Carvedilol Confers Ferroptosis Resistance in HL-1 Cells by Upregulating GPX4, FTH1, and FTL1 and Inducing Metabolic Remodeling Under Hypoxia/Reoxygenation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Hypoxia and HR Conditions, and Carvedilol Treatment
2.2. Analysis for Cellular ROS, Lipid Peroxidation, and Cell Death
2.3. Western Blot Analysis
2.4. Analysis for Cell Viability
2.5. Analysis of Extracellular Metabolites by Nuclear Magnetic Resonance (NMR)
2.6. Analysis for Fatty Acid Oxidation (FAO) Oxygen Consumption Rate (OCR) by Seahorse XF24 Analyzer
2.7. Analysis of Targeted Intracellular Metabolites by Liquid Chromatography Coupled with Tandem Mass Spectrometry (LC-MS/MS)
2.8. Analysis of Untargeted Intracellular Metabolites by Ultrahigh-Performance Liquid Chromatography Time-of-Flight Mass Spectrometry (UPLC-TOF-MS)
2.9. Quantitative Polymerase Chain Reaction (qPCR)
2.10. Statistical Analysis
3. Results
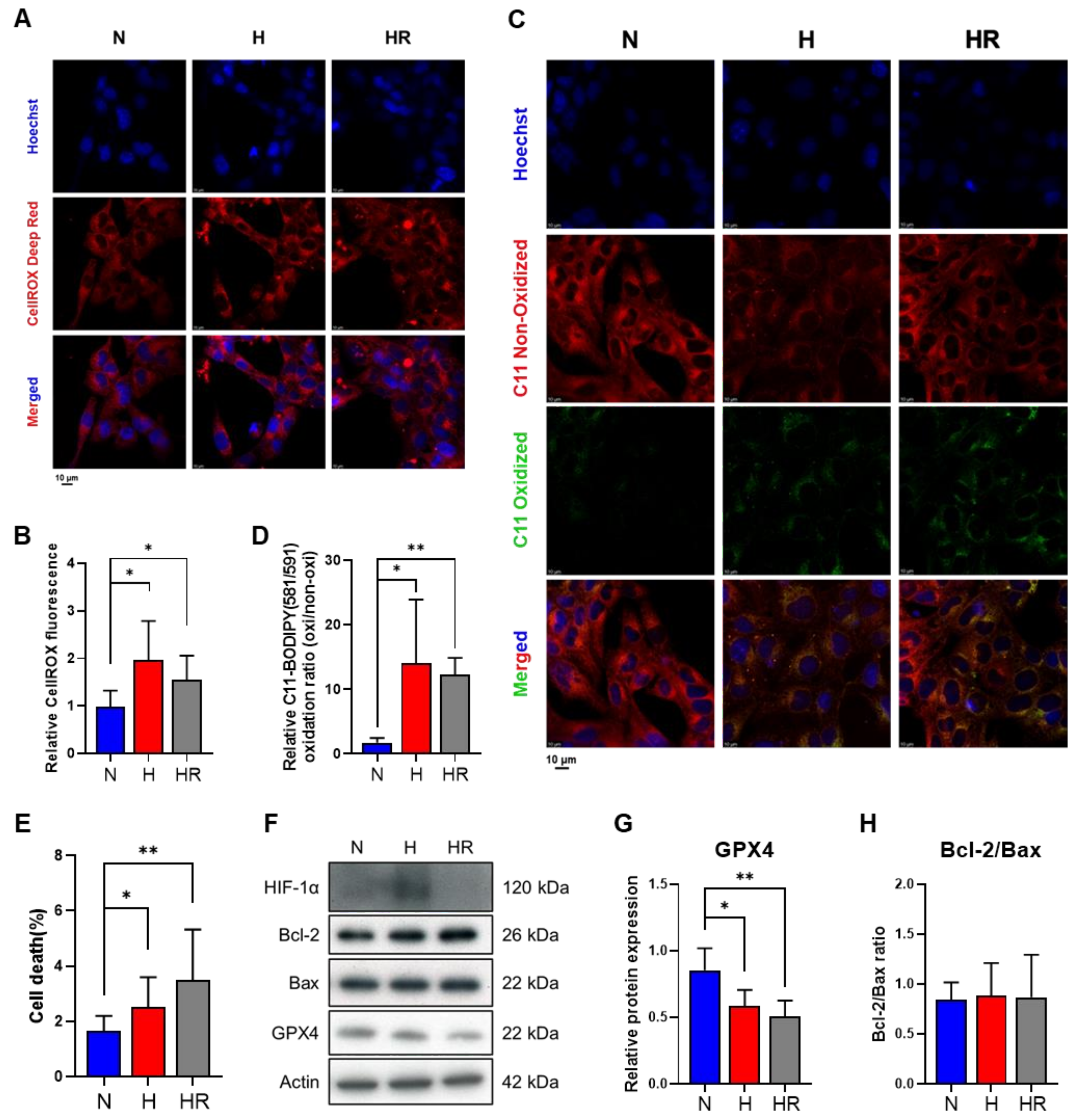
3.1. Hypoxia and HR Induced Oxidative Stress and Lipid Peroxidation
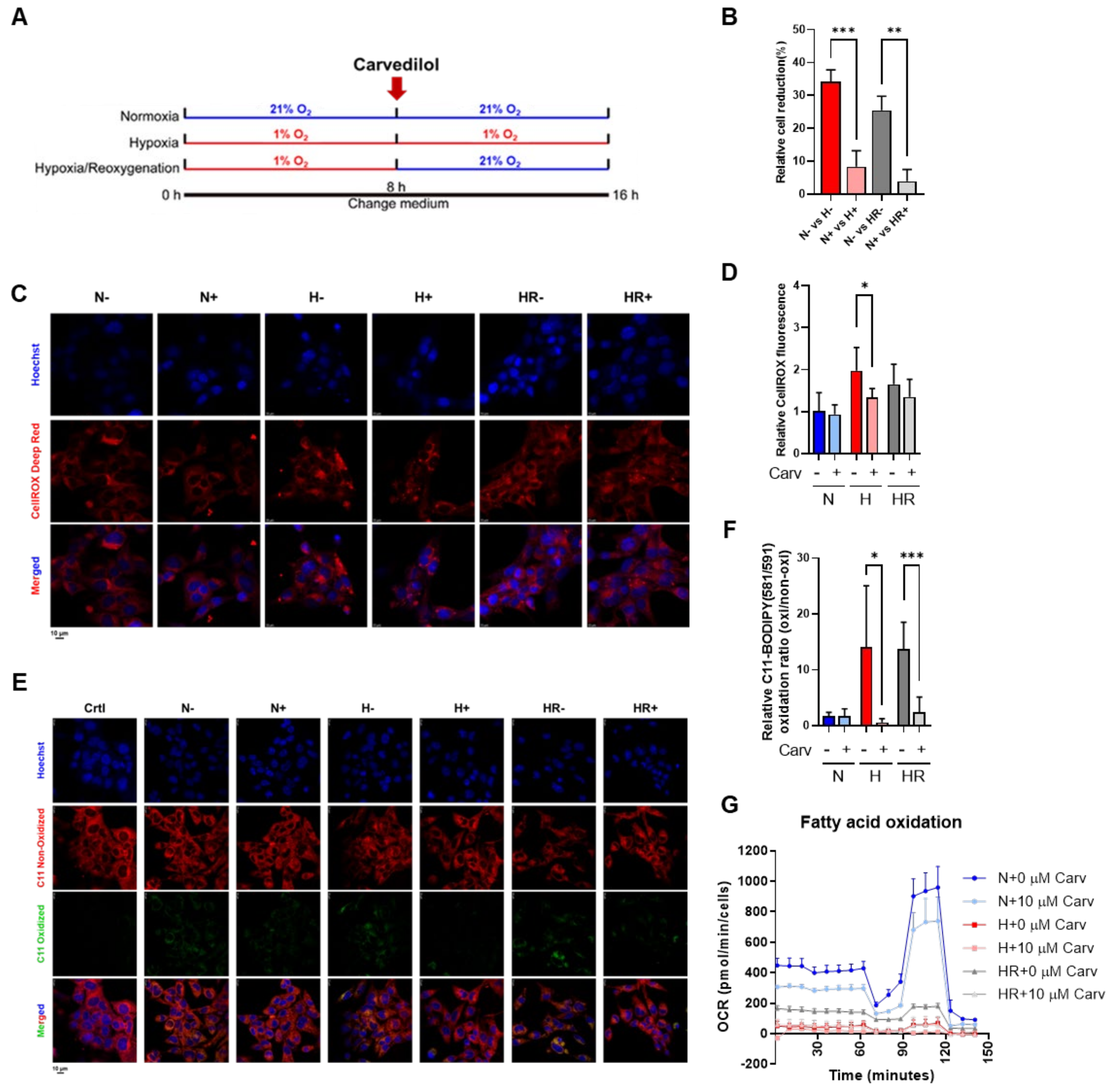
3.2. Carvedilol Promoted Lipid Peroxidation Reduction Under HR
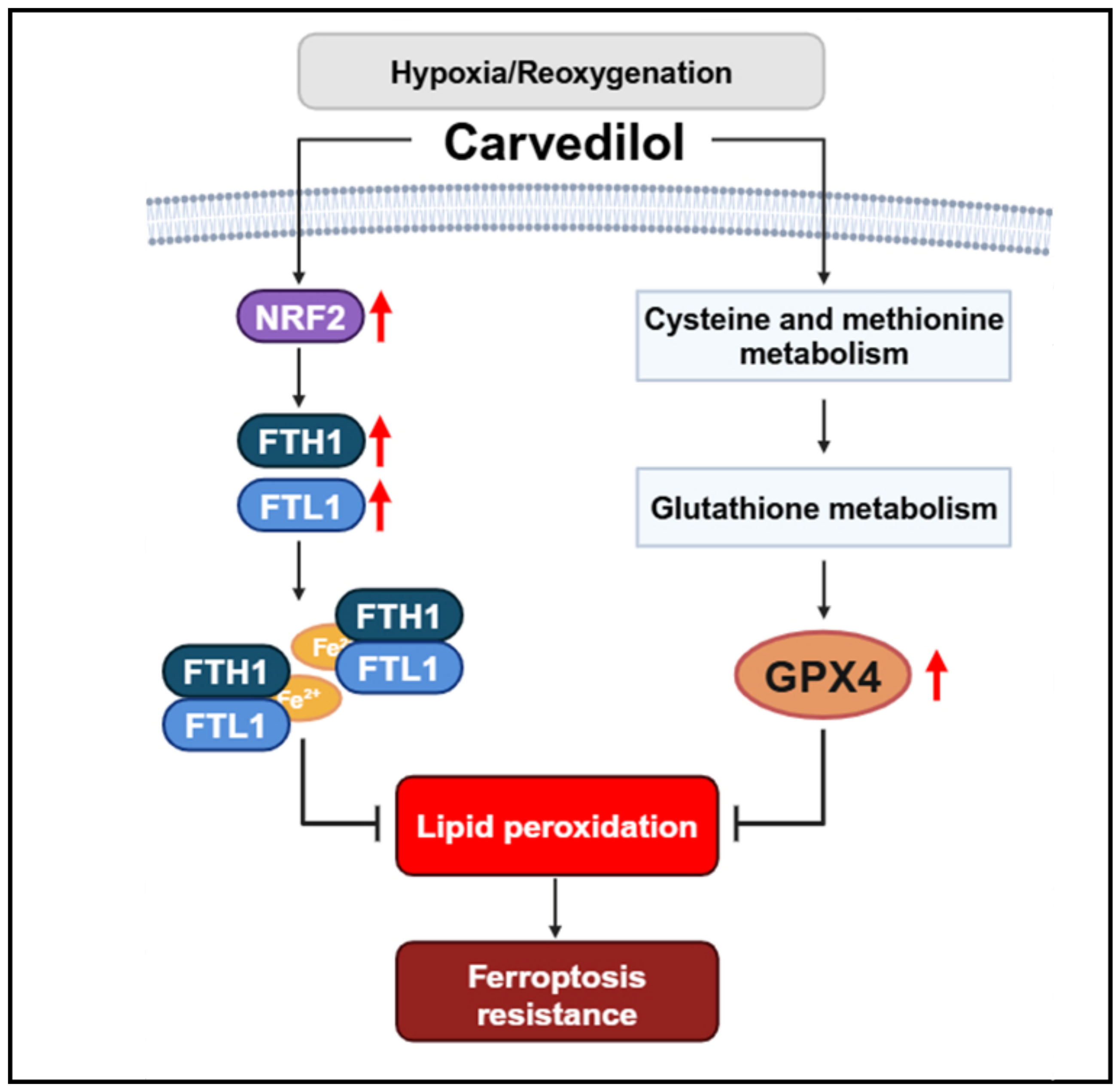
3.3. Carvedilol Activated GPX4 Expression by Upregulating Cysteine and Methionine Metabolism and GSH Metabolism to Counteract Oxidative Stress Under HR
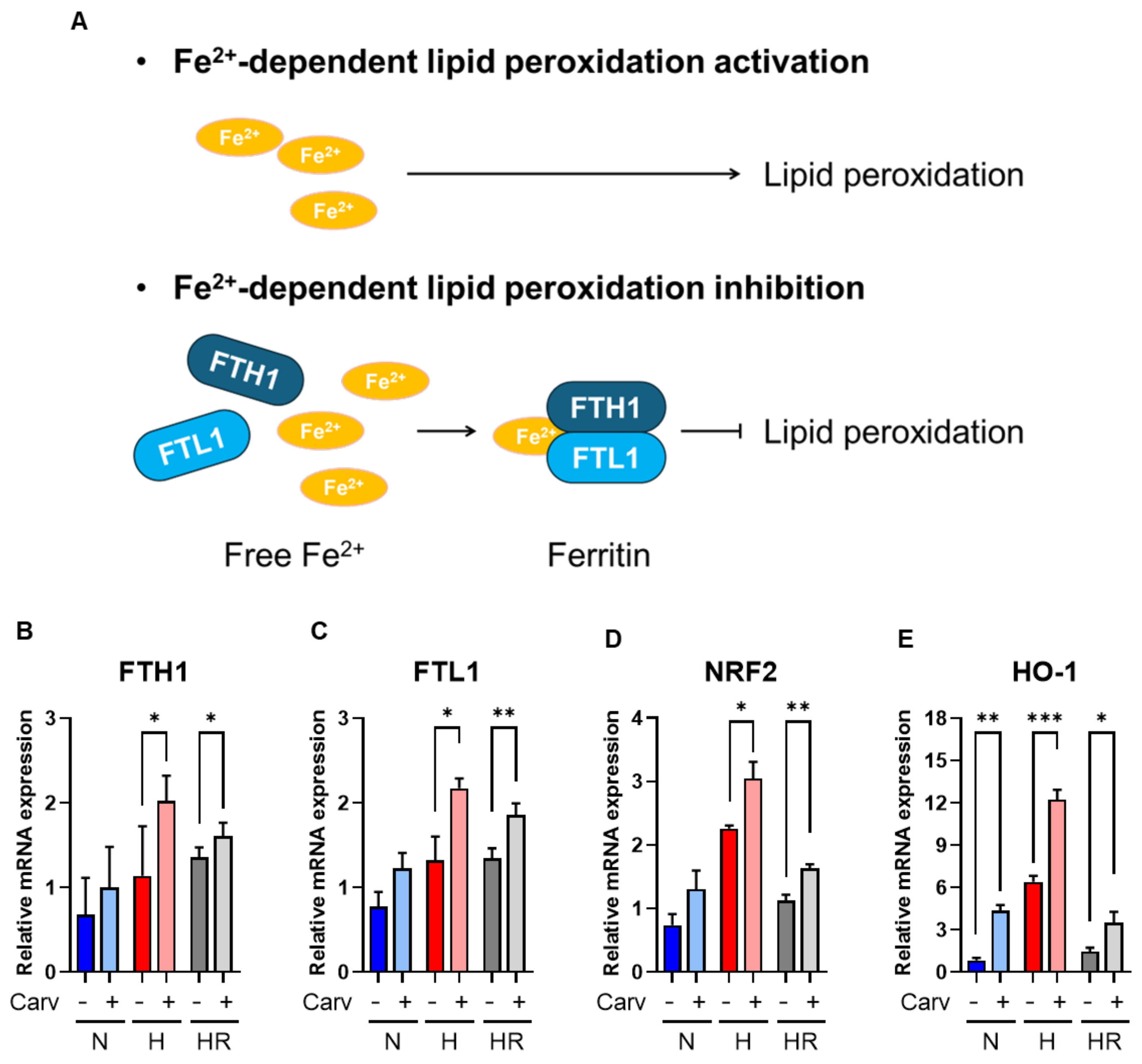
3.4. Carvedilol Activated FTH1 and FTL1 to Resist Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuomainen, T.; Tavi, P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Exp. Cell Res. 2017, 360, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mirtschink, P.; Krek, W. Hypoxia-driven glycolytic and fructolytic metabolic programs: Pivotal to hypertrophic heart disease. