Abstract
Carbon dot (CD) nanozymes have enzyme-like activity. Compared with natural enzymes, CD nanozymes offer several advantages, including simple preparation, easy preservation, good stability and recycling, which has made them a popular research topic in various fields. In recent years, researchers have prepared a variety of CD nanozymes for biosensing detection, medicine and tumor therapy, and many of them are based on oxidative stress regulation and reactive oxygen species clearance. Particularly to expand their potential applications, elemental doping has been utilized to enhance the catalytic capabilities and other properties of CD nanozymes. This review discusses the prevalent techniques utilized in the synthesis of CD nanozymes and presents the diverse applications of CD nanozymes based on their doping characteristics. Finally, the challenges encountered in the current utilization of CD nanozymes are presented. The latest research progress of synthesis, application and the challenges outlined in the review can help and encourage the researchers for the future research on preparation, application and other related researches of CD nanozymes.
1. Introduction
Enzymes, as a kind of biocatalyst with high efficient catalytic ability to catalyze various biochemical reactions specifically, are widely used in the field of medicine and industrial production, etc. [1,2,3]. The vast majority of natural enzymes are composed of proteins, and only a small number of them are ribozymes composed of RNA, all of which have highly efficient and specific catalytic properties [4,5]. The catalytic ability of enzymes will be affected by the reaction conditions; in the optimal temperature, pH and other reaction conditions, the enzyme can exert the maximum catalytic ability. On the contrary, the enzyme activity is reduced, and in extreme conditions it can even lead to the loss of enzyme activity [6]. The poor stability of natural enzymes, the expensive cost of preparation and preservation, the sensitivity to extreme reaction conditions and the difficulty of recovery and recycling all limit the application of natural enzymes in practice [7,8,9,10,11]. Nanomaterials based artificial mimicking enzymes, also named as nanozymes, have attracted considerable attention due to their higher stability and lower cost than that of natural enzymes [12,13,14,15,16]. In recent years, nanozymes have been used in biomedical applications, including diabetes treatment, oral infection treatment, anti-aging and tumor therapy, due to their excellent properties, including catalytic activities [17,18,19,20,21]. The preparation of nanozymes with enzyme-like activity based on carbon nanomaterials has attracted great interest from researchers [22,23]. The enzyme-like activity of nanozymes is affected by various factors, including structural features and the external environment [24]. There are many types of nanozymes based on carbon nanomaterials, including CDs, graphene, fullerenes and carbon nanorods, etc.
CDs have a small size of less than 10 nm, resulting in a large surface area and an increased abundance of surface groups, and the existence of carbon-rich, nitrogen-rich and oxygen-rich groups on the surface of CDs has been proposed [25,26,27]. Graphene CDs, carbon quantum dots, carbon nanodots and carbonized polymer dots are the four most common classifications of CDs, and different types of CDs have different structures and different functions [28]. Due to their small size, CDs can easily cross the cell membrane into the cytoplasm, and then into other organelles for action [29]. CDs can also cross structures that protect the organism from aggression, such as the blood–brain barrier, and different sizes, surface modifications and environments can lead to altered entry mechanisms [30]. CDs have unique fluorescent properties due to surface conjugation and other reasons and are a relatively new type of nanomaterial with better stability compared to other organic molecular dyes [31,32,33]. In addition, CDs also play a role in fighting against COVID-19 and other antiviral diseases [34]. CDs have also been applied in biomedicine [35]. CD nanozymes, compared to other materials, have better biocompatibility, lower toxicity and excellent stability and have been widely used in medicine, bio-detection and disease treatment, becoming a hot topic in current research [36,37,38,39].
CD nanozymes have strong mimetic enzyme activities and the proposed enzyme-like activities of CD nanozymes mainly include oxidase-like (OXD-like), peroxidase-like (POD-like), superoxide dismutase-like (SOD-like) and oxidoreductase-like activities [40,41]. The enzyme-like activities of CD nanozymes also exhibit catalytic properties similar to natural enzymes, such as how peroxidase-like enzymes of CDs were able to rapidly convert 3,3′,5,5′-tetramethylbenzidine (TMB) into colored products in the presence of hydrogen peroxide (H2O2), generating a large amount of reactive oxygen species (ROS) such as hydroxyl radicals (•OH), which can be used for antimicrobial therapy [42,43,44]. Compared with natural enzymes, CD nanozymes have more stable enzyme-like activity and photocatalytic properties. Surface modification, size and shape, and doping elements can also be used to further enhance the enzyme-like activity and other properties of CD nanozymes [45,46,47]. However, in the case of doping metal ions, the optical properties of the CD nanozymes may also be affected, which may lead to the decrease in the fluorescence quantum yield and other situations [48]. The smaller-sized CD nanozymes can be better dispersed in the solvent and promote the reaction in the solvent [49]. CD nanozymes can also be combined with other materials to form composites, which can inherit and improve the catalytic ability and other related properties of CD nanozymes, making the applications of CD nanozymes more extensive [50,51,52].
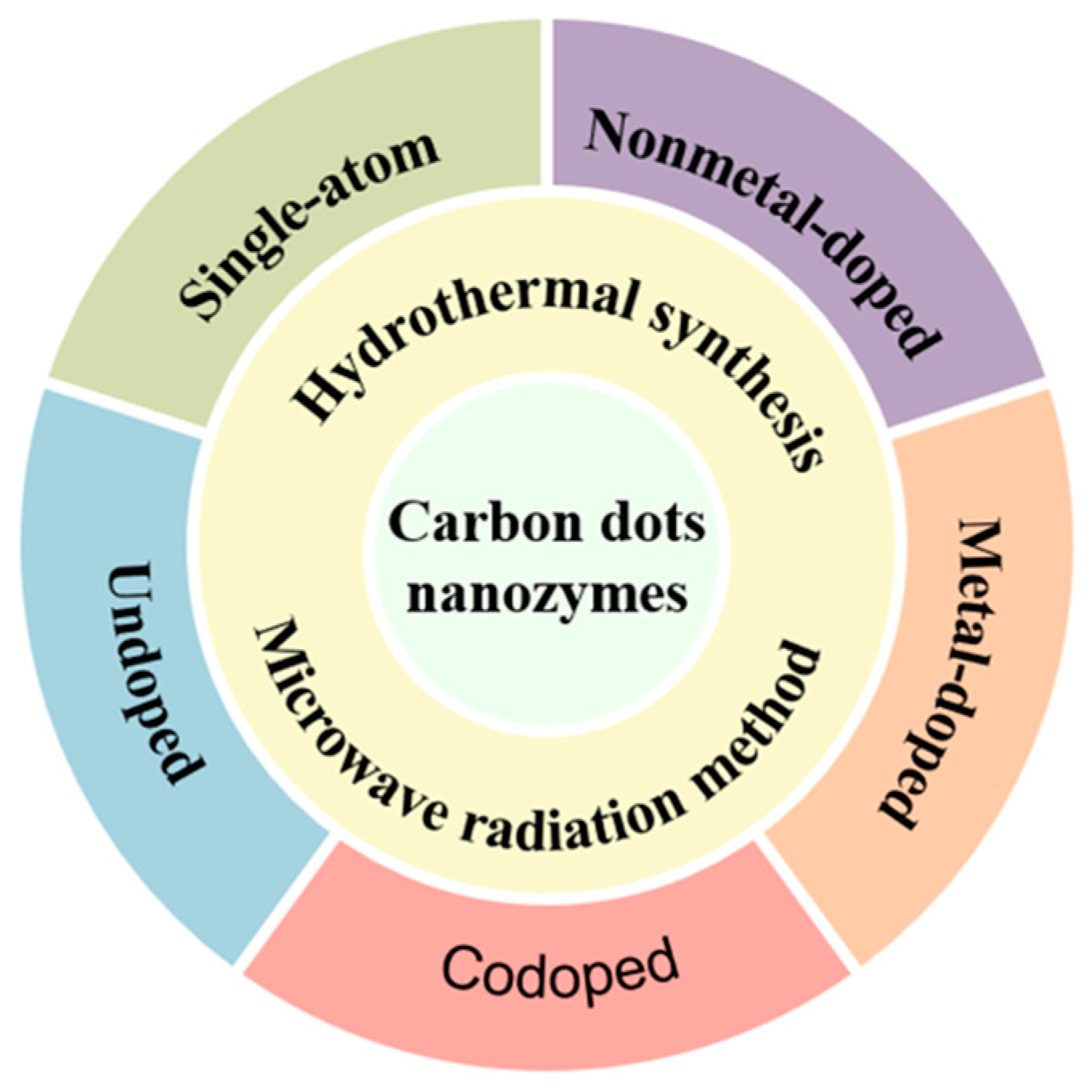
This review mainly introduces the main preparation methods of CD nanozymes, hoping to help understand the preparation methods of CD nanozymes and provide ideas for the design and development of CD nanozymes in the future. In addition, the applications of CD nanozymes are also introduced based on different doping types, so as to understand the direction of different doped CD nanozymes in practical applications and to achieve the precise design and applications of CD nanozymes(Figure 1).
Figure 1.
Preparation of CD nanozymes and their doping types.
2. Preparation of CDs Nanozymes
Currently, the preparation of CDs can be divided into two strategies: one is the “top-down” method; the other is the “bottom-up” method [53,54]. The top-down method mainly involves decomposing or cutting the larger carbon materials through physical and chemical methods to obtain carbon nanoparticles, and the sizes of the obtained carbon nanoparticles vary, which refer to electrochemical techniques, laser ablation techniques, etc. The bottom-up method is mainly to obtain the CDs by fusing the molecular precursors or other polymerization precursors, and the CDs with controllable sizes and rich carbon sources can be obtained by this method, and the main methods include hydrothermal synthesis techniques and microwave radiation techniques [55,56,57]. The top-down method for synthesizing CDs requires strict reaction conditions, longer reaction time, lower efficiency and more by-products. The “bottom-up” method can control the size of CDs, making the materials easy to obtain and the reaction operation simple [58,59,60]. Currently, the preparation of CDs is mainly based on the bottom-up strategy, mainly obtaining the desired CDs through hydrothermal synthesis or microwave radiation [61]. The same synthesis strategy can be used for CD nanozymes. Due to the advantages of the bottom-up approach, this article mainly reviews the preparation of CD nanozymes using this strategy. Table 1 summarizes the recent studies on the preparation of CD nanozymes and related enzyme-like activity by the “bottom-up” method.

Table 1.
Preparation materials and enzyme-like activities of various CD nanozymes under different preparation methods.
2.1. Hydrothermal Synthesis
Hydrothermal synthesis is the most commonly used method for the preparation of CD nanozymes. The general procedure of hydrothermal synthesis is to dissolve the molecular precursors or other polymerization precursors in ultrapure water or other solvents to form a solution, transfer it to a reactor, react under high temperature and pressure for a period of time, cool the mixture to room temperature, and then purify it by column chromatography to obtain the prepared carbon-dot nanozymes. Hydrothermal synthesis is relatively simple, low-cost and environmentally friendly and non-toxic compared with other preparation methods. Moreover, the optical properties of CDs obtained by the hydrothermal method are excellent, and the post-processing operation is convenient and fast [83,84,85]. However, the reaction time and temperature have a direct influence on the properties and functions of the prepared CDs, so the reaction time and temperature should be strictly controlled during the reaction process [86].
