Early Life Stress Influences Oxidative Stress Enzyme Activities in Liver, Heart, Kidney, Suprarenal Glands, and Pancreas in Male and Female Rat Pups
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Scheme
2.3. Tissue Homogenate Preparation
2.4. Protein Concentration
2.5. Superoxide Dismutase (SOD) Activity
2.6. Catalase (CAT)
2.7. Glutathione Peroxidase (GPX)
2.8. Glutathione Reductase (GR)
2.9. Glutathione S Transferase (GST)
2.10. Nitric Oxide Synthase (NOS)
2.11. Malondialdehyde
2.12. FRAP (Ferric-Reducing Ability Potential)
2.13. Statistical Analysis
3. Results
- Total Superoxide Dismutase (SOD) activity
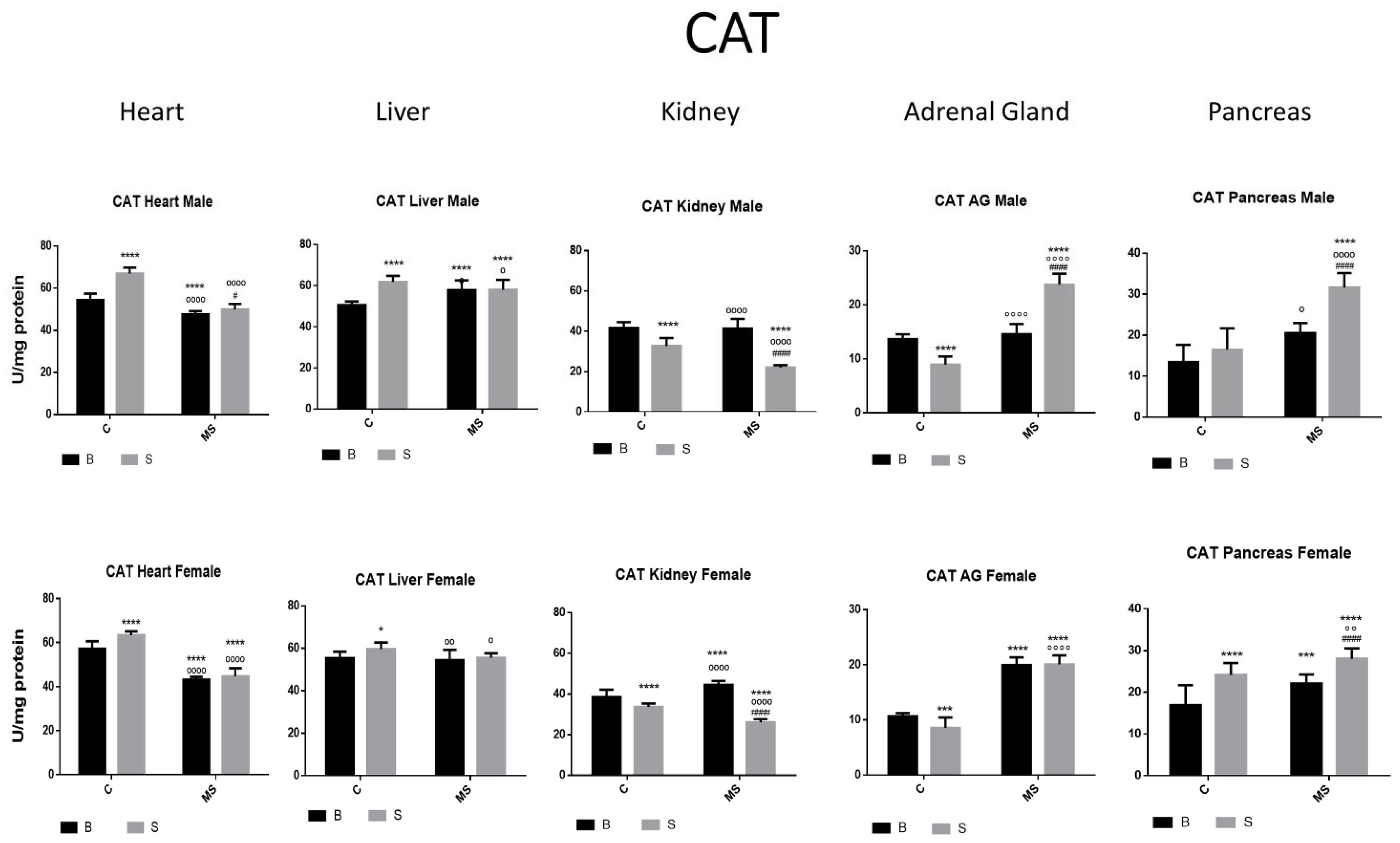
- Catalase (CAT) activity
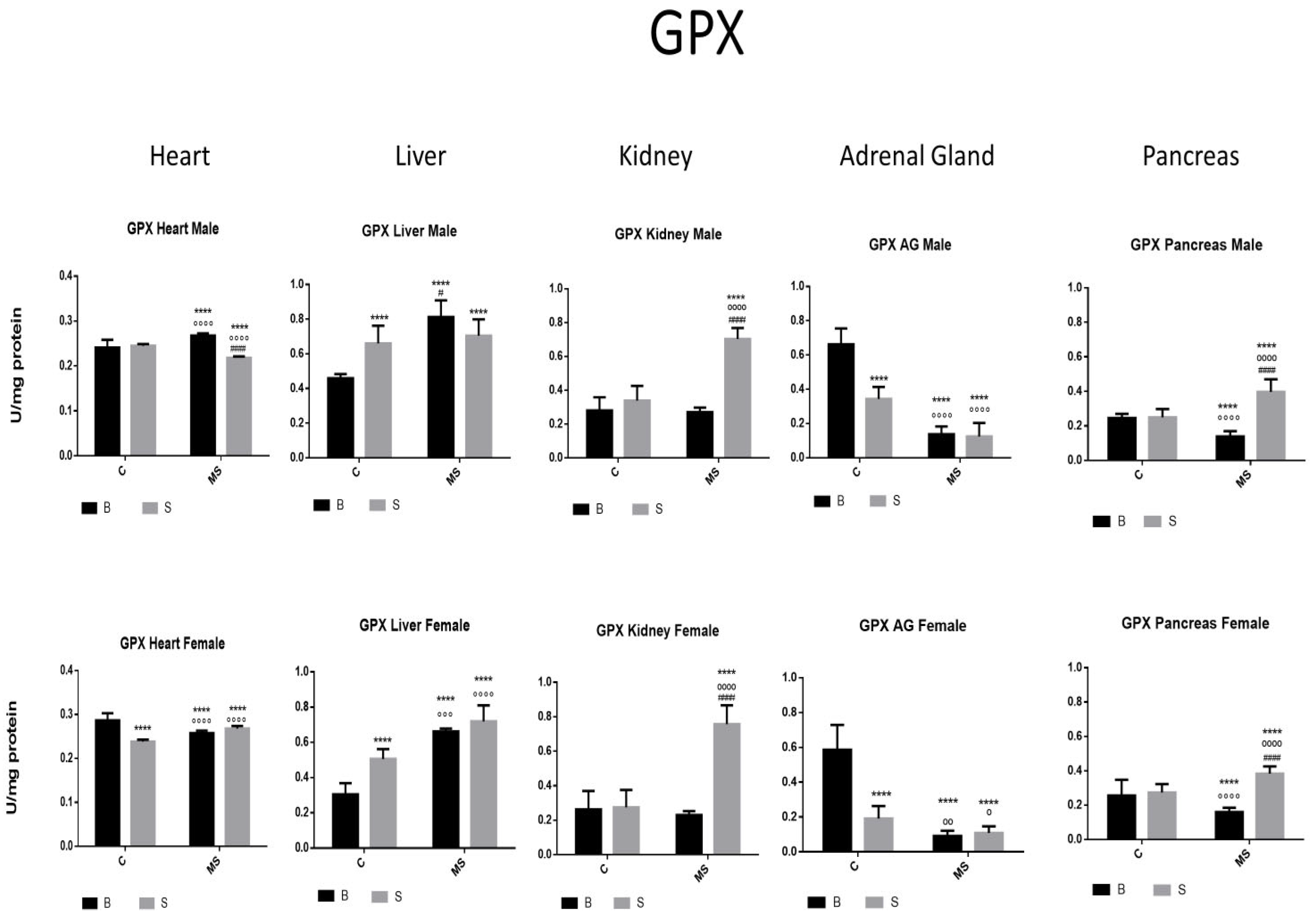
- Glutathione peroxidase (GPX)
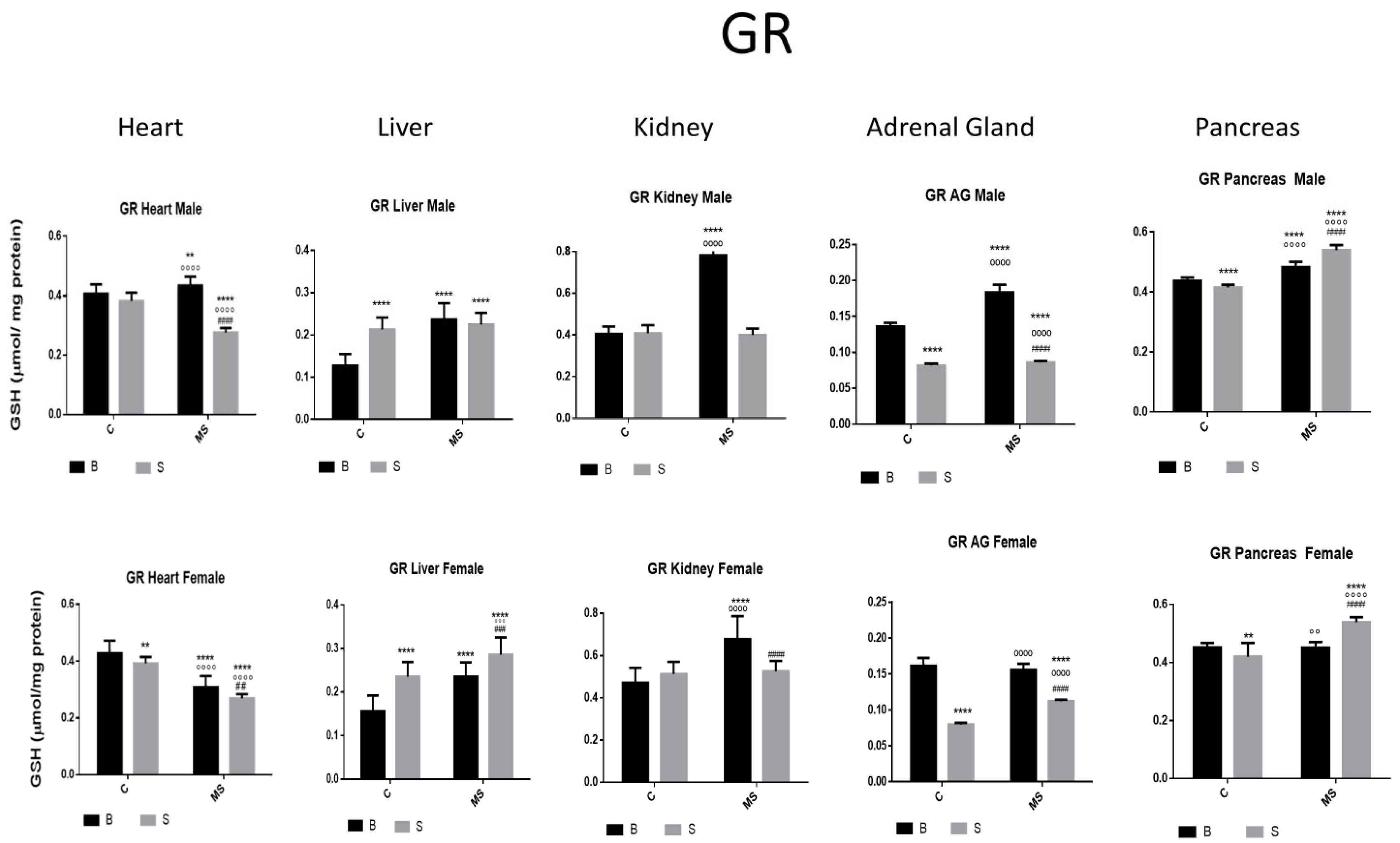
- Glutathione Reductase (GR)
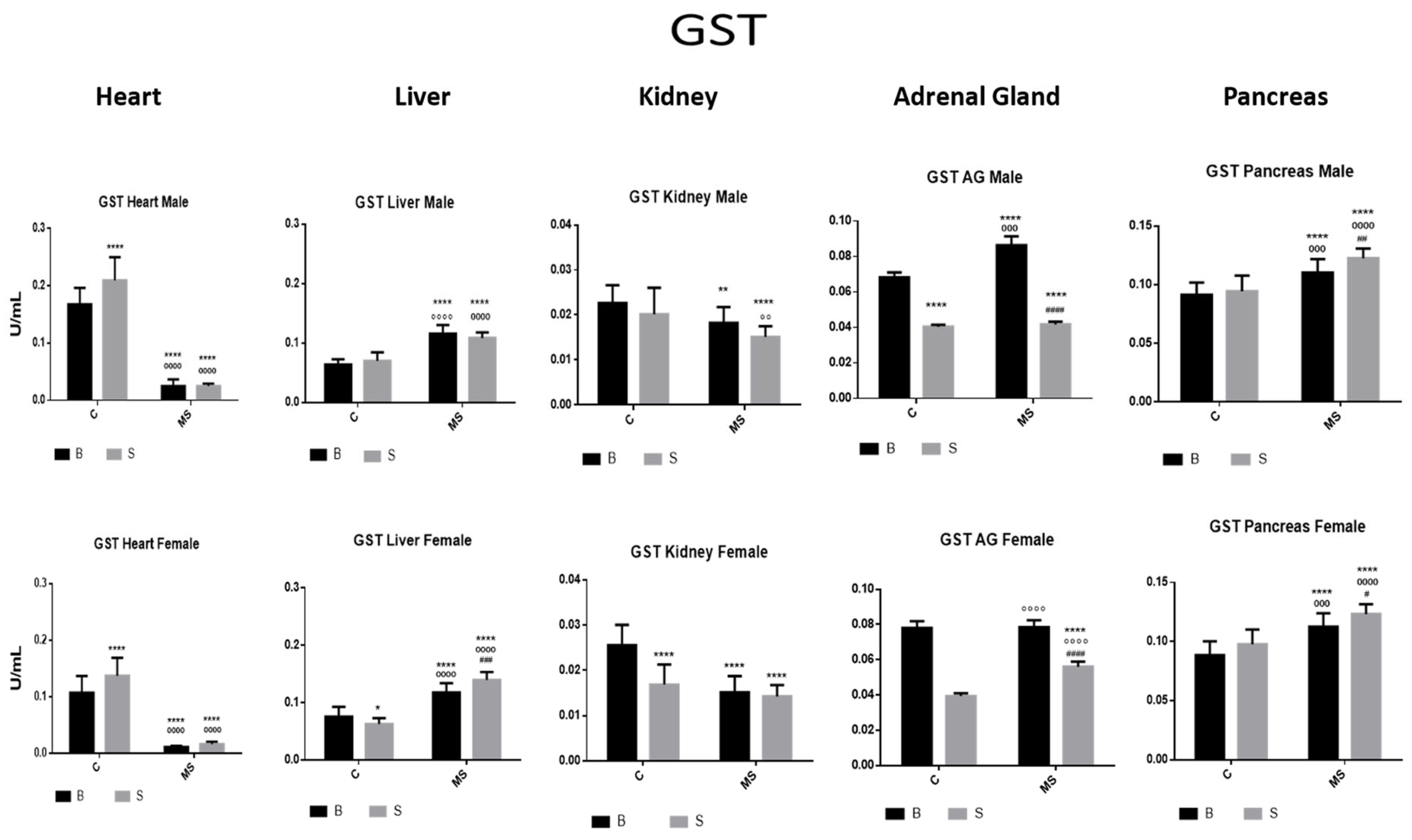
- Glutathione S Transferase (GST)
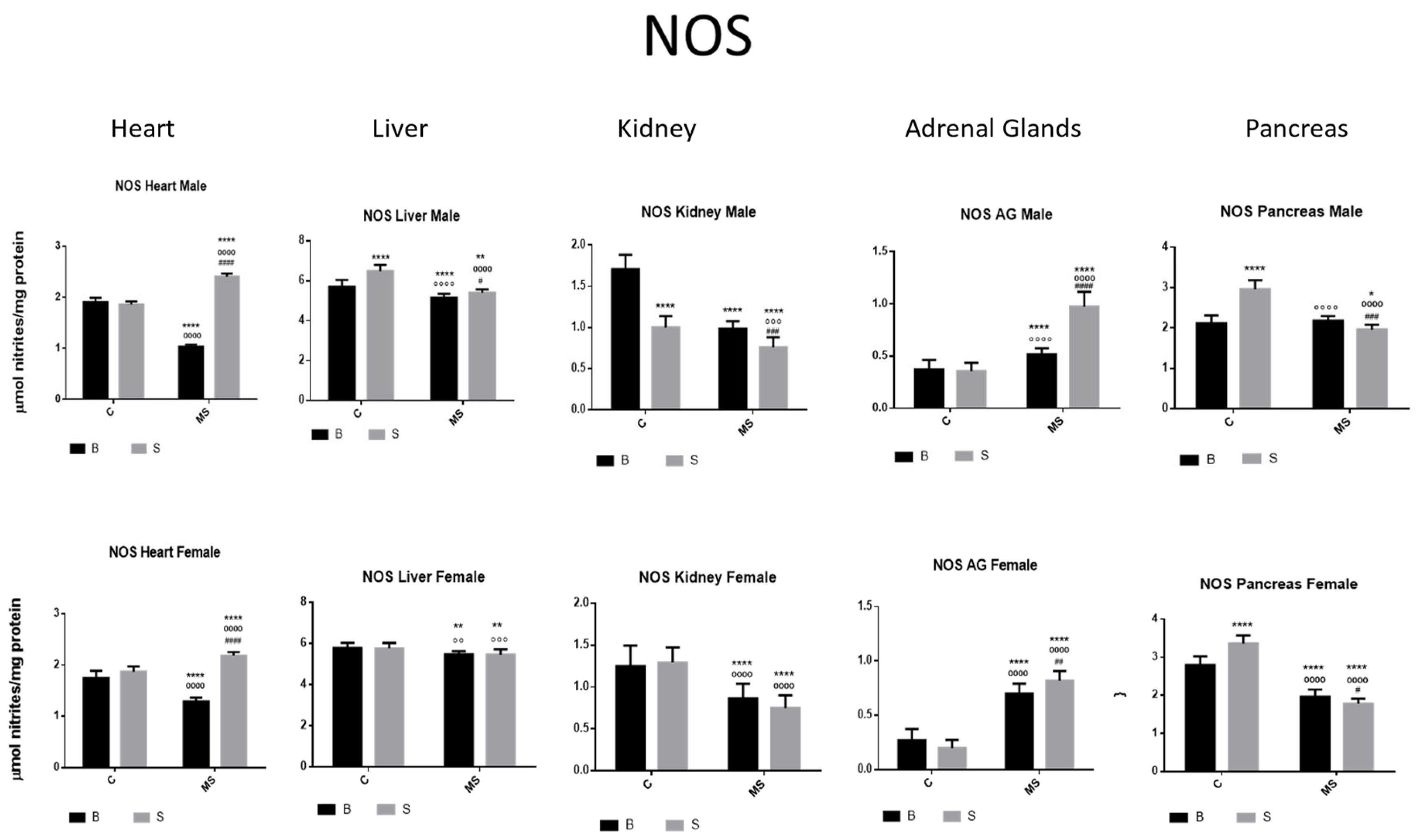
- Nitric Oxide Synthase (NOS)
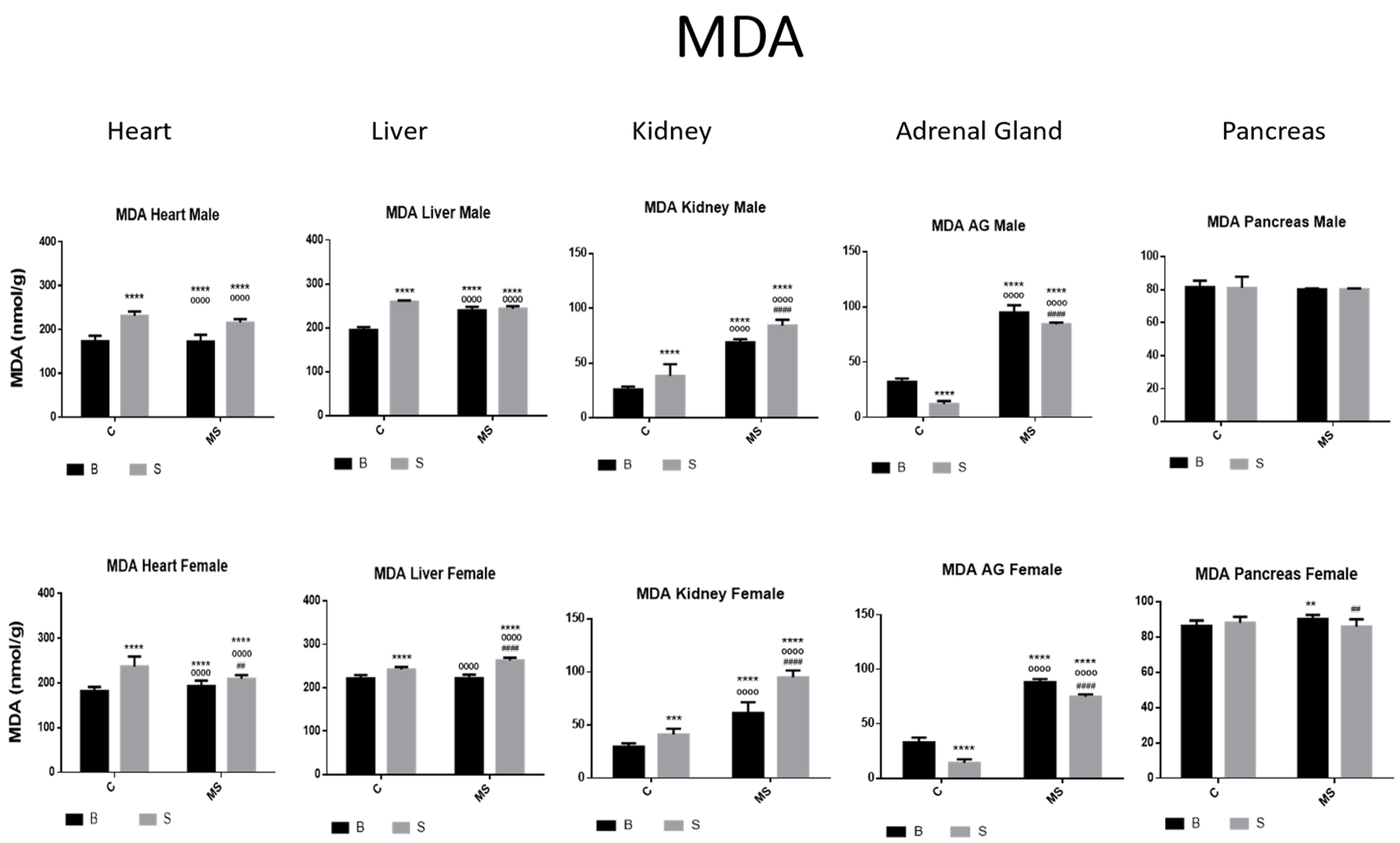
- Malondialdehyde (MDA)
- Ferric-Reducing Ability Potential (FRAP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, M.O.; Cohn, D.M.; Loria, A.S. Developmental origins of cardiovascular disease: Impact of early life stress in humans and rodents. Neurosci. Biobehav. Rev. 2017, 74, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.D.; Farias, J.S.; de