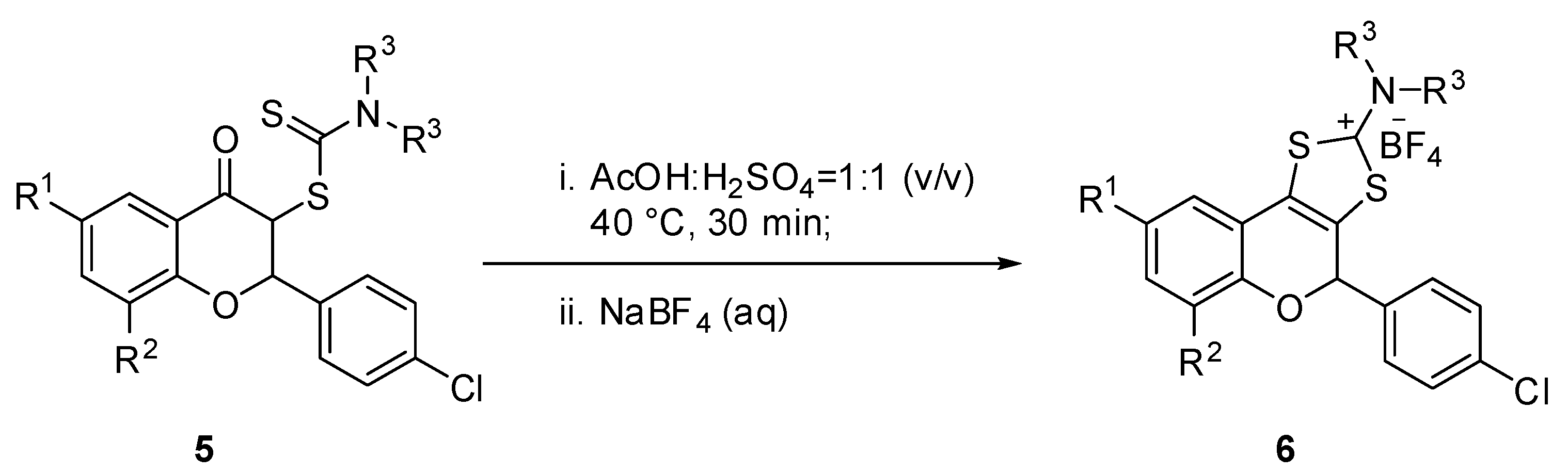Abstract
The antioxidant properties of 3-dithiocarbamic flavanones have been investigated. The influence of the halogen substituents on ring A of the flavanones and the nature of the secondary amine from the dithiocarbamic moiety have been accounted. The results indicated that the presence of a halogen substituent at the C-8 position of the benzopyran ring induce better antioxidant properties against DPPH and ABTS than butylated hydroxytoluene (BHT) and ascorbic acid. The presence of a halogen substituent at the mentioned position appears to induce a higher stability for a free radical intermediate at the C-3 position of the benzopyran ring. A free radical enolate is most likely to be involved in the antioxidant activity of this dithiocarbamic flavanone. It is a stable intermediate that supports the influence of dithiocarbamic moiety on the antioxidant properties of the reported flavanones.
1. Introduction
Reactive oxygen species (ROS) are unstable derivatives of molecular oxygen that display high reactivity. ROS are divided into two main categories, namely radical ROS, such as superoxide (O2•−), peroxyl (HO•) or hydroxyl radicals (ROO•), and non-radical ROS, such as singlet oxygen (1O2) and hydrogen peroxide (H2O2). In humans, ROS are generated mainly by mytochondrial processes, as well as by various enzymes during normal cell metabolism [1,2]. At low concentrations, ROS can act as mediators in a number of signaling processes; however, at higher concentrations, ROS indiscriminately damage biomacromolecules such as DNA, proteins, lipids and carbohydrates [3,4]. In the human body, this kind of oxidative stress can be counteracted to some degree with the help of antioxidants. These, in turn, can be produced by the cells (endogenous) and include enzymes, non-enzymatic proteins, thiols, etc., or acquired from the diet (exogenous), such as vitamins, carotenoids, polyphenols, etc. [5].
