14-Deoxy-11,12-didehydroandrographolide Alleviates IL-1β-Induced Insulin Resistance by Modulating NOX2-Driven ROS Generation and Restoring Insulin Signaling in 3T3-L1 Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of deAND
2.3. Cell Culture and Differentiation
2.4. Target Prediction
2.5. PPI Network Construction and Analysis
2.6. GO and KEGG Enrichment Analysis
2.7. Cell Viability Assay
2.8. Oil Red O Staining
2.9. Subcellular Fractionation
2.10. Western Blotting Analysis
2.11. Glucose Uptake Assay
2.12. Reactive Oxygen Species Measurement
2.13. Statistical Analysis
3. Results
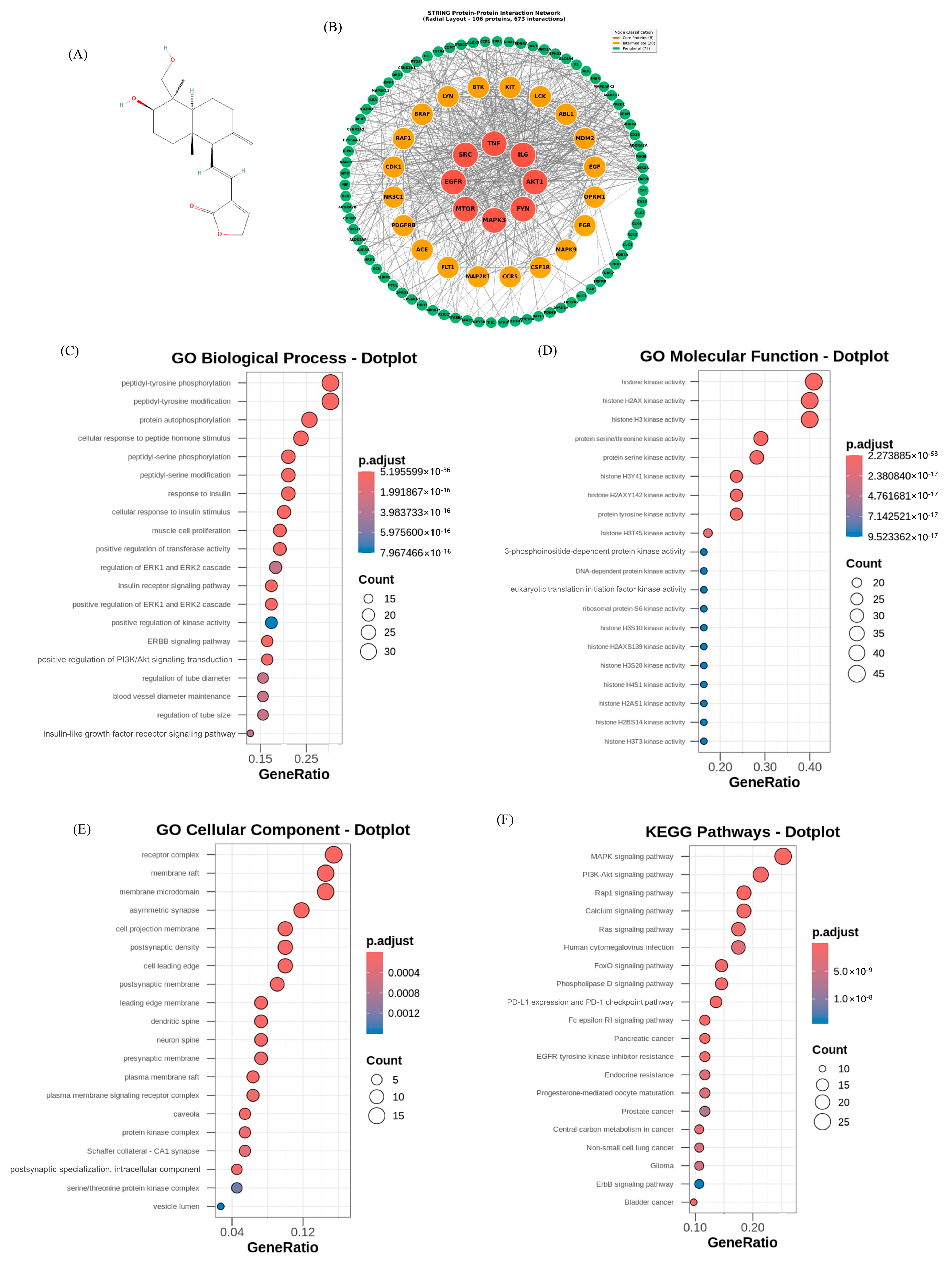
3.1. Retrieval of Targets and Construction of PPI Network
3.2. GO and KEGG Pathway Enrichment
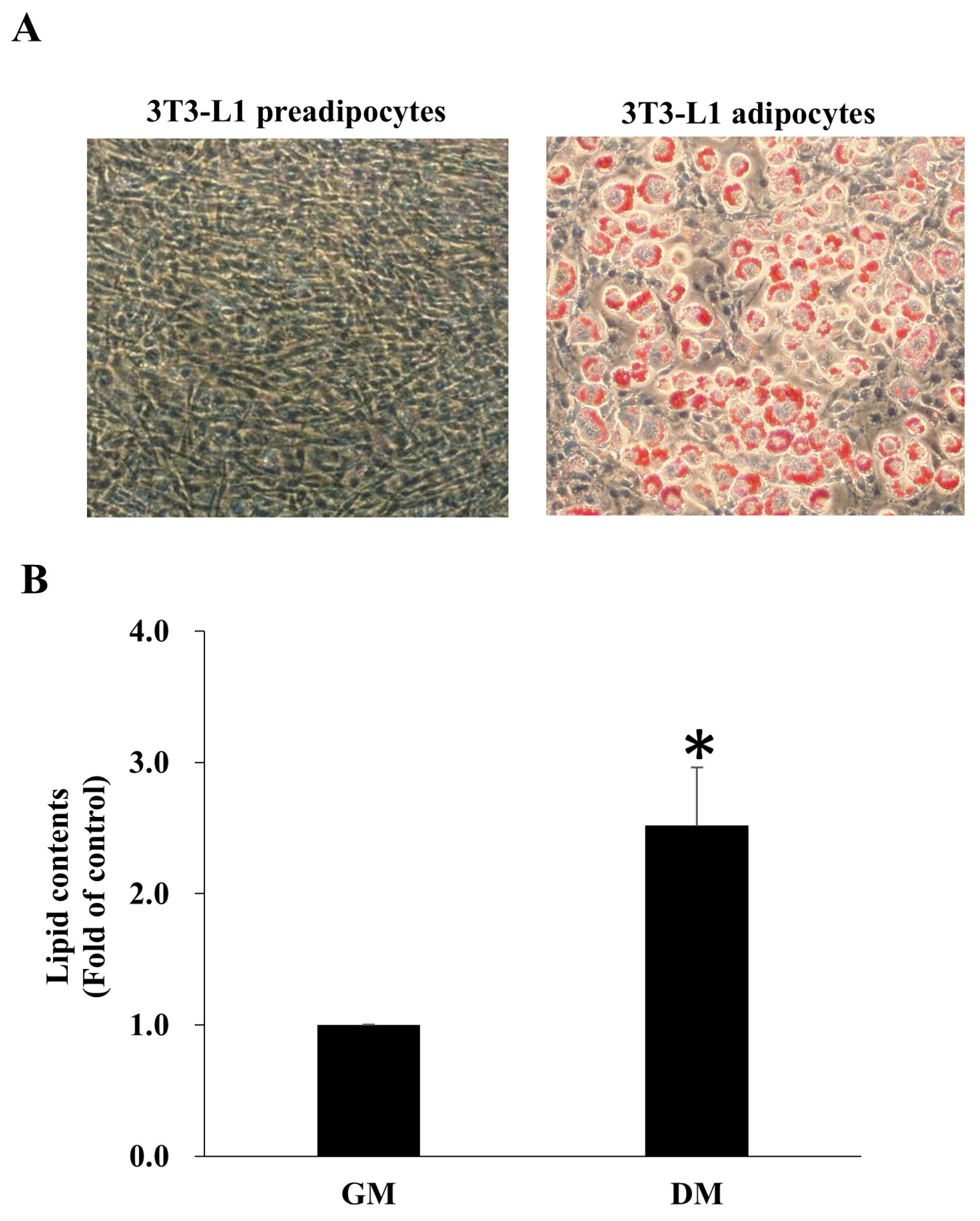
3.3. 3T3-L1 Preadipocyte Differentiation
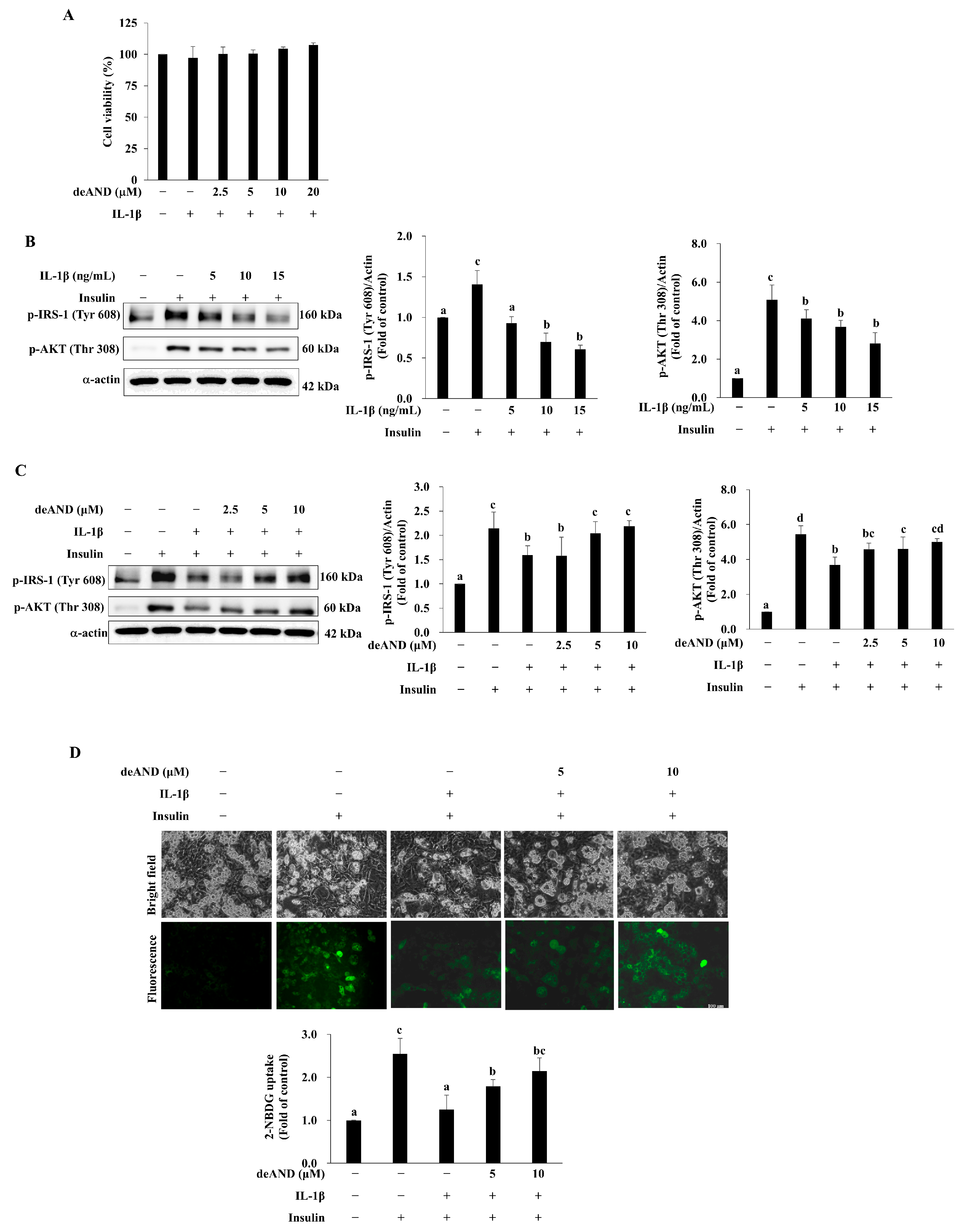
3.4. deAND Shows No Detrimental Effect on Cell Viability but Inhibits Insulin Resistance Induced by IL-1β
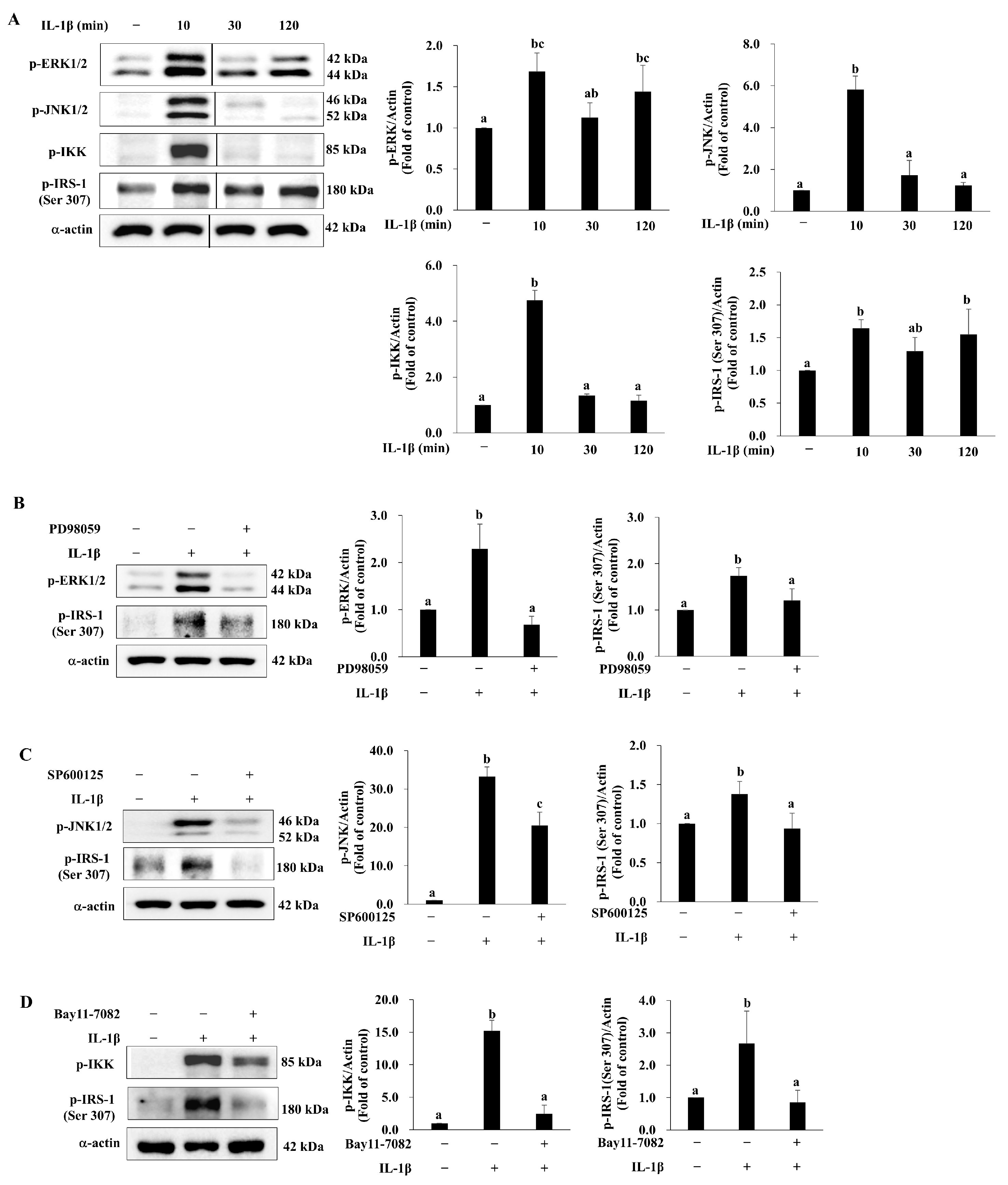
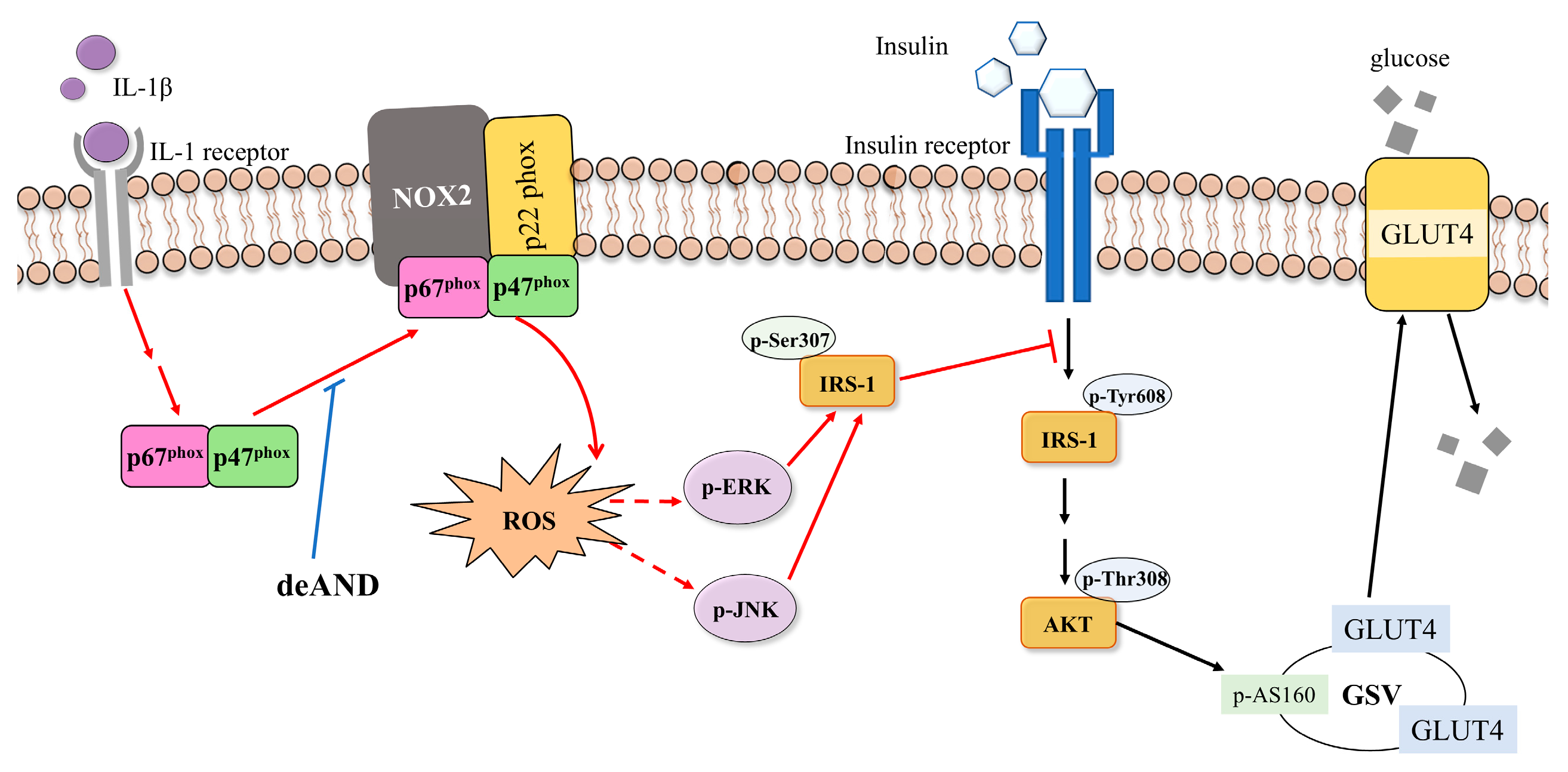
3.5. deAND Attenuates IL-1β-Induced Phosphorylation of IRS-1 at Serine 307 Through Inhibition of ERK and JNK Activation in 3T3-L1 Adipocytes
3.6. deAND Attenuates IL-1β-Induced ROS Generation by Inhibiting NOX2 Complex Formation in 3T3-L1 Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IR | Insulin resistance |
| T2DM | Type 2 diabetes mellitus |
| IL-1β | Interleukin-1β |
| deAND | 14-Deoxy-11,12-didehydroandrographolide |
| AND | Andrographolide |
| NOX2 | NADPH oxidase 2 |
| ROS | Reactive oxygen species |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| IBMX | 3-Isobutyl-1-methylxanthine |