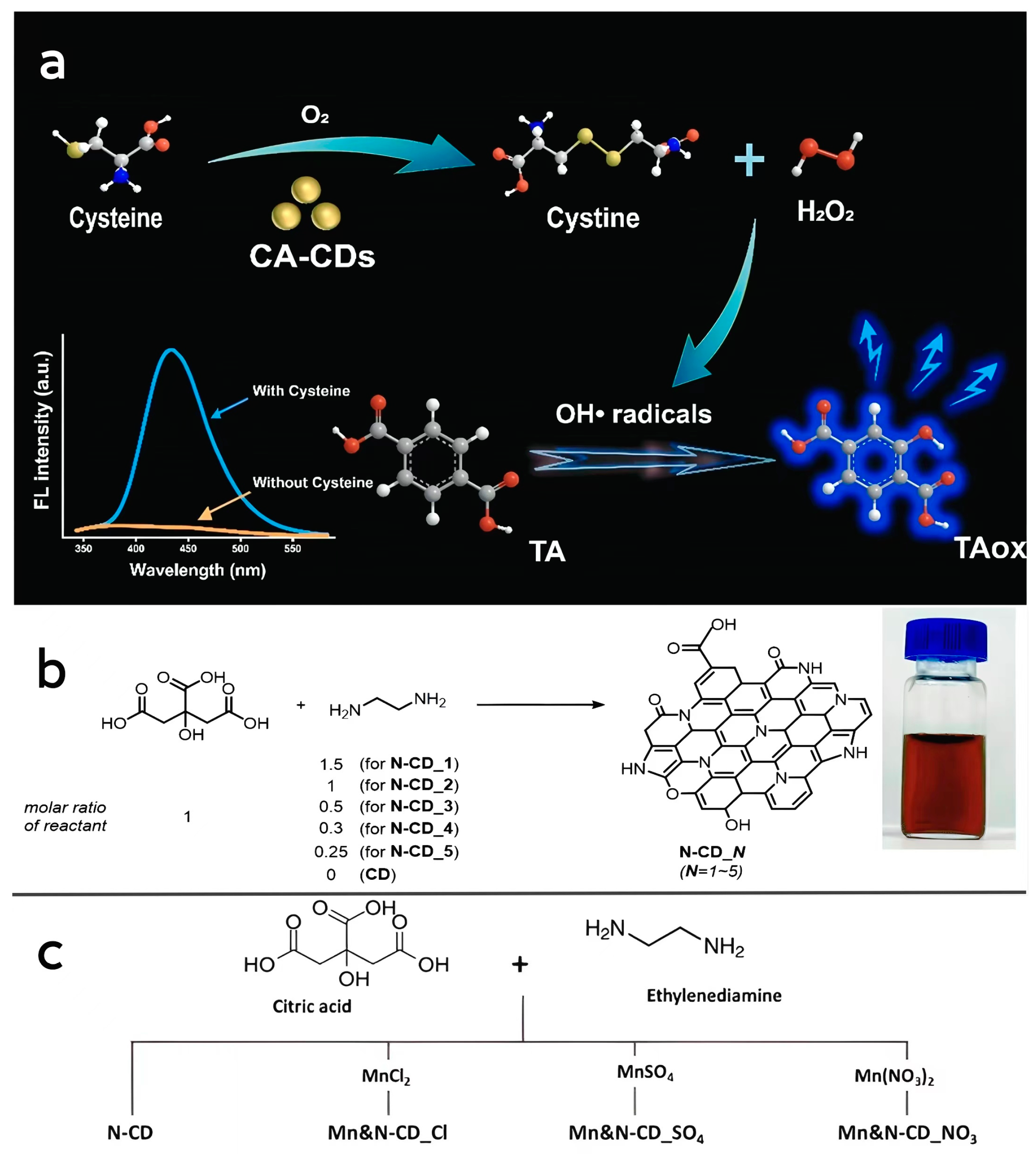
Lin et al. [87] dissolved citric acid in water and reacted it at 200 °C for 12 h. Undoped citric acid CD nanozymes (CA-CDs) were obtained after dialysis purification. The obtained CA-CDs were uniformly distributed with an average particle size of 1.65 nm, and Fourier-transform infrared analysis (FTIR) revealed that C=C double bonds were formed on their surfaces, and carbon-13 nuclear magnetic resonance (13C-NMR) analysis showed that the newly synthesized CA-CDs contained aromatic structures. The prepared CA-CDs have cysteine oxidase-like activity, which can catalyze the oxidative decomposition of cysteine to obtain cystine and H2O2, and the prepared CA-CDs have a higher affinity for cysteine compared to natural cysteine oxidase (Figure 2a).
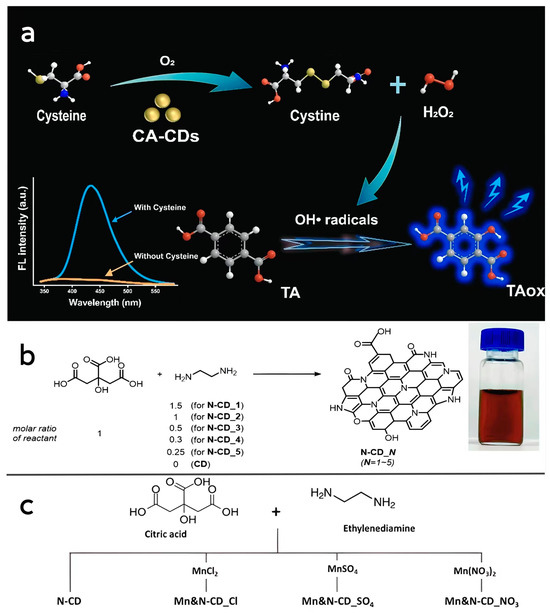
Figure 2.
(a) Schematic representation of cysteine-like oxidase activity of CA-CDs. Reprinted with permission from ref. [87]. Copyright 2022, Elsevier. (b) Photographs of the preparation process of N-CDs and the prepared N-CD samples suspended in water. Reprinted with permission from ref. [64]. Copyright 2021, Royal Society of Chemistry. (c) Schematic diagram of the preparation process of Mn, N-CDs. Reprinted with permission from ref. [88]. Copyright 2023, Royal Society of Chemistry.
Lee et al. [64] used citric acid and ethylenediamine as precursors to obtain N-CDs with peroxidase-like activity by reacting the formulated solvents at a high pressure of 200 °C for 1 h, cooling to 25 °C and purifying by column chromatography (Figure 2b). Due to the different ratios of carbon and nitrogen sources used in the reaction, the size of all types of synthesized CD nanozymes ranges from 3.4 to 17.8 nm. By comparing the optical properties of CD nanozymes with different ratios of carbon and nitrogen sources, it was found that the N-doping content of the CD nanozymes played a key role in influencing their optical properties. Compared with the undoped CD nanozymes, the N-CDs can oxidize TMB more rapidly, and the maximum enzymatic reaction velocity (Vmax) was also higher than that of the undoped CD nanozymes in all cases. It is noteworthy that the value of Km, the affinity between the enzyme and the substrate, changes only slightly with the change in the N-doped content, which also suggests that doping of heteroatoms plays an important optimizing role on the enzymatic activity of CD nanozymes. Kang et al. [88] also obtained Mn, N-codoped CD nanozymes by hydrothermal synthesis with the addition of a manganese source, and the codoped CD nanozymes had higher Vmax and lower Km values than N-CDs, showed higher affinity for the substrate and possessed a more excellent peroxidase-like catalytic activity, which also indicates that metal doping has a significant enhancement effect on the activity of CD nanozymes. This also indicates that metal doping has a significant enhancement effect on the activity of CD nanozymes, but the related mechanism has not been thoroughly studied yet (Figure 2c).
For comparison, Zhuo et al. [68] used citric acid and ethylenediamine as precursors to synthesize Mn-CDs and bare CDs through hydrothermal synthesis and also prepared pure Mn samples for comparison. The preparation process involved dissolving citric acid, ethylenediamine and manganese sources in ultrapure water, reacting the obtained mixture in an autoclave at 180 °C for 10 h and purifying the supernatant by centrifugation followed by dialysis for 24 h to obtain the prepared Mn-CDs. The synthesized Mn-CDs possessed oxidase-like activities and the oxidase-like activities possessed were higher than those of the CD nanozymes synthesized using only citric acid or ethylenediamine as a single precursor. The possible reason is due to the synergistic effect of Mn doping on the CDs, which enhances the enzyme activity.
The solvent pyrolysis and hydrothermal synthesis methods for the preparation of CD nanozymes are not very different in nature, and thus both can be categorized as hydrothermal synthesis methods for the preparation of CD nanozymes.
2.2. Microwave Radiation Method
The microwave radiation method is a green and convenient method to obtain CD nanozymes, through which CD nanozymes containing dopant elements can be quickly and efficiently obtained from the prepared organic mixtures [55]. The microwave radiation method is faster compared to the hydrothermal method for the preparation of CD nanozymes, but the morphological regularity of the prepared CD nanozymes is poor [89]. The specific procedure is to react the precursor mixture in a microwave synthesis reactor at a certain power and temperature, followed by cooling, purifying, and removing impurities through dialysis and other methods to obtain the pure CD nanozymes. The CD nanozymes obtained by the microwave radiation method can enhance their fluorescence properties by surface passivation or purification and decontamination to improve the breadth of their application in practical applications.
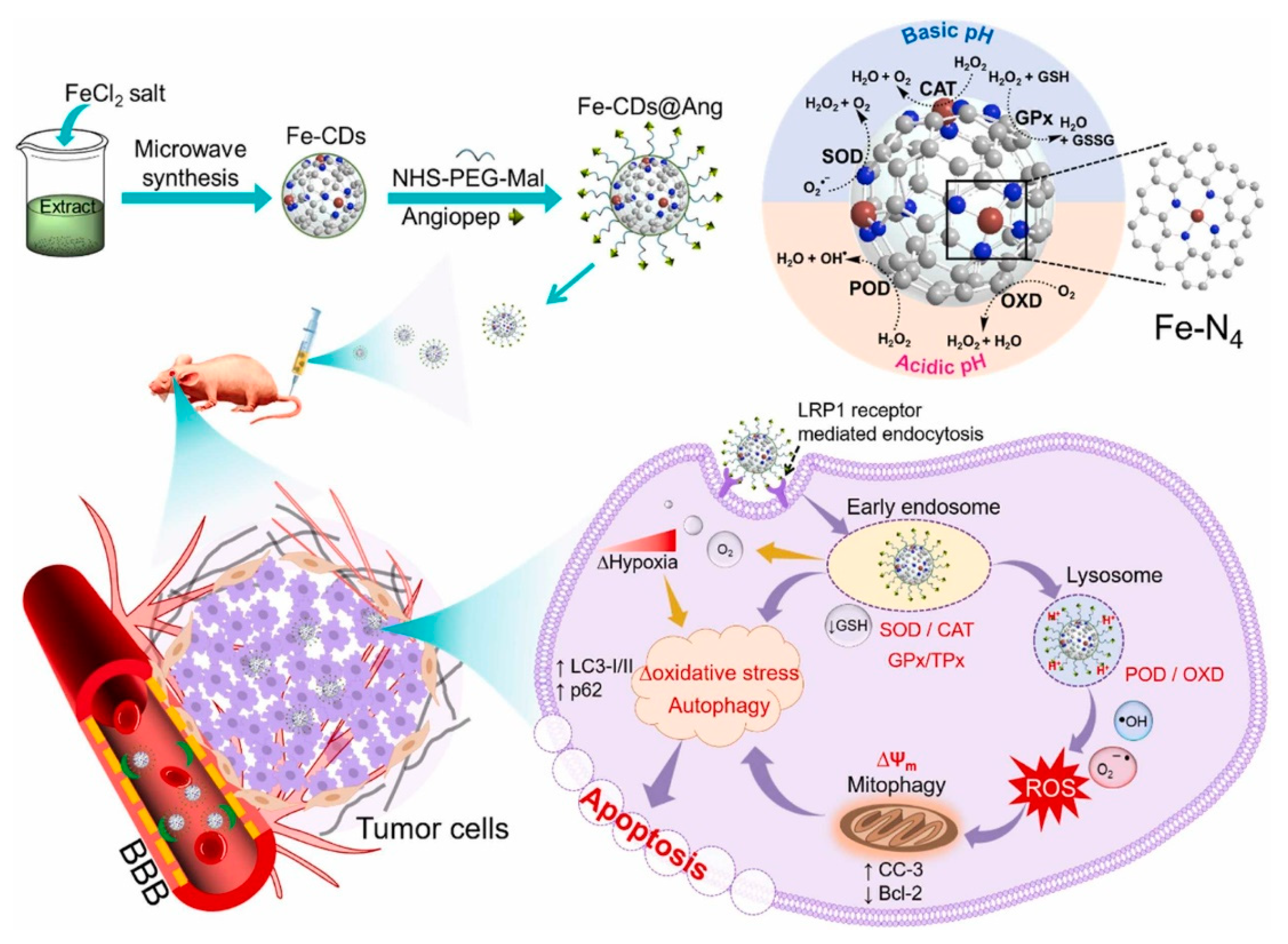
Muhammad et al. [80] used the leaves of bougainvillea plants as a carbon source precursor material for the synthesis of CD nanozymes. The leaves were pulverized and a mixture of 10 g of pulverized leaves with 100 mL of ethanol-water solute on miscible at 1:1 ratio was configured, after which ferric chloride was added as a dopant element, and the obtained mixture was processed in a 630 W microwave oven to obtain the different sizes of the Fe-CDs, which were further purified by centrifugation and other steps to obtain ultra-small CD-loaded iron monoatomic nanozymes (Figure 3). It was found that Fe-CDs have oxidase-like, catalase-like, superoxide dismutase-like, peroxidase-like, glutathione peroxidase-like and thiol peroxidase-like activities and thus could be used to eliminate the damage caused by reactive oxygen species generated in the body to keep the organism in a relatively stable state. By comparing Fe-CDs with non-metal doped N-CDs, it could be observed that Fe-CDs had relatively high oxidase-like, peroxidase-like and superoxide dismutase-like activities, but their catalase-like activities were significantly lower compared to N-CDs. N-CDs exhibited almost nonexistent glutathione peroxidase-like and thiol peroxidase-like activities, which also reflected the influence that different elemental dopings have on CD nanozymes’ activity.
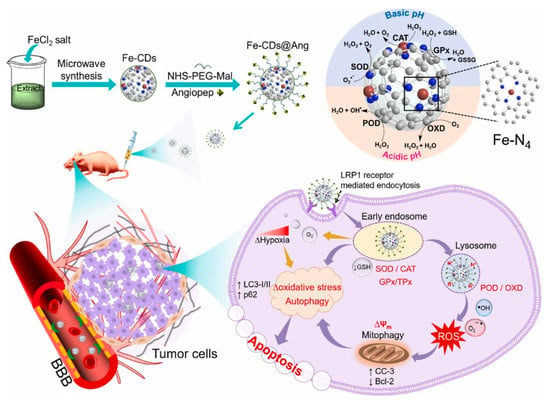
Figure 3.
Schematic illustration of the preparation of Fe-CD nanozyme and the enzymatic cascade initiated by angiopep-2-modified Fe-CD nanozyme for ROS regulation to induce the autophagy–lysosome pathway for GBM therapy. Reprinted with permission from ref. [80]. Copyright 2022, Elsevier.