Flavonoids are a group of polyphenols found in nature throughout the plant kingdom. In plants, they play a wide variety of roles, acting as antioxidants, modulators, pollinator attractors or pest-deterrents [6,7,8,9,10,11]. Flavonoids inevitably make their way into our diets and it is estimated that the dietary intake of flavonoids is up to 800 mg/day [5]. As such, considerable effort has been dedicated to study the effects of flavonoids on the human body. Flavonoids have been shown to display cardioprotective, neuroprotective, antiproliferative, antiviral and antimicrobial properties [12,13,14,15,16,17,18,19]. In the last few years, our group reported on the synthesis, antimicrobial and cytotoxic properties of several novel synthetic flavonoids bearing a 1,3-dithiolium ring fused to the C ring of the flavan core [20,21]. Herein, we report on the antioxidant properties of several synthetic dithiocarbamic flavanones, used as precursors in the synthesis of the above mentioned tricyclic synthetic flavonoids.
2. Materials and Methods
2.1. Chemistry
Melting points were obtained using a KSPI melting point meter (A. KRÜSS Optronic, Hamburg, Germany) and are uncorrected. IR spectra were recorded on a Bruker Tensor 27 instrument (Bruker Optik GmbH, Ettlingen, Germany). NMR spectra were recorded on a Bruker 500 MHz spectrometer (Bruker BioSpin, Rheinstetten, Germany). Chemical shifts are reported in ppm downfield from TMS. Mass spectra were recorded on a Thermo Scientific ISQ LT instrument (Thermo Fisher Scientific Inc., Waltham, MA, USA). UV-vis measurements were carried out using a Varian BioChem 100 spectrophotometer (Varian, Sydney, Australia) equipped with a 8 × 6 multicell block water thermostatted. All reagents were commercially available and used without further purification. Elemental analysis and copies of 13C-NMR spectra are available in the Supplementary Material.
2.1.1. General Procedure for 6,8-Dibromo-2-(4-chlorophenyl)-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5h)
To a solution of 1-(3,5-dibromo-2-hydroxyphenyl)-1-oxoethan-2-yl-pyrrolidine-1-carbodithioate (3h) (0.44 g, 1 mmol) in a mixture of MeOH/CHCl3 (15 mL, 1:1 v/v), aminal 4 (0.3 g, 1 mmol) was added and the reaction mixture was heated under reflux for 3 h. After cooling, the solid material was filtered off and purified by recrystallization from ethanol to give 5h (0.45 g, 80%) as colorless crystals. M.p. 184–185 °C. IR (ATR, cm−1) 2893, 1700, 1586, 1431, 1088, 1431, 1088, 800, 640, 539, 458. 1H NMR (CDCl3, selected data for the major syn isomer) δ 8.03 (d, J = 2.1 Hz, 1H), 7.90 (d, J = 2.1 Hz, 1H), 7.47 (d, J = 8.3 Hz, 2H), 7.37 (d, J = 8.3 Hz, 2H), 6.26 (d, J = 3.5 Hz, 1H), 5.99 (d, J = 3.5 Hz, 1H), 3.91 (m, 1H), 3.87 (m, 1H), 3.66 (m, 1H), 3.52 (m, 1H), 2.03 (m, 2H), 1.97 (m, 2H). 13C NMR (CDCl3, selected data for the major syn isomer) δ 187.4, 186.4, 155.5, 141.5, 134.7, 133.3, 129.5, 128.8, 128.1, 122.2, 114.8, 113.2, 80.8, 56.7, 56.6, 50.8, 26.0, 24.2. MS (EI) m/z: 558.95 (M+, 3%) for C20H1679Br2ClNO2S2.
