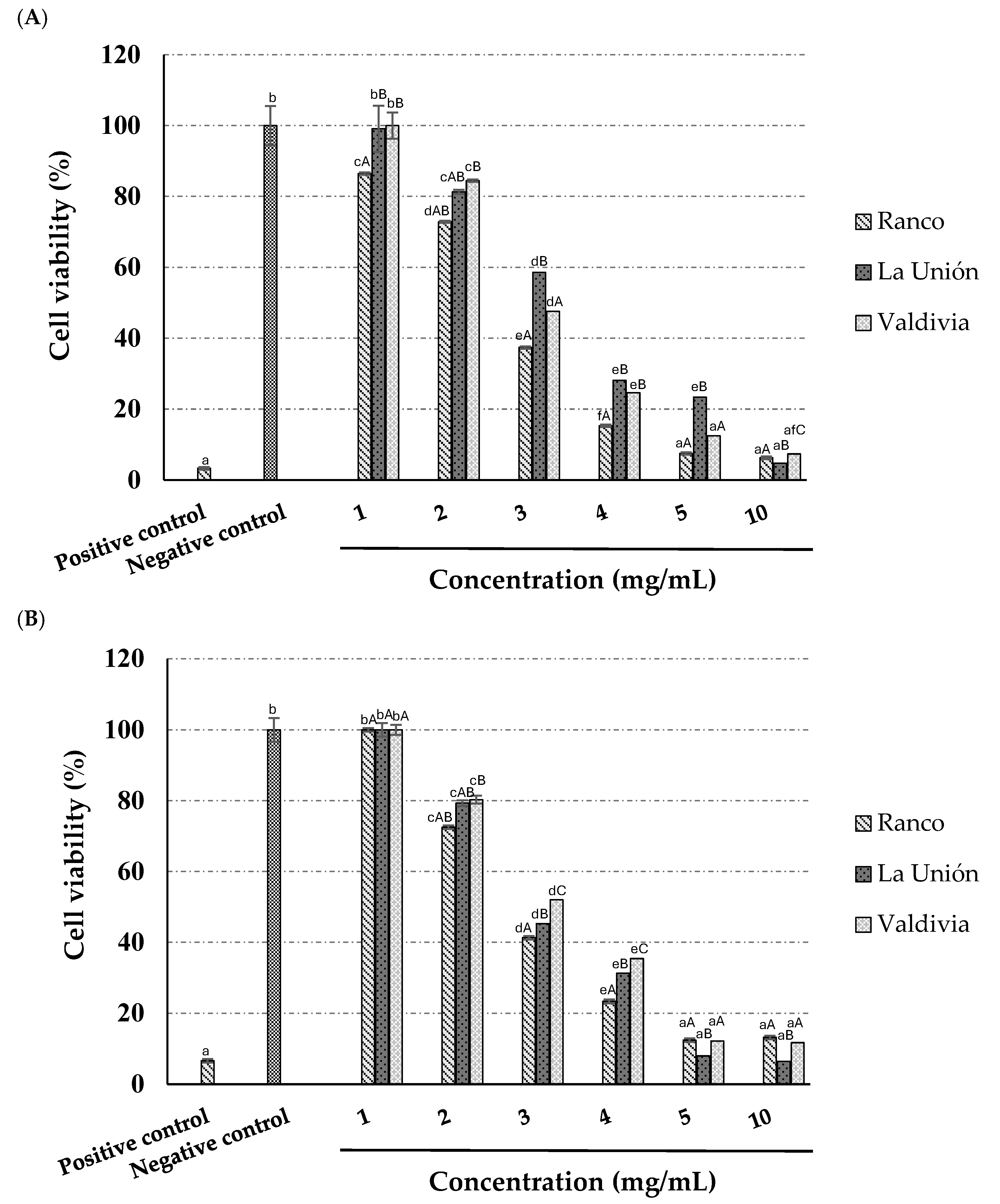
3.8. Anti-Tumoral Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ćirić, A.; Soković, M.; Calhelha, R.C.; Torija-Isasa, E.; de Cortes Sánchez-Mata, M.d.C.; Ferreira, I.C. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crops Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Dos Santos, B.; Sousa, M. Review hops-bioactive compounds and their applications. World. J. Pharm. Sci. 2023, 12, 1854–1886. [Google Scholar]
- Bocquet, L.; Sahpaz, S.; Rivière, C. An Overview of the Antimicrobial Properties of Hop. In Natural Antimicrobial Agents. Sustainable Development and Biodiversity; Mérillon, J.M., Riviere, C., Eds.; Springer: Cham, Switzerland, 2018; Volume 19, pp. 31–54. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Rivera, O.; Teuber, O.; Palma, M.T. Supercritical CO2 extraction of Chilean hop (Humulus lupulus) ecotypes. J. Sci. Food Agric. 2003, 83, 1349–1356. [Google Scholar] [CrossRef]
- Kausel, G.; Behn, A. Cerveceros artesanales de la Región de los Ríos, Chile—Diagnóstico y perspectivas para apoyar su desarrollo sustentable. Agro. Sur. 2016, 44, 3–12. [Google Scholar] [CrossRef]
- Behn, A.; Jerez, A.; Villatoro, J.M.; Celedón, M.; Lüer, C.; Kausel, G. Caracterización del ecotipo de lúpulo (Humulus lupulus L.) chileno Ranco en la Región de Los Ríos. Agro. Sur. 2022, 49, 33–44. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, N.; Yang, A.; Huang, J.; Ren, X.; Xian, M.; Zou, H. Hop bitter acids: Resources, biosynthesis, and applications. Appl. Microbiol. Biotechnol. 2021, 105, 4343–4356. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, K.; Liang, B.; Huang, X. Anticancer effect of xanthohumol induces growth inhibition and apoptosis of human liver cancer through NF-B/p53-apoptosis signaling pathway. Oncol. Rep. 2016, 35, 669–675. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and immune function: Relevance to inflammatory bowel diseases. Int. Rev. Immunol. 2009, 28, 506–534. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for cancer prevention and treatment. Iubmb. Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef]
