Abstract
Carissa carandas L. (Apocynaceae) is widely distributed in tropical and subtropical regions of Asia including Pakistan, India, Afghanistan, and Sri Lanka. C. carandas is considered as an integral component of traditional medicinal systems to combat several health ailments. The present study aimed to assess this plant’s phytochemical contents and biological potential by performing sequential extraction, adopting a bioassay-guided approach. C. carandas powder was extracted with n-hexane to remove fatty substances and then residues were sequentially extracted with dichloromethane, methanol, and 50% methanol. All the sequential crude extracts were evaluated for phytochemical contents (total phenolics, flavonoids, and anthocyanins), in vitro antioxidant activity (FRAP, DPPH), in vitro anti-inflammatory activity (serum and egg albumin denaturation), in vivo anti-inflammatory activity (carrageenan- and formaldehyde-induced paw edema), and in vitro antimicrobial activity. Active crude extract was then partitioned using the liquid-liquid separation method followed by further separation of the active fraction by RP-HPLC. The active fraction was then subjected to LC-ESI-MS/MS analysis for tentative identification of bioactive metabolites responsible for its bioactive properties, followed by HPLC quantification. The analysis revealed methanol extract to have more phytochemical contents, radical scavenging properties, reduced inflammation in both models (in vitro and in vivo), and antimicrobial properties against urinary tract infection-causing agents as compared to dichloromethane and 50% methanol extracts. The ethyl acetate fraction obtained after liquid-liquid partitioning (LLP) of the active methanol extract exhibited more activity as compared to C. carandas methanol extract. RP-HPLC sub-fractionation yielded seven sub-fractions, but a slight decrease in biological potential was recorded. Therefore, LLP fraction B was subjected to further analysis. LC-ESI-MS/MS analysis led to the tentative identification of phenolic acids (chlorogenic acid, quinic acid), flavonoids (quercetin), and anthocyanins (peonidin-3-arabinoside, delphinidin-3-galactoside, delphinidin-3-rutinoside) in the active LLP ethyl acetate fraction. Chlorogenic acid, ellagic acid, and quinic acid were quantified as 17.6 µg/mg, 5.90 µg/mg, and 3.30 µg/mg, respectively, on a dry weight basis by HPLC. C. carandas may be considered a promising therapeutic plant, and the results of the current study provide more evidence to support the assertions made in ancient medical traditions. These findings highlight its promising applications in health, medicine, cosmetics, preservatives, and as a natural coloring agent.
1. Introduction
Oxidative stress, which affects lipids, proteins, and DNA, is linked to cardiovascular, inflammatory, and neurodegenerative ailments, and the process of ageing [1,2]. Synthetic antioxidants, including butylated hydroxyanisole and butylated hydroxy-toluene are taken in food, medicines, and cosmetics to avoid oxidative reactions, as well as to treat maladies associated with oxidative stress. Nevertheless, there is increasing apprehension over their safety due to several studies that have demonstrated mutagenic and carcinogenic properties connected to some synthetic antioxidants [3]. Similarly, nonsteroidal drugs (NSAIDS) and corticosteroids are widely used to provide relief from inflammation-related pain, but when taken for longer periods of time can induce gastric problems and disturb the normal functioning of the kidney and liver [4,5]. Additionally, bacterial diseases, mainly urinary tract infections (UTIs), are very common; it is estimated that around 150 million people across the world suffer UTIs every year [6]. For instance, over 11 million people in the United States receive treatments for UTIs annually, with a total cost of USD 6 million. UTIs comprise a significant portion of healthcare-associated infections (HAIs) in Europe, accounting for 19.0% of all HAIs [7,8]. Prevalence of UTIs is greater in women than men, and per reported data, 50% of women and 12% of men develop UTIs in the course of their life [9]. Escherichia coli, Klebsiella pneumoniae, Citrobacter, Staphylococcus aureus, Streptococcus bovis, Candida albicans, and Candida glabrata have been established as the main causative agents [10,11]. The treatment includes medicines and hospitalization, resulting in great economic burden. Other than that, frequent use of antibiotics, i.e., antibacterials (imipenem, ciprofloxacin, amikacin, nitrofurantoin) and antifungals (nystatin, fluconazole) can result in antibiotic resistance [12,13]. Presently, multidrug-resistant (MDR) uropathogenic Escherichia coli (UPEC) is a major clinical concern, particularly in developing nations. This resistance requires extensive use of broad-spectrum antibiotics, which prolongs hospitalizations and raises treatment expenses. The fast rise of antibiotic resistance in UPEC strains threatens world health care systems [10]. Therefore, non-antibiotic antimicrobial techniques for UTI treatment and prevention are becoming more popular. Alternatives considered are the use of vaccines, bacteriophages, probiotics, and secondary metabolites, the last of which is the main current focus worldwide [14].
Carissa carandas (C. carandas) belongs to the Apocynaceae family and is an evergreen shrub that remains a less well-known plant with significant economic, commercial, clinical, and nutritional potential. It is also known as “Crane berry” (English), Karonda (Devanagari), Karonda (Hindi), Karamcha (Bengali), and Ci-Huang-Guo (Mandarin Chinese). It is found to be widely distributed in tropical and subtropical regions of Pakistan, India, Sri Lanka, Myanmar, Bangladesh, and Nepal [15]. C. carandas berries are widely recognized as a crucial element in ancient medicinal systems for the treatment of various health conditions such as epilepsy, edema, diarrhea, and myopathic spasms [16]. Fruits of C. carandas are known to have potential in treating hemorrhoids, appetite loss, calming nervous disorders (as a nervine), colic, splenomegaly, hepatomegaly, amenorrhea, CVDs, and psychiatric anorexia in humans [15]. Its roots are utilized as a bitter stomachic, a vermifuge, and for itching. Traditional medicinal claims regarding C. carandas are now substantiated by pharmacological studies that have confirmed its antioxidant [17], anti-inflammatory [18], anticancer [19], antidiabetic [20], and antimicrobial effects [21]. C. carandas fruit extracts are reported to contain anthocyanins, phenolic acids, flavonols, alkaloids, and terpenoids [22,23]. These compounds contribute to the plant’s significant biological activity, making it a valuable source.
However, the aforementioned studies used crude extracts to evaluate the plant’s biological potential. In the present investigation, sequential extraction was performed followed by bioassay-guided fractionation of more potent crude extracts using liquid-liquid partitioning and reverse phase-high performance liquid chromatography to identify the compound or group of compounds responsible for a range of bioactivities including antioxidant, anti-inflammatory, and anti-UTI. Liquid chromatography-mass spectrometry was used to tentatively identify the compounds followed by quantification using external standards by HPLC.
