Abstract
Aflatoxin (AFT) contamination poses a significant global public health and safety concern, prompting widespread apprehension. Of the various AFTs, aflatoxin B1 (AFB1) stands out for its pronounced toxicity and its association with a spectrum of chronic ailments, including cardiovascular disease, neurodegenerative disorders, and cancer. Lycopene, a lipid-soluble natural carotenoid, has emerged as a potential mitigator of the deleterious effects induced by AFB1 exposure, spanning cardiac injury, hepatotoxicity, nephrotoxicity, intestinal damage, and reproductive impairment. This protective mechanism operates by reducing oxidative stress, inflammation, and lipid peroxidation, and activating the mitochondrial apoptotic pathway, facilitating the activation of mitochondrial biogenesis, the endogenous antioxidant system, and the nuclear factor erythroid 2-related factor 2 (Nrf2)/kelch-like ECH-associated protein 1 (KEAP1) and peroxisome proliferator-activated receptor-γ coactivator-1 (PGC-1) pathways, as well as regulating the activities of cytochrome P450 (CYP450) enzymes. This review provides an overview of the protective effects of lycopene against AFB1 exposure-induced toxicity and the underlying molecular mechanisms. Furthermore, it explores the safety profile and potential clinical applications of lycopene. The present review underscores lycopene’s potential as a promising detoxification agent against AFB1 exposure, with the intent to stimulate further research and practical utilization in this domain.
1. Introduction
Mycotoxins are secondary toxic metabolites naturally produced by certain filamentous fungi. Approximately 500 mycotoxins have been identified, including aflatoxins (AFTs), deoxynivalenol (DON), T-2 toxin, HT-2 toxin, ochratoxin A (OTA), zearalenone (ZEN), nivalenol (NIV), and fumonisins (FBs) [1,2,3]. These mycotoxins can contaminate a variety of food items, encompassing fruits, grain crops, and processed products such as beer, dried fruits, cereals, and animal feed [1,4,5]. A recent report has indicated mycotoxins could be detected in Chinese herbal medicine [6]. Prior to 1985, the Food and Agriculture Organization (FAO) estimated that global food crop contamination from mycotoxins was around 25% annually. Currently, the figure may be up to 60–80%, based on detectable levels [2,7]. Developing countries face higher rates of mycotoxin contamination. For instance, Xu et al. found 100% positivity for DON, 68.7% for ZEA, and 99.5% for deoxynivalenol-3-glucoside (DON-3-G) among 370 wheat samples in Anhui Province, China [8]. Exposure to mycotoxins has been linked to various chronic diseases, including neurodegenerative issues, cardiovascular disease, chronic enteritis, and endemic diseases [9,10,11]. Acute mycotoxin poisoning can be fatal to both animals and humans [12]. Given the widespread contamination and significant health risks associated with mycotoxins, efforts to prevent, control, and detoxify these contaminants have emerged as a global imperative.
Of particular concern among mycotoxins are AFTs, known for their potent toxicity and carcinogenic properties [13,14]. The primary producers of AFTs are Aspergillus (A.) flavus and A. parasiticus [15]. Over recent decades, a total of 21 AFTs have been identified, with the most prevalent variants being aflatoxin B1 (AFB1), B2 (AFB2), G1 (AFG1), G2 (AFB2), aflatoxin B1-8,9-epoxide (AFBO), M1 (AFM1), and M2 (AFM2) [1,16]. As early as 1987, the International Agency for Research on Cancer (IARC) had classified AFB1 and AFM1 as human Group 1 and Group 2B carcinogens, respectively [17]. Epidemiological data have shown a positive association between AFB1 exposure and liver cancer incidence, with 4.6–28.2% of liver cancer cases linked to AFB1 exposure [18,19,20]. Previous in vitro and animal studies have demonstrated that AFB1 exposure can lead to a range of toxic effects, including neurotoxicity, hepatotoxicity, cardiotoxicity, and gastrointestinal toxicity in mammals and poultry [21,22,23,24,25,26,27,28,29]. Mechanistic studies have revealed that AFB1-induced toxic effects involve various pathways, such as lipid peroxidation, inflammatory response, oxidative stress, and cell death (e.g., pyroptosis, apoptosis, necroptosis, and ferroptosis) mechanisms [1,30,31,32,33,34,35,36,37]. Further exploration by scientists reveals multiple signaling pathways, including the NOD-like receptor (NLR) family pyrin domain-containing 3 (NLRP3), aryl hydrocarbon receptor (AHR), phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), toll-like receptors (TLRs), adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), the mammalian target of rapamycin (mTOR), tumor protein P53 (p53), mitogen-activated protein kinase (MAPK), peroxisome proliferator-activated receptor gamma (PPARγ) coactivator 1 alpha (PGC-1α), phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1)/Parkin, Wnt/β-catenin, nuclear factor erythroid 2-related factor 2 (Nrf2)/kelch-like ECH-associated protein 1 (KEAP1), nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs), nuclear factor-kappa B (NF-κB), and mitochondrial apoptotic pathways [1,30,31,32,33,34,35,36,37,38]. By targeting these critical signaling pathways, some compounds, including resveratrol, curcumin, caffeic acid, gallic acid, proanthocyanidin, and quercetin, have been demonstrated to own the potential detoxification effects against AFB1 exposure-induced toxicity by using in vitro cell or experimental animal models [1,39,40,41,42,43,44,45]. Several Chinese herbal extracts or probiotics could also effectively improve AFB1 exposure-induced toxic effects [42,46]. Recent studies have highlighted the protective effects of lycopene (Figure 1), a natural carotenoid, against AFB1 exposure-induced toxicity in animal models [47,48,49,50,51,52,53,54]. Lycopene, derived mainly from the diet, has shown promising safety profiles in both humans and animals and is commercially available in health care products in the U.S., China, and Europe [55,56,57]. This review aims to provide a comprehensive overview of lycopene’s protective effects against AFB1 toxicity, including its molecular mechanisms and clinical implications, to inform future research and interventions aimed at mitigating the health risks posed by AFB1 exposure.
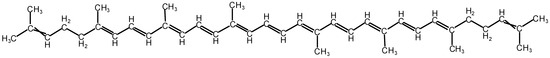
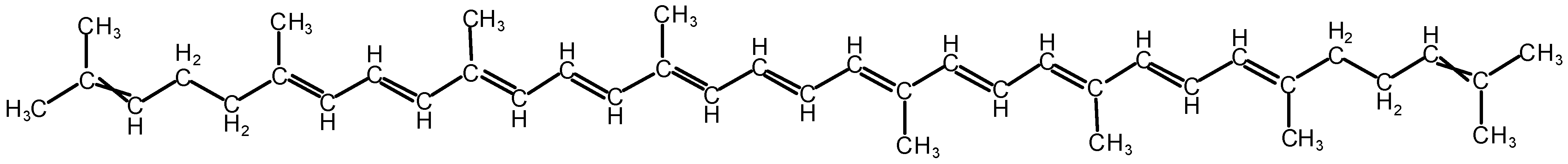
Figure 1.
The chemical molecular structure of lycopene (i.e., C40H56).
2. An Overview of Lycopene’s Protections against Aflatoxin B1 Toxicity
Lycopene is a common non-provitamin, comprising a 40-carbon acyclic carotenoid with 13 double bonds and 11 linearly arranged conjugated double bonds. It is typically abundant in various vegetables and fruits, including watermelons, tomatoes, apricots, pink grapefruits, cranberries, papayas, guavas, and peaches [57,58]. Lycopene exhibits diverse biological activities such as anti-oxidative stress, anti-aging, anti-inflammatory, anti-cancer, and immune regulation functions [55,59,60,61,62]. The beneficial effects of lycopene in mitigating the harmful effects induced by AFB1 exposure are outlined in Table 1. Studies have demonstrated that the potential molecular mechanisms through which lycopene protects against AFB1-induced toxicity involve the inhibition of reactive oxygen species (ROS) production, the suppression of the inflammatory response, the mitigation of mitochondrial dysfunction, the enhancement of endogenous antioxidant levels and antioxidant enzyme activities, and the activation of the Nrf2/KEAP1 pathway and the PGC-1α pathway [47,48,49,50,51,52,53,54]. Furthermore, it was also reported that lycopene supplementation could influence the metabolism of AFTs by modulating cytochrome P450 (CYP450) isozymes in animals [63,64]. Subsequent sections will offer an in-depth examination of the precise molecular mechanisms responsible for the protective effects of lycopene.

Table 1.
A summary of lycopene protecting against AFB1 exposure-induced harmful effects in vivo and in vitro.
2.1. Inhibition of Oxidative Stress
Oxidative stress generally occurs due to an imbalance between the body’s oxidative and antioxidant systems, often resulting in the overproduction of reactive oxygen species (ROS) [67]. ROS comprise various free radicals, such as superoxide anion (O2•−), hydroxyl radical (•OH), peroxynitrite (ONOO−), and nitric oxide (NO) [68]. Under normal physiological conditions, these free radicals are efficiently neutralized by intracellular endogenous antioxidants or antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), and glutathione (GSH) [67]. Studies have shown that exposure to AFB1 can induce oxidative stress by promoting the generation of free radicals and reducing the levels of the antioxidants mentioned above or of antioxidant enzymes [40,69,70,71,72,73,74,75,76,77,78,79,80,81]. The generation of ROS by AFB1, like O2•– and •OH, is partially attributed to its metabolic processing in tissue [53]. Elevated levels of intracellular ROS can cause damage to lipids, proteins, DNA, and other cellular components, leading to various forms of cell death, such as apoptosis, autophagic cell death, ferroptosis, necroptosis, and necrosis, among others [82].
Lycopene has been reported to exhibit potent radical scavenging activities. Results from in vitro biochemical analyses have suggested that lycopene’s antioxidant properties are roughly twice as effective as curcumin, another powerful protective agent against AFB1 toxicity [1,83,84]. This potent radical scavenging activity is suggested to be associated with the number of conjugated double bonds in the structure of lycopene [85]. For example, a 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and O2•– radical scavenging test showed that the half maximal inhibitory concentrations (IC50s) of lycopene are approximately 20 and 5 µg/mL, respectively [86]. Lycopene supplementation has shown significant protective effects against AFB1-induced oxidative stress damage in various organs of mammals (e.g., mice and rats) and poultry (e.g., chicks and ducklings) [47,48,49,51,52,53,54,66]. For instance, El-Sheshtawy et al. demonstrated that a 25-day lycopene supplementation regimen markedly reduced AFB1-induced increases in intracellular malondialdehyde (MDA), a lipid peroxidation marker, while also enhancing the activities of glutathione S transferase (GST), catalase (CAT), and total antioxidant capacity (TAC), thereby attenuating AFB1-induced liver damage in ducklings [54]. Wan et al. observed that lycopene supplementation at 200 mg/kg via the basal diet significantly inhibited the production of reactive oxygen species (ROS) and lipid peroxidation products, such as MDA and 4-hydroxynonenal (4-HNE), in chicken liver tissue [48]. Huang et al. found that oral lycopene supplementation at 5 mg/kg per day for 30 days lowered MDA and H2O2 levels, while upregulating superoxide dismutase (SOD) and CAT activity, leading to a partial reduction in AFB1-induced testicular lesions in mice [47]. Furthermore, several studies indicated that lycopene supplementation effectively mitigated AFB1-induced oxidative stress in the livers, kidneys, and hearts of rodents by increasing GSH levels and the activities of GPX and thioredoxin reductase [22,48,51,53]. A recent study has shown that administering oral lycopene supplements at a dosage of 10 mg/kg body weight per day significantly reduced oxidative stress, mitochondrial dysfunction, and ferroptotic cell death caused by a combination of mycotoxins in the jejunum tissue of mice. These mycotoxins included ZEN at 10 mg/kg body weight, DOX at 1 mg/kg body weight, and AFB1 at 0.5 mg/kg body weight [87]. The study found that lycopene’s ability to activate the body’s antioxidant system played a crucial role in protecting against the toxic effects triggered by AFB1.