- Contin, D.R.; Habermann, E.; de Souza, B.C.; Dias de Oliveira, E.A.; Martinez, C.A.; Vieira, P.C.; Da Costa, F.B. Exploring the tropical acclimation of European and American hop cultivars (Humulus lupulus L.): Focus on physiology, productivity, and chemical composition. Eur. J. Agron. 2023, 151, 126990. [Google Scholar] [CrossRef]
- Krofta, K.; Patzak, J.; Sedlák, T.; Mikyška, A.; Štěrba, K.; Jurková, M. Kazbek—The First Czech Aroma “Flavor Hops” Variety: Characteristics and Utilization. Kvas. Prum. 2019, 65, 72–83. [Google Scholar] [CrossRef]
- Krofta, K.; Mikyška, A.; Vrzal, T. Comparison of phytochemical and brewing characteristics of Cascade and Kazbek hop cultivars. J. Food Compos. Anal. 2024, 135, 106680. [Google Scholar] [CrossRef]
- Chávez, V. La batalla contra las superbacterias: No más antimicrobianos, no hay ESKAPE. TIP Rev. Espec. Cienc. Químico-Biológicas 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Araya, M.; García, S.; Rengel, J.; Pizarro, S.; Álvarez, G. Determination of free and protein amino acid content in microalgae by HPLC-DAD with pre-column derivatization and pressure hydrolysis. Mar. Chem. 2021, 234, 103999. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Valenzuela, P.; Roco, T.; Pérez-Won, M.; Espinoza, J.; Bernal, C.; Martínez, R. Isolation and Characterization of Polyunsaturated Fatty Acid-Rich Oil from Jumbo Squid (Dosidicus gigas) Viscera: Antioxidant Potential and Anticancer Activity on Colorectal Cancer Cells. Waste Biomass Valorization 2025, 16, 3507–3517. [Google Scholar] [CrossRef]
- ASBC: American Society of Brewing Chemists. Hop 14. α-Acids and β-Acids in Hops and Hop Extracts by HPLC (International Method); ASBC: St. Paul, MN, USA, 2008. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Maliar, T.; Nemeček, P.; Ürgeová, E.; Maliarová, M.; Nesvadba, V.; Krofta, K.; Vulganová, K.; Krošlák, E.; Kraic, J. Secondary metabolites, antioxidant and anti-proteinase activities of methanolic extracts from cones of hop (Humulus lupulus L.) cultivars. Chem. Pap. 2017, 71, 41–48. [Google Scholar] [CrossRef]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024; Volume 34.
