Culture of Bovine Aortic Endothelial Cells in Galactose Media Enhances Mitochondrial Plasticity and Changes Redox Sensing, Altering Nrf2 and FOXO3 Levels
Abstract
1. Introduction
2. Materials and Methods
3. Results
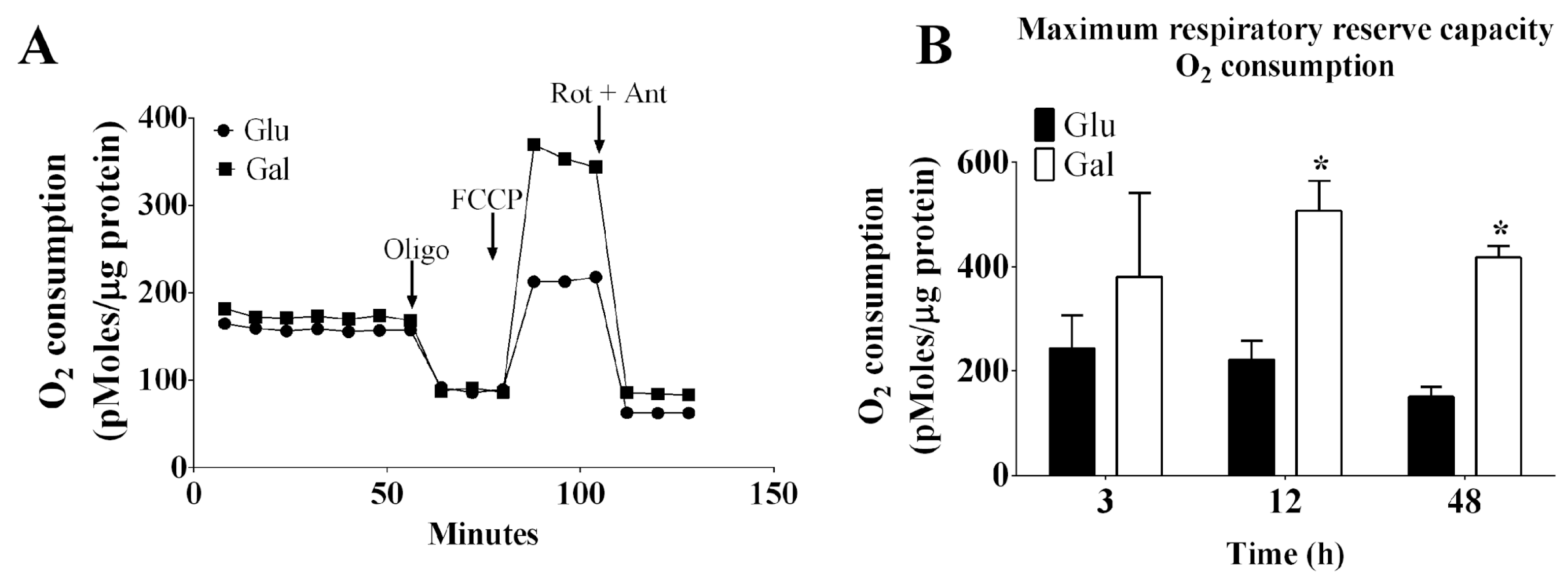
3.1. Effect of Glucose- or Galactose-Containing Medium in Cellular Respiration and Mitochondrial Superoxide Radical (O2•−) Levels in BAEC
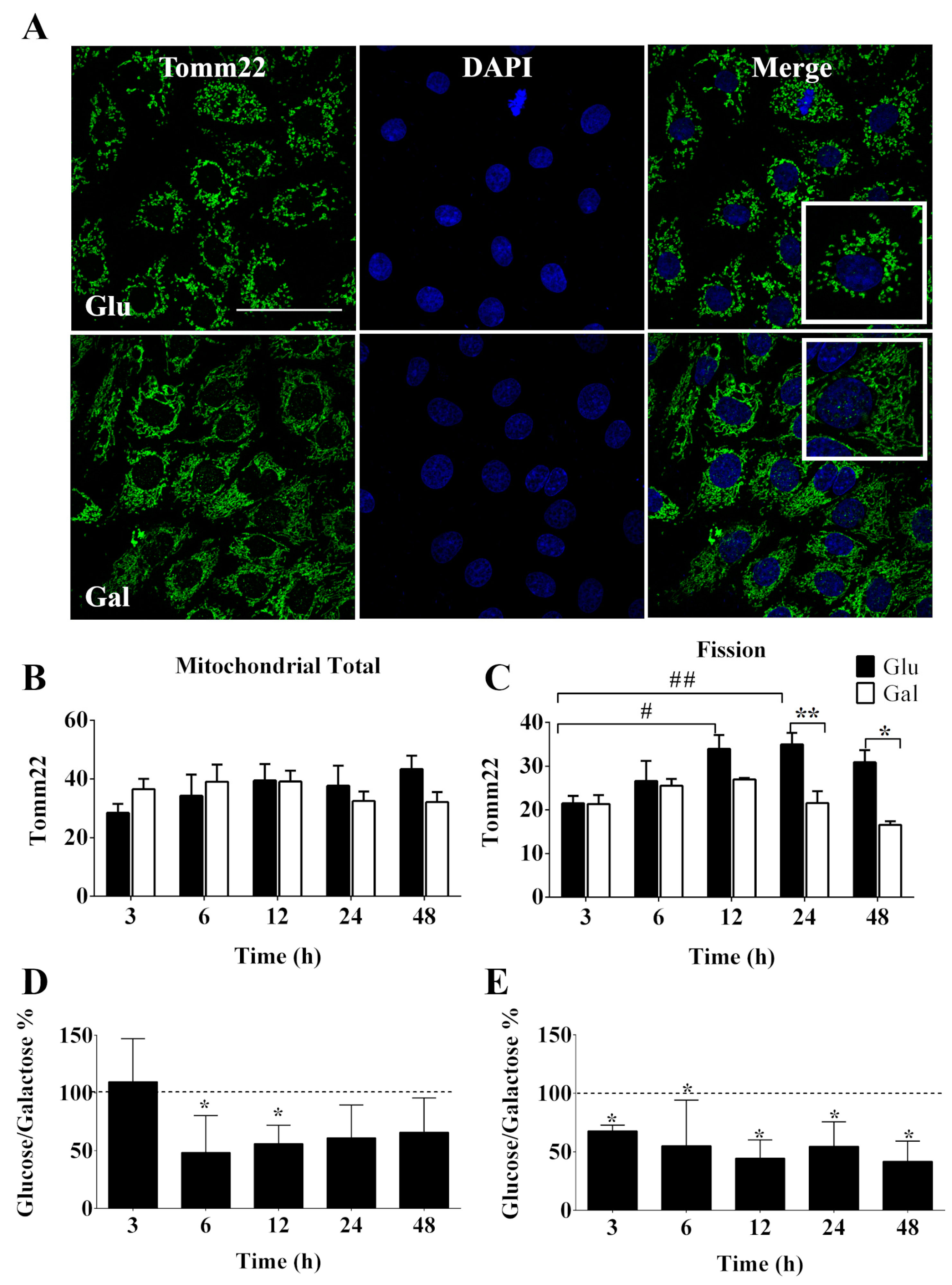
3.2. Mitochondrial Dynamics of BAEC Conditioned in Glucose or Galactose Medium
3.3. Effect of Glucose- or Galactose-Containing Medium on Translocation and Expression of Nrf2
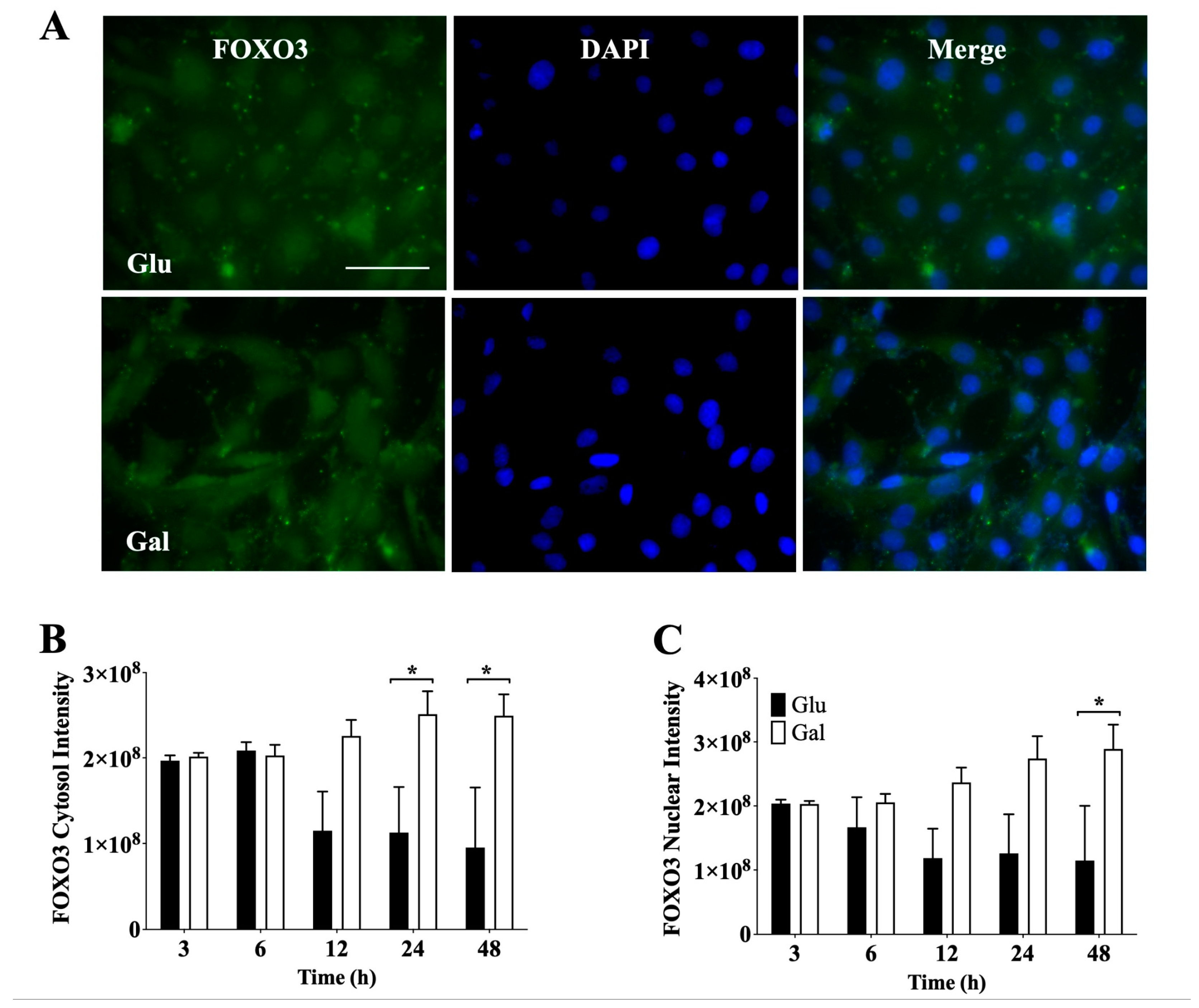
3.4. Effect of Glucose- or Galactose-Containing Medium on Translocation and Expression of FOXO3 Transcription Factor
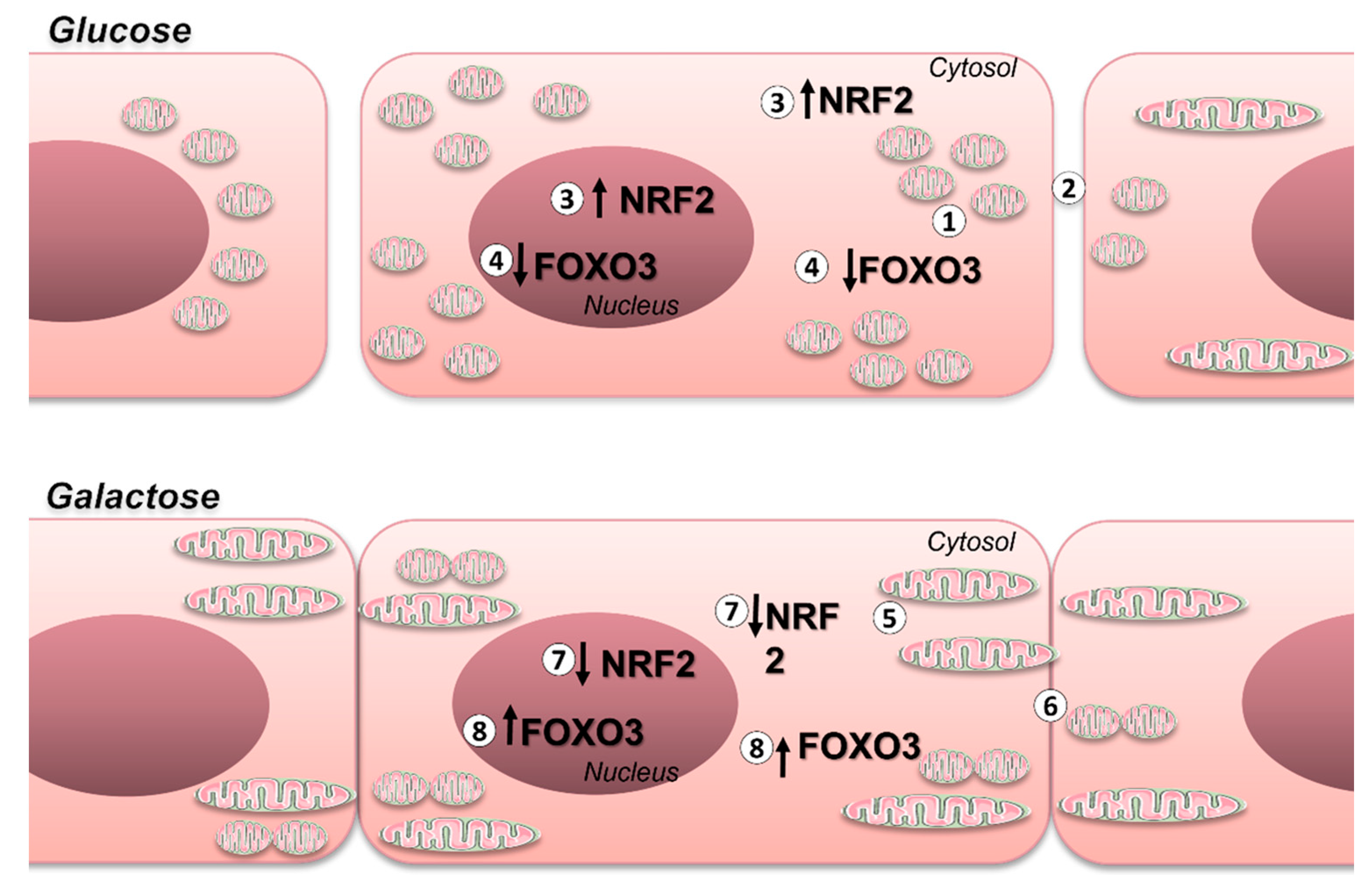
4. Discussion
5. Study Limitations
- This study was conducted solely on a single cell type and species; thus, the generalizability of the findings to other cell types and species remains to be established.
- The analytical procedure employed for assessing mitochondrial dynamics does not include high-resolution TEM analysis, and the derived conclusions would require further validation, including the analysis of proteins involved in the regulation of mitochondrial dynamics.
- Since we did not assess the antibodies used in immunofluorescence on knock-out cells, some signals may stem from non-specific staining of the samples.
- Further testing would be required to determine if the observed nuclear localization changes do actually impact on Nrf2 and FOXO3A transcriptional activity.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguer, C.; Gambarotta, D.; Mailloux, R.J.; Moffat, C.; Dent, R.; McPherson, R.; Harper, M.E. Galactose Enhances Oxidative Metabolism and Reveals Mitochondrial Dysfunction in Human Primary Muscle Cells. PLoS ONE 2011, 6, e28536. [Google Scholar] [CrossRef] [PubMed]
