Abstract
Cadmium (Cd) is a heavy metal that is highly toxic to humans and animals. Its adverse effects have been widely associated with mitochondrial alterations. However, there are not many treatments that target mitochondria. This study aimed to evaluate the impact of sulforaphane (SFN) pre-exposure against cadmium chloride (CdCl2)-induced toxicity and mitochondrial alterations in the nematode Caenorhabditis elegans (C. elegans), by exploring the role of the insulin/insulin-like growth factor signaling pathway (IIS). The results revealed that prior exposure to SFN protected against CdCl2-induced mortality and increased lifespan, body length, and mobility while reducing lipofuscin levels. Furthermore, SFN prevented mitochondrial alterations by increasing mitochondrial membrane potential (Δψm) and restoring mitochondrial oxygen consumption rate, thereby decreasing mitochondrial reactive oxygen species (ROS) production. The improvement in mitochondrial function was associated with increased mitochondrial mass and the involvement of the daf-16 and skn-1c genes of the IIS signaling pathway. In conclusion, exposure to SFN before exposure to CdCl2 mitigates toxic effects and mitochondrial alterations, possibly by increasing mitochondrial mass, which may be related to the regulation of the IIS pathway. These discoveries open new possibilities for developing therapies to reduce the damage caused by Cd toxicity and oxidative stress in biological systems, highlighting antioxidants with mitochondrial action as promising tools.
1. Introduction
Cadmium (Cd) is a highly toxic heavy metal with no biological function. It is primarily found in its divalent form (Cd2+) and is constantly released into the environment from anthropogenic and natural sources [1,2]. Exposure can occur either occupationally or environmentally. Workers in electroplating, battery production, and pigment industries are at the highest risk of Cd exposure [3]. In addition, environmental exposure occurs through air pollution, consumption of contaminated food and water, and smoking [4,5]. Attempts have been made to control exposure sources; however, Cd pollution remains a significant issue in many developing countries due to its accumulation [6].
Cd has a long half-life and bioaccumulates in plants, invertebrates, and vertebrates [5]. Toxic effects following exposure include growth retardation and toxicity in different body systems [5,7]. It causes obstructive respiratory diseases, emphysema, end-stage renal failure, diabetes, blood pressure disorders, bone diseases, immunosuppression, premature aging, cancer, and even death [8,9,10]. Numerous studies have shown that mitochondria play a fundamental role in Cd toxicity [11,12]. Cd enters mitochondria and affects the electron transport system (ETS), increasing the reactive oxygen species (ROS) production and decreasing the mitochondrial membrane potential (Δψm), which induces alterations in mitochondrial dynamics, mutations in mitochondrial deoxyribonucleic acid (mtDNA), and decreases in mitochondrial biogenesis [11,12,13,14]. Currently, there is no specific or effective treatment for Cd poisoning [7]. Furthermore, among the few treatments available, none is targeted to treat mitochondrial dysfunction.
Conversely, sulforaphane (SFN) is an isothiocyanate derived from the hydrolysis of glucosinolates found in cruciferous vegetables such as broccoli, cauliflower, and cabbage [15]. In recent years, SFN has garnered interest in mitochondrial target studies due to growing evidence suggesting that SFN decreases alterations in mitochondrial dynamics, Δψm, and bioenergetics [16]. The main SFN mechanism of action is the activation and nuclear translocation of the nuclear factor erythroid 2-related factor 2 (Nrf2), which stimulates the transcription of the enzymatic antioxidant battery [17,18]. Previously, SFN has been observed to protect against Cd toxicity in different organs and cell types, including the liver [19], testes [20], Leydig cells (TM3) [21], Sertoli cells [22], mesenchymal stem cells [23], and peripheral blood lymphocytes and monocytes [24]. However, whether the SFN protective effects on Cd-induced damage are related to mitochondrial protection is still unclear. Furthermore, the molecular mechanism has not yet been elucidated.
Finally, the nematode Caenorhabditis elegans (C. elegans) stands as a valuable and extensively utilized model for investigating the impact of phytochemicals on mitochondrial function and the toxicity of environmental chemicals [25,26]. The use of this animal has helped the discovery of several conserved signaling pathways involved in Cd detoxification mechanisms [27,28]. Notably, it has also been found that human genes responsible for various mitochondrial diseases have orthologous genes in this nematode, making it possible to use C. elegans as a model organism to study different mitochondrial alterations [29]. Similarly, oxidative stress signaling pathways are highly conserved, especially the insulin/insulin-like growth factor signaling (IIS) pathway [30,31]. Hence, it would be of predictive value to study the mechanisms of action of pro-oxidant agents, such as Cd, and antioxidants, such as SFN, in this model. This study aimed to investigate the protective potential of SFN against Cd-induced toxicity and mitochondrial alterations using the nematode C. elegans as a model. Additionally, the role of the IIS pathway in this interaction was analyzed to better understand the underlying mechanisms of SFN action in this process.
2. Materials and Methods
2.1. Reagents
Sulforaphane (SFN-S8044) was purchased from LKT Laboratories, Inc. (St Paul, MN, USA). Cadmium chloride (CdCl2-C-2544), cholesterol (C3045), yeast extract (70161), 5-fluoro-2′-desoxiuridina (FUdR-F0503), dimethyl sulfoxide (DMSO), streptomycin sulfate salt (S6501), sodium azide (S2002), antimycin A (A8674), and rotenone (R8875) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium hypochlorite (NaClO) was purchased from Cloralex (Oakland, CA, USA). 12% Parmisole (Levamisole HCl 12 g-Q-0021-006) was purchased from PARFAM, S.A. (Ciudad de México, México). MitoTrackerTM Green FM (M7514), MitoSOXTM Red mitochondrial superoxide indicator (M36008), 5,5,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimi-dazoylcarbocyanine iodide (JC-1-T3168), 6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA-C6827), bacto-agar (214010), bactoTM peptone, and bactoTM tryptone were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Magnesium sulfate heptahydrate (MgSO4•7H2O-2500-01), potassium phosphate monobasic (KH2PO4-3246-01), sodium hydrogen phosphate (Na2HPO4-3828-01), sodium hydroxide (NaOH-46697-2002), and sodium chloride (NaCl-7647-14-5) were purchased from JT Baker (Xalostoc, Edo. Mex., Mexico). Potassium phosphate dibasic (K2HPO4-7088) and potassium chloride (KCl-6858) were purchased from Mallinckrodt, AR (St. Louis, MO, USA).
2.2. C. elegans Strains
The following C. elegans strains used in this study were purchased from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA), including N2 (wild-type, Bristol) [32], VC128 mtl-2 (gk125) V [33], RB1623 cdr-2 (ok1996) V [33], TK22 mev-1 (kn1) III [34,35], TJ1052 age-1(hx546) II [33,36], QV225 skn-1 (zj15) IV [37], GR2245 skn-1 (mg570) IV [38,39], GR1307 daf-16(mgDf50)I [35], and the reporter strain SJ4143 zcIs17 (Pges-1::GFPmt) [40,41], QQ202 daf-2(cv20[daf-2::GFP]) III, and OH16024 daf-16(ot971[daf-16::GFP]) I [42]. The characteristics of the strains are summarized in Table S1.
2.3. C. elegans Culture
C. elegans was cultured in Nematode Growth Medium complete (NGM: 0.3% NaCl, 1.7% agar, 2.5% peptone, 0.1% potassium phosphate buffer (1 M, pH 6), 5 µg/mL cholesterol, 1 mM CaCl2, and 1 mM MgSO4) on plastic plates seeded with a lawn of E. coli OP50-1 bacteria (CGC, University of Minnesota, Minneapolis, MN, USA). E. coli OP50-1 bacteria were cultured overnight at 37 °C in liquid Lysogeny broth (LB: 10 g bactoTM tryptone, 5 g yeast extract, 5 g sodium chloride, and 1000 mL ddH2O) and diluted to an OD600 of approximately 0.3, as previously described [43]. Subsequently, 1000 µL of the E. coli OP50-1 suspension was seeded onto 60 mm NGM plates and dried overnight at room temperature. The worms were maintained according to previous standard protocols [44] with an incubation temperature of 20 °C.
2.4. Experimental Design
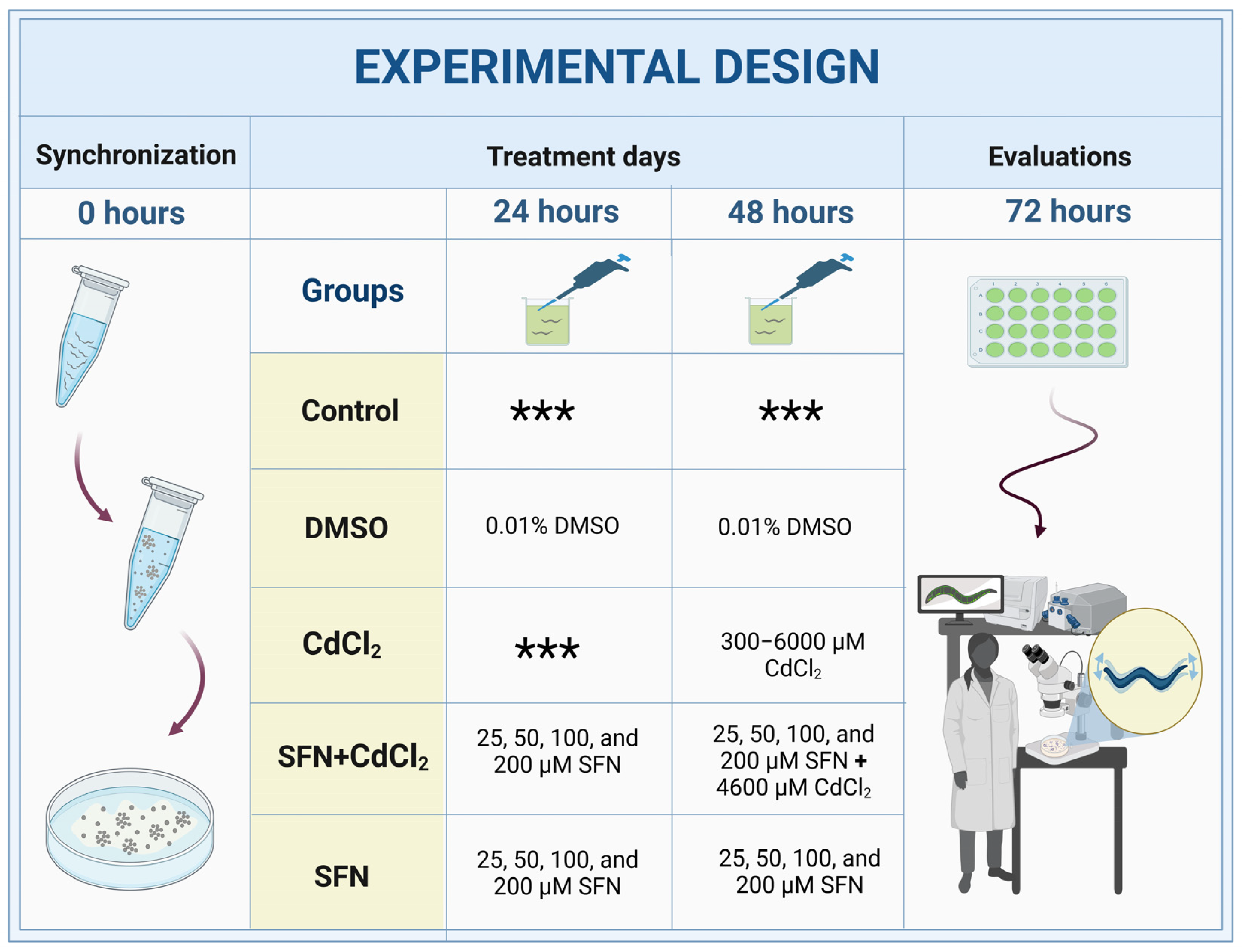
C. elegans were collected from NGM plates using M9 buffer (6 g Na2HPO4, 3 g KH2PO4, 5 g NaCl, 0.25 g MgSO4•7H2O, and 1000 mL ddH2O), and cultures were synchronized using a bleaching solution (NaOH 0.5 M and 0.5% NaClO) [45]. Subsequently, the eggs were allowed to hatch in NGM boxes with E. coli OP50-1 bacteria. Twenty-four hours after synchronization, worms in the L1 stage were transferred to 24-well plates with K medium (52 mM NaCl and 32 mM KCl), OP50-1 bacteria (1:10 dilution), and the different treatments. There were five experimental groups: (I) Control, without treatment; (II) DMSO, 48 h with 0.1% DMSO; (III) CdCl2, 24 h with the different concentrations of CdCl2 (300–6000 μM); (IV) SFN+CdCl2, 24 h with SFN (25, 50, 100, and 200 μM) plus 24 h with SFN and CdCl2 (4600 μM); and (V) SFN, 48 h with the different concentrations of SFN (25, 50, 100, and 200 μM) (Figure 1). All evaluations were carried out at the end of the exposures when most of the worms were in the adult stage. To prepare the SFN concentrations, a 100 mM stock solution was prepared in DMSO and diluted 1:1000 in K medium. For CdCl2 concentrations, a 1 M stock solution was prepared in milliQ water and then diluted to 50 mM in K medium [46,47]. Note: To evaluate mitochondria-associated oxygen consumption, the exposures to the treatments were not carried out in a liquid medium (review Section 2.10).
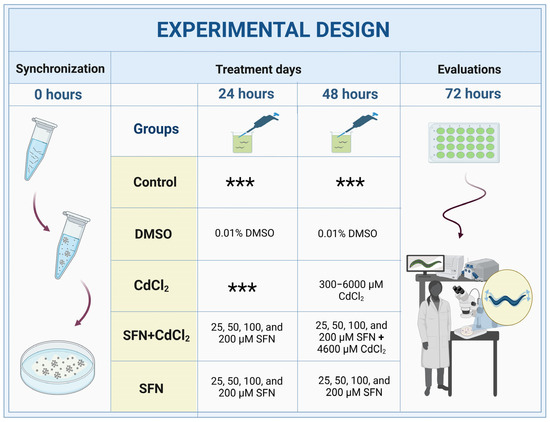
Figure 1.
Experimental design with C. elegans. DMSO: dimethyl sulfoxide, CdCl2: cadmium chloride, SFN: sulforaphane. *** indicates that nematodes are cultured only with K medium and OP50-1 bacteria (1:10 dilution).
2.5. Survival
Nematodes from the N2, VC128, RB1623, TK22, TJ1052, GR1307, GR2245, and QV225 strains were exposed to the different treatments in 24-well clear plates at 20 °C (30 ± 2 nematodes per well). After exposure, nematodes were classified, under a dissecting microscope, as either alive or dead. Dead nematodes did not move after a gentle touch and showed no pharyngeal pumping [48]. Three to five independent assays were conducted, with three replicates per concentration in each experiment. Concentration-response curves were obtained for N2 worms exposed to CdCl2.
2.6. Lifespan Analysis
The lifespan assays were conducted as described by Takahashi et al. [49]. Following exposure to the different treatments, random samples of 20 N2 nematodes were transferred to NGM plates containing 25 μM FUdR and E. coli OP50-1 and incubated at 20 °C. Day 0 was considered the day in which worms were transferred to NGM plates. Worms that no longer responded to gentle touch with a platinum wire were classified as dead and removed from the plates. The endpoint was considered the day when all worms were dead. At least five independent experiments were conducted. Finally, the Kaplan–Meier method was used to calculate the survival percentage using GraphPad Prism software version 10.
2.7. Lipofuscin Assay
Lipofuscin is a senescence marker in nematodes that can be analyzed using fluorescence microscopy [50,51]. After exposure to the different treatments, random samples of 20 N2 nematodes were placed in black, clear-bottomed 96-well plates. Nematodes were paralyzed with 5 mM Levamisole, and images were taken with the Cytation™ 5 using a blue fluorescence filter to DAPI. Images were analyzed using Fiji 1.54f (See Section 2.14) [52]. At least three independent experiments were conducted.
2.8. Body Length
After exposure to the different treatments, random samples of 20 N2 nematodes were placed in black, clear-bottom 96-well plates. Nematodes were paralyzed with 5 mM Levamisole, and images were taken with the Cytation™ 5. Subsequently, body length was measured using Fiji 1.54f [52]. Three independent experiments were conducted.
2.9. Mobility/Body-Bending Assay
Body bends are used to reflect worm mobility [47]. After exposure to the different treatments, random samples of 10 N2 nematodes per group were transferred to freshly prepared NGM plates without food and left at 20 °C for 2 min. Subsequently, they were individually examined for body bends, defined as a change in the direction of the posterior bulb or regular head oscillation. Body bends were counted for 60 s. Three independent experiments were conducted.
