Abstract
Peroxiredoxin 6 (Prdx6), a unique 1-Cys member of the peroxiredoxin family, exhibits peroxidase activity, phospholipase activity, and lysophosphatidylcholine acyltransferase (LPCAT) activity. Prdx6 has been known to be an important enzyme for the maintenance of lipid peroxidation repair, cellular metabolism, inflammatory signaling, and antioxidant damage. Growing research has demonstrated that the altered activity of this enzyme is linked with various pathological processes including central nervous system (CNS) disorders. This review discusses the distinctive structure, enzyme activity, and function of Prdx6 in different CNS disorders, as well as emphasizing the significance of Prdx6 in neurological disorders.
1. Introduction
Peroxiredoxins (Prdx) are a ubiquitous family of highly conserved antioxidant enzymes featuring a cysteine (cys) residue involved in peroxide reduction. In Homo sapiens, to date, six isoforms of Prdx (Prdx1–Prdx6) have been reported, which are categorized into three subgroups based on the position and number of cysteine residues participating in catalysis: intermolecular (typical 2-Cys, Prdx1–4), intramolecular (atypical 2-Cys, Prdx5) disulfide bonds, and noncovalent interactions (1-Cys, Prdx6). Distinct from other family members, Prdx6 is a unique 1-Cys peroxidase and does not utilize thioredoxin as a reducing agent [1,2]. Prdx6 is a multifunctional enzyme with peroxidase activity, antioxidant-acting acidic calcium-independent phospholipase A2 (aiPLA2) activity, and lysophosphatidylcholine acyltransferase (LPCAT) activity [3,4,5]. These unique enzyme activities make it interesting to explore the physiological and pathological functions of Prdx6 in different diseases. Over the past several years, Prdx6 has been extensively investigated in brain diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [6,7]. Nevertheless, the underlying mechanisms of action for Prdx6 in neurological disorders have largely remained elusive. Therefore, this review seeks to unveil the significance of Prdx6 in CNS disorders, which will contribute to a greater understanding about the potential value of Prdx6 as a new therapeutic target for neurological diseases.
2. Structure of Prdx6
Human Prdx6 protein consists of 224 amino acids and is encoded by an 11,542 base-pair gene with five exons. It is located on human chromosome 1, as verified by the cloning and sequencing of the Prdx6 cDNA. It has a relative molecular weight of 25–29 kDa on SDS/PAGE and an isoelectric point (pI) of 5.1, which varies depending on the oxidation and phosphorylation status of the protein [8,9]. The comparison of Prdx6 proteins among mammals (humans, mice, and rats) reveals an 95% identity in amino acid sequences (Figure 1). Compared with other proteins in the Prdx family, Prdx6 has a single conserved cysteine residue, known as the 1-Cys Prdx subgroup, and an additional Cys residue presented in the N-terminus (C47) of humans and mice, whereas this residue is absent in rats and bovines [10]. The secondary structure of Prdx6 contains a typical thioredoxin fold, consisting of approximately 80 amino acids arranged as four inverted β-sheets between two α-helices [11,12]. Furthermore, a low pH results in the conformational transformation of Prdx6 into high-order oligomers, and oligomer formation underlies the resistance of human Prdx6 at the lysosomal pH and high temperatures [13]. Some studies have shown that Prdx6 is a pH-dependent enzyme, and this pH specificity is attributed to the differential substrate preference at different pH values, such as peroxided phospholipids at neutral pH and reduced phospholipids at acidic pH [14]. Prdx6 regulates oxidative stress, and this function has been identified in the cerebral cortex and hippocampus of young and old mice [15]. Evidence suggests that Prdx6 serves as a rheostat in regulating cell physiology by scavenging reactive oxygen species (ROS) to optimize gene regulation. Prdx6 ameliorates ROS-based oxidative damage and NF-κB-mediated aberrant signaling in human cortical neuronal cells, HCN-2, and mouse hippocampal cells, HT22. Therefore, Prdx6 expression is critical for the protection of neuronal cells from oxidative stress-evoked damage [16]. In a word, the function of Prdx6 is closely related to its structure and enzyme activity.

Figure 1.
Prdx6 amino acid sequence for a human (H), mouse (M), and rat (R).
3. Enzyme Activities and Function of Prdx6
Prdx6 can bind and reduce phospholipid hydroperoxides; this process involves peroxidase activity. The peroxidase activity in Prdx6 is associated with distinct active sites (C47, R132, H39) [8]. Prdx6 expresses only a conserved Cys and uses glutathione (GSH) and GSH S-transferase (GST) for the reduction and resolution of its oxidized peroxidatic Cys [17]. The activity of phospholipid hydroperoxidase GSH peroxidase (PHGPx) is mediated by the catalytic cysteine at position 47 (C47). The mutation of cysteine to serine (C47S) in Prdx6 can eliminate its ability to reduce hydroperoxides [18,19]. Prdx6, as a peroxidase, has substrate specificity, and its substrates include H2O2, short-chain hydroperoxides, and phospholipid hydroperoxides [20,21]. Prdx6 binds to oxidized lipid substrates (oxidized membrane phospholipids) and reduces the generation of phospholipid hydroperoxides induced by oxidative stress [22,23]. Also, Prdx6 interacts with phospholemman in a glutathione-dependent manner and depalmitoylates phospholemman via reactive thiol [24]. The peroxidase activity of Prdx6 is a conformation-driven process based on the redox state, which essentially involves monomer-dimer transition [9].
The enzyme activity of Prdx6, Ca2+-independent intracellular phospholipase A2 (aiPLA2) activity, was discovered successively in rat and bovine lung tissues. The aiPLA2 activity plays important roles in the synthesis and phospholipid conversion of the lung surfactant. The aiPLA2 activity has been confirmed in the deduced amino acids from the nucleotide sequences of clones isolated from a cDNA library of human adult myeloid cells [17,25]. The catalytic triad (S32, H26, and D140) of phospholipase activity resides on the surface of the Prdx6 protein, which plays a pivotal role in the reduction of oxidized phospholipids and cell-membrane remodeling [26]. Research has indicated that the mutations in the His26 and Ser32 of Prdx6 lead to the loss of the ability to bind phospholipids and the aiPLA2 activity. Nevertheless, the Asp140 mutation causes a loss of aiPLA2 activity but does not affect binding to phospholipids [10]. Prdx6 can exert aiPLA2 activity in the cytoplasm at a neutral pH and in the lumen of acidic lysosomes. The aiPLA2 activity is highest when phosphatidylcholine is used as the substrate, but gradually decreased when phosphatidylethanolamine, phosphatidylglycerol, inositol, and serine are the substrates [27]. The aiPLA2 catalyzes the hydrolysis of the sn-2 fatty acyl ester bond of glycerophospholipids to produce free fatty acids and lysophospholipids. In addition, it has an essential physiological role in the repair of peroxidized cell membranes and the activation of NADPH oxidase 2 (Nox2) [10,28,29].
Initially, it was thought that Prdx6 is a bifunctional enzyme, but recent studies reported that Prdx6 also has LPCAT activity. The current investigation provides evidence that Prdx6 exhibits acyltransferase activity, a function dependent on the presence of the amino acid D31 site involved in lipid metabolism [5]. The LPCAT activity is relatively specific for lysophosphatidylcholine and palmitoyl-CoA. There is no release of intermediates when LPCAT activity is combined with the aiPLA2 activity of Prdx6 [30]. In addition, LPCAT activity is a critical component of the phospholipid remodeling pathway. The reduction of phospholipid hydroperoxides depends on the synergistic activation of the LPCAT and aiPLA2 activities of Prdx6. Therefore, it can play a role in the lipid synthesis remodeling pathway and act as an integral enzyme to repair peroxidized cell-membrane phospholipids [31].