Queiroz, D.B.; Cabral, E.V.; Lima-Filho, M.M.; Sant’Helena, B.R.; Aires, R.S.; Ribeiro, V.S.; Santos-Rocha, J.; Xavier, F.E.; et al. Oxidative stress induced by prenatal LPS leads to endothelial dysfunction and renal haemodynamic changes through angiotensin II/NADPH oxidase pathway. BBA—Mol. Basis Dis. 2018, 1864, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Chen, H.J.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2014, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, E.; Daskalakis, N.P.; Agorastos, A. Oxidative Dysregulation in Early Life Stress and Posttraumatic Stress Disorder: A Comprehensive Review. Brain Sci. 2021, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-Y.; Lee, T.-H. Antioxidant enzymes as redox-based biomarkers: A brief review. BMB Rep. 2015, 48, 200–208. Available online: www.bmbreports.org (accessed on 10 April 2023). [CrossRef] [PubMed]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Slot, J.W.; Geuze, H.J.; Crapo, J.D. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J. Cell Biol. 1988, 107, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants—Quo vadis? Trends Pharmacol. Sci. 2011, 32, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Malys, N.; Gaskell, S.J.; Barber, J. Partition and turnover of glutathione reductase from Saccharomyces cerevisiae: A proteomic approach. J. Proteome Res. 2013, 12, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.L.; Olivenza, R.; Moro, M.A.; Lizasoain, I.; Lorenzo, P.; Rodrigo, J.; Leza, J.C. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 2001, 24, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Rasheed, N.; Banu, N.; Palit, G. Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress 2010, 13, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Gawryluk, J.W.; Wang, J.F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased levels of glutathione, the major brain antioxidant, in postmortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sorce, S.; Krause, K.-H. NOX enzymes in the central nervous system: From signaling to disease. Antioxid. Redox Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef] [PubMed]
- Schliess, F.; Görg, B.; Fischer, R.; Desjardins, P.; Bidmon, H.J.; Herrmann, A.; Butterworth, R.F.; Zilles, K.; Häussinger, D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J. 2002, 16, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Stamler, J.S. Enzymatic mechanisms regulating protein S-nitrosylation: Implications in health and disease. J. Mol. Med. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Frossard, J.L.; Quadri, R.; Hadengue, A.; Morel, P.; Pastor, C.M. Endothelial nitric oxide synthase regulation is altered in pancreas from cirrhotic rats. World J. Gastroenterol. 2006, 12, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ghibaudi, M.; Bonfanti, L. How Widespread Are the “Young” Neurons of the Mammalian Brain? Front. Neurosci. 2022, 16, 918616. [Google Scholar] [CrossRef] [PubMed]
- Mount, P.F.; Power, D.A. Nitric oxide in the kidney: Functions and regulation of synthesis. Acta Physiol. 2006, 187, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Afework, M.; Tomlinson, A.; Burnstock, G. Distribution and colocalization of nitric oxide synthase and NADPH-diaphorase in adrenal gland of developing, adult and aging Sprague-Dawley rats. Cell Tissue Res. 1994, 276, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana 062-ZOO. Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio. Diario oficial. 1999. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 10 April 2023).
- Saavedra, P.L.M.; Fenton-Navarro, B.; Torner, L. Early life stress activates glial cells in the hippocampus but attenuates cytokine secretion in response to an immune challenge in rat pups. Neuroimmunomodulation 2018, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ganske, F. BMG LABTECH, Offenburg, Germany E.J. Dell, BMG LABTECH, Durham, USA. Bradford Assay Performed on BMG LABTECH Microplate Readers. 2024. Available online: https://www.thermofisher.com/mx/es/home/life-science/protein-biology/protein-assays-analysis/protein-assays/bradford-assays.html (accessed on 12 July 2023).