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Barrera, J.C.A.; Ondo-Mendez, A.; Giera, M.; Kostidis, S. Metabolomic and Lipidomic Analysis of the Colorectal Adenocarcinoma Cell Line HT29 in Hypoxia and Reoxygenation. Metabolites 2023, 13, 875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.T.; Miller, J.H.; Day, M.M.; Munger, J.C.; Brookes, P.S. Accumulation of Succinate in Cardiac Ischemia Primarily Occurs via Canonical Krebs Cycle Activity. Cell Rep. 2018, 23, 2617–2628. [Google Scholar] [CrossRef]
- Hu, H.; Li, X.; Ren, D.; Tan, Y.; Chen, J.; Yang, L.; Chen, R.; Li, J.; Zhu, P. The cardioprotective effects of carvedilol on ischemia and reperfusion injury by AMPK signaling pathway. Biomed. Pharmacother. 2019, 117, 109106. [Google Scholar] [CrossRef]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Yajima, D.; Motani, H.; Hayakawa, M.; Sato, Y.; Sato, K.; Iwase, H. The relationship between cell membrane damage and lipid peroxidation under the condition of hypoxia-reoxygenation: Analysis of the mechanism using antioxidants and electron transport inhibitors. Cell Biochem. Funct. 2009, 27, 338–343. [Google Scholar] [CrossRef]
- Behn, C.; Araneda, O.F.; Llanos, A.J.; Celedón, G.; González, G. Hypoxia-related lipid peroxidation evidences, implications and approaches. Respir. Physiol. Neurobiol. 2007, 158, 143–150. [Google Scholar] [CrossRef]
- Zheng, X.; Liang, Y.; Zhang, C. Ferroptosis Regulated by Hypoxia in Cells. Cells 2023, 12, 1050. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Sickinger, S.; Frotschnig, S.; Grimm, M. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 276–284. [Google Scholar] [CrossRef]
- Komai, K.; Kawasaki, N.K.; Higa, J.K.; Matsui, T. The Role of Ferroptosis in Adverse Left Ventricular Remodeling Following Acute Myocardial Infarction. Cells 2022, 11, 1399. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xiong, Y.; Zhang, Y.; Leng, Y.; Tao, J.; Li, L.; Qiu, Z.; Xia, Z. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia–reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones 2022, 27, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Weaver, K.; Skouta, R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines 2022, 10, 891. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, J.; Gu, L.; Wang, S.; Yang, T.; Wei, T.; Shan, T.; Wang, H.; Wang, L. Programmed Cell Death: Complex Regulatory Networks in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 794879. [Google Scholar] [CrossRef]
- Wang, L.-M.; Zhang, W.-W.; Qiu, Y.-Y.; Wang, F. Ferroptosis regulating lipid peroxidation metabolism in the occurrence and development of gastric cancer. World J. Gastrointest. Oncol. 2024, 16, 2781–2792. [Google Scholar] [CrossRef]
- Remme, W.J. Which Beta-Blocker is Most Effective in Heart Failure? Cardiovasc. Drugs Ther. 2010, 24, 351–358. [Google Scholar] [CrossRef]
- Park, M.; Steinberg, S.F. Carvedilol Prevents Redox Inactivation of Cardiomyocyte Β1-Adrenergic Receptors. JACC Basic. Transl. Sci. 2018, 3, 521–532. [Google Scholar] [CrossRef]
- Elmorsy, E.A.; Saber, S.; Hamad, R.S.; Abdel-Reheim, M.A.; El-Kott, A.F.; AlShehri, M.A.; Morsy, K.; Negm, S.; Youssef, M.E. Mechanistic insights into carvedilol’s potential protection against doxorubicin-induced cardiotoxicity. Eur. J. Pharm. Sci. 2024, 200, 106849. [Google Scholar] [CrossRef] [PubMed]
- Sgobbo, P.; Pacelli, C.; Grattagliano, I.; Villani, G.; Cocco, T. Carvedilol inhibits mitochondrial complex I and induces resistance to H2O2-mediated oxidative insult in H9C2 myocardial cells. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Wang, W.; He, S. Carvedilol activates nuclear factor E2-related factor 2/antioxidant response element pathway to inhibit oxidative stress and apoptosis of retinal pigment epithelial cells induced by high glucose. Bioengineered 2022, 13, 735–745. [Google Scholar] [CrossRef]