| APE | A. paniculata ethanolic extract |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BP | Biological Process |
| MF | Molecular Function |
| CC | Cellular Component |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate-buffered saline |
| SDS | Sodium dodecyl sulfate |
| PVDF | Polyvinylidene difluoride |
| DCFDA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| 2-NBDG | 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose |
| MEK | MAPK/ERK kinase |
| MKK | MAPK kinase |
| IKK | IκB kinase |
| PI3K | Phosphoinositide 3-kinase |
| PDK1 | 3-Phosphoinositide-dependent protein kinase-1 |
| GLUT4 | Glucose transporter 4 |
| GSV | GLUT4 storage vesicle |
| HFD | High-fat diet |
References
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Johnson, C.L. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002, 288, 1723–1727. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef]
- Shah, A.; Mehta, N.; Reilly, M.P. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J. Parenter. Enter. Nutr. 2008, 32, 638–644. [Google Scholar] [CrossRef]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F.H. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Vanegas, S.; Maddux, B.A.; Mendoza, N.; Zhu, S.; Goldfine, I.D.; Mirza, A.M. XOMA 052, an anti-IL-1beta monoclonal antibody, prevents IL-1beta-mediated insulin resistance in 3T3-L1 adipocytes. Obesity 2013, 21, 306–309. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The role of NADPH oxidases in the etiology of obesity and metabolic syndrome: Contribution of individual Isoforms and cell biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef]