2.10. Mitochondria-Associated Oxygen Consumption
Mitochondrial respiration measurement was conducted as described by Branicky et al. [53] with slight modifications. Briefly, N2 nematodes were exposed to different treatments: (I) control, (II) DMSO (0.01%), (III) CdCl2 (4600 μM), (IV) SFN (100 μM) + CdCl2 (4600 μM), and (V) SFN (100 μM), on NGM plates with E. coli OP50-1 bacteria. The DMSO, SFN, and CdCl2 were prepared in OP50-1 bacteria DO600 = 0.3. Subsequently, 2 mL was placed on the 100 mm × 15 mm NGM plates and allowed to dry for 24 h before placing the worms. After exposure to the different treatments, the worms were washed with M9 buffer and loaded into the 2 mL chamber of the Oroboros Oxygraph 2K. The number of worms was counted in 3 aliquots of 20 µL of the worm suspension to determine the total number of worms in the chamber. Basal respiration was measured after N2 nematode addition at 20 °C, and residual respiration (ROX) was obtained by the titration with 25 µL of 2M sodium azide, 10 µL of 5 mM antimycin, plus 5 µL of 1 mM rotenone. The ROX value was subtracted from the basal respiration to determine the value of the mitochondrial respiration rate. The mitochondrial respiration rate was normalized to the number of worms in the chamber, which averaged around 1000 nematodes. Three independent experiments were conducted.
2.11. Use of Fluorescent Probes
Fluorescent probes were used to assess mitochondrial mass, Δψm, and intracellular and mitochondrial ROS (Table 1). N2 nematodes were exposed to the different treatments according to the scheme in Figure 1. However, before completing the exposure time, the corresponding probe was added to the K medium, considering the incubation time of each of the probes (Example: the H2DCFDA probe was added 4 h before completing treatments). This was done to ensure simultaneous completion of treatment exposure and probe incubation. Subsequently, nematodes were washed with M9 buffer to remove excess dye and then plated on NGM plates with E. coli bacteria for 1 h at 20 °C to remove excess probe from the intestine. Nematodes were paralyzed with 5 mM Levamisole in black, clear-bottom 96-well plates. Images were taken with the Cytation™ 5 and analyzed using Fiji 1.54f (See Section 2.14) [52]. The MitoSOXTM Red/MitoTracker® Green fluorescence ratio was determined for mitochondrial ROS. At least three independent experiments were conducted.

Table 1.
Conditions of use of fluorescent probes.
2.12. Quantification of Green Fluorescent Proteins (GFP)
After exposure to the different treatments, random samples of nematodes from the SJ4143 and QQ202 strains were taken and placed in black, clear-bottom 96-well plates. Nematodes were paralyzed with 5 mM Levamisole, and images were taken with the Cytation™ 5 using a green fluorescence filter. Images were analyzed using Fiji 1.54f (See Section 2.14) [52]. At least three independent experiments were conducted.
2.13. Nuclear Localization of DAF-16
The nuclear localization of DAF-16 was measured as described previously [54]. After exposure to the different treatments, random samples of 15 nematodes from the transgenic strain OH16024, expressing a DAF-16::GFP fusion protein, were taken and placed in black, clear-bottom 96-well plates. Nematodes were paralyzed with 5 mM Levamisole, and images were taken with the Cytation™ 5 using a green fluorescence filter. The nuclear localization of DAF-16::GFP was classified as cytosolic, intermediate, and nuclear, as proposed by Oh et al. [55]. At least three independent experiments were conducted.
2.14. Image Analyses
Image analyses were performed in Fiji 1.54f [52]. A macro for executing the following procedures was written. We segmented each worm by automatically thresholding the 8-bit image and manually selecting the contours of the worms. Each contour was added to the region of interest (ROI) manager, and the fluorescence intensity for each ROI was determined in the channel of interest. Background fluorescence for each image was also determined by adding random background rectangles to the ROI manager. Data were extracted, the background subtracted for each worm, and the fluorescence normalized to the control group.
2.15. Statistical Analysis and Calculation of the Mean Lethal Concentration (LC50)
The results are presented as mean ± standard error of the mean (SEM). Normality and homoscedasticity of variances were assessed using the Shapiro–Wilk and Bartlett tests, respectively. Differences between groups were determined using one-way analysis of variance (ANOVA) followed by Tukey’s test. If normality was not met, the Kruskal–Wallis test was used, followed by Dunn’s test. When comparing the two groups, an unpaired t-test was performed. Data from lifespan analysis were analyzed using Kaplan–Meier analysis and a log-rank test. Statistical analyses were conducted using GraphPad Prism 10™ software (GraphPad Software, Inc., San Diego, CA, USA). Differences were considered significant at p ≤ 0.05. To calculate the LC50, two different analyses were performed: a simple logistic regression with GraphPad Prism 10™ and a Probit analysis with Miller–Tainter corrections. Finally, for nuclear translocation of DAF-16, a multinomial logistic regression model was fitted, treatment effect analyzed through a chi-square test, and pairwise comparisons made using Tukey's method (R 4.3.0 software).
3. Results
3.1. SFN Prevents CdCl2-Induced Decrease in Survival
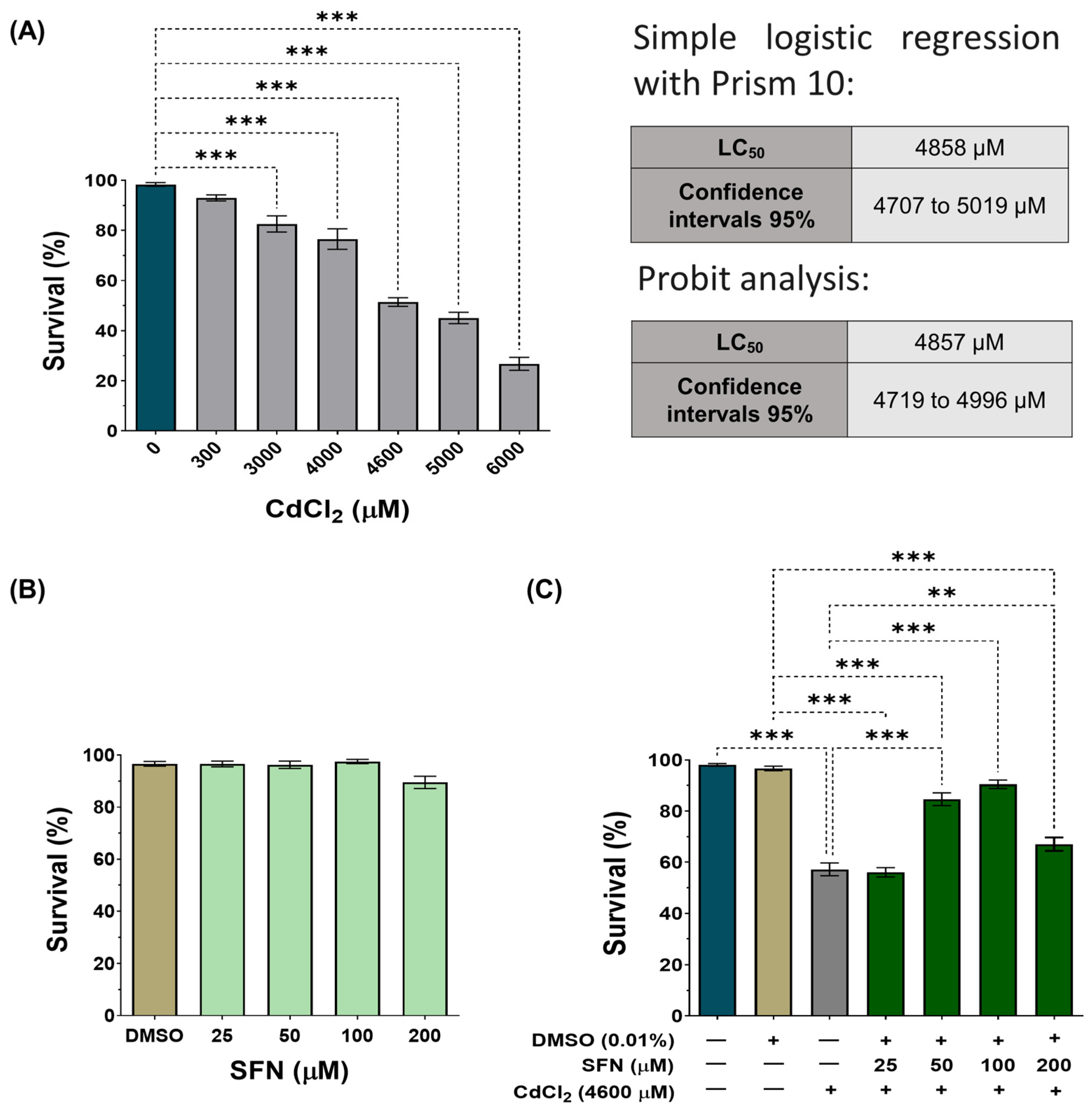
To validate our model, wild-type C. elegans (Bristol-N2) were exposed to various concentrations of CdCl2 (300–6000 μM). A concentration-dependent effect was observed, with the concentration of 6000 μM decreasing survival by 73%, while at the concentration of 300 μM, the decrease was only 7% (Figure 2A). To determine the LC50, we performed two different analyses: a simple logistic regression with GraphPad Prism 10™ and a Probit analysis that showed an LC50 of 4858 μM (IC95: 4707–4019 μM) and 4857 μM (IC95: 4719–4996 μM), respectively (Figure 2A and Supplementary Figure S1). These concentrations are similar to each other and to those previously reported by other authors [46,56]. Subsequently, nematodes were exposed to concentrations of SFN between 25 and 200 μM to demonstrate that SFN alone had no toxic effects. As expected, SFN had no significant effect on the survival of C. elegans (Figure 2B). Finally, we evaluated whether pre-exposure to SFN prevents the death of nematodes caused by exposure to 4600 μM of CdCl2 (a concentration close to the lower limit of the LC50 of CdCl2 and the one chosen to carry out the remaining experiments). To do this, the nematodes were exposed for 24 h to different concentrations of SFN, followed by another 24 h of coincubation with SFN and CdCl2. Exposure to 50 and 100 μM SFN increased survival by 27.45 and 34.01%, respectively, compared to the group treated only with CdCl2. It should be noted that pre-exposure of 100 μM of SFN achieved a survival rate of 90.5% (Figure 2C). Since protection was highest at 100 μM, subsequent experiments were performed using only this concentration of SFN. These data demonstrate that pre-exposure of SFN prevents CdCl2-induced death in C. elegans.
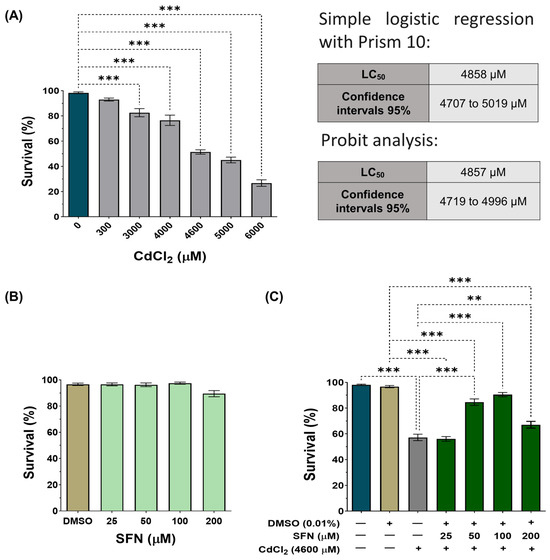
Figure 2.
Effect of sulforaphane (SFN) pre-exposure on the survival of Caenorhabditis elegans (C. elegans) exposed to cadmium chloride (CdCl2). Bristol N2 nematodes in the L1 stage were exposed to different concentrations of (A) CdCl2 for 24 h, (B) SFN for 48 h, and (C) SFN with 4600 μM of CdCl2 (24 h of SFN alone plus 24 h of SFN+CdCl2). After the treatments, nematodes were scored under a dissecting microscope as alive if they were moving or as dead if they did not respond to gentle probing. Percentage calculations were made based on the obtained data. Data are presented as mean ± SEM, n = 3 to 5 independent bioassays with 3 technical replicates each. ** p < 0.01, *** p < 0.001.
3.2. SFN Prevents the Toxic Effects Induced by CdCl2
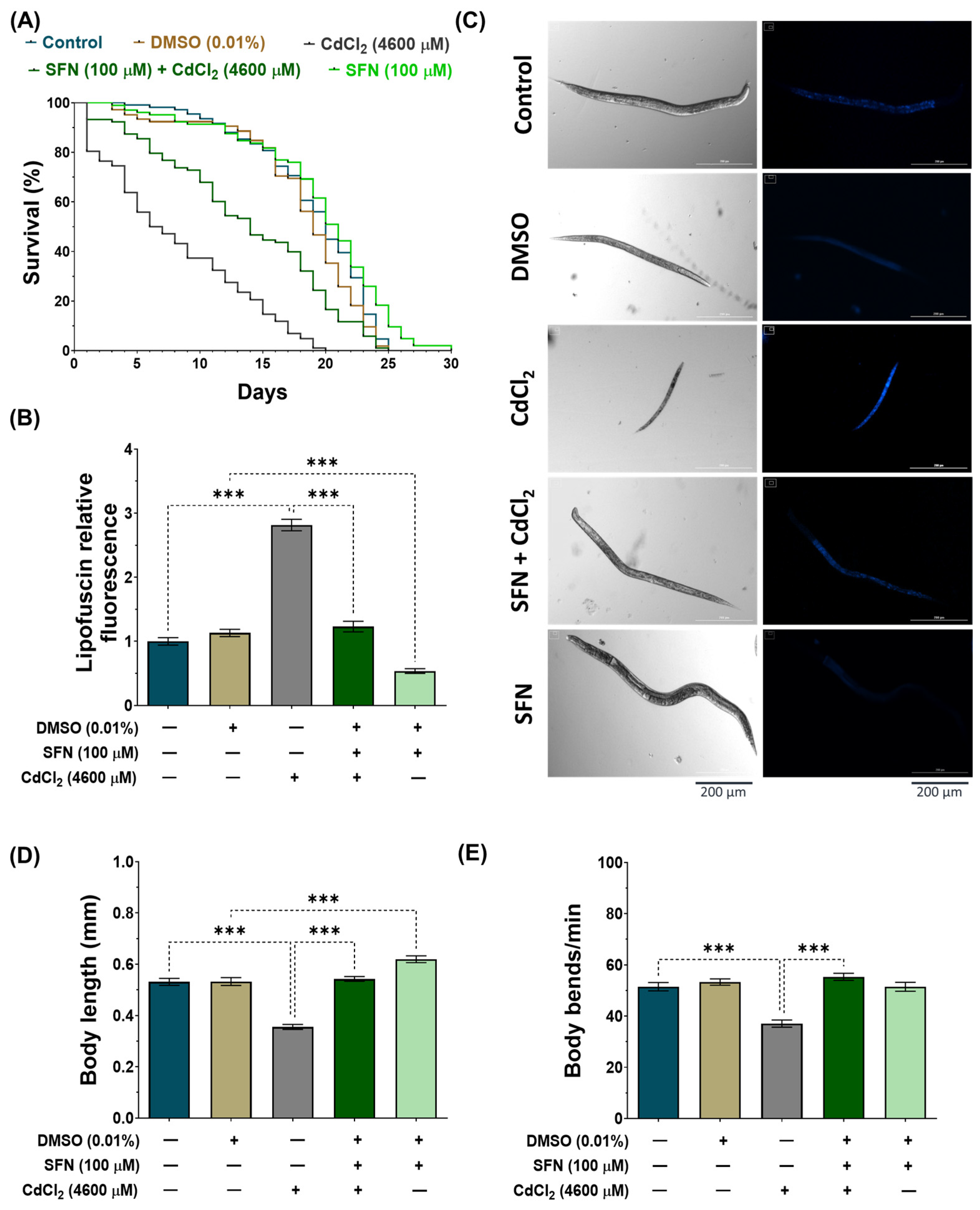
To evaluate whether SFN pre-exposure mitigates the toxic effects of CdCl2 by increasing the lifespan and improving the health of C. elegans, we analyzed the mean and maximum lifespan, lipofuscin levels, body size, and body bends of the nematodes. In the lifespan evaluation (Figure 3A and Table 2), it was found that exposure to CdCl2 drastically decreased the lifespan to a mean of 8 ± 0.56 days (maximum lifespan of 20 days) compared to the control group, which was 19 ± 0.45 days (maximum lifespan of 25 days). Meanwhile, in the SFN+CdCl2 group, the decrease in nematode lifespan was prevented, as the mean lifespan was 14 ± 0.68 days (maximum lifespan of 25 days), corresponding to a 42.9% increase compared to the group treated with CdCl2 alone. Furthermore, and consistent with previous reports [47], exposure to SFN alone increased the mean lifespan of C. elegans to 20 ± 0.58 days (maximum lifespan of 30 days) compared to the DMSO group. On the other hand, lipofuscin accumulation levels were evaluated (Figure 3B,C), a pigment associated with aging or cellular deterioration. Lipofuscin is a complex mixture of oxidized protein and lipid degradation residues, along with minor amounts of carbohydrates and metals. It originates from an incomplete lysosomal digestion of the phagocyte and self-phagocyte material, for example, from incomplete lysosomal degradation of damaged mitochondria [57,58]. In addition, it has been observed that additional oxidative stress promotes its formation [59]. In this study, we observed a significant increase in lipofuscin levels in the CdCl2-exposed group compared to the control group, indicating a physiological aging state possibly associated with increased oxidative stress. However, in the SFN+CdCl2-exposure group, this increase was prevented by 21.1%, suggesting a protective effect of SFN against Cd-induced lipofuscin accumulation. Furthermore, exposure to SFN alone reduced lipofuscin levels by 52.51% compared to the DMSO group. Other toxicity parameters evaluated were body size (Figure 3C,D) and body bends (Figure 3E). We found that the nematodes in the CdCl2-exposed group were smaller, and the number of body bends per minute decreased significantly compared with the control group. Meanwhile, in the SFN+CdCl2 group, nematodes statistically increased their body length and bends by 34.5% and 33%, respectively, compared to the group exposed to CdCl2 alone. Finally, the SFN-exposed group only increased body size compared to the DMSO group and did not affect body bends. Overall, these results indicate that pre-exposure of SFN prevents toxic effects and increases the lifespan of C. elegans exposed to CdCl2.
