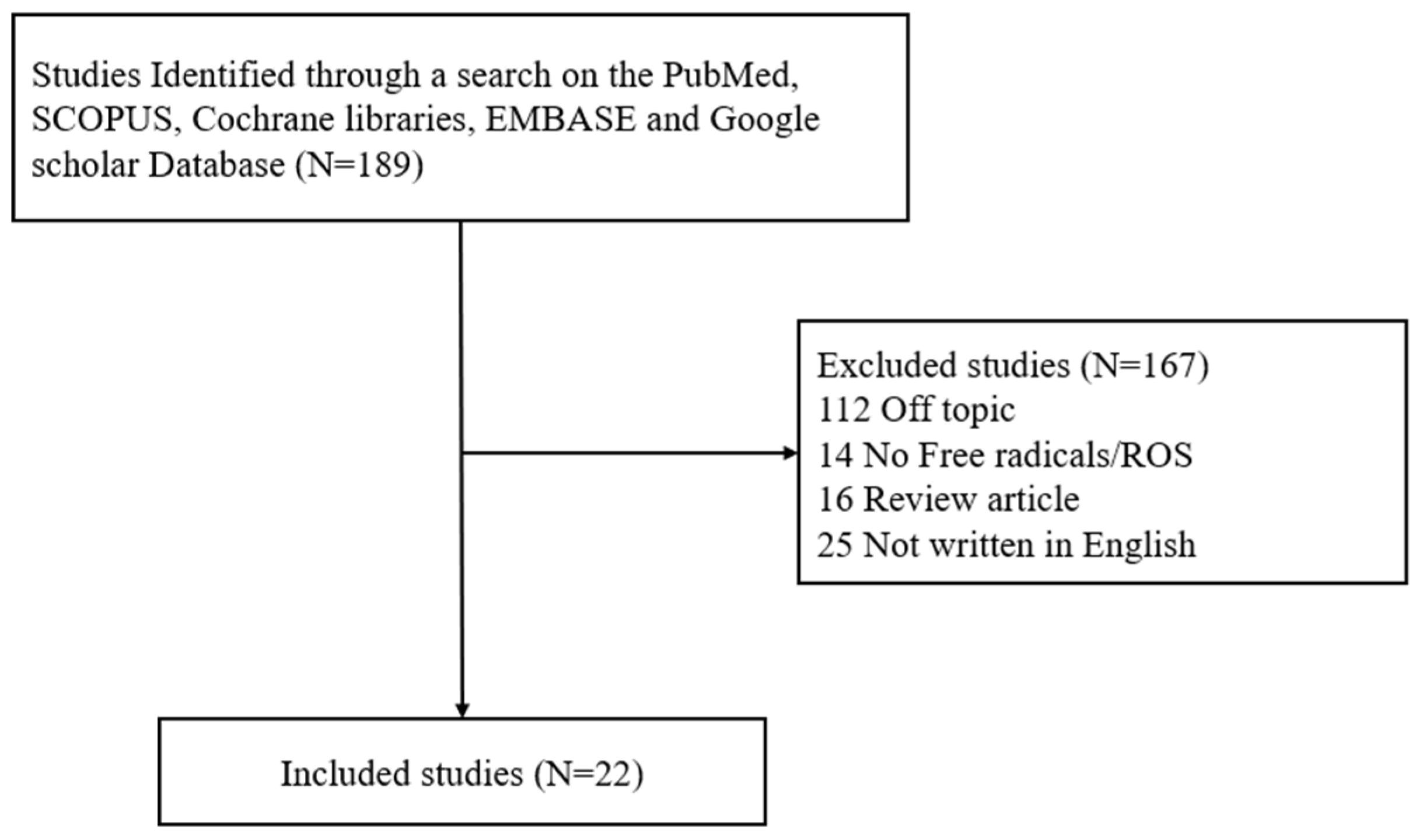
Narrative Review of Free Radicals and Reactive Oxygen Species in Otitis Media
Abstract
1. Introduction
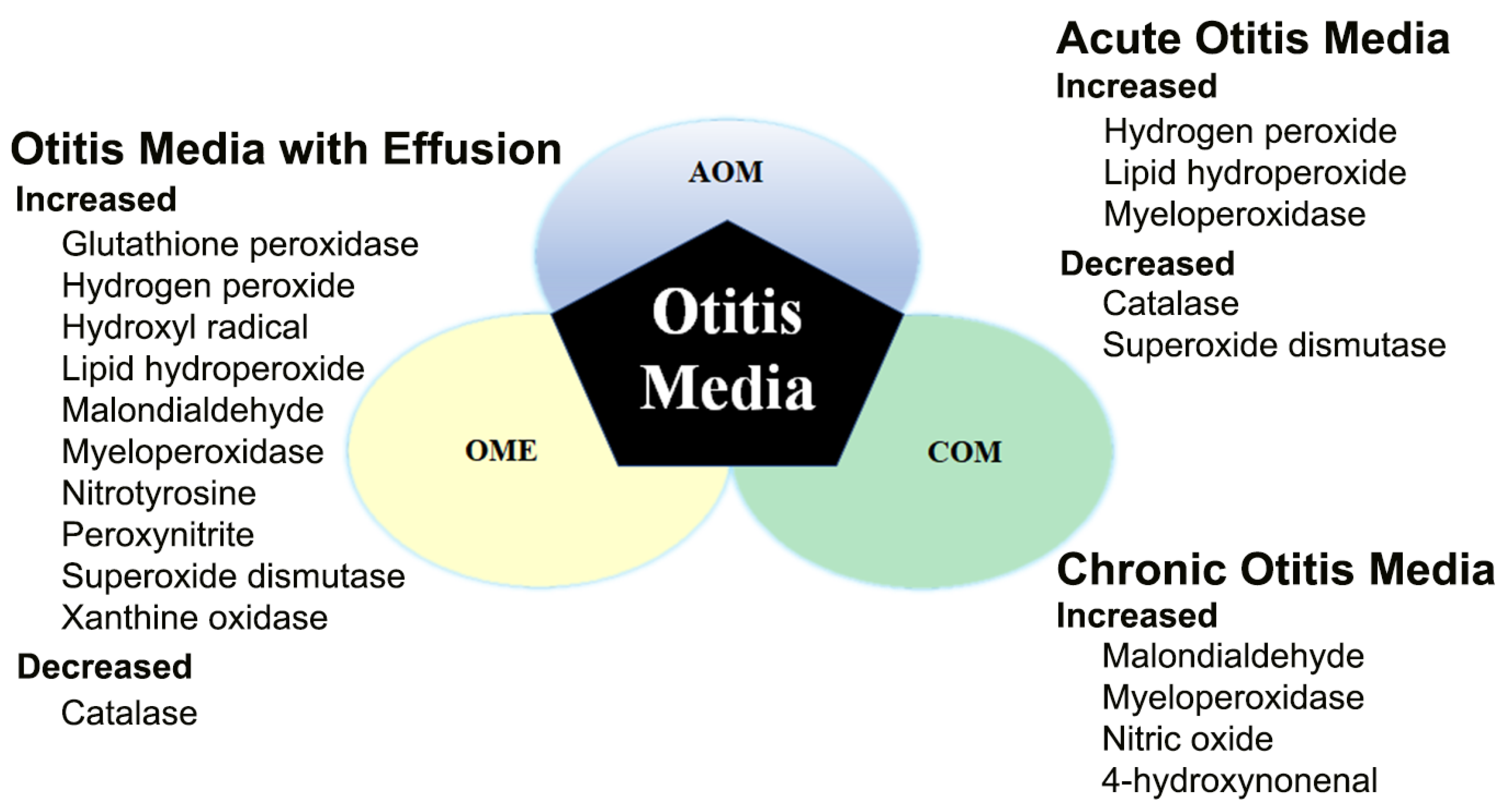
1.1. Otitis Media—Acute Otitis Media, Otitis Media with Effusion, and Chronic Otitis Media
1.2. Free Radicals/Reactive Oxygen Species (ROS)
2. Expression and Role of Free Radicals/ROS in Otitis Media
2.1. Free Radicals/ROS in Acute Otitis Media (AOM)
2.2. Free Radicals/ROS in Otitis Media with Effusion (OME)
2.3. Free Radicals/ROS in AOM + OME
2.4. Free Radicals/ROS in COM
2.5. Limitations
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeo, S.G. Acute otitis media. In Korean Society of Otorhinolaryngology-Head and Neck Surgery, 3rd ed.; KoonJa: Seoul, Republic of Korea, 2018; pp. 363–383. [Google Scholar]
- Margaretha, L.C.; Ellen, M.M. Acute otitis media and otitis media with effusion. In Cummings Otolaryngology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3019–3037. [Google Scholar]
- Jang, J.H. Acute otitis media. Korean Otol. Soc. 2022, 27, 433–441. [Google Scholar]
- Bluestone, C.D. Epidemiology and pathogenesis of chronic suppurative otitis media; implications for prevention and treatment. Int. J. Pediatr. Otorhinolaryngol. 1998, 42, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.J.; Daly, K.A.; Brainbridge, K.E. Epidemiolody, natural history, and risk factors. Otolaryngol. Head Neck Surg. 2013, 148, E1–E25. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.W. Otitis media with effudion. Korean Otol. Soc. 2022, 28, 443–460. [Google Scholar]
- Bluestone, C.D.; Klein, J.O. Otitis media and eustachian tube dysfunction. In Pediatric Otolaryngology, 4th ed.; Saunders: Philadelphia, PA, USA, 2003; pp. 474–685. [Google Scholar]
- Gleesion, M.; Browning, G.G.; Burton, M.J. Scott-Brown’s Otorhinolaryngology: Head and Neck Surgery, 7th ed.; Routledge: London, UK, 2007; Volume 3, pp. 3395–3452. [Google Scholar]
- Park, Y.H. Chronic otitis media. Korean Otol. Soc. 2022, 30, 469–480. [Google Scholar]
- Verhoeff, M.; Van der Veen, E.L.; Rovers, M.M. Chrinic suppurative otitis media: A review. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1–12. [Google Scholar] [CrossRef]
- Wiatr, M.; Skladzien, J.; Strek, P.; Przeklasa-Muszynska, A. Understanding the etiology and resolution of chronic oriris media from animal and human studies. J. Int. Adv. Otol. 2019, 15, 12–17. [Google Scholar] [CrossRef]
- Cho, S.I. Cholesteatomatous otitis media. Korean Otol. Soc. 2022, 31, 481–494. [Google Scholar]
- Van der Toom, H.F.E.; van der Schroeff, M.P.; Pauw, R.J. Single-Stage Mastoid Obliteration in Cholesteatoma Surgery and Recurrent and Residual Disease Rates: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 440–446. [Google Scholar] [CrossRef]
- Jovanovic, I.; Zivkovic, M.; Djuric, T.; Stojkovic, L.; Jesic, S.; Stankovic, A. Perimatrix of middle ear cholesteatoma: A granulation tissue with a specific transcriptomic signature. Laryngoscope 2020, 130, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Choi, Y.H.; Jeon, E.S.; Yang, H.C.; Cho, Y.B. Extradural granulation complicated by chronic suppurative otitis media with cholesteatoma. In Vivo 2014, 28, 651–655. [Google Scholar] [PubMed]
- Patlevič, P.; Vašková, J.; Švorc, P., Jr.; Vaško, L.; Švorc, P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Di Mascio, P. Lipid hydroperoxides as a source of singlet molecular oxygen. Subcell. Biochem. 2014, 77, 3–20. [Google Scholar] [PubMed]