Beyond its enzymatic functions, research has explored the impact of how Prdx6 interacts with other proteins or drugs on pathological processes and homeostasis. In non-alcoholic steatohepatitis (NASH), the interaction between Miz1 and Prdx6 in hepatocytes is crucial. A loss in Miz1 leads to the inhibition of mitophagy mediated by Prdx6 and triggers the production of pro-inflammatory cytokines by hepatic macrophages [32]. Liu et al. demonstrated that the nuclear phosphoprotein (NPM) regulates the expression of Prdx6 and affects the level and distribution of ROS, particularly in tumor cells [33]. Daverey and Agrawal showed that curcumin protects astrocytes from oxidative stress by reducing astrocyte GFAP, decreasing waveform proteins, and inhibiting Prdx6 expression [34]. Another study reported that Bmal1 and Nrf2 could directly regulate the transcription of Prdx6 in human lens/lens epithelial cells (hLECs) by binding to the E-box element and ARE sites, respectively, which cooperated to activate the Prdx6 transcription in hLECs and facilitate the antioxidant defense to maintain redox homeostasis [35]. A Nrf2 activator, Sulforaphane (SFN), reactivates cellular antioxidant defense by inducing Prdx6 activity to influence the intracellular homeostasis [36,37].
4. Prdx6 Expression in the CNS
The Prdx6 protein is originally isolated from the bovine ciliary body [2]. In mammalians, Prdx6 is expressed in almost all organs, especially in the lungs, brain, kidneys, and testes [38,39]. In the CNS, the Prdx6 protein is expressed in olfactory areas, the cortex, the hippocampus, thalamic areas, the hypothalamus, the brainstem, the cerebellum, and the spinal cord [40]. At the cellular level, Prdx6 is mainly expressed in astrocytes and not in other glial cells [6]. The intracellular expression of Prdx6 is localized in the cytoplasm and acidic organelles, such as lysosomes and lysosome-associated organelles [4]. Prdx6 is expressed during brain development and increases after birth [41]. Therefore, the lack of oxidative-damage protection in early development may be related to the low expression of Prdx6. Prdx6 remains in a dormant state in a typical brain, while in some pathological states, such as glioma and AD, Prdx6 is selectively upregulated in astrocytes [42,43]. It is likely that the expression of Prdx6 in activated astrocytes may contribute to initiating oxidative stress. In experimental autoimmune encephalomyelitis (EAE) mice and multiple sclerosis (MS) patients, the expression of Prdx6 is markedly increased in spinal cord astrocytes, which may be related to high levels of nitric oxide (NO) and superoxide after EAE and MS [44].
5. Prdx6 and CNS Diseases
5.1. Alzheimer’s Disease (AD)
AD is a progressive neurodegenerative disease characterized by hyperphosphorylated tau and abnormal beta-amyloid deposition, as well as neuronal degeneration [45]. In the brains of AD patients, Prdx6 was significantly increased in astrocytes in both white and gray matter in the midfrontal cortex, cingulate, hippocampus, and amygdala, and astrocytes with a high expression of Prdx6 participated in the detoxification of diffuse plaque [43] (Table 1). Furthermore, significantly elevated Prdx6 expression in astrocytes was identified exclusively in AD patients, not in controls, which was related to disease-associated glial cell activation in AD [46]. However, a proteomic study found that compared to typical sporadic AD, the levels of Prdx6 in amyloid plaques in rapidly progressing AD (rpAD) was significantly reduced [47]. In addition, there is evidence pointing to a decrease in both Prdx6 mRNA and protein levels in Aβ1–42 (amyloid beta 1–42 peptide)-induced AD rats [48]. However, there was no change in the expression levels of Prdx6 between the 3xTg AD mice and the control group [49], which is consistent with the study in the postmortem brain tissue of AD patients [50]. The heterogeneity of Prdx6 expression may be related to different tissues, and in animal models of AD, it may be related to differences in the modeling methods of AD (Table 2).
Oxidative stress has been implicated in the pathogenesis of AD patients, and AD brains exhibited an increased thiol oxidation state of Prdx6. The increased expression of the Prdx6 protein in AD was closely related to the degree of oxidative stress [51]. According to reports, aiPLA2 plays a crucial role in the Aβ1–42-mediated disturbance of mitochondrial function and oxidative stress in astrocytes [52], which provides new insights into the mechanistic details of Prdx6 in AD. In a correlation study between tumors and AD, the activity of the aiPLA2 of Prdx6 was inhibited through γ-secretase, consequently suppressing the development of lung tumors in AD patients and transgenic mice with mutant presenilin 2 [53]. In Prdx6 transgenic mice infused with Aβ1–42, there was an elevation in aiPLA2 activity causing worse memory impairment compared to Aβ1–42-infused C57BL/6 mice. The increased aiPLA2 could be involved with the progression of AD. Moreover, Aβ1–42-infused Prdx6 transgenic mice exhibited a significant increase in β-secretase activity, protein carbonyl, and 4-HNE levels to increase amyloidogenesis. These data demonstrated that the overexpression of Prdx6 in AD may promote amyloidogenesis and oxidative stress, thereby expediting the progression of AD (Figure 2) [54]. However, increased Prdx6 levels induced by thiacremonone can improve memory dysfunction by interfering with oxidative stress and amyloidogenesis in Aβ1–42/H2O2-induced cultured neuronal cells and amyloid precursor protein/presenilin1 (APP/PS1) transgenic AD mice [55]. There was an upregulation of Prdx6 in astrocytes in P301S transgenic mice, and these astrocytes were infiltrated in the area with a large amount of hyperphosphorylated tau protein and neuron loss, which indicates that Prdx6 may play neuroprotective roles against tau toxicity [56]. Prdx6 was identified as a vital factor regulating astrocyte responses to Aβ plaques. The upregulation of Prdx6 attenuates Aβ pathology and may contribute to AD treatment in the hemizygous knock-in of Prdx6 in APPswe/PS1dE9 AD transgenic mice, which promotes the selective induction and penetration of astrocytes in Aβ plaques and promotes microglia phagocytic activation [6]. Prdx6 can protect rat PC12 cells from neurotoxicity and resist the oxidative burst in the microglial cell line BV2 stimulated by amyloids [57,58]. In addition, Prdx6 inhibits neurogenesis in neural precursor cells through the TLR4-dependent downregulation of WD-repeat- and FYVE-domain-containing protein 1 (WDFY1) [7]. Although the role of Prdx6 in AD has been extensively investigated, Prdx6 plays different roles in different AD models. Prdx6 can play a protective role in some AD models, such as P301S and APPswe/PS1dE9 transgenic mice, and may also accelerate the progression of AD, such as Aβ1–42-infused Prdx6 transgenic mice.
