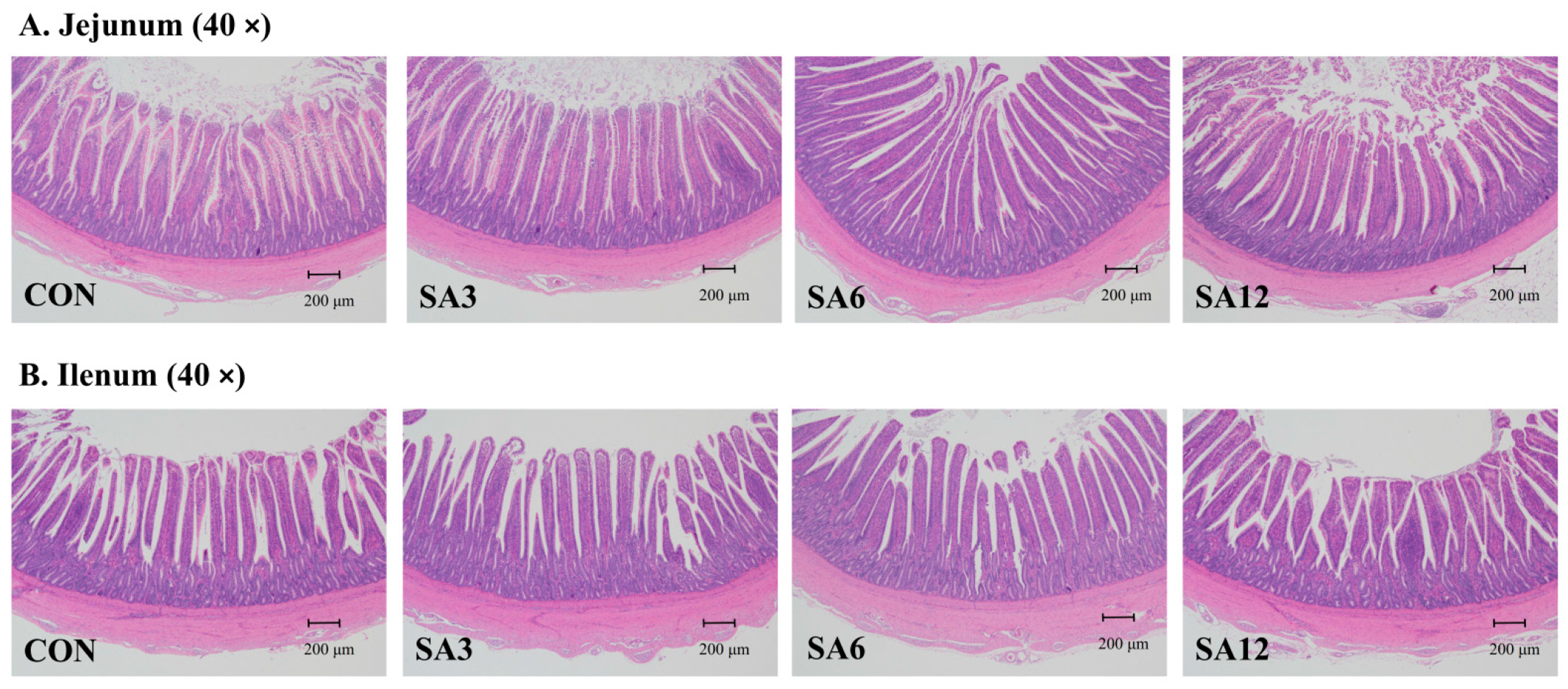
4.1. SA Improves Small Intestine Morphology and Nutrient Digestibility
Given that SA contains 33.15% cellulose and 27.13% hemicellulose, both of which are major components of dietary fiber, over half of SA’s composition consists of dietary fiber [
19]. The primary dietary modification from adding SA is an increase in dietary fiber. Compared to other monogastric animals, birds typically have a shorter and lighter gastrointestinal tract (GIT). While adapting to roughage, including herbaceous feed, dietary fiber significantly influences the intestinal development of Zhedong white geese. Villi, which are essential for nutrient absorption, consist of regions that include crypts and are replenished by stem cells [
20]. Consequently, any reduction in VH reduces the surface area available for nutrient absorption and the secretion of digestive enzymes. Conversely, an increase in CD, indicative of active cell replacement, results in decreased absorption [
21,
22]. Thus, the VH/CD ratio has become an important indicator of GIT development and digestive capacity. In this study, SA supplementation improved intestinal morphology; however, an excessive level of SA (12%) had adverse effects. This finding is consistent with Jiménez-Moreno et al. [
23], who reported that adding 2.5% pea hulls or sugar beet pulp improved the VH/CD ratio in broilers, whereas a 7.5% dietary inclusion led to villus shortening and increased mucus output. Similar results were observed by Koçer et al. [
24], where adding 47 g of sunflower meal per kg of the diet increased the VH/CD ratio, which then decreased with 97 g of sunflower meal per kg in laying hens. This phenomenon may be attributed to the negative impact of excessive fiber intake, which can lead to disruption of intestinal villi due to fiber particle abrasion [