- Marroquin, L.D.; Hynes, J.; Dykens, J.A.; Jamieson, J.D.; Will, Y. Circumventing the Crabtree Effect: Replacing Media Glucose with Galactose Increases Susceptibility of HepG2 Cells to Mitochondrial Toxicants. Toxicol. Sci. 2007, 97, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Orlicka-Płocka, M.; Gurda-Wozna, D.; Fedoruk-Wyszomirska, A.; Wyszko, E. Circumventing the Crabtree Effect: Forcing Oxidative Phosphorylation (OXPHOS) via Galactose Medium Increases Sensitivity of HepG2 Cells to the Purine Derivative Kinetin Riboside. Apoptosis 2020, 25, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Altamirano, L.; Paudel, S.; Welker, L.; Konkle, M.E.; Chakraborty, N.; Menze, M.A. Modulation of Cellular Energetics by Galactose and Pioglitazone. Cell Tissue Res. 2017, 369, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, M.; Prieto, I.; de Bem, A.F.; Olmos, Y. Methodological approach for the evaluation of FOXO as a positive regulator of antioxidant genes. Methods Mol. Biol. 2019, 1890, 61–76. [Google Scholar] [CrossRef]
- Caja, S.; Enríquez, J.A. Mitochondria in endothelial cells: Sensors and integrators of environmental cues. Redox Biol. 2017, 12, 821–827. [Google Scholar] [CrossRef]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial dysfunction in vascular endothelial cells and its role in atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef]
- García-Quintans, N.; Sánchez-Ramos, C.; Prieto, I.; Tierrez, A.; Arza, E.; Alfranca, A.; Redondo, J.M.; Monsalve, M. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis 2016, 19, 217–228. [Google Scholar] [CrossRef] [PubMed]
