Uncovering the Role of Selenite and Selenium Nanoparticles (SeNPs) in Adolescent Rat Adipose Tissue beyond Oxidative Balance: Transcriptomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. SeNPs Synthesis
2.3. Sample Harvesting and Tissue Homogenization
2.4. Oxidative Balance in WAT
2.4.1. Activity of Antioxidant Enzymes
2.4.2. Oxidative Stress Markers: Malondialdehyde and H2O2 Levels
2.5. Statistical Analysis
2.6. Transcriptomic Analysis
2.6.1. Isolation of RNA from Lipid Tissue
2.6.2. RNA Sequencing and Quality Control
2.6.3. Reads Mapping to the Reference Genome and Quantification of Gene Expression Level
2.6.4. Differential Gene Expression Analysis
2.6.5. Functional Analysis
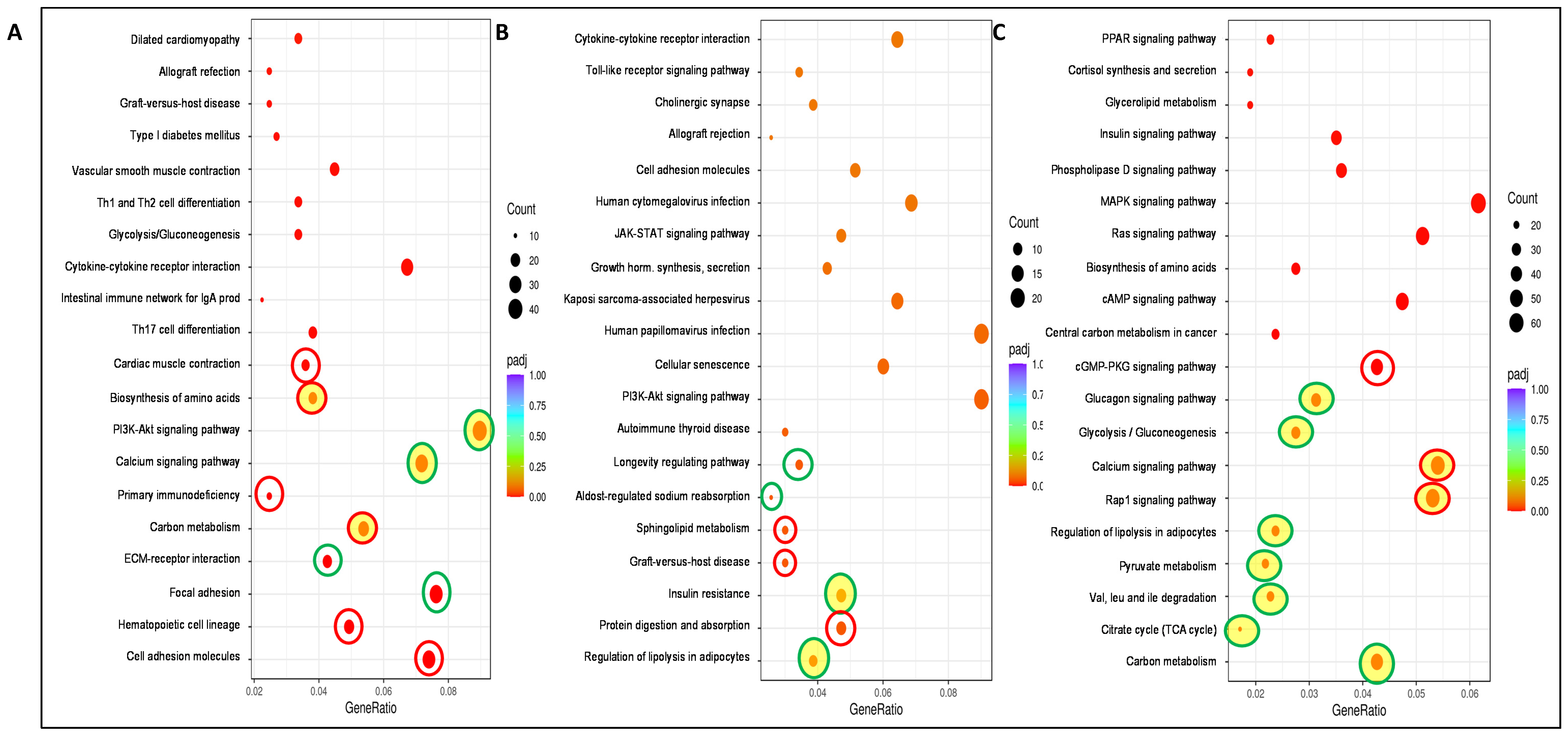
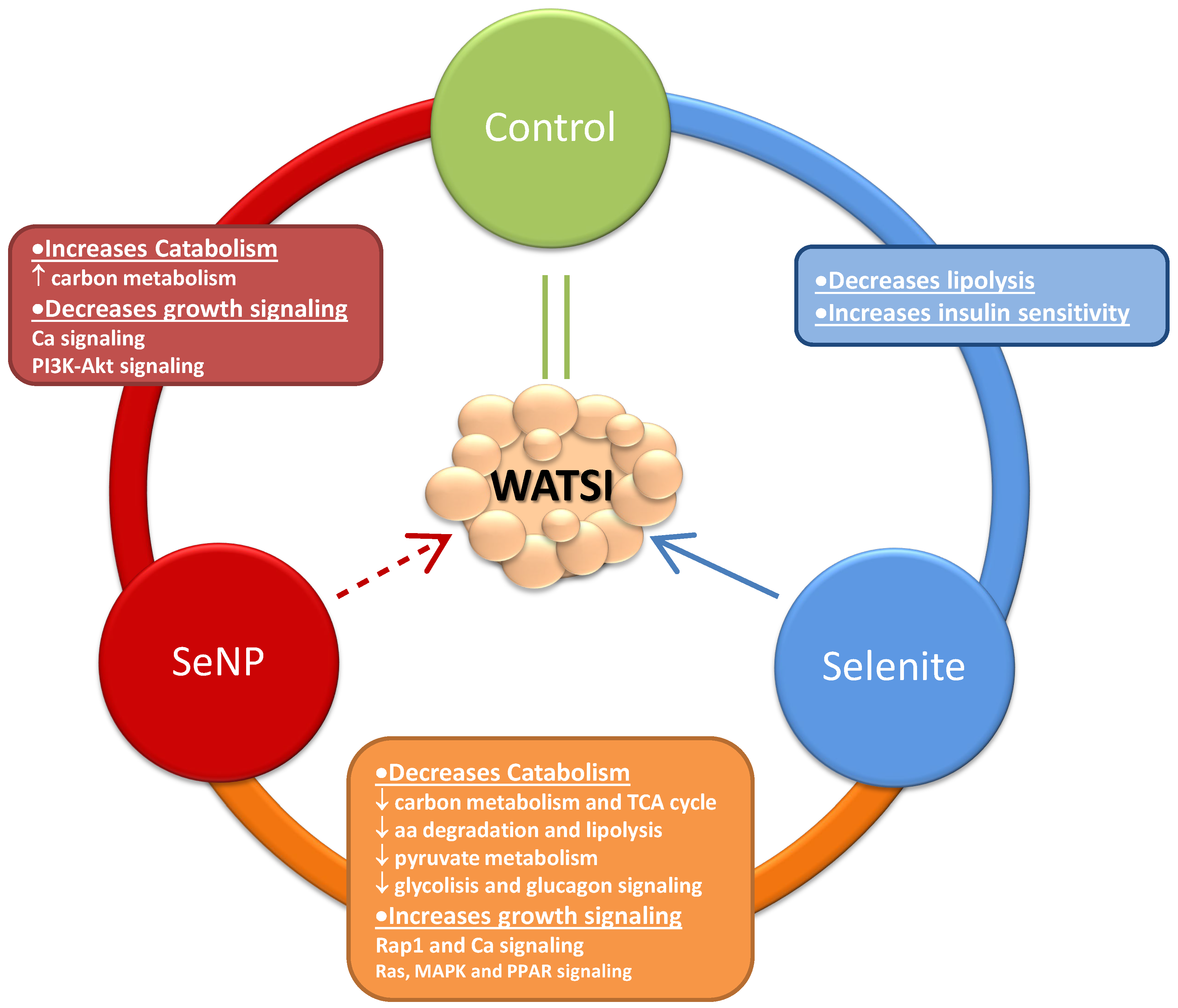
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The Role of Selenium in Type-2 Diabetes Mellitus and Its Metabolic Comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef] [PubMed]
- Vescini, F.; Chiodini, I.; Palermo, A.; Cesareo, R.; De Geronimo, V.; Scillitani, A.; Gennari, L.; Falchetti, A. Selenium: A Trace Element for a Healthy Skeleton-A Narrative Review. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Beckett, G.J.; Arthur, J.R. Selenium and Endocrine Systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of Mitochondrial Reactive Oxygen Species in Homeostasis Regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kasaikina, M.V.; Hatfield, D.L.; Gladyshev, V.N. Understanding Selenoprotein Function and Regulation through the Use of Rodent Models. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Magd, N.F.; Barbosa, P.O.; Nick, J.; Covalero, V.; Grignetti, G.; Bermano, G. Selenium, as Selenite, Prevents Adipogenesis by Modulating Selenoproteins Gene Expression and Oxidative Stress–Related Genes. Nutrition 2022, 93, 111424. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Nogales, F.; Carreras, O.; Pajuelo, E.; Gallego-López, M.d.C.; Romero-Herrera, I.; Begines, B.; Moreno-Fernández, J.; Díaz-Castro, J.; Alcudia, A. Different Effects of Low Selenite and Selenium-Nanoparticle Supplementation on Adipose Tissue Function and Insulin Secretion in Adolescent Male Rats. Nutrients 2022, 14, 3571. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Kari, Z.A.; Abdul Razab, M.K.A.; Ahmed, H.A.; Alagawany, M.; Gewaily, M.S. Selenium Nanoparticles as a Natural Antioxidant and Metabolic Regulator in Aquaculture: A Review. Antioxidants 2021, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the Cytotoxic Effect of Selenium Nanoparticles in Different Human Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Tzanninis, S.; Ntanasis-Stathopoulos, I.; Panotopoulos, G.; Kostopoulou, M.; Tzanninis, I.G.; Tsagianni, A.; Sergentanis, T.N. Prevention and Treatment of Childhood and Adolescent Obesity: A Systematic Review of Meta-Analyses. World J. Pediatr. 2019, 15, 350–381. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B. Adolescent Eating Disorders: Update on Definitions, Symptomatology, Epidemiology, and Comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Moreno, M.; Perez-Herrera, A.; Locia-Morales, D.; Dizzel, S.; Meyre, D.; Stearns, J.C.; Cruz, M. Association of Gut Microbiome with Fasting Triglycerides, Fasting Insulin and Obesity Status in Mexican Children Summary Background: The Association of Gut Microbiota with Obesity and Its Cardio-Metabolic. Pediatr. Obes. 2020, 16, e12748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dang, Y. Roles of Gut Microbiota and Metabolites in Overweight and Obesity of Children. Front. Endocrinol. 2022, 13, 994930. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Peng, Y.; Deehan, E.C.; Tan, Q.; Field, C.J.; Madsen, K.L.; Walter, J.; Prado, C.M.; Tun, H.M.; Haqq, A.M. Composition and Functions of the Gut Microbiome in Pediatric Obesity: Relationships with Markers of Insulin Resistance. Microorganisms 2021, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Huang, W.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T.; Au, C.K.R. Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease. Nutrients 2021, 13, 1104. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine Farnesoid X Receptor Agonist and the Gut Microbiota Activate G-Protein Bile Acid Receptor-1 Signaling to Improve Metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Pathak, P.; Liu, H.; Donepudi, A.; Ferrell, J.; Boehme, S. Intestinal Farnesoid X Receptor and Takeda G Protein Couple Receptor 5 Signaling in Metabolic Regulation. Dig. Dis. 2017, 35, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T.E.; Gariballa, S.; Parekh, K.A.; Thomas, S.A.; Saadi, H.; Al Kaabi, J.; Nagelkerke, N.; Gedulin, B.; Young, A.A. Rectal Taurocholate Increases L Cell and Insulin Secretion, and Decreases Blood Glucose and Food Intake in Obese Type 2 Diabetic Volunteers. Diabetologia 2012, 55, 2343–2347. [Google Scholar] [CrossRef]