- Pepping, J.K.; Freeman, L.R.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E392–E404. [Google Scholar] [CrossRef]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Hu, X.; Huang, X.J.; Chen, G.X. Current understanding of glucose transporter 4 expression and functional mechanisms. World J. Biol. Chem. 2020, 11, 76–98. [Google Scholar] [CrossRef]
- Werner, E.D.; Lee, J.; Hansen, L.; Yuan, M.; Shoelson, S.E. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J. Biol. Chem. 2004, 279, 35298–35305. [Google Scholar] [CrossRef]
- Chen, H.W.; Huang, C.S.; Li, C.C.; Lin, A.H.; Huang, Y.J.; Wang, T.S.; Yao, H.T.; Lii, C.K. Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats. Toxicol. Appl. Pharmacol. 2014, 280, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Li, C.C.; Yang, Y.C.; Chiu, T.H.; Liu, K.L.; Lii, C.K.; Chen, H.W. Andrographis paniculata diterpenoids and ethanolic extract inhibit TNFalpha-induced ICAM-1 expression in EA.hy926 cells. Phytomedicine 2019, 52, 157–167. [Google Scholar] [CrossRef]
- Chen, C.C.; Lii, C.K.; Lin, Y.H.; Shie, P.H.; Yang, Y.C.; Huang, C.S.; Chen, H.W. Andrographis paniculata Improves Insulin Resistance in High-Fat Diet-Induced Obese Mice and TNFalpha-Treated 3T3-L1 Adipocytes. Am. J. Chin. Med. 2020, 48, 1073–1090. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.K.; Karuppayil, S.M. Andrographis paniculata (Burm. F) Wall ex Nees: Antiviral properties. Phytother. Res. 2021, 35, 5365–5373. [Google Scholar] [CrossRef]
- Firdous, J.; Latif, N.A.; Mona, R.; Mansor, R.; Muhamad, N. Andrographis paniculata and its Endophytes: A Review on their Pharmacological Activities. Res. J. Pharm. Technol. 2020, 13, 2027–2030. [Google Scholar] [CrossRef]