Bijalwan et al. [81] prepared metal-doped Cu-CD nanozymes and Mn-CD nanozymes, as well as undoped CD nanozymes, through microwave radiation solid-phase synthesis. For the preparation of Cu-CDs, 0.75 g of urea, 0.25 g of citric acid and 0.03 g of copper acetate were used as carbon, nitrogen and dopant element precursors, respectively, the mixtures were pulverized and placed in a microwave reactor at 220 °C for 10 min, then cooled down to room temperature, purified by centrifugation and decontamination, and dried to obtain the Cu-doped CD nanozymes. At the same time, the same method was used to obtain the Mn-CD nanozymes with manganese tetrahydrate as a manganese precursor. Mn-doped CD nanozymes with manganese acetate as a manganese precursor and undoped CD nanozymes with urea and citric acid as precursors were also obtained by the same method. It is worth noting that solid-phase synthesis was used for the synthesis of CD nanozymes by this method, which eliminated unnecessary solvents and made the preparation of CD nanozymes more convenient and efficient compared with the previous synthesis methods. The three prepared CD nanozymes were all around 2–15 nm in size, and only the Cu-doped CD nanozymes possessed peroxidase-like activity in a neutral pH environment, and with the change in pH, the enzyme activity showed different degrees of changes, with the highest enzyme activity in an acidic environment at pH 4 and the lowest enzyme activity in an alkaline environment at pH 9, and so it was hypothesized that their peroxidase-like activity might result from the synergistic interactions between copper and CDs as well as the interaction of radical cations with charge transfer complexes. As the enzyme can function over a wide range of pHs, it can be used as a peroxidase-like mimetic nanozyme under physiological conditions.
3. Applications of CDs Nanozymes
The application of CD nanozymes was explored according to different doping types, as different doping elements during the preparation process may influence the properties of CD nanozymes. CD nanozymes were widely used in biosensing, crop growth, detection and therapy due to their unique physical properties and advantages, including having catalytic properties that mimic those of natural enzymes [76,90,91,92].
3.1. Single-Atom Nanozymes
Single-atom nanozymes stand out from conventional nanomaterials due to their unique geometrical and electronic structures, providing them with distinctive advantages. Unlike ordinary nanomaterials, single-atom nanozymes offer benefits such as a uniform distribution of active sites and minimal interference among individual atoms. These characteristics enable single-atom nanozymes to be effectively dispersed in solvents, enhancing their overall performance. These factors contribute to the widespread utilization of single-atom nanozymes across multiple sectors [93,94,95]. As a result of their enhanced properties, single-atom nanozymes exhibit superior performance and find applications in various fields, such as biomedicine. The properties exhibited by single-atom nanozymes vary in size, shape, temperature and pH [96]. Moreover, the higher efficiency of metal utilization in single-atom nanozymes contributes to reducing preparation costs [97,98].
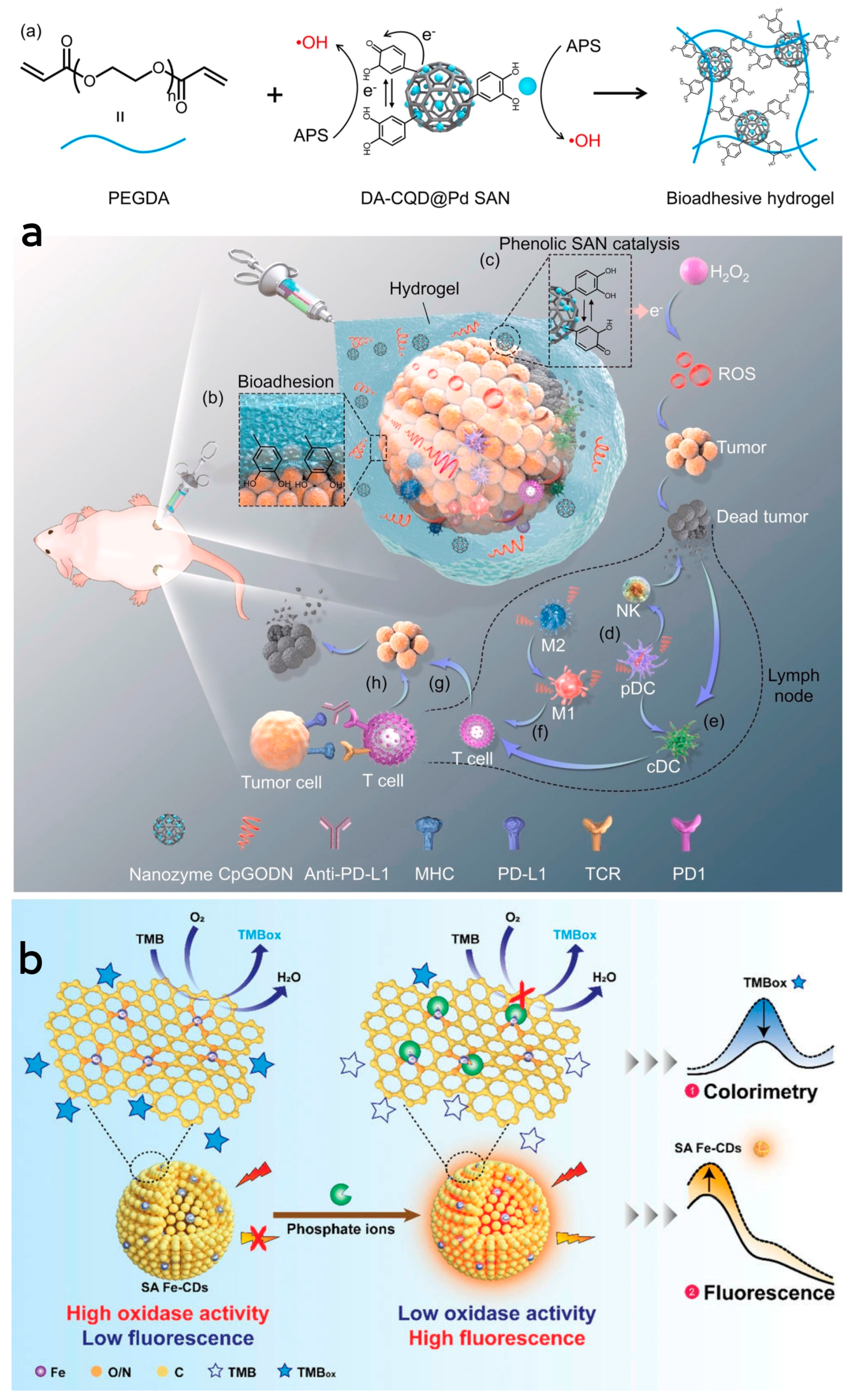
He et al. [99] synthesized phenolic platinum single-atom composite CD nanozymes by the hydrothermal synthesis method and subsequent treatment. This nanozyme exhibited better peroxidase-like and persulfatase-like activities, capable of catalyzing the generation of ROS from H2O2 in the tumor microenvironment, leading to the destruction of tumor cells. Subsequently, a bioadhesive hydrogel containing DA-CQD@Pd single-atom nanozymes and CpGODN was formed through a free radical polymerization reaction catalyzed by the single-atom nanozymes. Upon injection, this hydrogel formed a stable adhesion around the tumor, providing a localized site for immune response activation. The controlled release of CpGODN from the hydrogel sustained immune system activation, minimizing systemic exposure, reducing toxicity, and protecting CpGODN from degradation. This combined approach, along with the immune checkpoint inhibitor anti-PD-L1, enabled local immunomodulation, maximizing therapeutic efficacy and preventing tumor metastasis through catalytic immunotherapy. Dendritic cells take up CpGODN from the hydrogel, activating them to trigger a natural immune response by secreting interferon-alpha. Simultaneously, CpGODN induces polarization of M2 to M1 macrophages, facilitating the activation of antigen-presenting cells and T-cells, thereby initiating an adaptive immune response. The synergistic action of the hydrogel and anti-PD-L1 therapy significantly hindered primary and distal tumor growth, increased survival rates, and enhanced systemic anti-tumor immune responses. (Figure 4a). For the first time, Zhang et al. [100] prepared Pt single-atom nanozymes assembled with dual enzymatic peroxidase-like and glutathione oxidase activities. These assemblies synergize enzyme activity, photothermal effect and chemotherapeutic activity to inhibit the growth of tumor cells. Furthermore, they exhibit better biocompatibility, thus reducing damage to the organism’s cells. The enzyme makes better use of GSH to prevent the depletion of generated 1O2 and shows photothermal therapeutic effects at low temperatures. It also enables sustained drug release through depolymerization and drug release controlled by the tumor microenvironment or laser. Additionally, the enzyme assembly effectively regulates the intracellular redox balance, achieving highly efficient tumor growth inhibition.
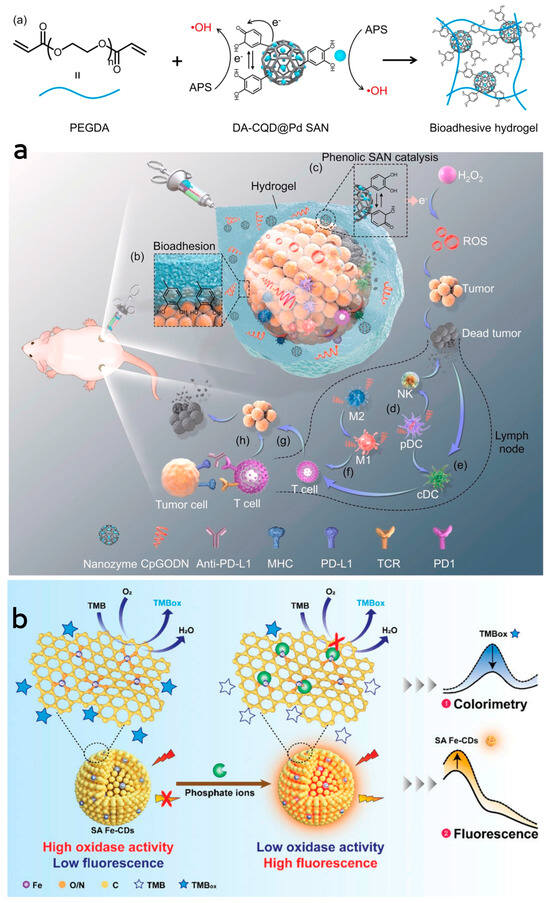
Figure 4.
(a) Schematic illustration of Pt single−atom nanozyme complexes combined with CpGODN hydrogels for catalytic immunotherapy of tumors. Reprinted with permission from ref. [99]. Copyright 2021, Elsevier. (b) Schematic illustration of the dual-mode colorimetric and fluorometric sensing of Pi based on SA Fe-CDs. Reprinted with permission from ref. [101]. Copyright 2022, Wiley-VCH GmbH.
Li et al. [101] synthesized a small-sized iron monoatomic CD nanozyme with a more stable oxidase-like activity (Figure 4b). Various characterization techniques revealed that iron atoms were uniformly dispersed as single atoms in the carbon matrix, forming abundant Fe-O/N active centers. Monatomic iron in the Fe-O/N active centers triggered the decomposition of dissociated O2 and generated highly reactive superoxide radicals which rapidly oxidized the TMB to its blue oxidation state (oxTMB). Phosphate ions (Pi) competitively inhibited the oxidase-like activity of the Fe-monatomic nano-enzymes because the adsorbed Pi occupied the catalytically active site. The sensitive and selective detection of Pi can be achieved through variations in UV-vis spectra and fluorescence spectra of the single-atomic iron-doped carbon dots. Muhammad et al. [80] developed ultra-small CD-loaded iron monoatomic nanozymes with multi-species enzymatic activity using the microwave radiation method. The CDs demonstrated OXD-like activity under physiological pH and temperature conditions. Remarkably, the enzyme exhibited enhanced POD activity in acidic environments, intensifying oxidative stress levels in tumor cells. The iron monoatomic nanozymes could convert superoxide anions into oxygen and H2O2, thereby regulating intracellular ROS balance. Moreover, these nanozymes accumulated in acidic lysosomes displayed OXD- and POD-like activities that disrupted lysosomal degradation and activated autophagy. By triggering autophagy, the iron monoatomic nanozymes facilitated the breakdown of damaged organelles and proteins within tumor cells, ultimately leading to cell death. Furthermore, the nanozymes were capable of modulating ROS levels in the tumor microenvironment, inhibiting tumor growth and incorporating peptides for BBB penetration through surface modification, effectively targeting GBM cells to enhance therapeutic efficacy. Notably, the enzyme combatted drug resistance in solid GBM tumors by engaging the ROS-mediated autophagy signaling pathway. Their GPx- and TPx-like activities enabled the regulation of ROS levels, consequently impacting the redox balance of tumor cells and promoting cell death to achieve therapeutic outcomes, establishing them as a promising novel nanodrug (Figure 3). Han et al. [102] introduced phenanthroline as a mediating ligand to synthesize ultra-small CD-loaded Fe monoatomic nanozymes by the hydrothermal method. The enzyme exhibited a higher degree of graphitization compared to conventionally synthesized Fe monoatomic nanozymes, resulting in enhanced electron transfer efficiency. This suggests a higher peroxidase activity within this enzyme class, facilitating the catalysis of enzymatic reactions. Analogous to metalloenzymes in organisms, isolated iron atoms present on the enzyme’s surface served as active centers, promoting the catalysis of H2O2 decomposition to produce highly toxic •OH, ultimately leading to cancer cell-specific apoptosis. The enzyme’s ultra-small size (3–5 nm) enabled it to effectively penetrate deep into tumor tissue, where it efficiently eradicated tumor cells. Additionally, when exposed to near-infrared light, the enzyme could convert light energy into heat energy, which synergistically complemented chemodynamic therapy (CDT) in inhibiting the growth of tumor cells.