The Nrf2 belongs to the cap ‘n’ collar subfamily of basic region leucine zipper transcription factors and is recognized as a housekeeping gene that responds to oxidative stress triggered by xenobiotics [88]. In unstressed cells, Nrf2 interacts with KEAP1 in the cytoplasm. Under oxidative stress conditions, ROS and electrophiles can directly bind to KEAP1 at multiple sites, including cysteines 151 (C151), 273 (C273), and 288 (C288), facilitating the release of Nrf2 from KEAP1-mediated degradation and its translocation into the cell nucleus. Subsequently, Nrf2 activates the expression of over 200 cytoprotective genes involved in anti-inflammatory and antioxidant responses, phase II detoxification enzymes, and xenobiotic metabolism [89,90]. Previous studies have indicated that exposure to AFB1 can markedly suppress Nrf2 gene expression, leading to an increased sensitivity to AFB1 in Nrf2 knockout mice and underscoring the critical role of Nrf2 as a target of AFB1 [91]. Multiple studies have demonstrated that supplementation with lycopene effectively activates the Nrf2/KEAP1 pathway, leading to the upregulation of downstream genes such as SOD, CAT, glutathione S-transferase (GST), heme oxygenase-1 (HO-1), and quinone oxidoreductase 1 (NQO1), among others. This activation provides protection against various drugs (including colistin, cisplatin, and atrazine), environmental toxins (such as aristolochic acid, atrazine, and chlorpyrifos, Di[2-ethylhexyl]phthalate), and tissue damage induced by ischemia-reperfusion [92,93,94,95,96,97]. Huang et al. recently reported that oral supplementation with 5 mg/kg of lycopene per day for 30 days significantly increased the expression of nuclear Nrf2 protein in the testicular tissues of mice [47]. Yu et al. found that lycopene supplementation at the same dosage for the same duration notably upregulated Nrf2 and its downstream targets, including CAT, NQO1, SOD1, glutathione synthetase (GSS), glutamate–cysteine ligase catalytic (GCLC), and glutamate–cysteine ligase modifier subunit (GCLM), thereby mitigating AFB1-induced renal damage in mice [22]. In a chick model, researchers demonstrated that lycopene supplementation at a basal diet level (200 mg/kg) significantly upregulated Nrf2 expression and downstream genes like HO-1, Cu/ZnSOD, MnSOD, CAT, and GPX mRNAs, leading to a pronounced reduction in AFB1-induced intestinal damage [52]. These results suggest that the activation of the Nrf2/KEAP1 pathway plays a role in the protective effects of lycopene against oxidative stress induced by AFB1 exposure. Previous studies have suggested that lycopene-induced activation of Nrf2/KEAP1 may be modulated by p62, AMP-activated protein kinase (AMPK), and silent information regulator 1 (SIRT1) [95,98,99]. Nonetheless, the direct interaction of lycopene with KEAP1 remains unclear, highlighting the need for further investigations to elucidate the precise molecular mechanisms of lycopene in activating the Nrf2/KEAP1 pathway.
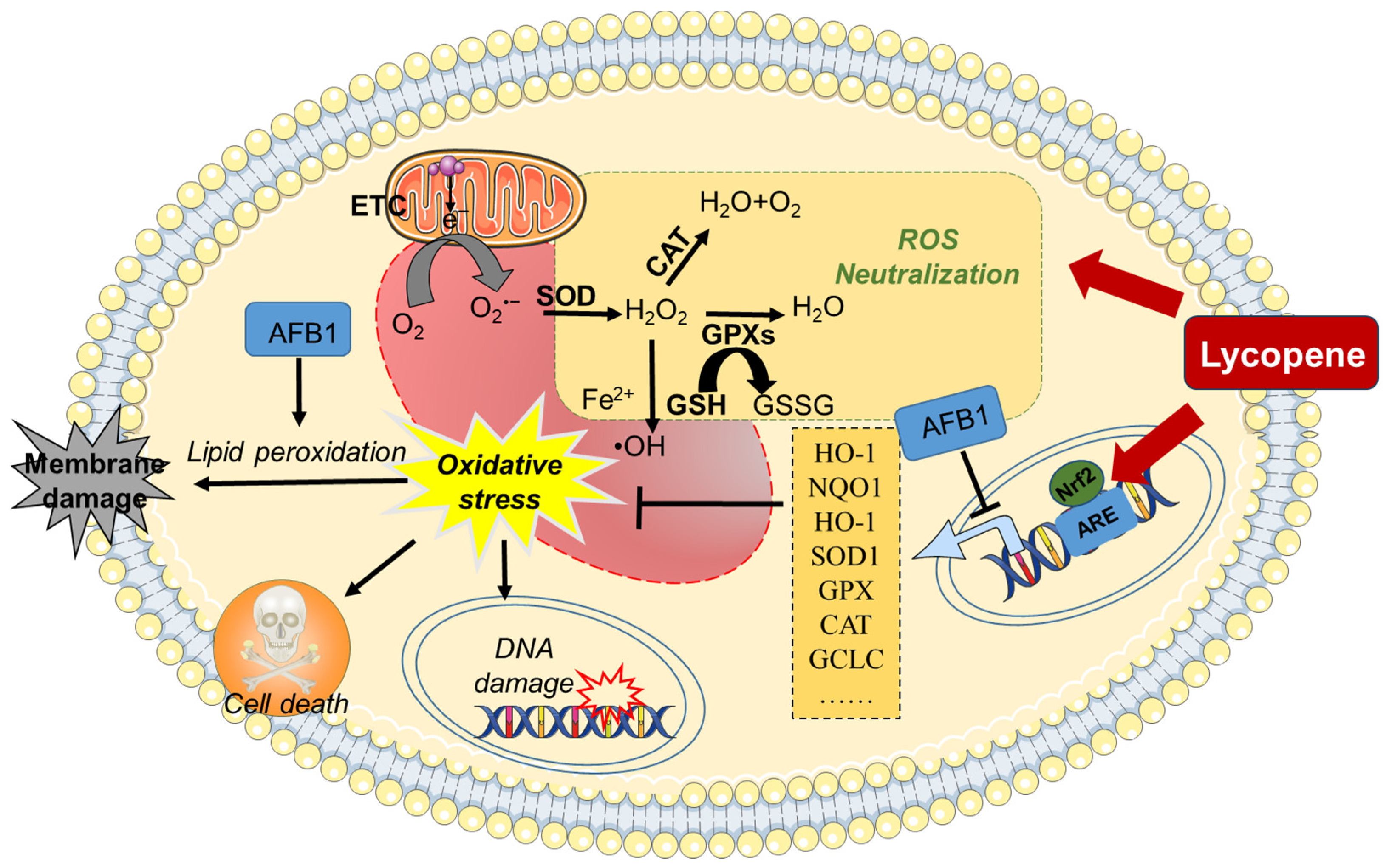
In summary, supplementation with lycopene may provide robust protection against AFB1-induced oxidative stress damage by eliminating ROS, enhancing antioxidant enzyme activity, and activating the Nrf2/Keap1 pathway (see Figure 2).
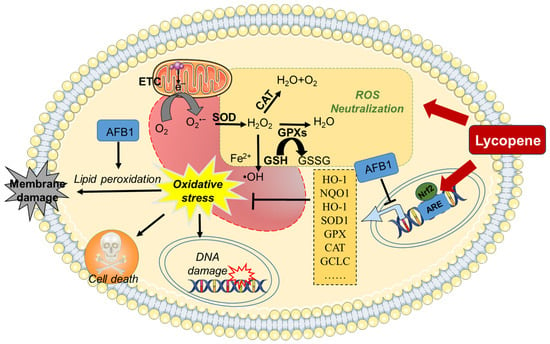
Figure 2.
A working model of lycopene protecting against AFB1-induced oxidative stress. AFB1 exposure could induce oxidative stress damage through the inhibition of antioxidant enzymes’ activities, via the inhibition of the Nrf2 pathway. Lycopene supplementation could offer a protection for AFB1 exposure-induced production of ROS and oxidative stress damage by activating the Nrf2 pathway and enhancing intracellular antioxidant enzyme activity and antioxidant levels. AFB1, aflatoxin B1; ARE, antioxidant response element; CAT, catalase; ETC, electron transport chain; GCLC, glutamate–cysteine ligase catalytic subunit; GSSG, oxidized glutathione; GPX, glutathione peroxidase; GSH, glutathione; H2O2, hydrogen peroxide; HO-1, heme oxygenase 1; NQO1, NAD (P)H quinone oxidoreductase 1; Nrf2, nuclear factor erythroid 2-related factor 2; O2•–, superoxide anion; •OH, hydroxyl radical; ROS, reactive oxygen species; SOD, superoxide dismutase.
2.2. Improvement in Inflammatory Response and Immune Function
Prior investigations have demonstrated that exposure to AFB1 can activate an inflammatory response [72,100,101,102]. Epidemiological studies have established a positive association between AFB1 exposure and various chronic diseases, such as neurodegenerative disease, chronic enteritis, and liver cancer [16,100,103,104]. Experimental evidence suggests that AFB1 exposure can enhance the development of lung tumorigenesis and liver cancer by activating inflammatory responses, particularly pronounced in individuals co-infected with hepatitis B and C viruses [38,105,106,107,108,109,110]. Furthermore, chronic exposure to AFB1 can lead to immunosuppression, as evidenced by decreased spleen weights, lymphocyte reduction (B and T cells), and decreases in immune cells and immune-related factors like IL-2, IL-10, and interferon-gamma (IFN-γ) [26,111,112]. The potential molecular mechanisms underlying AFB1-induced inflammatory responses and immunotoxicity may involve various pathways, such as the protein kinase C (PKC), AHR, NF-κB, toll-like receptor 4 (TLR4), NRLP3, MAPK, and receptor-interacting serine/threonine-specific protein kinase 1 (RIPK1) pathways [1,38,113,114,115].