- Shirazi-Tehrani, E.; Firouzabadi, N.; Tamaddon, G.; Bahramali, E.; Vafadar, A. Carvedilol Alters Circulating MiR-1 and MiR-214 in Heart Failure. Pharmacogenom. Pers. Med. 2020, 13, 375–383. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Li, X.; Li, Z.; Diao, H.; Liu, L.; Zhang, J.; Ju, J.; Wen, L.; Liu, X.; et al. MicroRNA-1 downregulation induced by carvedilol protects cardiomyocytes against apoptosis by targeting heat shock protein 60. Mol. Med. Rep. 2019, 19, 3527–3536. [Google Scholar] [CrossRef]
- Kametani, R.; Miura, T.; Harada, N.; Shibuya, M.; Wang, R.; Tan, H.; Fukagawa, Y.; Kawamura, S.; Matsuzaki, M. Carvedilol inhibits mitochondrial oxygen consumption and superoxide production during calcium overload in isolated heart mitochondria. Circ. J. 2006, 70, 321–326. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Chen, T.-P.; Wang, Y.-C.; Lin, Y.-M.; Fang, S.-W. Carvedilol treatment after myocardial infarct decreases cardiomyocytic apoptosis in the peri-infarct zone during cardioplegia-induced cardiac arrest. Shock 2013, 39, 343–352. [Google Scholar] [CrossRef]
- White, S.M.; Constantin, P.E.; Claycomb, W.C. Cardiac physiology at the cellular level: Use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H823–H829. [Google Scholar] [CrossRef]
- Claycomb, W.C.; Lanson, N.A., Jr.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J., Jr. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef]
- Yang, Z.; Su, W.; Wei, X.; Qu, S.; Zhao, D.; Zhou, J.; Wang, Y.; Guan, Q.; Qin, C.; Xiang, J.; et al. HIF-1α drives resistance to ferroptosis in solid tumors by promoting lactate production and activating SLC1A1. Cell Rep. 2023, 42, 112945. [Google Scholar] [CrossRef]