2.1.2. 6,8-Dibromo-2-(4-chlorophenyl)-4-oxochroman-3-yl-piperidine-1-carbodithioate (5i)
M.p. 170–171 °C, (0.45 g, 79%). IR (ATR, cm−1) 2945, 1693, 1585, 1452, 1268, 813, 653, 543. 1H NMR (CDCl3, selected data for the major syn isomer) δ 8.04 (d, J = 2.2 Hz, 1H), 7.98 (d, J = 2.2 Hz, 1H), 7.47 (d, J = 8.1 Hz, 2H), 7.37 (d, J = 8.1 Hz, 2H), 6.35 (d, J = 3.6 Hz, 1H), 6.00 (d, J = 3.6 Hz, 1H), 4.25 (m, 2H), 3.84 (m, 2H), 1.69 (m, 6H). 13C NMR (CDCl3, selected data for the major syn isomer) δ 190.6, 186.0, 155.8, 141.6, 135.0, 133.3, 129.5, 128.8, 128.7, 122.3, 114.6, 113.1, 80.9, 57.4, 54.2, 52.1, 26.1, 25.3, 24.1. MS (EI) m/z: 572.86 (M+, 1%) for C21H1879Br2ClNO2S2.
2.1.3. 6,8-Dibromo-2-(4-chlorophenyl)-4-oxochroman-3-yl-morpholine-4-carbodithioate (5j)
M.p. 167–168 °C, (0.43 g, 75%). IR (ATR, cm−1) 2914, 1702, 1587, 1447, 1259, 1214, 808, 644, 540. 1H NMR (CDCl3, selected data for the major syn isomer) δ 8.04 (d, J = 2.3 Hz, 1H), 7.90 (d, J = 2.3 Hz, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.37 (d, J = 8.4 Hz, 2H), 6.29 (d, J = 3.7 Hz, 1H), 6.01 (d, J = 3.7 Hz, 1H), 4.20 (m, 2H), 3.86 (m, 2H), 3.73 (m, 4H). 13C NMR (CDCl3, selected data for the major syn isomer) δ 192.2, 186.1, 155.4, 141.6, 134.9, 133.1, 129.5, 128.8, 128.2, 122.2, 114.9, 113.2, 80.7, 66.2, 66.1, 57.1, 51.0, 50.7. MS (EI) m/z: 574.88 (M+, 3%) for C20H1679Br2ClNO3S2.
2.1.4. 6,8-Diiodo-2-(4-chlorophenyl)-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5m)
M.p. 205–206 °C (0.42 g, 75%). IR (ATR, cm−1) 2880, 1694, 1426, 1154, 952, 808, 639, 537. 1H NMR (DMSO-d6, selected data for the major syn isomer) δ 8.42 (d, J = 2.1 Hz, 1H), 8.04 (d, J = 2.1 Hz, 1H), 7.57 (d, J = 8.6 Hz, 2H), 7.51 (d, J = 8.6 Hz, 2H), 6.41 (d, J = 2.8 Hz, 1H), 5.89 (d, J = 2.8 Hz, 1H), 3.71 (m, 1H), 3.66 (m, 1H), 3.52 (m, 1H), 3.43 (m, 1H), 1.98 (m, 2H), 1.84 (m, 2H). 13C NMR (DMSO-d6, selected data for the major syn isomer) δ 187.5, 186.4, 158.9, 152.3, 135.8, 135.6, 134.7, 129.0, 128.8, 121.8, 89.8, 87.3, 80.1, 56.4, 56.3, 51.5, 26.1, 24.2. MS (EI) m/z: 654.34 (M+, 4%) for C20H16ClI2NO2S2.
2.1.5. 6,8-Diiodo-2-(4-chlorophenyl)-4-oxochroman-3-yl-piperidine-1-carbodithioate (5n)
M.p. 195–196 °C (0.41 g, 72%). IR (ATR, cm−1) 2928, 1698, 1434, 1255, 963, 824, 623, 512, 458. 1H NMR (DMSO-d6, selected data for the major syn isomer) δ 8.40 (d, J = 1.4 Hz, 1H), 8.00 (d, J = 1.4 Hz, 1H), 7.52 (m, 4H), 6.41 (d, J = 2.5 Hz, 1H), 5.95 (d, J = 2.5 Hz, 1H), 4.10 (m, 2H), 3.77 (m, 2H), 1.50 (m, 6H). 13C NMR (DMSO-d6, selected data for the syn major isomer) δ 190.3, 186.2, 159.0, 152.4, 135.6, 135.5, 134.2, 130.2, 128.8, 121.9, 89.8, 86.8, 82.2, 57.1, 54.1, 52.2, 26.2, 25.5, 23.8. MS (EI) m/z: 668.94 (M+, 2%) for C20H18ClI2NO2S2.