Furthermore, single-atom nanozymes like Ir [103], Mn [104,105,106] and Co [107,108] are utilized for biomedical and environmental improvements, offering significant advantages over other nanomaterials.
3.2. Nonmetal-Doped CD Nanozymes
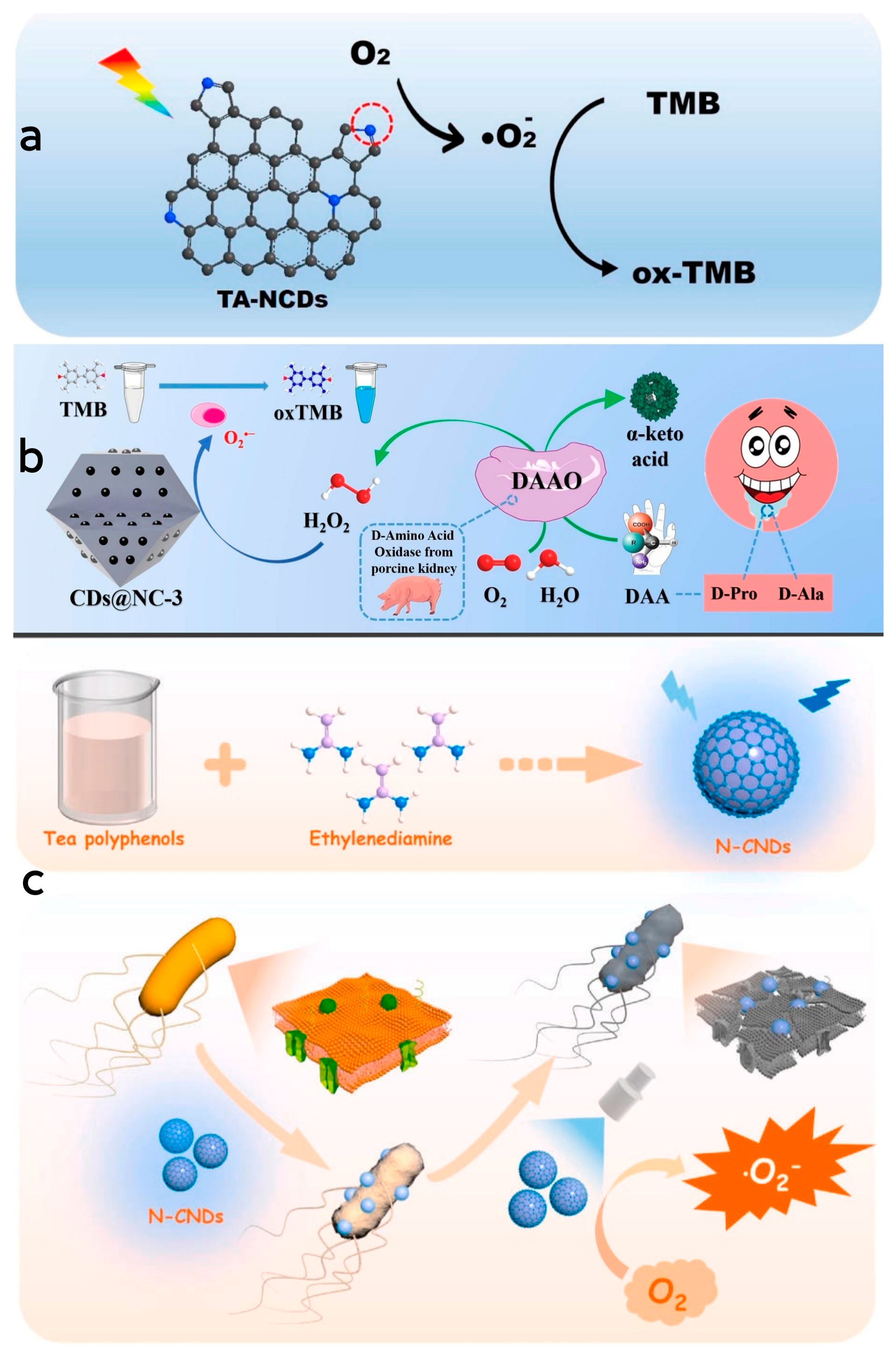
In the preparation process of CDs, doped CDs can be modified by incorporating a small quantity of dopant atoms, which serves to enhance their quantum yield and alter their physical and chemical properties [109]. By doping elements into CDs during their preparation, the resulting CDs exhibit improved fluorescence performance and catalytic capability when contrasted with CDs produced via conventional techniques, thereby broadening their range of applications [110]. Moreover, the introduction of doping elements is pivotal in regulating the properties and catalytic performance of mimetic enzymes, while non-metallic doping exhibits superior biocompatibility with organisms and causes less cellular damage, making them more suitable for applications in biomedicine or therapeutics [111,112]. Through hydrothermal synthesis, Wang et al. [66] successfully synthesized a series of nitrogen-doped CD nanozymes with various molar ratios of precursor substances. These nanozymes exhibited oxidase-like activity, wherein the pyrrolyl nitrogen serves as both the catalytically active site and the substrate-binding site. The oxidation of TMB was catalyzed by activating oxygen to form superoxide radicals. The generation of reactive oxygen species was attributed to the electrons being excited into the conduction band under light stimulation to facilitate the reduction in oxygen. Remarkably, under visible light irradiation, the enzyme demonstrated strong binding to specific targets in mitochondria, leading to its enrichment in the mitochondria of HeLa cells. This localization resulted in the killing of nearly 60% of HeLa cells, underscoring the enzyme’s significant biological activity and its potential for future applications in the treatment of cancer-related diseases (Figure 5a). Li et al. [113] designed and synthesized a carbon-doped nanozyme restricted in a nitrogen-doped carbon framework through pyrolysis of a ZIF-8 precursor filled with glucose (G@ZIF-8). The CD nanozyme demonstrated robust peroxidase-like activity, converting H2O2 into superoxide radicals and oxidizing TMB molecules into blue oxTMB during the chromogenic reaction. To explore the role of the carbon host and CD guest in the catalytic activity of CDs@NC-3, the authors synthesized the NC host and CDs separately and investigated their catalytic activities. The results indicated that the synergetic effect was not the dominate factor in the improvement of catalytic activity and instead was the confinement effect that played a pivotal role in the improvement of catalytic activity. Notably, the saliva of early gastric cancer patients was found to contain elevated levels of D-proline (D-Pro) and D-alanine (D-Ala), while D-amino acid oxidase exhibited chiral-specific catalytic properties in alkaline and neutral conditions, selectively catalyzing D-Pro and D-Ala in these patients. This specific catalysis involved the conversion of D-Pro and D-Ala into H2O2, followed by TMB oxidation based on the H2O2-related catalytic activity of CDs@NC, enabling the detection of D-Pro and D-Ala (Figure 5b). Wang et al. [65] utilized hydrothermal synthesis to produce a nitrogen-doped CD nanozyme with oxidase-like activity. This nanozyme was created by using tea polyphenol and ethylenediamine as precursor. Compared to tea polyphenol alone, the nanozyme displayed significantly enhanced antimicrobial properties. The introduction of nitrogen doping resulted in the formation of surface-active sites such as pyrrolyl nitrogen and graphitic nitrogen structures, allowing the enzyme to alter its electronic energy level and boost its catalytic activity. The enzyme catalyzed O2 to generate superoxide radicals under light radiation. These radicals subsequently oxidated TMB to oxTMB. The nanozyme effectively penetrated the bacterial cell membranes, interacting with intracellular proteins, lipids and nucleic acids, disrupted of cellular structure and function by generating superoxide radicals, thereby inhibiting or eradicating the bacteria. Moreover, the antimicrobial efficacy of the nanozyme was significantly higher under light exposure compared to dark conditions. Furthermore, the antibacterial activity exhibited a synergistic relationship between the concentration of the nanozyme and the duration of light exposure. As the concentration and duration of light increased, this synergistic effect resulted in improved antibacterial efficiency (Figure 5c).
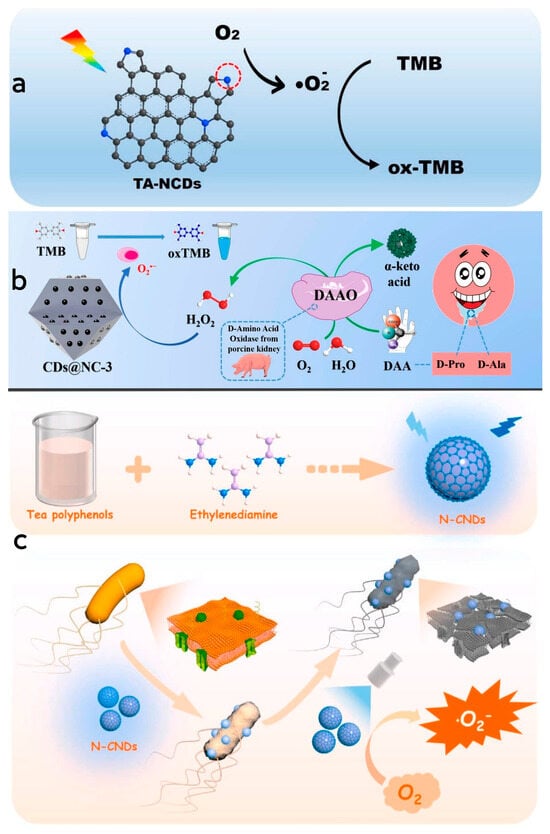
Figure 5.
(a) Schematic diagram of the mechanism of TMB catalyzed by N-CDs. Reprinted with permission from ref. [66]. Copyright 2021, Elsevier. (b) Schematic diagram of detection mechanism of D-Pro and D-Ala by using CDs@NC-3. Reprinted with permission from ref. [113]. Copyright 2021, Elsevier. (c) Schematic diagram of the preparation process of N-CDs and their mechanism in the sterilization process. Reprinted with permission from ref. [65]. Copyright 2023, Elsevier.
3.3. Metal-Doped CDs Nanozymes
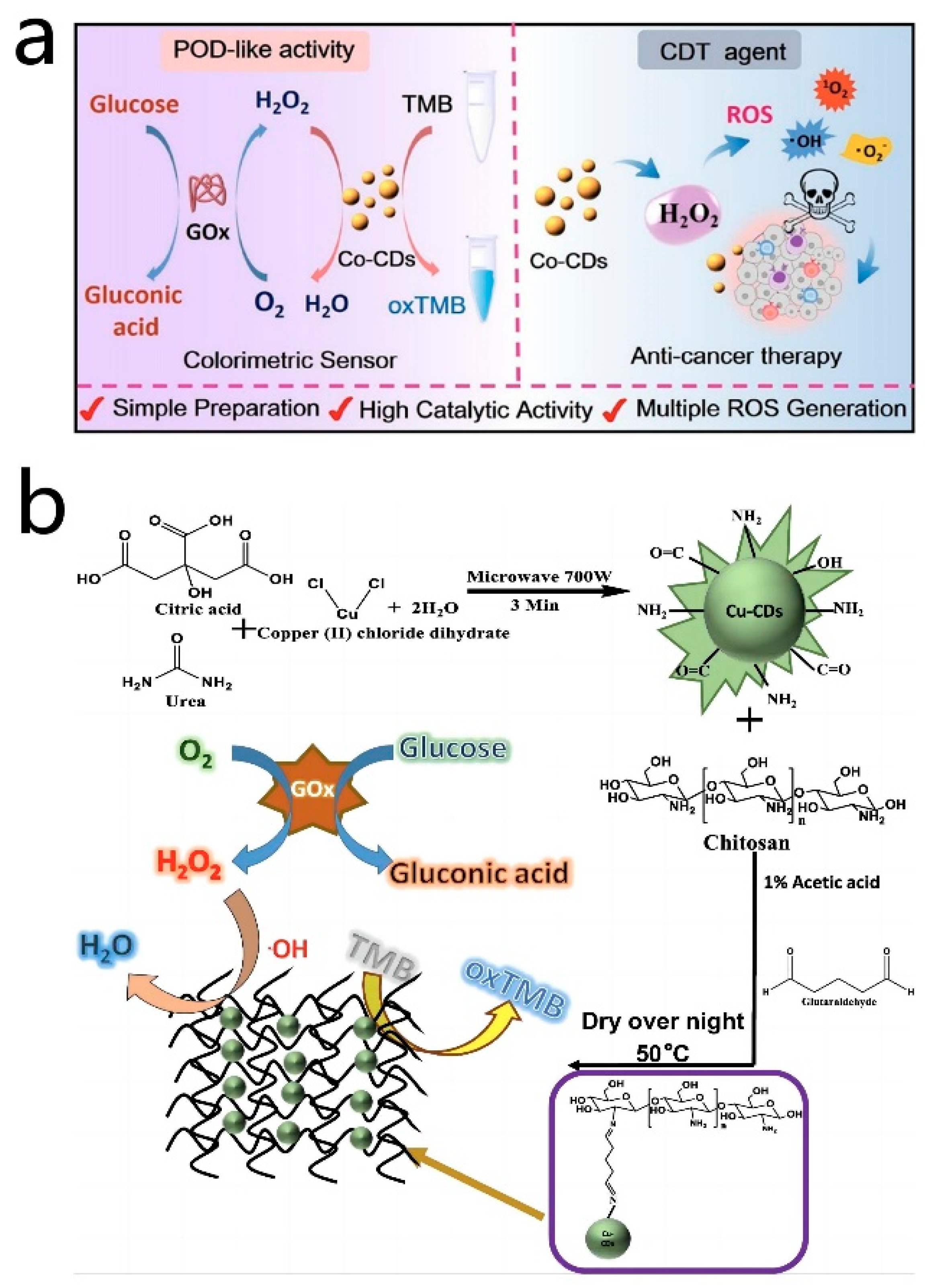
Doping metal atoms into CD nanozymes can significantly enhance their optical properties and catalytic abilities, owing to the unique structures of the metal atoms and other associated factors. Different metals utilized for doping can yield distinct effects on the properties and catalytic performances of CD nanozymes [114,115]. For instance, Zhuo et al. [68] developed a rapid and efficient colorimetric assay for the measurement of ascorbic acid using manganese-doped CD nanozymes. The Mn-CD nanozymes exhibit oxidase-like activity, whereby the dissolved oxygen could interact with the active sites on the surface of Mn-CD, leading to the generation of ROS. These ROS then catalyzed the conversion of TMB to blue oxTMB. Upon the addition of ascorbic acid (AA) to the catalytic system, AA reacted with the oxTMB, reducing it back to the colorless TMB. This catalytic system demonstrated high selectivity for AA and offered a significant advantage in detecting AA in real samples. Moreover, Lu et al. [69] synthesized Co-doped carbon nanozymes with strong peroxidase-like activity through hydrothermal synthesis using vitamin B12 and citric acid as precursors. In this process, cobalt ions were present in the form of Co(II) and Co(III). Co(II) ions demonstrated excellent Fenton reactivity, enabling the decomposition of H2O2 and the production of hydroxyl radicals (•OH), ultimately converting TMB to oxTMB. Simultaneously, in the presence of H2O2, Co-CDs could be oxidized, leading to the generation of peroxyl radicals following the Russell mechanism, which subsequently combined to form singlet oxygen (1O2). Moreover, Co-CDs exhibited the capability to interact with glucose oxidase (GOx), which oxidized glucose into H2O2 and gluconic acid. Subsequently, Co-CDs leverage this H2O2 to catalyze the oxidation of TMB, resulting in the formation of ox-TMB with distinct absorption peaks at 652 nm. The good linear correlation between the absorbance of oxTMB and the glucose concentration allowed for the precise and quantitative detection of glucose. Additionally, Tummala et al. [116] utilized the microwave radiation method to prepare copper-doped CD nanozymes, which were subsequently doped into chitosan membranes through a cross-linking reaction to create a composite material. The cross-linking reaction between amino groups and Cu-CDs played a crucial role in enhancing the stability of the membranes and preventing particle aggregation during the analysis process. The resulting composite exhibited peroxidase-like activity, facilitating the catalysis of the H2O2-mediated TMB oxidation reaction. This catalytic activity was particularly effective under acidic conditions, leading to the generation of blue oxTMB. A standard curve established by the colorimetric detection of TMB enabled the quantitative detection of H2O2 and glucose concentrations. This method not only allowed for the colorimetric detection of H2O2 and glucose but also mitigated issues related to particle aggregation arising from the presence of chitosan membranes. Ultimately, this approach proved valuable in the analysis of glucose sugars in human serum (Figure 6b). In addition, there were a variety of doping elements, such as Fe [67,79,117,118,119], Sc [120], Ce [121] and Mo [70]. Such metal-doped CD nanozymes have been used in analytical and biomedical applications, with promising application prospects.