- Taverne, Y.J.; Merkus, D.; Bogers, A.J.; Halliwell, B.; Duncker, D.J.; Lyons, T.W. Reactive Oxygen Species: Radical Factors in the Evolution of Animal Life: A molecular timescale from Earth’s earliest history to the rise of complex life. Bioessays 2018, 40, 1700158. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J. Biological defense against free radicals and the failure of defense. Food Technol. 1997, 10, 4–26. [Google Scholar]
- Hassan, H.A.; Ahmed, H.S.; Hassan, D.F. Free radicals and oxidative stress: Mechanisms and therapeutic targets: Review article. Hum. Antibodies 2024, 32, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Stuhr, R.; Bayer, P.; von Wangelin, A.J. The Diverse Modes of Oxygen Reactivity in Life & Chemistry. ChemSusChem 2022, 15, e202201323. [Google Scholar]
- Hong, J.K. Theoretical review of skin aging caused by reactive oxygen species and the efficacy of antioxidant vitamins. J. Korean Soc. Aesthetic Cosmetol. 2009, 7, 51–62. [Google Scholar]
- Joorabloo, A.; Liu, T. Recent advances in reactive oxygen species scavenging nanomaterials for wound healing. Exploration 2024, 4, 20230066. [Google Scholar] [CrossRef]
- Humayun, S.; Hayyan, M.; Alias, Y. A review on reactive oxygen species-induced mechanism pathways of pharmaceutical waste degradation: Acetaminophen as a drug waste model. J. Environ. Sci. 2025, 147, 688–713. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Yeo, S.G.; Jeong, M.H. Generation and action of reactive oxygen species. Biochem. Mol. Biol. News 2005, 12, 10–15. [Google Scholar]
- Kim, K.S. The role of antioxidants in the treatment of aging. In Proceedings of the Conference of the Korean Society of Clinical Geriatrics; 2004; pp. 379–392. [Google Scholar]
- Addor, F.A.S. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.R.; Huang, C.C.; Haddad, J., Jr. Superoxide dismutase in an animal model of otitis media. Eur. Arch. Otorhinolaryngol. 1995, 252, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.R.; Huang, C.C.; Haddad, J., Jr. Middle ear catalase distribution in an animal model of otitis media. Eur. Arch. Otorhinolaryngol. 1996, 253, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Takoudes, T.G.; Haddad, J., Jr. Hydrogen peroxide in acute otitis media in guinea pigs. Laryngoscope 1997, 107, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J., Jr. Lipoperoxidation as a measure of free radical injury in otitis media. Laryngoscope 1998, 108, 524–530. [Google Scholar] [CrossRef]
- Takoudes, T.G.; Haddad, J., Jr. Lipid peroxides in middle ear fluid after acute otitis media in guinea pigs. Ann. Otol. Rhinol. Laryngol. 1999, 108, 564–568. [Google Scholar] [CrossRef]
- Takoudes, T.G.; Haddad, J., Jr. Free radical production by antibiotic-killed bacteria in the guinea pig middle ear. Laryngoscope 2001, 111, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Jin, C.; Wang, W.; Wang, Z.; Huang, Y.; Fan, F.; Ma, Y.; Zhang, X.; Xu, W.; Yin, Y.; et al. The critical role of myeloperoxidase in Streptococcus pneumoniae clearance and tissue damage during mouse acute otitis media. Innate Immun. 2017, 23, 296–306. [Google Scholar] [CrossRef]
- Dong, Y.; Jin, C.; Ding, Z.; Zhu, Y.; He, Q.; Zhang, X.; Ai, R.; Yin, Y.; He, Y. TLR4 regulates ROS and autophagy to control neutrophil extracellular traps formation against Streptococcus pneumoniae in acute otitis media. Pediatr. Res. 2021, 89, 785–794. [Google Scholar] [CrossRef]