2.2. In Vitro Antioxidant Activities
2.2.1. 2,2-Diphenyl-1-picrylhydrazyl radical (DPPH) Assay
The DPPH method was performed according to the reported literature procedure [22,23]. Using a standard 4 mL quartz UV cell, flavanones 5a–o, at different concentrations (1.6–2.5 mM in ethanol), were added to a solution of DPPH (0.1 mM in ethanol, 2.75 mL). The flavanones were added in 80 µL batches until absorbance dropped below half of the initial value. The reaction for scavenging DPPH radicals was performed in the dark at room temperature and the absorbance was measured at 517 nm after 15 min of incubation at 37 °C. The IC50 (nM) was determined as the flavanones’ 5a–o concentration of 50% of the DPPH scavenging capacity (Table 1).

Table 1.
Radical scavenging activities of compounds 5a–o and 6 (IC50 values in nM) *.
2.2.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Assay
The ABTS assay was performed according to a slightly modified literature-reported protocol [24,25]. In short, ABTS radical cation (ABTS+) was prepared by reacting an ABTS solution (7 mM) in phosphate buffer (PBS, 0.1 M, pH 7.4) with a solution of potassium persulfate (2.45 mM) in water. The mixture was left to stand at room temperature for 16 h before use. After that, the mixture was diluted with PBS to reach an absorbance value of 0.70 ± 0.5 at 734 nm. Using a standard 4 mL quartz UV cell, flavanones 5a–o, at different concentrations (0.8–2.5 mM in ethanol), were added to a solution of ABTS+ (2.75 mL). The flavanones were added in 20 µL batches until absorbance dropped below half of the initial value. The reaction for scavenging ABTS+ radicals was performed in the dark at room temperature and the absorbance was measured at 734 nm after 10 min of incubation at 37 °C. The IC50 (nM) was determined as the flavanones’ 5a–o concentration of 50% of the ABTS+ scavenging capacity (Table 1).
2.2.3. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP method was performed according to the reported literature procedure. The FRAP reagent was prepared by mixing acetate buffer (300 mM, pH 3.6), a solution of 10 mM 2,4,6-tris(2-pyridyl)triazine (TPTZ) in 40 mM HCl, and 20 mM FeCl3 at 10:1:1 (v/v/v). Using a standard 4 mL quartz UV cell, flavanones 5a–o, at different concentrations (1.8–2.4 mM in ethanol), were added to a solution of FRAP (2.75 mL). The flavanones were added in 30 µL batches until absorbance dropped below half of the initial value. The reaction for scavenging radicals was performed in the dark at room temperature and the absorbance was measured at 593 nm after 20 min of incubation at 37 °C. The IC50 (nM) was determined as the flavanones’ 5a–o concentration of 50% of the FRAP scavenging capacity (Table 1).
