NoxO1 Determines the Level of ROS Formation by the Nox1-Centered NADPH Oxidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. mRNA Expression in Murine Tissue and in Cell Lines
2.3. Overexpression Systems
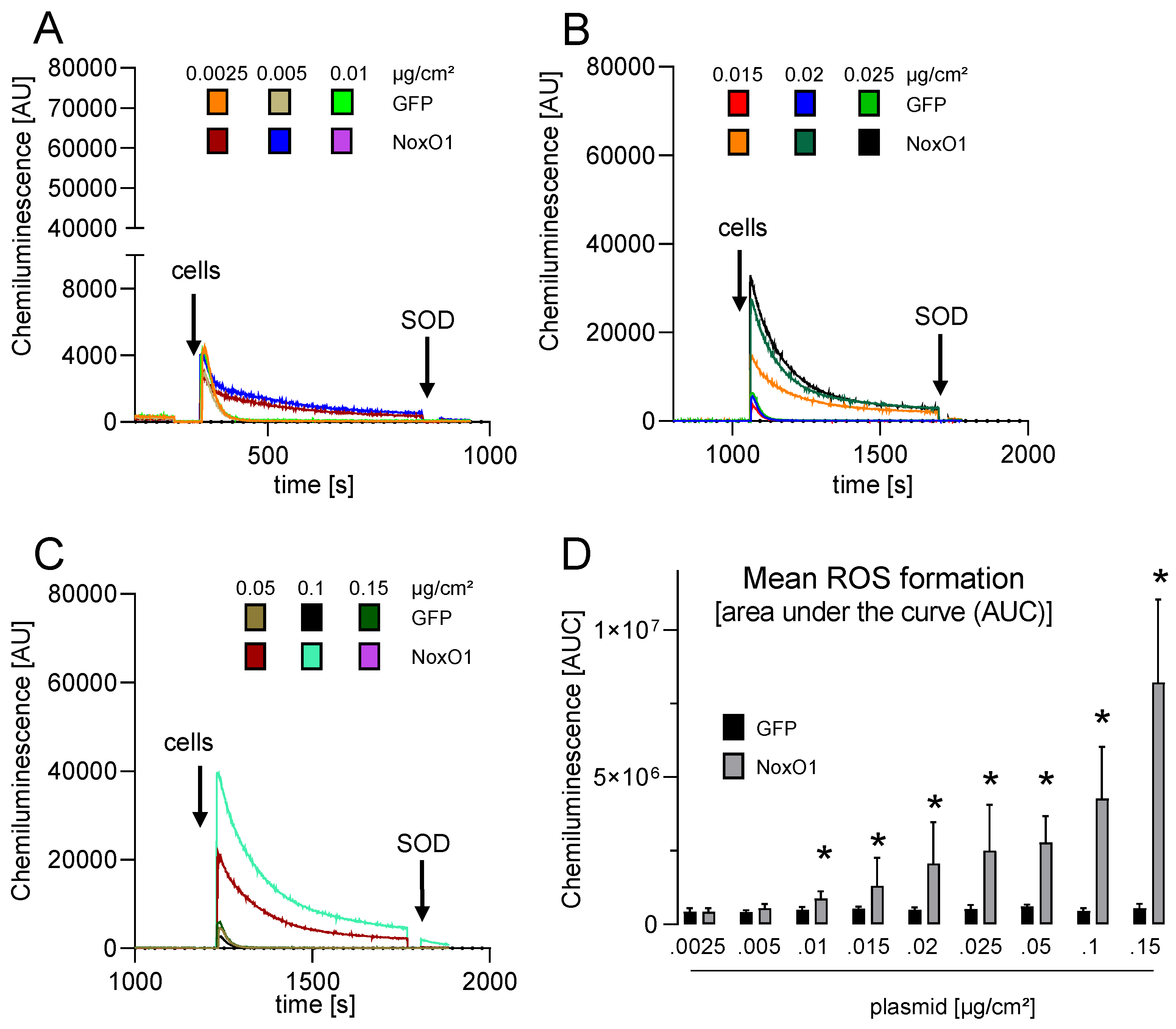
2.4. ROS Measurement with Chemiluminescence
2.5. Immunofluorescence and Confocal Microscopy
2.6. NoxO1 Protein Detection
2.7. Statistical Analysis
3. Results
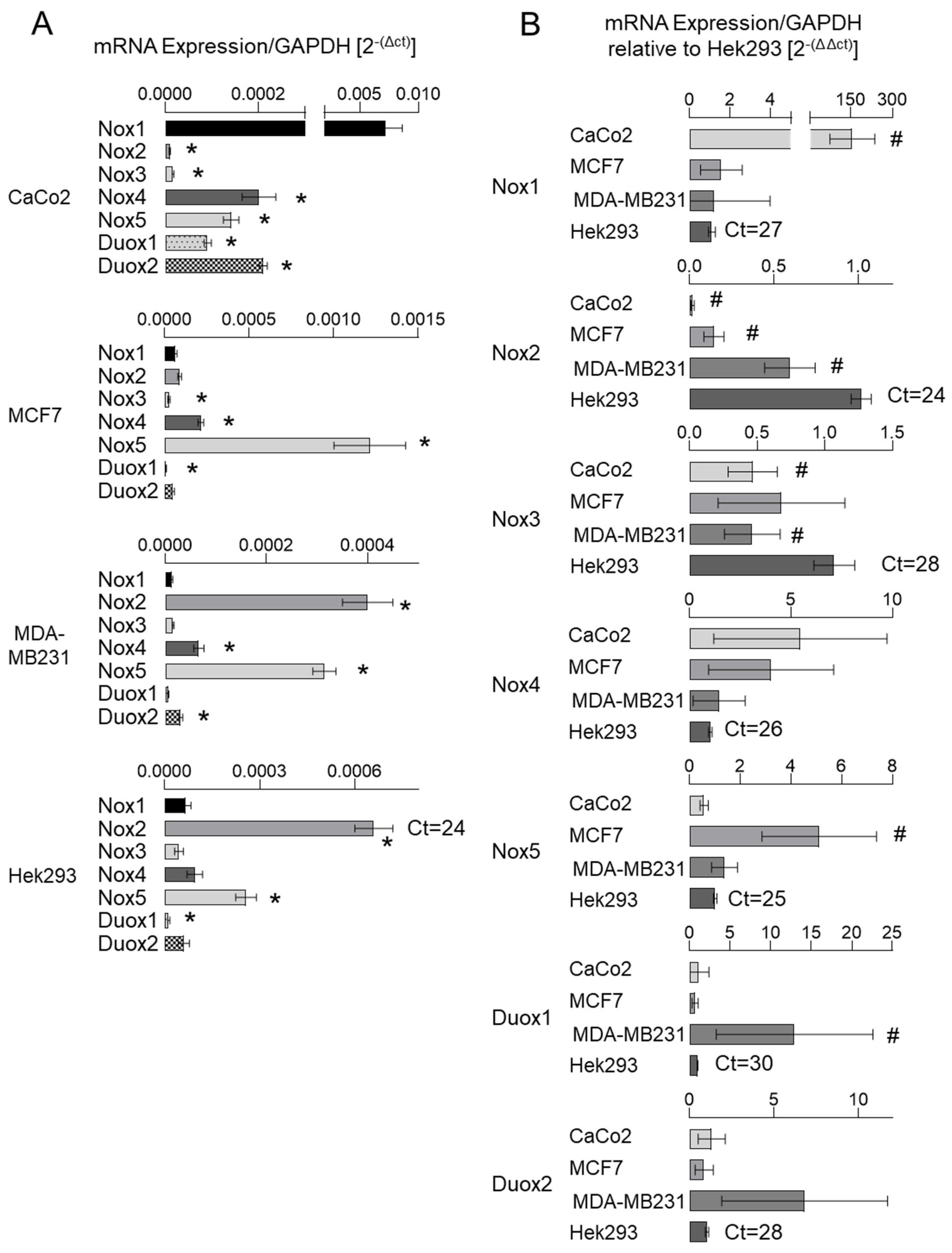
3.1. NADPH Oxidase Subunits Are Differentially Expressed in Cancer Cells
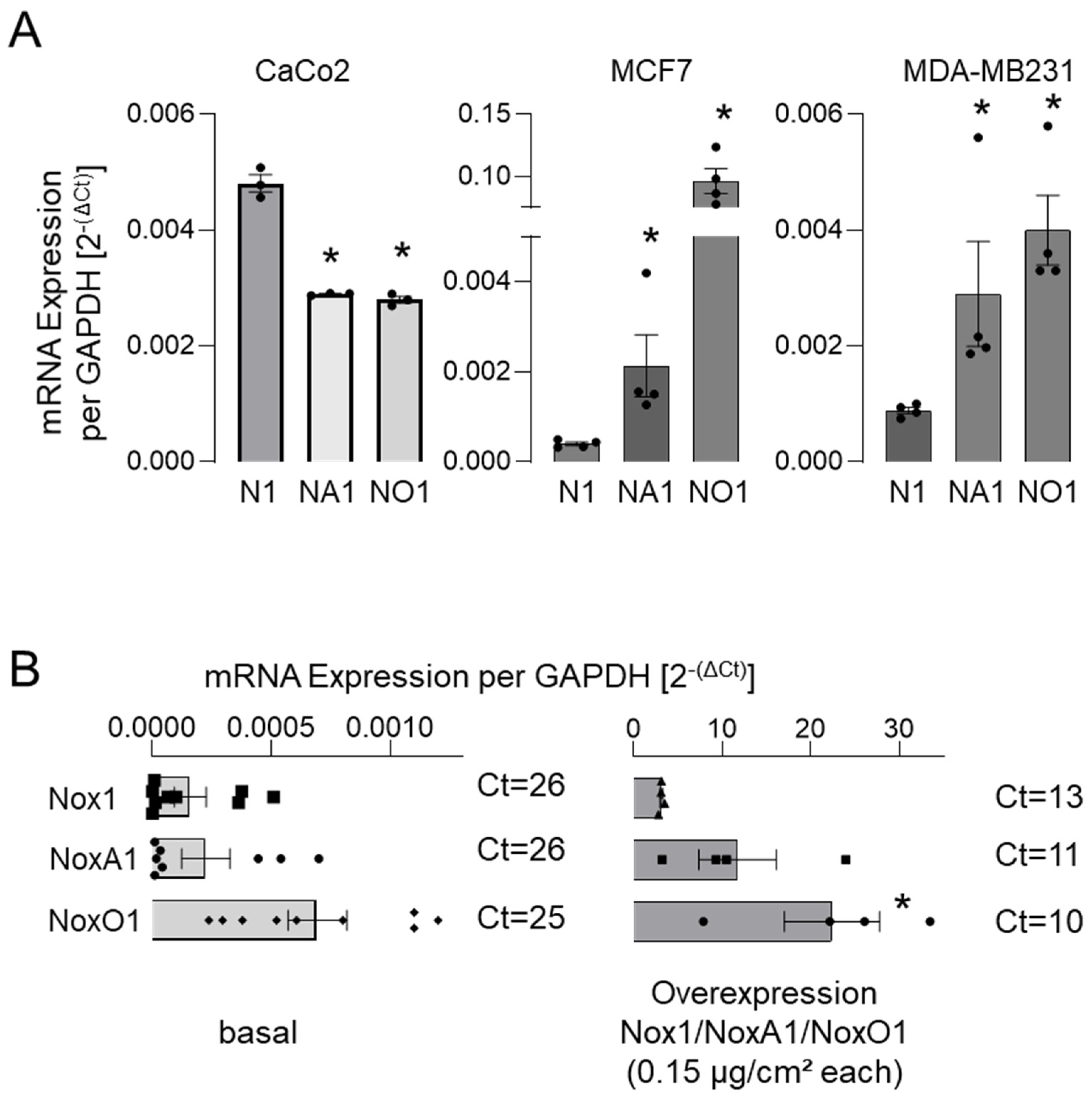
3.2. NoxO1 Expression Is Often Higher than That of Nox1 and NoxA1 Even in Overexpression Settings
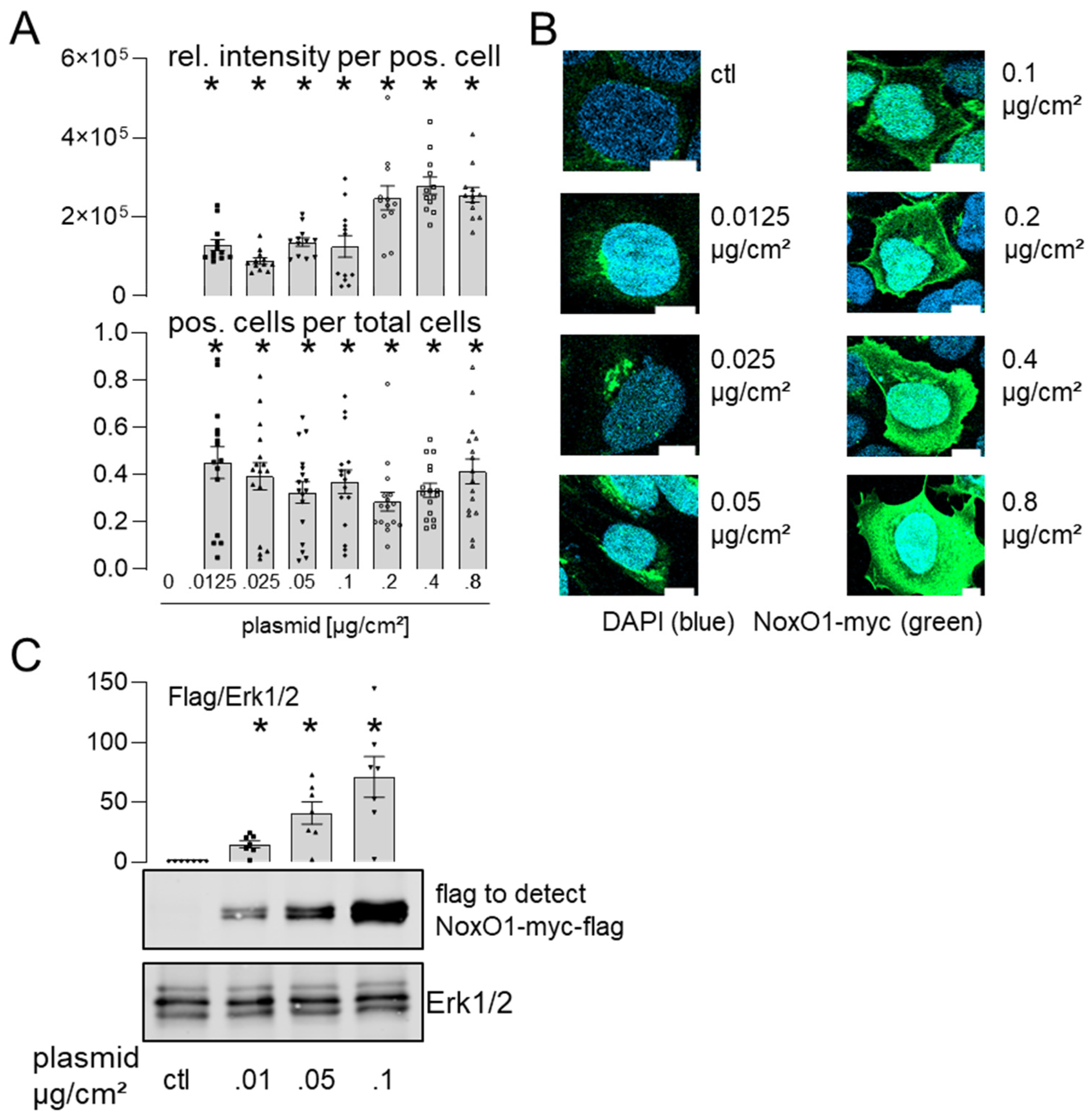
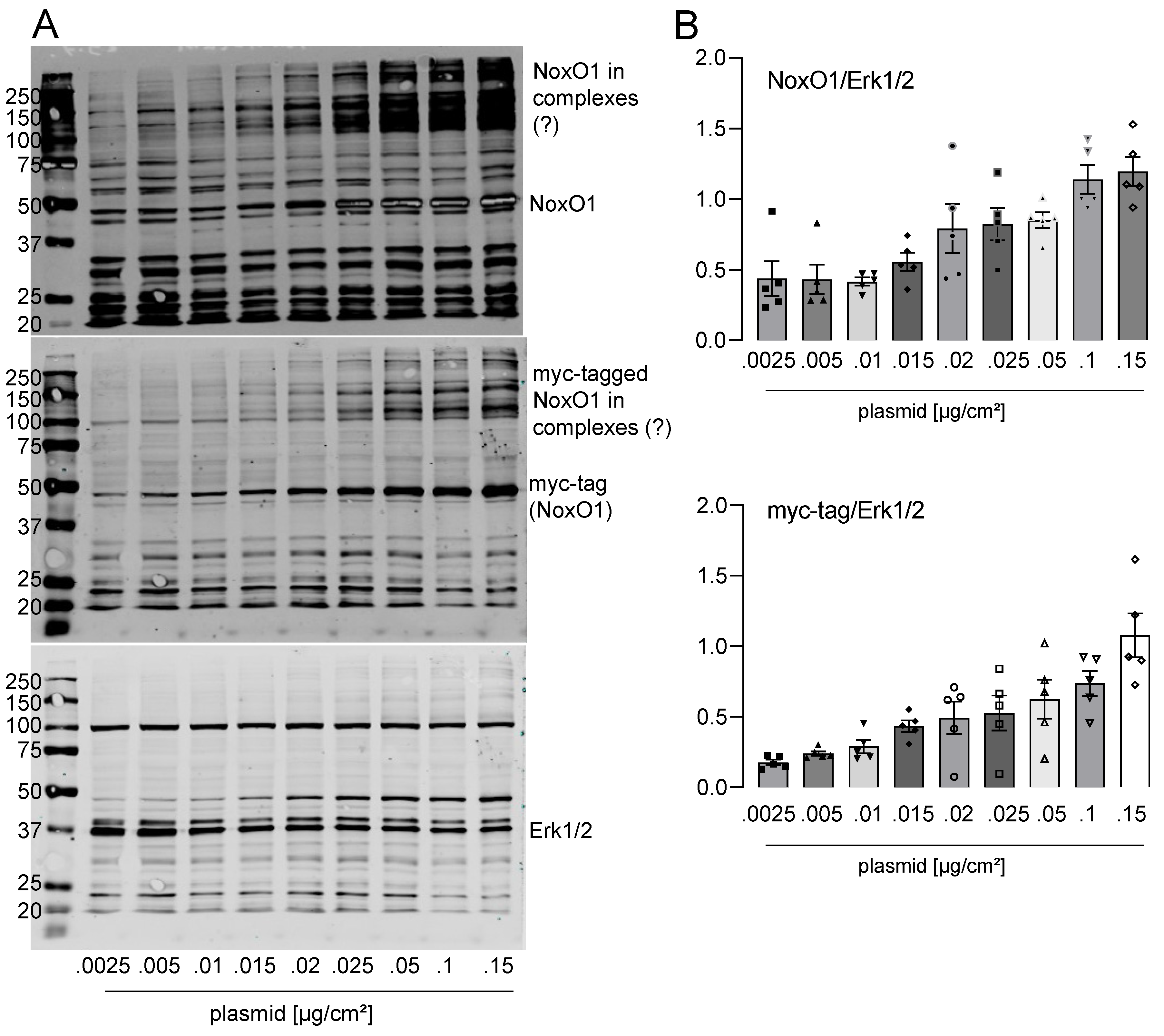
3.3. Overexpressed NoxO1 Is Functional and Dose Dependently Induces ROS Formation by the Nox1-Centered NADPH Oxidase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumimoto, H.; Minakami, R.; Miyano, K. Soluble Regulatory Proteins for Activation of NOX Family NADPH Oxidases. Methods Mol. Biol. 2019, 1982, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Buvelot, H.; Jaquet, V.; Krause, K.