- Lee, E.S.; Woo, J.S.; Hwang, S.J.; Lim, H.H.; Suh, H.K. Protective role of superoxide dismutase in rat eustachian tubal mucosa against acute otitis media induced by upper respiratory tract infection. J. Laryngol. Otol. 2000, 114, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, D.; Huang, Y. Fisetin administration improves LPS-induced acute otitis media in mouse in vivo. Int. J. Mol. Med. 2018, 42, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kawana, M.; Kawana, C.; Yokoo, T.; Quie, P.G.; Giebink, G.S. Oxidative Metabolic Products Released from Polymorphonuclear Leukocytes in Middle Ear Fluid during Experimental Pneumococcal Otitis Media. Infect. Immun. 1991, 59, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Testa, D.; Guerra, G.; Marcuccio, G.; Landolfo, P.G.; Motta, G. Oxidative stress in chronic otitis media with effusion. Acta Otolaryngol. 2012, 132, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Takoudes, T.G.; Haddad, J., Jr. Evidence of oxygen free radical damage in human otitis media. Otolaryngol.-Head Neck Surg. 1999, 120, 638–642. [Google Scholar] [CrossRef]
- Parks, R.R.; Huang, C.C.; Haddad, J., Jr. Evidence of oxygen radical injury in experimental otitis media. Laryngoscope 1994, 104, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, X.; Xu, H.; Xhao, J.; Li, W. Relationship of T lymphocytes, cytokines, immunoglobulin E and nitric oxide with otitis media with effusion in children and their clinical significances. Rev. Assoc. Med. Bras. 2019, 65, 971–976. [Google Scholar] [CrossRef]
- Aktan, B.; Taysi, S.; Gumustekin, K.; Baka, N.; Sutbeyaz, Y. Evaluation of oxidative stress in erythrocytes of guinea pigs with experimental otitis media and effusion. Ann. Clin. Lab. Sci. 2003, 33, 232–236. [Google Scholar]
- Hisamatsu, K.; Inoue, H.; Makiyama, K.; Homma, M. Nitrotyrosine in otitis media with effusion. Ann. Otol. Rhinol. Laryngol. 2005, 114, 804–808. [Google Scholar] [CrossRef]
- Yariktas, M.; Doner, F.; Dogru, H.; Yasan, H.; Delibas, N. The role of free oxygen radicals of the development of otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Sagiroglu, S.; Ates, S.; Tolun, F.I.; Oztarakci, H. Evaluation of oxidative stress and antioxidants effect on turning process acute otitis media to chronic otitis media with effusion. Niger. J. Clin. Pract. 2019, 22, 375–379. [Google Scholar] [CrossRef]
- Karhdag, T.; Ilhan, N.; Kaygusuz, I.; Keles, E.; Yalcin, S. Comparison of free radicals and antioxidant enzymes in chronic otitis media with and without tympanosclerosis. Laryngoscope 2004, 114, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Garca, M.F.; Aslan, M.; Tuna, B.; Kozan, A.; Cankaya, H. Serum Myeloperoxidase Activity, Total Antioxidant Capacity and Nitric Oxide Levels in Patients with Chronic Otitis Media. J. Membr. Biol. 2013, 246, 519–524. [Google Scholar] [CrossRef]
- Westerberg, J.; Granath, A.; Drakskog, C.; Tideholm, E.; Georen, S.K.; Weitzburg, E.; Cardell, L.O. Nitric Oxide Is Locally Produced in the Human Middle Ear and Is Reduced by Acquired Cholesteatoma. Otol. Neurotol. 2022, 43, 198–204. [Google Scholar] [CrossRef] [PubMed]

| Type | Element Name | Enzyme | Reaction Catalysed |
|---|---|---|---|
| O2·− | Superoxide radical (or anion) | Superoxide dismutase | O2·− + O2·− + 2H+ → 2H2O2 + O2 |
| HO2 | Perhydroxyl radical | Catalase | 2H2O2 → 2H2O + O2 |
| H2O2 | Hydrogen peroxide | Glutathione peroxidase | H2O2 + 2GSH → GSSG + 2H2O |
| HO· | Hydroxyl radical | Glutathione S-transferase | LOOH + 2GSH → GSSG + H2O + LOH |
| 1O2 | Singlet oxygen | Glutathione reductase | RX + GSH → RSG + HX * |
| OCL- | Hypochlorite | Thioredoxin | NADPH + GSSG ⇄ NADP++ 2GSH |
| O2 | Ozone | Cytochrome c peroxidase | Trx-(SH)2 + Protein-S2 ⇄ Trx-S2 + Protein-(SH)2 |
| Complex + 2cyt-c (Fe2+) → enzyme + 2cyt-c (Fe2+) + 2OH− | |||
| Non-radicals | Nonenzymatic scavengers | ||