2. Material and Methods
2.1. Collection of Berries
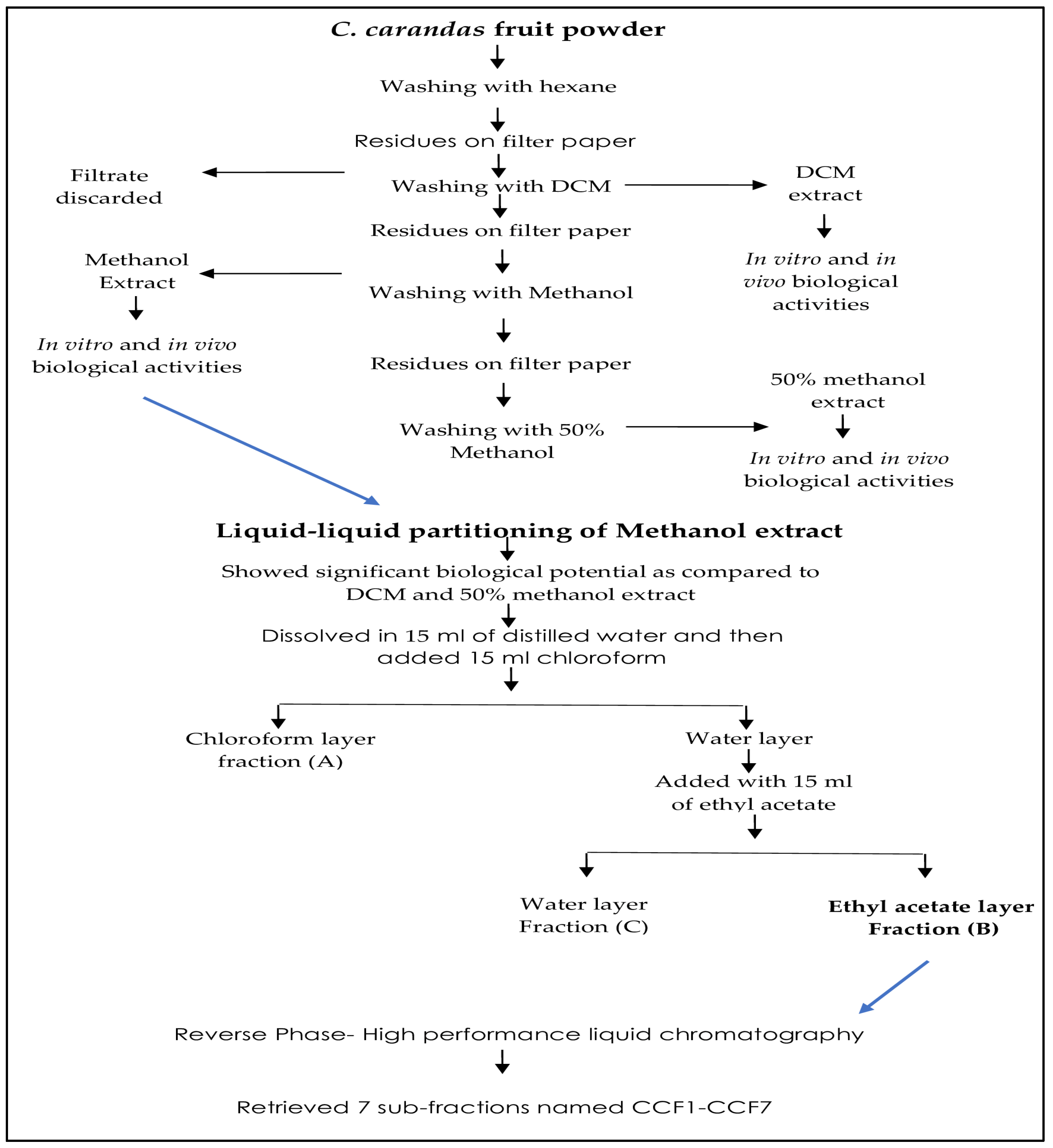
The collection C. Carandas fruits was done in late July 2023 from the gardens of Bahauddin Zakariya University, Multan, Pakistan, and was subjected to varietal identification. After washing the berries, they were cut into pieces and seeds were removed. C. carandas pulp extraction was performed using various solvents on the basis of increasing polarity, i.e., initially with hexane to remove fatty contents, followed by dichloromethane, methanol, and 50% methanol. To obtain the concentrated extracts, the solvents were evaporated in a rotary evaporator (Heidolph, Hei-Vap, Schwabach, Germany). Semi-solid extracts were stored at −70 °C in an ultralow freezer for future experiments. Sequential extraction is well outlined in Figure 1.
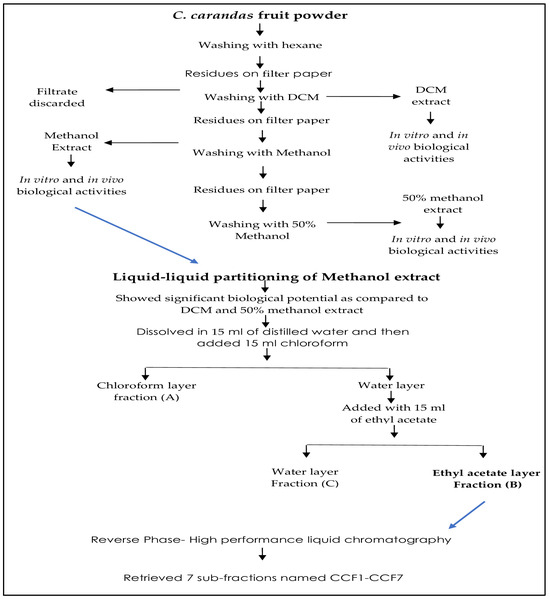
Figure 1.
Sequential extraction of C. carandas.
2.2. Solvents and Reagents
Standard solvents were employed in the extraction process including gallic acid, quercetin, cyanidin-3-glucoside, indomethacin, and diclofenac sodium, in addition to inflammatory mediators like carrageenan and formaldehyde, all sourced from the local supplier of Sigma Aldrich (Stockholm, Sweden). All chemicals, reagents, and solvents utilized in this study were of analytical grade unless specified otherwise.
2.3. Animals
The animal study was performed at the Department of Food Science and Technology, Faculty of Food Science and Nutrition, Multan under the protocol number 02-23 and title “In vivo anti-inflammatory potential of C. carandas sequential crude extracts” approved by the Bioethical Committee at Bahauddin Zakariya University for animal usage. Two animals/cage were maintained in a controlled environment (25 °C ± 4 °C, 12/12 light/dark cycles) with ample access to food and water (ad libitum).
2.4. Phytochemicals Characterization
Total phenolic contents were calculated adopting the Folin Ciocalteu (FC) colorimetric method using gallic acid as standard and ethanol as blank. Briefly, 0.5 mL of test sample was mixed with 1.5 mL Folin–Ciocalteu reagent and 1.2 mL of 7.5% sodium carbonate (Na2CO3) aqueous solution in test tubes. The absorbance was measured thrice using a spectrophotometer (V-3000; VWR, Darmstadt, Germany) at 756 nm after 30 min of incubation at room temperature. The results were expressed as mg gallic acid equivalent (GAE) per gram of dried extract [23].