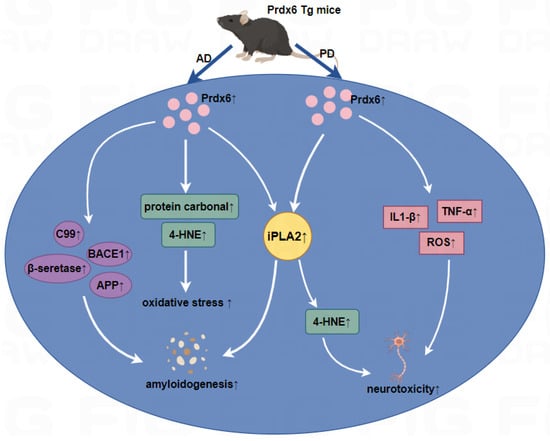
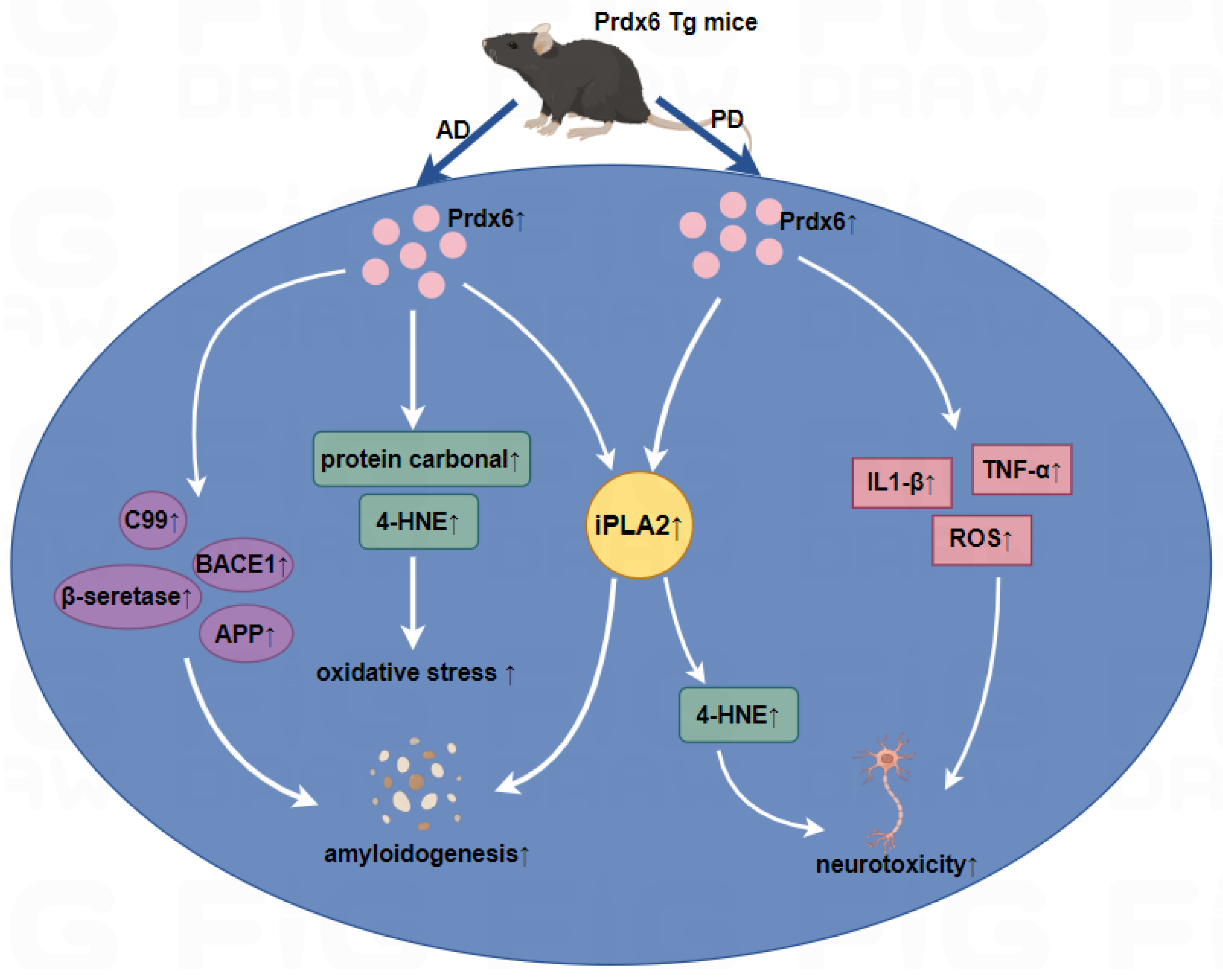
Figure 2.
Roles of Prdx6 in Aβ1–42-infused AD mice and MPTP-infused PD mice.

Table 1.
Prdx6 in different AD patients.
Table 1.
Prdx6 in different AD patients.
| Tissues | Species | Expression Level | Expression Tissue | Function | Reference |
|---|---|---|---|---|---|
| Postmortem brain | Human | ↑ | midfrontal cortex, cingulate, hippocampus, and amygdala | Prdx6 plays an anti-oxidant role in AD | [43] |
| Postmortem brain (female) | Human | ↑ | superior frontal gyrus | N/A | [46] |
| rpAD postmortem brain | Human | ↓ | hippocampus | N/A | [47] |
| Postmortem brain | Human | no change | frontal cortex and cerebellum | Prdx6 did not show significant changes in the brains of AD patients and possibly has no critical role in cellular defense against oxidative stress. | [50] |
| Postmortem brain | Human | ↑ | hippocampus | The increased expression of Prdx6 in AD was closely related to the degree of oxidative stress. | [51] |
↑, upregulation; ↓, downregulation; rpAD, rapidly progressive AD; N/A, not applicable.

Table 2.
Prdx6 in different AD models.
Table 2.
Prdx6 in different AD models.
| Tissues | Species | Expression Level | Expression Tissue | Function | Reference |
|---|---|---|---|---|---|
| APP/PS1 Prdx6 Tg female mice | Mice | ↑ | cortex and hippocampus | The upregulation of Prdx6 in AD mice can attenuate Aβ pathology. | [6] |
| 3xTg mice | Mice | no change | hippocampus | Prdx6 was not associated with cumulative oxidative stress in animal models of neurodegenerative disease. | [49] |
| PS2 (N141I) Tg mice | Mice | ↓ | lung | The PS2 mutation inhibits the aiPLA2 activity of Prdx6 through the γ-secretase cleavage mechanism to suppress lung-tumor development. | [53] |
| Aβ1–42-infused Prdx6 transgenic mice | Mice | ↑ | cortex and hippocampus | The overexpression of Prdx6 in AD mice promotes amyloidosis and increases oxidative stress, thereby expediting the progression of AD. | [54] |
| Tau (P301S) Tg mice | Mice | ↑ | anterior horn of the spinal cord | Prdx6 functions as a neuroprotective mechanism against tau toxicity. | [56] |
| Aβ25–35-treated BV2 cells | Mice | ↑ | N/A | Prdx6 is protective against oxidative stress in microglia and synergistically maintains the transition to a chronic neuroinflammatory phenotype, reinforcing the role of Prdx6 in AD | [57] |
| Aβ1–42-infused rat | Rat | ↑ | hippocampus | N/A | [48] |
| Aβ1–42-induced rat primary neuron | Rat | ↓ | N/A | Thiacremonone influences Prdx6 expression levels and oxidative stress, thereby protecting against amyloidosis and memory dysfunction and inhibiting the development and progression of AD. | [55] |
| Aβ25–35-treated rat PC12 cells | Rat | ↑ | N/A | Prdx6 can slow the progression of AD and limit the extent of AD-induced neuronal cell death. | [58] |
↑, upregulation; ↓, downregulation; APP, β-amyloid precursor protein; PS1, presenilin 1; N/A, not applicable; Tg, transgenic; PS2, presenilin 2.