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Sanz, V.; Torres, M.D.; Lopez Vilarino, J.M.; Dominguez, H. Green extraction of phenolic compounds from Perle Hallertau and Nuggets hop pellets. Food Biosci. 2022, 50, 102044. [Google Scholar] [CrossRef]
- Lafontaine, S.; Caffrey, A.; Dailey, J.; Varnum, S.; Hale, A.; Eichler, B.; Dennenlöhr, J.; Schubert, C.; Knoke, L.; Lerno, L.; et al. Evaluation of Variety, Maturity, and Farm on the Concentrations of Monoterpene Diglycosides and Hop Volatile/Nonvolatile Composition in Five Humulus lupulus Cultivars. J. Agric. Food Chem. 2021, 69, 4356–4370. [Google Scholar] [CrossRef]
- Behn, A.; Eibel, S.; Celedón, M.; Neugrodda, C.; Gastl, M.; Becker, T.; Kausel, G. Novel hop ecotypes revealed genetic variation in Chilean Humulus lupulus L. Genet. Resour. Crop. Evol. 2024, 72, 3027–3038. [Google Scholar] [CrossRef]
- Yamauchi, H.; Mukouzaka, Y.; Taniguchi, T.; Nakashima, K.; Furukubo, S.; Harada, M. Newly developed snp-based identification method of hop varieties. J. Am. Soc. Brew. Chem. 2014, 72, 239–245. [Google Scholar] [CrossRef]
- Gahr, A.; Forster, A.; De Clippeleer, J.; Van Opstaele, F.; Gahr, A. Reproducibility Trials in a Research Brewery and Effects on the Evaluation of Hop Substances in Beer Part 3: Transfer Rates of Aroma Compounds from Hops to Beer and their Ageing Behaviour. Brew. Sci. 2019, 12, 217–227. [Google Scholar] [CrossRef]
- Bruner, J.; Marcus, A.; Fox, G. Changes in Diacetyl and Amino Acid Concentration during the Fermentation of Dry-Hopped Beer: A Look at Twelve Saccharomyces Species and Strains. J. Am. Soc. Brew. Chem. 2023, 81, 242–254. [Google Scholar] [CrossRef]
- Kauffmann, A.C.; Castro, V.S. Phenolic Compounds in Bacterial Inactivation: A Perspective from Brazil. Antibiotics 2023, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Mohammad, A.R.; Karodia, N.; Rahman, A. Multimodal role of amino acids in microbial control and drug development. Antibiotics 2020, 9, 330. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Akers, K.S.; Romano, D.R.; Woodbury, R.L.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. D-amino acids enhance the activity of antimicrobials against biofilms of clinical wound isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 4353–4361. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z.; Yang, T.; Jiang, M.; Wang, J.; Cheng, Z.; Yang, M.-J.; Zhu, J.-X.; Zhang, T.-T.; Li, H.; et al. Glutamine promotes antibiotic uptake to kill multidrug-resistant uropathogenic bacteria. Sci. Transl. Med. 2021, 13, eabj0716. [Google Scholar] [CrossRef]
- Giordano, C.; Barnini, S. Glycine restores the sensitivity to antibiotics in multidrug-resistant bacteria. Microbiol. Spectr. 2024, 12, e0016424. [Google Scholar] [CrossRef] [PubMed]
- Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Pérez, L. Cationic surfactants derived from lysine: Effects of their structure and charge type on antimicrobial and hemolytic activities. J. Med. Chem. 2011, 54, 989–1002. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. In Virulence Mechanisms of Bacterial Pathogens; Wiley: Hoboken, NJ, USA, 2016; pp. 481–511. [Google Scholar] [CrossRef]
- Kostyanev, T.; Can, F. The Global Crisis of Antimicrobial Resistance. In Antimicrobial Stewardship; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–12. [Google Scholar] [CrossRef]
- Farag, M.A.; Porzel, A.; Schmidt, J.; Wessjohann, L.A. Metabolite profiling and fingerprinting of commercial cultivars of Humulus lupulus L. (hop): A comparison of MS and NMR methods in metabolomics. Metabolomics 2012, 8, 492–507. [Google Scholar] [CrossRef]
- Rettberg, N.; Thorner, S.; Garbe, A. Starting hop lipidomics-Isolation and characterization of non-polar, neutral and polar hop lipids. Brew. Sci. 2013, 6, 176–184. [Google Scholar]
- Fan, G.; Li, Y.; Liu, Y.; Suo, X.; Jia, Y.; Yang, X. Gondoic acid alleviates LPS-induced Kupffer cells inflammation by inhibiting ROS production and Pkcθ/Erk/Stat3 signaling pathway. Int. Immunopharmacol. 2022, 111, 109171. [Google Scholar] [CrossRef]
- Li, L.; Wang, N.; Wang, L.; Yang, F.; Wang, W.; Han, Y.; Yu, D. Characteristics, immobilization of linoleic acid isomerase from Bifidobacterium breve and its application in rice bran oil. Food Res. Int. 2025, 212, 116518. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Chu, C.; Chen, S.; Yue, D.; Hao, Y.J.; Sun, G.; Xia, H. Normal fat intake with high MUFA content and an appropriate SFA/MUFA/PUFA ratio improved the health of rats. Eur. J. Lipid Sci. Technol. 2024, 126, 2300214. [Google Scholar] [CrossRef]
- Sleha, R.; Radochova, V.; Malis, J.; Mikyska, A.; Houska, M.; Krofta, K.; Bogdanova, K.; Janovska, S.; Pejchal, J.; Kolar, M.; et al. Strong antimicrobial and healing effects of beta-acids from hops in methicillin-resistant Staphylococcus aureus-infected external wounds in vivo. Antibiotics 2021, 10, 708. [Google Scholar] [CrossRef]
- Nesvadba, V.; Charvátová, J.; Olšovská, J.; Trnková, S. Evaluation of variability in alpha and beta acid content in European hop varieties (Humulus lupulus L.). Kvas. Prum. 2024, 70, 919–926. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kocábek, T.; Nath, V.S.; Khan, A.; Matoušek, J.; Hazzouri, K.M.; Sudalaimuthuasari, N.; Krofta, K.; Ludwig-Müller, J.; Amiri, K.M. The multifaceted roles of R2R3 transcription factor HlMYB7 in the regulation of flavonoid and bitter acids biosynthesis, development and biotic stress tolerance in hop (Humulus lupulus L.). Plant Physiol. Biochem. 2023, 197, 107636. [Google Scholar] [CrossRef] [PubMed]
- Protsenko, L.; Liashenko, M.; Vlasenko, A.; Hryniuk, T.; Dobrovolny, O. Investigation of properties of biologically active substances and their content in cones of ukrainian hop varieties. Agric. Sci. Pract. 2018, 5, 52–63. [Google Scholar] [CrossRef]