Total flavonoid contents were recorded adopting the AlCl3 method using quercetin as standard [24]. Briefly, 0.5 mL of test sample was added to 0.5 mL distilled water, 0.15 mL of sodium nitrite (NaNO2) solution, 150 µL of AlCl3 solution (2%), and 0.2 mL of NaOH (1M). The sample values were recorded at an absorbance of 510 nm using a spectrophotometer after 30 min of incubation at room temperature and results were expressed as mg quercetin equivalents (QE) per gram of dried extract.
The pH differential method with few alterations was used to determine total anthocyanin contents in the sample [25]. The results were expressed as milligrams of cyanidin 3-glucoside (C3G) per kilogram of dried extract.
2.5. Antioxidant Activity
C. carandas fruit sequential crude extracts were evaluated for DPPH inhibition using the previously described method [26]. Methanol was used to make 1 mM DPPH stock solution, which was kept at −20 °C for analysis. Mixing 10 mL stock and 90 mL methanol yielded 0.1 mM DPPH working solution. To the test tubes, 2 mL DPPH, 2 mL methanol, and 0.2 mL extract (1 g/20 mL) were added. Test tubes were incubated for 30 min in the dark. Via spectrophotometer (UV-Vis 3000, ORI, Hille, Germany), absorbance was measured at 517 nm after incubation. For blank and standard recordings, methanol and quercetin (125 µg/mL) were employed. DPPH % inhibition was recorded using the following equation.
% inhibition = (Control OD − Sample OD/Control OD) × 100
C. carandas fruit sequential crude extracts were also tested for ferric-reducing antioxidant potential (FRAP) following the guidelines of Zahin et al. [27]. According to this method, 100 µL of extract (1 g/20 mL) was added to 300 µL of FRAP working solution (containing 300 mmol/L acetate buffer (pH 3.6), 10 mmol/L 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mmol/L HCl, and 20 mmol/L FeCl3 in a ratio of 10:1:1) and then incubated for 30 min at room temperature. Finally, the absorbance was measured at 596 nm with a spectrophotometer and the results were reported as Fe mmol/g of dried extract.
2.6. Antimicrobial Activity
The antimicrobial potential of C. carandas sequential crude extracts was tested against UTI-causing bacteria (Escherichia coli; ATCC 25922, Klebsiella pneumoniae; ATCC 13883), and fungi (Candida albicans; BNCC 187382, Candida glabrata; BNCC 337348) using the disc diffusion method, as described by Bauer et al. [28]. The bacterial strains were grown on a Nutrient Agar (NA) slant, incubated at 37 °C for 24 h, and kept at 4 °C until further use, whereas the fungal strains were cultured on Sabouraud dextrose agar (SDA) using the spread plate technique. In detail, Mueller-Hinton agar underwent sterilization by autoclaving at a temperature of 121 °C for a period of 15 min. The aseptic method was used to transfer the sterile medium onto pre-sterilized petri dishes, which were then allowed to cool in order to solidify. Bacterial and fungal strains were grown on solid agar using the spread plate method in a sterile environment. Following that, tiny apertures of 6 mm in diameter were generated in the agar and then infused with 30 µL of C. carandas dichloromethane, methanol, and 50% methanol extracts at doses of 50 mg/mL, 100 mg/mL, 300 mg/mL, and 400 mg/mL. In contrast, a traditional antibacterial (ciprofloxacin) and antifungal (nystatin) drug were given at a concentration of 5 µg/mL. The petri plates were correctly marked and put in an incubator set at a temperature of 37 °C for a period of 24–48 h for antibacterial activity and 48–72 h for antifungal activity. The activity was recorded as zone of inhibition (ZOI) in mm, wherein a ZOI greater than 8 mm is considered to demonstrate antibacterial and antifungal activity.
2.7. Anti-Inflammatory Activity
2.7.1. In Vitro Assessment
Membrane stabilization potential of C. carandas sequential crude extracts (50–300 µg/mL) was tested per the guidelines of Shinde et al. [29]. Blood samples from healthy humans’ cubital veins were promptly transferred to heparinized tubes. Following centrifugation at 3000 rpm for 5 min, the tubes were rinsed three times with normal saline. The blood volume was then measured in a 10% v/v suspension using an isotonic buffer solution consisting of 10 mM sodium phosphate buffer at pH 7.4 [30]. After that, 2 mL reaction mixture was made by addition of various crude extracts of C. carandas (1 mL) to red blood cell suspension. The method involves two centrifugations, the first performed immediately after mixing the fruit extract and red blood cells suspension, and the second after cooling the mixture. Finally, the supernatant was recovered to check absorbance using a spectrophotometer at 560 nm. The protocols of Mizushima and Kobayashi [31] and Sakat et al. [32] were followed to test the egg albumin and bovine serum albumin denaturation inhibition abilities of sequential crude extracts of C. carandas and subsequent fractions. In the egg albumin assay, 0.2 mL egg albumin, phosphate buffer saline, and plant extracts (50, 100, 200, 300 µg/mL) of several concentrations (2 mL) were mixed to produce a reaction mixture of 5 mL. Then, the reaction mixture was incubated, cooled, its absorbance was measured at 660 nm, and the results were expressed as % inhibition.
Likewise, in the serum albumin assay, 0.05 mL of plant extracts (50, 100, 200, 300 µg/mL) was added to 0.045 mL of serum albumin to produce 0.5 mL of reaction mixture. The reaction mixture was incubated, then a saline phosphate buffer was added, and finally the absorbance was read using a spectrophotometer at 660 nm.
In all three assays, the positive control was diclofenac sodium and the negative control was phosphate buffer, and the results were outlined as % inhibition. The following equation was used to calculate the % inhibition of plant extracts and standard drug:
% Inhibition = (Abs Control − Abs Treated)/Abs Control × 100
2.7.2. In Vivo Assessment
In the current study, we tested sequential crude extracts of C. carandas for in vivo anti-inflammatory activity using carrageenan- and formaldehyde-induced paw edema models, adhering to the guidelines of Morris (2003) [33]. Eight different groups were treated, each group containing 5 animals, wherein group 1 was the control that received normal saline, group 2 was the positive control that received indomethacin at 100 mg/kg b.w., and the remaining 6 groups were named active groups. Groups 3 and 4 received dichloromethane extract (200 and 400 mg/kg body weight (b.w.)), groups 5 and 6 received methanol extract (200 and 400 mg/kg b.w.), and groups 7 and 8 received 50% methanol extract (200 and 400 mg/kg b.w.). We measured the paw circumference of each rat before the treatment, fed them the aforementioned extracts, and intoxicated the right hind paw’s plantar aponeurosis surface with carrageenan after 30 min. The study used a plethysmometer (UGO-BASILE 7140, Comerio, Italy) to measure the change in paw diameter after 0, 1, 2, and 3 h. The study considered the rise in paw circumference as a sign of swelling.
Brownlee’s [34] guidelines were followed to assess formaldehyde-induced edema inhibition of the C. carandas sequential crude extracts. The study divided the albino mice into 8 groups, maintaining the same parameters as the carrageenan model. Before the treatment, the size of each rat’s paw was measured. The mice were then given the extracts, and after 30 min, formaldehyde (100 µL, 4%) was injected into the right hind paw’s plantar aponeurosis. The study used a plethysmometer to measure the change in paw diameter after 0, 3, 6, 12, and 24 h. The study considered the rise in paw circumference as a sign of swelling.