3. Results
3.1. Chemistry
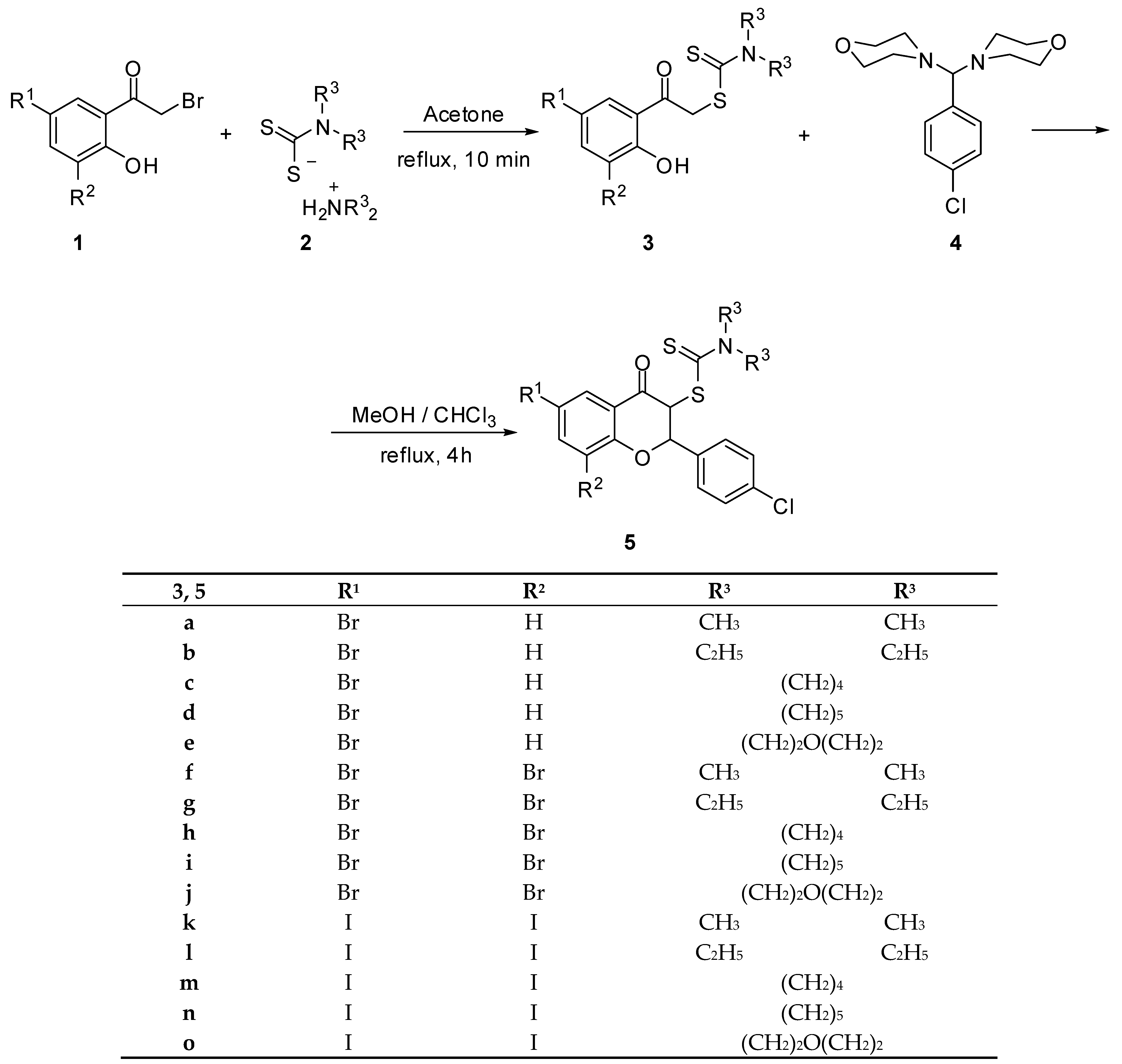
The synthesis of 3-dithiocarbamic flavanones 5a–o has been accomplished through a two-step procedure, as shown in Scheme 1: the synthesis of the corresponding phenacyl carbodithioates 3, followed by their cyclocondensation to the corresponding substituted benzopyran derivatives. The key precursors for phenacyl dithiocarbamates 3a–o are 2-bromo-1-(5-bromo-2-hydroxyphenyl)ethan-1-one [26], 2-bromo-1-(3,5-dibromo-2-hydroxyphenyl)ethan-1-one [26] and 2-bromo-1-(2-hydroxy-3,5-diiodophenyl)ethan-1-one [27], compounds that have been synthesized according to the reported procedures. The reactions of these compounds with the various salts of dialkyldithiocarbamic acid 2, which are easily available from the reaction of secondary amine with carbon disulfide, have provided the corresponding phenacyl dithiocarbamates 3a–o in good yields (70–80%). The treatment of the latter compounds with p-chloroaminal 4 has been performed using a recently reported experimental procedure by us, providing the desired 3-dithiocarbamic flavanones 5a–o (Scheme 1).