Left panel: In Aβ1–42-infused Prdx6 transgenic mice, the overexpression of Prdx6 could increase iPLA2 activity and oxidative stress in astrocytes, and increase the β-secretase activity and the expression of APP, BACE1, resulting in increased Aβ aggregation, then accelerate the development of AD. Right panel: In the MPTP mice model, the iPLA2 activity of Prdx6 was upregulated followed by an increase in the level of ROS and 4-HNE, and iPLA2 activity induces astrocytic activation and leads to the increased secretion of proinflammatory cytokines such as TNF-α and IL1-β, resulting in dopaminergic neurotoxicity. ↑, upregulation.
5.2. Parkinson’s Disease (PD)
PD is an age-related, progressive neurodegenerative disorder characterized by bradykinesia, resting tremor, muscle rigidity, and postural abnormalities [59]. The predominant molecular pathogenesis of PD includes the misfolding and aggregation of alpha-synuclein, mitochondrial dysfunction, impaired protein clearance, neuroinflammation, and oxidative stress [60]. Postmortem brain tissue of PD patients showed that Prdx6 was upregulated in the grey matter and white matter of the frontal and cingulated cortices [61,62] (Table 3). Proteomics analysis using Parkin−/− PD mice revealed a decrease in Prdx6 protein levels [63] (Table 4). aiPLA2 is involved in the development of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The aiPLA2 inhibitor quinacrine has a protective effect against MPTP- and 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in mice [64]. In addition, the aiPLA2 activity of Prdx6 is a critical crosstalk between neurons and glial cells in the nigrostriatal dopaminergic neuronal system. The aiPLA2 activity of Prdx6 was increased after MPTP administration in the Prdx6 transgenic mice and upregulated aiPLA2 was accompanied with increased ROS and 4-HNE levels, which results in a greater loss of dopaminergic neurons and increased behavioral damage. And, in MPP+-treated primary astrocytes from Prdx6 transgenic mice, aiPLA2 activity and the release of neurotoxic products as well as reactive oxygen species were increased in MPP+-treated primary astrocytes from Prdx6 transgenic mice to trigger dopaminergic neurotoxicity. These findings demonstrated that the iPLA2 activity of Prdx6 is associated with the progression of PD (Figure 2) [65,66]. In addition, a study showed that Pink1-Parkin-mediated mitochondrial autophagy is ROS-dependent, and Prdx6 is recruited to mitochondria after carbonyl cyanide m-chlorophenyl hydrazone (CCCP) treatment to balance excess ROS, thereby protecting cells from death due to excessive mitochondrial phagocytosis. This suggested that Prdx6 is a key factor in the initial step of mitochondrial clearance and is upstream of the PINK1-Parkin pathway [67]. To sum up, Prdx6 is closely related to the pathogenesis of PD [68], and understanding the enzyme activity of Prdx6 is of great significance for the novel therapeutic approach for PD treatment.

Table 3.
Prdx6 in different PD patients.

Table 4.
Prdx6 in different PD models.
5.3. Cerebral Ischemia
Ischemic stroke is a major threat to human health worldwide due to its high morbidity, mortality, and disability rates. Although the exact mechanism is still unclear, oxidative stress, apoptosis, and inflammation have been proven to be involved in the pathogenesis of ischemic stroke [69,70]. Prdx6 is an antioxidant protein that plays an important role in ischemic stroke. The transplantation of cerebral endothelial cells (hCMEC/D3) into cerebral ischemia rats may potentially suppress the expression of Prdx6 induced by ischemia injury to control neuroinflammation, which suggests a potential association between Prdx6 and neuroinflammation in cerebral ischemia [71] (Table 5). A research study analyzed the change in cortical protein profile at 24 h and 2 months after hemorrhagic stroke in white pigs and showed that Prdx6 was significantly increased. Therefore, Prdx6 was related to the activation of neuroprotective compensatory mechanisms [72]. However, in endothelial cells overexpressing endothelin-1 (TET-1) mice, middle cerebral artery occlusion (MCAO) for 30 min followed by reperfusion for 7 days increased the expression of Prdx6 around blood vessels in the ipsilateral hippocampus, leading to neuronal apoptosis, glial activation, and blood–brain barrier disruption [73]. A sustained upregulation of Prdx6 expression may protect hippocampal neurons from oxidative stress in a rat model of stroke (localized heat-induced brain injury in the left anterior cortical tectum) [74,75]. Curcumin treatment upregulated the expression of Prdx6 to attenuate neurological deficits and oxidative stress in cerebral ischemia/reperfusion (I/R) rats, suggesting that Prdx6 has a neuroprotective effect against oxidative stress in rats after stroke [76]. Prdx6 was increased with melatonin treatment to protect neuronal cells from ischemic damage and prevent cell death caused by ischemic injury in MCAO model rats [77,78]. The role of the aiPLA2 activity of Prdx6 following OGD/R has been investigated in a BV2 cell lines/CTX-TNA2 cell lines co-culture system, which showed that aiPLA2 induces ROS production in astrocytes via activating the NOX2 and Drp1 related mitochondrial pathways to promote neuroinflammation [79]. In addition, the aiPLA2 activity of Prdx6 was associated with the secretion of neurotoxic inflammatory factors and a high expression of Toll-like receptor 2/4 (TLR2/4) in cerebral ischemia/reperfusion injury. Inhibiting the aiPLA2 activity of Prdx6 decreased the neurologic deficits, cerebral infarction, and inflammatory molecules [80]. In a recent study, a reduction of Prdx6 activity in the MCAO model resulted in increased neuronal apoptosis through the enhancement of the PINK1/PARKIN pathway-mediated mitochondrial autophagy, therefore exacerbating cerebral ischemia-reperfusion injury and apoptosis [81]. Furthermore, 4-hydroxy-benzylalcohol (4-HBA) mediated the upregulation of Prdx6 to protect neurons against cerebral ischemic injury via the PI3K/Akt pathway [82,83]. However, Prdx6 was proven to be released from necrotic brain cells within 12 h after stroke onset, coinciding with the timing of leukocyte infiltration, then initiating destructive immune responses acting as an endogenous ligand for TLR2 and TLR4 [84,85]. Thus, Prdx6 may exhibit dual roles in cerebral ischemia, potentially linked to its divergent functions within and outside the cell. Hence, there is a need to improve our understanding of the role of Prdx6 in cerebral ischemia to provide reliable validation data for cerebral ischemia treatment.

Table 5.
Prdx6 in different stroke models.