2.8. Bioassay-Guided Fractionation
2.8.1. Liquid-Liquid Partitioning
C. carandas fruit methanol extract exhibiting higher activities was further fractionated using the liquid-liquid partitioning technique using ethyl acetate, dichloromethane, and water. This led to three fractions: fraction A (chloroform), fraction B (ethyl acetate), and fraction C (water).
2.8.2. Method Optimization for Fractionation
Fractionation was performed on an HPLC system with a diode array detector (DAD) (1100/1200 series, Agilent, Waldbronn, Germany) with an analytical column (Zorbax-SB-C18, 4.6 × 150 mm, 5 μm, Agilent, Waldbronn, Germany). Five milligrams of ethyl acetate fraction were dissolved in 1 mL of methanol, and after a staying time of 30 min passed through a syringe filter (0.45 µm). The mobile phase was composed of 0.1% trifluoroacetic acid (A), and acetonitrile with 0.1% trifluoracetic acid (B). The sample (10 µL) was injected into the HPLC column. The flow rate was set to 0.5 mL/min and the gradient elution was used: B (15%) in 0–10 min, B (15–30%) in 11–15 min, B (30–60%) in 15–22 min, B (60–90%) in 22–27 min, and B (100%) in 27–30 min. Several detection wavelengths were used including 354, 280, 310, 520 nm to obtain the chromatograms. The process retrieved seven sub-fractions.
2.8.3. RP-HPLC Sub-Fractionation
A volume of 70 µL of a sample with a concentration of 1 g/10 mL was loaded onto a Zorbax SB-C18 semi-preparative column (25 × 250 mm, 5 μm particle size, Agilent, Germany). As a consequence, seven sub-fractions, named CCF1-CCF7, were obtained from fraction B (ethyl acetate) of the methanol extract of C. carandas fruit. The RP-HPLC sub-fractions showed decreased activity in terms of in vitro antioxidant, anti-inflammatory, and antibacterial properties compared to the parent LLP fraction B and C. carandas methanol extract. Subsequently, LLP fraction B underwent additional examination using mass spectrometry.
2.9. LC-ESI-MS/MS Analysis
Active liquid-liquid partitioned (LLP) fraction or RP-HPLC sub-fractions exhibiting significant biological potential as compared to other fractions and external standards were then analyzed using the LC-MS/MS technique. The detection was performed using direct injection mode with Electron Spray Ionization, in both positive and negative ionization modes. The sample flow rate, temperature of the capillary tube, and mass range were kept to 8 μL/min, 280 °C, and m/z 50–1000, respectively [35]. Analysis of LC-ESI-MS/MS acquired data was performed using manual Thermo Xcalibur Qual Browser v.3 (Thermo Scientific, Waltham, MA, USA). Structural elucidation was performed using ChemDraw (ChemDraw Ultra 8.0) and then compared with previously published data.
2.10. Quantification of Tentatively Identified Compounds Using External Standards
An attempt was made to quantify the phenolic acids identified in the LC-MS/MS analysis of fraction B (C. carandas methanol extract). In the present study, chlorogenic acid and ellagic acid were quantified on the basis of standard curves. Sixty milligrams of C. carandas fraction B was dissolved in 1 mL methanol (60 mg/mL), and concentration of the phenolic (chlorogenic acid, ellagic acid) and organic acid (quinic acid) standards were 250 µg/mL. One hundred microliters of plant extract and standards was injected into the HPLC system. As stated in Section 2.8.2, all other parameters were identical. UV spectra and retention times of standards were compared to plant extracts for identification and quantification.
2.11. Statistical Analysis
The data from this research are presented as mean ± standard error of means (SEM) for 3 readings. The study compared control and treatment groups using analysis of variance (ANOVA) and Dunnett’s test, with significance set at p-value < 0.05 (* p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001). The software employed was Graphpad Prism (Graph Pad Software 8.0.2, San Diego, CA, USA).
3. Results
3.1. Phytochemical Characterization and Antioxidant Activity
The successive crude extracts of C. carandas were assessed for their total phenolic, flavonoid, and anthocyanin contents, as shown in Table 1. The methanol extract exhibited higher total phenolic contents, whereas the dichloromethane extract showed the lowest levels. The methanol extract had a higher affinity for extracting both total flavonoids and total anthocyanins compared to the 50% methanol and dichloromethane extracts. The quercetin (standard) exhibited the highest level of antioxidant activity in both the DPPH and FRAP experiments, followed by the methanol, 50% methanol, and dichloromethane extracts. Moreover, results are also expressed in terms of quercetin equivalents, determined by comparison to a standard curve generated using known concentrations of quercetin. For the DPPH assay, the C. carandas extract at a concentration of 50 mg/mL exhibited a significant % inhibition of 73.0%, which corresponds to a quercetin equivalent concentration of 59.87 µg/mL. Similarly, in the FRAP assay, the 50 mg/mL C. carandas extract concentration demonstrated a FRAP value of 51.5 mmol/g, which translates to a quercetin equivalent concentration of 84.53 µg/mL, underscoring its potential as a natural antioxidant source.

Table 1.
Phytochemical contents of C. carandas sequential crude extracts.
3.2. Anti-UTI Potential of Sequential Crude Extracts
C. carandas sequential crude extracts were tested for antimicrobial potential against several UTI-causing organisms, and their activity was compared to standard medicines including ciprofloxacin and nystatin (Table 2). The methanol extract showed significant antibacterial activity against Escherichia coli (ZOI: 21 ± 0.5 mm) and Klebsiella pneumoniae (ZOI: 26 ± 0.5 mm) at 50 µg/mL, surpassing the reference medication, i.e., ciprofloxacin at 5 µg/mL. The methanol extract was effective against Candida albicans (ZOI: 12 ± 0.5 mm) and Candida glabrata (ZOI: 10 ± 0.5 mm), while nystatin demonstrated strong antifungal activity against both Candida species.

Table 2.
Anti-urinary tract infection potential of C. carandas sequential crude extracts.
3.3. Anti-Inflammatory Potential of Sequential Crude Extracts
The current study investigated the anti-inflammatory activity of C. carandas sequential crude extracts including in vitro and in vivo models (Table 3). The findings were compared to common anti-inflammatory medicines, namely diclofenac sodium (in vitro) and indomethacin (in vivo). In vitro studies found that the methanol extract significantly inhibited egg albumin denaturation (76 ± 0.13%) and serum albumin denaturation (78 ± 0.5%) at 400 µg/mL. These inhibition rates were similar to the effectiveness of diclofenac sodium, which showed inhibition rates of 91 ± 0.1% and 93 ± 0.12% for egg and serum albumin denaturation, respectively, at 400 µg/mL. The methanol extract significantly inhibited heat-induced hemolysis (48 ± 0.58%) at 400 µg/mL, albeit to a lower degree than diclofenac sodium (86 ± 0.2%). In vivo experiments confirmed the results, with the methanol extract inhibiting carrageenan-induced edema (74%), as well as formaldehyde-induced paw edema (71%) at 400 mg/kg b.w. These inhibition rates are close to those of indomethacin, which inhibited carrageenan-induced edema and formaldehyde-induced paw edema at rates of 79% and 73%, respectively, at 100 mg/kg b.w.