- Cheng, M.-L.; Yang, C.-H.; Wu, P.-T.; Li, Y.-C.; Sun, H.-W.; Lin, G.; Ho, H.-Y. Malonyl-CoA Accumulation as a Compensatory Cytoprotective Mechanism in Cardiac Cells in Response to 7-Ketocholesterol-Induced Growth Retardation. Int. J. Mol. Sci. 2023, 24, 4418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-L.; Tang, H.-Y.; Wu, P.-T.; Yang, C.-H.; Lo, C.-J.; Lin, J.-F.; Ho, H.-Y. 7-Ketocholesterol Induces Lipid Metabolic Reprogramming and Enhances Cholesterol Ester Accumulation in Cardiac Cells. Cells 2021, 10, 3597. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Grüning, N.-M.; Wamelink, M.M.; Kerick, M.; Kirpy, A.; Parkhomchuk, D.; Bluemlein, K.; Schweiger, M.-R.; Soldatov, A.; Lehrach, H.; et al. The Pentose Phosphate Pathway Is a Metabolic Redox Sensor and Regulates Transcription During the Antioxidant Response. Antioxid. Redox Signal 2011, 15, 311–324. [Google Scholar] [CrossRef]
- Tracey, T.J.; Steyn, F.J.; Wolvetang, E.J.; Ngo, S.T. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Tavasoli, M.; Lahire, S.; Reid, T.; Brodovsky, M.; McMaster, C.R. Genetic diseases of the Kennedy pathways for membrane synthesis. J. Biol. Chem. 2020, 295, 17877–17886. [Google Scholar] [CrossRef]
- Carpenter, E.L.; Becker, A.L.; Indra, A.K. NRF2 and Key Transcriptional Targets in Melanoma Redox Manipulation. Cancers 2022, 14, 1531. [Google Scholar] [CrossRef]
- Yao, F.; Cui, X.; Zhang, Y.; Bei, Z.; Wang, H.; Zhao, D.; Wang, H.; Yang, Y. Iron regulatory protein 1 promotes ferroptosis by sustaining cellular iron homeostasis in melanoma. Oncol. Lett. 2021, 22, 657. [Google Scholar] [CrossRef]
- Mumbauer, S.; Pascual, J.; Kolotuev, I.; Hamaratoglu, F. Ferritin heavy chain protects the developing wing from reactive oxygen species and ferroptosis. PLoS Genet. 2019, 15, e1008396. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Cai, K.; Jiang, H.; Zou, Y.; Song, C.; Cao, K.; Chen, S.; Wu, Y.; Zhang, Z.; Geng, D.; Zhang, N.; et al. Programmed death of cardiomyocytes in cardiovascular disease and new therapeutic approaches. Pharmacol. Res. 2024, 206, 107281. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Zhang, J.; Ji, T. Cardioprotective effect of carvedilol: Inhibition of apoptosis in H9c2 cardiomyocytes via the TLR4/NF-κB pathway following ischemia/reperfusion injury. Exp. Ther. Med. 2014, 8, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Y.; Xu, M.; Yu, L.; Zhao, Y.; Chen, J.; Yuan, Y.; Zheng, Q.; Niu, X. FoxO3a Modulates Hypoxia Stress Induced Oxidative Stress and Apoptosis in Cardiac Microvascular Endothelial Cells. PLoS ONE 2013, 8, e80342. [Google Scholar] [CrossRef][Green Version]
- Bai, Y.-T.; Xiao, F.-J.; Wang, H.; Ge, R.-L.; Wang, L.-S. Hypoxia protects H9c2 cells against Ferroptosis through SENP1-mediated protein DeSUMOylation. Int. J. Med. Sci. 2021, 18, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, H.; Chen, Q.; Yang, Y.; Chen, M.; Huang, J.; Chen, M.; Lian, N. The role of ferroptosis in chronic intermittent hypoxia-induced lung injury. BMC Pulm. Med. 2022, 22, 488. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Wang, J.-X.; Chen, M.-H.; Xu, J.-J.; Jiang, M.-H.; Feng, Y.-L.; Gu, Y.-F. miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. 2020, 29, 101402. [Google Scholar] [CrossRef]
- Huang, J.; Xie, H.; Yang, Y.; Chen, L.; Lin, T.; Wang, B.; Lin, Q.-C. The role of ferroptosis and endoplasmic reticulum stress in intermittent hypoxia-induced myocardial injury. Sleep. Breath. 2023, 27, 1005–1011. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Leng, Y.; Xiong, Y.; Xia, Z. Ferroptosis Is Involved in Diabetes Myocardial IschemiaReperfusion Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol. 2020, 39, 210–225. [Google Scholar] [CrossRef]