Various studies have demonstrated the immune-regulatory and anti-inflammatory properties of lycopene. Its effectiveness is attributed to its lipophilic nature, which allows it to modulate signaling pathways of inflammatory mediators and induce the expression of antioxidant genes by interacting with cellular components [116]. Xu et al. demonstrated that oral lycopene supplementation at a dose of 5 mg/kg per day for 30 days significantly reduced AFB1-induced histopathological injuries in mouse spleens; meanwhile, lycopene supplementation notably increased spleen weight, spleen coefficient, T lymphocyte subsets, and upregulated the mRNA expressions of TNF-α, IFN-γ, and IL-2 genes in the spleen and the corresponding proteins in the bloodstream [26]. Another study by Sarker et al. showed that lycopene supplementation through diet (200 mg/kg feed) for 42 days effectively inhibited increases in IFN-γ, IL-1β proteins, and mRNA expression, caused by AFB1 exposure, upregulated levels of IL-10 protein in the intestine mucosa of chicks, enhanced intestinal barrier function, and improved intestinal health [52]. Lycopene is also an inhibitor of NF-κB, which is a critical transcription factor mediating the expression of multiple inflammatory factors, including TNF-α, IL-1β, COX2, and IL-6 [117]. The inhibitory effects of lycopene on the NF-κB pathway could be mediated via the blockade of the MAPK and TLR4 pathways [118,119]. In addition, excessive ROS production could exacerbate the generation of pro-inflammatory cytokines and chemokines via triggering the PKC, NLRP3, TLR4, NF-κB, and MAPK pathways [16,120].
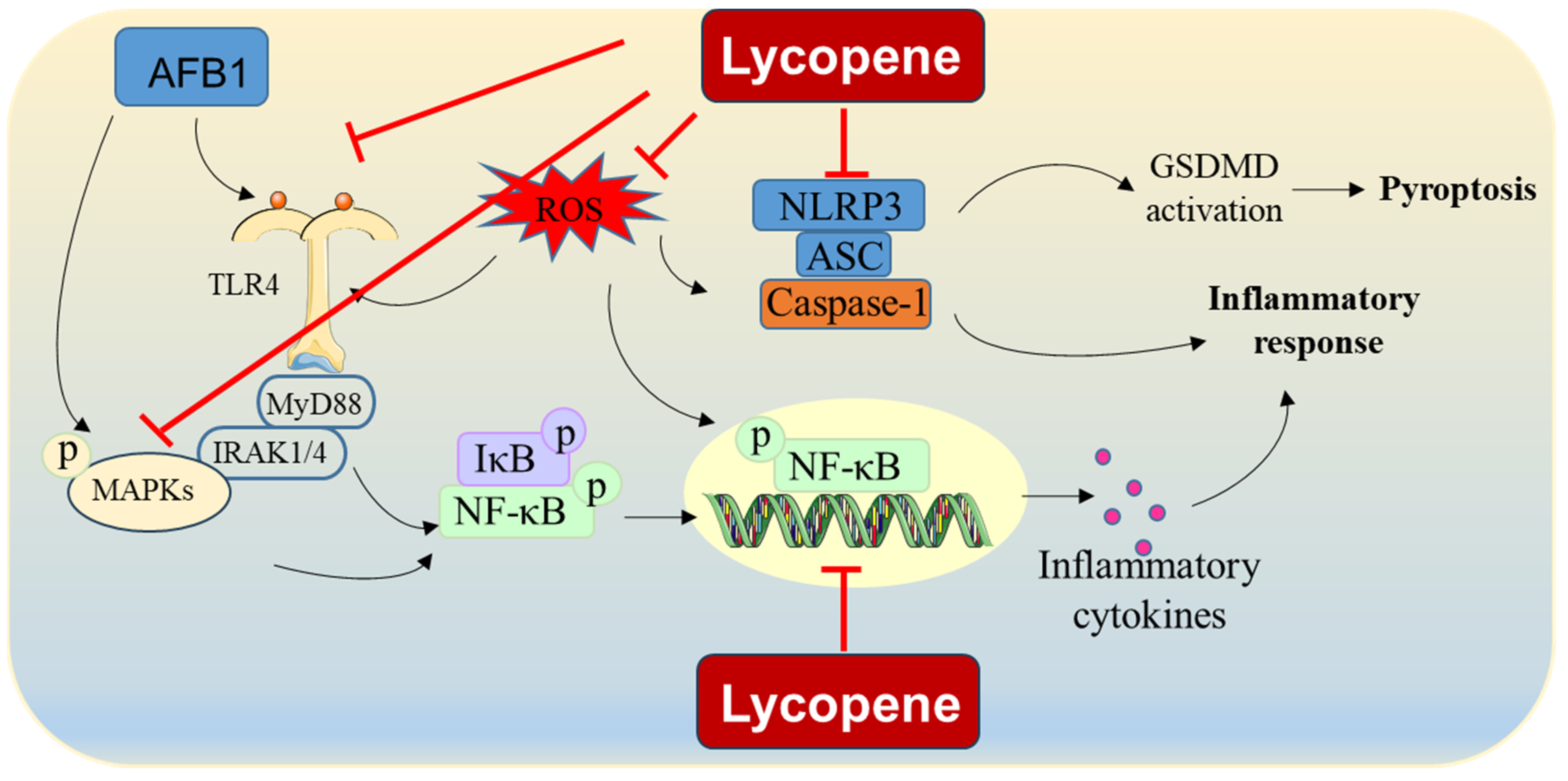
Taken together, these results indicate that lycopene may mitigate AFB1-induced inflammatory responses by inhibiting the TLR4, NF-κB, and MAPK pathways and reducing ROS production (Figure 3). However, the precise molecular mechanisms responsible for lycopene’s effectiveness in countering AFB1-induced inflammation remain incompletely understood, highlighting the need for further comprehensive investigations into these mechanisms.
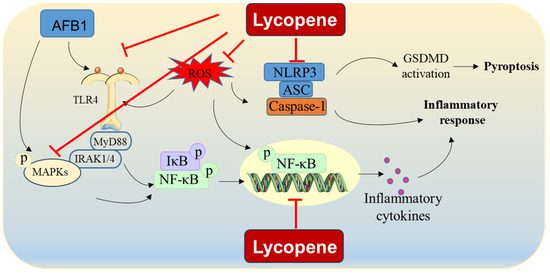
Figure 3.
AFB1-induced inflammatory response and proposed modulation of lycopene. Lycopene supplementation could reduce AFB1 exposure-induced inflammatory response via the inhibition of formation of NLRP3-mediated inflammasome, NF-κB pathway, MAPK pathway, and NLRP3 pathway. It also may be partly attributed to the inhibition of ROS production.
2.3. Inhibition of Mitochondrial Dysfunction and Apoptosis
Mitochondria play a crucial role in sustaining life by facilitating the production of adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS) [121]. They are also involved in regulating apoptotic cell death processes [121]. Dysfunctions in mitochondria, resulting from sustained damage, can affect various cellular processes such as mitochondrial membrane potential, respiration, and electron transfer [122,123]. This dysfunction can be triggered by factors like drugs, toxins, and excessive production of reactive oxygen species (ROS), ultimately leading to cell apoptosis and other forms of regulated cell death [121]. Studies have demonstrated that exposure to aflatoxin B1 (AFB1) can induce mitochondrial dysfunction, as evidenced by disruptions in mitochondrial DNA, electron transport chain, membrane potential, and biosynthesis [48,124,125,126]. Notably, even low doses of AFB1 exposure can lead to abnormal changes in mitochondrial structure, membrane potential, and expression of genes involved in electron transport chain complexes in the liver of mice [127]. Moreover, AFB1 exposure has been linked to the diminished mRNA expression of key regulators, such as nuclear respiratory factor 1 (Nrf1), mitochondrial transcription factor A (MTFA), PGC-1α, and peroxisome proliferator-activated receptor γ coactivator 1α (PPAR1α), important for mitochondrial biosynthesis [48,127,128]. Notably, MTFA is a direct regulator of mitochondrial DNA replication/transcription. The activation of PGC-1α could stimulate a powerful expression of Nrf1 and Nrf2 genes; it also binds to Nrf1 and coactivates the expression of MTFA, promoting mitochondrial DNA replication/transcription [129]. These data indicate that AFB1 exposure-induced abnormalities of mitochondrial biosynthesis may involve the inhibition of the PGC-1α/Nrf1/MTFA pathway.
Recently, Huang et al. found that oral lycopene supplementation at the dose of 5 mg/kg per day for 30 days could effectively improve AFB1 exposure to the damage in mitochondrial structure and upregulate the expression of PGC-1α, Nrf1, MTFA, and cytochrome C oxidase IV (COXIV) mRNAs in the testicular tissues of mice [47]. Similarly, Wan et al. found that lycopene supplementation at 200 mg/kg via the basal diet for 42 days significantly improved mitochondrial function, evidenced by reduced swelling, increased activities of mitochondrial complexes III, IV, and V, elevated ATP levels, and enhanced expression of PGC-1α, Nrf1, and MTFA mRNAs in AFB1-treated chickens’ livers [48]. A previous study found that lycopene-mediated upregulation of PGC-1α is partly dependent on the expression of sirtuin 1 (SIRT1), which could deacetylate PGC-1α at multiple lysine sites and consequently increase the activities of PGC-1α [130]. In addition, the activation of AMPK by lycopene could enhance PGC-1α-dependent transcription via the phosphorylation of SIRT1 [131]. These evidences indicated that the activation of PGC-1α pathways may be dependent on the activation of AMPK and SIRT1 proteins. Furthermore, Xu et al. demonstrated that 5 mg/kg per day of oral lycopene supplementation for 30 days mitigated AFB1-induced loss of mitochondrial membrane potential in mouse spleen tissues [26]. In conclusion, the evidence suggests that lycopene supplementation can ameliorate AFB1-induced mitochondrial damage, loss of membrane potential, and dysfunction of the ETC and biosynthesis processes. The precise molecular mechanisms still need to be further investigated.
Generally, an increase in the ratio of the pro-apoptotic protein Bax to the anti-apoptotic proteins Bcl-2 or Bcl-XL can lead to the formation of mitochondrial permeability transition pores (MPTPs), the subsequent release of CytC from the mitochondria, the activation of caspase-9 and caspase-3, and ultimately culminate in cell apoptosis [132]. Previous research has shown that exposure to AFB1 significantly elevates the Bax/Bcl-2 protein ratio, enhances caspase-9 and caspase-3 activities, and increases their mRNA expressions, ultimately resulting in cell apoptosis in HepG2 cells, IMR-32 cells (a neuroblastoma cell line), as well as in the brain, spleen, liver, and renal tissues of animals [26,80,133,134,135]. Numerous studies have indicated that lycopene supplementation can effectively mitigate apoptotic cell death induced by colistin, tert-butyl hydroperoxide, and lead through the inhibition of mitochondrial dysfunction and the mitochondrial pathway [94,136,137]. Consistent with these findings, researchers have observed that lycopene supplementation at different doses can successfully attenuate AFB1 exposure-induced cell apoptosis, as evidenced by the suppression of cytoplasmic CytC, Bax, and cleaved caspase-3 proteins’ expression, as well as the activities and mRNA expression of caspases-3 and -9, and the upregulation of Bcl-2 protein [26,47,87,138].