- Guan, S.P.; Kong, L.R.; Cheng, C.; Lim, J.C.; Wong, W.S. Protective role of 14-deoxy-11,12-didehydroandrographolide, a noncytotoxic analogue of andrographolide, in allergic airway inflammation. J. Nat. Prod. 2011, 74, 1484–1490. [Google Scholar] [CrossRef]
- Li, C.C.; Liu, K.L.; Lii, C.K.; Yan, W.Y.; Lo, C.W.; Chen, C.C.; Yang, Y.C.; Chen, H.W. Benzyl isothiocyanate inhibits TNFalpha-driven lipolysis via suppression of the ERK/PKA/HSL signaling pathway in 3T3-L1 adipocytes. Nutr. Res. 2024, 121, 95–107. [Google Scholar] [CrossRef]
- Yu, A.L.; Lu, C.Y.; Wang, T.S.; Tsai, C.W.; Liu, K.L.; Cheng, Y.P.; Chang, H.C.; Lii, C.K.; Chen, H.W. Induction of heme oxygenase 1 and inhibition of tumor necrosis factor alpha-induced intercellular adhesion molecule expression by andrographolide in EA.hy926 cells. J. Agric. Food Chem. 2010, 58, 7641–7648. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lii, C.K.; Wei, Y.L.; Li, C.C.; Lu, C.Y.; Liu, K.L.; Chen, H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-kappaB pathways. J. Nutr. Biochem. 2013, 24, 204–212. [Google Scholar] [CrossRef]
- Gao, P.; Hu, Y.; Wang, J.; Ni, Y.; Zhu, Z.; Wang, H.; Yang, J.; Huang, L.; Fang, L. Underlying mechanism of insulin resistance: A bioinformatics analysis based on validated related-genes from public disease databases. Med. Sci. Monit. 2020, 26, e924334. [Google Scholar] [CrossRef]
- Grimble, R.F. Inflammatory status and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 551–559. [Google Scholar] [CrossRef]
- Pickup, J.C.; Chusney, G.D.; Thomas, S.M.; Burt, D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000, 67, 291–300. [Google Scholar] [CrossRef]