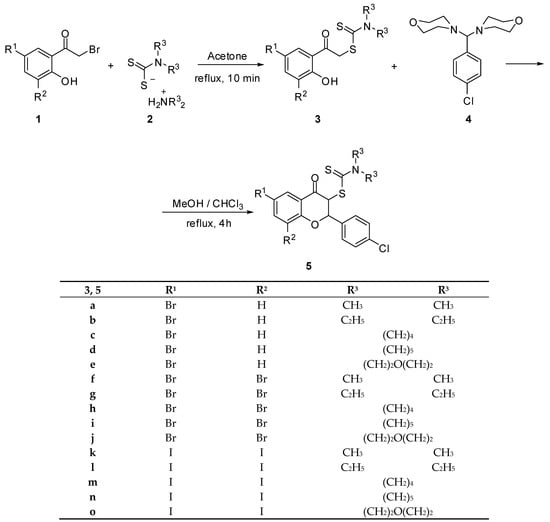
Scheme 1.
Synthesis of 3-dithiocarbamic flavanones 5a–o.
The synthesis of flavanones 5a–g, 5k,l [20] and 5o has been previously reported by us. The structure of newly synthesized flavanones has been proved by spectral and analytical data. The formation of the benzopyran ring is accompanied by important spectral changes. 1H-NMR spectra indicated the disappearance of the phenolic proton signal, as well as of the methylene protons signal, and the appearance of two new doublets, corresponding to the hydrogen atoms from the C-2 and C-3 positions of the pyran ring. Besides these, the NMR pattern of the para-substituted aromatic ring originating from aminal 4 can be observed. 13C NMR spectra indicate the appearance of a new signal provided by the thiocarbonyl carbon atom around 190–192 ppm. The presence of two new signals, one for the C-2 carbon atom, found to be around 80.0 ppm, and one for the C-3 carbon atom, found to be around 60.0 ppm, also support the closure of the pyrane ring.
As previously disclosed by us, the 3-dithiocarbamic flavanones are important precursors for a new class of tricyclic synthetic flavonoids of type 6, which exhibit remarkable antimicrobial properties with MIC and MBC values of up to 0.24 µg/mL. The experimental procedure consists in acid catalyzed cyclocondensation of 3-dithiocarbamic flavanones, using a mixture of acetic acid/sulfuric acid (1:1 v/v) as cyclization agent, under mild conditions (Scheme 2). In order to investigate the antioxidant properties of tricyclic flavonoids, we synthesized a series of these compounds, namely 6a–g, 6k, 6l and 6o, whose synthesis and structural characterization have been previously reported by us.
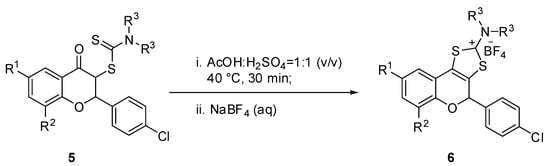
Scheme 2.
Synthesis of tricyclic flavonoids 6.
3.2. In Vitro Antioxidant Activities
In order to investigate the antioxidant properties of the above synthesized compounds, we determined the DPPH, ABTS+• and FRAP scavenging activities of these. Ascorbic acid and BHT, two common antioxidants, were used as standards for the antioxidant potential comparison [28,29,30]. The experimental procedure is described in Section 2.2; this was also used to determine the antioxidant properties of tricyclic flavonoids of type 6. The results are presented in Table 1.
4. Discussion
In order to investigate the antioxidant properties of 3-dithiocarbamic flavanones of type 5, we decided to undertake a structure–activity relationship study by varying different substituents at ring A and dithiocarbamic moiety, while keeping the same substituent (chlorine) at para position of ring B. There are three sets of dithiocarbamic flavanones, namely 6-bromo, 6,8-dibromo and 6,8-diiodo, each one with five derivatives according to the nature of secondary amine moiety, namely dimetylamino, diethylamino, pyrrolidinyl, piperidinyl and morpholinyl.