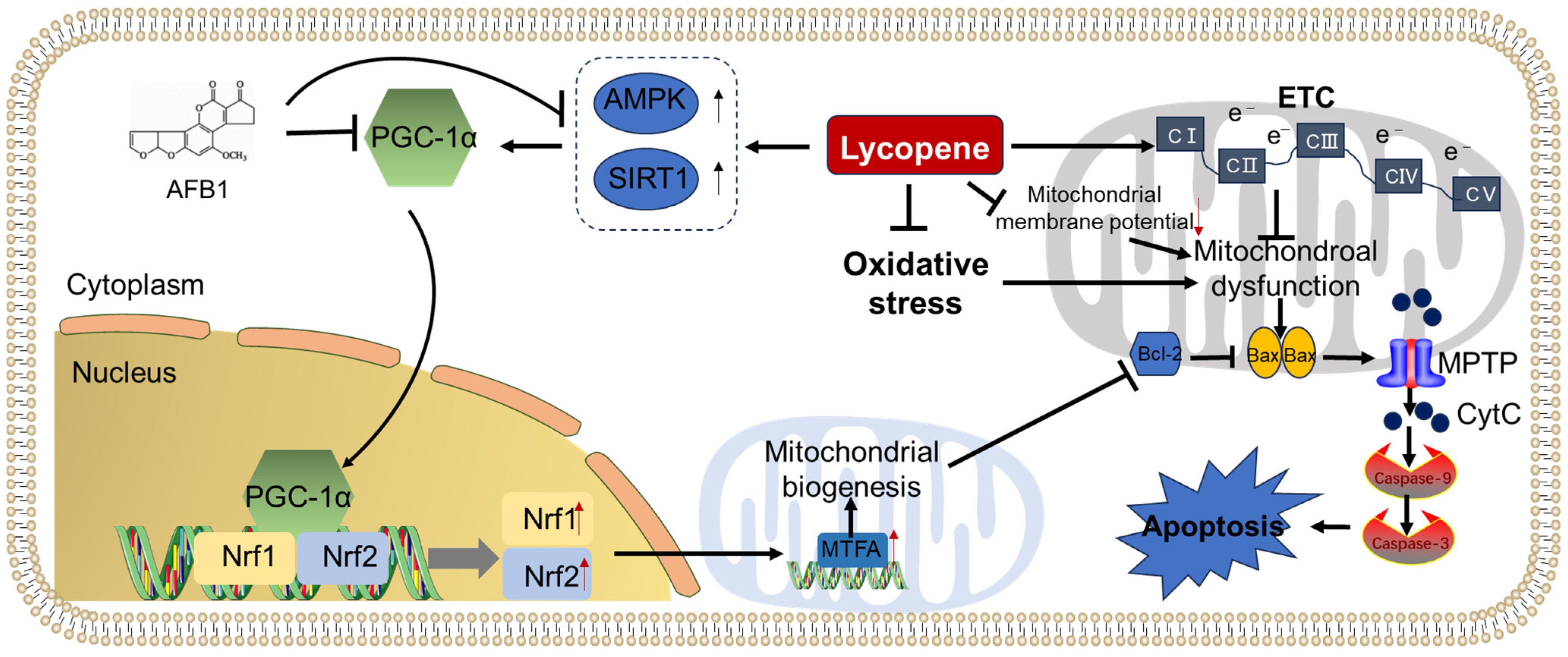
A proposed model illustrating the protective effect of lycopene supplementation against AFB1 exposure-induced mitochondrial dysfunction and mitochondrial apoptotic pathway is presented in Figure 4. Notably, the ameliorated effects of lycopene supplementation on AFB1 exposure-induced mitochondrial dysfunction may be partly attributed to the activation of PGC-1α/Nrf1/MTFA pathway-mediated mitochondrial biosynthesis, the improvement in ETC function, and the increases in mitochondrial membrane potential. Additionally, oxidative stress is a critical contributor for mitochondrial dysfunction [139]. Therefore, the inhibitory effects of lycopene on the production of ROS may also play a critical role. Nevertheless, the specific molecular mechanisms underlying lycopene’s efficacy against AFB1-induced mitochondrial dysfunction remain largely unclear, the detailed exploration for these mechanisms are still required.
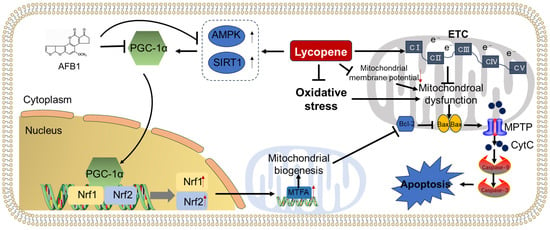
Figure 4.
Lycopene supplementation attenuated AFB1-induced mitochondrial dysfunction and apoptotic cell death. AFB1 exposure could inhibit the activation of PGC-1α directly or indirectly via the inhibition of AMPK and SIRT1 proteins, then reduce the mitochondrial biogenesis via the Nrf1/Nrf2/MTFA pathway, and, finally, ameliorate mitochondrial dysfunction and apoptosis. Lycopene supplementation could protect AFB1 exposure-induced mitochondrial dysfunction and apoptosis via the activation of the PGC-1α pathway, the inhibition of oxidative stress and the loss of mitochondrial membrane potential, and the activation of the mitochondrial apoptotic pathway.
2.4. Metabolic Intervention
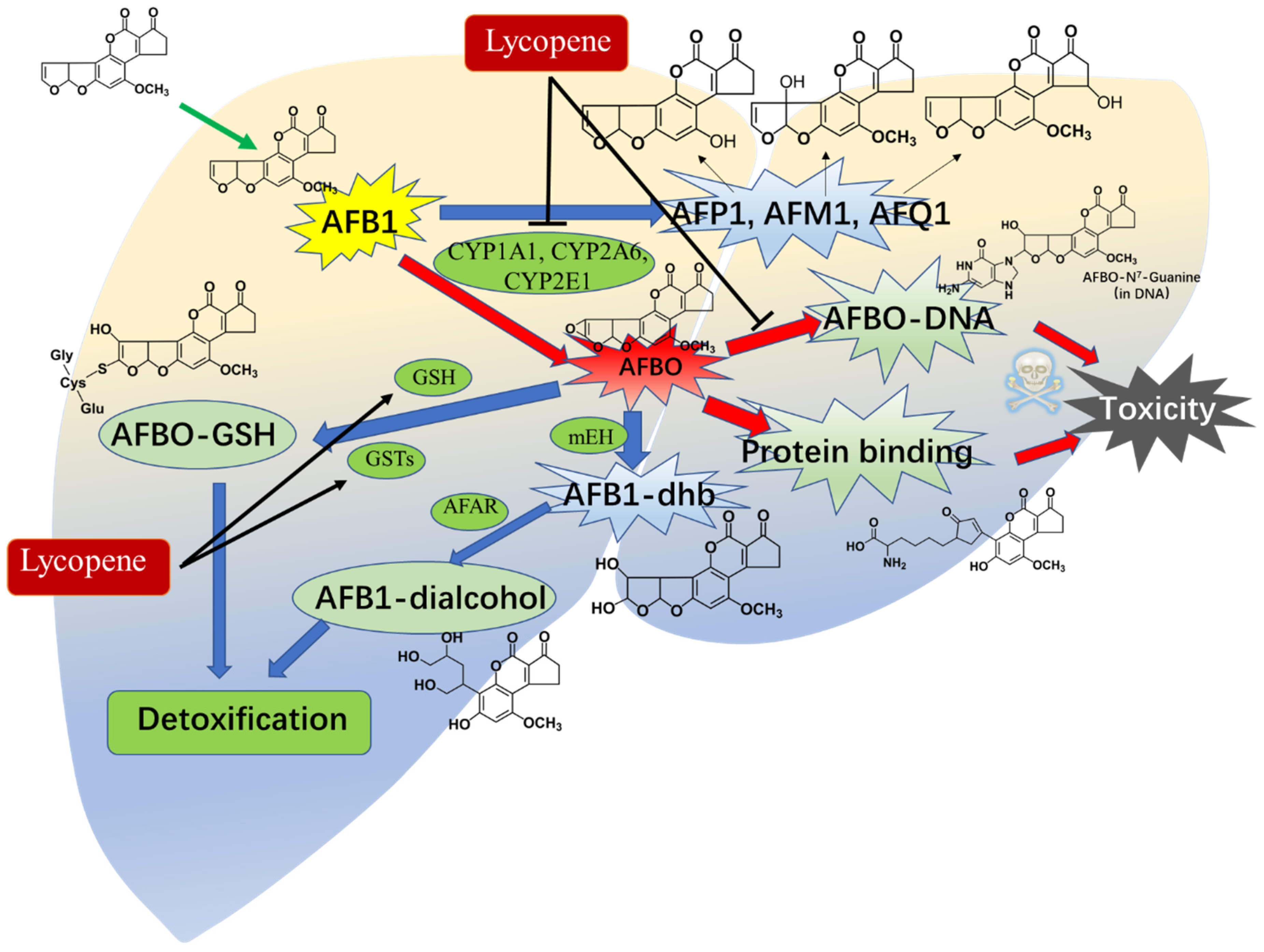
The liver plays a dual role in the metabolism of aflatoxin B1 (AFB1), serving as both a target for its toxic effects and a crucial organ for its detoxification in humans and animals [1,140]. Variations in the metabolism of AFB1 among species and organs are attributed to differences in the expression and content of metabolic enzymes [141]. Dohnal et al. provide a comprehensive review of these differences. AFB1 undergoes four major pathways of metabolism, including hydroxylation, ketoreduction, O-dealkylation, and epoxidation [142]. Broadly, four major pathways have been identified in the metabolism of AFB1, including hydroxylation, ketoreduction, O-dealkylation, and epoxidation [141]. Approximately 95% of AFB1 undergoes transformation into highly toxic AFBO and AFM1, as well as other less toxic forms (e.g., AFP1, AFK1, or AFB2a), by cytochrome P450 (CYP450) enzymes (such as CYP1A1, CYP1A2, CYP1A5, CYP2A6, CYP2A13, CYP3A37, and CYP3A4) in the liver tissues [141,143,144,145,146]. Notably, AFBO is considered the primary toxic metabolite of AFB1 due to its direct interaction with DNA, leading to multiple toxic effects within cells [147]. AFBO can be detoxified through conjugation with glutathione (GSH) or through hydrolysis by epoxide hydrolase enzymes, to produce the highly cytotoxic AFB1–8,9-dihydro diol (AFB1–dhd) [141].