- Mirzoyan, Z.; Valenza, A.; Zola, S.; Bonfanti, C.; Arnaboldi, L.; Ferrari, N.; Pollard, J.; Lupi, V.; Cassinelli, M.; Frattaroli, M.; et al. A Drosophila model targets Eiger/TNFalpha to alleviate obesity-related insulin resistance and macrophage infiltration. Dis. Model. Mech. 2023, 16, dmm050388. [Google Scholar] [CrossRef] [PubMed]
- de Alvaro, C.; Teruel, T.; Hernandez, R.; Lorenzo, M. Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 2004, 279, 17070–17078. [Google Scholar] [CrossRef] [PubMed]
- Sykiotis, G.P.; Papavassiliou, A.G. Serine phosphorylation of insulin receptor substrate-1: A novel target for the reversal of insulin resistance. Mol. Endocrinol. 2001, 15, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Chi, R.; Zhang, L.; Wang, N.J.; Lu, Y.L. Effects of paeoniflorin on tumor necrosis factor-alpha-induced insulin resistance and changes of adipokines in 3T3-L1 adipocytes. Fitoterapia 2013, 91, 44–50. [Google Scholar] [CrossRef]
- Fujishiro, M.; Gotoh, Y.; Katagiri, H.; Sakoda, H.; Ogihara, T.; Anai, M.; Onishi, Y.; Ono, H.; Abe, M.; Shojima, N.; et al. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes. Mol. Endocrinol. 2003, 17, 487–497. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef]
- Yuan, M.; Konstantopoulos, N.; Lee, J.; Hansen, L.; Li, Z.W.; Karin, M.; Shoelson, S.E. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001, 293, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Hakim, Z.S.; Madamanchi, N.R.; Rojas, M.; Madamanchi, C.; Runge, M.S. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arter. Thromb. Vasc. Biol. 2007, 27, 2714–2721. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, H. T Cells in Adipose Tissue: Critical Players in Immunometabolism. Front. Immunol. 2018, 9, 2509. [Google Scholar] [CrossRef]