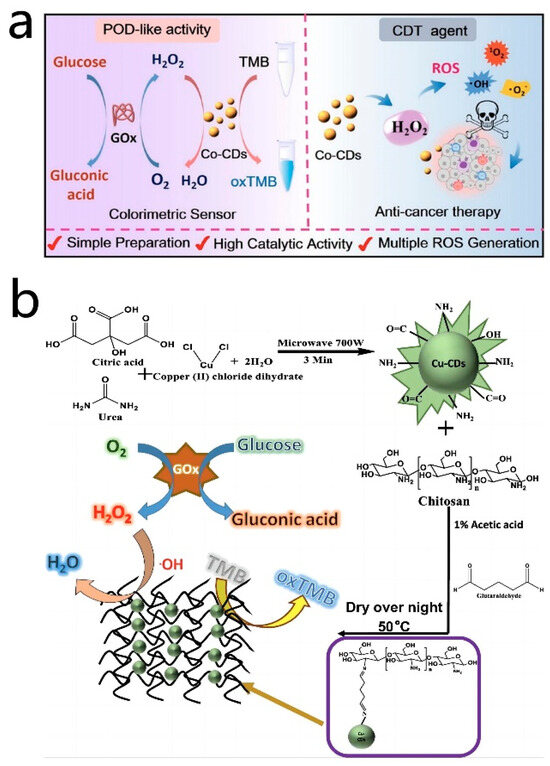
Figure 6.
(a) Illustration of the Co-CD nanozyme for colorimetric determination of glucose and anticancer cell effect. Reprinted with permission from ref. [69]. Copyright 2022, American Chemical Society. (b) Schematic representation for the synthesis of Cu-CDs/chitosan film and the detection of H2O2 and glucose. Reprinted with permission from ref. [116]. Copyright 2022, Srikrishna Tummala et al.
3.4. Codoped CD Nanozymes
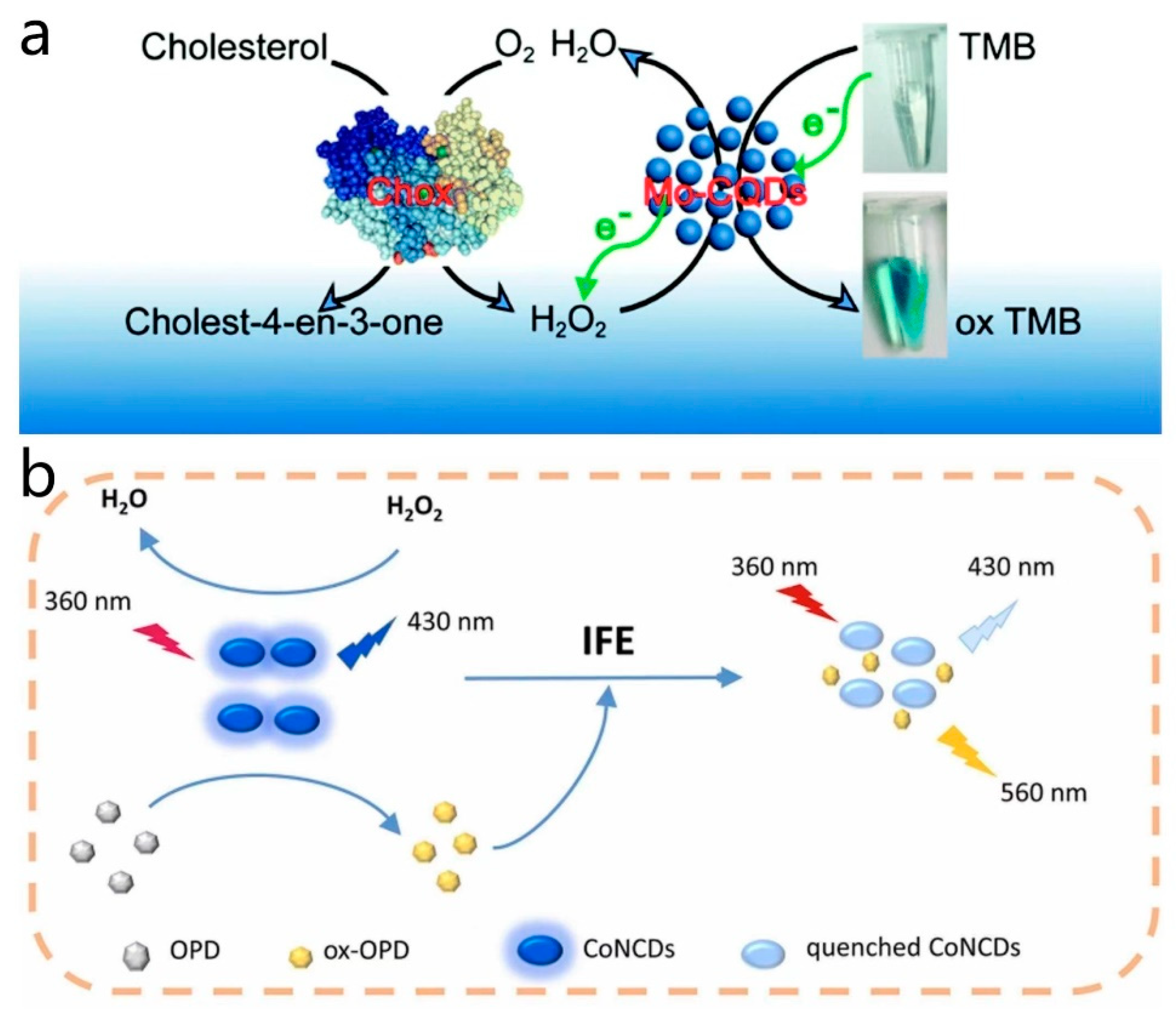
Codoped CD nanozymes, which are prepared by doping two or more elements to enhance the properties as well as the catalytic ability of CD nanozymes, have been a focus of research in recent years. Zhao et al. [71] synthesized a novel Mo and S codoped CD nanozyme with strong peroxidase-like activity using a hydrothermal synthesis method. The codoping of Mo and S significantly increased the production yield of the fabricated CD nanozyme. The functional groups on the surface of the enzyme could interact with the amino groups of TMB, facilitating the transfer of electron pairs from TMB to the enzyme. This enhanced the electron density and mobility of the enzyme, thereby boosting its catalytic activity. Due to its peroxidase-like activity, this enzyme can catalyze the color reaction of TMB to oxTMB in the presence of H2O2. When combined with the action of cholesterol oxidase (Chox), which generates H2O2 through the oxidation of cholesterol, the codoped CD nanozyme catalyzes the reaction between H2O2 and TMB, resulting in a color change. This color change allows for the visual detection of cholesterol concentration, enabling highly sensitive cholesterol testing (Figure 7a). Su et al. [77] developed and prepared a cobalt and nitrogen codoped CD nanozyme, which exhibited excellent peroxidase-like activity and fluorescence characteristics in neutral and alkaline environments. The Co(II) center in Co, N-CDs served as the active site that interacted with H2O2. In the presence of H2O2, the Co(II) center was oxidized to Co(III), initiating the catalytic cycle. Under the influence of the Co(II) center, o-phenylenediamine (OPD) molecules were oxidized to the corresponding product oxOPD, involving electron transfer where the Co(II) center accepts electrons from OPD while transferring electrons to H2O2, facilitating its decomposition into reactive hydroxyl radicals (•OH). As OPD was oxidized and oxOPD was formed, the Co(III) center was reduced back to Co(II), preparing for the next catalytic cycle. Co, N-CDs exhibited higher catalytic activity under neutral to alkaline conditions but lower activity under acidic conditions, likely due to the charge state of the Co, N-CDs surface. In neutral to alkaline environments, the negatively charged surface of Co, N-CDs favors electrostatic adsorption with the positively charged OPD substrate, promoting catalytic reactions. The enzyme displayed selectivity towards the OPD substrate, possibly attributed to the surface properties of Co, N-CDs and the electronic structure of the Co(II) center, enhancing its affinity and catalytic efficiency towards OPD. By catalyzing the oxidation of OPD in the presence of H2O2, the enzyme underwent fluorescence quenching, allowing for the construction of the colorimetric and ratiometric fluorescence method for determining H2O2 based on the absorbance of ox-OPD at 420 nm and the fluorescence intensity ratio of ox-OPD to CoNCDs (I560/I430). The colorimetric and fluorescent detection method was based on the absorbance and fluorescence intensity ratio of the corresponding substances to determine the H2O2 content. Utilizing this H2O2 measurement method, in conjunction with cholesterol oxidase or xanthine oxidase catalyzing the production of H2O2 from cholesterol or xanthine, enabled the detection of cholesterol and xanthine in human serum samples. This method offered high sensitivity in detecting cholesterol or xanthine and produced multiple fluorescence signals, reducing potential interference during the detection process and increasing the precision of the method (Figure 7b). In a similar vein, Dong et al. [78] synthesized cobalt and nitrogen codoped CD nanozymes. Unlike other enzymes, this enzyme exhibited peroxidase-like activity. The Co doping created the active center, which interacted with the surface or internal structure of the CDs, providing an appropriate electron environment for catalyzing oxygen activation. The presence of cobalt ions enhanced the affinity of Co, N-CDs for oxygen by providing unpaired electrons, thereby promoting oxygen activation. Initially, oxygen (O2) molecules were adsorbed by the active center of Co, N-CDs. The interaction between oxygen molecules and cobalt ions altered the electronic structure of the oxygen molecules, reducing their oxidation potential and facilitating the release of reactive oxygen species. Facilitated by the active center, Co, N-CDs convert oxygen molecules into singlet oxygen (1O2) through an electron transfer process. The reaction between 1O2 and extracellular DNA (eDNA) in bacterial biofilms leads to DNA strand breakage, disrupting the structure and function of the biofilm, rendering bacteria vulnerable. Furthermore, 1O2 not only damaged the biofilm but also reacted directly with bacterial cell membranes and internal components, causing oxidative damage, compromising the integrity of the cell membrane and ultimately resulting in bacterial death. This enzyme exhibited broad-spectrum antibiofilm activity against both Gram-positive and Gram-negative bacteria.