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y. Effect of temperature preconditioning on catalase, peroxidase, and superoxide dismutase in chilled zucchini squash. Postharvest. Biol. Technol. 1994, 5, 67–76. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalase and Peroxidase. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B. The isozymes of Glutatione Transferase. Adv. Enzymol. Relat. Areas Mol. Biol. 1985, 57, 357–417. [Google Scholar] [CrossRef] [PubMed]
- Huari Marín, G.; Jiménez Rodríguez, Y.; Julca Majo, A.; Lpoez Díaz, S.; Llapo Miñano, A.; Mendoza Sicche, J.; Luis Reyna, M.; Becerra Urquizo, A.; Guzmán Ruiz, L.; Silva Correa, C. Effect of Latrodectus mactans venom in plasma nitric oxide levels and sexual behavior in Oryctolagus cunniculus. Rev. Pharm. 2014, 2, 24–31. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Alireza Rezaeizadeh, A.R.; Zuki, A.B.Z.; Maryam Abdollahi, M.A.; Meng GohYong, M.G.; Noordin Mohamed Mustapha, N.M.M.; Muhajir, H.; Tengku Azmi, T.I. Antioxidant and antihyperglycaemic effects of an aqueous extract from Momordica charantia fruit in a type II diabetic rat model. J. Med. Plants Res. 2011, 5, 2990–3001. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘‘Antioxidant Power’’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Habibi, E.; Arab-Nozari, M.; Elahi, P.; Ghasemi, M.; Shaki, F. Modulatory effects of Viola odorata flower and leaf extracts upon oxidative stress-related damage in an experimental model of ethanol-induced hepatotoxicity. Appl. Physiol. Nutr. Metab. 2019, 44, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Courtiol, E.; Wilson, D.A.; Shah, R.; Sullivan, R.M.; Teixeira, C.M. Maternal Regulation of Pups’ Cortical Activity: Role of Serotonergic Signaling. eNeuro 2018, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.; Marasco, V.; Møller, A.P. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2011, 181, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peña, C.J.; Kronman, H.G.; Walker, D.M.; Cates, H.M.; Bagot, R.C.; Purushothaman, I.; Issler, O.; Loh, Y.-H.E.; Leong, T.; Kiraly, D.D.; et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 2017, 356, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; Umeoka, E.H.L. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Tank, A.W.; Lee, W.D. Peripheral and central effects of circulating catecholamines. Compr. Physiol. 2015, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Mousikou, M.; Kyriakou, A.; Skordis, N. Stress and Growth in Children and Adolescents. Horm. Res. Paediatr. 2023, 96, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Geerling, J.C.; Shin, J.W.; Chimenti, P.C.; Loewy, A.D. Paraventricular hypothalamic nucleus: Axonal projections to the brainstem. J. Comp. Neurol. 2010, 518, 1460–1499. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; McEwen, B.S. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nat. Neurosci. 2012, 15, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Mccorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R. Functional profile of the binary brain corticosteroid receptor system: Mediating, multitasking, coordinating, integrating. Eur. J. Pharmacol. 2013, 719, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yu, Y.; Chung, R.K.; Lian, X.; Wang, X.; Cheung, W.M.; Tsang, H.W.H. The relationship between liver function and neurophysiological factors in depressed individuals: A cross-sectional study using an integrated “East meets West” medicine approach. Front. Psychiatry 2023, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, K.L.K.; Sakai, R.R. Stress and Energy Homeostasis. Encycl. Behav. Neurosci. 2010, 322–327. [Google Scholar] [CrossRef]
- Breen, M.S.; Beliakova-Bethell, N.; Mujica-Parodi, L.R.; Carlson, J.M.; Ensign, W.Y.; Woelk, C.H.; Rana, B.K. Acute psychological stress induces short-term variable immune response. Brain Behav. Immun. 2016, 53, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Diduk, R.; Galano, A. Adrenaline and Noradrenaline: Protectors against Oxidative Stress or Molecular Targets? J. Phys. Chem. B 2015, 119, 3335–3620. [Google Scholar] [CrossRef] [PubMed]
- Gagan, B.N.; Chainy, G.B.; Kumar, D.S. Hormones and oxidative stress: An overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef]
- Bayo, J.M.T.; Frenisb, K.; Hahada, O.; Stevena, S.; Guy Cohene, G.; Cuadradog, A.; Münzela, T.; Daibera, A. Protective actions of nuclear factor erythroid 2-related factor 2 (NRF2) and downstream pathways against environmental stressor. Free Radic. Biol. Med. 2022, 187, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, D.; Rai, P.K.; Mehta, S.; Chatterji, S.; Shukla, S.; Rai, D.K.; Sharma, G.; Sharma, B.; Khair, S.; Watal, G. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac. J. Trop. Med. 2013, 6, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Shakya, G.; Charanraj Goud, A.; Pajaniradje, S.; Rajagopalan, R. Protective role of wheatgrass on oxidative stress in streptozotocin induced type 2 diabetic rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 415–423. Available online: https://www.researchgate.net/publication/285202031 (accessed on 18 March 2024).