Previous studies have indicated that lycopene can modulate the activities of metabolic enzymes, including CYP3As, CYP2C, CYP2D, and CYP2E [148]. Nosková et al. reported that oral supplementation of lycopene at doses ranging from 4 to 100 mg/kg per day for 10 days could enhance the activities of CYP2B, CYP2D, and CYP3A [149]. Wan et al. demonstrated that lycopene supplementation at a dose of 200 mg/kg in the basal diet for 42 days significantly decreased the activities of CYP1A1 and CYP2A6, albeit not affecting CYP1A2 and CYP3A4, thereby leading to a reduction in the formation of AFB1–DNA adducts and DNA damage in the liver tissues of chickens [64]. Lin et al. observed that pretreatment with lycopene at a dose of 10 mg/kg per day through oral supplementation for 14 days markedly decreased the expression of hepatic CYP2E1 protein, consequently lowering AFB1-induced liver toxicity [150]. Moreover, Tang et al. found that oral administration of lycopene at a dose of 100 mg/kg/day for 15 days significantly reduced the formation of AFB–DNA adducts in liver tissues, as well as the levels of AFM1, AFQ1, and AFP1 excreted in urine, along with AFB1–albumin adducts in serums of rats [49]. Additionally, AFB1-induced AFB1–N7-guanine adducts could lead to apoptotic cell death and the suppression of the p53 protein expression [138]. Reddy et al. reported that lycopene supplementation at a dose of 0.5 μg/mL notably diminished the formation of AFB1–N7-guanine and its excretion in HepG2 cells [138]. Correspondingly, lycopene supplementation also markedly decreased the levels of AFB1–N7-guanine in rat urine and elevated levels of AFB–NAC in urine excretion [49]. AFB–NAC is the primary detoxifying metabolic product of AFBO, and its formation is reliant on the activities of phase II enzymes, including GPX and GST [151]. This observation aligns with prior studies indicating that lycopene can elevate GSH levels and the activity of enzymes such as GPX, GST, and glutathione reductase (GR) in tissues exposed to AFB1 (e.g., kidney, heart, liver, and intestine) across various animal models including chickens, ducklings, rats, and mice [22,51,52,53,54]. These findings suggest that lycopene may protect against AFB1 toxicity by modulating the activities of CYP450 enzymes and phase II detoxification enzymes (see Figure 5).
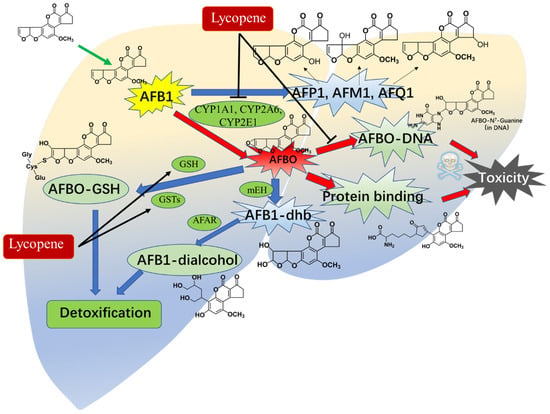
Figure 5.
An overview of lycopene supplementation modulating the metabolism of AFB1 in animals’ livers. Lycopene supplementation could reduce the formation of AFBO, AFP1, AFM1, AFQ1, and AFB1–DNA adducts in the body via the inhibition of CYP1A1, CYP2A6, and CYP2E1 enzyme activities in the liver tissues of animals. It also could upregulate the level of GSH and phase II enzyme GSTs, enhance the formation of AFBO–GSH and AFB1–dialcohol, and, finally, promote the detoxification of AFB1.
3. Safety of Lycopene and Its Clinical Application
Lycopene, a potent antioxidant found in tomatoes and tomato-based products, is a significant component of human dietary intake, typically ranging from 0.7 to 25.2 mg/day [152,153]. The absorption of lycopene from the diet typically ranges from 10% to 30% of the intake amount, with the remainder excreted [153]. Studies have indicated that, in humans, the time to reach maximum concentration (tmax) and the elimination half-life (t1/2) of lycopene are 0.5 and 48 days, respectively. Numerous clinical trials have demonstrated that lycopene supplementation offers various health benefits in the prevention and treatment of chronic diseases, such as cancer, high-density lipoprotein (HDL)-associated inflammation, and heart disease [154,155,156]. Experimental studies have shown that oral supplementation of lycopene, at doses ranging from 5 to 200 mg/kg/day, can have multiple biological effects, such as antioxidant, anti-aging, anti-inflammatory, immune regulation, and antimicrobial functions [157,158,159,160]. Previous studies have shown that lycopene has a high safety profile. An older study reported that a subcutaneous injection of lycopene at a dose of 3 g/kg body weight led to only a temporary decrease in muscle tone in mice, while oral and intraperitoneal administration of this dose had no effect [161]. Moreover, recent research by Michael and colleagues demonstrated that rats treated with lycopene beadlet formulations, at doses up to 500 mg/kg body weight/day for 14 weeks or 1000 mg/kg body weight/day for 4 weeks, showed no significant toxicity, establishing a non-observable adverse effect level (NOAEL) of 500 mg/kg body weight/day for lycopene [162]. These findings suggest that the effective dose of lycopene is below its safety threshold, making it a viable option in both medical and food industries [154,155,156,163].
While animal studies have shown the effectiveness of lycopene in mitigating AFB1 exposure-induced toxicity, limited clinical evidence exists regarding its detoxification effects against AFB1 in humans. Therefore, further clinical trials or subclinical investigations are necessary to fully understand the detoxification potential of lycopene against AFB1.
4. Conclusions and Future Directions
The global concern regarding AFB1 contamination arises from its significant toxicity and carcinogenicity in both human and animal populations. Extensive studies, encompassing both animal experimentation and epidemiological inquiries, have elucidated the various adverse effects associated with AFB1 exposure. These effects include, but are not limited to, neurotoxicity, immune toxicity, reproductive toxicity, genotoxicity, hepatotoxicity, nephrotoxicity, and gastrointestinal toxicity in mammals and poultry. Notably, lycopene, a lipid-soluble natural carotenoid obtained from the daily diet, has shown promise in protecting against the multiple toxic effects induced by AFB1 exposure, such as immune toxicity, reproductive toxicity, genotoxicity, hepatotoxicity, nephrotoxicity, cardiac toxicity, and hematologic toxicity. The underlying molecular mechanisms of this protection involve the inhibition of oxidative stress, inflammation, lipid peroxidation, CYP450 enzyme activity, and the mitochondrial apoptotic pathway, as well as the activation of mitochondrial biogenesis, the endogenous antioxidant system, and the Nrf2/KEAP1 and PGC-1 pathways. Moreover, supplementation with lycopene has demonstrated the ability to enhance gut health and immune function.
These findings collectively suggest that lycopene holds promise as a potent therapeutic agent against AFB1-induced toxic effects in mammals and poultry. However, despite these promising results, the precise protective mechanisms of lycopene remain incompletely understood, and there is a dearth of effective clinical evaluations regarding its protective effects against AFB1-induced harm in both human and animal populations. Furthermore, addressing the issue of lycopene’s bioavailability is crucial. Consequently, additional research efforts are warranted to gain a comprehensive understanding of the molecular basis of lycopene’s protective capabilities, its bioavailability, and its clinical efficacy.
Author Contributions
Investigation, C.D.; writing—original draft preparation, review, and editing, C.D., J.L. and J.S.; methodology, X.P., Z.H., C.D., M.L., S.T., G.S., S.Y. and J.S.; formal analysis, C.D. and G.S.; validation, C.D. and M.L.; supervision, C.D. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the National Natural Science Foundation of China (32102724) and the Pinduoduo–China Agricultural University Research Fund (Grant No. PC2023A01002).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 toxicity and protective effects of curcumin: Molecular mechanisms and clinical implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- FAO Joint. Evaluation of certain mycotoxins in food. Fifty-sixth report of the joint FAO/WHO expert committee on food additives. World Health Organ. Tech. Rep. Ser. 2002, 906, i–viii. [Google Scholar]
- Ayelign, A.; De Saeger, S. Mycotoxins in ethiopia: Current status, implications to food safety and mitigation strategies. Food Control 2020, 113, 107163. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nabi, B.G.; Ansar, S.; Bhat, Z.F.; Aadil, R.M.; Khaneghah, A.M. Mycotoxins and consumers’ awareness: Recent progress and future challenges. Toxicon 2023, 232, 107227. [Google Scholar] [CrossRef]
- Wei, G.; Guo, X.; Liang, Y.; Liu, C.; Zhang, G.; Liang, C.; Huang, Z.; Zheng, Y.; Chen, S.; Dong, L. Occurrence of fungi and mycotoxins in herbal medicines and rapid detection of toxin-producing fungi. Environ. Pollut. 2023, 333, 122082. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Xu, W.; Han, X.; Li, F. Co-occurrence of multi-mycotoxins in wheat grains harvested in anhui province, china. Food Control 2019, 96, 180–185. [Google Scholar] [CrossRef]
- Assunção, R.; Viegas, S. Mycotoxin exposure and related diseases. Toxins 2020, 12, 172. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Sun, F.; Zhang, Y.; Hoyer, D.; Shen, J.; Tang, S.; Velkov, T. T-2 toxin neurotoxicity: Role of oxidative stress and mitochondrial dysfunction. Arch. Toxicol. 2019, 93, 3041–3056. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Stoloff, L. Carcinogenicity of aflatoxins. Science 1987, 237, 1283–1284. [Google Scholar] [CrossRef]
- Gourd, E. High concentrations of aflatoxin in ugandan grains sparks public health concern. Lancet Oncol. 2023, 24, 315. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Zhang, S.; Zhu, M.; Yang, Q.; Dong, J.; Zhang, Q.; Feng, P. Research progress related to aflatoxin contamination and prevention and control of soils. Toxins 2023, 15, 475. [Google Scholar] [CrossRef]
- Dai, C.; Sharma, G.; Liu, G.; Shen, J.; Shao, B.; Hao, Z. Therapeutic detoxification of quercetin for aflatoxin B1-related toxicity: Roles of oxidative stress, inflammation, and metabolic enzymes. Environ. Pollut. 2024, 345, 123474. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the iarc monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Wu, H.C.; Wang, Q.; Yang, H.I.; Ahsan, H.; Tsai, W.Y.; Wang, L.Y.; Chen, S.Y.; Chen, C.J.; Santella, R.M. Aflatoxin B1 exposure, hepatitis b virus infection, and hepatocellular carcinoma in taiwan. Cancer Epidemiol. Biomark. Prev. 2009, 18, 846–853. [Google Scholar] [CrossRef]