- Han, C.Y. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab. J. 2016, 40, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schroder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef]
- Ling, L.; Louis, H.; Isang, B.B.; Emori, W.; Benjamin, I.; Ahuekwe, E.F.; Cheng, C.R.; Manicum, A.L.E. Inflammatory Studies of Dehydroandrographolide: Isolation, Spectroscopy, Biological Activity and Theoretical Modeling. Appl. Biochem. Biotechnol. 2024, 196, 417–435. [Google Scholar] [CrossRef]
- Liu, Y.T.; Chen, H.W.; Lii, C.K.; Jhuang, J.H.; Huang, C.S.; Li, M.L.; Yao, H.T. A diterpenoid, 14-deoxy-11,12-didehydroandrographolide, in andrographis paniculata reduces steatohepatitis and liver injury in mice fed a high-fat and high-cholesterol diet. Nutrients 2020, 12, 523. [Google Scholar] [CrossRef]
- Chen, C.C.; Lii, C.K.; Lo, C.W.; Lin, Y.H.; Yang, Y.C.; Huang, C.S.; Chen, H.W. 14-deoxy-11,12-didehydroandrographolide ameliorates glucose intolerance enhancing the LKB1/AMPKα/TBC1D1/GLUT4 signaling pathway and inducing GLUT4 expression in myotubes and skeletal muscle of obese mice. Am. J. Chin. Med. 2021, 49, 1473–1491. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Bouzakri, K.; Zierath, J.R. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J. Biol. Chem. 2007, 282, 7783–7789. [Google Scholar] [CrossRef]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell. Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Liu, K.L.; Lin, Y.R.; Kuo, W.C. The mechanisms of carnosic acid attenuates tumor necrosis factor-alpha-mediated inflammation and insulin resistance in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2014, 58, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Jager, J.; Gremeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T.Y. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Nishikawa, T.; Kukidome, D.; Sonoda, K.; Fujisawa, K.; Matsuhisa, T.; Motoshima, H.; Matsumura, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res. Clin. Pract. 2007, 77 (Suppl. S1), S161–S164. [Google Scholar] [CrossRef]
- Taniyama, Y.; Hitomi, H.; Shah, A.; Alexander, R.W.; Griendling, K.K. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arter. Thromb. Vasc. Biol. 2005, 25, 1142–1147. [Google Scholar] [CrossRef]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002, 277, 1531–1537. [Google Scholar] [CrossRef]
- Bloch-Damti, A.; Potashnik, R.; Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F.; Rudich, A.; Bashan, N. Differential effects of IRS1 phosphorylated on Ser307 or Ser632 in the induction of insulin resistance by oxidative stress. Diabetologia 2006, 49, 2463–2473. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The role of oxidative stress in atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Quinn, M.T. Assembly of the phagocyte NADPH oxidase: Molecular interaction of oxidase proteins. J. Leukoc. Biol. 1996, 60, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Moreno-Fernandez, M.E.; Giles, D.A.; Cappelletti, M.; Stankiewicz, T.E.; Chan, C.C.; Divanovic, S. Nicotinamide adenine dinucleotide phosphate (reduced) oxidase 2 modulates inflammatory vigor during nonalcoholic fatty liver disease progression in mice. Hepatol. Commun. 