- Bhanot, R.; Singh Hundal, S. Effect of untreated sewage water on antioxidant enzymes of fish Labeo rohita. Int. J. Chem. Stud. 2019, 7, 3111–3117. [Google Scholar]
- Baş, H.; Apaydin, F.G.; Kalender, S.; Kalender, Y. Lead nitrate and cadmium chloride induced hepatotoxicity and nephrotoxicity: Protective effects of sesamol on biochemical indices and pathological changes. J. Food Biochem. 2021, 45, e13769. [Google Scholar] [CrossRef]
- Ajjuri, R.R.; O’Donnell, J.M. Novel Whole-tissue Quantitative Assay of Nitric Oxide Levels in Drosophila Neuroinflammatory Response. J. Vis. Exp. 2013, 82, e50892. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Bjelaković, G.; Beninati, S.; Pavlović, D.; Kocić, G.; Jevtović, T.; Kamenov, Β.; Šaranac, L.J.; Bjelaković, B.; Stojanović, I.; Bašić, J. Glucocorticoids and oxidative stress. JBPCC J. Basic Clin. Physiol. Pharmacol. 2007, 18, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Birnie-Gauvin, K.; Peiman, K.S.; Larsen, M.H.; Aarestrup, K.; Willmore, W.G.; Cooke, S.J. Shortterm and long-term effects of transient exogenous cortisol manipulation on oxidative stress in juvenile brown trout. J. Exp. Biol. 2017, 220, 1693–1700. [Google Scholar] [PubMed]
- Tan, H.; Zhou, H.; Chen, J.; Ren, H.; Guo, Y.; Jiang, X. Association of early life adversity with cardiovascular disease and its potential mechanisms: A narrative review. Front. Public Health 2024, 12, 1341266. [Google Scholar] [CrossRef] [PubMed]
- Marais, L.; van Rensburg, S.J.; van Zyl, J.M.; Stein, D.J.; Daniels, W.M.U. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci. Res. 2008, 61, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, F.; Zardooz, H.; Khodagholi, F.; Hedayati, M. Maternal separation aggravated pancreatic oxidative and inflammatory damage in chronic socially defeated adult male rats. Diabetes Res. Clin. Pract. 2022, 185S, 109602. [Google Scholar] [CrossRef]
- Nishida, M.; Ishikawa, T.; Saiki, S.; Sunggip, C.; Aritomi, S.; Harada, E.; Kuwahara, K.; Hirano, K.; Mori, Y.; Kim-Mitsuyama, S. Voltage-dependent N-type Ca2+ channels in endothelial cells contribute to oxidative stress-related endothelial dysfunction induced by angiotensin II in mice. Biochem. Biophys. Res. Commun. 2013, 434, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Mei, Y.; Weisbrod, R.M.; Silver, M.; Shukla, P.C.; Bolotina, V.M.; Cohen, R.A.; Tong, X. Glutathione adducts on sarcoplasmic/endoplasmic reticulum Ca2+ ATPase Cys-674 regulate endothelial cell calcium stores and angiogenic function as well as promote ischemic blood flow recovery. J. Biol. Chem. 2014, 289, 19907–19916. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem. Soc. Trans. 2007, 35, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.C.; Sosnowski, D.W.; Marchesoni, J.; Grenier, C.; Thorp, J.; Murphy, S.K.; Johnson, S.B.; Schlief, B.; Hoyo, C. Maternal adverse childhood experiences (ACEs) and offspring imprinted gene DMR methylation at birth. Epigenetics 2024, 19, 2293412. [Google Scholar] [CrossRef] [PubMed]
- Silberman, D.M.; Acosta, G.B.; Zorrilla Zubilete, M.A. Long-term effects of early life stress exposure: Role of epigenetic mechanisms. Pharmacol. Res. 2016, 109, 64–73. [Google Scholar] [CrossRef] [PubMed]

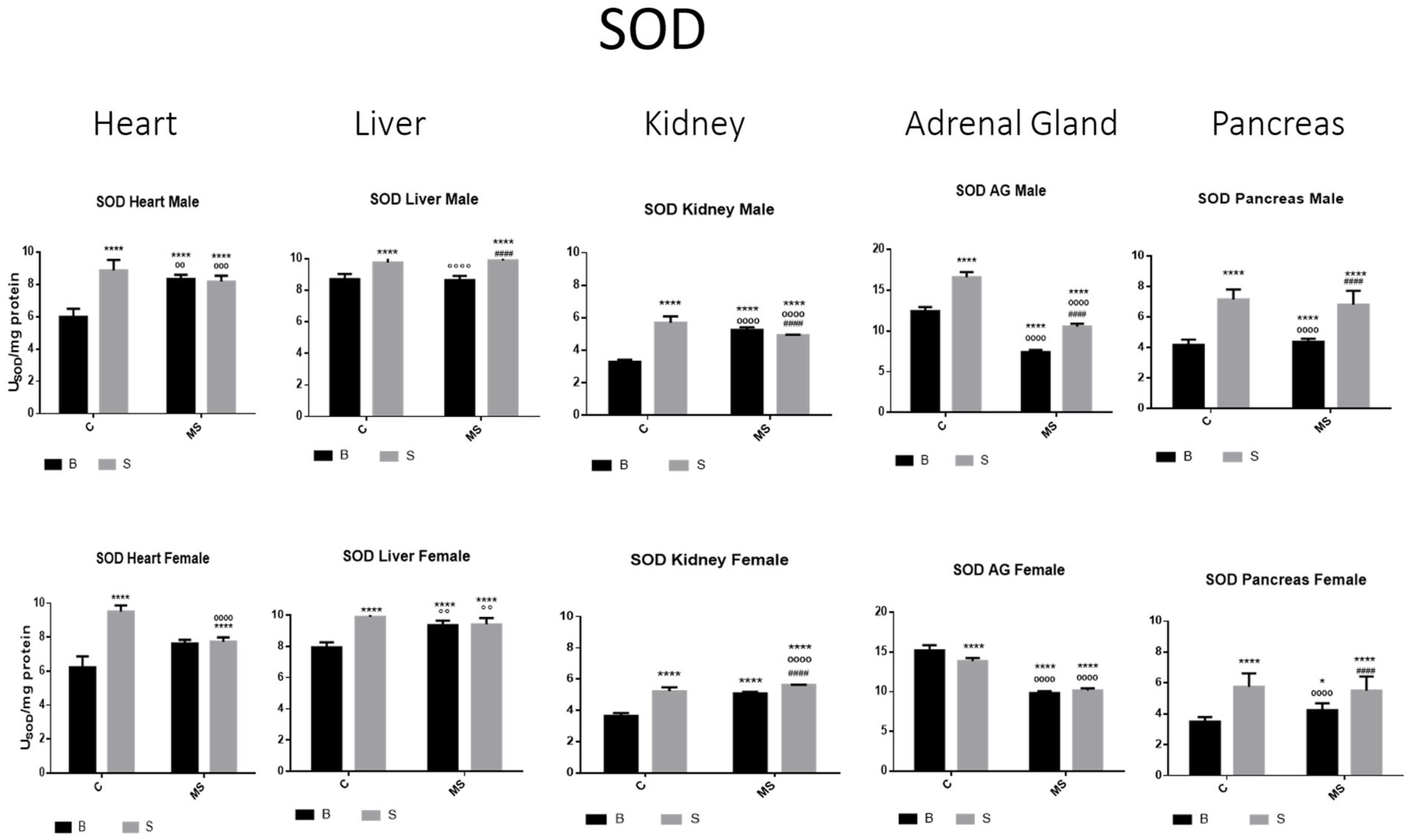

| A | ||||
| SOD | ||||
| Factor 1 | Factor 2 | Factor 3 | ||
| Treatment | Euthanasia | Gender | Interaction | |
| Heart | ||||
| Male | F (1, 76) = 170.7, p < 0.0001 | F (1, 76) = 64.51, p < 0.0001 | NS | F (1, 76) = 215.8, p < 0.0001 |
| Female | F (1, 68) = 314.0, p < 0.0001 | NS | NS | F (1, 68) = 273.8, p < 0.0001 |
| Liver | ||||
| Male | F (1, 64) = 160.3, p < 0.0001 | NS | NS | NS |
| Female | F (1, 54) = 116.5, p < 0.0001 | F (1, 54) = 26.10, p < 0.0001 | NS | F (1, 54) = 104.9, p < 0.0001 |
| Kidney | ||||
| Male | F (1, 64) = 448.2, p < 0.0001 | F (1, 64) = 152.3, p < 0.0001 | NS | F (1, 64) = 775.3, p < 0.0001 |
| Female | F (1, 54) = 628.0, p < 0.0001 | F (1, 54) = 470.2, p < 0.0001 | NS | F (1, 54) = 148.0, p < 0.0001 |
| Adrenal Gland | ||||
| Male | F (1, 76) = 1222, p < 0.0001 | F (1, 76) = 2842, p < 0.0001 | NS | F (1, 76) = 24.84, p < 0.0001 |
| Female | F (1, 68) = 27.49, p < 0.0001 | F (1, 68) = 2219, p < 0.0001 | NS | F (1, 68) = 73.57, p < 0.0001 |
| Pancreas | ||||
| Male | F (1, 76) = 424.0, p < 0.0001 | NS | NS | F (1, 76) = 4.275, p = 0.0421 |
| Female | F (1, 68) = 108.0, p < 0.0001 | NS | NS | F (1, 68) = 8.316, p = 0.0053 |