- Pšenáková, I.; Hetešová, L.; Nemeček, P.; Faragó, J.; Kraic, J. Genotype and seasonal variation in antioxidant activity of hop extracts. Agriculture (Pol’nohospodárstvo) 2010, 56, 106–113. [Google Scholar]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial properties of spent hops extracts, flavonoids isolated therefrom, and their derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef]
- He, L.; Cheng, H.; Chen, F.; Song, S.; Zhang, H.; Sun, W.; Bao, X.; Zhang, H.; He, C. Oxidative Stress-Mediated Antibacterial Activity of the Total Flavonoid Extracted from the Agrimonia pilosa Ledeb. in Methicillin-Resistant Staphylococcus aureus (MRSA). Vet. Sci. 2022, 9, 71. [Google Scholar] [CrossRef]
- Wu, C.N.; Sun, L.C.; Chu, Y.L.; Yu, R.C.; Hsieh, C.W.; Hsu, H.Y.; Hsu, F.-C.; Cheng, K.-C. Bioactive compounds with anti-oxidative and anti-inflammatory activities of hop extracts. Food. Chem. 2020, 330, 127244. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Menghini, L.; Ferrante, C.; Recchia, E.; Castro-Amorim, J.; Gameiro, P.; Cellini, L.; Bessa, L.J. Hop Extract: An Efficacious Antimicrobial and Anti-biofilm Agent Against Multidrug-Resistant Staphylococci Strains and Cutibacterium acnes. Front. Microbiol. 2020, 11, 1852. [Google Scholar] [CrossRef] [PubMed]
- Khaliullina, A.; Kolesnikova, A.; Khairullina, L.; Morgatskaya, O.; Shakirova, D.; Patov, S.; Nekrasova, P.; Bogachev, M.; Kurkin, V.; Trizna, E.; et al. The Antimicrobial Potential of the Hop (Humulus lupulus L.) Extract against Staphylococcus aureus and Oral Streptococci. Pharmaceuticals 2024, 17, 162. [Google Scholar] [CrossRef]
- Bogdanova, K.; Kolar, M.; Langova, K.; Dusek, M.; Mikyska, A.; Bostikova, V.; Bostik, P.; Olsovska, J. Inhibitory effect of hop fractions against gram-positive multi-resistant bacteria. A pilot study. Biomed. Pap. 2018, 164, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Biehler, K.; Schwabe, K.; Haarhaus, B.; Quirin, K.W.; Frank, U.; Schempp, C.M.; Wölfle, U. Hop extract acts as an antioxidant with antimicrobial effects against Propionibacterium acnes and Staphylococcus aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef]
- Piasecki, B.; Biernasiuk, A.; Ludwiczuk, A. Anti-Coccal Activity and Composition of the Essential Oils and Methanolic Extracts Obtained from Brewing Quality Humulus lupulus L. Hop Pellets. Pharmaceuticals 2023, 16, 1098. [Google Scholar] [CrossRef]
- Kolenc, Z.; Langerholc, T.; Hostnik, G.; Ocvirk, M.; Štumpf, S.; Pintarič, M.; Košir, I.J.; Čerenak, A.; Garmut, A.; Bren, U. Antimicrobial Properties of Different Hop (Humulus lupulus) Genotypes. Plants 2023, 12, 120. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Cömert, F.; Ay, M.; Aydoğan Türkoğlu, S.; Tura Köçkar, F.; Çelik, A. Antiproliferative activity of Humulus lupulus extracts on human hepatoma (Hep3B), colon (HT-29) cancer cells and proteases, tyrosinase, β-lactamase enzyme inhibition studies. J. Enzym. Inhib. Med. Chem. 2016, 31, 90–98. [Google Scholar] [CrossRef]