| H2O2 | Hydrogen peroxide | Vitamin A | |
| HOCI | Hypochlorous acid | Vitamin C (ascorbic acid) | |
| Vitamin E (-tocopherol)-carotene | |||
| ONOO | Peroxynitrite | Cystein | |
| 1O2 | Singlet oxygen | Coenzyme Q | |
| Uric acid | |||
| Flavonoids | |||
| Sulfhydryl group | |||
| Thioether compounds |
| Author/ Year/ Reference | Study Design | Species and/or Sample | Type of OM | Detection Method | Target Substance(s) Associated with Free Radicals | Results/Conclusions |
|---|---|---|---|---|---|---|
| Takodues T.G. et al., 1997 [30] | Animal study | 32 guinea pigs injected transtympanically with Streptococcus pneumoniae | AOM | Histologic analysis, hydrogen peroxide assay | Hydrogen peroxide | Hydrogen peroxide levels increased with time after inoculation into infected ears (p < 0.01), and hydrogen peroxide levels were significantly higher in infected than in control middle ears at each time point < 0.05). Hydrogen peroxide, a component of oxygen free radical damage, was shown to be elevated in infected middle ear fluid in pneumococcal AOM. /This indicates that a neutrophil response to transtympanic injection was likely responsible for the generation of small amounts of H2O2 in these middle ears. |
| Haddad, J., Jr. et al., 1998 [31] | Animal study | 82 guinea pigs injected transtympanically with Streptococcus pneumoniae | AOM | Histologic analysis, hydroperoxide assay | Lipid hydroperoxide | Free radical damage, evidenced by lipoperoxidation, which was previously found to contribute to the inflammatory changes associated with AOM. Lipoperoxidation was highest on days 5 (p < 0.03) and 10 (p < 0.04), significantly decreasing by days 20 (p < 0.04) and 30 (p < 0.01). /This indicates that lipoperoxidation may contribute to middle ear inflammation for a significant period of time after acute infection. |
| Takoudes T.G. et al., 2001 [32] | Animal study | 78 guinea pigs that underwent bilateral middle ear inoculation with sterile saline (control), amoxicillin, Streptococcus pneumoniae killed with amoxicillin, and S. pneumoniae | AOM | Protein assays and histological evaluation | Lipid hydroperoxide | Mucosal lipid hydroperoxide significantly higher in the mucosa of both the antibiotic-killed S. pneumoniae group and the S. pneumoniae-infected group than in the control group on day 1 and significantly higher in the mucosa of the S. pneumoniae-infected group than in the mucosa of the antibiotic-killed and control groups on day 5. /This indicates that free radical damage to the middle ear mucosa may occur in otitis media, despite appropriate antibiotic therapy. |
| Takoudes T.G. et al., 1999 [33] | Animal study | 21 Hartley guinea pigs injected with a suspension of type 3 S. pneumoniae. | AOM | Lipid peroxide assay, histologic evaluation | Lipid peroxide | Total LPO levels significantly higher in fluid from infected than control animals (p = 0.04). LPO concentration in the middle ear fluid, defined as total LPO divided by the weight of the fluid collected, was significantly higher (p = 0.01) in specimens from infected than control guinea pigs. Histologic examination confirmed leukocyte infiltration and mucosal edema that were consistent with mucosal damage. /Free radicals, which are likely to arise from both pathogens and neutrophils during infection, appear to attack mucosal cell membranes. |