Table 3.
Anti-inflammatory potential of C. carandas sequential crude extracts.
3.4. Bioassay-Guided Fractionation
C. carandas methanol extract demonstrating notable antioxidant, anti-inflammatory, and antimicrobial properties as compared to other extracts was fractionated adopting the liquid-liquid partitioning (LLP) method as mentioned in Section 2.8. LLP resulted in three different fractions: A (chloroform), B (ethyl acetate), and C (water), and all of these fractions were assessed for the aforementioned biological activities. As can be seen in Table 4, an increase in the biological potential was observed in case of fraction B when compared to the parent methanol extract, but a decline was noticed for fraction A and C. Furthermore, fraction C was then further subjected to RP-HPLC and seven sub-fractions were obtained named CCF1-CCF7. All the sub-fractions were evaluated again, but a decline in biological potential was observed as compared to fraction C and the parent methanol extract. As a result, fraction B was selected for LC-MS/MS for the tentative identification of bioactive metabolites responsible for its biological activities (Table 4).

Table 4.
Biological activities of bioassay-guided liquid-liquid partitioned fractions and RP-HPLC sub-fractions.
3.5. LC-ESI-MS/MS Analysis and HPLC Quantification
Liquid-liquid partitioned fraction B of C. carandas methanol extract demonstrating in vitro biological potential in the aforementioned assays was further tested on LC-ESI-MS/MS for tentative identification of bioactive metabolites (Table 5). The analysis led to the identification of phenolic acids (ellagic acid, quinic acid, chlorogenic acid) and anthocyanins (cyanidin-3-galactoside, peonidin-3-arabinoside, delphinidin-3-galactoside, delphinidin-3-rutinoside). The identification was confirmed using the previous literature which identified quinic acid [36], chlorogenic acid [37], ellagic acid [38], and anthocyanins [39]. Respective to the availability of standards, the present study quantified chlorogenic acid, ellagic acid, and quinic acid at 17.6 µg/mg, 5.9 µg/mg, 3.3 µg/mg, respectively, on a dry weight basis.

Table 5.
LC-ESI-MS/MS analysis of bioactive metabolites from active liquid-liquid partitioned fraction B of C. carandas methanol extract.
4. Discussion
The fruit of C. carandas underwent sequential extraction using n-hexane to remove fatty substances, followed by dichloromethane, methanol, and 50% methanol extractions. This approach was used since the choice of solvent and its polarity may affect the extraction of phytochemicals. C. carandas methanol extract was recorded to have higher total phenolic (261 ± 0.8 mg GAE/g), flavonoids (1.2 ± 0.4 mg QE/g), and anthocyanins (112 ± 2.1 mg/kg) contents as compared to 50% methanol and dichloromethane extracts. Previously, C. carandas methanol extract was reported to contain notable amounts of phenolic and flavonoid compounds, totaling 15.0 mg GAE/g and 2.92 mg RE/g, respectively [19]. C. carandas fruit methanol extract was found to offer greater affinity towards extracting total phenolic (841 mg GAE/100 g) and flavonoid (848 mg CE/100 g) contents as compared to 50% methanol, and 70% methanol extract, which supports the results of the current investigation [40].
The antioxidant activity was evaluated using DPPH and FRAP assays. The DPPH test is a straightforward, precise, and efficient way to check the radical scavenging capacities of plant-based extracts. The approach entails quantifying the shift in DPPH coloration, transitioning from violet to light yellow, as a consequence of the presence of antioxidant compounds [41]. In the present study, antioxidant activity was found consistent with phytochemical potential. Table 1 shows that stable free radical-scavenging inhibition potential varied between 21% and 73%, wherein the C. carandas methanol extract was recorded to have higher phytochemical contents and also exhibited the highest inhibition in comparison with the standard quercetin (88% inhibition), while dichloromethane extract showed the lowest results. The findings of Siddiqi et al. [42] and Sarma et al. [43] observed C. carandas fruit methanol extract to cause notable inhibition of DPPH at 66% and with an IC50 of 27.4 µg/mL, respectively. In another study, C. carandas fruit methanol extract scavenged DPPH radicals with an IC50 of 46.1 µg/mL as compared to an IC50 of 12 µg/mL for standard BHT, which supports the findings of the current investigation. The FRAP (ferric-reducing antioxidant power) test is often used to assess the overall antioxidant abilities of experimental extracts. The FRAP test quantifies the aptitude of an experimental extract to donate electrons by measuring the reduction of ferric ions to ferrous ions [44]. In the present study, FRAP varied between 10.2–51 mmol/g wherein methanol extract displayed more potential as compared to 50% methanol and dichloromethane extracts, and in comparison to the standard quercetin (59.2 mmol/g). C. carandas fruit methanol extract was reported to have FRAP inhibition of 90% as compared to 97% inhibition for standard ascorbic acid [16].
The study tested C. carandas sequential crude extracts for their antimicrobial potential against UTI-causing organisms (Table 2). Antimicrobial potential was found in order with the phytochemical potential and antioxidant activity. Secondary metabolites, especially flavonoids, can prevent formation of nucleic acid by interacting with enzymes like DNA gyrase and topoisomerase, upset cytoplasmic membrane function, leak intracellular contents, affect energy metabolism pathways like the electron transport chain and ATP synthesis, avert biofilm formation, and modify membrane permeability, causing ion gradients and osmotic pressure imbalances, thereby reducing the likelihood of resistance development [45]. In the present study, C. carandas methanol extract exhibited substantial antibacterial activity against Escherichia coli and Klebsiella pneumoniae with ZOI of 21 mm and 26 mm, respectively, in a dose-dependent manner. These values were even higher than those of the standard ciprofloxacin, which had ZOI of 15 mm and 19 mm, respectively. In addition, C. carandas methanol extract also showed strong antifungal activity against Candida albicans (ZOI 12 mm) and Candida glabrata (ZOI 10 mm) but considerably lower than standard nystatin. This study supports previous findings in the literature that the antimicrobial activity has a direct relation to increasing extract concentration [46]. In contrast, dichloromethane extract had no activity, while 50% methanol extract showed intermediate activity but proved ineffective against fungal strains. The results of the current investigation are in accordance with the previous findings of Agarwal et al. [47] wherein a methanol extract of C. carandas leaves demonstrated inhibition of Escherichia coli (ZOI 19 mm), i.e., more than standard tetracycline inhibition (ZOI 16.1 mm). C. carandas fruit water and methanol extracts were recorded to have significant activity against Escherichia coli and Klebsiella pneumonia [20,48]. C. carandas fruit methanol, ethanol, and water extracts were reported to have significant antibacterial activity against a wide range of pathogenic bacteria including Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus mutans, Pseudomonas fluorescens, and Salmonella Typhi, which further strengthens its antibacterial reputation [20,47,48,49]. C. carandas leaves methanol extract was found to have significant inhibition against Candida albicans with MIC of 0.312 mg/mL as compared to petroleum ether and water extracts wherein no activity was observed [50]. C. carandas fruit methanol extract was reported to have slightly higher antifungal activity against Candida albicans as compared to dichloromethane extract [51], but considerably lower than the results recorded in the current investigation.