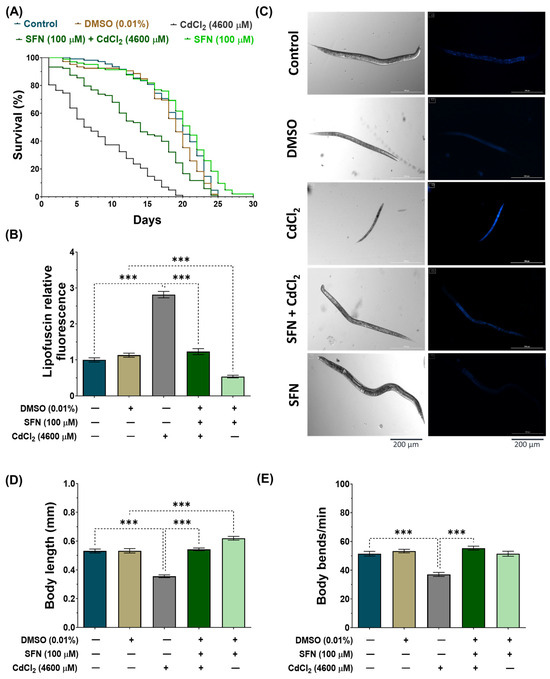
Figure 3.
Protective effect of sulforaphane (SFN) exposure against cadmium chloride (CdCl2)-induced toxicity in Caenorhabditis elegans (C. elegans). Bristol N2 nematodes at the L1 stage were initially exposed to SFN. After 24 h, they were re-exposed, this time to a combination of SFN and CdCl2 for another 24 h. Additional groups were created and exposed to 0.1% dimethyl sulfoxide (DMSO, SFN vehicle) for 48 h, CdCl2 (4600 μM) for 24 h, and SFN (100 μM) for 48 h. Once the exposure times were over, the corresponding evaluations were carried out. (A) Kaplan–Meier curves for lifespan (n ≥ 100 nematodes divided into 5 independent experiments), (B) lipofuscin levels corrected for background in each image and normalized to the control group (n = 60 nematodes distributed in 3 independent experiments), (C) representative images of lipofuscin levels and body size, (D) body size (n = 60 nematodes distributed in 3 independent experiments), and (E) body bends (n = 30 nematodes distributed in 3 independent experiments). Data are presented as mean ± SEM, *** p < 0.001.

Table 2.
Summary of lifespan data.
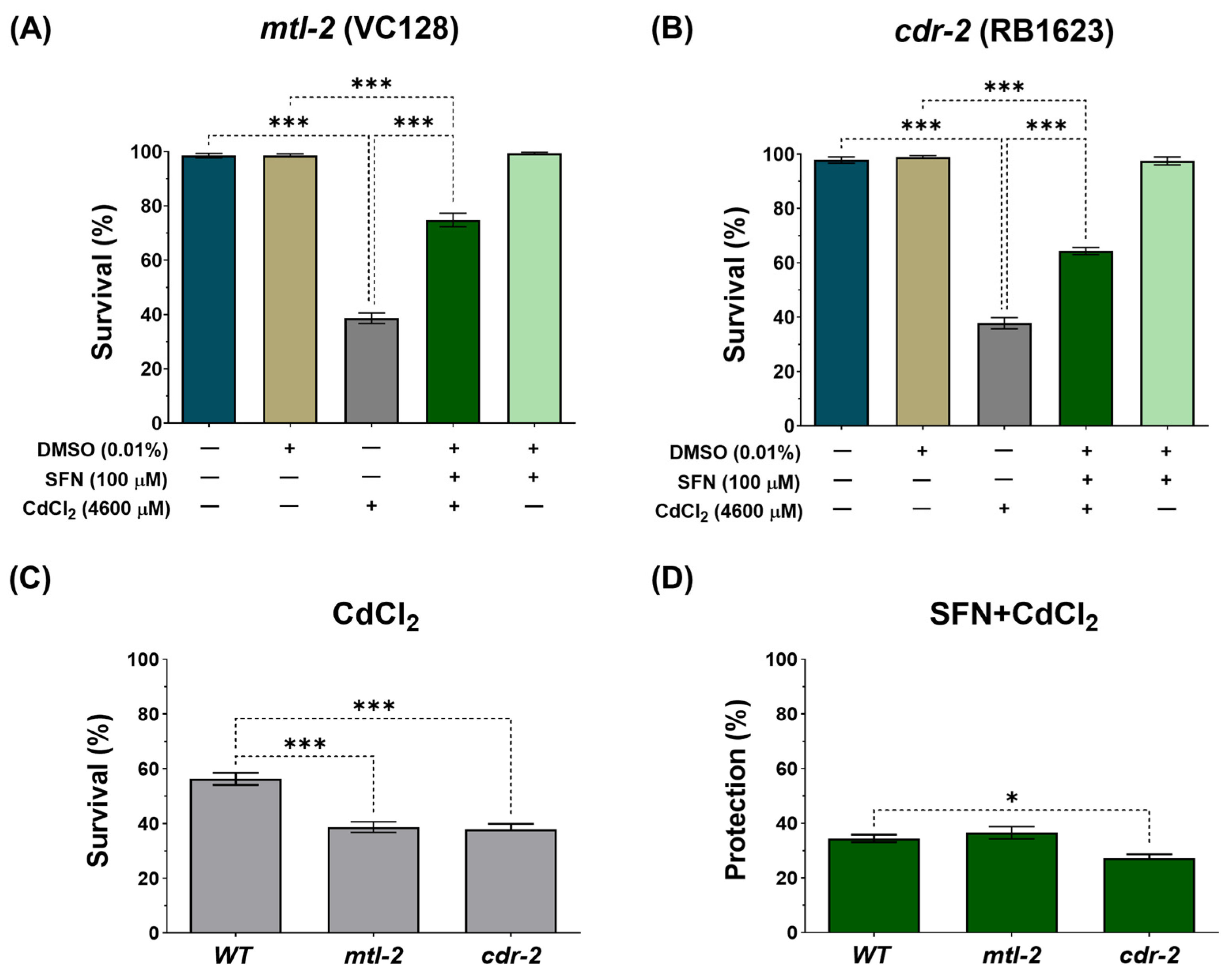
It has been reported that in response to Cd, C. elegans upregulates several hundred genes, including two metallothioneins, 1 and 2 (mtl-1 and mtl-2), and the Cd-responsive genes, cdr-1 to cdr-7 [60,61]. To assess whether these genes play a role in the protection conferred by SFN against CdCl2 toxicity, survival was evaluated in the mutant strains VC128 and RB1623, which have mutated genes mtl-2 and cdr-2, respectively. It was observed that SFN pre-exposure protects from death induced by CdCl2 exposure in both strains (Figure 4A,B). However, deleting these genes made C. elegans more hypersensitive to CdCl2 toxicity (Figure 4C). Furthermore, protection in the RB1623 strain decreased by 7.49% compared to the protection observed in the wild-type (WT) strain (Figure 4D). These data suggest that the cdr-2 gene might be associated with SFN protection against Cd toxicity.
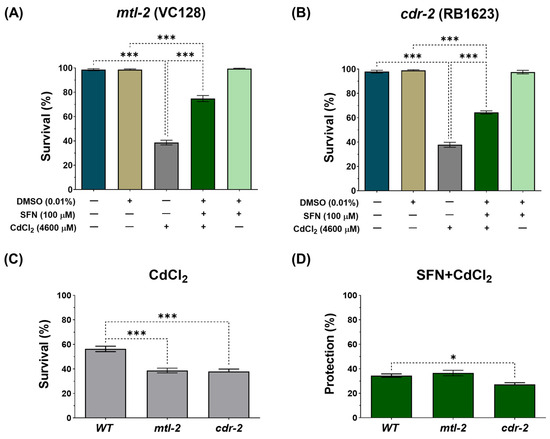
Figure 4.
Effect of pre-exposure to sulforaphane (SFN) on response genes to cadmium chloride (CdCl2) toxicity in Caenorhabditis elegans (C. elegans). Nematodes of strains VC128 (mtl-2 mutant) and RB1623 (cdr-2 mutant) in stage L1 were initially exposed to SFN. After 24 h, they were exposed again, this time to a combination of SFN and CdCl2 for another 24 h. Additional groups were created and exposed to 0.1% dimethyl sulfoxide (DMSO, SFN vehicle) for 48 h, CdCl2 (4600 μM) for 24 h, and SFN (100 μM) for 48 h. Once the exposure times were completed, survival was evaluated. (A) Survival percentage of strain VC128 (mtl-2 mutant), (B) Survival percentage of strain RB1623 (cdr-2 mutant), (C) Effect of CdCl2 on the survival of mutant strains compared to the wild type (WT), and (D) Percent survival protection in the SFN+CdCl2 mutant strain group compared to WT. mtl-2: metallothionein 2, cdr-2: cadmium response gene 2. Data are presented as mean ± SEM, n = 3 independent bioassays with 3 technical replicates each. * p < 0.05, *** p < 0.001.
3.3. SFN Prevents CdCl2-Induced Mitochondrial Dysfunction
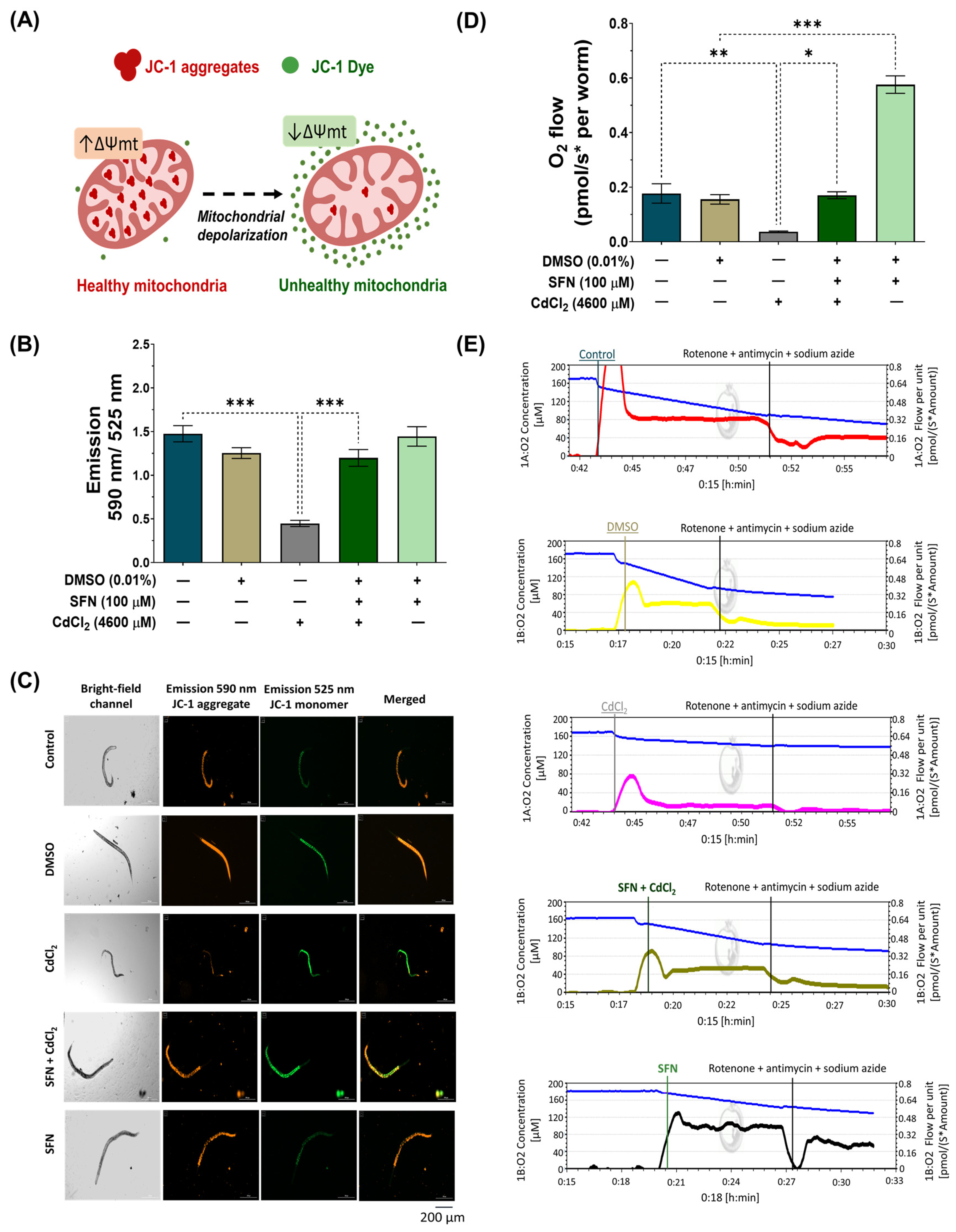
Cd directly affects mitochondrial homeostasis [11]. Thus, to assess whether SFN prevents CdCl2-induced mitochondrial dysfunction, Δψm was evaluated using the JC-1 probe, and mitochondrial-associated oxygen consumption rate was evaluated by high-resolution respirometry. For Δψm measurements, nematodes were exposed to the different treatments together with the JC-1 probe, followed by capturing images with red and green fluorescence. JC-1 is a lipophilic, cationic dye that can selectively enter mitochondria and reversibly change color from green to red with increasing membrane potential. In polarized mitochondria, JC-1 spontaneously forms complexes known as J-aggregates with intense red fluorescence. In contrast, JC-1 remains in the monomeric form in depolarized mitochondria, exhibiting only green fluorescence (Figure 5A). Our results demonstrate that in the group exposed to CdCl2, Δψm decreases by 69.67% compared to the control group. However, exposure to SFN prevents the decrease in Δψm, as there was a 62.65% increase compared to the group exposed to CdCl2, which is statistically significant. Finally, the group exposed only to SFN showed no significant effects (Figure 5B,C).
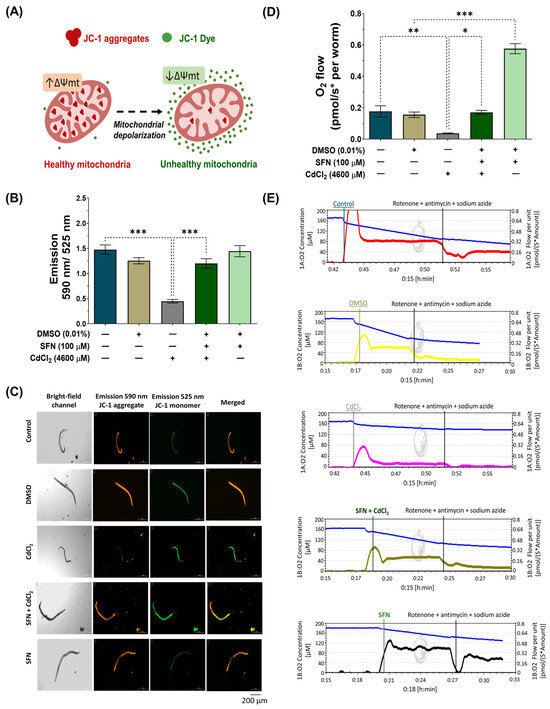
Figure 5.