| Dong Y. et al., 2021 [34] | Animal study, in vitro | Mouse model of AOM established by transbullar injection of S. pneumoniae | AOM | Western blotting, cell culture, confocal microscopy, fluorescence microscopy | NETs, TLR4, ROS | Otitis media caused mainly by Streptococcus pneumoniae (Spn) NETs present in the middle ear of animals with Spn-induced AOM. TLR4 contributed to the regulation of NET formation via autophagy activation and ROS production. TLR4 partly mediated NET formation in response to Spn in vitro and in vivo during AOM, producing NETs able to engulf and kill Spn both in vivo and in vitro. /TLR4 regulates ROS and autophagy to control the formation of NETs against Spn during the course of AOM. |
| Xiang Y. et al., 2017 [35] | Animal study | Model of AOM in C57BL/6 mice by direct bilateral transtympanic inoculation of S. pneumoniae into middle ears | AOM | MPO kinetic–colorimetric assay, flow cytometry, histopathologic analysis, lactate dehydrogenase assay, ELISA, immunofluorescence assay | MPO, ROS | MPO production by recruited neutrophils significantly higher in Spn-infected than in control mice, with neutrophils killing Spn in an MPO-dependent manner. MPO facilitated the generation of ROS, promoted Spn clearance at an early stage and exacerbated tissue damage. /MPO has dual roles in AOM, contributing to the removal of bacteria in the middle ear cavity, while also causing tissue damage partly associated with its ability to increase ROS generation. |
| Parks R.R. et al., 1995 [36] | Animal study | 20 Hartley guinea pigs injected in the right ear with S. pneumoniae and in the left ear with sterile normal saline | AOM | Immunohistochemistry, Western blotting, tissue assay | SOD concentration significantly higher in normal (1.77 ± 0.48 μg/mg protein) than in infected (1.02 ± 0.28 μg/mg protein) mucosa (p < 0.05). /Infected ears showed a disproportionate increase in the ratio of submucosa to overlying epithelium, increasing the ratio of relatively SOD-poor to SOD-rich tissue. | |
| Lee E.S. et al., 2000 [37] | Animal study | 40 Sprague Dawley rats injected with S. pneumoniae and divided into three groups according to their tympanic cavity conditions: no-AOM, AOM, and recovery groups. | AOM | Immunohistochemistry, Western blot | SOD | Western blotting of samples from the Non-AOM, recovery, and control groups showed that optical density (213.5 ± 22.4 vs. 219.3 ± 18.7 vs. 223.5 ± 26.2) and surface area (13.2 ± 0.8.mm2 vs. 14.8 ± 0.7 mm2 vs. 16.7 ± 0.4 mm2) were similar, but that both optical density (167.6 ± 19.3) and surface area (6.5 ± 0.9 mm2) were markedly lower in the AOM group. /These findings suggest that superoxide dismutase may play a role in protecting tubal mucosa from free radical injury during AOM. |
| Parks R.R. et al., 1996 [38] | Animal study | 20 mature guinea pigs injected with S. pneumoniae. | AOM | Immunohistochemistry, Western blotting, ELISA | Catalase | The submucosal tissues in the infected middle ears stained poorly for catalase, suggesting that their catalase contents were lower than those of the epithelium. This staining pattern was similar to that of SOD. This result was expected, as the two enzymes have complementary functions. /The antioxidant enzymes catalase, glutathione peroxidase, and superoxide dismutase protect tissues from the destructive effects of free radicals, with these enzymes having complementary functions. |
| Li et al., 2018 [39] | Animal study | 60 male C57BL/6 mice injected with LPS into the middle ear via the tympanic membrane, followed by intragastric administration of fisetin. | AOM | H&E staining, ELISA, RT-qPCR, Western blotting, flow cytometry, immunohistochemistry | ROS, MDA, SOD | The concentrations of the pro-inflammatory cytokines, IL-1β, TNF-α, IL-6, and VEGF, were high in LPS-treated mice, with all of these concentrations being reduced by fisetin administration. This process was dependent on TLR4/NF-kB inactivation. LPS reduced serum SOD and MDA activities, reductions reversed by fisetin administration. /Fisetin can improve the symptoms of AOM by inhibiting inflammatory responses and reducing oxidative stress. |