The analysis of the data presented in Table 1 disclosed interesting facts about structure–activity relationship of flavanones 5a–o. Thus, the presence of a second halogen substituent at the C-8 position of the benzopyran ring of flavanones 5f–o increased both DPPH and ABTS+• scavenging activity as compared to the monohalogenated flavanones 5a–e. In fact, the latter flavanones do not exhibit better antioxidant properties than both ascorbic acid and BHT. By introducing a second halogen substituent on the benzopyran ring, better antioxidant properties have been recorded. Thus, DPPH radical scavenging of flavanones 5f–j and 5k–o indicated better antioxidant activities as compared with BHT. All five 6,8-dibromoflavanones 5f–j display smaller IC50 values than BHT, with pyrrolidinyl 5h and N-morpholinyl 5j derivatives being the most active with IC50 values of around 120 nM. From the 6,8-diiodoflavanones 5k–o set, N-morpholinyl 5o derivative displayed the best antioxidant properties with IC50 of 127 nM.
The ABTS+• radical scavenging of flavanones 5f–j and 5k–o indicated better antioxidant activity as compared with both ascorbic acid and BHT. With the exception of 5i, among the 6,8-dibromoflavanones 5f–j set, all compounds displayed smaller IC50 values than the used standards, with special attention paid to the pyrrolidinyl derivative 5h with an IC50 value of 10 nM. As compared with ascorbic acid, 6,8-diiodoflavanones 5l and 5o displayed better antioxidant properties. Again, N-morpholinyl derivative 5o, with an IC50 of 13 nM, showed increased antioxidant activity as compared with both ascorbic acid and BHT.
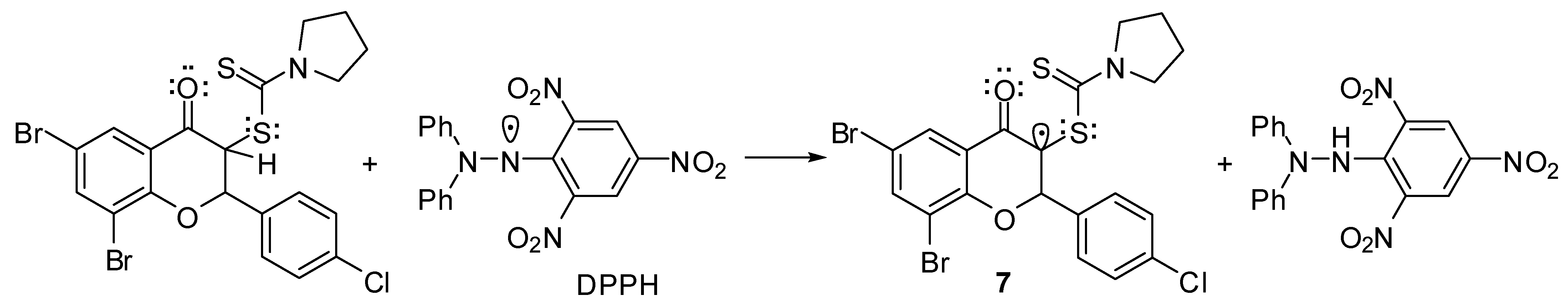
Concerning the reaction mechanism for radical scavenging of flavanones 5, important information have been provided by the investigation of antioxidant properties of tricyclic flavonoids of type 6. DPPH, ABTS+• and FRAP scavenging activities of tricyclic flavonoids revealed practically no antioxidant properties as compared with standards. Whereas the heterocyclocondensation of flavanones 5 to tricyclic flavonoids 6 (Scheme 2) is accompanied by the elimination of a hydrogen atom from the C-3 position of the benzopyran ring, it might be concluded that this atom makes the major contribution to the antioxidant properties of flavanones. Thus, a free hydrogen radical transfer to DPPH or ABTS+• should provide a stable enolate radical of type 7 (e.g., Scheme 3).