- Vesaghhamedani, S.; Ebrahimzadeh, F.; Najafi, E.; Shabgah, O.G.; Askari, E.; Shabgah, A.G.; Mohammadi, H.; Jadidi-Niaragh, F.; Navashenaq, J.G. Xanthohumol: An underestimated, while potent and promising chemotherapeutic agent in cancer treatment. Prog. Biophys. Mol. Biol. 2022, 172, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Sun, T.; Du, J.; Zhang, B.; Xiang, D.; Li, W. Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro. Oncol. Rep. 2018, 40, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Ambrož, M.; Lněničková, K.; Matoušková, P.; Skálová, L.; Boušová, I. Antiproliferative effects of hop-derived prenylflavonoids and their influence on the efficacy of oxaliplatine, 5-fluorouracil and irinotecan in human colorectalC cells. Nutrients 2019, 11, 879. [Google Scholar] [CrossRef] [PubMed]

| Ecotype | Moisture (% d.m.) | Ash (% d.m.) | Fat (% d.m.) | Protein (% d.m.) | Total Carbohydrates (% d.m.) |
|---|---|---|---|---|---|
| Ranco | 5.45 ± 0.01 c | 7.39 ± 0.07 a | 1.90 ± 0.02 a | 13.14 ± 0.11 b | 77.58 ± 0.12 b |
| La Unión | 4.52 ± 0.04 b | 7.89 ± 0.16 b | 2.37 ± 0.10 b | 14.02 ± 0.26 c | 75.71 ± 0.20 a |
| Valdivia | 3.63 ± 0.02 a | 8.05 ± 0.09 c | 1.94 ± 0.04 a | 11.12 ± 0.18 a | 78.90 ± 0.18 c |
| Ecotype | Ranco (g/100 g d.m.) | La Unión (g/100 g d.m.) | Valdivia (g/100 g d.m.) |
|---|---|---|---|
| ASP | 1.80 ± 0.26 a | 1.70 ± 0.06 a | 1.35 ± 0.55 a |
| GLU | 0.65 ± 0.01 a | 0.52 ± 0.00 a | 0.48 ± 0.22 a |
| SER | 0.31 ± 0.63 a | 0.29 ± 0.06 a | 0.30 ± 0.06 a |
| HIS | 0.06 ± 0.11 a | 0.14 ± 0.00 a | 0.13 ± 0.00 a |
| GLY | 0.25 ± 0.16 a | 0.25 ± 0.05 a | 0.32 ± 0.05 a |
| THR | 0.39 ± 0.18 a | 0.47 ± 0.59 a | 0.60 ± 0.01 a |
| ARG | 0.27 ± 0.01 a | 0.27 ± 0.00 a | 0.27 ± 0.00 a |
| ALA | 0.25 ± 0.08 a | 0.31 ± 0.08 a | 0.28 ± 0.04 a |
| TYR | 0.14 ± 0.00 a | 0.13 ± 0.00 a | 0.13 ± 0.00 a |
| CY2 | 1.50 ± 0.23 a | 1.45 ± 0.22 a | 1.14 ± 0.24 a |
| MET | 0.24 ± 0.06 a | 0.15 ± 0.06 a | 0.15 ± 0.07 a |
| PHE | 0.32 ± 0.08 a | 0.24 ± 0.00 a | 0.19 ± 0.08 a |
| ILE | 0.57 ± 0.08 a | 0.49 ± 0.08 a | 0.50 ± 0.00 a |
| LEU | 0.07 ± 0.03 a | 0.10 ± 0.01 a | 0.10 ± 0.00 a |
| LYS | 0.77 ± 0.05 b | 0.55 ± 0.02 ab | 0.48 ± 0.10 a |
| Fatty Acids | Ecotype | ||
|---|---|---|---|
| Ranco | La Unión | Valdivia | |
| Undecanoic | 5.26 ± 0.01 a | 5.17 ± 1.21a | 5.00 ± 0.05 a |
| Palmitic | 33.35 ± 0.18 a | 33.85 ± 1.48 a | 30.67 ± 1.26 a |
| Stearic | 19.88 ± 0.53 a | 17.32 ± 2.13 a | 16.12 ± 1.70 a |
| Total SFA | 53.22 ± 0.35 a | 56.35 ± 2.23 a | 51.78 ± 2.91 a |
| Linoleic | 16.13 ± 1.17 a | 16.31 ± 0.84 a | 17.76 ± 0.47 a |
| Eicosenoic | 31.41 ± 0.83 a | 31.86 ± 1.74 a | 35.83 ± 2.45 a |
| Total MUFA and PUFA | 46.95 ± 0.83 a | 48.17 ± 2.23 a | 51.86 ± 2.91 a |