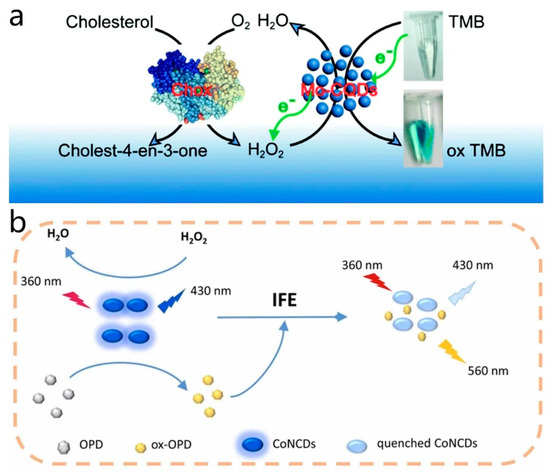
Figure 7.
(a) Schematic diagram of cholesterol detection by Mo, S-CDs and cholesterol oxidase. Reprinted with permission from ref. [71]. Copyright 2019, Royal Society of Chemistry. (b) Ratio fluorescence multimodal analysis strategies for H2O2 detection. Reprinted with permission from ref. [77]. Copyright 2021, Elsevier.
3.5. Undoped CD Nanozymes
Undoped CD nanozymes are prepared by using a single carbon source as a precursor substance, and the prepared CDs are free from elemental doping and have certain enzyme-like catalytic activities. Lin et al. [87] prepared undoped CD nanozymes by hydrothermal synthesis, using citric acid as a single carbon source. These nanozymes exhibited cysteine oxidase-like activity, catalyzing the oxidation of cysteine to cysteamine and H2O2. Compared to natural cysteine oxidase, the CD nanozymes demonstrated higher catalytic activity. The active sites on the enzyme surface catalyzed the further decomposition of H2O2 to generate highly reactive hydroxyl radicals (•OH). The hydroxyl radicals (•OH) possessed strong oxidizing properties, facilitating the oxidation of terephthalic acid (TA) to produce highly fluorescent hydroxyterephthalic acid (TA-OH). The TA-OH emitted strong fluorescence at specific wavelengths, allowing for indirect quantification of cysteine concentration by measuring the fluorescence intensity. This method offered low detection limits, high selectivity and excellent stability, presenting a novel tool for biomedical analysis and clinical diagnosis (Figure 2a). Geng et al. [122] developed undoped CD nanozymes with highly efficient superoxide dismutase-like enzyme activity. The catalytic active center of this enzyme contained specific chemical groups that can interact with •O2− and H2O2, providing an electron to •O2− to reduce it to oxygen. Simultaneously, other groups on the enzyme could bind to H2O2, further converting it into water and oxygen. In addition to SOD-like enzyme activity, this enzyme also exhibited antioxidant properties by decomposing H2O2 into water and oxygen, thereby helping to reduce the toxicity of reactive oxygen species (ROS). By scavenging •O2− around mitochondria, this enzyme protected mitochondrial function, inhibiting cell apoptosis and reducing levels of inflammatory cytokines, effectively mitigating oxidative stress and inflammatory responses within the body and protecting cellular damage. Through regulating the hepatic inflammatory network and suppressing apoptosis, it achieved the therapeutic effect of treating liver ischemia/reperfusion injury.
4. Summary and Prospects
The absorption of light energy in the catalytic process of enzymes may result in a modification of the internal electronic state of the enzyme molecule, consequently influencing its activity. Photoluminescence is the phenomenon where a substance absorbs photons and emits photons. CD nanozymes, distinguished by their unique photoluminescent attributes, can exhibit catalytic behavior comparable to natural enzymes under light excitation, while they do not display thermocatalytic activity in the absence of light. By introducing new functional groups or structures onto the surface of CDs, the dots’ activity can be altered. For example, by incorporating functional groups like amino, carboxyl and hydroxyl onto the dot’s surface, these can attach to the dots in a covalent or non-covalent manner, thereby imparting the dots with specific bioactivity or catalytic property. Furthermore, surface functionalization has repercussions on the optical properties of CDs. The addition of various fluorescent molecules or metal nanoparticles can alter the luminescence wavelength and quantum yield of CDs, alongside enhancing water solubility and biocompatibility, rendering them more suitable as carriers for bioimaging and drug-delivery purposes.
Graphene quantum dots (GQDs) and CDs are nanomaterials that demonstrate enzyme-like catalytic activity. GQDs exhibit peroxidase-like activity, particularly GQDs possessing a higher oxygen content, leading to increased peroxidase-like activity with lower Km for the substrate H2O2. This denotes a higher catalytic efficiency. Conversely, CDs display SOD-like activity, enabling them to scavenge superoxide anions. The catalytic activity of CDs is intricately linked to the presence of surface functional groups such as carbonyl and hydroxyl. These functional groups interact with superoxide anions via hydrogen bonding, capturing electrons to yield oxygen and reduced-state CDs, manifesting their SOD-like activity. Although both GQDs and CDs exhibit enzyme-like properties, variations exist in their catalytic mechanisms and active sites. GQDs’ peroxidase-like activity is predominantly associated with their oxygen-containing functional groups, whereas the SOD-like activity of CDs is contingent upon the abundance of functional groups on their surfaces and specific chemical alterations.
The study of CD nanozymes is still in its infancy compared with quantum dots and other carbon materials. Although there have been numerous descriptions of the mechanism of action of CD nanozymes, the detailed catalytic function mechanism remains not fully understood. Further research is required to comprehend the relationship between their structure and function and to achieve precise development and application of their functions. Currently, limitations still exist in the characterization of CDs, necessitating the development of more effective characterization techniques to identify the luminescence center or mechanism. Despite the relatively simple preparation of CDs, the urgent challenge of the large-scale production of high-quality and enzyme-specific CDs remains.
To accurately classify the various types of CD nanozymes, it is essential to establish a more systematic classification system. Currently, most of the discovered CD nanozymes predominantly exhibit oxidoreductase activity. Expanding research efforts to develop CD nanozymes with a wider range of catalytic types is crucial to broaden their application potential. Despite demonstrating lower toxicity in vitro compared to other materials, it is imperative to consider the biological safety of CD nanozymes during their application. Detailed investigations into their pharmacokinetics and other aspects are necessary to ensure the safe utilization of CD nanozymes in body treatments and other applications.
CD nanozymes are relatively simple to prepare and offer strong enzyme-like activity and optical properties, making them valuable for applications in sensing, medicine and therapy. The introduction of doping elements can further enhance the properties and catalytic activity of CD nanozymes. Due to their superior biocompatibility, stability, lower cost compared to other nanomaterials and relatively low toxicity, CD nanozymes hold significant promise for advancements in biomedicine and therapy. This paper provides a comprehensive overview of recent research progress on CD nanozymes, detailing common preparation methods and discussing their diverse applications based on doping types. With the continuous progress of research on CD nanoenzymes, their potential for widespread application is expected to be further expanded.
Author Contributions
Conceptualization, J.K.; Manuscript preparation, J.K. and F.Z.; Review and editing, J.K. and F.Z.; Funding acquisition, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundamental Research Funds for the Universities of Henan Province, grant number NSFRF220439, and key scientific research projects of colleges and universities in Henan Province, grant number 23B350001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lou, R.; Pan, W.; Li, N.; Tang, B. Nanoenzymes in Disease Diagnosis and Therapy. Chem. Commun. 2020, 56, 15513–15524. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Liu, S.; Chen, Y.; Liang, D.; Liu, Y.; Chen, W.; Du, L.; Wu, C. Application of Nanozymes in Environmental Monitoring, Management, and Protection. Biosensors 2023, 13, 314. [Google Scholar] [CrossRef]
- Chen, X.; Liao, J.; Lin, Y.; Zhang, J.; Zheng, C. Nanozyme’s Catalytic Activity at Neutral pH: Reaction Substrates and Application in Sensing. Anal. Bioanal. Chem. 2023, 415, 3817–3830. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Zhao, Y. Nanozymes: Versatile Platforms for Cancer Diagnosis and Therapy. Nano Micro Lett. 2022, 14, 95. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Qu, X. Biosystem-Inspired Engineering of Nanozymes for Biomedical Applications. Adv. Mater. 2023, 36, 2211147. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, C.; Xu, Y.; Cheng, D.; Lu, Q.; Gao, J.; Yang, W.; Zhu, X.; Liu, M.; Li, H.; et al. Group IV Nanodots: Newly Emerging Properties and Application in Biomarkers Sensing. TrAC Trends Anal. Chem. 2020, 131, 116007. [Google Scholar] [CrossRef]
- Jin, J.; Li, L.; Zhang, L.; Luan, Z.; Xin, S.; Song, K. Progress in the Application of Carbon Dots-Based Nanozymes. Front. Chem. 2021, 9, 748044. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Yan, M.; Sun, P.; Sun, Y.; Qu, L.; Li, Z. Recent Progress in Carbon-Dots-Based Nanozymes for Chemosensing and Biomedical Applications. Chin. Chem. Lett. 2021, 32, 2994–3006. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Z.; Deng, N.; Fu, K.; Zhu, C.; Hong, Q.; Shen, Y.; Liu, S.; Zhang, Y. Molecular Insights of Nanozymes from Design to Catalytic Mechanism. Sci. China Chem. 2023, 66, 1318–1335. [Google Scholar] [CrossRef]
- Shamsabadi, A.; Haghighi, T.; Carvalho, S.; Frenette, L.C.C.; Stevens, M.M.M. The Nanozyme Revolution: Enhancing the Performance of Medical Biosensing Platforms. Adv. Mater. 2023, 36, 2300184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Darland, D.C.; Zhao, J.X. Nanozymes-Hitting the Biosensing “Target”. Sensors 2021, 21, 5201. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Hu, B.; Zhang, B.; Zhang, H.; Yan, X.; Nie, G.; Liang, M. Carbon-Based Nanozymes for Biomedical Applications. Nano Res. 2021, 14, 570–583. [Google Scholar] [CrossRef]
- Yang, W.; Yang, X.; Zhu, L.; Chu, H.; Li, X.; Xu, W. Nanozymes: Activity Origin, Catalytic Mechanism, and Biological Application. Coord. Chem. Rev. 2021, 448, 214170. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in Bionanotechnology: From Sensing to Therapeutics and Beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, X.; Pan, Q.; Zhang, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. A Review of Carbon Dots in Synthesis Strategy. Coordin. Chem. Rev. 2024, 498, 215468. [Google Scholar] [CrossRef]
- Jiang, J.; Li, X.; Li, H.; Lv, X.; Xu, Y.; Hu, Y.; Song, Y.; Shao, J.; Li, S.; Yang, D. Recent Progress in Nanozymes for the Treatment of Diabetic Wounds. J. Mat. Chem. B 2023, 11, 6746–6761. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, Y.; Zhang, J.; Tang, N.; Bao, X.; Liu, Z. New Horizons for Therapeutic Applications of Nanozymes in Oral Infection. Particuology 2023, 80, 61–73. [Google Scholar] [CrossRef]
- Chakraborty, N.; Gandhi, S.; Verma, R.; Roy, I. Emerging Prospects of Nanozymes for Antibacterial and Anticancer Applications. Biomedicines 2022, 10, 1378. [Google Scholar] [CrossRef]
- Cong, W.; Meng, L.; Pan, Y.; Wang, H.; Zhu, J.; Huang, Y.; Huang, Q. Mitochondrial-Mimicking Nanozyme-Catalyzed Cascade Reactions for Aging Attenuation. Nano. Today 2023, 48, 101757. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Wang, H.; Zhao, G.; Li, J.; Wang, Y. Advances in the Application of Single-Atom Nanozymes for Heavy Metal Ion Detection, Tumor Therapy and Antimicrobial Therapy. Microchem. J. 2023, 191, 108817. [Google Scholar] [CrossRef]