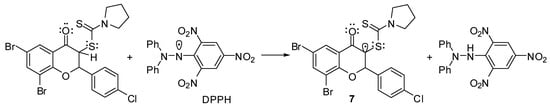
Scheme 3.
Plausible radical intermediate for flavanones’ radical scavenging mechanism.
The presence of the sulfur atom, with its non-participating electrons, next to the radical center also contributes to an increased stabilization due to a parallel overlapping with the free radical orbital. This intermediate also accounts for the influence of secondary amine moiety on radical scavenging of 3-dithiocarbamic flavanones, with N-pyrrolidinyl and N-morpholinyl carbodithioates being the most active. The formation of such a radical intermediate also explains the increased antioxidant activities of 6,8-disubstituted flavanones 5f–o towards monosubstituted flavanones 5a–e, apparently due to a supplementary stabilization induced by the C-8 bromine or iodine substituent. However, the formation of a free radical at the C-2 position, which is a benzylic one, cannot be ruled out.
5. Conclusions
A structure–activity relationship study on the antioxidant properties of 3-dithiocarbamic flavanones was performed. It was found that 6,8-dihalogenated flavanones exhibit better free radical scavenging than 6-monohalogenated flavanones. The influence of secondary amine moiety from the dithiocarbamic substituent has been also investigated, with N-pyrrolidinyl and N-morpholinyl carbodithioate derivatives exhibiting the highest antioxidant properties. Further investigation will target the influence of the substituents at the ring B on the antioxidant properties of dithiocarbamic flavanones provided by the hydrogen atom at the 2-position of the benzopyran scaffold. This is a preliminary report on the antioxidant properties of these 3-dithiocarmamic flavanoids and further studies will be reported soon.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13080963/s1. Elemental analysis and copies of 13C-NMR spectra.
Author Contributions
M.L.B. and L.G.S. equally contributed to conceptualization, methodology, investigation and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
Thanks to the CERNESIM Center in the Interdisciplinary Research Institute at the “Alexandru Ioan Cuza” University of Iasi for recording the NMR experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants and Human Disease: A General Introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kuang, Z.; Zhang, D.; Gao, Y.; Ying, M. Reactive oxygen species in immune cells: A new antitumor target. Biomed. Pharmacother. 2021, 133, 110978. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Friends in Arms: Flavonoids and the Auxin/Cytokinin Balance in Terrestrialization. Plants 2023, 12, 517. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Arista, M.; Buide, M.L.; Ortiz, P.L. Major Flower Pigments Originate Different Colour Signals to Pollinators. Front. Ecol. Evol. 2021, 9, 743850. [Google Scholar] [CrossRef]
- Ramaroson, M.L.; Koutouan, C.; Helesbeux, J.J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Apostu, M.O.; Birsa, L.M.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorg. Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Sarbu, L.G.; Hopf, H.; Jones, P.G.; Babii, C.; Stefan, M.; Birsa, M.L. The influence of halogen substituents on the biological properties of sulfur-containing flavonoids. Bioorg. Med. Chem. 2016, 24, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Gursoy, N.; Sarikurkcu, C.; Cengiz, M.; Halil Solak, M. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem. Toxicol. 2009, 47, 2381–2388. [Google Scholar] [CrossRef]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Voss, H.-P.; Bast, A. Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food Chem. Toxicol. 2004, 42, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Buu-Hoi, N.P.; Lavit, D. The bromination of o- and p-hydroxyaryl ketones. J. Chem. Soc. 1955, 18–20. [Google Scholar] [CrossRef]
- Sandulache, A.; Cascaval, A.; Toniutti, N.; Giumanini, A.G. New flavones by a novel synthetic route. Tetrahedron 1997, 53, 9813–9822. [Google Scholar] [CrossRef]
- Kano, M.; Takayanagi, T.; Harada, K.; Makino, K.; Ishikawa, F. Antioxidative activity of anthocyanins from purple sweet potato Ipomoera batatas cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005, 69, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bozin, B.; Sokovic, M.; Simin, N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).