| Author/ Year/ Reference | Study Design | Species and/or Sample | Type of OM | Detection Method | Target Substance(s) Associated with Free Radicals | Results/Conclusions |
|---|---|---|---|---|---|---|
| Testa D. et al., 2012 [40] | Comparative study | 59 children with a history of middle ear effusion unresponsive to repeated medical treatments who underwent myringotomy. | OME | Flow cytometry | Lipid peroxides, free radicals, glutathione | Lipid peroxide levels in all samples were high (mean 11.5 nmole/million cells), indicating a high level of oxidative stress and a high percentage of inflammatory cells. /Inflammatory cells in chronic OME generate high levels of oxygen-derived free radicals. The improvement induced by GSH treatment while applying ventilation tubes and after surgery can prevent this possible oxidative stress. |
| Takoudes T.G. et al., 1999 [41] | Comparative study | 35 specimens of middle ear fluid from children with chronic otitis media classified as mucoid (n = 19), purulent (n = 10), or serous (n = 6). | OME | LPO assay | Lipid hydroperoxide | The significantly higher levels of total LPO in mucoid than in serous effusions and of LPO concentrations in purulent than in serous effusions may be explained by the pathogenesis of these effusions. Purulent effusions may contain both increased neutrophils and Streptococcus organisms, which produce FR-like hydrogen peroxide, increasing LPO levels. Serous effusions are likely to contain fewer neutrophils, resulting in a lower level of lipid peroxidation. /LPOs are present in fluid recovered from middle ears of pediatric patients with OM. The total amount of LPO (in nanomoles) is significantly higher in mucoid than in serous effusions, and the LPO concentration (nanomoles per microgram of fluid) is significantly higher in purulent than in serous effusions. |
| Parks R.R. et al., 1994 [42] | Animal study | 20 Hartley guinea pigs injected with Streptococcus pneumoniae | Experimental Otitis Media | Histologic analysis, tissue assays; lipid hydroperoxide and thiobarbituric acid (TBA) assays | LPO and MDA | The concentrations of LPO (p < 0.01) and MDA (p < 0.05) were found to be significantly higher in infected mucosa than in saline-injected controls. /Free radicals play a role in the pathogenesis of otitis media. |
| Kawana M. et al., 1991 [43] | Animal study, in vitro | 46 chinchillas injected with 7F S. pneumoniae | OME | Biochemical and colorimetric assays | MPO, Superoxide anion | In vitro stimulated production of MPO and O2− was significantly lower from middle ear than from peripheral blood neutrophils 24 h after inoculation of nonviable pneumococci, although these levels were similar after 48 h. 24 h after inoculation of viable pneumococci, middle ear neutrophils stimulated in vitro produced less MPO but the same amount of O2− as did blood neutrophils. /Oxidative metabolic products are released from phagocytic cells into the MEF during OM. |
| Fan W. et al., 2019 [44] | Comparative study | 50 children with OME, 50 healthy children as controls | OME | Flow cytometry and ELISA | Immunoglobulin E, NO, cytokines, T lymphocytes | The percentages of peripheral blood CD4+ and CD8+, the CD4+/CD8+ ratio, and IgE and NO levels were significantly higher in children with than without OME (p < 0.01 each). In children with OME, the levels of IL-2, IL-6, IgE, and NO were significantly higher in the MEE than in peripheral blood (p < 0.01 each). /The levels of CD4+ and CD8+ T lymphocytes in peripheral blood and of IL-2, IL-6, IgE, and NO in MEE are increased in children with OME. |