- Ren, X.; Liu, J.; Ren, J.; Tang, F.; Meng, X. One-Pot Synthesis of Active Copper-Containing Carbon Dots with Laccase-like Activities. Nanoscale 2015, 7, 19641–19646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, S.; Lu, X.; Wu, P.; Liu, J. Manganese as a Catalytic Mediator for Photo-Oxidation and Breaking the pH Limitation of Nanozymes. Nano Lett. 2019, 19, 3214–3220. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Liu, X.; Ye, X.; Dai, M.; Fang, B.; Shen, X.; Liu, L. Nanozyme-Based Remodeling of Disease Microenvironments for Disease Prevention and Treatment: A Review. ACS Appl. Nano Mater. 2023, 6, 13792–13823. [Google Scholar] [CrossRef]
- Farshidfar, N.; Fooladi, S.; Nematollahi, M.H.; Iravani, S. Carbon Dots with Tissue Engineering and Regenerative Medicine Applications. RSC Adv. 2023, 13, 14517–14529. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Li, Q.; Qiu, S.; Li, M.; Shin, J.; Wu, P.; Singh, N.; Li, J.; Ding, Q.; Hu, C.; et al. Recent Developments in Carbon Dots: A Biomedical Application Perspective. J. Mat. Chem. B 2023, 11, 3038–3053. [Google Scholar] [CrossRef]
- Alafeef, M.; Srivastava, I.; Aditya, T.; Pan, D. Carbon Dots: From Synthesis to Unraveling the Fluorescence Mechanism. Small 2023, 20, 2303937. [Google Scholar] [CrossRef] [PubMed]
- Szczepankowska, J.; Khachatryan, G.; Khachatryan, K.; Krystyjan, M. Carbon Dots—Types, Obtaining and Application in Biotechnology and Food Technology. Int. J. Mol. Sci. 2023, 24, 14984. [Google Scholar] [CrossRef] [PubMed]
- Jorns, M.; Pappas, D. A Review of Fluorescent Carbon Dots, Their Synthesis, Physical and Chemical Characteristics, and Applications. Nanomaterials 2021, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Adeola, A.O.; Clermont-Paquette, A.; Piekny, A.; Naccache, R. Advances in the Design and Use of Carbon Dots for Analytical and Biomedical Applications. Nanotechnology 2024, 35, 012001. [Google Scholar] [CrossRef]
- Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications. Polymers 2021, 13, 3190. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Wang, X.; Bao, Y.; Sun, Z. Recent Advance of Carbon Dots in Bio-Related Applications. J. Phys. Mater. 2020, 3, 022003. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z. The Formation Mechanism and Fluorophores of Carbon Dots Synthesized via a Bottom-up Route. Mat. Chem. Front. 2020, 4, 400–420. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S.; Nair, A.B.; Deb, P.K. Exploring the Potential of Carbon Dots to Combat COVID-19. Front. Mol. Biosci. 2020, 7, 616575. [Google Scholar] [CrossRef]
- Fang, M.; Wang, B.; Qu, X.; Li, S.; Huang, J.; Li, J.; Lu, S.; Zhou, N. State-of-the-Art of Biomass-Derived Carbon Dots: Preparation, Properties, and Applications. Chin. Chem. Lett. 2024, 35, 108423. [Google Scholar] [CrossRef]
- Liu, C.; Fan, W.; Cheng, W.; Gu, Y.; Chen, Y.; Zhou, W.; Yu, X.; Chen, M.; Zhu, M.; Fan, K.; et al. Red Emissive Carbon Dot Superoxide Dismutase Nanozyme for Bioimaging and Ameliorating Acute Lung Injury. Adv. Funct. Mater. 2023, 33, 2213856. [Google Scholar] [CrossRef]
- Loukanov, A.; Chichova, M.; Filipov, C.; Shkodrova, M.; Mishonova, M.; Mladenova, K.; Doumanov, J.; Gagov, H. Photo-Oxidase Carbon Dot-Based Nanozyme for Breast Cancer Theranostics under Normoxia Condition. J. Photochem. Photobiol. A Chem. 2023, 439, 114632. [Google Scholar] [CrossRef]
- Pan, T.; Chen, H.; Gao, X.; Wu, Z.; Ye, Y.; Shen, Y. Engineering Efficient Artificial Nanozyme Based on Chitosan Grafted Fe-Doped-Carbon Dots for Bacteria Biofilm Eradication. J. Hazard. Mater. 2022, 435, 128996. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Yang, X.; Yang, L.; Yang, D.; Yang, Y.; Zhu, Y. Cu, I-Doped Carbon Dots as Simulated Nanozymes for the Colorimetric Detection of Morphine in Biological Samples. Anal. Biochem. 2023, 680, 115313. [Google Scholar] [CrossRef]
- Gao, W.; He, J.; Chen, L.; Meng, X.; Ma, Y.; Cheng, L.; Tu, K.; Gao, X.; Liu, C.; Zhang, M.; et al. Deciphering the Catalytic Mechanism of Superoxide Dismutase Activity of Carbon Dot Nanozyme. Nat. Commun. 2023, 14, 160. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, R.; Wang, Y. One-Pot Hydrothermal Synthesis of Metal-Doped Carbon Dot Nanozymes Using Protein Cages as Precursors. RSC Adv. 2023, 13, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Zandieh, M.; Liu, J. Nanozymes: Definition, Activity, and Mechanisms. Adv. Mater. 2023, 36, 2211041. [Google Scholar] [CrossRef]
- Pei, J.; Zhao, R.; Mu, X.; Wang, J.; Liu, C.; Zhang, X.-D. Single-Atom Nanozymes for Biological Applications. Biomater. Sci. 2020, 8, 6428–6441. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, R.; Yan, X.; Fan, K. Nanozyme: A New Approach for Anti-Microbial Infections. J. Inorg. Mater. 2023, 38, 43–54. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Hua, K.; Yang, D.; Yang, Y. Iodine-Doped Carbon Dots with Inherent Peroxidase Catalytic Activity for Photocatalytic Antibacterial and Wound Disinfection. Anal. Bioanal. Chem. 2021, 413, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Rahimizadeh, K.; Shafiee, A.; Rabiee, N.; Iravani, S. Nanozymes and Their Emerging Applications in Biomedicine. Process. Biochem. 2023, 131, 154–174. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J. Facile Synthesis of Iron and Nitrogen Co-Doped Carbon Dot Nanozyme as Highly Efficient Peroxidase Mimics for Visualized Detection of Metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, H.; Zhang, C.; Xiao, X.; He, Z. UV-Induced Oxidase Activity of Carbon Dots in Visible UVA Dosage, Escherichia Coli Quantification and Bacterial Typing. Anal. Chim. Acta 2024, 1288, 342140. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, Z.; Li, Y.; Xi, F.; Liu, J. Magnetic Nanozyme Based on Loading Nitrogen-Doped Carbon Dots on Mesoporous Fe3O4 Nanoparticles for the Colorimetric Detection of Glucose. Molecules 2023, 28, 4573. [Google Scholar] [CrossRef]
- Yang, D.; Li, Q.; Zhang, Q.; Wang, Y.; Li, H.; Tammina, S.K.; Yang, Y. A Multifunctional Nanozyme-Based Enhanced System for Tert-Butyl Hydroquinone Assay by Surface-Enhanced Raman Scattering. Microchim. Acta 2022, 189, 29. [Google Scholar] [CrossRef]
- Bandi, R.; Alle, M.; Park, C.-W.; Han, S.-Y.; Kwon, G.-J.; Kim, N.-H.; Kim, J.-C.; Lee, S.-H. Cellulose Nanofibrils/Carbon Dots Composite Nanopapers for the Smartphone-Based Colorimetric Detection of Hydrogen Peroxide and Glucose. Sens. Actuator B Chem. 2021, 330, 129330. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Ren, L.; Wang, T.; Tan, X.; Cui, F.; Li, T.; Li, J. Chitosan-Chelated Carbon Dots-Based Nanozyme of Extreme Stability with Super Peroxidase Activity and Antibacterial Ability for Wound Healing. Int. J. Biol. Macromol. 2024, 258, 129098. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, L.; Sun, C.; Zhou, N. Research on Preparation Methods of Carbon Nanomaterials Based on Self-Assembly of Carbon Quantum Dots. Molecules 2022, 27, 1690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-X.; Tao, S.-Y.; Yang, B. The Classification of Carbon Dots and the Relationship between Synthesis Methods and Properties. Chin. J. Chem. 2023, 41, 2206–2216. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Fan, H.; Zhang, Y.; Wang, X.; Zhang, C. A Minireview on Doped Carbon Dots for Photocatalytic and Electrocatalytic Applications. Nanoscale 2020, 12, 13899–13906. [Google Scholar] [CrossRef]
- Ross, S.; Wu, R.-S.; Wei, S.-C.; Ross, G.M.; Chang, H.-T. The Analytical and Biomedical Applications of Carbon Dots and Their Future Theranostic Potential: A Review. J. Food Drug Anal. 2020, 28, 677–695. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Hallaji, Z.; Najafi Nobar, S.; Bagheri, Z. Carbon Dots with pH-Responsive Fluorescence: A Review on Synthesis and Cell Biological Applications. Microchim. Acta 2020, 187, 150. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, K.; Lu, K.; Li, N.; Sun, S.; Dong, Z. Recent Advances in Solid-State Fluorescent of Red Carbon Dots: A Comprehensive Review. J. Alloy Compd. 2024, 971, 172688. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in Fluorescent Carbon Dots for Biomedical Applications. Adv. Phys. X 2020, 5, 1758592. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Wu, S.; Hou, X.; Zheng, C.; Wu, P.; Liu, J. Self-Photo-Oxidation for Extending Visible Light Absorption of Carbon Dots and Oxidase-like Activity. Carbon 2021, 182, 537–544. [Google Scholar] [CrossRef]
- Li, P. The Valine-based N-doped Carbon Dots with High Peroxidase-like Activity. Luminescence 2022, 37, 1725–1732. [Google Scholar] [CrossRef]
- Lee, A.; Yun, S.; Kang, E.S.; Kim, J.W.; Park, J.H.; Choi, J. Effect of Heteroatoms on the Optical Properties and Enzymatic Activity of N-Doped Carbon Dots. RSC Adv. 2021, 11, 18776–18782. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Wang, Q.; Dong, W.; Liu, Y.; Hu, Q.; Song, X.; Shuang, S.; Dong, C.; Gong, X. Metal-Free Nitrogen-Doped Carbon Nanodots as an Artificial Nanozyme for Enhanced Antibacterial Activity. J. Clean. Prod. 2023, 411, 137337. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Wei, K.; Zhao, Y.; Nie, H.; Ma, Y.; Zhou, Y.; Huang, H.; Liu, Y.; Shao, M.; et al. Pyrrolic Nitrogen Dominated the Carbon Dot Mimic Oxidase Activity. Carbon 2021, 179, 692–700. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, B.; Lu, M.; Li, S.; Guo, J.; Chen, F.; Xiong, X.; Yin, Z.; Liu, H.; Zhou, D. Ultrasmall Fe-Doped Carbon Dots Nanozymes for Photoenhanced Antibacterial Therapy and Wound Healing. Bioact. Mater. 2022, 12, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Fang, J.; Li, M.; Wang, J.; Zhu, C.; Du, J. Manganese(II)-Doped Carbon Dots as Effective Oxidase Mimics for Sensitive Colorimetric Determination of Ascorbic Acid. Microchim. Acta 2019, 186, 745. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Guo, Y.; Zhang, J.; Yue, Y.; Fan, L.; Li, F.; Dong, C.; Shuang, S. A High Catalytic Activity Nanozyme Based on Cobalt-Doped Carbon Dots for Biosensor and Anticancer Cell Effect. ACS Appl. Mater. Interfaces 2022, 14, 57206–57214. [Google Scholar] [CrossRef]
- Lu, W.; Guo, Y.; Yue, Y.; Zhang, J.; Fan, L.; Li, F.; Zhao, Y.; Dong, C.; Shuang, S. Smartphone-Assisted Colorimetric Sensing Platform Based on Molybdenum-Doped Carbon Dots Nanozyme for Visual Monitoring of Ampicillin. Chem. Eng. J. 2023, 468, 143615. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, Z.; Liu, G.; Lu, H.; Gao, Y.; Liu, F.; Wang, C.; Cui, J.; Lu, G. High-Activity Mo, S Co-Doped Carbon Quantum Dot Nanozyme-Based Cascade Colorimetric Biosensor for Sensitive Detection of Cholesterol. J. Mat. Chem. B 2019, 7, 7042–7051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Song, J.; Zhao, L. Metallic Deep Eutectic Solvents-Assisted Synthesis of Cu, Cl-Doped Carbon Dots as Oxidase-like and Peroxidase-like Nanozyme for Colorimetric Assay of Hydroquinone and H2O2. Colloid Surf. S Physicochem. Eng. Asp. 2022, 648, 129390. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Ouyang, D.; Ye, K.; Chen, Y.; Li, Q.; Xia, Q.; Wu, X.; Yang, Y. Copper- and Iodine-Doped Nanozymes with Simulated Enzyme Activity and Efficient Antifungal Activity against Candida Albicans. Biochem. Eng. J. 2023, 191, 108791. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Yuan, T.; Zhu, J.; Yang, Y. Influence of the Iodine Content of Nitrogen- and Iodine-Doped Carbon Dots as a Peroxidase Mimetic Nanozyme Exhibiting Antifungal Activity against C. Albicans. Biochem. Eng. J. 2021, 175, 108139. [Google Scholar] [CrossRef]
- Alqahtani, Y.S.; Mahmoud, A.M.; El-Wekil, M.M. Bifunctional Nanoprobe for Dual-Mode Detection Based on Blue Emissive Iron and Nitrogen Co-Doped Carbon Dots as a Peroxidase-Mimic Platform. Talanta 2023, 253, 124024. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Yang, Z.; Wang, Y.; Rao, H.; Yue, G.; Wu, C.; Lu, C.; Wang, X. A Ratiometric Fluorescence and Colorimetric Dual-Mode Assay for H2O2 and Xanthine Based on Fe, N Co-Doped Carbon Dots. Dyes Pigments 2020, 180, 108486. [Google Scholar] [CrossRef]