19].
The small intestine is the principal organ responsible for nutrient digestion and absorption. Changes in digestibility closely follow the trends observed in small intestine morphology. Excessive fiber consumption can erode the villi of the small intestine, leading to a decrease in VH and consequently reducing the surface area for nutrient digestion. The alterations in the morphology of the jejunum and ileum due to SA supplementation are likely the main contributors to the differences in nutrient digestibility observed in Zhedong white geese. Additionally, the solubility and water-holding capacity of fiber can significantly affect nutrient utilization. A diet high in fiber content may increase viscosity, slowing the diffusion rate of endogenous enzymes into the digesta, and potentially reducing nutrient digestion [
25].
4.2. SA Increases Cecum Length and Improves Gut Microbial Structure
SA supplementation influenced the relative length of the cecum. This is partially consistent with the findings of Li et al. [
26], who indicated that feeding geese β-glucan influenced not only the cecum but also the duodenum, ileum, and colon. The cecum is the primary site of dietary fiber fermentation. An increase in dietary fiber intake leads to digestive bulk and physical distension of the intestinal walls, resulting in an increase in size. Despite evidence indicating a link between fiber type and quantity with organ development [
27], our results revealed that SA supplementation had no effect on the development of organs other than the GIT. This aligns with Li et al. [
28], who reported that diverse dietary fibers (2.5–6.1%) did not influence the relative weight of the liver, spleen, bursa, thymus, etc.
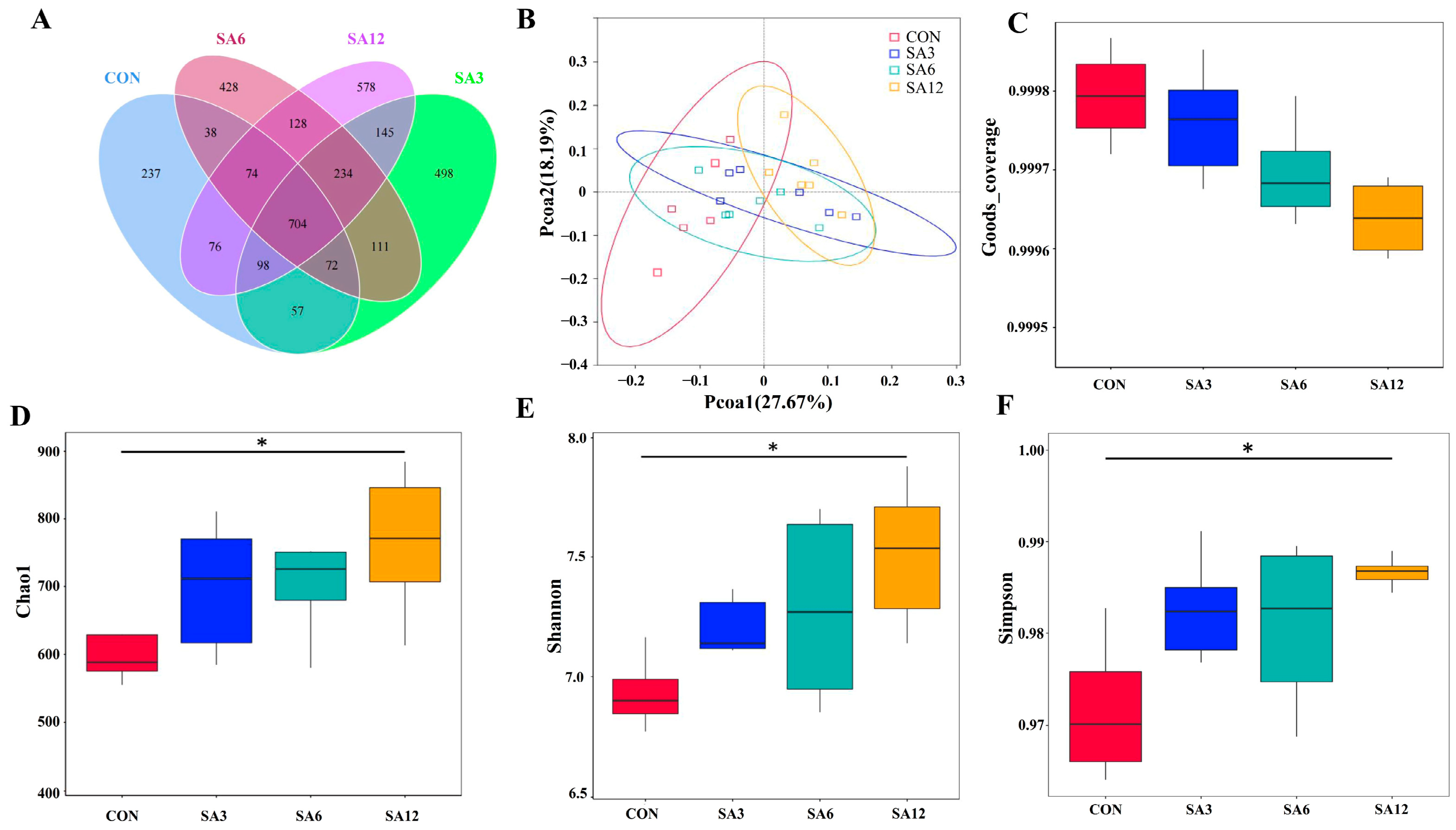
The poultry microbiota comprises a myriad of bacteria, including beneficial species involved in critical biological processes, such as the production of digestive enzymes, regulation of leukocyte activity, and amino acid metabolism. These beneficial species contrast with harmful bacteria that can invade the GIT and disrupt normal functioning by producing toxins [
29]. A balanced and stable microbiota in the GIT is crucial for maintaining intestinal health. The Shannon and Simpson indices are routinely employed to evaluate bacterial diversity, while Chao1 measures the richness of the gut microbiome. A diverse gut microbiome enhances resistance to colonization by pathogens and reduces the risk of GIT diseases [
30,
31]. In this study, a notable increase in the diversity and abundance of cecum microbiota was observed in the SA12 group. Li et al. [
32] reported similar findings, showing that a reduction in dietary fiber levels diminished the Shannon and Chao1 indices of cecum microbiota in geese on day 70. SCFAs are the main metabolites produced by gut microbiota during dietary fiber fermentation in the cecum and are closely associated with the abundance of gut microbiota [
33,
34,
35]. Multiple studies have documented that SCFA levels in the cecum increase with higher levels of dietary fiber [
33,
36]. The heightened SCFAs in SA supplementation groups suggest that SA supplementation could improve the diversity of cecum microbiota in Zhedong white geese.