| Hisamatsu K. et al., 2005 [45] | Comparative study | 90 patients with OME, including 50 with acute and 40 with chronic OME. Patients were also classified by age: <16 years (group A, n = 13), 16–50 years (group B, n = 14), and >50 years (group C, n = 63). | OME | ELISA and colorimetric assays | Nitrotyrosine, NO, SOD, LDH | NT concentration was significantly higher in group A than in group C (p < 0.05), and significantly higher in patients with acute than chronic OME (p < 0.05). NO concentration did not differ significantly among patient groups. SOD activity correlated significantly with NT and NO concentrations and LDH activity (p < 0.05 each). LDH activity was significantly greater in group A than in group C (p < 0.05). /The NO–superoxide system is involved in the pathogenesis of OME, providing evidence for protein and/or cell injury in the middle ear. |
| Yariktas M. et al., 2004 [46] | Comparative study | 74 subjects, including 26 OME patients who underwent adenoidectomy with ventilation tube replacement (Group 1), 28 age-matched OME patients who underwent adenoidectomy without ventilation tube insertion (Group 2), and 20 healthy controls (Group 3). | OME | Blood parameters | MDA, SOD, CAT, GSH-Px enzymes | Erythrocyte MDA level and GSH-Px enzyme activity in blood samples were significantly higher in Group 1 than Groups 2 and 3 (p < 0.05 each). SOD enzyme activity in blood samples was significantly lower in Group 1 than in Group 2 (p < 0.05) and significantly higher in Group 1 than in Group 3 (p < 0.05). CAT enzyme activity was significantly higher in Group 1 than Group 3 (p < 0.05). /Inflammation of the middle ear increases the levels of FORs in erythrocytes. FOR levels are normally maintained at steady state by antioxidant enzymes. Weakening of the antioxidant defense system increases FORs, contributing to OME. |
| Aktan B. et al., 2003 [47] | Animal study | 12 guinea pigs, six injected with histamine solution and six injected with normal saline. | OME | Biochemical parameters | SOD, CAT, XO, MDA, TSSA | The MDA level, TSSA, SOD, and XO activities in the erythrocytes of the experimental OME group were significantly higher than those of the control group (p < 0.05, for the first three parameters; p < 0.01, for the last one). The TSSA and CAT activities in erythrocytes of the experimental OME group did not differ significantly from those of the control group. /This suggests that OFRs may play an important role in cell and tissue damage due to OME. |
| Author/ Year/ Reference | Study Design | Species and/or Sample | Type of OM | Detection Method | Target Substance(s) Associated with Free Radicals | Results/Conclusions |
|---|---|---|---|---|---|---|
| Sagiroglu S. et al., 2018 [48] | Comparative study | 31 AOM, 39 COME patients, and 37 control subjects. | AOM, OME | Blood parameters | MPO, GPx, CAT, NO, MDA, SOD | MPO (p = 0.040), NO (p = 0.001), and CAT (p = 0.044) were significantly higher in the AOM and COME groups than in the control groups. CAT was low during the acute phase and high during the chronic phase of OM. MPO and CAT distributions were significantly greater in the COME group. CAT was lower in the AOM than in the COME and control groups. MDA, GPx, and SOD distributions were not significantly different in the three groups. /Serum levels of oxidative stress markers and antioxidants play an active role in the pathogenesis of COME and AOM. |