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Trace Element Selenium Effectively Alleviates Intestinal Diseases. Int. J. Mol. Sci. 2021, 22, 1708. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 292. [Google Scholar]
- Hu, X.; Chandler, J.D.; Orr, M.L.; Hao, L.; Liu, K.; Uppal, K.; Go, Y.M.; Jones, D.P. Selenium Supplementation Alters Hepatic Energy and Fatty Acid Metabolism in Mice. J. Nutr. 2018, 148, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Nogales, F.; Carrasco López, J.A.; Gallego-López, M.d.C.; Carreras, O.; Alcudia, A.; Pajuelo, E. Microbiota-Liver-Bile Salts Axis, a Novel Mechanism Involved in the Contrasting Effects of Sodium Selenite and Selenium-Nanoparticle Supplementation on Adipose Tissue Development in Adolescent Rats. Antioxidants 2023, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Huang, F.; Zhao, L.; Zhang, Y.; Yang, W.; Wang, S.; Li, M.; Han, X.; Ge, K.; Qu, C.; et al. A Dysregulated Bile Acid-Gut Microbiota Axis Contributes to Obesity Susceptibility. EBioMedicine 2020, 55, 102766. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. The Adolescent Brain and Age-Related Behavioral Manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.G.; Hill, K.E.; Burk, R.F. Dietary Selenium Intake Controls Rat Plasma Selenoprotein P Concentration. J. Nutr. 1989, 119, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Gangadoo, S.; Stanley, D.; Hughes, R.J.; Moore, R.J.; Chapman, J. The Synthesis and Characterisation of Highly Stable and Reproducible Selenium Nanoparticles. Inorg. Nano-Met. Chem. 2017, 47, 1568–1576. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randal, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Romero-Herrera, I.; Nogales, F.; Gallego-López, M.d.C.; Díaz-Castro, J.; Moreno-Fernandez, J.; Ochoa, J.J.; Carreras, O.; Ojeda, M.L. Adipose Tissue Homeostasis Orchestrates the Oxidative, Energetic, Metabolic and Endocrine Disruption Induced by Binge Drinking in Adolescent Rats. J. Physiol. 2023, 601, 5617–5633. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione Peroxidase Activity in Selenium-Deficient Rat Liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Fridovich, I.; Cytochrome, C. CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 213–216. ISBN 9781351072922. [Google Scholar]
- Beers, R.F.; Sizer, I.W. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde Determination as Index of Lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Ultrafast One-Pass FASTQ Data Preprocessing, Quality Control, and Deduplication Using Fastp. iMeta 2023, 2, e107. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS 2012, 16, 284. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Hauffe, R.; Stein, V.; Chudoba, C.; Flore, T.; Rath, M.; Ritter, K.; Schell, M.; Wardelmann, K.; Deubel, S.; Kopp, J.F.; et al. GPx3 Dysregulation Impacts Adipose Tissue Insulin Receptor Expression and Sensitivity. JCI Insight 2020, 5, e136283. [Google Scholar] [CrossRef] [PubMed]
- Hauffe, R.; Rath, M.; Agyapong, W.; Jonas, W.; Vogel, H.; Schulz, T.J.; Schwarz, M.; Kipp, A.P.; Blüher, M.; Kleinridders, A. Obesity Hinders the Protective Effect of Selenite Supplementation on Insulin Signaling. Antioxidants 2022, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Faki, Y.; Er, A. Different Chemical Structures and Physiological/Pathological Roles of Cyclooxygenases. Rambam Maimonides Med. J. 2021, 12, e0003. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K. Prostaglandins as PPARγ Modulators in Adipogenesis. PPAR Res. 2012, 2012, 527607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, C.K.; Zhang, C.L.; Huang, Y. Interplay Between Oxidative Stress, Cyclooxygenases, and Prostanoids in Cardiovascular Diseases. Antioxid. Redox Signal. 2021, 34, 784–799. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Kermouni, A.; Abdel-Hafez, M.; Lau, D.C.W. Role of Cyclooxygenases COX-1 and COX-2 in Modulating Adipogenesis in 3T3-L1 Cells. J. Lipid Res. 2003, 44, 424–429. [Google Scholar] [CrossRef]
- Gartung, A.; Zhao, J.; Chen, S.; Mottillo, E.; VanHecke, G.C.; Ahn, Y.H.; Maddipati, K.R.; Sorokin, A.; Granneman, J.; Lee, M.J. Characterization of Eicosanoids Produced by Adipocyte Lipolysis: IMPLICATION OF CYCLOOXYGENASE-2 IN ADIPOSE INFLAMMATION. J. Biol. Chem. 2016, 291, 16001–16010. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Luo, L.; Luo, Y.; Yang, X.; Ding, X.; Wang, L.; Le, H.; Feldman, L.E.R.; Men, X.; et al. Adipocyte-Derived PGE2 Is Required for Intermittent Fasting-Induced Treg Proliferation and Improvement of Insulin Sensitivity. JCI Insight 2022, 7, e153755. [Google Scholar] [CrossRef]
- Scoditti, E.; Carpi, S.; Massaro, M.; Pellegrino, M.; Polini, B.; Carluccio, M.A.; Wabitsch, M.; Verri, T.; Nieri, P.; De Caterina, R. Hydroxytyrosol Modulates Adipocyte Gene and MiRNA Expression Under Inflammatory Condition. Nutrients 2019, 11, 2493. [Google Scholar] [CrossRef]
- Becker, M.; Levings, M.K.; Daniel, C. Adipose-Tissue Regulatory T Cells: Critical Players in Adipose-Immune Crosstalk. Eur. J. Immunol. 2017, 47, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