- Peng, Y.; Liao, B.; Zhou, Y.; Zeng, W.; Zeng, Z.-Y. Atorvastatin Inhibits Ferroptosis of H9C2 Cells by regulatingSMAD7/Hepcidin Expression to Improve Ischemia-Reperfusion Injury. Cardiol. Res. Pract. 2022, 2022, 3972829. [Google Scholar] [CrossRef]
- Miyamoto, H.D.; Ikeda, M.; Ide, T.; Tadokoro, T.; Furusawa, S.; Abe, K.; Ishimaru, K.; Enzan, N.; Sada, M.; Yamamoto, T.; et al. Iron Overload via Heme Degradation in the Endoplasmic Reticulum Triggers Ferroptosis in Myocardial Ischemia-Reperfusion Injury. JACC Basic. Transl. Sci. 2022, 7, 800–819. [Google Scholar] [CrossRef]
- Li, Y.; Feng, D.; Wang, Z.; Zhao, Y.; Sun, R.; Tian, D.; Liu, D.; Zhang, F.; Ning, S.; Yao, J.; et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019, 26, 2284–2299. [Google Scholar] [CrossRef]
- Dandona, P.; Ghanim, H.; Brooks, D.P. Antioxidant activity of carvedilol in cardiovascular disease. J. Hypertens. 2007, 25, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, L.; Yuan, W.; Sun, L.; Xia, Z.; Zhang, Z.; Yao, W. Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J. Cell. Mol. Med. 2020, 24, 6670–6679. [Google Scholar] [CrossRef] [PubMed]
- Szponar, J.; Gorska, A.; Ostrowska-Lesko, M.; Korga-Plewko, A.; Tchorz, M.; Ciechanski, E.; Dabrowska, A.; Poleszak, E.; Burdan, F.; Dudka, J.; et al. Assessment of the Impact of Carvedilol Administered Together with Dexrazoxan and Doxorubicin on Liver Structure and Function, Iron Metabolism, and Myocardial Redox System in Rats. Int. J. Mol. Sci. 2024, 25, 2219. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer. 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Xia, Y.; Choi, H.K.; Lee, K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur. J. Med. Chem. 2012, 49, 24–40. [Google Scholar] [CrossRef]
- Cheong, H.I.; Asosingh, K.; Stephens, O.R.; Queisser, K.A.; Xu, W.; Willard, B.; Hu, B.; Dermawan, J.K.T.; Stark, G.R.; Prasad, S.V.N.; et al. Hypoxia sensing through β-adrenergic receptors. JCI Insight 2016, 1, e90240. [Google Scholar] [CrossRef]



| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| mouse GSS(NM_008180) | 5′-CAAAGCAGGCCATAGACAGGG-3′ | 5′-AAAAGCGTGAATGGGGCATAC-3′ |
| mouse GSR(NM_010344) | 5′-GACACCTCTTCCTTCGACTACC-3′ | 5′-CCCAGCTTGTGACTCTCCAC-3′ |
| mouse G6PD(NM_008062) | 5′-CACAGTGGACGACATCCGAAA-3′ | 5′-AGCTACATAGGAATTACGGGCAA-3′ |
| mouse NRF2(BC026943) | 5′-CTGAACTCCTGGACGGGACTA-3′ | 5′-CGGTGGGTCTCCGTAAATGG-3′ |
| mouse HO-1(NM_010442) | 5′-GCCGAGAATGCTGAGTTCATG-3′ | 5′-TGGTACAAGGAAGCCATCACC-3′ |
| mouse FTH1(NM_010239) | 5′-CAAGTGCGCCAGAACTACCA-3′ | 5′-GCCACATCATCTCGGTCAAAA-3′ |
| mouse FTL1(NM_010240) | 5′-CCATCTGACCAACCTCCGC-3′ | 5′-CGCTCAAAGAGATACTCGCC-3′ |
| mouse beta-Actin(NM_007393) | 5′-GGCTGTATTCCCCTCCATCG-3′ | 5′-CCAGTTGGTAACAATGCCATG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-C.; Cheng, M.-L. Carvedilol Confers Ferroptosis Resistance in HL-1 Cells by Upregulating GPX4, FTH1, and FTL1 and Inducing Metabolic Remodeling Under Hypoxia/Reoxygenation. Antioxidants 2025, 14, 7. https://doi.org/10.3390/antiox14010007
Li Y-C, Cheng M-L. Carvedilol Confers Ferroptosis Resistance in HL-1 Cells by Upregulating GPX4, FTH1, and FTL1 and Inducing Metabolic Remodeling Under Hypoxia/Reoxygenation. Antioxidants. 2025; 14(1):7. https://doi.org/10.3390/antiox14010007
Chicago/Turabian StyleLi, Yi-Chin, and Mei-Ling Cheng. 2025. "Carvedilol Confers Ferroptosis Resistance in HL-1 Cells by Upregulating GPX4, FTH1, and FTL1 and Inducing Metabolic Remodeling Under Hypoxia/Reoxygenation" Antioxidants 14, no. 1: 7. https://doi.org/10.3390/antiox14010007
APA StyleLi, Y.-C., & Cheng, M.-L. (2025). Carvedilol Confers Ferroptosis Resistance in HL-1 Cells by Upregulating GPX4, FTH1, and FTL1 and Inducing Metabolic Remodeling Under Hypoxia/Reoxygenation. Antioxidants, 14(1), 7. https://doi.org/10.3390/antiox14010007