-H. Mammalian NADPH Oxidases. Methods Mol. Biol. 2019, 1982, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Bánfi, B.; Clark, R.A.; Steger, K.; Krause, K.-H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003, 278, 3510–3513. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, S.; Shi, D.; Zhang, Y.; Bai, C.; Ye, R.D. A predicted structure of NADPH Oxidase 1 identifies key components of ROS generation and strategies for inhibition. PLoS ONE 2023, 18, e0285206. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, X.; Li, J.; Yao, X.-C.; Liu, W.-L.; Xu, P.-S.; Tan, G.-S. Cytotoxic effects of the biflavonoids isolated from Selaginella trichoclada on MCF-7 cells and its potential mechanism. Bioorg. Med. Chem. Lett. 2022, 56, 128486. [Google Scholar] [CrossRef]
- Haq, S.; Sarodaya, N.; Karapurkar, J.K.; Suresh, B.; Jo, J.K.; Singh, V.; Bae, Y.S.; Kim, K.-S.; Ramakrishna, S. CYLD destabilizes NoxO1 protein by promoting ubiquitination and regulates prostate cancer progression. Cancer Lett. 2022, 525, 146–157. [Google Scholar] [CrossRef]
- Echizen, K.; Horiuchi, K.; Aoki, Y.; Yamada, Y.; Minamoto, T.; Oshima, H.; Oshima, M. NF-κB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells. Oncogene 2019, 38, 4250–4263. [Google Scholar] [CrossRef]