| B | ||||
| CAT | ||||
| Factor 1 | Factor 2 | Factor 3 | ||
| Treatment | Euthanasia | Gender | Interaction | |
| Heart | ||||
| Male | F (1, 76) = 158.7, p < 0.0001 | F (1, 76) = 406.2, p < 0.0001 | NS | F (1, 76) = 75.63, p < 0.0001 |
| Female | F (1, 68) = 32.76, p < 0.0001 | F (1, 68) = 617.0, p < 0.0001 | NS | F (1, 68) = 12.77, p < 0.0007 |
| Liver | ||||
| Male | F (1, 32) = 19.30, p = 0.0001 | NS | NS | F (1, 32) = 18.29, p = 0.0002 |
| Female | F (1, 40) = 6.978, p = 0.0117 | F (1, 40) = 6.377, p = 0.0156 | NS | F (1, 40) = 2.315, p = 0.1360 |
| Kidney | ||||
| Male | F (1, 64) = 314.2, p < 0.0001 | F (1, 64) = 27.75, p < 0.0001 | NS | F (1, 64) = 57.50, p < 0.0001 |
| Female | F (1, 54) = 362.1, p < 0.0001 | NS | NS | F (1, 54) = 132.0, p < 0.0001 |
| Adrenal Gland | ||||
| Male | F (1, 76) = 37.37, p < 0.0001 | F (1, 76) = 460.0, p < 0.0001 | NS | F (1, 76) = 360.7, p < 0.0001 |
| Female | F (1, 68) = 8.168, p = 0.0057 | F (1, 68) = 855.5, p < 0.0001 | NS | F (1, 68) = 9.710, p = 0.0027 |
| Pancreas | ||||
| Male | F (1, 76) = 63.12, p < 0.0001 | F (1, 76) = 157.1, p < 0.0001 | NS | F (1, 76) = 20.70, p < 0.0001 |
| Female | F (1, 68) = 76.72, p < 0.0001 | F (1, 68) = 35.77, p < 0.0001 | NS | NS |
| A | ||||
| GPX | ||||
| Factor 1 | Factor 2 | Factor 3 | ||
| Treatment | Euthanasia | Gender | Interaction | |
| Heart | ||||
| Male | F (1, 76) = 745.4, p < 0.0001 | F (1, 76) = 304.2, p < 0.0001 | NS | F (1, 76) = 841.4, p < 0.0001 |
| Female | F (1, 68) = 81.53, p < 0.0001 | NS | NS | F (1, 68) = 194.9, p < 0.0001 |
| Liver | ||||
| Male | NS | F (1, 28) = 44.03, p < 0.0001 | NS | F (1, 28) = 26.69, p < 0.0001 |
| Female | F (1, 33) = 20.07, p < 0.0001 | F (1, 33) = 97.54, p < 0.0001 | NS | F (1, 33) = 6.239, p = 0.0177 |
| Kidney | ||||
| Male | F (1, 64) = 197.2, p < 0.0001 | F (1, 64) = 103.0, p < 0.0001 | NS | F (1, 64) = 114.8, p < 0.0001 |
| Female | F (1, 54) = 110.9, p < 0.0001 | F (1, 54) = 77.42, p < 0.0001 | NS | F (1, 54) = 101.6, p < 0.0001 |
| Adrenal Gland | ||||
| Male | F (1, 76) = 43.24, p < 0.0001 | F (1, 76) = 216.7, p < 0.0001 | NS | F (1, 76) = 36.39, p < 0.0001 |
| Female | F (1, 68) = 97.29, p < 0.0001 | F (1, 68) = 226.7, p < 0.0001 | NS | F (1, 68) = 115.7, p < 0.0001 |
| Pancreas | ||||
| Male | F (1, 76) = 152.8, p < 0.0001 | NS | NS | F (1, 76) = 142.5, p < 0.0001 |
| Female | F (1, 68) = 81.43, p < 0.0001 | NS | NS | F (1, 68) = 59.27, p < 0.0001 |
| B | ||||
| GR | ||||
| Factor 1 | Factor 2 | Factor 3 | ||
| Treatment | Euthanasia | Gender | Interaction | |
| Heart | ||||
| Male | F (1, 76) = 38.44, p < 0.0001 | F (1, 76) = 35.57, p < 0.0001 | NS | F (1, 76) = 121.9, p < 0.0001 |
| Female | F (1, 68) = 19.90, p < 0.0001 | F (1, 68) = 22.54, p < 0.0001 | NS | NS |
| Liver | ||||
| Male | F (1, 74) = 274.1, p < 0.0001 | F (1, 74) = 7.397, p = 0.0081 | NS | F (1, 74) = 19.47, p < 0.0001 |
| Female | F (1, 66) = 301.4, p < 0.0001 | F (1, 66) = 4.934, p = 0.0298 | NS | F (1, 66) = 64.92, p < 0.0001 |
| Kidney | ||||
| Male | F (1, 64) = 193.0, p < 0.0001 | F (1, 64) = 181.4, p < 0.0001 | NS | F (1, 64) = 199.1, p < 0.0001 |
| Female | F (1, 54) = 9.826, p = 0.0028 | F (1, 54) = 38.73, p < 0.0001 | NS | F (1, 54) = 30.48, p < 0.0001 |
| Adrenal Gland | ||||
| Male | F (1, 76) = 2889, p < 0.0001 | F (1, 76) = 336.7, p < 0.0001 | NS | F (1, 76) = 237.7, p < 0.0001 |
| Female | F (1, 68) = 1561, p < 0.0001 | F (1, 68) = 71.25, p < 0.0001 | NS | F (1, 68) = 146.5, p < 0.0001 |
| Pancreas | ||||
| Male | F (1, 76) = 2.902, p = 0.0925 | F (1, 76) = 75.19, p < 0.0001 | NS | F (1, 76) = 16.26, p = 0.0001 |
| Female | F (1, 68) = 15.81, p = 0.0002 | F (1, 68) = 73.68, p < 0.0001 | NS | F (1, 68) = 74.69, p < 0.0001 |
| A | ||||
| GST | ||||
| Factor 1 | Factor 2 | Factor 3 | ||
| Treatment | Euthanasia | Gender | Interaction | |
| Heart | ||||
| Male | F (1, 76) = 13.93, p = 0.0004 | F (1, 76) = 858.0, p < 0.0001 | NS | F (1, 76) = 14.01, p = 0.0004 |
| Female | F (1, 68) = 67.55, p < 0.0001 | F (1, 68) = 465.3, p < 0.0001 | NS | F (1, 68) = 67.71, p < 0.0001 |
| Liver | ||||
| Male | NS | F (1, 76) = 292.1, p < 0.0001 | NS | F (1, 76) = 6.485, p = 0.0129 |
| Female | NS | F (1, 68) = 312.7, p < 0.0001 | NS | F (1, 68) = 26.43, p < 0.0001 |
| Kidney | ||||
| Male | F (1, 64) = 8.369, p = 0.0052 | F (1, 64) = 23.44, p < 0.0001 | NS | NS |
| Female | F (1, 54) = 17.88, p < 0.0001 | F (1, 54) = 32.87, p < 0.0001 | NS | F (1, 54) = 11.79, p = 0.0011 |
| Adrenal Gland | ||||
| Male | F (1, 76) = 49.68, p < 0.0001 | NS | NS | NS |
| Female | F (1, 68) = 14.07, p = 0.0004 | NS | NS | NS |
| Pancreas | ||||
| Male | F (1, 76) = 9.529, p = 0.0028 | F (1, 76) = 93.09, p < 0.0001 | NS | NS |
| Female | F (1, 68) = 14.88, p = 0.0003 | F (1, 68) = 91.27, p < 0.0001 | NS | NS |
| B | ||||