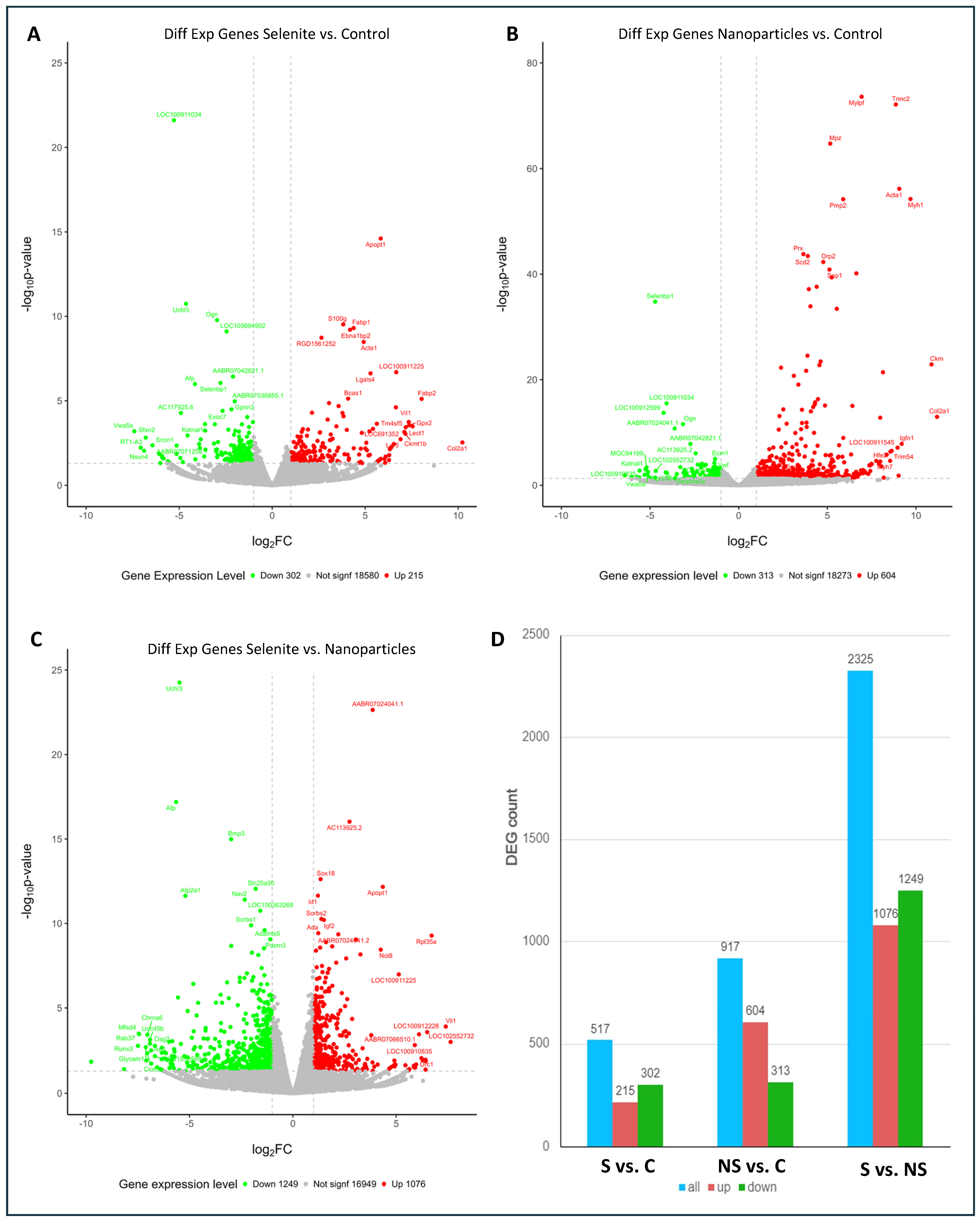
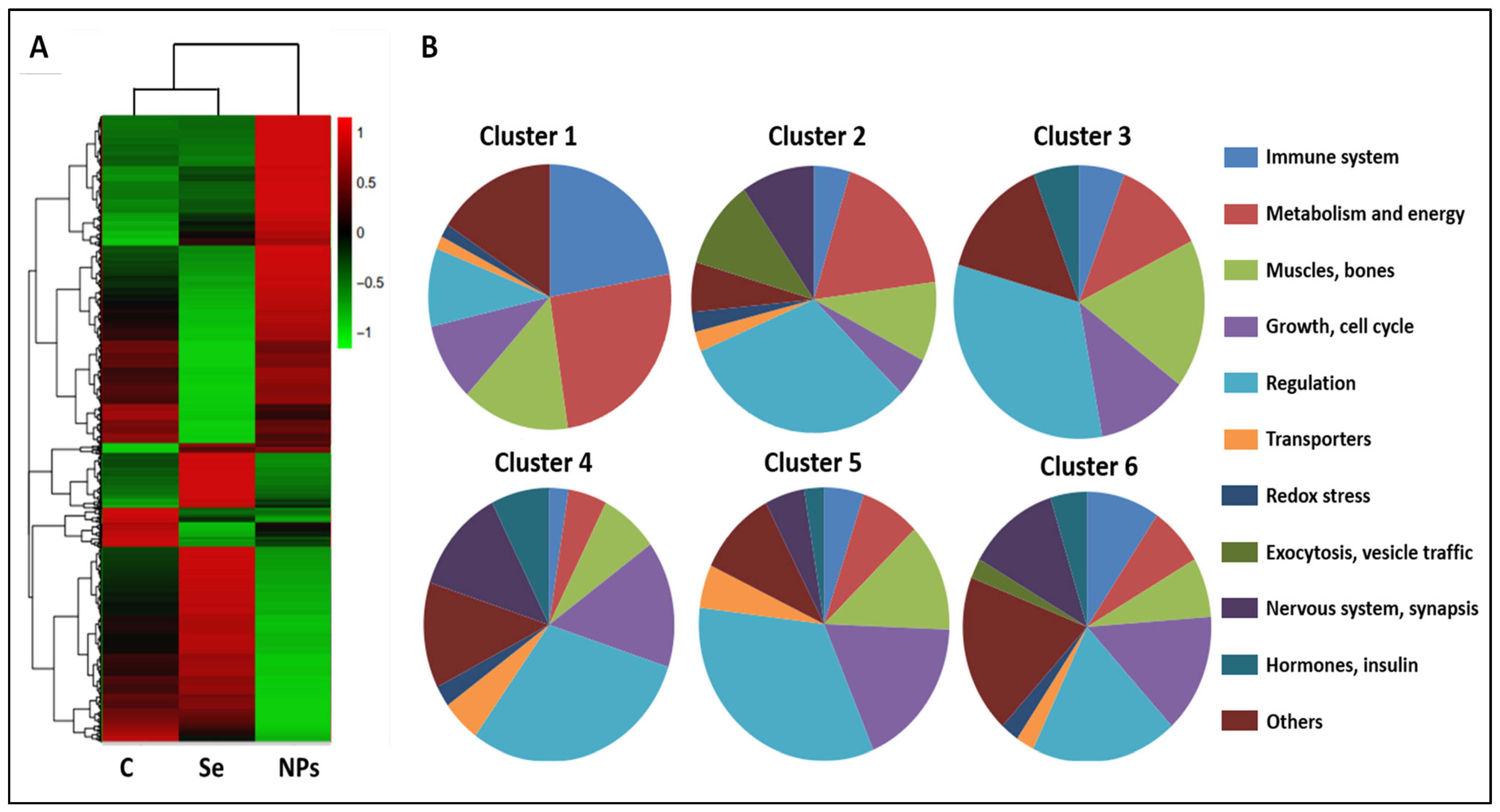
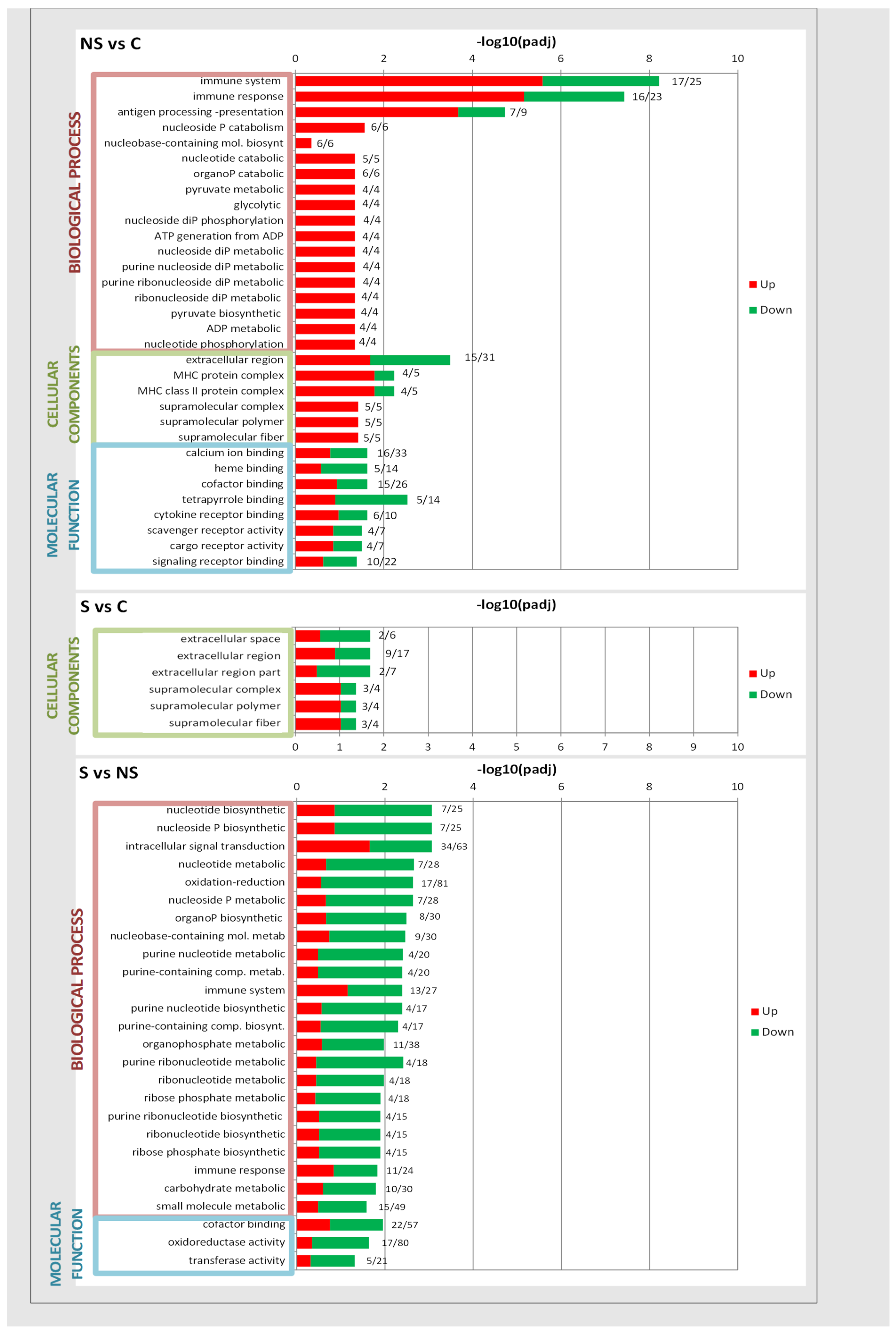
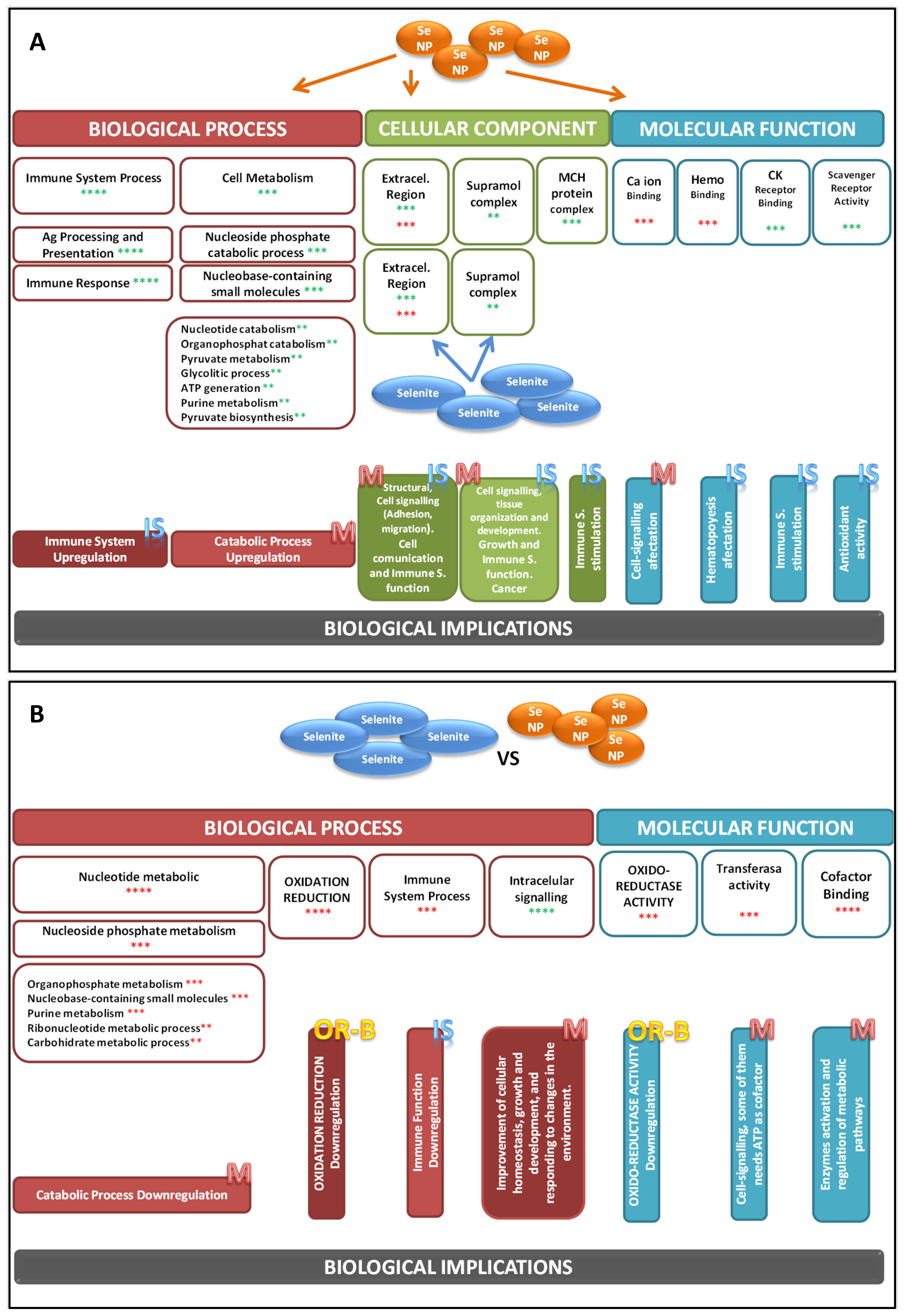
| C | S | NS | |
|---|---|---|---|
| Increased body weight (g/d) | 6.01 ± 0.1 | 6.07 ± 0.2 | 5.98 ± 0.2 |
| Total Se intake (µg/d) | 3.48 ± 0.08 | 6.81 ± 0.14 ** | 6.59 ± 0.09 ** |
| WATSI (%) | 0.62 ± 0.02 | 0.59 ± 0.03 | 0.50 ± 0.02 * |
| SOD activity (U/mg protein) | 12.6 ± 0.8 | 12.9 ± 0.3 | 36.9 ± 2.5 ***, sss |
| CAT activity (U/mg protein) | 101 ± 9 | 108 ± 9 | 184 ± 9 ***, sss |
| GPx activity (mU/mg protein) | 201 ± 4 | 254 ± 13 ** | 256 ± 11 ** |
| SOD/CAT-GPx ratio | 4.2 ± 0.1 | 3.5 ± 0.1 | 8.3 ± 0.2 ***, sss |
| H2O2 (µM) | 73.8 ± 2.9 | 76.3 ± 3.9 | 91.9 ± 2.9 **, s |
| MDA (mol/g wet tissue) | 21.0 ± 1.3 | 26.0 ± 1.6 | 28.3 ± 2.1 * |
| Status | Related Genes with | Gene_Symbol | Log2FC |
|---|---|---|---|
| UPREGULATED BY SELENITE | Energy metabolism | Me3, Ndufa5 | [0.93]–[0.55] |
| Oxidative stress | Cyp26b1, Aox1, GPx3, Nos3, Mt1, Msrb3,Cyp4b1, Cyp2e1, Cyp2ab1, Cyp27a1, Cyp2j4, LOC100912391 | [1.57]–[0.42] | |
| Others | Ptgs1, Alox15, Alox12 | [1.22]–[1.07] | |
| DOWNREGULATED BY SELENITE | Energy metabolism | Acadsb, Hadh, Acox3, Acad10, Acadm, Acadl, Mecr, Gapdh, Ldha, G6pdx, LOC303448, Gpd1, Aldh7a1, Aldh6a1, Aldh9a1, Aldh2, D2hgdh, Ldha, Pdpr, Idh3g, Fh, Mdh2, Idh3B, Acad9, Mdh1, Pcyox1, Ivd, Dld, Hibadh, Cdo1, LOC100911156, Pycr1, Rrm2b, RGD1565368, Idh3a | [−1.56]–[−0.37] |
| Oxidative stress | Msra, Mpo, Prdx3, Tp53i3, Aox4,Cyp51, Sc5d, Cyp1b1, Nsdhl, Cyp4f39, Dhcr24, Cyb5r3, Sqle, Sod2 | [−1.53]–[−0.43] | |