5.4. Spinal Cord Injury (SCI)
SCI is a devastating trauma in the CNS, including primary and secondary damages, with pathological changes such as inflammation, hemorrhage, edema, and oxidative stress, leading to motor, sensory, and functional impairment [86,87]. In contusion SCI rats, a downregulation of Prdx6 was observed, accompanied by an upregulation of TNF-α and an inhibition of motor function. However, the transduction of the TNF-α RNAi vector into the spinal cord increased Prdx6 expression, suggesting that TNF-α inhibition may work as a mechanism for improving motor function via the upregulation of Prdx6 in SCI [88]. A recent study demonstrated that low levels of Prdx6 in reperfusion injury led to increased white-matter inflammation and apoptosis in a rat SCI model, implicating a protective role of Prdx6 in spinal cord hypoxic-reperfusion injury. In addition, Prdx6 activity in white matter was regulated by its cellular distribution and possible interactions of Prdx6 with TNF-α and Nrf2. Nrf2 negatively regulated Prdx6 by inhibiting the aiPLA2 activity of Prdx6, which reduces axonal and astrocyte injury [89]. Therefore, Prdx6 provides a new strategy and target for the clinical treatment of SCI in the future.
5.5. Traumatic Brain Injury (TBI)
TBI, which is characterized as an intangible wound, triggers a series of intracerebral events, including hypoxia, oxidative stress, necrosis, apoptosis, and chronic inflammation [90,91]. Evidence from a previous study demonstrated that Prdx6 is a physiologically important redox-sensitive antioxidant component in cerebrospinal fluid. The TBI-induced oxidation of Prdx6 and its specific phospholipid peroxidase activity were correlated with trauma prognosis. The recovery of Prdx6 activity in patients 24 h after the onset of TBI was associated with a good prognosis [92,93,94]. Proteomics analysis revealed the Prdx6 expression was decreased in the brain tissue of diffuse TBI patients compared with those with focal TBI [95]. Other evidence suggests that the Prdx6 level is elevated in the peripheral blood of TBI patients; however, no association was identified between the Prdx6 levels and poor neurological prognosis or mortality at 6 months after surgery in TBI patients, indicating that Prdx6 may serve as a diagnostic marker for acute TBI, but its prognostic ability may be limited [96]. An autoimmunoassay indicated that Prdx6 could be used as a target for autoantibodies induced in response to TBI, and there were high Prdx6 levels in the perivascular area based on the immunohistochemical analysis of the rat cerebral cortex. Studies have shown a dramatic increase in Prdx6 during mild to moderate TBI in a rat TBI model, which indicated that Prdx6 may be a candidate marker of acute mild brain injury [97].
5.6. Prion Disease
Transmissible spongiform encephalopathies (TSEs), also known as prion diseases, are fatal neurodegenerative diseases caused by protein misfolding, mitochondrial dysfunction, and oxidative stress, leading to the loss of motor control, paralysis, wasting, and eventually death [98,99]. TSE is caused by the conversion of cellular prion protein (PrPC) to its abnormal isoform (PrPSc) [100,101]. Sporadic Creutzfeldt–Jakob disease (sCJD) is one of many prion diseases characterized by the spontaneous formation of misfolded prion proteins in the brain, and the expression of Prdx6 was increased in the frontal cortex of patients with sCJD [102]. The glycoproteome analysis of a brain revealed that the expression of Prdx6 was continuously increased during the late stages of prion infection in mice [49]. The loss of the Prdx6 protein exacerbated the prion disease, which mainly manifested as astrogliosis and an accumulation of proteinase K-resistant PrPSc, while the overexpression of Prdx6 improved cognitive behavior and attenuated prion-related astrocytosis [103]. Studies have shown that Prdx6 is consumed by peroxides produced by prion-induced oxidative stress, which prevents the emergence of prion-related neuropathology in Prdx6 transgenic mice with prion disease. Enhanced quantities of Prdx6 were identified in the brains of prion-infected mice and neuronal cell lines. Simultaneously, the level of PrPC raised with an increase in PrPSc transformation [104]. Overall, although most studies elucidate the elevated levels of Prdx6 in prion diseases, its role remains somewhat controversial, so additional studies are needed to provide insights into the potential value of Prdx6 in prion diseases.
5.7. Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease of the CNS. The primary pathological features are immune-cell infiltration, myelin loss, axonal degeneration, and reactive astrocyte proliferation [105,106]. Evidence from experimental autoimmune encephalomyelitis (EAE) mice and MS patients showed increased expression of Prdx6 in the astrocytes of the spinal cord in comparison with the control group, respectively. At the same time, the upregulation of Prdx6 in EAE mice reduced the loss of myelin, MMP9 expression, and microglia activation to prevent brain-barrier destruction and immune-cell infiltration [44]. Serum levels of Prdx6 in patients with MS were higher than that in control patients with amyotrophic lateral sclerosis and spinocerebellar degeneration. In addition, the serum Prdx6 levels were associated with the albumin quotient, which suggested that Prdx6 is associated with blood–brain barrier dysfunction to some extent in MS [107]. Therefore, Prdx6 may play an important role in the regulation of inflammation and the blood–brain barrier in MS and may be a therapeutic target or biomarker for MS.
5.8. Amyotrophic Lateral Sclerosis (ALS)
ALS, also known as motor neuron disease, involves the degeneration of upper and lower motor neurons, resulting in muscle weakness and eventual paralysis [108]. Studies have shown that neuroinflammation and redox dysregulation are important contributors to ALS pathogenesis [109,110]. Prdx6 was uniquely upregulated in mouse ALS models, suggesting that Prdx6 may be a defense against SOD1G93A-induced oxidative stress [102]. However, in a study on the spatiotemporal dynamics in ALS mice, the activity of Prdx6 increases as the disease progresses [111]. Studies on Prdx6 in ALS are limited, and further elucidation of the role of Prdx6 in ALS is needed.
5.9. Gliomas
A glioma is the most prevalent primary brain tumor in adults and has an extremely unfavorable prognosis and overall survival [112]. A proteomic study of a patient with oligodendroglioma (ODG) which rapidly developed into anagenic oligodendroglioma (AODG) showed a higher expression of the Prdx6 protein in ODG than in AODG, suggesting that Prdx6 is related to the malignant transformation of ODG and can be regarded as a biomarker of ODG progression [113]. Research from glioma patients revealed a positive correlation between the Prdx6 level and increasing grades of gliomas [114,115]. A gene-expression database has been employed to assess Prdx expression across various glioma subtypes and non-tumor brain tissues. The findings reveal a general increase in Prdx6 expression with the malignant grades of brain gliomas. Elevated Prdx6 expression is notably associated with a lower survival rate among patients, underscoring its pro-cancer effects in gliomas [116]. Hence, Prdx6 may play pro-tumor roles in glioma development and could serve as a potential therapeutic target for gliomas.
5.10. Epilepsy
Epilepsy is a chronic neurological disease caused by abnormal paroxysmal neuronal discharges, with cognitive impairment and potential risk of dementia [117]. Proteomic profiling showed increased levels of prdx6 in hippocampuses of patients with mesial temporal lobe epilepsy. But, reduced Prdx6 expression was observed in childhood cortical dysplasia (CCD) patients, a common cause of childhood seizures [118]. A similar tendency of prdx6 expression was found in stargazer (stg) mutant mice, which exhibited several neurological disorders including spontaneous absence seizures [119]. Recent studies indicated that Prdx6 upregulation induced by specificity protein 1 (Sp1) in epileptic hippocampuses may act as aiPLA2 rather than GPx, leading to autophagic astroglial degeneration [120]. Furthermore, the increased aiPLA2 activity of Prdx6 may abrogate GPx1-mediated glutamine synthase (GS) preservation and lead to extended seizure duration due to impaired glutamate–glutamine conversion regulated by GS [121]. These studies suggested that Prdx6 plays an important role in the pathogenesis of epilepsy. Our unpublished studies also showed that the Prdx6 protein was increased in epileptic hippocampus astrocytes, and the knockdown of astrocytic prdx6 relieved neuronal damage via regulating connexin-43-mediated hemichannel activity.