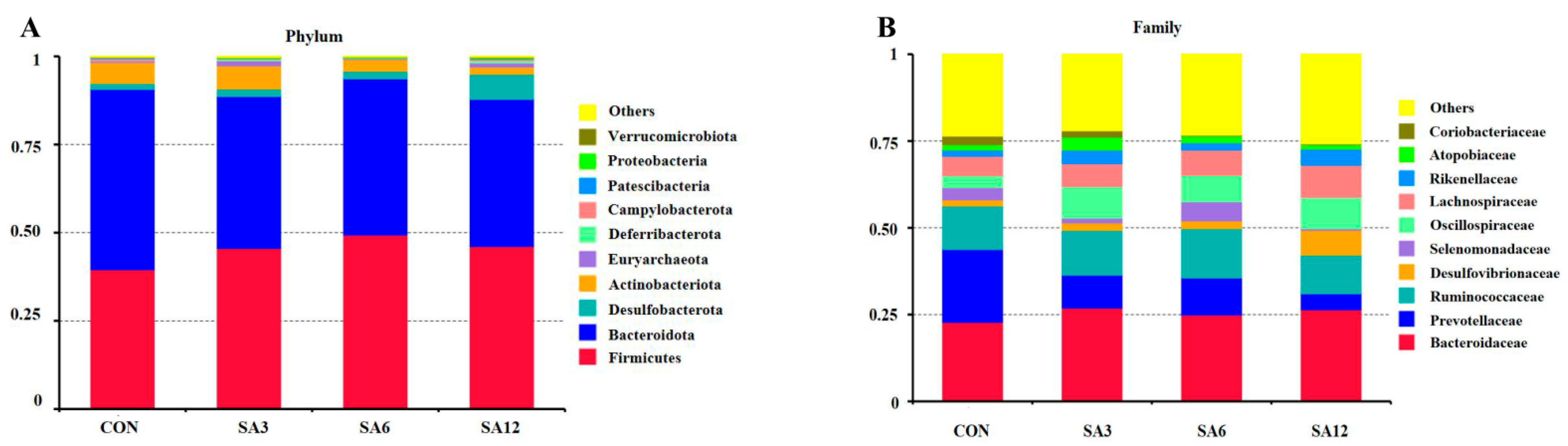
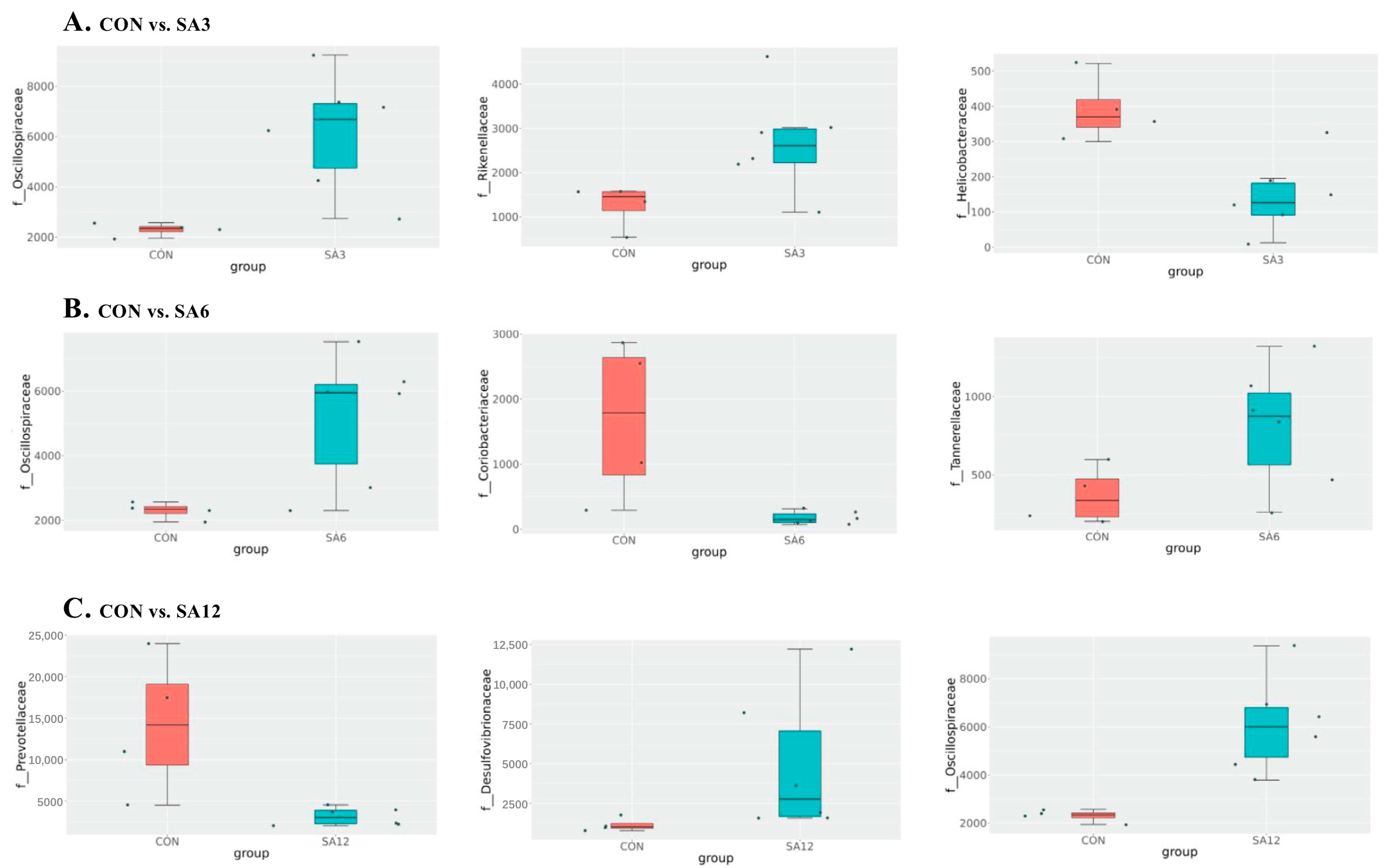
Spartina anglica supplementation triggered changes in the cecum’s length, which in turn influenced microbial composition. In the cecum of Zhedong white geese, the dominant bacteria were Bacteroidetes, Firmicutes, and the Desulfobacterota phylum. This contradicts the findings of Li et al. [
32], who identified Proteobacteria as the third most common bacteria in geese. Desulfobacterota, a sulfate-reducing bacteria, can inhibit enzymes involved in nicotinamide adenine dinucleotide recycling and ultimately remove hydrogen, which restricts SCFA production [
37]. This may also explain why the SA12 group had the highest SCFA levels. Regardless of the proportion of SA added, the greatest effect on microorganisms at the genus level was an increase in Oscillospiraceae. Oscillospiraceae represents important producers of SCFAs in the gut, particularly butyric acid, which not only provides energy to the intestinal epithelium and enhances intestinal barrier function but also possesses anti-inflammatory properties that help reduce inflammatory responses and improve intestinal immune function [
38]. The production of SCFAs reduces the pH level of the cecum, creating an acidic environment that inhibits harmful bacteria and promotes the growth of beneficial bacteria, thus maintaining a healthy microbial community. One noticeable change induced by 12% SA supplementation was the reduction of Prevotellaceae at the family level. Many studies have identified the degradation of plant-derived polysaccharides as an energy source for Prevotellaceae [
39,
40]. Although SA contains plant polysaccharides, its significant impact is an increase in insoluble fiber. This is corroborated by our findings and by Gálvez et al. [
41], who observed stark reductions in the abundance of murine Prevotellaceae when replacing a diet rich in plant-derived polysaccharides with a diet containing starch and indigestible cellulose as fiber. Overall, SA supplementation altered the cecum microbiota and may influence pro-inflammatory cytokines, intestinal permeability, and gut barrier function by modulating the corresponding microbiota [
42,
43].