- García-Quintans, N.; Prieto, I.; Sánchez-Ramos, C.; Luque, A.; Arza, E.; Olmos, Y.; Monsalve, M. Regulation of endothelial dynamics by PGC-1α relies on ROS control of VEGF-A signaling. Free Radic. Biol. Med. 2016, 93, 41–51. [Google Scholar] [CrossRef]
- Baker, N.; Patel, J.; Khacho, M. Linking Mitochondrial Dynamics, Cristae Remodeling and Supercomplex Formation: How Mitochondrial Structure Can Regulate Bioenergetics. Mitochondrion 2019, 49, 259–268. [Google Scholar] [CrossRef]
- Zorov, D.B.; Vorobjev, I.A.; Popkov, V.A.; Babenko, V.A.; Zorova, L.D.; Pevzner, I.B.; Silachev, D.N.; Zorov, S.D.; Andrianova, N.V.; Plotnikov, E.Y. Lessons from the Discovery of Mitochondrial Fragmentation (Fission): A Review and Update. Cells 2019, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Doblado, L.; Lueck, C.; Rey, C.; Samhan-arias, A.K.; Prieto, I.; Stacchiotti, A.; Monsalve, M. Mitophagy in Human Diseases. Int. J. Mol. Sci. 2021, 22, 3903. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Hyde, B.; Shirihai, O.S. Mitochondrial Fusion, Fission and Autophagy as a Quality Control Axis: The Bioenergetic View. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Jendrach, M.; Mai, S.; Pohl, S.; Vöth, M.; Bereiter-Hahn, J. Short- and Long-Term Alterations of Mitochondrial Morphology, Dynamics and MtDNA after Transient Oxidative Stress. Mitochondrion 2008, 8, 293–304. [Google Scholar] [CrossRef]
- Paltauf-Doburzynska, J.; Malli, R.; Graier, W.F. Hyperglycemic Conditions Affect Shape and Ca2+ Homeostasis of Mitochondria in Endothelial Cells. J. Cardiovasc. Pharmacol. 2004, 44, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Giedt, R.J.; Yang, C.; Zweier, J.L.; Matzavinos, A.; Alevriadou, B.R. Mitochondrial Fission in Endothelial Cells after Simulated Ischemia/Reperfusion: Role of Nitric Oxide and Reactive Oxygen Species. Free Radic. Biol. Med. 2012, 52, 348–356. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic. Biol. Med. 2022, 189, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Krafczyk, N.; Lars-Oliver Klotz, L.O. FOXO transcription factors in antioxidant defense. IUBMB Life 2022, 74, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Tirapo, J.; Bayo Jiménez, M.T.; Yuste-García, P.; Cordova, I.; Peñas, A.; García-Borda, F.J.; Quintela, C.; Prieto, I.; Sánchez-Ramos, C.; Ferrero-Herrero, E.; et al. Evaluation of Mitochondrial Function in Blood Samples Shows Distinct Patterns in Subjects with Thyroid Carcinoma from Those with Hyperplasia. Int. J. Mol. Sci. 2023, 24, 6453. [Google Scholar] [CrossRef]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018, 28, 224–236. [Google Scholar] [CrossRef]
- Dott, W.; Mistry, P.; Wright, J.; Cain, K.; Herbert, K.E. Modulation of Mitochondrial Bioenergetics in a Skeletal Muscle Cell Line Model of Mitochondrial Toxicity. Redox Biol. 2014, 2, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Chan, D.K.; Haugrud, A.B.; Miskimins, W.K. Mechanisms by Which Low Glucose Enhances the Cytotoxicity of Metformin to Cancer Cells Both in Vitro and in Vivo. PLoS ONE 2014, 9, e108444. [Google Scholar] [CrossRef] [PubMed]
- Abuarab, N.; Munsey, T.S.; Jiang, L.H.; Li, J.; Sivaprasadarao, A. High Glucose-Induced ROS Activates TRPM2 to Trigger Lysosomal Membrane Permeabilization and Zn2+-Mediated Mitochondrial Fission. Sci. Signal. 2017, 10, eaal4161. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Long, J.; Wang, J.; Haudek, S.B.; Overbeek, P.; Chang, B.H.J.; Schumacker, P.T.; Danesh, F.R. Mitochondrial Fission Triggered by Hyperglycemia Is Mediated by ROCK1 Activation in Podocytes and Endothelial Cells. Cell Metab. 