Effect of sulforaphane (SFN) pre-exposure on mitochondrial dysfunction in Caenorhabditis elegans (C. elegans) exposed to cadmium chloride (CdCl2). Bristol N2 nematodes at the L1 stage were initially exposed to SFN. After 24 h, they were exposed again, this time to a combination of SFN and CdCl2 for another 24 h. Additional groups were created and exposed to 0.1% dimethyl sulfoxide (DMSO, SFN vehicle) for 48 h, CdCl2 (4600 μM) for 24 h, and SFN (100 μM) for 48 h. Once the exposure times were completed, the corresponding evaluations were carried out. (A) Schematic illustration depicting the entry of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) into mitochondria and the generation of J-aggregates; JC-1, a cationic carbocyanine dye (green), accumulates in mitochondria in a potential-dependent manner, where it forms J aggregates (red), and upon depolarization, it remains as a monomer showing green fluorescence, (B) Evaluation of Δψm with JC-1 probe by calculating the fluorescence ratio at 590 nm/525 nm, (C) Representative images of JC-1 probe fluorescence (scale bar indicates 200 μM), (D) Quantification of oxygen consumption (basal oxygen consumption minus oxygen consumption in the presence of electron transport system inhibitors), and (E) Representative graphs of oxygen consumption measurement by Oroboros Oxygraph 2K. n = 45 nematodes distributed in 3 independent experiments. Data are presented as mean ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.001.
ΔΨm is a key indicator of mitochondrial activity, as it reflects the process of electron transport that determines the oxidative phosphorylation as a driving force behind adenosine triphosphate (ATP) production [62]. Therefore, the reduction in mitochondrial respiration rate triggers lower ΔΨm, favoring the depletion in ATP production. The mitochondrial oxygen-consumption rate was evaluated to determine if SFN preserves ΔΨm by ETS maintaining. It was found that exposure to CdCl2 decreased mitochondrial oxygen consumption by 79.11% compared to the control group. Similarly to the ΔΨm assessment, the SFN+CdCl2 group preserved mitochondrial function, showing an increase of 78.29% compared to the CdCl2-exposed group. Interestingly, it was observed that the group treated only with SFN increased the mitochondrial oxygen-consumption rate above control values (Figure 5D,E).
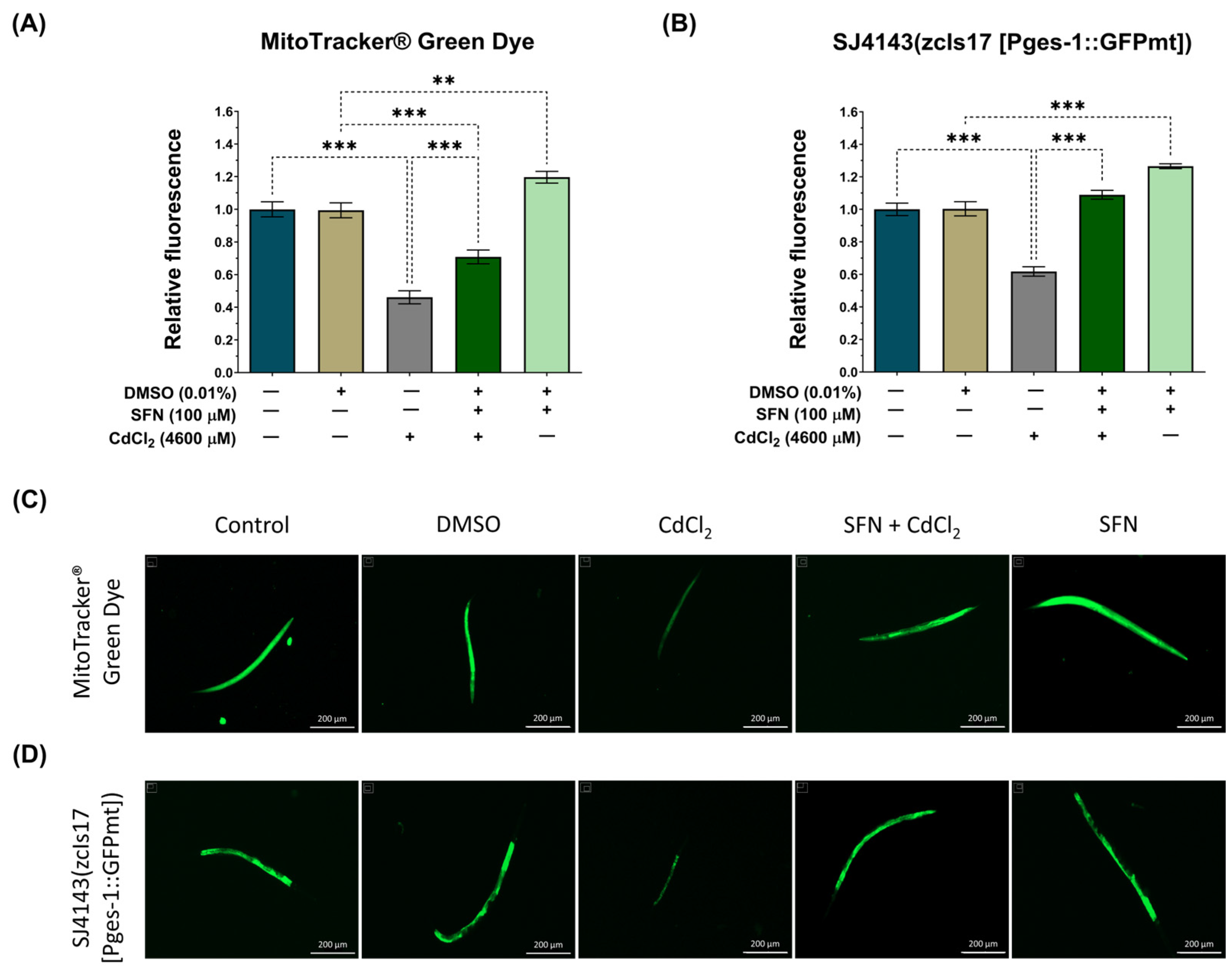
3.4. SFN Prevents the Decrease in Mitochondrial Mass Induced by CdCl2
It has been demonstrated that SFN increases mitochondrial mass in HHL-5 cells and rats fed a high-fat diet, enhancing ETS function and increasing ΔΨm [63]. Therefore, to elucidate whether the preservation of mitochondrial bioenergetics by SFN in nematodes exposed to CdCl2 was associated with increased mitochondrial mass, we utilized the MitoTracker® Green probe to assess the mitochondrial mass of the entire worm. Additionally, considering that the intestine is the primary organ exposed to this metal, where more significant damage would be expected [64], we employed the transgenic reporter strain SJ4143 zcIs17(Pges-1::GFPmt) to evaluate mitochondrial mass in the worm’s intestine. Our results show that exposure to CdCl2 decreases total and intestinal mitochondrial mass compared to the control group. Meanwhile, in the SFN+CdCl2 group, preservation of mitochondrial mass was observed. In the evaluation with the MitoTracker® Green probe, an increase of 24.39% was observed (Figure 6A,C), and in the measurement with the SJ4143 strain, an increase of 44.04% was observed (Figure 6B,D) compared to the CdCl2 group. These results suggest that one of the mechanisms by which SFN prevents mitochondrial dysfunction is by increasing mitochondrial mass. Interestingly, in the group treated only with SFN, a statistically significant increase in mitochondrial mass compared to the DMSO group was observed. This can explain the increase in the mitochondrial oxygen-consumption rate reported in Figure 5D, where a higher number of mitochondria leads to higher oxygen consumption. With the MitoTracker® Green probe, the increase was 19%, and with the SJ414 strain, the increase was 26%.
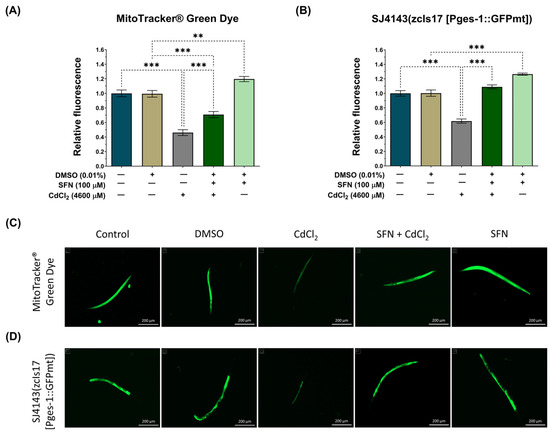
Figure 6.
Effect of sulforaphane (SFN) pre-exposition on mitochondrial mass in Caenorhabditis elegans (C. elegans) exposed to cadmium chloride (CdCl2). Bristol N2 nematodes and SJ4143 zcIs17 (Pges-1::GFPmt) nematodes at the L1 stage were initially exposed to SFN. After 24 h, they were exposed again, this time to a combination of SFN and CdCl2 for another 24 h. Additional groups were created and exposed to 0.1% dimethyl sulfoxide (DMSO, SFN vehicle) for 48 h, CdCl2 (4600 μM) for 24 h, and SFN (100 μM) for 48 h. Once the exposure times were completed, the nematodes were paralyzed with levamisole, and images were taken using the Cytation™ 5. The fluorescence intensity was evaluated using Fiji 1.54f. (A) Relative fluorescence of the MitoTracker® Green probe (n = 36 nematodes divided into 3 independent experiments), (B) Relative fluorescence of the strain SJ4143 zcIs17 (Pges-1::GFPmt) (n = 80 nematodes divided into four independent experiments), (C,D) Representative fluorescence images of the MitoTracker® Green probe and the SJ4143 zcIs17 (Pges-1::GFPmt) strain, respectively (scale bar indicates 200 μM). GFP: green fluorescent protein, mt: mitochondria. Data are presented as mean ± SEM, ** p < 0.01, *** p < 0.001.
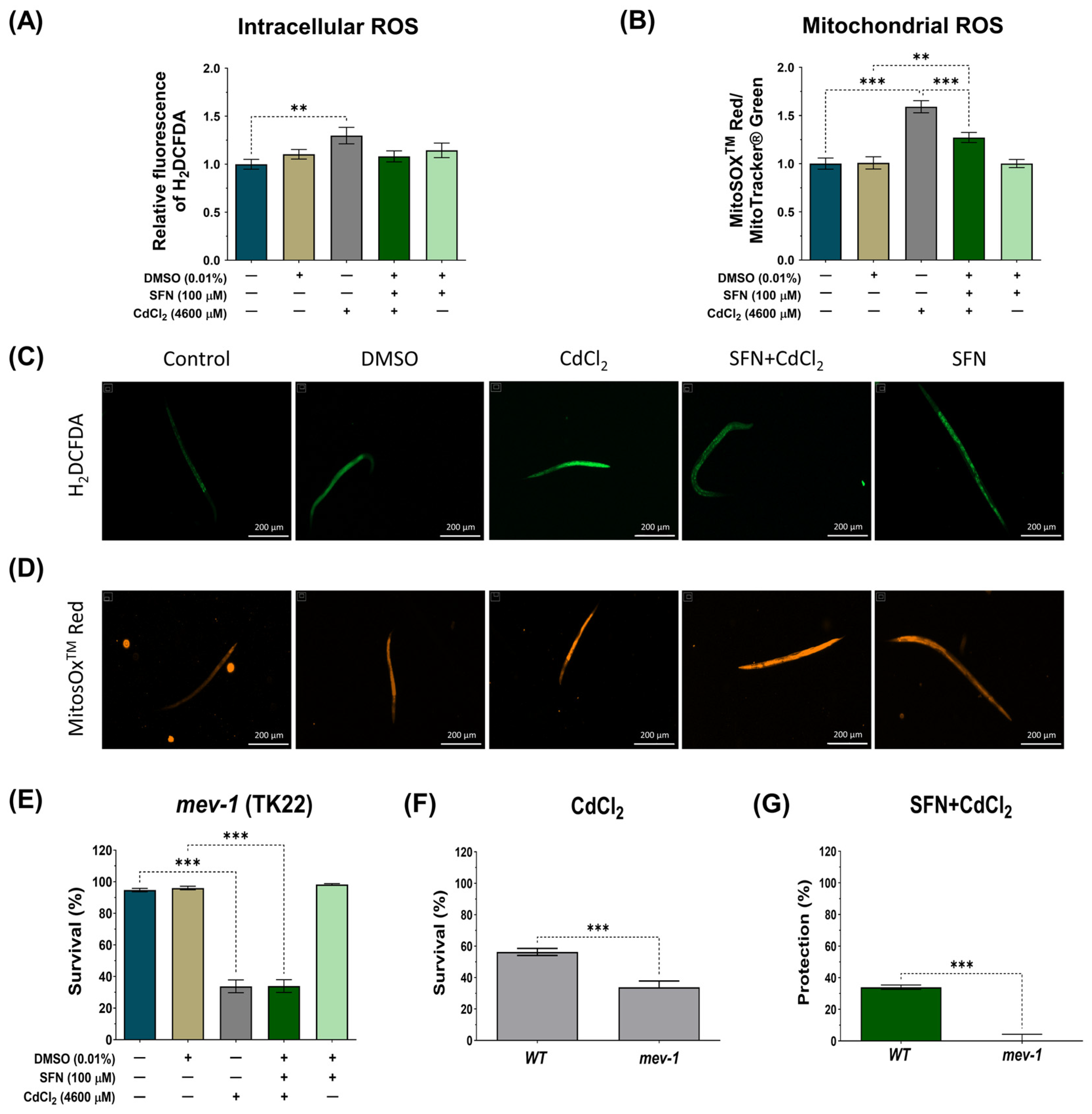
3.5. SFN Prevents Oxidative Damage Induced by CdCl2
The Cd toxicity is primarily manifested as an increase in oxidative stress [65]. Mitochondrial dysfunction is one of the main mechanisms by which Cd increases ROS levels, thereby exacerbating mitochondrial alterations and generating a vicious cycle [11]. The effect of SFN on intracellular and mitochondrial ROS levels was evaluated to determine if protecting mitochondrial function could prevent CdCl2-induced ROS production. N2 nematodes and the probes H2DCFDA and MitoSOXTM Red were used to assess intracellular and mitochondrial ROS levels, respectively. Mitochondrial ROS levels were calculated by the ratio of MitoSOXTM Red probe fluorescence to MitoTracker® Green probe fluorescence to ensure that the number of mitochondria did not mask the effects on ROS levels. In the case of intracellular ROS, CdCl2 was found to significantly increase ROS by 10.4% compared to the control group. However, although there was a trend towards decreased intracellular ROS levels in the SFN+CdCl2 group, these data were not statistically significant (Figure 7A,C). On the contrary, when mitochondrial ROS were evaluated, it was found that CdCl2 increased levels by 59.2% compared to the control group. In the SFN+CdCl2 group, mitochondrial ROS levels decreased by 20.11% compared to those exposed to CdCl2 alone (Figure 7B,D). These data suggest that SFN prevents ROS production, mainly in the mitochondria.
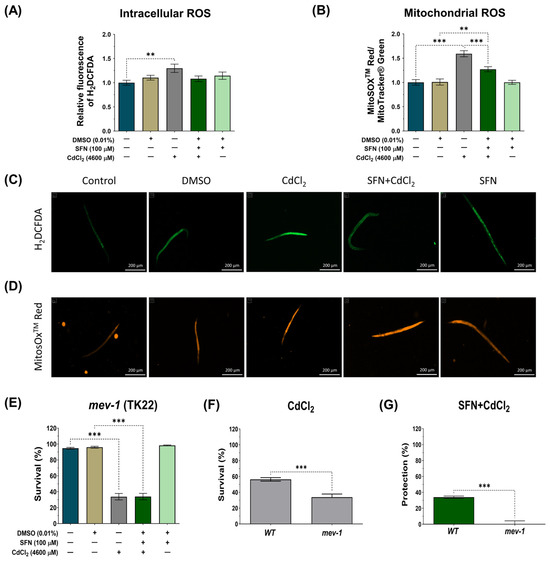
Figure 7.