| Author/ Year/ Reference | Study Design | Species and/or Sample | Type of OM | Detection Method | Target Substance(s) Associated with Free Radicals | Results/Conclusions |
|---|---|---|---|---|---|---|
| Karhdag T. et al., 2004 [49] | Comparative study | 65 patients with COM, including 34 with tympanosclerotic plaques on the tympanic membrane, middle ear mucosa, ossicular chain, or mastoid bone, and 31 without these plaques, along with 30 controls. | COM | Venous blood, middle ear mucosa, and tympanic membrane | NO, SOD, Malondialdehyde, Catalase | NO and MDA levels in middle ear mucosa (p = 0.001) and tympanic membrane (p = 0.01), as well as plasma MDA and CAT levels (p = 0.001 each), were significantly higher in COM patients with than those without tympanosclerotic plaques. SOD levels were similar in the two groups (p > 0.05). /Increased oxidative stress seems to be associated with decreased antioxidant levels in patients with COM, suggesting a role for increased oxidative stress in the pathogenesis of COM. |
| Garca M.F. et al., 2013 [50] | Comparative study | 61 patients, 21 with and 40 without cholesteatoma. | COM | Spectrophotometer, chromatographic spectrometer, ELISA | MPO, NO, MDA, 4-HNE | Serum MPO activity and MDA, 4-HNE, and NO levels were significantly higher, and TAC levels significantly lower, in COM patients than in controls (p < 0.001 each). Serum MPO activity and MDA, 4-HNE, and NO levels were higher in COM patients with than without cholesteatoma, but between-group differences were not statistically significant (p > 0.05). /Increased oxidative stress may play a role in the pathogenesis of COM. |
| Johanna Westerberg J. et al. 2022 [51] | Case–control study | Gaseous NO from 11 patients with unilateral perforations. Middle ear mucosa from 48 patients with COM and 26 with cholesteatoma. | COM | Chemiluminescence | NOS | The gaseous NO concentration was higher in ears with a unilateral tympanic membrane perforation or grommet than in controls (p = 0.04). eNOS levels were lower in pooled samples from COM patients than controls (p = 0.010), and nNOS levels were lower in COM patients with cholesteatoma than in controls (p = 0.011). /NOS enzymes in the middle ear mucosa are indicative of ongoing NO production. Reduced NOS in ears with cholesteatoma and in pooled samples from ears with COM suggests a role for locally produced NO in middle ear disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Hong, S.M.; Choi, Y.S.; Lee, J.; Yeo, J.H.; Kim, S.S.; Lee, J.M.; Yon, D.K.; Yeo, S.G. Narrative Review of Free Radicals and Reactive Oxygen Species in Otitis Media. Antioxidants 2025, 14, 3. https://doi.org/10.3390/antiox14010003
Lee J, Hong SM, Choi YS, Lee J, Yeo JH, Kim SS, Lee JM, Yon DK, Yeo SG. Narrative Review of Free Radicals and Reactive Oxygen Species in Otitis Media. Antioxidants. 2025; 14(1):3. https://doi.org/10.3390/antiox14010003
Chicago/Turabian StyleLee, Jeongmin, Seok Min Hong, Yong Sung Choi, Jinseok Lee, Joon Hyung Yeo, Sung Soo Kim, Jae Min Lee, Dong Keon Yon, and Seung Geun Yeo. 2025. "Narrative Review of Free Radicals and Reactive Oxygen Species in Otitis Media" Antioxidants 14, no. 1: 3. https://doi.org/10.3390/antiox14010003
APA StyleLee, J., Hong, S. M., Choi, Y. S., Lee, J., Yeo, J. H., Kim, S. S., Lee, J. M., Yon, D. K., & Yeo, S. G. (2025). Narrative Review of Free Radicals and Reactive Oxygen Species in Otitis Media. Antioxidants, 14(1), 3. https://doi.org/10.3390/antiox14010003