Protein denaturation is a primary source of inflammation, and reliable studies previously described support this connection [52,53]. Anti-inflammatory drugs are known to impart stabilization effects towards human red blood cell membranes against hypotonicity-induced lysis [54]. C. carandas fruit methanol extract showed significant inhibition against heat-induced hemolysis, serum albumin denaturation, and egg albumin denaturation inhibition, i.e., 55% (p < 0.01), 78% (p < 0.001), 76% (p < 0.001) at 400 µg/mL, in a dose-dependent manner, whereas standard diclofenac sodium exhibited potent (p < 0.0001) inhibition of 86%, 93%, and 91% at the same dose, respectively. A positive correlation was observed between anti-inflammatory activity, phytochemical potential, and antioxidant activity. Similarly, concentration-reliant anti-inflammatory properties of commercial drugs including salicylic acid and phenylbutazone has been previously reported [31]. C. carandas methanol extract was reported to show significant anti-inflammatory activity in egg albumin denaturation (58% inhibition), serum albumin denaturation (IC50 188 mg/mL), and heat-induced hemolysis (75% inhibition), i.e., comparable to standard diclofenac sodium [55]. Modifications in the cell surface charges of erythrocyte membranes are believed to be the mechanism behind the anti-inflammatory potential of plant-based extracts and commercial drugs. Antioxidants are known to play an important role in the stabilization of red blood cell membranes by decreasing their chances of collision with other substances that may cause aggregation [56]. Previous studies reported a significant in vitro and in vivo potential of secondary metabolites including phenolic acids, flavonoids, flavonols, and saponins owing to their erythrocyte membrane-stabilizing features and strong affinity for binding cations [30].
In vitro results were then further confirmed in carrageenan- and formaldehyde-induced paw edema animal models. C. carandas fruit methanol extract (400 mg/kg b.w.) caused a significant inhibition of carrageenan- and formaldehyde-intoxicated paw edema, at 74% (p < 0.0001) and 71% (p < 0.0001) when recorded after 3 and 4 h, respectively, comparable to the standard indomethacin (100 mg/kg b.w.) inhibition of 79% and 73%, respectively (p < 0.0001). The findings are in accordance with Anupama and Madhumitha [57], wherein C. carandas fruit methanol extract at 400 mg/kg reduced carrageenan-induced inflammation up to 76% after 2 h in rats as compared to 80% inhibition of standard Indomethacin. Recently, C. carandas ripe fruit methanol extract demonstrated notable inhibition against carrageenan-induced edema (66%) as compared to standard inhibition of 78% at the end of 3 h in rats due to the presence of flavonoids, phenolic acids, and anthocyanins [58]. In contrast, the present study observed dichloromethane extract to have non-significant effects, and 50% methanol extract to have moderate anti-inflammatory potential.
Activity-guided fractionation is an effective and successful technique in identification, isolation, and purification of biologically active metabolites [59,60]. In the present study, liquid-liquid partitioned fraction B (chloroform) exhibited more antioxidant and anti-inflammatory activity while no difference was observed in antimicrobial potential as compared to the parent methanol extract. RP-HPLC sub-fractionation of fraction B further yielded seven fractions, but the activity was declined. As a consequence, fraction B was subjected to LC-MS analysis and tentatively identified to have phenolic acids and anthocyanins, with the activity attributed to the presence of these compounds. Reliable literature has been cited to show the biological potential of compounds tentatively identified in the present study. Chlorogenic acid was reported to inhibit carrageenan-induced oxidative stress and inflammatory reactions by inhibiting pro-inflammatory mediators [61]. Ellagic acid was reported to provide protection against carrageenan-induced acute inflammation by inhibiting nuclear factor kappa B, inducible cyclooxygenase, and proinflammatory cytokines. It also enhanced interleukin-10 via an antioxidant mechanism [62]. Likewise, antibacterial activities of chlorogenic acid, ellagic acid, and quinic acid have been reported previously [63,64,65] mediated by membrane disruption properties. Anthocyanins-rich extracts of berry fruits were also found to have significant antioxidant, anti-inflammatory, and antimicrobial properties [66,67,68]. It can be concluded that biological properties of C. carandas fruit methanol extract (fraction B) is due to the synergistic effect of the aforementioned phenolic acids and anthocyanins.
5. Conclusions
An in-depth analysis of C. carandas fruit revealed its phytochemical makeup, which includes phenolic acids and anthocyanins. Furthermore, it exhibited promising biological potential such as antioxidant, anti-inflammatory, and antibacterial effects, thereby validating its ancient use in therapeutic procedures. C. carandas’ antioxidant characteristics make it a desirable option for utilization in cosmetics and as a natural preservative for fats, oils, and the meat industry as a green-label alternative to synthetic antioxidants like BHT and BHA. In addition, the use of anthocyanins, which are renowned for their vibrant hues, presents promising opportunities as organic pigments in the food and cosmetics sectors. Robust anti-inflammatory activity, both in vitro and in vivo, suggests their utility in managing inflammatory conditions. Additionally, their antibacterial efficacy against common urinary tract infection pathogens underscores their potential as therapeutic agents in combating microbial infections and also as natural preservatives. Further exploration into the integration of these extracts into functional foods or nutraceuticals could pave the way for innovative solutions in both sectors, addressing the growing demand for natural and effective health-promoting ingredients. Future studies should also give priority to the isolation of specific bioactive compounds, the clarification of their mechanisms of action, and the exploration of their potential in the development of pharmaceuticals, cosmetics, and food products.
Author Contributions
Conceptualization, T.I. and M.Q.; methodology, M.Q.; software, T.I.; validation, T.I. and M.Q.; formal analysis, W.S.; investigation, W.S.; resources, T.I.; data curation, M.Q.; writing—original draft preparation, W.S., M.Q. and T.I.; writing—review and editing, T.I., M.Q. and T.E.; visualization, M.Q.; supervision, T.I. and M.Q.; project administration, T.I.; funding acquisition, T.E. All authors have read and agreed to the published version of the manuscript.
Funding
The publication of this article was funded by the Open Access Fund of Leibniz Universität Hannover.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are presented in the manuscript.