6. Conclusions
Prdx6 is a multifunctional enzyme that exerts different effects in CNS diseases. Several studies have shown that Prdx6 is associated with oxidative stress, phospholipid homeostasis, and redox balance. At the same time, Prdx6 can exacerbate or attenuate neuronal damage during disease processes. Therefore, Prdx6 may represent a potential therapeutic agent and target for CNS diseases. Under certain conditions, one or more enzymes have been considered to be the contributing factors in particular diseases. The current research on Prdx6 faces the following challenges: first, Prdx6 has multiple enzyme activities, and the specific enzyme involved in different diseases should be considered. Second, Prdx6 plays diverse roles in various CNS diseases, capable of either protecting or causing damage to the nervous system. Therefore, it may play contradictory roles in different models of the same disease, which makes it more difficult and complex to study the function of Prdx6 in CNS disorders. In addition, Prdx6 may function by interacting with other proteins, rather than relying on its enzyme activity. Further studies are needed to identify the Prdx6-interacting proteins and elucidate their regulatory mechanisms.
Although previous studies about the roles of Prdx6 in brain diseases are somewhat controversial, Prdx6 appears to work primarily via Gpx and aiPLA2 activities to reduce oxidative stress. The human CNS, especially the brain, is extremely sensitive to changes in blood oxygen levels, so it is crucial to study CNS diseases that involve oxidative stress, such as acute brain injury and ischemic stroke. Simultaneously, it can be used as a specific indicator for prognosis in brain diseases. Additional studies are still needed to explore whether Prdx6 can be used as a neuroprotective agent in CNS diseases. Importantly, it is essential to understand the possible roles and underlying mechanisms of Prdx6.
Here, we summarized the studies about CNS diseases and Prdx6 that have been identified over the past two decades, hoping to contribute to future research on this topic. However, due to the specificity of the enzyme activity of the Prdx6, there is a gap in our understanding of it, especially the issue of the signal transduction pathway between the diseases and the protein. Therefore, Prdx6 might represent a potential and alternative target for therapeutic intervention in brain diseases.
Author Contributions
Writing—original draft preparation, M.X. and X.H.; writing—review and editing, Y.S., T.Z., L.Z. and H.Y.; funding acquisition, L.F. and M.X. All authors have read and agreed to the published version of the manuscript.
Funding
This review article was supported by grants from the National Natural Science Foundation of China (grant no. 81973337); the Research Fund of Anhui Institute of translational medicine (grant no. 2023zhyx-C01); the Key Project of University Excellent Talents Support Program in Anhui (grant no. gxyqZD2020010); the Scientific Research Level Promotion Project of Anhui Medical University (grant no. 2020xkjT002); and the Scientific Research Foundation of Anhui Medical University (grant no. 2023xkj001).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Peshenko, I.V.; Novoselov, V.I.; Evdokimov, V.A.; Nikolaev Yu, V.; Shuvaeva, T.M.; Lipkin, V.M.; Fesenko, E.E. Novel 28-kDa secretory protein from rat olfactory epithelium. FEBS. Lett. 1996, 381, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Shichi, H.; Demar, J.C. Non-selenium glutathione peroxidase without glutathione S-transferase activity from bovine ciliary body. Exp. Eye. Res. 1990, 50, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Dodia, C.; Feinstein, S.I.; Jain, M.K.; Fisher, A.B. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J. Biol. Chem. 2000, 275, 28421–28427. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal. 2011, 15, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Sorokina, E.M.; Li, H.; Zhou, S.; Raabe, T.; Feinstein, S.I. A novel lysophosphatidylcholine acyl transferase activity is expressed by peroxiredoxin 6. J. Lipid Res. 2016, 57, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, J.E.; Diaz, J.R.; Martá-Ariza, M.; Lizińczyk, A.M.; Franco, L.A.; Sadowski, M.J. Peroxiredoxin 6 mediates protective function of astrocytes in Aβ proteostasis. Mol. Neurodegener. 2020, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.J.; Park, M.H.; Son, D.J.; Kim, J.Y.; Nam, K.T.; Hyun, B.K.; Kim, S.Y.; Jung, M.H.; Song, M.J.; Chun, H.O.; et al. PRDX6 Inhibits Neurogenesis through Downregulation of WDFY1-Mediated TLR4 Signal. Mol. Neurobiol. 2019, 56, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, H.; Herojit, K.; Singh, L.R.; Haobam, R.; Fisher, A.B. Structural and Functional Diversity of the Peroxiredoxin 6 Enzyme Family. Antioxid. Redox Signal. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, R.K.; Rahaman, H.; Singh, L.R. Structural basis of peroxidase catalytic cycle of human Prdx6. Sci. Rep. 2020, 10, 17416. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Goncharov, R.G.; Parfenyuk, S.B.; Glushkova, O.V.; Novoselov, V.I. The Role of Phospholipase Activity of Peroxiredoxin 6 in Its Transmembrane Transport and Protective Properties. Int. J. Mol. Sci. 2022, 23, 15265. [Google Scholar] [CrossRef] [PubMed]
- Shahnaj, S.; Potshangbam, A.M.; Chowhan, R.K.; Parray, Z.A.; Kakchingtabam, P.; Kumari, A.; Islam, A.; Khan, A.; Singh, L.R.; Rahaman, H. The anti-oxidant enzyme, Prdx6 might have cis-acting regulatory sequence(s). Int. J. Biol. Macromol. 2020, 149, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Sureshan, M.; Prabhu, D.; Aruldoss, I.; Saraboji, K. Potential inhibitors for peroxiredoxin 6 of W. bancrofti: A combined study of modelling, structure-based drug design and MD simulation. J. Mol. Graph. Model. 2022, 112, 108115. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, R.K.; Hotumalani, S.; Rahaman, H.; Singh, L.R. pH induced conformational alteration in human peroxiredoxin 6 might be responsible for its resistance against lysosomal pH or high temperature. Sci. Rep. 2021, 11, 9657. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Chen, C.; Li, S.; Zhang, Z.; Xu, H.; Zhu, F.; Liu, J.; Spencer, P.S.; Dai, Z.; et al. Proteomic Profile of Mouse Brain Aging Contributions to Mitochondrial Dysfunction, DNA Oxidative Damage, Loss of Neurotrophic Factor, and Synaptic and Ribosomal Proteins. Oxidative Med. Cell. Longev. 2020, 2020, 5408452. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Chhunchha, B.; Fatma, N.; Kubo, E.; Singh, S.P.; Singh, D.P. Delivery of a protein transduction domain-mediated Prdx6 protein ameliorates oxidative stress-induced injury in human and mouse neuronal cells. Am. J. Physiol. Cell Physiol. 2016, 310, C1–C16. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Antioxidants Special Issue: Peroxiredoxin 6 as a Unique Member of the Peroxiredoxin Family. Antioxidants 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Derewenda, Z.S.; Sharp, A.M. News from the interface: The molecular structures of triacylglyceride lipases. Trends Biochem. Sci. 1993, 18, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Paluchova, V.; Cajka, T.; Durand, T.; Vigor, C.; Dodia, C.; Chatterjee, S.; Fisher, A.B.; Kuda, O. The role of peroxiredoxin 6 in biosynthesis of FAHFAs. Free Radic. Biol. Med. 2022, 193, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef] [PubMed]