- Xiang, X.; Qin, H.G.; You, X.M.; Wang, Y.Y.; Qi, L.N.; Ma, L.; Xiang, B.D.; Zhong, J.H.; Li, L.Q. Expression of p62 in hepatocellular carcinoma involving hepatitis b virus infection and aflatoxin B1 exposure. Cancer Med. 2017, 6, 2357–2369. [Google Scholar] [CrossRef]
- Qiao, B.; He, Y.; Gao, X.; Liu, H.; Rao, G.; Su, Q.; Ruan, Z.; Tang, Z.; Hu, L. Curcumin attenuates AFB1-induced duck liver injury by inhibiting oxidative stress and lysosomal damage. Food Chem. Toxicol. 2023, 172, 113593. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, J.; Cao, Z.; Ji, Q.; Han, Y.; Song, M.; Shao, B.; Li, Y. Lycopene attenuates AFB1-induced renal injury with the activation of the nrf2 antioxidant signaling pathway in mice. Food Funct. 2018, 9, 6427–6434. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wu, Y.; Gao, S.; Zhou, M.; Liu, Z.; Xiong, Q.; Jiang, L.; Yuan, G.; Li, L.; Yang, L. Deciphering the hazardous effects of AFB1 and T-2 toxins: Unveiling toxicity and oxidative stress mechanisms in pk15 cells and mouse kidneys. Toxins 2023, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Abdin, M.Z.; Javed, S. Protective effect of esculin against prooxidant aflatoxin B1-induced nephrotoxicity in mice. Mycotoxin Res. 2014, 30, 25–32. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Wang, T.; Liao, G.; Gu, J.; Hou, R.; Qiu, J. Lipidomic profiling study on neurobehavior toxicity in zebrafish treated with aflatoxin B1. Sci. Total Environ. 2023, 898, 165553. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, P.; Yao, Q.; Shao, B.; Yu, H.; Yu, K.; Li, Y. Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct. 2019, 10, 3868–3879. [Google Scholar] [CrossRef]
- Jin, X.; Li, Q.H.; Sun, J.; Zhang, M.; Xiang, Y.Q. Porcine β-defensin-2 alleviates AFB1-induced intestinal mucosal injury by inhibiting oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2023, 262, 115161. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, Y.; Xu, Z.J.; Zhang, N.Y.; Zhang, W.P.; Zuo, G.; Khalil, M.M.; Sun, L.H. Selenium mitigated aflatoxin B1-induced cardiotoxicity with potential regulation of 4 selenoproteins and ferroptosis signaling in chicks. Food Chem. Toxicol. 2021, 154, 112320. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, Y.; Hu, X.; Ren, L.; Zhang, C.; Wang, X.; Wang, W.; Zhang, Z.; Hao, J.; Guo, M.; et al. Melatonin protects in vitro matured porcine oocytes from toxicity of aflatoxin B1. J. Pineal Res. 2019, 66, e12543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jia, F.; Guo, C.; Wang, Y.; Zhang, X.; Cui, Y.; Song, M.; Cao, Z.; Li, Y. Pink1/parkin-mediated mitophagy as a protective mechanism against AFB1-induced liver injury in mice. Food Chem. Toxicol. 2022, 164, 113043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Liu, M.; Zhou, X.; Wang, M.; Cao, K.; Jin, S.; Shan, A.; Feng, X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the nlrp3 inflammasome and nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef]
- Liu, S.; Kang, W.; Mao, X.; Ge, L.; Du, H.; Li, J.; Hou, L.; Liu, D.; Yin, Y.; Liu, Y.; et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal fxr/liver tlr4 signaling axis in mice. J. Pineal Res. 2022, 73, e12812. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Chen, Y.; Ni, C.; Chen, X.; Zhang, L.; Xu, X.; Chen, M.; Ma, X.; Zhan, H.; et al. Aflatoxin B1 impairs leydig cells through inhibiting ampk/mtor-mediated autophagy flux pathway. Chemosphere 2019, 233, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cao, Z.; Zhang, J.; Ji, Q.; Li, Y. Aflatoxin B1 promotes autophagy associated with oxidative stress-related pi3k/akt/mtor signaling pathway in mice testis. Environ. Pollut. 2019, 255, 113317. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Feng, Y.; Wu, T.; Srinivas, S.; Yang, W.; Fan, J.; Yang, C.; Wang, S. Aflatoxin B1 negatively regulates wnt/β-catenin signaling pathway through activating mir-33a. PLoS ONE 2013, 8, e73004. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Sasaki, Y.; Terasaki, N.; Kawataki, T.; Takekawa, K.; Iwase, Y.; Shimizu, T.; Sanoh, S.; Ohta, S. Comparison of drug metabolism and its related hepatotoxic effects in heparg, cryopreserved human hepatocytes, and hepg2 cell cultures. Biol. Pharm. Bull. 2018, 41, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Ban, F.; Peng, S.; Xu, D.; Li, H.; Mo, H.; Hu, L.; Zhou, X. Exogenous iron induces nadph oxidases-dependent ferroptosis in the conidia of aspergillus flavus. J. Agric. Food Chem. 2021, 69, 13608–13617. [Google Scholar] [CrossRef]
- Zhu, Q.; Ma, Y.; Liang, J.; Wei, Z.; Li, M.; Zhang, Y.; Liu, M.; He, H.; Qu, C.; Cai, J.; et al. Ahr mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct. Target. Ther. 2021, 6, 299. [Google Scholar] [CrossRef]
- El-Agamy, D.S. Comparative effects of curcumin and resveratrol on aflatoxin B1-induced liver injury in rats. Arch. Toxicol. 2010, 84, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Wei, G.; Guo, G.; Yu, H.; Zhang, Y.; Ishfaq, M.; Fazilani, S.A.; Zhang, X. Curcumin protects against aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol. Environ. Saf. 2021, 208, 111725. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Xia, S.; Wei, G.; Ishfaq, M.; Zhang, Y.; Zhang, X. Protective role of curcumin on aflatoxin B1-induced tlr4/ripk pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol. Environ. Saf. 2022, 233, 113319. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Chung, W.T.; Kwon, J.K.; Yu, J.Y.; Jang, Y.S.; Park, S.M.; Lee, S.Y.; Lee, J.C. Inhibitory effects of quercetin on aflatoxin B1-induced hepatic damage in mice. Food Chem. Toxicol. 2010, 48, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Zhang, Y.; Li, P.; Yang, S.H.; Zhang, W.K.; Han, J.X.; Wang, Y.; He, J.B. Intervention of grape seed proanthocyanidin extract on the subchronic immune injury in mice induced by aflatoxin B1. Int. J. Mol. Sci. 2016, 17, 516. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.; Najophe, E.S.; Farombi, E.O.; Oyelere, A.K. Gallic acid protects against aflatoxin B1-induced oxidative and inflammatory stress damage in rats kidneys and liver. J. Food Biochem. 2020, 44, e13316. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Irozuru, C.E.; Arunsi, U.O.; Oyelere, A.K. Caffeic acid protects against DNA damage, oxidative and inflammatory mediated toxicities, and upregulated caspases activation in the hepatorenal system of rats treated with aflatoxin B1. Toxicon 2022, 207, 1–12. [Google Scholar] [CrossRef]
- Aytekin Sahin, G.; Karabulut, D.; Unal, G.; Sayan, M.; Sahin, H. Effects of probiotic supplementation on very low dose AFB1-induced neurotoxicity in adult male rats. Life Sci. 2022, 306, 120798. [Google Scholar] [CrossRef]
- Huang, W.; Cao, Z.; Cui, Y.; Huo, S.; Shao, B.; Song, M.; Cheng, P.; Li, Y. Lycopene ameliorates aflatoxin B1-induced testicular lesion by attenuating oxidative stress and mitochondrial damage with nrf2 activation in mice. Ecotoxicol. Environ. Saf. 2023, 256, 114846. [Google Scholar] [CrossRef]
- Wan, X.L.; Li, N.; Chen, Y.J.; Chen, X.S.; Yang, Z.; Xu, L.; Yang, H.M.; Wang, Z.Y. Protective effects of lycopene on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1-exposed broilers. Poult. Sci. 2021, 100, 101441. [Google Scholar] [CrossRef]
- Tang, L.; Guan, H.; Ding, X.; Wang, J.S. Modulation of aflatoxin toxicity and biomarkers by lycopene in f344 rats. Toxicol. Appl. Pharmacol. 2007, 219, 10–17. [Google Scholar] [CrossRef]
- Hidayat, D.F.; Mahendra, M.Y.N.; Kamaludeen, J.; Pertiwi, H. Lycopene in feed as antioxidant and immuno-modulator improves broiler chicken’s performance under heat-stress conditions. Vet. Med. Int. 2023, 2023, 5418081. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.; Yilmaz, S.; Kaya, E.; Altun, S. The effect of lycopene on hepatotoxicity of aflatoxin B1 in rats. Arch. Physiol. Biochem. 2021, 127, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.T.; Wan, X.; Yang, H.; Wang, Z. Dietary lycopene supplementation could alleviate aflatoxin B1 induced intestinal damage through improving immune function and anti-oxidant capacity in broilers. Animals 2021, 11, 3165. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Kaya, E.; Karaca, A.; Karatas, O. Aflatoxin B1 induced renal and cardiac damage in rats: Protective effect of lycopene. Res. Vet. Sci. 2018, 119, 268–275. [Google Scholar] [CrossRef] [PubMed]
- El-Sheshtawy, S.M.; El-Zoghby, A.F.; Shawky, N.A.; Samak, D.H. Aflatoxicosis in pekin duckling and the effects of treatments with lycopene and silymarin. Vet. World 2021, 14, 788–793. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A potent antioxidant for the amelioration of type ii diabetes mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, S.; Tokarczyk, G. Lycopene in the prevention of cardiovascular diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Khongthaw, B.; Dulta, K.; Chauhan, P.K.; Kumar, V.; Ighalo, J.O. Lycopene: A therapeutic strategy against coronavirus disease 19 (COVID-19). Inflammopharmacology 2022, 30, 1955–1976. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Yang, C.H.; Sohn, D.W.; Kim, S.W.; Kang, S.H.; Cho, Y.H. Synergistic effect between lycopene and ciprofloxacin on a chronic bacterial prostatitis rat model. Int. J. Antimicrob. Agents 2008, 31 (Suppl. S1), S102–S107. [Google Scholar] [CrossRef]
- Tang, C.; Li, Q.; Lin, T. Lycopene attenuates staphylococcus aureus-induced inflammation via inhibiting α-hemolysin expression. Microbes Infect. 2021, 23, 104853. [Google Scholar] [CrossRef]
- Song, X.; Luo, Y.; Ma, L.; Hu, X.; Simal-Gandara, J.; Wang, L.S.; Bajpai, V.K.; Xiao, J.; Chen, F. Recent trends and advances in the epidemiology, synergism, and delivery system of lycopene as an anti-cancer agent. Semin. Cancer Biol. 2021, 73, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.; Li, Y. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with nrf2 activation. J. Funct. Foods 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Wan, X.; Ji, H.; Ma, H.; Yang, Z.; Li, N.; Chen, X.; Chen, Y.; Yang, H.; Wang, Z. Lycopene alleviates aflatoxin B1 induced liver damage through inhibiting cytochrome 450 isozymes and improving detoxification and antioxidant systems in broiler chickens. Ital. J. Anim. Sci. 2022, 21, 31–40. [Google Scholar] [CrossRef]