- Su, L.; Qin, S.; Cai, Y.; Wang, L.; Dong, W.; Mao, G.; Feng, S.; Xie, Z.; Zhang, H. Co, N-Doped Carbon Dot Nanozymes with Acid pH-Independence and Substrate Selectivity for Biosensing and Bioimaging. Sens. Actuators B Chem. 2022, 353, 131150. [Google Scholar] [CrossRef]
- Dong, W.; Xu, L.; Chen, M.; Jiang, T.; Su, L.; Ma, J.; Chen, C.; Zhang, G. Co-, N-Doped Carbon Dot Nanozymes Based on an Untriggered ROS Generation Approach for Anti-Biofilm Activities and In Vivo Anti-Bacterial Treatment. J. Mater. Chem. B 2024, 12, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.A.; Hassan, R.O. Newly Synthesized Fe-Doped CDs for Colorimetric and Fluorometric Nanozyme-Based Levodopa Sensing. Luminescence 2023, 38, 437–449. [Google Scholar] [CrossRef]
- Muhammad, P.; Hanif, S.; Li, J.; Guller, A.; Rehman, F.U.; Ismail, M.; Zhang, D.; Yan, X.; Fan, K.; Shi, B. Carbon Dots Supported Single Fe Atom Nanozyme for Drug-Resistant Glioblastoma Therapy by Activating Autophagy-Lysosome Pathway. Nano Today 2022, 45, 101530. [Google Scholar] [CrossRef]
- Bijalwan, K.; Kumari, A.; Kaushal, N.; Indra, A.; Saha, A. Solid-State Synthesis of Cu Doped CDs with Peroxidase-Mimicking Activity at Neutral pH and Sensing of Antioxidants. ChemNanoMat 2022, 8, e202200044. [Google Scholar] [CrossRef]
- Kaushal, N.; Jain, A.; Kumar, A.; Sarraf, S.; Basu, A.K.; Raje, C.I.; Saha, A. Solvent-Free Synthesis of S,N-Doped Carbon Dots for Extended Visible-Light-Induced Oxidase-Mimicking Activities and Antimicrobial Applications. ChemPlusChem 2023, 88, e202300125. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Wang, X.; Luo, Y.; Jiang, Z. An Ultrasensitive K+ Fluorescence/Absorption Di-Mode Assay Based on Highly Co-Catalysiscarbon Dot Nanozyme and DNAzyme. Microchem. J. 2020, 159, 105508. [Google Scholar] [CrossRef]
- Saengsrichan, A.; Khemthong, P.; Wanmolee, W.; Youngjan, S.; Phanthasri, J.; Arjfuk, P.; Pongchaikul, P.; Ratchahat, S.; Posoknistakul, P.; Laosiripojana, N.; et al. Platinum/Carbon Dots Nanocomposites from Palm Bunch Hydrothermal Synthesis as Highly Efficient Peroxidase Mimics for Ultra-Low H2O2 Sensing Platform through Dual Mode of Colorimetric and Fluorescent Detection. Anal. Chim. Acta 2022, 1230, 340368. [Google Scholar] [CrossRef]
- Zhang, R.; Hou, Y.; Sun, L.; Liu, X.; Zhao, Y.; Zhang, Q.; Zhang, Y.; Wang, L.; Li, R.; Wang, C.; et al. Recent Advances in Carbon Dots: Synthesis and Applications in Bone Tissue Engineering. Nanoscale 2023, 15, 3106–3119. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Sedighi, O.; Rezaei, N.T.; Abedini, A.A.; Khachatourian, A.M.; Toprak, M.S.; Seifalian, A. Recent Advances in the Modification of Carbon-Based Quantum Dots for Biomedical Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111756. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zeng, Q.; Deng, Q.; Yao, W.; Deng, H.; Lin, X.; Chen, W. Citric Acid-Derived Carbon Dots as Excellent Cysteine Oxidase Mimics for Cysteine Sensing. Sens. Actuator B Chem. 2022, 359, 131563. [Google Scholar] [CrossRef]
- Kang, W.; Lee, A.; Tae, Y.; Lee, B.; Choi, J. Enhancing Catalytic Efficiency of Carbon Dots by Modulating Their Mn Doping and Chemical Structure with Metal Salts. RSC Adv. 2023, 13, 8996–9002. [Google Scholar] [CrossRef]
- Maholiya, A.; Ranjan, P.; Khan, R.; Murali, S.; Nainwal, R.C.; Chauhan, P.S.; Sathish, N.; Chaurasia, J.P.; Srivastava, A.K. An Insight into the Role of Carbon Dots in the Agriculture System: A Review. Environ. Sci. Nano 2023, 10, 959–995. [Google Scholar] [CrossRef]
- Dong, C.; Ma, X.; Huang, Y.; Zhang, Y.; Gao, X. Carbon Dots Nanozyme for Anti-Inflammatory Therapy via Scavenging Intracellular Reactive Oxygen Species. Front. Bioeng. Biotechnol. 2022, 10, 943399. [Google Scholar] [CrossRef]
- Yu, H.; Tang, K.; Cai, Z.; Lin, X.; Huang, Y.; Yu, T.; Zhang, Q.; Wang, Q.; Wu, L.; Yang, L.; et al. Carbon Dots-Based Nanozyme for Drug-Resistant Lung Cancer Therapy by Encapsulated Doxorubicin/siRNA Cocktail. Int. J. Nanomed. 2023, 18, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Liu, Q.; Cai, L.; Yang, Q.; Tong, Y.; Chen, X.; Kotha, S.; Mao, X.; He, W. Multimechanism Collaborative Superior Antioxidant CDzymes To Alleviate Salt Stress-Induced Oxidative Damage in Plant Growth. ACS Sustain. Chem. Eng. 2023, 11, 4237–4247. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhou, S. Progress and Perspective on Carbon-Based Nanozymes for Peroxidase-like Applications. J. Phys. Chem. Lett. 2021, 12, 11751–11760. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Guo, Z.; Liang, M. Recent Progress in Single-Atom Nanozymes Research. Nano Res. 2022, 16, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhang, Z.; Fauconnier, M.-L.; Li, C.; Chen, L.; Zheng, X.; Zhang, D. Bimodal single-atom iron nanozyme biosensor for volatile amine and food freshness detection. Nano Today 2023, 53, 102025. [Google Scholar] [CrossRef]
- Han, J.; Gu, Y.; Yang, C.; Meng, L.; Ding, R.; Wang, Y.; Shi, K.; Yao, H. Single-Atom Nanozymes: Classification, Regulation Strategy, and Safety Concerns. J. Mater. Chem. B 2023, 11, 9840–9866. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Pang, R.; Li, J.; Wang, E. Current Advances on the Single-Atom Nanozyme and Its Bioapplications. Adv. Mater. 2023, 36, 2211724. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Wang, C.; Cheng, Z.; Zheng, W.; Xu, P.; Chen, Q.; Zhao, Y. Missing-Linker-Confined Single-Atomic Pt Nanozymes for Enzymatic Theranostics of Tumor. Angew. Chem. Int. Ed. 2023, 62, e202217995. [Google Scholar] [CrossRef]
- He, H.; Fei, Z.; Guo, T.; Hou, Y.; Li, D.; Wang, K.; Ren, F.; Fan, K.; Zhou, D.; Xie, C.; et al. Bioadhesive Injectable Hydrogel with Phenolic Carbon Quantum Dot Supported Pd Single Atom Nanozymes as a Localized Immunomodulation Niche for Cancer Catalytic Immunotherapy. Biomaterials 2022, 280, 121272. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Q.; Hao, Y.; Wang, Z.; Dong, W.; Liu, Y.; Dong, Y.; Wu, H.; Shuang, S.; Dong, C.; et al. Drug-Primed Self-Assembly of Platinum-Single-Atom Nanozyme to Regulate Cellular Redox Homeostasis Against Cancer. Adv. Sci. 2023, 10, 2302703. [Google Scholar] [CrossRef]
- Li, X.; Ding, S.; Lyu, Z.; Tieu, P.; Wang, M.; Feng, Z.; Pan, X.; Zhou, Y.; Niu, X.; Du, D.; et al. Single-Atomic Iron Doped Carbon Dots with Both Photoluminescence and Oxidase-Like Activity. Small 2022, 18, 2203001. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ge, K.; Zhao, Y.; Bottini, M.; Fan, D.; Wu, W.; Li, L.; Liu, F.; Gao, S.; Liang, X.; et al. Modulating the Coordination Environment of Carbon-Dot-Supported Fe Single-Atom Nanozymes for Enhanced Tumor Therapy. Small 2023, 20, 2306656. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, B.; Zhu, J.; Xu, X.; Zhou, B.; Yang, Y. Single-Atom Nanozyme with Asymmetric Electron Distribution for Tumor Catalytic Therapy by Disrupting Tumor Redox and Energy Metabolism Homeostasis. Adv. Mater. 2023, 35, 2208512. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, G.; Xue, L.; Wang, Y.; Liu, M.; Liu, J.; Zhang, S.; Hu, W. Single-Atom Nanozyme Based on Mn-Center with Enhanced Peroxidase-like Activity for Organic Dye Degradation. ACS Appl. Nano Mater. 2023, 6, 4844–4853. [Google Scholar] [CrossRef]
- Wang, C.; Xue, H.; Zhuang, L.; Sun, H.; Zheng, H.; Wang, S.; He, S.; Luo, X. Developing Single-Atomic Manganese Nanozymes for Synergistic Mild Photothermal/Multienzymatic Therapy. ACS Omega 2023, 8, 49289–49301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, X.; Yang, J.; Zhao, Z.; Zhang, X.; Yang, F.; Zhang, Z.; Chen, P.; Li, L.; Zhang, R. Mn-Single-Atom Nano-Multizyme Enabled NIR-II Photoacoustically Monitored, Photothermally Enhanced ROS Storm for Combined Cancer Therapy. Biomater. Res. 2023, 27, 125. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Q.; He, X.; Yang, G.; Chen, X.; Meng, J.; Hu, B.; Qian, Y.; Shen, J.; Jin, L.; et al. NIR-II-Enhanced Single-Atom-Nanozyme for Sustainable Accelerating Bacteria-Infected Wound Healing. Appl. Surf. Sci. 2023, 612, 155866. [Google Scholar] [CrossRef]
- Ren, G.; Lu, M.; Zhao, Z.; Qin, F.; Li, K.; Chen, W.; Lin, Y. Cobalt Single-Atom Nanozyme Co-Administration with Ascorbic Acid Enables Redox Imbalance for Tumor Catalytic Ablation. ACS Biomater. Sci. Eng. 2023, 9, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Fernandez, G.; Cigales Canga, J.; Soldado, A.; Ruiz Encinar, J.; Costa-Fernandez, J.M. Functionalized Heteroatom-Doped Carbon Dots for Biomedical Applications: A Review. Anal. Chim. Acta 2023, 1284, 341874. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, S.; Li, N.; Lu, K.; Dong, Z. Based on Halogen-Doped Carbon Dots: A Review. Mater. Today Chem. 2023, 34, 101769. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, W.; Yang, R. Emerging Single-Atom Nanozymes for Catalytic Biomedical Uses. Nano Res. 2023, 16, 13056–13076. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L. Design of Carbon Dots as Nanozymes to Mediate Redox Biological Processes. J. Mater. Chem. B 2023, 11, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.; Ni, P.; Zhang, C.; Wang, B.; Duan, G.; Chen, C.; Jiang, Y.; Lu, Y. Carbon Dots Confined in N-Doped Carbon as Peroxidase-like Nanozyme for Detection of Gastric Cancer Relevant D-Amino Acids. Chem. Eng. J. 2022, 428, 131396. [Google Scholar] [CrossRef]
- Yuxin, X.; Laipeng, S.; Kang, L.; Haipeng, S.; Zonghua, W.; Wenjing, W. Metal-Doped Carbon Dots as Peroxidase Mimic for Hydrogen Peroxide and Glucose Detection. Anal. Bioanal. Chem. 2022, 414, 5857–5867. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, X.; Wang, Y.; Shen, B.; Yang, Y.; Huang, H. Heteroatom-Doped Nanozyme Progress and Perspectives: From Synthesis Strategies to Biomedical Applications. Chem. Eng. J. 2023, 468, 143703. [Google Scholar] [CrossRef]
- Tummala, S.; Bandi, R.; Ho, Y.-P. Synthesis of Cu-doped Carbon Dot/Chitosan Film Composite as a Catalyst for the Colorimetric Detection of Hydrogen Peroxide and Glucose. Microchim. Acta 2022, 189, 284. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Nie, Y.; Lu, J.; Bi, Q.; Cai, Z.; Song, X.; Ai, H.; Jin, R. Iron Doped Carbon Dots Based Nanohybrids as a Tetramodal Imaging Agent for Gene Delivery Promotion and Photothermal-Chemodynamic Cancer Synergistic Theranostics. Mater. Des. 2021, 208, 109878. [Google Scholar] [CrossRef]
- Yang, Y.M.; Yan, Y.; Zhou, J.Y.; Huang, C.Z.; Zhen, S.J.; Zhan, L. Fe-Doped Carbon Dots: A Novel Fluorescent Nanoprobe for Cellular Hypochlorous Acid Imaging. Anal. Sci. 2023, 40, 511–518. [Google Scholar] [CrossRef]
- Li, T.; Lin, L.; Wang, D.; Fang, H.; Zhang, Z.; Wang, Y.; Chen, Y.; Feng, L. Injectable Hydrogel Incorporated with Iron-Doped Carbon Dots Exhibiting Peroxidase-Like Activity for Antibacterial Therapy and Wound Healing. Adv. Ther. 2024, 7, 2300368. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Y.; Liao, J.; Zhang, J.; Zheng, C. Light-Activated Carbon Dot Nanozyme with Scandium for a Highly Efficient and pH-Universal Bio-Nanozyme Cascade Colorimetric Assay. J. Mat. Chem. B 2023, 11, 6697–6703. [Google Scholar] [CrossRef]
- Li, S.; Pang, E.; Gao, C.; Chang, Q.; Hu, S.; Li, N. Cerium-Mediated Photooxidation for Tuning pH-Dependent Oxidase-like Activity. Chem. Eng. J. 2020, 397, 125471. [Google Scholar] [CrossRef]
- Geng, H.; Chen, J.; Tu, K.; Tuo, H.; Wu, Q.; Guo, J.; Zhu, Q.; Zhang, Z.; Zhang, Y.; Huang, D.; et al. Carbon Dot Nanozymes as Free Radicals Scavengers for the Management of Hepatic Ischemia-Reperfusion Injury by Regulating the Liver Inflammatory Network and Inhibiting Apoptosis. J. Nanobiotechnol. 2023, 21, 500. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).