| (MUFA PUFA)/SFA | 0.88 | 0.85 | 1.00 |
| ω-3/ω-6 | 0 | 0 | 0 |
| Ecotype | Cohumulone (%w/w) | Adhumulone (%w/w) | Total Alpha Acids (%w/w) | Colupulone (%w/w) | Adlupulone (%w/w) | Total Beta Acids (%w/w) |
|---|---|---|---|---|---|---|
| Ranco | 0.56 ± 0.01 a | 1.75 ± 0.02 a | 2.31 ± 0.03 a | 2.49 ± 0.05 a | 3.14 ± 0.06 a | 5.63 ± 0.12 a |
| La Unión | 0.65 ± 0.05 b | 2.03 ± 0.16 b | 2.68 ± 0.21 b | 2.50 ± 0.08 a | 3.10 ± 0.09 a | 5.59 ± 0.17 a |
| Valdivia | 0.71 ± 0.01 c | 2.16 ± 0.06 b | 2.87 ± 0.07 b | 2.88 ± 0.08 b | 3.60 ± 0.12 b | 6.49 ± 0.21 b |
| Hop Ecotypes | mM Trolox/g d.m. | ||
|---|---|---|---|
| DPPH | FRAP | ABTS | |
| Ranco | 13.05 ± 0.05 a | 9.52 ± 0.18 a | 9.19 ± 0.03 a |
| La Unión | 12.69 ± 0.02 a | 8.22 ± 0.21 b | 9.09 ± 0.07 a |
| Valdivia | 14.67 ± 0.12 b | 10.10 ± 0.42 c | 10.00 ± 0.18 b |
| Microorganism | mg/mL | ||
|---|---|---|---|
| Ranco | La Unión | Valdivia | |
| K. pneumoniae ATCC 700603 | 5.0 | 5.0 | 5.0 |
| K. pneumoniae R-carbapenem | 5.0 | 5.0 | 5.0 |
| E. faecalis ATCC 29212 | 5.0 | 5.0 | 2.5 |
| E. faecalis R-vancomicina | 2.5 | 2.5 | 2.5 |
| P. aeruginosa ATCC 27853 | 5.0 | 5.0 | 5.0 |
| P. aeruginosa R-carbapenem | 5.0 | 5.0 | 2.5 |
| E. coli ATCC 25922 | 5.0 | 5.0 | 5.0 |
| E. coli R-carbapenem | 5.0 | 5.0 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancur, M.C.; Salazar, F.; Araya, M.; Behn, A.; López, J.; Quesille-Villalobos, A.; Villatoro, J.M.; Poblete, J.; Zambrano, A. Comprehensive Characterization of Bioactive Properties in Extracts from Different Chilean Hop Ecotypes (Humulus lupulus L.): Antioxidant, Antimicrobial and Antitumor Activities. Antioxidants 2025, 14, 1224. https://doi.org/10.3390/antiox14101224
Betancur MC, Salazar F, Araya M, Behn A, López J, Quesille-Villalobos A, Villatoro JM, Poblete J, Zambrano A. Comprehensive Characterization of Bioactive Properties in Extracts from Different Chilean Hop Ecotypes (Humulus lupulus L.): Antioxidant, Antimicrobial and Antitumor Activities. Antioxidants. 2025; 14(10):1224. https://doi.org/10.3390/antiox14101224
Chicago/Turabian StyleBetancur, María C., Fernando Salazar, Michael Araya, Anita Behn, Jéssica López, Ana Quesille-Villalobos, José M. Villatoro, Jacqueline Poblete, and Angara Zambrano. 2025. "Comprehensive Characterization of Bioactive Properties in Extracts from Different Chilean Hop Ecotypes (Humulus lupulus L.): Antioxidant, Antimicrobial and Antitumor Activities" Antioxidants 14, no. 10: 1224. https://doi.org/10.3390/antiox14101224
APA StyleBetancur, M. C., Salazar, F., Araya, M., Behn, A., López, J., Quesille-Villalobos, A., Villatoro, J. M., Poblete, J., & Zambrano, A. (2025). Comprehensive Characterization of Bioactive Properties in Extracts from Different Chilean Hop Ecotypes (Humulus lupulus L.): Antioxidant, Antimicrobial and Antitumor Activities. Antioxidants, 14(10), 1224. https://doi.org/10.3390/antiox14101224