4.3. SA Improves Antioxidant Capacity and Growth Performance
Under normal physiological conditions, organisms cyclically produce reactive oxygen species (ROS) and eliminate them through both enzymatic and non-enzymatic antioxidant systems, maintaining dynamic equilibrium. The enzymatic antioxidant system produces enzymes such as SOD, CAT, and GSH-Px, which convert ROS into H
2O and O
2, thereby protecting the body from oxidative damage [
44]. Total antioxidant capacity reflects the effectiveness of an animal’s non-enzymatic antioxidant defense system [
45]. As the level of SA supplementation increases, the antioxidant capacity of Zhedong white geese improves, peaking at a 6% supplementation level, but antioxidant performance remains essentially unchanged at the 12% SA supplementation level. Although research on the use of SA in animal feed is limited, it is widely accepted that plant-based feed ingredients enhance antioxidant capabilities. Liu et al. [
14] demonstrated that supplementing broiler diets with 80 mg/kg of natural capsaicin extract increased antioxidant capacity. Similarly, the inclusion of Artemisia annua in feed has been shown to reduce MDA levels and upregulate antioxidant enzyme expression [
46]. Plant-derived fibers are typically rich in polyphenolic compounds, which can inhibit free radical formation and reduce their activity [
47,
48]. Additionally, an increase in SCFAs, particularly butyrate, provides energy to intestinal epithelial cells, maintaining their integrity and health. This effectively prevents toxins and pathogens from entering the bloodstream, reducing oxidative stress sources. SCFAs also promote the expression of antioxidant enzymes via cellular signaling pathways, such as the Nrf2 pathway, thereby enhancing overall antioxidant capacity. However, compared to the SA6 group, the SA12 group shows no additional improvement in antioxidant performance. This phenomenon aligns with previous findings that polyphenolic compounds can exhibit antioxidant effects at low doses but pro-oxidant effects at high doses [
49,
50]. Furthermore, a 12% SA supplementation may cause intestinal villus abrasion, potentially offsetting the antioxidant benefits provided by polymeric polyphenols and other compounds.
Serum biochemical parameters are critical indicators of organ health and metabolic function [
51]. ALT, AST, and TP levels reflect liver tissue and cell permeability, while BUN indicates the state of amino acid metabolism [
52]. Our study found no significant effect of SA supplementation on the serum biochemical parameters of geese at any supplementation level. This suggests that dietary SA does not adversely affect liver or kidney function or related metabolic processes.
Growth performance is an integrated trait influenced by multiple factors, warranting its discussion toward the end. Studies have reported conflicting findings regarding the impact of dietary crude fiber on ADFI in poultry. While some studies [
37,
53] found no significant effects of fiber levels on feed intake, others, including Li et al. [
26] and our research, observed an influence of dietary fiber on feed consumption. These discrepancies may stem from differences in the composition of soluble and insoluble fibers, which vary in water-holding and swelling capacities, influencing digesta viscosity and retention time in the GIT [
19]. Supporting our findings, Zhang et al. [
54] reported the highest ADG in geese fed a diet containing 6% crude fiber, among six graded levels (3%, 4.5%, 6%, 7.5%, 9%, and 10.5%).
Body growth largely depends on intestinal development, immune function, and nutrient absorption. Moderate SA supplementation (6%) significantly improves intestinal morphology and cecal microbiota composition. However, excessive supplementation (12%) impairs intestinal morphology and nutrient digestion, with the detrimental effects outweighing the beneficial ones. Additionally, antioxidant capacity, a key health indicator, significantly influences animal growth and metabolism, with SA6 being the most effective. Therefore, a 6% SA supplementation level is recommended for optimal performance. These findings hold promise for enhancing the nutritional strategies in goose production and contribute to the broader understanding of how novel high-fiber feed ingredients can improve poultry health and productivity.