- Mirbagheri, S.Z.; Bakhtiari, R.; Fakhre Yaseri, H.; Rahimi Foroushani, A.; Eshraghi, S.S.; Alebouyeh, M. Transcriptional alteration of genes linked to gastritis concerning Helicobacter pylori infection status and its virulence factors. Mol. Biol. Rep. 2021, 48, 6481–6489. [Google Scholar] [CrossRef]
- Makhezer, N.; Ben Khemis, M.; Liu, D.; Khichane, Y.; Marzaioli, V.; Tlili, A.; Mojallali, M.; Pintard, C.; Letteron, P.; Hurtado-Nedelec, M.; et al. NOX1-derived ROS drive the expression of Lipocalin-2 in colonic epithelial cells in inflammatory conditions. Mucosal Immunol. 2019, 12, 117–131. [Google Scholar] [CrossRef]
- Moll, F.; Walter, M.; Rezende, F.; Helfinger, V.; Vasconez, E.; de Oliveira, T.; Greten, F.R.; Olesch, C.; Weigert, A.; Radeke, H.H.; et al. NoxO1 Controls Proliferation of Colon Epithelial Cells. Front. Immunol. 2018, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Proteomics DB. Available online: https://www.proteomicsdb.org/proteomicsdb/#human/proteinDetails/Q8NFA2/expression (accessed on 22 February 2023).