Acknowledgments
This paper is from the PhD thesis of Wisha Saeed.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shayganni, E.; Bahmani, M.; Asgary, S.; Rafieian-Kopaei, M. Inflammaging and cardiovascular disease: Management by medicinal plants. Phytomedicine 2016, 23, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, J.; Wang, H.; Zhang, X.; Zhang, Y.; He, C.; Li, H. Antioxidant activity of ethanolic extract of Cortex fraxini and use in peanut oil. Food Chem. 2007, 103, 913–918. [Google Scholar] [CrossRef]
- Rodriguez, V.L.; Davoudian, T. Clinical Measurement of Pain, Opioid Addiction, and Functional Status. In Treating Comorbid Opioid Use Disorder in Chronic Pain; Springer: Cham, Switzerland, 2016; pp. 47–56. [Google Scholar]
- Kazemi, S.; Shirzad, H.; Rafieian-Kopaei, M. Recent findings in molecular basis of inflammation and anti-inflammatory plants. Curr. Pharm. Des. 2018, 24, 1551–1562. [Google Scholar] [CrossRef]
- Stamm, W.E.; Norrby, S.R. Urinary tract infections: Disease panorama and challenges. J. Infect. Dis. 2001, 183, S1–S4. [Google Scholar] [CrossRef]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Fridkin, S.K. Multistate point-prevalence survey of health care–associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Tandogdu, Z.; Wagenlehner, F.M. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016, 29, 73–79. [Google Scholar] [CrossRef]
- Bader, M.S.; Loeb, M.; Brooks, A.A. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 2017, 129, 242–258. [Google Scholar] [CrossRef]
- Olin, S.J.; Bartges, J.W. Urinary tract infections: Treatment/comparative therapeutics. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 721–746. [Google Scholar] [CrossRef]
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef]
- McCann, E.; Sung, A.H.; Ye, G.; Vankeepuram, L.; Tabak, Y.P. contributing factors to the clinical and economic burden of patients with laboratory-confirmed carbapenem-nonsusceptible Gram-negative urinary tract infections. Clin. Outcomes Res. CEOR 2020, 12, 191–200. [Google Scholar] [CrossRef]
- Alaoui Mdarhri, H.; Benmessaoud, R.; Yacoubi, H.; Seffar, L.; Guennouni Assimi, H.; Hamam, M.; Kettani-Halabi, M. Alternatives therapeutic approaches to conventional antibiotics: Advantages, limitations and potential application in medicine. Antibiotics 2022, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Syed, S.A.; Siddiqui, B.S.; Sattar, S.A.; Choudhary, M.I. Carandinol: First isohopane triterpene from the leaves of Carissa carandas L. and its cytotoxicity against cancer cell lines. Phytochem. Lett. 2013, 6, 91–95. [Google Scholar] [CrossRef]
- Jayakumar, K.; Muthuraman, B. Traditional uses and nutrient status of Indian native plant fruit (Carissa carandas Linn.). World Sci. News 2018, 96, 217–224. [Google Scholar]
- Madhuri, S.; Neelagund, S.E. Antioxidant, anti-diabetic activity and DNA damage inhibition activity of Carissa carandas fruit. Int. J. Adv. Res. Dev. 2019, 4, 75–82. [Google Scholar]
- Vysakh, A.; Ratheesh, M.; Rajmohanan, T.P.; Pramod, C.; Premlal, S.; Sibi, P.I. Polyphenolics isolated from virgin coconut oil inhibits adjuvant induced arthritis in rats through antioxidant and anti-inflammatory action. Int. Immunopharmacol. 2014, 20, 124–130. [Google Scholar] [CrossRef]
- David, M.; Karekalammanavar, G. Spectrographic analysis and in vitro study of antibacterial, anticancer activity of aqueous ethanolic fruit extract of Carissa carandas L. J. Adv. Sci. Res. 2015, 6, 10–13. [Google Scholar]
- Itankar, P.R.; Lokhande, S.J.; Verma, P.R.; Arora, S.K.; Sahu, R.A.; Patil, A.T. Antidiabetic potential of unripe Carissa carandas Linn. fruit extract. J. Ethnopharmacol. 2011, 135, 430–433. [Google Scholar] [CrossRef]
- Siddiqi, R.; Naz, S.; Ahmad, S.; Sayeed, S.A. Antimicrobial activity of the polyphenolic fractions derived from Grewia asiatica, Eugenia jambolana and Carissa carandas. Int. J. Food Sci. Technol. 2011, 46, 250–256. [Google Scholar] [CrossRef]
- Le, X.T.; Huynh, M.T.; Pham, T.N.; Than, V.T.; Toan, T.Q.; Bach, L.G.; Trung, N.Q. Optimization of total anthocyanin content, stability and antioxidant evaluation of the anthocyanin extract from Vietnamese Carissa carandas L. fruits. Processes 2019, 7, 468. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Galik, S. Determination of the anthocyanin concentration in table wines and fruit juices using visible light spectrophotometry. Cell Biol. 2012, 2, 1–12. [Google Scholar]
- Alara, O.; Abdurahman, N.; Mudalip, S.A.; Olalere, O. Effect of drying methods on the free radicals scavenging activity of Vernonia amygdalina growing in Malaysia. J. King Saud Univ. Sci. 2019, 31, 495–499. [Google Scholar] [CrossRef]
- Zahin, M.; Aqil, F.; Ahmad, I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 703, 99–107. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Shinde, U.A.; Phadke, A.S.; Nair, A.M.; Mungantiwar, A.A.; Dikshit, V.J.; Saraf, M.N. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Sadique, J.; Al-Rqobahs, W.A.; Bughaith, E.I.; Gindi, A.R. The bioactivity of certain medicinal plants on the stabilization of RBC membrane system. Fitoterapia 1989, 60, 525–532. [Google Scholar]
- Mizushima, Y.; Kobayashi, M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 1968, 20, 169–173. [Google Scholar] [CrossRef]
- Sakat, S.; Juvekar, A.R.; Gambhire, M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003, 225, 115–121. [Google Scholar]
- Brownlee, G. Effect of deoxycortone and ascorbic acid on formaldehyde-induced arthritis in normal and adrenalectomised rats. Lancet 1950, 255, 157–159. [Google Scholar] [CrossRef]
- Steinmann, D.; Ganzera, M. Recent advances on HPLC/MS in medicinal plant analysis. J. Pharm. Biomed. Anal. 2011, 55, 744–757. [Google Scholar] [CrossRef]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Rasool, N.; Iqbal, M.; Tawab, A.; E-Habib, F.; Khan, A.; Farman, M. Liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) analysis of Russelia equisetiformis extract. Bulg. Chem. Commun. 2017, 49, 354–359. [Google Scholar]
- Yan, L.; Yin, P.; Ma, C.; Liu, Y. Method development and validation for pharmacokinetic and tissue distributions of ellagic acid using ultrahigh performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Molecules 2014, 19, 18923–18935. [Google Scholar] [CrossRef]