Protective effect of sulforaphane (SFN) pre-exposure against oxidative damage induced by cadmium chloride (CdCl2) in Caenorhabditis elegans (C. elegans). Bristol N2 and nematodes of strain TK22 (mev-1 mutants) were initially exposed to SFN. After 24 h, they were exposed again, this time to a combination of SFN and CdCl2 for another 24 h. Additional groups were created and exposed to 0.1% dimethyl sulfoxide (DMSO, SFN vehicle), CdCl2 (4600 μM) for 24 h, and SFN (100 μM) for 48 h. Once the exposure times were completed, the corresponding evaluations were carried out. (A) Relative fluorescence of the 2′,7′-dichlorofluorescein diacetate (H2DCFDA) probe representing intracellular reactive oxygen species (ROS) (n= 45 nematodes divided into 3 independent experiments), (B) Ratio of MitoSOXTM Red/MitoTracker® Green to determine the Mitochondrial ROS (n = 36 nematodes divided into 3 independent experiments), (C,D) Representative images of the fluorescence of the H2DCFDA and MitoSOXTM Red probes, respectively (scale bar indicates 200 μM), (E) Percent survival of strain TK22 (mev-1 mutant) (n = three independent bioassays with 3 technical replicates each), (F) Effect of CdCl2 on strain survival of strain TK22 (mev-1 mutant) compared to wild strain (WT) represented in percentage, and (G) Percentage of survival protection in the SFN+CdCl2 group of strain TK22 (mev-1 mutant) compared to WT. Data are presented as mean ± SEM, ** p < 0.01, *** p < 0.001.
On the other hand, in C. elegans, mev-1 encodes a subunit of succinate-coenzyme Q oxidoreductase in complex II of the electron transport chain [66,67]. Mutation of mev-1 increases superoxide levels and hypersensitivity to oxygen, indicating that mev-1 may modulate the cellular response to oxidative stress [66,67]. It has also been observed that mev-1 governs the rate of aging by modulating the cellular response to oxidative stress in C. elegans [66]. Therefore, we investigated survival in the TK22 strain with a loss-of-function mutation in mev-1 exposed to CdCl2 and evaluated the effect of SFN on it to determine its protective capacity against oxidative damage. As shown in Figure 7F, the mev-1 mutation resulted in hypersensitivity to CdCl2; however, this effect was not attenuated with SFN treatment, as indicated in Figure 7E,G. These data suggest that the protective effect of SFN against CdCl2-induced oxidative damage may occur through a mev-1-dependent pathway in nematodes.
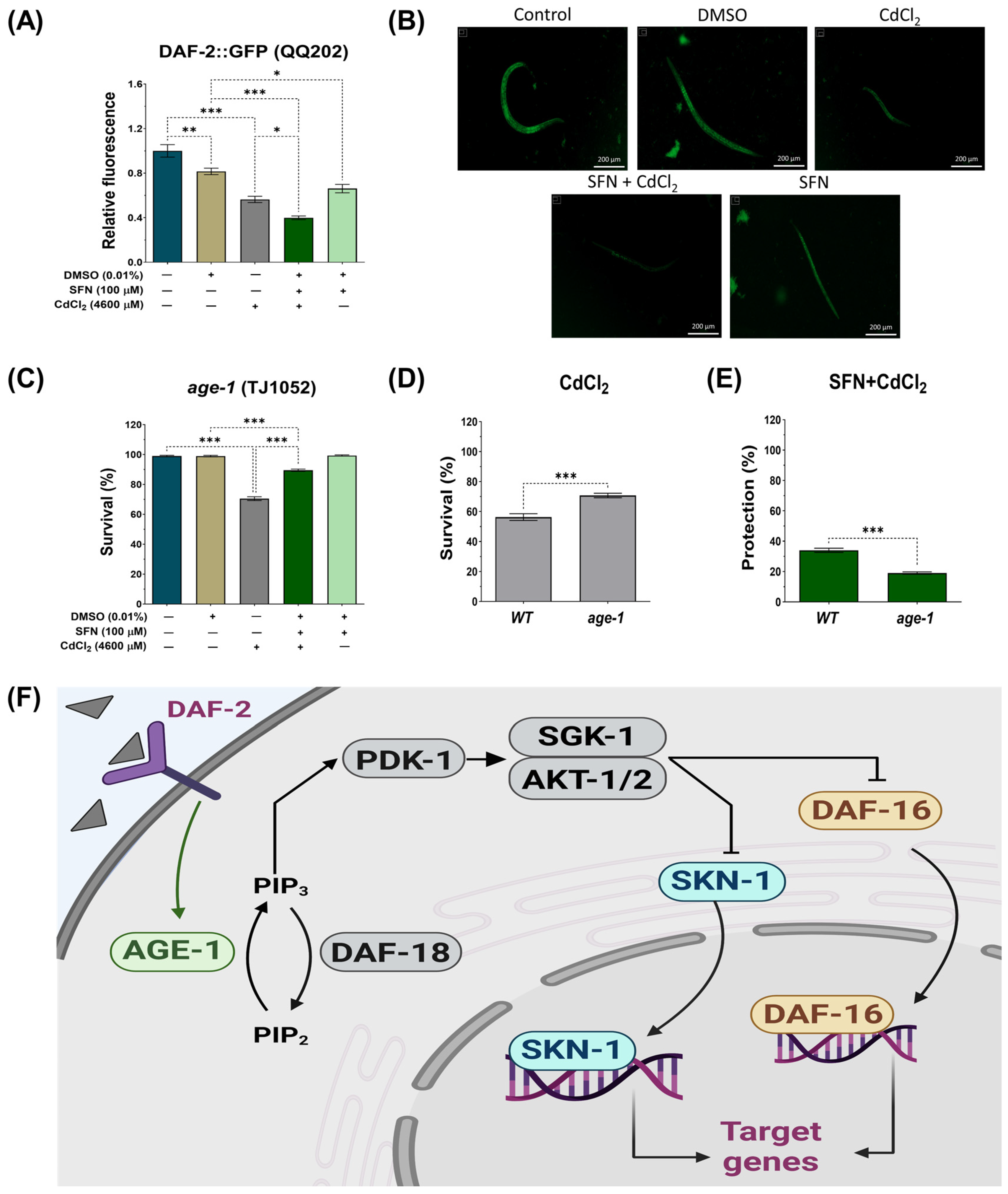
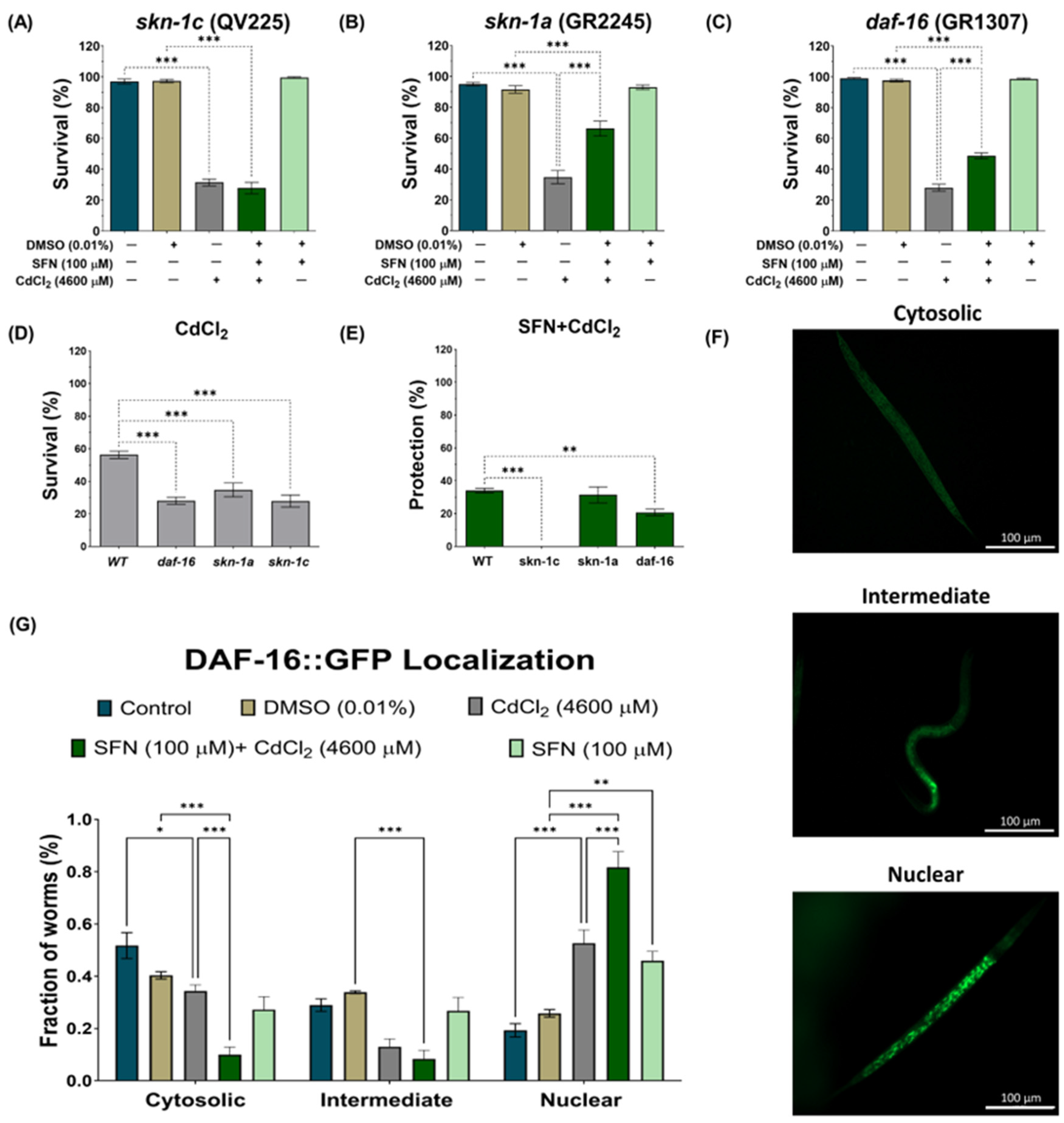
3.6. SFN-Mediated Protection against CdCl2 Toxicity Is Mediated by the IIS Pathway
The IIS pathway in C. elegans regulates various biological processes, including metabolism, growth, development, longevity, stress response, and behavior [31]. This essential pathway operates through the interaction of insulin-like peptide ligands with the transmembrane receptor DAF-2, an analog of the insulin/insulin-like growth factor-1 (IGF-1) receptor (IGFR). Activation of DAF-2/IGFR triggers a series of events, including activation of phosphatidylinositol 3-kinase (AGE-1), which generates phosphatidylinositol 3,4,5-trisphosphate (PIP3). In turn, PIP3 activates 3-phosphoinositide-dependent protein kinase 1 (PDK-1), which phosphorylates and activates protein kinases B (AKT1-2) and glucocorticoid- and serum-regulated kinase 1 (SGK-1). This complex process culminates in regulating transcription factors such as DAF-16, which controls most of the functions of this pathway, and skn-1 [31] (Figure 8F). To determine whether the IIS pathway is involved in protecting SFN against Cd toxicity, the expression of DAF-2 was determined using strain QQ202 (daf-2 reporter) and the survival of worms from strain TJ1052 (age-1 mutant). It was observed that exclusive exposure to CdCl2 caused a 44% reduction in DAF-2 expression compared to the control group. However, this decrease was even more pronounced in the SFN+CdCl2 group, where a 50.72% reduction was recorded compared to the DMSO group and 32.82% compared to the group exposed only to CdCl2 (Figure 8A,B). These findings indicate that pre-exposure of SFN to CdCl2 exerts a synergistic effect in reducing DAF-2 expression.
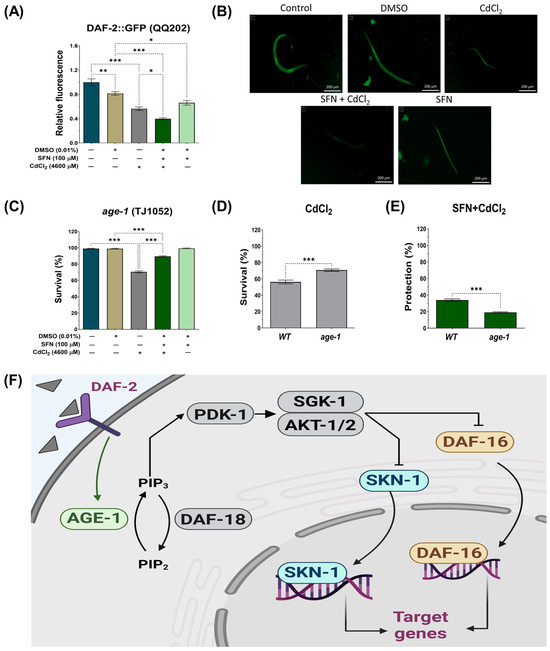
Figure 8.
Effect of sulforaphane (SFN) pre-exposure on the insulin/insulin-like growth factor (IIS) signaling pathway in Caenorhabditis elegans (C. elegans) exposed to cadmium chloride (CdCl2). Nematodes of strains QQ202 (daf-2 reporter) and TJ1052 (age-1 mutant) at the L1 stage were initially exposed to SFN. After 24 h, they were exposed again, this time to a combination of SFN and CdCl2 for another 24 h. Additional groups were created and exposed to 0.1% dimethyl sulfoxide (DMSO, SFN vehicle) for 48 h, CdCl2 (4600 μM) for 24 h, and SFN (100 μM) for 48 h. Once the exposure times were completed, the corresponding evaluations were carried out. (A) Relative fluorescence of DAF-2::GFP (n = 60 nematodes divided into 4 independent experiments), (B) representative fluorescence images of strain QQ202 (daf-2(cv20[daf-2::GFP]) (scale bar indicates 200 μM), III), (C) percent survival of strain TJ1052 (age-1 mutant), (D) effect of CdCl2 on the survival of strain TJ1052 (age-1 mutant) compared to wild type (WT), (E) percentage of survival protection in the SFN+CdCl2 group of strain TJ1052 (age-1 mutant) compared to WT (n= 3 independent bioassays with 3 technical replicates each), and (F) schematic representation of signaling the IIS path. age-1: phosphatidylinositol 3-kinase orthologous gene, AGE-1: phosphatidylinositol 3-kinase gene protein, AKT1-2: protein kinases B, daf-16: forkhead box O (FoxO) orthologous gene, DAF-18: PTEN/human tumor suppressor homolog, daf-2: a receptor tyrosine kinase (IGFR) homolog, DAF-2: receptor tyrosine kinase (IGFR) homologous gene protein, PDK-1: 3-phosphoinositide-dependent protein kinase 1, PIP3: phosphatidylinositol 3,4,5-trisphosphate, SGK-1: glucocorticoid-regulated kinase 1, SKN-1c: ortholog of nuclear factor erythroid 2-related factor 2 (Nrf2). Data are presented as mean ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.001.
On the other hand, when evaluating the survival of the age-1 mutant, it was observed that pre-exposure to SFN continued to protect against CdCl2-induced death. Although this protection was decreased compared to the WT strain, survival still reached almost 90% (Figure 8C,E). Notably, in worms exposed exclusively to CdCl2, survival increased compared to the WT strain, suggesting that age-1 mutants are more resistant to CdCl2 (Figure 8D).