2012, 15, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pan, Q.; Wang, X.; Li, D.; Lin, Y.; Man, F.; Xiao, F.; Guo, L. Impaired Mitochondrial Fusion and Oxidative Phosphorylation Triggered by High Glucose Is Mediated by Tom22 in Endothelial Cells. Oxid. Med. Cell Longev. 2019, 2019, 4508762. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 Represses Nuclear Activation of Antioxidant Responsive Elements by Nrf2 through Binding to the Amino-Terminal Neh2 Domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription Factor Nrf2 Coordinately Regulates a Group of Oxidative Stress-Inducible Genes in Macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Afzal-Ahmed, I.; Mann, G.E.; Shennan, A.H.; Poston, L.; Naftalin, R.J. Preeclampsia Inactivates Glucose-6-Phosphate Dehydrogenase and Impairs the Redox Status of Erythrocytes and Fetal Endothelial Cells. Free Radic. Biol. Med. 2007, 42, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.-S. Longevity Factor FOXO3: A Key Regulator in Aging-Related Vascular Diseases. Front. Cardiovasc. Med. 2021, 8, 778674. [Google Scholar] [CrossRef]
- Peng, C.; Ma, J.; Gao, X.; Tian, P.; Li, W.; Zhang, L. High Glucose Induced Oxidative Stress and Apoptosis in Cardiac Microvascular Endothelial Cells Are Regulated by FoxO3a. PLoS ONE 2013, 8, e79739. [Google Scholar] [CrossRef]
- Chen, J.; Gomes, A.R.; Monteiro, L.J.; Wong, S.Y.; Wu, L.H.; Ng, T.T.; Karadedou, C.T.; Millour, J.; Ip, Y.C.; Cheung, Y.N.; et al. Constitutively Nuclear FOXO3a Localization Predicts Poor Survival and Promotes Akt Phosphorylation in Breast Cancer. PLoS ONE 2010, 5, e12293. [Google Scholar] [CrossRef] [PubMed]