- Sarkar, R.; Kundu, A.; Banerjee, K.; Saha, S. Anthocyanin composition and potential bioactivity of karonda (Carissa carandas L.) fruit: An Indian source of biocolorant. LWT 2018, 93, 673–678. [Google Scholar] [CrossRef]
- Dhar, G.; Akther, S.; Sultana, A.; May, U.; Islam, M.M.; Dhali, M.; Sikdar, D. Effect of extraction solvents on phenolic contents and antioxidant capacities of Artocarpus chaplasha and Carissa carandas fruits from Bangladesh. J. Appl. Biol. Biotechnol. 2017, 5, 39–44. [Google Scholar]
- Ngonda, F. In-vitro Anti-oxidant Activity and Free Radical Scavenging Potential of roots of Malawian Trichodesma zeylanicumm (burm. f.). Asian J. Biomed. Pharm. Sci. 2013, 3, 21. [Google Scholar]
- Siddiqi, R.; Naz, S.; Sayeed, S.A.; Ishteyaque, S.; Haider, M.S.; Tarar, O.M.; Jamil, K. Antioxidant potential of the polyphenolics in Grewia asiatica, Eugenia jambolana and Carissa carandas. J. Agric. Sci. 2013, 5, 217. [Google Scholar] [CrossRef]
- Sarma, A.; Sarmah, P.; Kashyap, D.; Dutta, S.; Mahanta, M. Antioxidant activity and nutraceutical property of the fruits of an ethno-medicinal plant: Carissa carandas L. found in Brahmaputra valley agro-climatic condition. J. Pharm. Sci. Res. 2015, 7, 55. [Google Scholar]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Shukla, V.J. Antibacterial and antifungal activities from leaf extracts of Cassia fistula L.: An ethnomedicinal plant. J. Adv. Pharm. Technol. Res. 2011, 2, 104–109. [Google Scholar] [CrossRef]
- Agarwal, T.; Singh, R.; Shukla, A.D.; Waris, I. In vitro study of antibacterial activity of Carissa carandas leaf extracts. Asian J. Plant Sci. Res. 2012, 2, 36–40. [Google Scholar]
- Pilasombut, K.; Laosinwattana, C.; Nguyen, T.T.; Ngamyeeesoon, N.; Teerarak, M. Antimicrobial properties of extracts from Carissa carandas L. fruits and its application in chilled and frozen ground pork. Int. J. Agric. Technol. 2019, 15, 91–102. [Google Scholar]
- Shifa, S.; Begum, T.; Afroze, F.; Shraboni, M.K. Preliminary phytochemical screening, antibacterial activity and cytotoxic activity of leaves extract of Carissa carandas Linn. J. Pharmacogn. Phytochem. 2019, 8, 801–804. [Google Scholar]
- Fartyal, M. Comparative study of antifungal potential of various extracts of leaves of Carissa carandas Linn., Nerium oleander Linn. and Allamanda cathartica Linn. against human fungal pathogen Candida albicans. Vegetos 2023, 1–8. [Google Scholar] [CrossRef]
- Sudjaroen, Y. Lack of in vitro anticancer and antimicrobial activities from Karanda (Carissa carandas) fruit extracts. J. Pharm. Negat. Results 2017, 8, 31–36. [Google Scholar] [CrossRef]
- Opie, E.L. On the relation of necrosis and inflammation to denaturation of proteins. J. Exp. Med. 1962, 115, 597–608. [Google Scholar] [CrossRef]
- Williams, L.A.D.; O’Connar, A.; Latore, L.; Dennis, O.; Ringer, S.; Whittaker, J.A.; Conrad, J.; Vogler, B.; Rosner, H.; Kraus, W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med. J. 2008, 57, 327–331. [Google Scholar] [PubMed]
- Kumari, C.S.; Yasmin, N.; Hussain, M.R.; Babuselvam, M. In vitro anti-inflammatory and anti-arthritic property of Rhizopora mucronata leaves. Intern. J. Pharm. Sci. Res. 2015, 6, 482–485. [Google Scholar]
- Thida, M.; Aung, H.M.; Wai, N.P.; Su, M. In vitro evaluation of antioxidant, antiglycation and anti-protein denaturation potentials of indigenous Myanmar medicinal plant extracts. J. Herbs Spices Med. Plants 2024, 30, 278–291. [Google Scholar] [CrossRef]
- Moussaid, M.; Elamrani, A.E.; Bourhim, N.; Benaissa, M. In vivo anti-inflammatory and in vitro antioxidant activities of Moroccan medicinal plants. Nat. Prod. Commun. 2011, 6, 1441–1443. [Google Scholar]
- Anupama, N.; Madhumitha, G.; Rajesh, K.S. Role of dried fruits of Carissa carandas as anti-inflammatory agents and the analysis of phytochemical constituents by GC-MS. BioMed Res. Int. 2014, 2014, 512369. [Google Scholar] [CrossRef]
- Saher, S.; Narnawre, S.; Patil, J. Evaluation of phytochemical and pharmacological activity of Carissa carandas L. fruits at three different stages of maturation. Drug Res. 2020, 70, 80–85. [Google Scholar] [CrossRef]
- Malheiros, A.; Filho, V.C.; Schmitt, C.B.; Yunes, R.A.; Escalante, A.; Svetaz, L.; Zacchino, S.; Monache, F.D. Antifungal activity of drimane sesquiterpenes from Drimys brasiliensis using bioassay-guided fractionation. J. Pharm. Pharm. Sci. 2005, 8, 335–339. [Google Scholar]
- Zhang, X.; Han, F.; Gao, P.; Yu, D.; Liu, S. Bioassay-guided fractionation of antifertility components of castorbean (Ricinus communis L.) seed extracts. Nat. Prod. Res. 2007, 21, 982–989. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Satti, N.K.; Sharma, V.K.; Dutt, P.; Suri, K.A.; Bani, S. Amelioration of inflammatory responses by chlorogenic acid via suppression of pro-inflammatory mediators. J. Appl. Pharm. Sci. 2011, 1, 67–75. [Google Scholar]
- El-Shitany, N.A.; El-Bastawissy, E.A.; El-desoky, K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int. Immunopharmacol. 2014, 19, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Miao, Y. The antibacterial activity and mechanism of chlorogenic acid against foodborne pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Ma, J.N.; Ma, C.M. Antifungal inhibitory activities of caffeic and quinic acid derivatives. In Coffee in Health and Disease Prevention; Academic Press: Washington, DC, USA, 2015; pp. 635–641. [Google Scholar]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Kumazawa, S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008, 56, 4457–4462. [Google Scholar] [CrossRef] [PubMed]
- Pertuzatti, P.B.; Barcia, M.T.; Rebello, L.P.G.; Gómez-Alonso, S.; Duarte, R.M.T.; Duarte, M.C.T.; Hermosín-Gutiérrez, I. Antimicrobial activity and differentiation of anthocyanin profiles of rabbiteye and highbush blueberries using HPLC–DAD–ESI-MSn and multivariate analysis. J. Funct. Foods 2016, 26, 506–516. [Google Scholar] [CrossRef]
- Speciale, A.; Bashllari, R.; Muscarà, C.; Molonia, M.S.; Saija, A.; Saha, S.; Cimino, F. Anti-inflammatory activity of an in vitro digested anthocyanin-rich extract on intestinal epithelial cells exposed to TNF-α. Molecules 2022, 27, 5368. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).