2018, 2, 546–560. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-C.; Lo, C.-W.; Lee, J.-L.; Liu, K.-L.; Li, C.-C.; Lii, C.-K.; Hsu, C.-E.; Yang, Y.-C.; Chen, H.-W. 14-Deoxy-11,12-didehydroandrographolide Alleviates IL-1β-Induced Insulin Resistance by Modulating NOX2-Driven ROS Generation and Restoring Insulin Signaling in 3T3-L1 Adipocytes. Antioxidants 2025, 14, 1155. https://doi.org/10.3390/antiox14101155
Yen C-C, Lo C-W, Lee J-L, Liu K-L, Li C-C, Lii C-K, Hsu C-E, Yang Y-C, Chen H-W. 14-Deoxy-11,12-didehydroandrographolide Alleviates IL-1β-Induced Insulin Resistance by Modulating NOX2-Driven ROS Generation and Restoring Insulin Signaling in 3T3-L1 Adipocytes. Antioxidants. 2025; 14(10):1155. https://doi.org/10.3390/antiox14101155
Chicago/Turabian StyleYen, Chih-Ching, Chia-Wen Lo, Jyun-Lin Lee, Kai-Li Liu, Chien-Chun Li, Chong-Kuei Lii, Chia-En Hsu, Ya-Chen Yang, and Haw-Wen Chen. 2025. "14-Deoxy-11,12-didehydroandrographolide Alleviates IL-1β-Induced Insulin Resistance by Modulating NOX2-Driven ROS Generation and Restoring Insulin Signaling in 3T3-L1 Adipocytes" Antioxidants 14, no. 10: 1155. https://doi.org/10.3390/antiox14101155
APA StyleYen, C.-C., Lo, C.-W., Lee, J.-L., Liu, K.-L., Li, C.-C., Lii, C.-K., Hsu, C.-E., Yang, Y.-C., & Chen, H.-W. (2025). 14-Deoxy-11,12-didehydroandrographolide Alleviates IL-1β-Induced Insulin Resistance by Modulating NOX2-Driven ROS Generation and Restoring Insulin Signaling in 3T3-L1 Adipocytes. Antioxidants, 14(10), 1155. https://doi.org/10.3390/antiox14101155