- Hornsveld, M.; Dansen, T.B.; Derksen, P.W.; Burgering, B.M.T. Re-Evaluating the Role of FOXOs in Cancer. Semin. Cancer Biol. 2018, 50, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Tenbaum, S.P.; Ordóñez-Morán, P.; Puig, I.; Chicote, I.; Arqués, O.; Landolfi, S.; Fernández, Y.; Herance, J.R.; Gispert, J.D.; Mendizabal, L.; et al. β-Catenin Confers Resistance to PI3K and AKT Inhibitors and Subverts FOXO3a to Promote Metastasis in Colon Cancer. Nat. Med. 2012, 18, 892–901. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galant, L.S.; Doblado, L.; Radi, R.; de Bem, A.F.; Monsalve, M. Culture of Bovine Aortic Endothelial Cells in Galactose Media Enhances Mitochondrial Plasticity and Changes Redox Sensing, Altering Nrf2 and FOXO3 Levels. Antioxidants 2024, 13, 873. https://doi.org/10.3390/antiox13070873
Galant LS, Doblado L, Radi R, de Bem AF, Monsalve M. Culture of Bovine Aortic Endothelial Cells in Galactose Media Enhances Mitochondrial Plasticity and Changes Redox Sensing, Altering Nrf2 and FOXO3 Levels. Antioxidants. 2024; 13(7):873. https://doi.org/10.3390/antiox13070873
Chicago/Turabian StyleGalant, Leticia Selinger, Laura Doblado, Rafael Radi, Andreza Fabro de Bem, and Maria Monsalve. 2024. "Culture of Bovine Aortic Endothelial Cells in Galactose Media Enhances Mitochondrial Plasticity and Changes Redox Sensing, Altering Nrf2 and FOXO3 Levels" Antioxidants 13, no. 7: 873. https://doi.org/10.3390/antiox13070873
APA StyleGalant, L. S., Doblado, L., Radi, R., de Bem, A. F., & Monsalve, M. (2024). Culture of Bovine Aortic Endothelial Cells in Galactose Media Enhances Mitochondrial Plasticity and Changes Redox Sensing, Altering Nrf2 and FOXO3 Levels. Antioxidants, 13(7), 873. https://doi.org/10.3390/antiox13070873