- GTEx Portal. Available online: https://www.gtexportal.org/home/gene/NOXO1 (accessed on 22 February 2023).
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 24 January 2024).
- Zhao, S.; Ye, Z.; Stanton, R. Misuse of RPKM or TPM normalization when comparing across samples and sequencing protocols. RNA 2020, 26, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Rezende, F.; Prior, K.-K.; Löwe, O.; Wittig, I.; Strecker, V.; Moll, F.; Helfinger, V.; Schnütgen, F.; Kurrle, N.; Wempe, F.; et al. Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radic. Biol. Med. 2017, 102, 57–66. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Grant, J.; Santos, A.R.C.; Hernandez, E.; Ivanov, D. Neuronal NAD(P)H oxidases contribute to ROS production and mediate RGC death after ischemia. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2823–2830. [Google Scholar] [CrossRef]
- Hill, A.J.; Drever, N.; Yin, H.; Tamayo, E.; Saade, G.; Bytautiene, E. The role of NADPH oxidase in a mouse model of fetal alcohol syndrome. Am. J. Obstet. Gynecol. 2014, 210, 466.e1–466.e5. [Google Scholar] [CrossRef]
- Mittal, M.; Roth, M.; König, P.; Hofmann, S.; Dony, E.; Goyal, P.; Selbitz, A.-C.; Schermuly, R.T.; Ghofrani, H.A.; Kwapiszewska, G.; et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ. Res. 2007, 101, 258–267. [Google Scholar] [CrossRef]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef]
- Helfinger, V.; Freiherr von Gall, F.; Henke, N.; Kunze, M.M.; Schmid, T.; Rezende, F.; Heidler, J.; Wittig, I.; Radeke, H.H.; Marschall, V.; et al. Genetic deletion of Nox4 enhances cancerogen-induced formation of solid tumors. Proc. Natl. Acad. Sci. USA 2021, 118, e2020152118. [Google Scholar] [CrossRef]
- Meijles, D.N.; Sahoo, S.; Al Ghouleh, I.; Amaral, J.H.; Bienes-Martinez, R.; Knupp, H.E.; Attaran, S.; Sembrat, J.C.; Nouraie, S.M.; Rojas, M.M.; et al. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci. Signal. 2017, 10, eaaj1784. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, G.K.; Schürmann, C.; Warwick, T.; Schulz, M.H.; Spaeth, M.; Müller, O.J.; Schröder, K.; Jo, H.; Weissmann, N.; Brandes, R.P. Deletion of NoxO1 limits atherosclerosis development in female mice. Redox Biol. 2020, 37, 101713. [Google Scholar] [CrossRef] [PubMed]