To further elucidate our findings, we examined whether SFN could target the transcription factor DAF-16/FOXO and SKN-1/Nrf downstream of DAF-2. SKN-1/Nrf proteins are members of the cap ‘n’ collar (CNC) family of transcription factors, which act as master regulators of oxidative stress resistance and longevity [68]. The skn-1 gene encodes four major predicted isoforms, of which, three are expressed in vivo (skn-1a to skn-1c) [69]. In particular, the skn-1c isoform has been described as the ortholog of Nrf2 in mammals, one of the main targets of SFN [17]. Furthermore, skn-1c has been reported to be important for mitochondrial biogenesis and health in C. elegans [70]. To determine whether skn-1c plays a role in protecting SFN against CdCl2 toxicity, survival was assessed in strain QV225 (skn-1c mutant). As expected, deletion of skn-1c caused nematodes to be hypersensitive to CdCl2 toxicity (Figure 9D). Furthermore, it was observed that SFN lost its ability to prevent the decline in survival in nematodes exposed to CdCl2, as protection decreased from 34.01% in WT to −3.604% in skn-1c mutants (Figure 9A,E). These results indicate that skn-1c is crucial in protecting SFN against CdCl2 toxicity. Subsequently, we evaluated whether the skn-1a isoform could also be involved in the protection of SFN against CdCl2 toxicity. skn-1a is the ortholog of Nrf1 in mammals and has been detected in preparations of endoplasmic reticulum (ER) and mitochondria. Its main functions have been associated with the ER stress response and support of proteostasis [69]. This evaluation was included because Cd has been shown to alter protein folding in the ER and induce an unfolded protein response [71], which has also been associated with mitochondrial alterations [72]. However, our results showed that skn-1a is unrelated to SFN protection, as similar protection is still observed in strain GR2245 (skn-1a mutant) compared to the WT strain (Figure 9B,E). In particular, nematodes were more sensitive to CdCl2 toxicity in the mutant strain than in the WT (Figure 9D). Another gene involved in resistance to oxidative stress is daf-16, a human homolog of FoxO [73,74]. It was recently shown that SFN increased the nuclear translocation of DAF-16, thereby increasing the longevity of C. elegans [47]. Furthermore, DAF-16 is important in responding to Cd toxicity [28]. Therefore, to determine the role of DAF-16 in protecting SFN against CdCl2 toxicity, the survival of the mutant strain GR1307 was evaluated. We found that daf-16 deletion nematodes showed increased sensitivity to CdCl2 toxicity (Figure 9D), while exposure to SFN prior to CdCl2 increased survival by 19.61% compared to exclusive exposure to CdCl2 (Figure 9C). Despite this, the protection offered is significantly lower than that observed in the WT strain (Figure 9E). Additionally, we determined the nuclear translocation of DAF-16 using strain OH16024 with GFP markers. The nuclear localization of DAF-16::GFP was classified as cytosolic, intermediate, or nuclear, as proposed by Oh et al. [55] (Figure 9F). Exposure to CdCl2 alone was found to increase DAF-16 nuclear translocation compared to the control group; however, in the SFN+CdCl2 group, the increase in nuclear translocation was much more significant compared to the CdCl2 group (Figure 9G). Together, these findings point out that regulation of the IIS pathway is critical for SFN-mediated protection against CdCl2 toxicity in C. elegans, particularly in the activation of DAF-16/FOXO and SKN-1c/Nrf2.
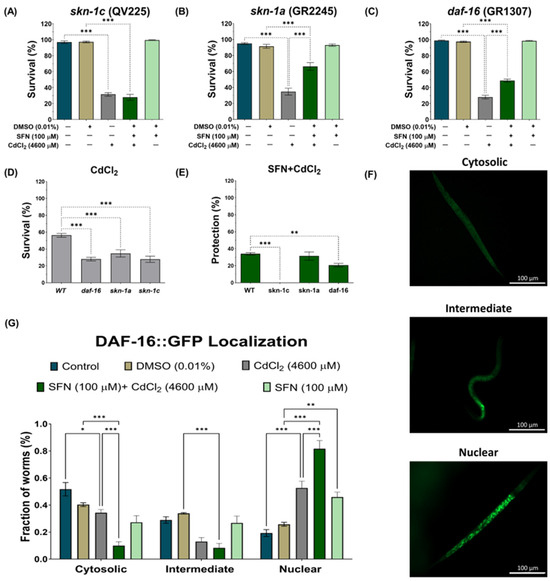
Figure 9.
Exploration of the participation of skn-1 and daf-16 in the protection of sulforaphane (SFN) pre-exposure against cadmium chloride (CdCl2) toxicity in Caenorhabditis elegans (C. elegans). Nematodes of strains QV225 (skn-1c mutant), GR2245 (skn-1a mutant), GR1307 (daf-16 mutant), and OH16024 (daf-16 reporter) at the L1 stage were initially exposed to SFN. After 24 h, they were exposed again, this time to a combination of SFN and CdCl2 for another 24 h. Additional groups were created and exposed to 0.1% dimethyl sulfoxide (DMSO, SFN vehicle) for 48 h, CdCl2 (4600 μM) for 24 h, and SFN (100 μM) for 48 h. Once the exposure times were completed, the corresponding evaluations were carried out. (A) Percentage survival of strain QV225 (skn-1c mutant), (B) percentage survival of strain GR2245 (skn-1a mutant), (C) percentage survival of strain GR1307 (daf-16 mutant), (D) effect of CdCl2 on the survival of mutant strains compared to wild type (WT), (E) percentage of survival protection in the SFN+CdCl2 mutant strain group compared to WT, (F) representative images of the cytosolic, intermediate, and nuclear categories, used to evaluate the nuclear translocation of DAF-16 and (G) nuclear localization of DAF-16 presented as the fraction of nematodes classified in each category (cytosolic, intermediate and nuclear). n of the survival assays = 3 independent bioassays with 3 technical replicates, and n of the nuclear translocation assay = 60 nematodes divided into three independent experiments. daf-16: forkhead box O orthologous gene (FoxO), DAF-16: FoxO orthologous gene protein, GFP: green fluorescent proteins, skn-1c: nuclear factor erythroid-related 2 (Nrf2) ortholog, skn1a: ortholog of Nrf1. Data are presented as mean ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
The purpose of this study was to examine whether exposure to SFN can influence the toxicity and mitochondrial dysfunction induced by Cd in the nematode C. elegans, as well as to explore any possible involvement of the IIS pathway in this process. Our results revealed that prior exposure to SFN prevents the toxic effects induced by CdCl2, since it was observed that the SFN+CdCl2 group maintained survival above 90%. Furthermore, there was a significant increase in lifespan, body length, and mobility, as well as a decrease in lipofuscin levels, compared to the group exposed exclusively to CdCl2 (Figure 2 and Figure 3). Although previous studies had demonstrated the ability of SFN to mitigate Cd cytotoxicity [19,20,21,22,23,24], this is the first study to demonstrate a similar effect in the nematode C. elegans.
It is currently known that mitochondrial dysfunction is key in Cd toxicity, and previous studies have shown that SFN can improve mitochondrial malfunction [75,76]. However, there is limited information on whether SFN specifically prevents Cd-induced mitochondrial dysfunction. Exposure to Cd triggers oxidative stress, damaging mitochondria and affecting their energy efficiency, which impacts cellular health and organism functioning. Various studies indicate that Cd also directly affects mitochondrial function, altering internal metabolic processes [11,14]. Cd enters mitochondria through the voltage-dependent anion channel (VDAC), divalent metal transporter 1 (DMT1), and mitochondrial calcium uniporter (MCU) [77,78]. Once inside, it can disrupt the electron transport system, increase ROS production, and dissipate ΔΨm, triggering mitochondrial uncoupling [11,13]. Additionally, it reduces enzymatic activity in the Krebs cycle, damages mitochondrial DNA, and decreases mitochondrial biogenesis [79,80]. The Cd also affects the expression of key genes such as sirtuin 1 (Sirt1), nuclear respiratory factors 1 (NRF1), peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α), and mitochondrial transcription factor A (TFAM), influencing the regulation and transcription of key mitochondrial proteins [81]. Our results indicate that exposure to SFN prevents the decrease in ΔΨm and the mitochondrial oxygen consumption rate, both affected by CdCl2 exposure (Figure 5). Additionally, pre-exposure to SFN before CdCl2 significantly decreases the level of mitochondrial ROS compared to the group exposed exclusively to CdCl2 (Figure 7B). Furthermore, a significant increase in mitochondrial mass was observed in the SFN+CdCl2 group (Figure 6). The beneficial effects on mitochondrial health that we observed suggest that exposure to SFN prevents CdCl2-induced mitochondrial dysfunction, and this could be primarily due to the increase in mitochondrial mass. Previous studies have suggested that SFN increases mitochondrial biogenesis in mice with accelerated senescence, which could explain the improvement in ETS activity, restoring ΔΨm [82]. It has also been demonstrated that SFN enhances multiple mitochondrial bioactivities and bioenergetic parameters such as ΔΨm, ATP, and ETS by promoting PGC-1α-dependent mitochondrial biogenesis and improving Nrf2-dependent mitochondrial redox regulation in HHL-5 cells and in rats fed a high-fat diet [63]. Additionally, among the mechanisms of compounds studied as potential strategies to protect against Cd damage is the restoration of mitochondrial mass, primarily by restoring mitochondrial biogenesis and regulating redox balance [80,83]. Furthermore, improvement in mitochondrial biogenesis has been associated with decreased ROS production and increased longevity [84]. Therefore, the results of our study suggest that SFN promotes mitochondrial restoration by increasing mitochondrial mass, which could contribute to its ability to mitigate Cd toxicity.
It is important to note that when evaluating intracellular ROS, no significant effect was observed in the SFN+CdCl2 group (Figure 7A). This lack of effect could be attributed to a masking phenomenon due to the increased mitochondrial mass. In other words, intracellular ROS might be slightly elevated in the SFN+CdCl2 group due to the increased mitochondrial mass observed in this study. Therefore, although there is a trend towards decreased intracellular ROS in this group, this effect did not reach statistical significance. This phenomenon was not observed in the assessment of mitochondrial ROS, as the fluorescence of MitoSOXTM Red was corrected with MitoTracker® Green fluorescence, which was used to measure mitochondrial mass to avoid this issue.
The specificity of probes for ROS detection could also be important in understanding the mechanisms of action of compounds, such as SFN, in protecting against heavy metal-induced oxidative stress like Cd. It has been observed that the H2DCFDA probe is more sensitive to hydrogen peroxide (H2O2), hydroxyl radicals (•OH), and peroxyl radicals (ROO), while the MitoSOXTM Red probe primarily targets superoxide anion (O2•−) [30]. This differentiation is crucial since different ROS species may impact cellular and tissue health differently. In our study, the MitoSOXTM Red probe’s observation of a decrease in O2•− levels in the SFN+CdCl2 group could suggest a protective effect of SFN, specifically, against this ROS species. The enzyme superoxide dismutase (SOD) is responsible for reducing O2•− to H2O2. In the case of C. elegans, this organism possesses five SOD genes encoding two cytosolic CuZn-SOD enzymes (sod-1 and sod-5), two mitochondrial Mn-SOD enzymes (sod-2 and sod-3), and one extracellular CuZn-SOD enzyme (sod-4) [85]. It is known that Cd affects all isoforms. In Mn-SOD isoforms, Cd can replace the manganese ion, thereby reducing its activity, while in CuZn-SOD isoforms, momentary inhibition is attributed to a Cd/enzyme interaction [86]. Additionally, Cd directly damages the structure of the antioxidant enzymes catalase and glutathione peroxidase, which are responsible for reducing H2O2 to water [65,87]. SFN can eliminate both the O2•− and H2O2 through a double hydrogen transfer without barriers or with low energy barriers, a process that requires the presence of Fe-SOD [88]. Additionally, it has been previously demonstrated that SFN increases the expression of antioxidant enzymes, either depending on the transcription factor Nrf2 or, in the case of C. elegans, through skn-1c, its orthologue [89,90]. Furthermore, in C. elegans, it has been observed that the transcription factor daf-16 also activates key antioxidant enzymes [91,92]. It is important to note that in situations of excessive damage, such as a mutation in the mev-1 gene, the protective potential of SFN is lost (Figure 7G), suggesting that the integrity of the succinate dehydrogenase enzyme is crucial in SFN’s protective effect against Cd toxicity. It has been reported that the mev-1 gene plays an important role in regulating ROS and stress tolerance [93]. Mutation in the mev-1 gene has been shown to increase Cd toxicity [94,95]. Genetic variation in mev-1 is associated with increased in O2•− levels and greater susceptibility to oxygen [66,67].
SFN acts as an indirect antioxidant, exerting its primary effect on oxidative stress and mitochondrial dysfunction through the activation of transcription factors such as skn-1/Nrf2 and daf-16/FoXO [47,96]. In C. elegans, both transcription factors belong to the pathway IIS. Therefore, the role of this pathway in SFN’s ability to protect against mitochondrial damage and Cd-induced toxicity has been investigated (Figure 8 and Figure 9). Firstly, we assessed the expression of DAF-2 and a mutant strain of the age-1 gene. Both DAF-2 and AGE-1 are upstream regulators of SKN-1 and DAF-16, responsible for phosphorylating these transcription factors and preventing their translocation into the nucleus [31]. Our results indicate a decrease in DAF-2 expression in the SFN+CdCl2-treated group. Additionally, upon evaluating the survival of the age-1 mutant strain, we found that SFN continued to protect against nematode mortality compared to the group exposed solely to CdCl2 (Figure 8). These findings suggest that skn-1 and daf-16 may not be phosphorylated and, therefore, may be activated. To support these hypotheses, we assessed survival in the QV225 (skn-1c) and GR1307 (daf-16) mutant strains, where we observed a decrease in the protection provided by SFN against Cd toxicity in the SFN+CdCl2 group compared to the WT strain. Furthermore, an increase in nuclear translocation of DAF-16 was observed in this group. These results indicate that the presence of these genes is essential for the protection conferred by SFN against Cd and that SFN may be activating them by regulating the IIS pathway. It has been previously reported that SFN extends lifespan and improves health in C. elegans by regulating IIS pathway signaling and activating the nuclear transcription factor DAF-16/FoxO [47]. Recent studies confirm our assumptions and show that various natural compounds use insulin signaling to enhance the oxidative stress response [97,98]. Notably, in mammals, SFN also regulates Nrf2 activation, the ortholog of the skn-1c gene. Mechanically, SFN binds to cysteine residues of Kelch-like ECH-associated protein 1 (Keap1), the inhibitor of Nrf2, inducing conformational changes that promote the release of “sequestered” Nrf2 and its subsequent translocation to the nucleus [99,100].
Additionally, the regulation of the IIS pathway by SFN, leading to the activation of skn-1 and daf-16, would account for the observed protection against mitochondrial alterations. It has been observed that skn-1c is crucial for the expression of various genes related to mitochondrial biogenesis and mitochondrial health in C. elegans. It was discovered that skn-1 associates with proteins of the outer mitochondrial membrane [101]. The transcriptional activity of SKN-1 is essential for mitohormesis-mediated longevity and for maintaining mitochondrial homeostasis. Indeed, the reduction of skn-1 alters the morphology of the mitochondrial network, causes mitochondrial membrane depolarization, and increases cytoplasmic Ca2+ concentration [70]. Particularly, the reduction of SKN-1 decreases mitochondrial DNA content, highlighting its role in maintaining mitochondrial integrity [70].
Finally, another gene that plays a role in protection against Cd toxicity is cdr-2. Our findings revealed that the cdr-2 gene might be implicated in the protection provided by SFN, as a decrease in SFN protection against CdCl2 was observed in the cdr-2 mutant strain compared to the WT strain (Figure 4D). Ji-Yeon and colleagues demonstrated that the removal of cdr-2 increased mortality and reduced reproductive potential in Cd-exposed C. elegans nematodes [56]. The cdr genes encode integral membrane proteins, primarily localized to the lysosomal membrane [60,102]. It has been postulated that alterations in the biological activity of cdr genes may trigger imbalances in fluids or disruptions in the osmoregulation of C. elegans [60,102]. Disruption in osmolarity has been shown to have various adverse effects on mitochondria, interfering with their normal function in energy production and other essential cellular processes. This includes affecting ΔΨm, ion transport, membrane permeability, and ATP generation, leading to reduced efficiency of cellular respiration and increased ROS production [103,104,105]. Despite sufficient evidence that Cd increases cdr-2 expression as a protective mechanism, this study represents the first to establish a connection between SFN and cdr-2 gene function, generating the hypothesis that cdr-2 may be relevant to the protection conferred by this antioxidant. Conducting additional transcriptomic and proteomic experiments would be crucial to validate these associations. Furthermore, it would be interesting to explore the impact of SFN on cdr-1, which is the most specifically Cd-sensitive gene within the cdr family.