| Enzymes of the mitochondrial respiratory | Maob, Sdha, Ndufs1, Ndufb6, LOC685596, Foxred1, Uqcrfs1 | [−1.15]–[−0.38] | |
| Others | Ptgs2, Aifm1, Loxl4, Loxl2, Tas1r2, AC098459.1, AABR07012795.1, Aifm2 | [−2.05]–[−0.46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogales, F.; Pajuelo, E.; Romero-Herrera, I.; Carreras, O.; Merchán, F.; Carrasco López, J.A.; Ojeda, M.L. Uncovering the Role of Selenite and Selenium Nanoparticles (SeNPs) in Adolescent Rat Adipose Tissue beyond Oxidative Balance: Transcriptomic Analysis. Antioxidants 2024, 13, 750. https://doi.org/10.3390/antiox13060750
Nogales F, Pajuelo E, Romero-Herrera I, Carreras O, Merchán F, Carrasco López JA, Ojeda ML. Uncovering the Role of Selenite and Selenium Nanoparticles (SeNPs) in Adolescent Rat Adipose Tissue beyond Oxidative Balance: Transcriptomic Analysis. Antioxidants. 2024; 13(6):750. https://doi.org/10.3390/antiox13060750
Chicago/Turabian StyleNogales, Fátima, Eloísa Pajuelo, Inés Romero-Herrera, Olimpia Carreras, Francisco Merchán, José A. Carrasco López, and María Luisa Ojeda. 2024. "Uncovering the Role of Selenite and Selenium Nanoparticles (SeNPs) in Adolescent Rat Adipose Tissue beyond Oxidative Balance: Transcriptomic Analysis" Antioxidants 13, no. 6: 750. https://doi.org/10.3390/antiox13060750
APA StyleNogales, F., Pajuelo, E., Romero-Herrera, I., Carreras, O., Merchán, F., Carrasco López, J. A., & Ojeda, M. L. (2024). Uncovering the Role of Selenite and Selenium Nanoparticles (SeNPs) in Adolescent Rat Adipose Tissue beyond Oxidative Balance: Transcriptomic Analysis. Antioxidants, 13(6), 750. https://doi.org/10.3390/antiox13060750