- Schader, T.; Reschke, C.; Spaeth, M.; Wienstroer, S.; Wong, S.; Schröder, K. NoxO1 Knockout Promotes Longevity in Mice. Antioxidants 2020, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Harenkamp, S.; Schürmann, C.; Josipovic, I.; Rashid, B.; Rezende, F.; Löwe, O.; Moll, F.; Epah, J.; Eresch, J.; et al. The Cytosolic NADPH Oxidase Subunit NoxO1 Promotes an Endothelial Stalk Cell Phenotype. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1558–1565. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
| mRNA (Accession No.) | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| GAPDH (NM_002046.7) | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| mNoxo1 (NM_027988.4) | TGGAGGAGGTAGCAACGTGC | AGAGCGACTGCCCTCGTAGG |
| hNox1 (NM_007052.5) | TCTTATGTGGCCCTCGGACT | CCAGACTGGAATATCGGTGACA |
| hNoxA1 (NM_006647.2) | TGGGAGGTGCTACACAATGTG | TTGGACATGGCCTCCCTTAG |
| hNoxO1 (NM_144603.4) | GAGATCTGACCGCGTTCTCC | CAGCAGCCTCCGAGAATAGG |
| p47phox (NM_000265.7) | ACGAGTTCCATAAAATGCTGAAGG | GAGATCTTCACGGGCAGTCC |
| p67phox (NM_000433.4) | ACCTTGAACCAGTTGAGTTGCG | GTCGGACTGCGGAGAGCTT |
| Protein Expressed | Backbone | Tag for Detection | |
|---|---|---|---|