- Sarker, M.T.; Wang, Z.Y.; Yang, H.; Wan, X.; Emmanuel, A. Evaluation of the protective effect of lycopene on growth performance, intestinal morphology, and digestive enzyme activities of aflatoxinB1 challenged broilers. Anim. Sci. J. 2021, 92, e13540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Xu, F.; Huang, W.; Ji, Q.; Han, Y.; Shao, B.; Li, Y. Protective effects of lycopene against AFB1-induced erythrocyte dysfunction and oxidative stress in mice. Res. Vet. Sci. 2020, 129, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; San, J.; Pang, H.; Du, Y.; Li, W.; Zhou, X.; Yang, X.; Hu, J.; Yang, J. Taurine attenuates AFB1-induced liver injury by alleviating oxidative stress and regulating mitochondria-mediated apoptosis. Toxicon 2022, 215, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of oral exposure to aflatoxin B1-induced renal dysfunction, oxidative stress, and cell apoptosis in mice kidney by curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.X.; Cao, Q.Q.; Zhang, C.D.; Xu, T.T.; Yue, K.; Li, Q.; Liu, F.; Wang, X.; Dong, H.J.; Huang, S.C.; et al. Aflatoxin B1 causes oxidative stress and apoptosis in sheep testes associated with disrupting rumen microbiota. Ecotoxicol. Environ. Saf. 2022, 232, 113225. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, T.; Li, P.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 can alleviate aflatoxin B1-induced kidney oxidative stress and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 218, 112286. [Google Scholar] [CrossRef]
- Xu, D.; Peng, S.; Guo, R.; Yao, L.; Mo, H.; Li, H.; Song, H.; Hu, L. Egcg alleviates oxidative stress and inhibits aflatoxin B1 biosynthesis via mapk signaling pathway. Toxins 2021, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Li, Z.; Nabi, F.; Hu, Y.; Hu, Z.; Liu, J. Penthorum chinense pursh compound ameliorates AFB1-induced oxidative stress and apoptosis via modulation of mitochondrial pathways in broiler chicken kidneys. Front. Vet. Sci. 2021, 8, 750937. [Google Scholar] [CrossRef] [PubMed]
- Saad-Hussein, A.; Shahy, E.M.; Shaheen, W.; Ibrahim, K.S.; Mahdy-Abdallah, H.; Taha, M.M.; Hafez, S.F. Hepatotoxicity of aflatoxin B1 and its oxidative effects in wood dust egyptian exposed workers. Arch. Environ. Occup. Health 2021, 76, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.A.; Shaukat, A.; Wu, K.; Rajput, I.R.; Baloch, D.M.; Akhtar, R.W.; Raza, M.A.; Najda, A.; Rafał, P.; Albrakati, A.; et al. Luteolin alleviates aflatoxinB1-induced apoptosis and oxidative stress in the liver of mice through activation of nrf2 signaling pathway. Antioxidants 2021, 10, 1268. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Discovering the protective effects of resveratrol on aflatoxin B1-induced toxicity: A whole transcriptomic study in a bovine hepatocyte cell line. Antioxidants 2021, 10, 1225. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Transcriptional profiling of aflatoxin B1-induced oxidative stress and inflammatory response in macrophages. Toxins 2021, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, Z.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 can alleviate liver apoptosis and oxidative stress induced by aflatoxin B1. Food Chem. Toxicol. 2021, 151, 112124. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, E.E.; Burd, R. Quercetin as a systemic chemopreventative agent: Structural and functional mechanisms. Mini Rev. Med. Chem. 2011, 11, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Stinco, C.M.; Meléndez-Martínez, A.J. Free radical scavenging properties of phytofluene and phytoene isomers as compared to lycopene: A combined experimental and theoretical study. J. Phys. Chem. B 2014, 118, 9819–9825. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion complexes of lycopene and β-cyclodextrin: Preparation, characterization, stability and antioxidant activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zuo, C.; Liang, T.; Huang, Y.; Kang, P.; Xiao, K.; Liu, Y. Lycopene alleviates multiple-mycotoxin-induced toxicity by inhibiting mitochondrial damage and ferroptosis in the mouse jejunum. Food Funct. 2022, 13, 11532–11542. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. Nrf2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Takaku, M.; Egner, P.A.; Morita, M.; Kaneko, T.; Mashimo, T.; Kensler, T.W.; Yamamoto, M. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin B1 toxicity. Toxicol. Sci. 2016, 152, 40–52. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Ma, J.; Xv, Q.; Gao, H.; Yin, H.; Yan, G.; Jiang, X.; Yu, W. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the nrf2 antioxidant system. Redox Biol. 2022, 57, 102494. [Google Scholar] [CrossRef]
- Abdel-Naim, A.B.; Hassanein, E.H.M.; Binmahfouz, L.S.; Bagher, A.M.; Hareeri, R.H.; Algandaby, M.M.; Fadladdin, Y.A.J.; Aleya, L.; Abdel-Daim, M.M. Lycopene attenuates chlorpyrifos-induced hepatotoxicity in rats via activation of nrf2/ho-1 axis. Ecotoxicol. Environ. Saf. 2023, 262, 115122. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Deng, S.; Zhang, S.; Zhou, Y.; Velkov, T.; Li, J.; Xiao, X. Lycopene attenuates colistin-induced nephrotoxicity in mice via activation of the nrf2/ho-1 pathway. Antimicrob. Agents Chemother. 2015, 59, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xia, J.; Zhao, H.S.; Hou, R.; Talukder, M.; Yu, L.; Guo, J.Y.; Li, J.L. Lycopene triggers nrf2-ampk cross talk to alleviate atrazine-induced nephrotoxicity in mice. J. Agric. Food Chem. 2018, 66, 12385–12394. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Zhao, Y.; Dai, X.Y.; Talukder, M.; Li, J.L. Lycopene ameliorates dehp exposure-induced renal pyroptosis through the nrf2/keap-1/nlrp3/caspase-1 axis. J. Nutr. Biochem. 2023, 113, 109266. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Tuzcu, M.; Sahin, N.; Ali, S.; Kucuk, O. Nrf2/ho-1 signaling pathway may be the prime target for chemoprevention of cisplatin-induced nephrotoxicity by lycopene. Food Chem. Toxicol. 2010, 48, 2670–2674. [Google Scholar] [CrossRef]
- Guo, W.; Huang, D.; Li, S. Lycopene alleviates oxidative stress-induced cell injury in human vascular endothelial cells by encouraging the sirt1/nrf2/ho-1 pathway. Clin. Exp. Hypertens. 2023, 45, 2205051. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Zhu, S.Y.; Chen, J.; Li, M.Z.; Zhao, Y.; Talukder, M.; Li, J.L. Lycopene alleviates di(2-ethylhexyl) phthalate-induced splenic injury by activating p62-keap1-nrf2 signaling. Food Chem. Toxicol. 2022, 168, 113324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, L.; Wang, J.S. Aflatoxin B1 induces gut-inflammation-associated fecal lipidome changes in f344 rats. Toxicol. Sci. 2021, 183, 363–377. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.; Tsim, K.W.K.; Shen, X.; Li, X.; Li, X.; Lei, H.; Liu, Y. Aflatoxin B1 induces inflammatory liver injury via gut microbiota in mice. J. Agric. Food Chem. 2023, 71, 10787–10797. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, Y.; Zhang, M.; Lan, H. Aflatoxin B1 exposure triggers inflammation and premature skin aging via ERMCS/Ca2+/ROS signaling cascade. Int. Immunopharmacol. 2023, 124, 110961. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The compromised intestinal barrier induced by mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Toshima, T.; Taketomi, A.; Taguchi, K.; Yoshizumi, T.; Uchiyama, H.; Harimoto, N.; Kajiyama, K.; Egashira, A.; Maehara, Y. Hepatic aflatoxin B1-DNA adducts and tp53 mutations in patients with hepatocellular carcinoma despite low exposure to aflatoxin B1 in southern japan. Liver Int. 2011, 31, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Yeh, S.H.; Chen, P.J. Androgen enhances aflatoxin-induced genotoxicity and inflammation to liver cancer in male hepatitis b patients. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, H.; Yi, L.; Shao, P.; Soulika, A.M.; Meng, X.; Xing, L.; Yan, X.; Zhang, X. Oral administration of aflatoxin g1 induces chronic alveolar inflammation associated with lung tumorigenesis. Toxicol. Lett. 2015, 232, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Gao, Y.T.; Dean, M.; Egner, P.; Nepal, C.; Jones, K.; Wang, B.; Rashid, A.; Luo, W.; Van Dyke, A.L.; et al. Association of aflatoxin and gallbladder cancer. Gastroenterology 2017, 153, 488–494.e1. [Google Scholar] [CrossRef]
- Xiang, X.; Hao, Y.; Cheng, C.; Hu, H.; Chen, H.; Tan, J.; Wang, Y.; Liu, X.; Peng, B.; Liao, J.; et al. A tgf-β-dominant chemoresistant phenotype of hepatoblastoma associated with aflatoxin exposure in children. Hepatology 2024, 79, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.J.; Yang, H.I.; Wu, H.C.; Lee, M.H.; Liu, J.; Wang, L.Y.; Lu, S.N.; Jen, C.L.; You, S.L.; Santella, R.M.; et al. Aflatoxin B1 exposure increases the risk of hepatocellular carcinoma associated with hepatitis c virus infection or alcohol consumption. Eur. J. Cancer 2018, 94, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, Y.; Wang, Y.; Wang, Q.; Huo, S.; Zhang, X.; Cao, Z.; Song, M.; Li, Y. Pink1/parkin-mediated mitophagy is activated to protect against AFB1-induced immunosuppression in mice spleen. Toxicol. Lett. 2022, 366, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Bai, S.; Ding, X.; Zhang, K. Pathological impairment, cell cycle arrest and apoptosis of thymus and bursa of fabricius induced by aflatoxin-contaminated corn in broilers. Int. J. Environ. Res. Public Health 2017, 14, 77. [Google Scholar] [CrossRef]