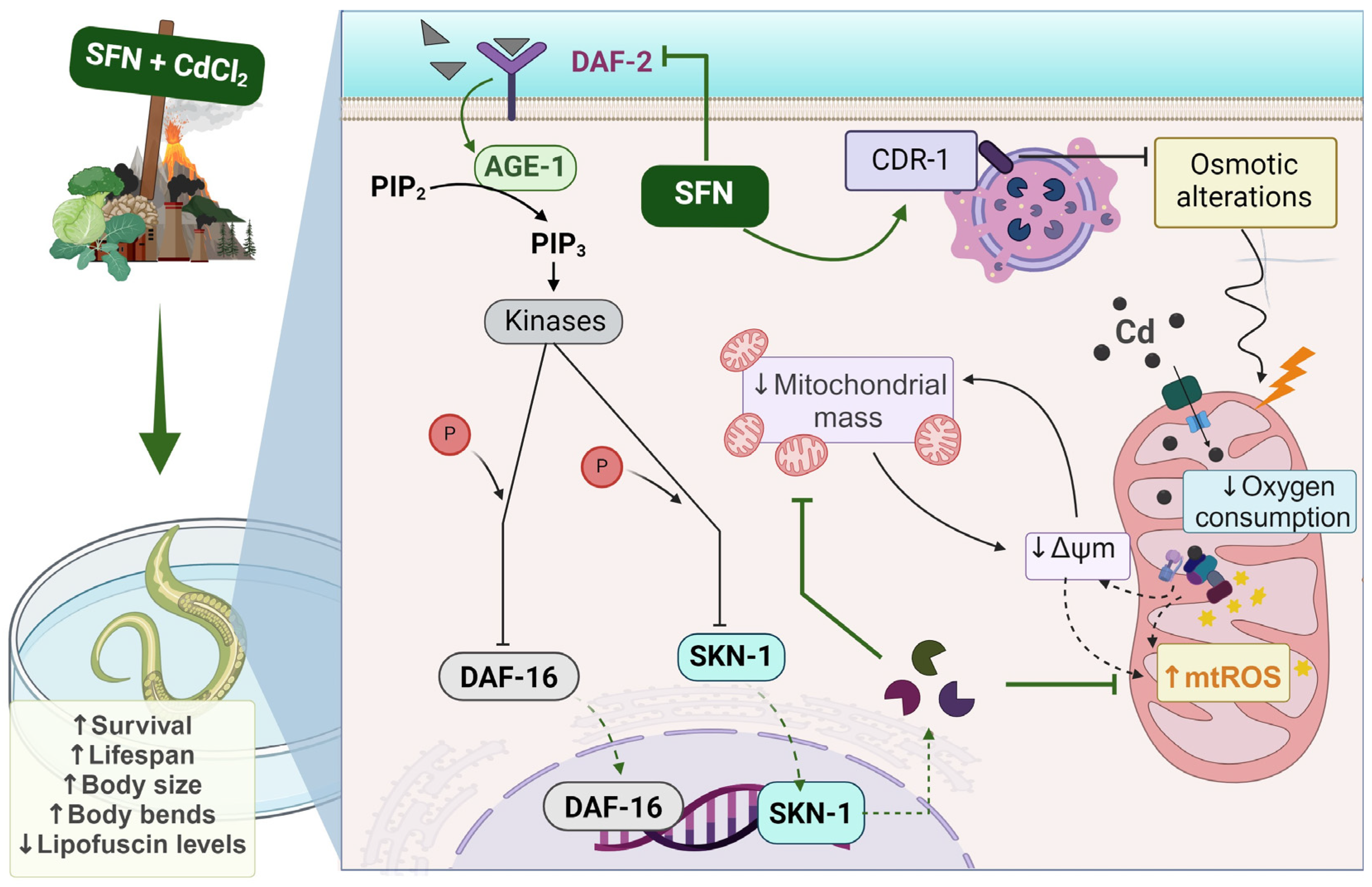
As shown in the integrative scheme (Figure 10), our results suggest that exposure to Cd induces toxicity and alterations in mitochondrial function, such as decreased ΔΨm, mitochondrial oxygen consumption, and increased ROS levels, which may be attributable to reduced mitochondrial mass. On the other hand, SFN may prevent mitochondrial alterations by increasing mitochondrial mass through the regulation of the IIS pathway.
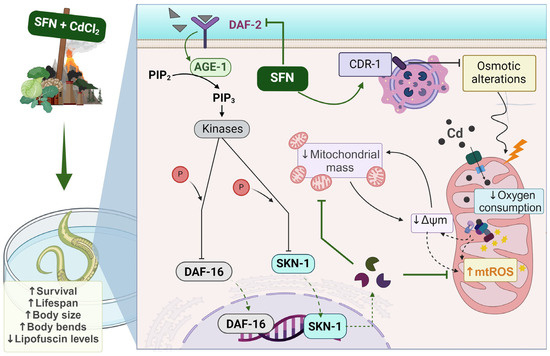
Figure 10.
Integrative scheme. Cadmium (Cd) ingresses into the mitochondria and induces toxicity and alterations in mitochondrial function, such as the reduction of mitochondrial membrane potential (ΔΨm), mitochondrial oxygen consumption, and the increase of mitochondrial reactive oxygen species (ROS), which may be attributable to the reduction of mitochondrial mass. Conversely, sulforaphane (SFN) can prevent mitochondrial alterations by increasing mitochondrial mass through the regulation of the IIS pathway. Furthermore, the Cd response gene (CDR-1) could be implicated in Cd protection. Ultimately, the effect of SFN on mitochondrial alterations is evidenced by enhanced health in the nematode Caenorhabditis elegans (C. elegans). AGE-1: phosphatidylinositol 3-kinase gene protein, CdCl2: cadmium chloride, DAF-16: forkhead box O (FoxO) orthologous gene, DAF-2: receptor tyrosine kinase (IGFR) homologous gene protein, P: phosphate group, PDK-1: 3-phosphoinositide-dependent protein kinase 1, PIP3: phosphatidylinositol 3,4,5-trisphosphate, SKN-1c: ortholog of nuclear factor erythroid 2-related factor 2 (Nrf2). Created with biorender.com (published with permission from biorender.com).
5. Conclusions
Exposure to SFN prior to CdCl2 exposure mitigates toxic effects and mitochondrial alterations, possibly by increasing mitochondrial mass, which may be related to the regulation of the IIS pathway. Additionally, the possibility arises that the genes mev-1 and cdr2 are important for the protection provided by SFN. These findings open up new possibilities for developing therapies to mitigate damage caused by Cd toxicity and oxidative stress in animals, highlighting mitochondria-targeted antioxidants as a promising tool.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13050584/s1, Figure S1: Calculation of the mean lethal concentration (LC50). Table S1: Characterization of C. elegans wildtype and mutant strains.
Author Contributions
Conceptualization, E.Y.H.-C., V.J.V. and J.P.-C.; methodology, E.Y.H.-C., O.E.A.-T. and M.Z.-L.; formal analysis, E.Y.H.-C., O.E.A.-T., D.E.-P., E.J.-P. and M.Z.-L.; investigation, E.Y.H.-C.; resources, V.J.V. and J.P.-C.; writing—original draft preparation, E.Y.H.-C.; writing—review and editing, E.Y.H.-C., V.J.V. and J.P.-C.; supervision, E.Y.H.-C., V.J.V. and J.P.-C.; project administration, E.Y.H.-C., V.J.V. and J.P.-C.; funding acquisition, V.J.V. and J.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT) México, Grants Number A1-S-7495, by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), Grant Numbers IN202219 and IN200922 of the Universidad Nacional Autonoma de México (UNAM), and by Programa de Apoyo a la Investigacion y el Posgrado (PAIP), Grant Number 5000-9105 to J.P.-C. Also, CONAHCYT Ciencia Basica 0284867 and PAPIIT IN203820 and IN217824 to V.J.V.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
All the authors are thankful to their respective affiliated institutions for providing research facilities to carry out this work. We thank the Caenorhabditis Genetics Center (CGC) at the University of Minnesota for its support and Nallely Cano-Domínguez for her technical support. Estefani Yaquelin Hernández-Cruz is a doctoral student from Programa de Doctorado en Ciencias Biológicas from Universidad Nacional Autónoma de México (UNAM). She received fellowships 779741 (No. CVU: 853816) from CONAHCyT.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
| age-1 | orthologous gene of phosphatidylinositol 3-kinase |
| C. elegans | Caenorhabditis elegans |
| Cd | cadmium |
| CdCl2 | cadmium chloride |
| cdr-1 o 2 | cadmium response genes |
| CI95 | confidence interval 95% |
| daf-16 | orthologous gene of forkhead box O (FoxO) |
| DAF-16 | protein of the orthologous gene of forkhead box O (FoxO) |
| DAF-18 | human tumor suppressor homologue/PTEN |
| daf-2 | homolog of tyrosine kinase receptor (IGFR) |
| DAF-2 | protein of gen homolog de tyrosine kinase receptor (IGFR) |
| DMSO | dimethyl sulfoxide |
| mtDNA | mitochondrial deoxyribonucleic acid |
| ER | Endoplasmic reticulum |
| ETS | electron transport system |
| FoxO | forkhead box O |
| FUdR | 5-fluoro-2′-desoxiuridina |
| GFP | green fluorescent protein |
| H2DCFDA | 6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate |
| IC95 | 95% confidence interval |
| IIS | insulin/insulin-like growth factor signaling pathway |
| IGF-1 | insulin-like growth factor-1 |
| K2HPO4 | potassium phosphate dibasic |
| KCl | potassium chloride |
| KH2PO4 | potassium phosphate monobasic |
| LC50 | Lethal concentration 50 |
| MgSO4•7H2O | magnesium sulfate heptahydrate |
| mtl-1 or 2 | Metallothionein-1 or 2 |
| Na2HPO4 | sodium hydrogen phosphate |
| NaCl | sodium chloride |
| NaClO | sodium hypochlorite |
| NaOH | sodium hydroxide |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| PDK-1 | 3-phosphoinositide-dependent protein kinase 1 |
| ROS | reactive oxygen species |
| SFN | sulforaphane |
| SGK-1 | glucocorticoid-regulated kinase 1 |
| skn1a | ortholog of Nrf1 |
| skn-1c | ortholog of nuclear factor erythroid 2–related factor 2 (Nrf2) |
| ΔΨm | mitochondrial membrane potential |
| Sirt1 | sirtuin 1 |
| NRF1 | nuclear respiratory factors 1 |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator 1 alpha |
| TFAM | mitochondrial transcription factor A |
| VDAC | voltage-dependent anion channel |
| DMT1 | divalent metal transporter 1 |
| MCU | mitochondrial calcium uniporter |
| PIP3 | phosphatidylinositol 3,4,5-triphosphate |
| JC-1 | 5,5,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimi-dazoylcarbocyanine iodide |
| AKT1-2 | protein kinases B |
References
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental Hazards of Cadmium: Past, Present, and Future. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–183. [Google Scholar]
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Kumar, A.; et al. Bio-Remediation Approaches for Alleviation of Cadmium Contamination in Natural Resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef] [PubMed]
- Elinder, C.G.; Kjellstrom, T.; Hogstedt, C.; Andersson, K.; Spang, G. Cancer Mortality of Cadmium Workers. Occup. Environ. Med. 1985, 42, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Cadmium Sources and Toxicity. Toxics 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium Exposure in Living Organisms: A Short Review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- ATSDR ATSDR—Toxicological Profile. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15 (accessed on 19 April 2024).
- Bernhoft, R.A. Cadmium Toxicity and Treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef] [PubMed]
- Tägt, J.; Helte, E.; Donat-Vargas, C.; Larsson, S.C.; Michaëlsson, K.; Wolk, A.; Vahter, M.; Kippler, M.; Åkesson, A. Long-Term Cadmium Exposure and Fractures, Cardiovascular Disease, and Mortality in a Prospective Cohort of Women. Environ. Int. 2022, 161, 107114. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cruz, E.Y.; Amador-Martínez, I.; Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza Chaverri, J. Renal Damage Induced by Cadmium and Its Possible Therapy by Mitochondrial Transplantation. Chem. Biol. Interact. 2022, 361, 109961. [Google Scholar] [CrossRef]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and Mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Pacini, A.; Gulisano, M.; Taddei, N.; Fiorillo, C.; Becatti, M. Cadmium-Induced Cytotoxicity: Effects on Mitochondrial Electron Transport Chain. Front. Cell Dev. Biol. 2020, 8, 604377. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Human Metabolism and Excretion of Cancer Chemoprotective Glucosinolates and Isothiocyanates of Cruciferous Vegetables. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 1091–1100. [Google Scholar] [PubMed]
- Zyla, K.; Plafker, S.M. Sulforaphane and Mitochondria. In Mitochondrial Physiology and Vegetal Molecules; de Oliveira, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 233–246. [Google Scholar]
- Arana-Hidalgo, D.; Silva-Palacios, A. Role of Sulforaphane in Endoplasmic Reticulum Homeostasis through Regulation of the Antioxidant Response. Life Sci. 2022, 299, 120554. [Google Scholar] [CrossRef]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of Phase II Enzymes by Sulforaphane: Implications for Its Cardioprotective Potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Luo, Y.; Xie, Z. Sulforaphane Ameliorates Cadmium Induced Hepatotoxicity through the Up-Regulation of /Nrf2/ARE Pathway and the Inactivation of NF-ΚB. J. Funct. Foods 2021, 77, 104297. [Google Scholar] [CrossRef]
- Yang, S.-H.; Long, M.; Yu, L.-H.; Li, L.; Li, P.; Zhang, Y.; Guo, Y.; Gao, F.; Liu, M.-D.; He, J.-B. Sulforaphane Prevents Testicular Damage in Kunming Mice Exposed to Cadmium via Activation of Nrf2/ARE Signaling Pathways. Int. J. Mol. Sci. 2016, 17, 1703. [Google Scholar] [CrossRef]
- Yang, S.-H.; Li, P.; Yu, L.-H.; Li, L.; Long, M.; Liu, M.-D.; He, J.-B. Sulforaphane Protect Against Cadmium-Induced Oxidative Damage in Mouse Leydigs Cells by Activating Nrf2/ARE Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 630. [Google Scholar] [CrossRef]
- Yang, S.-H.; Yu, L.-H.; Li, L.; Guo, Y.; Zhang, Y.; Long, M.; Li, P.; He, J.-B. Protective Mechanism of Sulforaphane on Cadmium-Induced Sertoli Cell Injury in Mice Testis via Nrf2/ARE Signaling Pathway. Molecules 2018, 23, 1774. [Google Scholar] [CrossRef] [PubMed]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Assessment of Sulforaphane-Induced Protective Mechanisms against Cadmium Toxicity in Human Mesenchymal Stem Cells. Environ. Sci. Pollut. Res. 2018, 25, 10080–10089. [Google Scholar] [CrossRef]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Sulforaphane Mitigates Cadmium-Induced Toxicity Pattern in Human Peripheral Blood Lymphocytes and Monocytes. Environ. Toxicol. Pharmacol. 2017, 55, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cruz, E.Y.; Eugenio-Pérez, D.; Ramírez-Magaña, K.J.; Pedraza-Chaverri, J. Effects of Vegetal Extracts and Metabolites against Oxidative Stress and Associated Diseases: Studies in Caenorhabditis elegans. ACS Omega 2023, 8, 8936–8959. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.K.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An Emerging Model in Biomedical and Environmental Toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef]
- Koga, M.; Zwaal, R.; Guan, K.-L.; Avery, L.; Ohshima, Y. A Caenorhabditis elegans MAP Kinase Kinase, MEK-1, Is Involved in Stress Responses. EMBO J. 2000, 19, 5148–5156. [Google Scholar] [CrossRef]
- Keshet, A.; Mertenskötter, A.; Winter, S.A.; Brinkmann, V.; Dölling, R.; Paul, R.J. PMK-1 P38 MAPK Promotes Cadmium Stress Resistance, the Expression of SKN-1/Nrf and DAF-16 Target Genes, and Protein Biosynthesis in Caenorhabditis elegans. Mol. Genet. Genom. 2017, 292, 1341–1361. [Google Scholar] [CrossRef] [PubMed]
- Lemire, B. Mitochondrial Genetics. In WormBook; The C. elegans Research Community; WormBook: Minneapolis, MN, USA, 2005; Available online: http://www.wormbook.org/chapters/www_mitogenetics/mitogenetics.pdf (accessed on 19 April 2024).
- Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caenorhabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules 2020, 25, 3194. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T. Insulin/Insulin-Like Growth Factor Signaling in C. elegans. In WormBook; The C. elegans Research Community; WormBook: Minneapolis, MN, USA, 2013; pp. 1–43. Available online: https://www.ncbi.nlm.nih.gov/books/NBK179230/ (accessed on 19 April 2024). [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- The C. elegans Deletion Mutant Consortium. Large-Scale Screening for Targeted Knockouts in the Caenorhabditis elegans Genome. G3 Genes|Genomes|Genet. 2012, 2, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Senoo-Matsuda, N.; Yasuda, K.; Tsuda, M.; Ohkubo, T.; Yoshimura, S.; Nakazawa, H.; Hartman, P.S.; Ishii, N. A Defect in the Cytochrome b Large Subunit in Complex II Causes Both Superoxide Anion Overproduction and Abnormal Energy Metabolism in Caenorhabditis elegans. J. Biol. Chem. 2001, 276, 41553–41558. [Google Scholar] [CrossRef]
- Shukla, V.; Phulara, S.C.; Yadav, D.; Tiwari, S.; Kaur, S.; Gupta, M.M.; Nazir, A.; Pandey, R. Iridoid Compound 10-O-Trans-p-Coumaroylcatalpol Extends Longevity and Reduces Alpha Synuclein Aggregation in Caenorhabditis elegans. CNS Neurol. Disord. Drug Targets 2013, 11, 984–992. [Google Scholar] [CrossRef]
- Chen, J.; Carey, J.R.; Ferris, H. Comparative Demography of Isogenic Populations of Caenorhabditis elegans. Exp. Gerontol. 2001, 36, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Dodd, W.; Choe, K. Isolation of a Hypomorphic Skn-1 Allele That Does Not Require a Balancer for Maintenance. G3 Genes|Genomes|Genet. 2016, 6, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Lehrbach, N.J.; Ruvkun, G. Proteasome Dysfunction Triggers Activation of SKN-1A/Nrf1 by the Aspartic Protease DDI-1. eLife 2016, 5, e17721. [Google Scholar] [CrossRef] [PubMed]
- Lehrbach, N.J.; Ruvkun, G. Endoplasmic Reticulum-Associated SKN-1A/Nrf1 Mediates a Cytoplasmic Unfolded Protein Response and Promotes Longevity. eLife 2019, 8, e44425. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, C.A.; Kern, C.C.; Butkeviciute, E.; McCarthy, E.; Dockrell, H.M.; Moreno-Altamirano, M.M.B.; Aguilar-López, B.A.; Bhosale, G.; Wang, H.; Gems, D.; et al. Mitochondrial Signature in Human Monocytes and Resistance to Infection in C. elegans During Fumarate-Induced Innate Immune Training. Front. Immunol. 2020, 11, 01715. [Google Scholar] [CrossRef] [PubMed]
- Angeli, S.; Foulger, A.; Chamoli, M.; Peiris, T.H.; Gerencser, A.; Shahmirzadi, A.A.; Andersen, J.; Lithgow, G. The Mitochondrial Permeability Transition Pore Activates the Mitochondrial Unfolded Protein Response and Promotes Aging. eLife 2021, 10, e63453. [Google Scholar] [CrossRef]
- Aghayeva, U.; Bhattacharya, A.; Hobert, O. A Panel of Fluorophore-Tagged Daf-16 Alleles. MicroPubl. Biol. 2020, 2020, 000210. [Google Scholar] [CrossRef]
- Ke, T.; Santamaría, A.; Tinkov, A.A.; Bornhorst, J.; Aschner, M. Generating Bacterial Foods in Toxicology Studies with Caenorhabditis elegans. Curr. Protoc. Toxicol. 2020, 84, e94. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook; The C. elegans Research Community; WormBook: Minneapolis, MN, USA, 2006; pp. 1–11. Available online: http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.html (accessed on 19 April 2024). [CrossRef]
- Donkin, S.G.; Williams, P.L. Influence of Developmental Stage, Salts and Food Presence on Various End Points Using Caenorhabditis elegans for Aquatic Toxicity Testing. Environ. Toxicol. Chem. 1995, 14, 2139–2147. [Google Scholar] [CrossRef]
- Song, S.; Han, Y.; Zhang, Y.; Ma, H.; Zhang, L.; Huo, J.; Wang, P.; Liang, M.; Gao, M. Protective Role of Citric Acid against Oxidative Stress Induced by Heavy Metals in Caenorhabditis elegans. Environ. Sci. Pollut. Res. 2019, 26, 36820–36831. [Google Scholar] [CrossRef]
- Qi, Z.; Ji, H.; Le, M.; Li, H.; Wieland, A.; Bauer, S.; Liu, L.; Wink, M.; Herr, I. Sulforaphane Promotes C. elegans Longevity and Healthspan via DAF-16/DAF-2 Insulin/IGF-1 Signaling. Aging 2021, 13, 1649–1670. [Google Scholar] [CrossRef]
- Duran-Izquierdo, M.; Taboada-Alquerque, M.; Sierra-Marquez, L.; Alvarez-Ortega, N.; Stashenko, E.; Olivero-Verbel, J. Hydroalcoholic Extract of Haematoxylum Brasiletto Protects Caenorhabditis elegans from Cadmium-Induced Toxicity. BMC Complement. Med. Ther. 2022, 22, 184. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Daitoku, H.; Hirota, K.; Tamiya, H.; Yokoyama, A.; Kako, K.; Nagashima, Y.; Nakamura, A.; Shimada, T.; Watanabe, S.; et al. Asymmetric Arginine Dimethylation Determines Life Span in C. elegans by Regulating Forkhead Transcription Factor DAF-16. Cell Metab. 2011, 13, 505–516. [Google Scholar] [CrossRef]
- Kampkötter, A.; Gombitang Nkwonkam, C.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the Flavonoids Kaempferol and Fisetin on Thermotolerance, Oxidative Stress and FoxO Transcription Factor DAF-16 in the Model Organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A Living Model for Obesity and Aging Research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Branicky, R.; Wang, Y.; Khaki, A.; Liu, J.-L.; Kramer-Drauberg, M.; Hekimi, S. Stimulation of RAS-Dependent ROS Signaling Extends Longevity by Modulating a Developmental Program of Global Gene Expression. Sci. Adv. 2022, 8, eadc9851. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, B.; Zhang, S.; Fan, M.; Ji, X.; Zhang, S.; Wang, Z.; Qiao, K. Lentinan Protects Caenorhabditis elegans against Fluopyram-Induced Toxicity through DAF-16 and SKN-1 Pathways. Ecotoxicol. Environ. Saf. 2023, 265, 115510. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK Regulates Lifespan in Caenorhabditis elegans by Modulating Nuclear Translocation of Forkhead Transcription Factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef]
- Roh, J.-Y.; Park, Y.-J.; Choi, J. A Cadmium Toxicity Assay Using Stress Responsive Caenorhabditis elegans Mutant Strains. Environ. Toxicol. Pharmacol. 2009, 28, 409–413. [Google Scholar] [CrossRef]
- Różanowska, M.B. Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina. Antioxidants 2023, 12, 2111. [Google Scholar] [CrossRef]
- Gray, D.A.; Woulfe, J. Lipofuscin and Aging: A Matter of Toxic Waste. Sci. Aging Knowl. Environ. 2005, 2005, re1. [Google Scholar] [CrossRef] [PubMed]
- Maydata, A.G.; Espina, A.L.; Argilagos, M.E.R.; Núñez, E.M. Estrés Oxidativo, Alimentación y Suplementación Antioxidante En Patología Ocular: Historia Breve y Visión Futura. Rev. Cubana. Oftalmol. 2007, 20, 1–13. [Google Scholar]
- Dong, J.; Song, M.O.; Freedman, J.H. Identification and Characterization of a Family of Caenorhabditis elegans Genes That Is Homologous to the Cadmium-Responsive Gene Cdr-1. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2005, 1727, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Haas, K.L.; Freedman, J.H. Role of MTL-1, MTL-2, and CDR-1 in Mediating CadmiumSensitivity in Caenorhabditis elegans. Toxicol. Sci. 2012, 128, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Tian, S.; Teng, C.; Huang, L.; Liu, X.; Wang, J.; Zhang, Y.; Li, B.; Shan, Y. Sulforaphane Improves Lipid Metabolism by Enhancing Mitochondrial Function and Biogenesis In Vivo and In Vitro. Mol. Nutr. Food Res. 2019, 63, 1800795. [Google Scholar] [CrossRef]
- Höss, S.; Schlottmann, K.; Traunspurger, W. Toxicity of Ingested Cadmium to the Nematode Caenorhabditis elegans. Environ. Sci. Technol. 2011, 45, 10219–10225. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium Stress: An Oxidative Challenge. BioMetals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Ishii, N.; Fujii, M.; Hartman, P.S.; Tsuda, M.; Yasuda, K.; Senoo-Matsuda, N.; Yanase, S.; Ayusawa, D.; Suzuki, K. A Mutation in Succinate Dehydrogenase Cytochrome b Causes Oxidative Stress and Ageing in Nematodes. Nature 1998, 394, 694–697. [Google Scholar] [CrossRef]
- Melov, S.; Ravenscroft, J.; Malik, S.; Gill, M.S.; Walker, D.W.; Clayton, P.E.; Wallace, D.C.; Malfroy, B.; Doctrow, S.R.; Lithgow, G.J. Extension of Life-Span with Superoxide Dismutase/Catalase Mimetics. Science 2000, 289, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 Transcription Factor Can Protect against Oxidative Stress and Increase Lifespan in C. elegans by Distinct Mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, Stress Responses, and Aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of Mitophagy and Mitochondrial Biogenesis during Ageing in C. elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef]
- Le, Q.G.; Ishiwata-Kimata, Y.; Kohno, K.; Kimata, Y. Cadmium Impairs Protein Folding in the Endoplasmic Reticulum and Induces the Unfolded Protein Response. FEMS Yeast Res. 2016, 16, fow049. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. Biomed. Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-X.; Sen, I.; Janssens, G.E.; Zhou, X.; Fonslow, B.R.; Edgar, D.; Stroustrup, N.; Swoboda, P.; Yates, J.R.; Ruvkun, G.; et al. DAF-16/FOXO and HLH-30/TFEB Function as Combinatorial Transcription Factors to Promote Stress Resistance and Longevity. Nat. Commun. 2018, 9, 4400. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M.; Huerta-Yepez, S.; Tapia, E.; Pedraza-Chaverri, J. Modulation of Mitochondrial Functions by the Indirect Antioxidant Sulforaphane: A Seemingly Contradictory Dual Role and an Integrative Hypothesis. Free Radic. Biol. Med. 2013, 65, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Jardim, F.R.; de Almeida, F.J.S.; Luckachaki, M.D.; de Oliveira, M.R. Effects of Sulforaphane on Brain Mitochondria: Mechanistic View and Future Directions. J. Zhejiang Univ. Sci. B 2020, 21, 263–279. [Google Scholar] [CrossRef]
- Thévenod, F.; Fels, J.; Lee, W.-K.; Zarbock, R. Channels, Transporters and Receptors for Cadmium and Cadmium Complexes in Eukaryotic Cells: Myths and Facts. BioMetals 2019, 32, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M. The VDAC Channel: Molecular Basis for Selectivity. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol Rescues Cadmium-Induced Mitochondrial Injury by Enhancing Transcriptional Regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a Pathway in TCMK-1 Cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, C.; Sun, Y.-C.; Zhang, Q.; Lv, M.-W.; Guo, K.; Li, J.-L. Cadmium Exposure Triggers Mitochondrial Dysfunction and Oxidative Stress in Chicken (Gallus Gallus) Kidney via Mitochondrial UPR Inhibition and Nrf2-Mediated Antioxidant Defense Activation. Sci. Total Environ. 2019, 689, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kasai, S.; Yamazaki, H.; Tatara, Y.; Mimura, J.; Engler, M.J.; Tanji, K.; Nikaido, Y.; Inoue, T.; Suganuma, H.; et al. Sulforaphane Increase Mitochondrial Biogenesis-Related Gene Expression in the Hippocampus and Suppresses Age-Related Cognitive Decline in Mice. Int. J. Mol. Sci. 2022, 23, 8433. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Pi, H.; Xu, S.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; Li, R.; et al. Melatonin Improves Mitochondrial Function by Promoting MT1/SIRT1/PGC-1 Alpha-Dependent Mitochondrial Biogenesis in Cadmium-Induced Hepatotoxicity In Vitro. Toxicol. Sci. 2014, 142, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 00435. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Imai, H. Hydrogen Peroxide Produced by Superoxide Dismutase SOD-2 Activates Sperm in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 14804–14813. [Google Scholar] [CrossRef]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, C. Molecular Inhibitory Mechanisms of Antioxidant Enzymes in Rat Liver and Kidney by Cadmium. Toxicology 2002, 179, 37–50. [Google Scholar] [CrossRef]
- Newairy, A.A.; El-Sharaky, A.S.; Badreldeen, M.M.; Eweda, S.M.; Sheweita, S.A. The Hepatoprotective Effects of Selenium against Cadmium Toxicity in Rats. Toxicology 2007, 242, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.K.; Mishra, P.C. Mechanism of Action of Sulforaphane as a Superoxide Radical Anion and Hydrogen Peroxide Scavenger by Double Hydrogen Transfer: A Model for Iron Superoxide Dismutase. J. Phys. Chem. B 2015, 119, 7825–7836. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Talalay, P. Antioxidant Functions of Sulforaphane: A Potent Inducer of Phase II Detoxication Enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zheng, B.; Li, T.; Liu, R.H. Raspberry Extract Ameliorates Oxidative Stress in Caenorhabditis elegans via the SKN-1/Nrf2 Pathway. J. Funct. Foods 2020, 70, 103977. [Google Scholar] [CrossRef]
- Lee, S.S.; Kennedy, S.; Tolonen, A.C.; Ruvkun, G. DAF-16 Target Genes That Control C. elegans Life-Span and Metabolism. Science 2003, 300, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Song, Y.; Xie, J.; Xie, J.; Chen, Y.; Li, P.; Liu, D.; Hu, X.; Yu, Q. Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans. Foods 2022, 11, 1590. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.T.G.; Rodrigues, L.B.L.; Salgueiro, W.G.; de Castro Dal Forno, A.H.; Rodrigues, C.F.; Sacramento, M.; Franco, J.; Alves, D.; de Paula Oliveira, R.; Pinton, S.; et al. Organoselenotriazoles Attenuate Oxidative Damage Induced by Mitochondrial Dysfunction in Mev-1 Caenorhabditis elegans Mutants. J. Trace Elem. Med. Biol. 2019, 53, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xing, X. Pre-Treatment with Mild UV Irradiation Suppresses Reproductive Toxicity Induced by Subsequent Cadmium Exposure in Nematodes. Ecotoxicol. Environ. Saf. 2010, 73, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, P.; Yang, Y.; Shen, L. Formation of a Combined Ca/Cd Toxicity on Lifespan of Nematode Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2010, 73, 1221–1230. [Google Scholar] [CrossRef]
- Ji, H.; Qi, Z.; Schrapel, D.; Le, M.; Luo, Y.; Yan, B.; Gladkich, J.; Schaefer, M.; Liu, L.; Herr, I. Sulforaphane Targets TRA-1/GLI Upstream of DAF-16/FOXO to Promote C. elegans Longevity and Healthspan. Front. Cell Dev. Biol. 2021, 9, 784999. [Google Scholar] [CrossRef]
- Wang, S.; Lin, D.; Cao, J.; Wang, L. APPA Increases Lifespan and Stress Resistance via Lipid Metabolism and Insulin/IGF-1 Signal Pathway in Caenorhabditis elegans. Int. J. Mol. Sci. 2023, 24, 13682. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lai, C.; Tsai, M.; Wu, J.; Ho, C. Molecular Mechanisms for Anti-aging by Natural Dietary Compounds. Mol. Nutr. Food Res. 2012, 56, 88–115. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Egner, P.A.; Agyeman, A.S.; Visvanathan, K.; Groopman, J.D.; Chen, J.-G.; Chen, T.-Y.; Fahey, J.W.; Talalay, P. Keap1-Nrf2 Signaling: A Target for Cancer Prevention by Sulforaphane. Top. Curr. Chem. 2013, 329, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef]
- Paek, J.; Lo, J.Y.; Narasimhan, S.D.; Nguyen, T.N.; Glover-Cutter, K.; Robida-Stubbs, S.; Suzuki, T.; Yamamoto, M.; Blackwell, T.K.; Curran, S.P. Mitochondrial SKN-1/Nrf Mediates a Conserved Starvation Response. Cell Metab. 2012, 16, 526–537. [Google Scholar] [CrossRef]
- Dong, J.; Boyd, W.A.; Freedman, J.H. Molecular Characterization of Two Homologs of the Caenorhabditis elegans Cadmium-Responsive Gene Cdr-1: Cdr-4 and Cdr-6. J. Mol. Biol. 2008, 376, 621–633. [Google Scholar] [CrossRef]
- Garcia-Neto, W.; Cabrera-Orefice, A.; Uribe-Carvajal, S.; Kowaltowski, A.J.; Alberto Luévano-Martínez, L. High Osmolarity Environments Activate the Mitochondrial Alternative Oxidase in Debaryomyces Hansenii. PLoS ONE 2017, 12, e0169621. [Google Scholar] [CrossRef] [PubMed]
- Lewiston, N.; Newman, A.; Robin, E.; Holtzman, D. Shark Heart Mitochondria: Effects of External Osmolality on Respiration. Science 1979, 206, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Grauso, M.; Lan, A.; Andriamihaja, M.; Bouillaud, F.; Blachier, F. Hyperosmolar Environment and Intestinal Epithelial Cells: Impact on Mitochondrial Oxygen Consumption, Proliferation, and Barrier Function in Vitro. Sci. Rep. 2019, 9, 11360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).