- Shahnaj, S.; Chowhan, R.K.; Meetei, P.A.; Kakchingtabam, P.; Herojit Singh, K.; Rajendrakumar Singh, L.; Nongdam, P.; Fisher, A.B.; Rahaman, H. Hyperoxidation of Peroxiredoxin 6 Induces Alteration from Dimeric to Oligomeric State. Antioxidants 2019, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Bidooki, S.H.; Sánchez-Marco, J.; Martínez-Beamonte, R.; Herrero-Continente, T.; Navarro, M.A.; Rodríguez-Yoldi, M.J.; Osada, J. Endoplasmic Reticulum Protein TXNDC5 Interacts with PRDX6 and HSPA9 to Regulate Glutathione Metabolism and Lipid Peroxidation in the Hepatic AML12 Cell Line. Int. J. Mol. Sci. 2023, 24, 17131. [Google Scholar] [CrossRef] [PubMed]
- Villar, S.F.; Ferrer-Sueta, G.; Denicola, A. The multifaceted nature of peroxiredoxins in chemical biology. Curr. Opin. Chem. Biol. 2023, 76, 102355. [Google Scholar] [CrossRef] [PubMed]
- Howie, J.; Tulloch, L.B.; Brown, E.; Reilly, L.; Ashford, F.B.; Kennedy, J.; Wypijewski, K.J.; Aughton, K.L.; Mak, J.K.C.; Shattock, M.J.; et al. Glutathione-dependent depalmitoylation of phospholemman by peroxiredoxin 6. Cell Rep. 2024, 43, 113679. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Sundaresh, C.S.; Feinstein, S.I.; Dodia, C.; Skach, W.R.; Jain, M.K.; Nagase, T.; Seki, N.; Ishikawa, K.; Nomura, N.; et al. Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J. Biol. Chem. 1997, 272, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Dong, C.; Li, B. Anti-Oxidant and Pro-Oxidant Effects of Peroxiredoxin 6: A Potential Target in Respiratory Diseases. Cells 2023, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dodia, C.; Feinstein, S.I.; Harper, S.; Forman, H.J.; Speicher, D.W.; Fisher, A.B. Oxidation of Peroxiredoxin 6 in the Presence of GSH Increases its Phospholipase A₂ Activity at Cytoplasmic pH. Antioxidants 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Chatterjee, S. A Peptide Inhibitor of Peroxiredoxin 6 Phospholipase A(2) Activity Significantly Protects against Lung Injury in a Mouse Model of Ventilator Induced Lung Injury (VILI). Antioxidants 2021, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Qausain, S.; Khan, F.I.; Khan, M.K.A. Conserved acidic second shell residue modulates the structure, stability and activity of non-seleno human peroxiredoxin 6. Int. J. Biol. Macromol. 2023, 242, 124796. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Benipal, B.; Zhou, S.; Dodia, C.; Chatterjee, S.; Tao, J.Q.; Sorokina, E.M.; Raabe, T.; Feinstein, S.I.; Fisher, A.B. Critical role of peroxiredoxin 6 in the repair of peroxidized cell membranes following oxidative stress. Free Radic. Biol. Med. 2015, 87, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Wahlig, S.; Lovatt, M.; Mehta, J.S. Functional role of peroxiredoxin 6 in the eye. Free Radic. Biol. Med. 2018, 126, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Shi, Y.; Zhang, H.; Zhangyuan, G.; Wang, F.; Li, S.; Chen, C.; Zhang, J.; Wang, H.; Zhang, W.; et al. A TNFα/Miz1-positive feedback loop inhibits mitophagy in hepatocytes and propagates non-alcoholic steatohepatitis. J. Hepatol. 2023, 79, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Shi, J.X.; Shi, S.L.; Liu, F.; Rui, G.; Li, X.; Gao, L.B.; Deng, X.L.; Li, Q.F. Nucleophosmin Regulates Intracellular Oxidative Stress Homeostasis via Antioxidant PRDX6. J. Cell. Biochem. 2017, 118, 4697–4707. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Agrawal, S.K. Curcumin alleviates oxidative stress and mitochondrial dysfunction in astrocytes. Neuroscience 2016, 333, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Clock Protein Bmal1 and Nrf2 Cooperatively Control Aging or Oxidative Response and Redox Homeostasis by Regulating Rhythmic Expression of Prdx6. Cells 2020, 9, 1861. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Sulforaphane-Induced Klf9/Prdx6 Axis Acts as a Molecular Switch to Control Redox Signaling and Determines Fate of Cells. Cells 2019, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. The phospholipase A(2) activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef]
- Patel, P.; Chatterjee, S. Peroxiredoxin6 in Endothelial Signaling. Antioxidants 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Goemaere, J.; Knoops, B. Peroxiredoxin distribution in the mouse brain with emphasis on neuronal populations affected in neurodegenerative disorders. J. Comp. Neurol. 2012, 520, 258–280. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.Y.; Kim, H.S.; Kim, E.K.; Choi, J.H. Expression of peroxiredoxin 1, 2, and 6 in the rat brain during perinatal development and in response to dexamethasone. Free Radic. Res. 2012, 46, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Wang, J.; Guo, X.; Song, L. Expressions of peroxiredoxin 1, peroxiredoxin 6 and GFAP in human brain astrocytoma and their clinical significance. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2012, 32, 1255–1259. [Google Scholar]
- Power, J.H.; Asad, S.; Chataway, T.K.; Chegini, F.; Manavis, J.; Temlett, J.A.; Jensen, P.H.; Blumbergs, P.C.; Gai, W.P. Peroxiredoxin 6 in human brain: Molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta Neuropathol. 2008, 115, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Kim, E.C.; Hong, J.T. PRDX6 controls multiple sclerosis by suppressing inflammation and blood brain barrier disruption. Oncotarget 2015, 6, 20875–20884. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Lu, A.; Craessaerts, K.; Pavie, B.; Sala Frigerio, C.; Corthout, N.; Qian, X.; Laláková, J.; Kühnemund, M.; Voytyuk, I.; et al. Spatial Transcriptomics and In Situ Sequencing to Study Alzheimer’s Disease. Cell 2020, 182, 976–991.e919. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Nayak, S.; Faustin, A.; Pires, G.; Hickman, R.A.; Askenazi, M.; Cohen, M.; Haldiman, T.; Kim, C.; Han, X.; et al. Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer’s disease. Acta Neuropathol. 2017, 133, 933–954. [Google Scholar] [CrossRef] [PubMed]
- Panahzadeh, F.; Mirnasuri, R.; Rahmati, M. Exercise and Syzygium aromaticum reverse memory deficits, apoptosis and mitochondrial dysfunction of the hippocampus in Alzheimer’s disease. J. Ethnopharmacol. 2022, 286, 114871. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, L.; Simon, S.L.R.; Waitt, B.; Knox, J.D. Proteomic Screen of Brain Glycoproteome Reveals Prion Specific Marker of Pathogenesis. Proteomics 2018, 18, 1700296. [Google Scholar] [CrossRef] [PubMed]