- Mehrzad, J.; Bahari, A.; Bassami, M.R.; Mahmoudi, M.; Dehghani, H. Immunobiologically relevant level of aflatoxin B1 alters transcription of key functional immune genes, phagocytosis and survival of human dendritic cells. Immunol. Lett. 2018, 197, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Feng, Y.; Theve, E.J.; Raczynski, A.R.; Fiala, J.L.; Doernte, A.L.; Williams, M.; McFaline, J.L.; Essigmann, J.M.; Schauer, D.B.; et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut 2010, 59, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, D.; Zhang, J.; Tang, H.; Li, F.; Peng, Y.; Duan, X.; Meng, E.; Zhang, C.; Zeng, T.; et al. Role of macrophage ahr/tlr4/stat3 signaling axis in the colitis induced by non-canonical ahr ligand aflatoxin B1. J. Hazard. Mater. 2023, 452, 131262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food sources, biological activities, and human health benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Dai, X.; Liu, T.; Zhang, Y.; Wang, S.; Shi, H.; Yin, J.; Xu, T.; Zhu, R.; et al. Lycopene ameliorates islet function and down-regulates the tlr4/myd88/nf-κb pathway in diabetic mice and min6 cells. Food Funct. 2023, 14, 5090–5104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.W.; Guo, J.Y.; Lin, J.; Zhu, S.Y.; Dai, X.Y.; Saleem, M.A.U.; Zhao, Y.; Li, J.L. Mapk/nf-κb signaling mediates atrazine-induced cardiorenal syndrome and antagonism of lycopene. Sci. Total Environ. 2024, 922, 171015. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Li, D.; Xie, S.; Xiao, X.; Velkov, T. Curcumin attenuates colistin-induced neurotoxicity in n2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol. Neurobiol. 2018, 55, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Mishra, S.K.; Tripathi, S.; de Alencar, M.; JMC, E.S.; Rolim, H.M.L.; de Medeiros, M.; Ferreira, P.M.P.; Rouf, R.; Uddin, S.J.; et al. Mycotoxin-assisted mitochondrial dysfunction and cytotoxicity: Unexploited tools against proliferative disorders. IUBMB Life 2018, 70, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.L.; Chen, S.; Shen, M.Y.; Huang, Q.Y.; Li, H.G.; Sun, S.C.; Wang, J.L.; Luo, X.Q. Aflatoxin B1 impairs porcine oocyte quality via disturbing intracellular membrane system and atp production. Ecotoxicol. Environ. Saf. 2023, 263, 115213. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, B.G.; Bhat, N.K.; Avadhani, N.G. Preferential attack of mitochondrial DNA by aflatoxin B1 during hepatocarcinogenesis. Science 1982, 215, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Katayama, H.; Oikawa, A.; Negishi, L.; Ichikawa, T.; Suzuki, M.; Murase, K.; Takayama, S.; Sakuda, S. Dioctatin activates clpp to degrade mitochondrial components and inhibits aflatoxin production. Cell Chem. Biol. 2020, 27, 1396–1409.e10. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.; Cao, Z.; Zhang, J.; Huang, W. AFB1-induced mice liver injury involves mitochondrial dysfunction mediated by mitochondrial biogenesis inhibition. Ecotoxicol. Environ. Saf. 2021, 216, 112213. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Abdallah, M.F.; Grootaert, C.; Van Nieuwerburgh, F.; Rajkovic, A. New insights into the combined toxicity of aflatoxin B1 and fumonisin B1 in Hepg2 cells using seahorse respirometry analysis and RNA transcriptome sequencing. Environ. Int. 2023, 175, 107945. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator pgc-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, H.X.; Luo, Y.; Cui, J.G.; Talukder, M.; Li, J.L. Lycopene mitigates dehp-induced hepatic mitochondrial quality control disorder via regulating sirt1/pink1/mitophagy axis and mitochondrial unfolded protein response. Environ. Pollut. 2022, 292, 118390. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Luo, Y.; Yan, H.; Yu, J. Lycopene increases the proportion of slow-twitch muscle fiber by ampk signaling to improve muscle anti-fatigue ability. J. Nutr. Biochem. 2021, 94, 108750. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Sharma, G.; Dai, C. Neuroprotective properties of berberine: Molecular mechanisms and clinical implications. Antioxidants 2023, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lv, Y.; Huang, K.; Luo, Y.; Xu, W. Zinc inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in human hepatocytes (hepg2 cells). Food Chem. Toxicol. 2016, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yan, W.R.; Tang, J.K.; Jin, X.; Xue, H.H.; Wang, T.; Zhang, L.W.; Sun, Q.Y.; Liang, Z.X. Dietary phillygenin supplementation ameliorates aflatoxin B1-induced oxidative stress, inflammation, and apoptosis in chicken liver. Ecotoxicol. Environ. Saf. 2022, 236, 113481. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Abdeen, A.; Jalouli, M.; Abdelkader, A.; Megahed, A.; Alkahtane, A.; Almeer, R.; Alhoshani, N.M.; Al-Johani, N.S.; Alkahtani, S.; et al. Fucoidan supplementation modulates hepato-renal oxidative stress and DNA damage induced by aflatoxin B1 intoxication in rats. Sci. Total Environ. 2021, 768, 144781. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Ni, Y.; Guo, B.; Feng, X.; Jiang, Z. Lycpene antagonizes lead toxicity by reducing mitochondrial oxidative damage and mitochondria-mediatd apoptosis in cultured hippocampal neurons. MedComm 2020, 1, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, C.; Yang, M.; Gan, D.; Fan, C.; Li, A.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Lycopene protects against t-bhp-induced neuronal oxidative damage and apoptosis via activation of the pi3k/akt pathway. Mol. Biol. Rep. 2019, 46, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Odhav, B.; Bhoola, K. Aflatoxin B1-induced toxicity in hepg2 cells inhibited by carotenoids: Morphology, apoptosis and DNA damage. Biol. Chem. 2006, 387, 87–93. [Google Scholar] [CrossRef]
- Pope, S.; Land, J.M.; Heales, S.J. Oxidative stress and mitochondrial dysfunction in neurodegeneration; cardiolipin a critical target? Biochim. Biophys. Acta 2008, 1777, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Jaćević, V.; Dumanović, J.; Alomar, S.Y.; Resanović, R.; Milovanović, Z.; Nepovimova, E.; Wu, Q.; Franca, T.C.C.; Wu, W.; Kuča, K. Research update on aflatoxins toxicity, metabolism, distribution, and detection: A concise overview. Toxicology 2023, 492, 153549. [Google Scholar] [CrossRef] [PubMed]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, M.; Bordin, K.; Souto, P.; Corassin, C.; Oliveira, C. Comparative biotransformation of aflatoxin b 1 in swine, domestic fowls, and humans. Toxin Rev. 2015, 34, 142–150. [Google Scholar] [CrossRef]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M. Cytochrome p450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult. Sci. 2010, 89, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Johnson, W.W.; Shimada, T.; Ueng, Y.F.; Yamazaki, H.; Langouët, S. Activation and detoxication of aflatoxin B1. Mutat. Res. 1998, 402, 121–128. [Google Scholar] [CrossRef]
- Kamdem, L.K.; Meineke, I.; Gödtel-Armbrust, U.; Brockmöller, J.; Wojnowski, L. Dominant contribution of p450 3a4 to the hepatic carcinogenic activation of aflatoxin B1. Chem. Res. Toxicol. 2006, 19, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.W.; Guengerich, F.P. Reaction of aflatoxin B1 exo-8,9-epoxide with DNA: Kinetic analysis of covalent binding and DNA-induced hydrolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 6121–6125. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Song, C.; Ye, L.; Xu, J.; Guo, D.; Shi, Q. The effect of lycopene on cytochrome p450 isoenzymes and p-glycoprotein by using human liver microsomes and caco-2 cell monolayer model. Int. J. Food Sci. Nutr. 2018, 69, 835–841. [Google Scholar] [CrossRef]
- Nosková, K.; Dovrtělová, G.; Zendulka, O.; Strakošová, M.; Peš, O.; Juřica, J. Lycopene increases metabolic activity of rat liver cyp2b, cyp2d and cyp3a. Pharmacol. Rep. 2020, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liang, T.; Huang, Y.; Zuo, C.; Wang, D.; Liu, Y. Co-occurrence of mycotoxin-induced hepatotoxicity in mice inhibited by lycopene: Mitochondrial impairment and early hepatic fibrosis. Mol. Nutr. Food Res. 2023, 67, e2200671. [Google Scholar] [CrossRef] [PubMed]
- Scholl, P.F.; Musser, S.M.; Groopman, J.D. Synthesis and characterization of aflatoxin B1 mercapturic acids and their identification in rat urine. Chem. Res. Toxicol. 1997, 10, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of parkinson’s disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Karabelas, A.J. Natural origin lycopene and its “green” downstream processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 686–709. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves hdl functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.A.; Richmond, R.C.; Ferreira, D.L.S.; Ness, A.R.; May, M.; Smith, G.D.; Vincent, E.E.; Adams, C.; Ala-Korpela, M.; Würtz, P.; et al. Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: The prodiet randomised controlled trial. Int. J. Cancer 2019, 144, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Biddle, M.J.; Lennie, T.A.; Bricker, G.V.; Kopec, R.E.; Schwartz, S.J.; Moser, D.K. Lycopene dietary intervention: A pilot study in patients with heart failure. J. Cardiovasc. Nurs. 2015, 30, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, T.; Li, M.; Fu, Z.; Chen, L.; Shi, D.; Qiu, F.; Tan, X. Lycopene attenuates oxidative stress-induced hepatic dysfunction of insulin signal transduction: Involvement of fgf21 and mitochondria. J. Nutr. Biochem. 2022, 110, 109144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, R.K.; Zhu, S.Y.; Talukder, M.; Cui, J.G.; Zhang, H.; Li, X.N.; Li, J.L. Lycopene prevents dehp-induced hepatic oxidative stress damage by crosstalk between ahr-nrf2 pathway. Environ. Pollut. 2021, 285, 117080. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Nagata, N.; Xu, L.; Yamamoto, S.; Fuke, N.; Ushida, Y.; Suganuma, H.; Kaneko, S.; et al. Lycopene prevents the progression of lipotoxicity-induced nonalcoholic steatohepatitis by decreasing oxidative stress in mice. Free Radic. Biol. Med. 2020, 152, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, H.; Wang, J.; Liu, P.; Tan, X.; Ren, B.; Liu, Z.; Liu, X. Lycopene supplementation attenuates oxidative stress, neuroinflammation, and cognitive impairment in aged cd-1 mice. J. Agric. Food Chem. 2018, 66, 3127–3136. [Google Scholar] [CrossRef]
- Milani, C.; Maccari, M.; Mosconi, P. Action of lycopene in the experimental gastric ulcer. Pharmacology 1970, 4, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Michael McClain, R.; Bausch, J. Summary of safety studies conducted with synthetic lycopene. Regul. Toxicol. Pharmacol. 2003, 37, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).