| Nox1 | transient | pCMV.6-entry | c-myc, Flag-DDK |
| NoxA1 | |||
| NoxO1 | |||
| eGFP | pEGFP-C1 | GFP | |
| (empty vector) | constitutive | pLV-EF1a-IRES-Hygro | - |
| NoxO1 | |||
| Nox1 + NoxA1 | EF1aFull-hOct4-F2A-hKlf4-IRES-hSox2-P2A-hcMyc-W-loxP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hebchen, D.M.; Spaeth, M.; Müller, N.; Schröder, K. NoxO1 Determines the Level of ROS Formation by the Nox1-Centered NADPH Oxidase. Antioxidants 2024, 13, 1113. https://doi.org/10.3390/antiox13091113
Hebchen DM, Spaeth M, Müller N, Schröder K. NoxO1 Determines the Level of ROS Formation by the Nox1-Centered NADPH Oxidase. Antioxidants. 2024; 13(9):1113. https://doi.org/10.3390/antiox13091113
Chicago/Turabian StyleHebchen, Dana Maureen, Manuela Spaeth, Niklas Müller, and Katrin Schröder. 2024. "NoxO1 Determines the Level of ROS Formation by the Nox1-Centered NADPH Oxidase" Antioxidants 13, no. 9: 1113. https://doi.org/10.3390/antiox13091113
APA StyleHebchen, D. M., Spaeth, M., Müller, N., & Schröder, K. (2024). NoxO1 Determines the Level of ROS Formation by the Nox1-Centered NADPH Oxidase. Antioxidants, 13(9), 1113. https://doi.org/10.3390/antiox13091113