| NOS | ||||
| Factor 1 | Factor 2 | Factor 3 | ||
| Treatment | Euthanasia | Gender | Interaction | |
| Heart | ||||
| Male | F (1, 74) = 14.31, p = 0.0003 | F (1, 74) = 63.35, p < 0.0001 | NS | NS |
| Female | F (1, 66) = 9.204, p = 0.0035 | F (1, 66) = 53.84, p < 0.0001 | NS | NS |
| Liver | ||||
| Male | NS | F (1, 74) = 40.64, p < 0.0001 | NS | F (1, 74) = 353.3, p < 0.0001 |
| Female | NS | NS | NS | NS |
| Kidney | ||||
| Male | F (1, 64) = 311.1, p < 0.0001 | F (1, 64) = 275.9, p < 0.0001 | NS | F (1, 64) = 71.66, p < 0.0001 |
| Female | NS | F (1, 54) = 102.2, p < 0.0001 | NS | NS |
| Adrenal Gland | ||||
| Male | NS | NS | NS | NS |
| Female | NS | NS | NS | NS |
| Pancreas | ||||
| Male | F (1, 76) = 370.1, p < 0.0001 | F (1, 76) = 285.4, p < 0.0001 | NS | F (1, 76) = 90.65, p < 0.0001 |
| Female | F (1, 68) = 5.207, p = 0.0256 | F (1, 68) = 71.21, p < 0.0001 | NS | NS |
| A | ||||
| MDA | ||||
| Factor 1 | Factor 2 | Factor 3 | ||
| Treatment | Euthanasia | Gender | Interaction | |
| Heart | ||||
| Male | F (1, 76) = 98.47, p < 0.0001 | F (1, 76) = 594.5, p < 0.0001 | NS | F (1, 76) = 61.47, p < 0.0001 |
| Female | F (1, 68) = 40.28, p < 0.0001 | F (1, 68) = 383.7, p < 0.0001 | NS | F (1, 68) = 91.20, p < 0.0001 |
| Liver | ||||
| Male | F (1, 76) = 54.75, p < 0.0001 | F (1, 76) = 413.4, p < 0.0001 | NS | F (1, 76) = 54.56, p < 0.0001 |
| Female | F (1, 68) = 20.19, p < 0.0001 | F (1, 68) = 126.1, p < 0.0001 | NS | F (1, 68) = 160.5, p < 0.0001 |
| Kidney | ||||
| Male | F (1, 64) = 100.0, p < 0.0001 | F (1, 64) = 1036, p < 0.0001 | NS | NS |
| Female | F (1, 54) = 157.3, p < 0.0001 | F (1, 54) = 570.7, p < 0.0001 | NS | F (1, 54) = 37.56, p < 0.0001 |
| Adrenal Gland | ||||
| Male | F (1, 76) = 300.1, p < 0.0001 | F (1, 76) = 5804, p < 0.0001 | NS | F (1, 76) = 26.93, p < 0.0001 |
| Female | F (1, 68) = 439.3, p < 0.0001 | F (1, 68) = 5699, p < 0.0001 | NS | F (1, 68) = 12.70, p = 0.0007 |
| Pancreas | ||||
| Male | F (1, 76) = 21.60, p < 0.0001 | F (1, 76) = 1690, p < 0.0001 | NS | F (1, 76) = 57.87, p < 0.0001 |
| Female | F (1, 68) = 156.9, p < 0.0001 | F (1, 68) = 647.1, p < 0.0001 | NS | F (1, 68) = 18.52, p < 0.0001 |
| B | ||||
| FRAP | ||||
| Factor 1 | Factor 2 | Factor 3 | ||
| Treatment | Euthanasia | Gender | Interaction | |
| Heart | ||||
| Male | F (1, 76) = 237.1, p < 0.0001 | F (1, 76) = 106.0, p < 0.0001 | NS | F (1, 76) = 96.81, p < 0.0001 |
| Female | F (1, 68) = 22.77, p < 0.0001 | F (1, 68) = 269.5, p < 0.0001 | NS | F (1, 68) = 175.5, p < 0.0001 |
| Liver | ||||
| Male | F (1, 76) = 235.1, p < 0.0001 | F (1, 76) = 165.0, p < 0.0001 | NS | F (1, 76) = 150.5, p < 0.0001 |
| Female | F (1, 68) = 36.95, p < 0.0001 | F (1, 68) = 55.07, p < 0.0001 | NS | F (1, 68) = 76.85, p < 0.0001 |
| Kidney | ||||
| Male | F (1, 64) = 181.9, p < 0.0001 | F (1, 64) = 115.2, p < 0.0001 | NS | F (1, 64) = 6.096, p = 0.0162 |
| Female | F (1, 54) = 8.938, p = 0.0042 | F (1, 54) = 9.625, p = 0.0031 | NS | NS |
| Adrenal Gland | ||||
| Male | F (1, 76) = 226.4, p < 0.0001 | F (1, 76) = 390.8, p < 0.0001 | NS | F (1, 76) = 134.7, p < 0.0001 |
| Female | F (1, 68) = 21.24, p < 0.0001 | F (1, 68) = 55.94, p < 0.0001 | NS | F (1, 68) = 56.44, p < 0.0001 |
| Pancreas | ||||
| Male | F (1, 76) = 15.82, p = 0.0002 | F (1, 76) = 158.0, p < 0.0001 | NS | F (1, 76) = 75.20, p < 0.0001 |
| Female | NS | F (1, 68) = 47.23, p < 0.0001 | NS | F (1, 68) = 29.28, p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenton Navarro, B.; Casimiro Aguayo, A.A.; Torres Gómez, Y.L.; Cervantes Alfaro, M.; Torner, L. Early Life Stress Influences Oxidative Stress Enzyme Activities in Liver, Heart, Kidney, Suprarenal Glands, and Pancreas in Male and Female Rat Pups. Antioxidants 2024, 13, 802. https://doi.org/10.3390/antiox13070802
Fenton Navarro B, Casimiro Aguayo AA, Torres Gómez YL, Cervantes Alfaro M, Torner L. Early Life Stress Influences Oxidative Stress Enzyme Activities in Liver, Heart, Kidney, Suprarenal Glands, and Pancreas in Male and Female Rat Pups. Antioxidants. 2024; 13(7):802. https://doi.org/10.3390/antiox13070802
Chicago/Turabian StyleFenton Navarro, Bertha, Alexis Abraham Casimiro Aguayo, Yayr Luis Torres Gómez, Miguel Cervantes Alfaro, and Luz Torner. 2024. "Early Life Stress Influences Oxidative Stress Enzyme Activities in Liver, Heart, Kidney, Suprarenal Glands, and Pancreas in Male and Female Rat Pups" Antioxidants 13, no. 7: 802. https://doi.org/10.3390/antiox13070802
APA StyleFenton Navarro, B., Casimiro Aguayo, A. A., Torres Gómez, Y. L., Cervantes Alfaro, M., & Torner, L. (2024). Early Life Stress Influences Oxidative Stress Enzyme Activities in Liver, Heart, Kidney, Suprarenal Glands, and Pancreas in Male and Female Rat Pups. Antioxidants, 13(7), 802. https://doi.org/10.3390/antiox13070802