- Krapfenbauer, K.; Engidawork, E.; Cairns, N.; Fountoulakis, M.; Lubec, G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003, 967, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Shults, N.V.; Gychka, S.G.; Harris, B.T.; Suzuki, Y.J. Protein Expression of Angiotensin-Converting Enzyme 2 (ACE2) is Upregulated in Brains with Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Lai, Y.; Shelat, P.B.; Hu, C.; Sun, G.Y.; Lee, J.C. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 11111–11119. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Yun, H.M.; Hwang, C.J.; Park, S.I.; Han, S.B.; Hwang, D.Y.; Yoon, D.Y.; Kim, S.; Hong, J.T. Presenilin Mutation Suppresses Lung Tumorigenesis via Inhibition of Peroxiredoxin 6 Activity and Expression. Theranostics 2017, 7, 3624–3637. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Jin, P.; Han, J.Y.; Lee, M.S.; Han, S.B.; Oh, K.W.; Hong, S.H.; Jung, E.Y.; Hong, J.T. Acceleration of the development of Alzheimer’s disease in amyloid beta-infused peroxiredoxin 6 overexpression transgenic mice. Mol. Neurobiol. 2013, 48, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Jin, P.; Park, K.R.; Hwang, J.; Jeong, H.S.; Kim, E.C.; Jung, J.K.; Oh, K.W.; Hwang, B.Y.; Han, S.B.; et al. Thiacremonone Potentiates Anti-Oxidant Effects to Improve Memory Dysfunction in an APP/PS1 Transgenic Mice Model. Mol. Neurobiol. 2016, 53, 2409–2420. [Google Scholar] [CrossRef] [PubMed]
- Yata, K.; Oikawa, S.; Sasaki, R.; Shindo, A.; Yang, R.; Murata, M.; Kanamaru, K.; Tomimoto, H. Astrocytic neuroprotection through induction of cytoprotective molecules; a proteomic analysis of mutant P301S tau-transgenic mouse. Brain Res. 2011, 1410, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Correani, V.; Di Francesco, L.; Cera, I.; Mignogna, G.; Giorgi, A.; Mazzanti, M.; Fumagalli, L.; Fabrizi, C.; Maras, B.; Schininà, M.E. Reversible redox modifications in the microglial proteome challenged by beta amyloid. Mol. Biosyst. 2015, 11, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Lee, K.J.; Rhee, S.; Seo, S.B.; Pak, J.H. Protective effects of peroxiredoxin 6 overexpression on amyloid β-induced apoptosis in PC12 cells. Free Radic. Res. 2013, 47, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, R.P.; Cheng, W.H.; Zhu, J.H. Prioritized brain selenium retention and selenoprotein expression: Nutritional insights into Parkinson’s disease. Mech. Ageing Dev. 2019, 180, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Power, J.H.; Shannon, J.M.; Blumbergs, P.C.; Gai, W.P. Nonselenium glutathione peroxidase in human brain: Elevated levels in Parkinson’s disease and dementia with lewy bodies. Am. J. Pathol. 2002, 161, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Connelly, E.M.; Frankel, K.S.; Shaw, G.S. Parkin and mitochondrial signalling. Cell. Signal. 2023, 106, 110631. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, H.A.; Al Moutaery, K.; Al Deeb, S. Protective effect of quinacrine on striatal dopamine levels in 6-OHDA and MPTP models of Parkinsonism in rodents. Brain Res. Bull. 2001, 54, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Choi, D.Y.; Oh, K.W.; Hong, J.T. PRDX6 Exacerbates Dopaminergic Neurodegeneration in a MPTP Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2015, 52, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Xu, J.; Jensen, M.D.; Simonyi, A. Phospholipase A2 in the central nervous system: Implications for neurodegenerative diseases. J. Lipid Res. 2004, 45, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, X.; Zheng, L.; Li, Z.; Zhao, X.; Lai, W.; Shen, H.; Lv, J.; Yang, G.; Wang, Q.; et al. Peroxiredoxin 6 Is a Crucial Factor in the Initial Step of Mitochondrial Clearance and Is Upstream of the PINK1-Parkin Pathway. Antioxid. Redox Signal. 2016, 24, 486–501. [Google Scholar] [CrossRef]
- Davison, E.J.; Pennington, K.; Hung, C.C.; Peng, J.; Rafiq, R.; Ostareck-Lederer, A.; Ostareck, D.H.; Ardley, H.C.; Banks, R.E.; Robinson, P.A. Proteomic analysis of increased Parkin expression and its interactants provides evidence for a role in modulation of mitochondrial function. Proteomics 2009, 9, 4284–4297. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B. The global burden of stroke: Persistent and disabling. Lancet Neurol. 2019, 18, 417–418. [Google Scholar] [CrossRef]
- Ma, R.; Xie, Q.; Li, Y.; Chen, Z.; Ren, M.; Chen, H.; Li, H.; Li, J.; Wang, J. Animal models of cerebral ischemia: A review. Biomed. Pharmacother. 2020, 131, 110686. [Google Scholar] [CrossRef]
- Choi, T.M.; Yun, M.; Lee, J.K.; Park, J.T.; Park, M.S.; Kim, H.S. Proteomic Analysis of a Rat Cerebral Ischemic Injury Model after Human Cerebral Endothelial Cell Transplantation. J. Korean Neurosurg. Soc. 2016, 59, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Sidyakin, A.A.; Kaysheva, A.L.; Kopylov, A.T.; Lobanov, A.V.; Morozov, S.G. Proteomic Analysis of Cerebral Cortex Extracts from Sus scrofa with Induced Hemorrhagic Stroke. J. Mol. Neurosci. MN 2018, 65, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, P.K.; McAlonan, G.M.; Chung, S.S.; Chung, S.K. Transgenic mice over-expressing endothelial endothelin-1 show cognitive deficit with blood-brain barrier breakdown after transient ischemia with long-term reperfusion. Neurobiol. Learn. Mem. 2013, 101, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Ni, W.; Zhou, J.; Ling, Y.; Niu, D.; Lu, X.; Chen, T.; Ramalingam, M.; Hu, J. Peroxiredoxin 6 secreted by Schwann-like cells protects neuron against ischemic stroke in rats via PTEN/PI3K/AKT pathway. Tissue Cell 2021, 73, 101635. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Liu, H.; Zhou, J.; Liu, Z.; Yang, Y.; Peng, Y.; You, H.; Yang, D.; Xie, P. Ipsilateral hippocampal proteomics reveals mitochondrial antioxidative stress impairment in cortical-lesioned chronic mild stressed rats. Curr. Mol. Med. 2014, 14, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Tan, B.; Ma, J.; Zhang, L.; Jin, X.; Li, C. Prdx6 Upregulation by Curcumin Attenuates Ischemic Oxidative Damage via SP1 in Rats after Stroke. BioMed Res. Int. 2017, 2017, 6597401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Zhao, Y.; Guo, N.; Han, C.; Wu, Q.; Mu, C.; Zhang, Y.; Tan, S.; Zhang, J.; et al. Systematic review of melatonin in cerebral ischemia-reperfusion injury: Critical role and therapeutic opportunities. Front. Pharmacol. 2024, 15, 1356112. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Cho, E.H.; Kim, M.O.; Koh, P.O. Identification of proteins differentially expressed by melatonin treatment in cerebral ischemic injury—A proteomics approach. J. Pineal Res. 2009, 46, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Ji, Y.; Li, Y.; You, Y.; Zhou, Y. PRDX6-iPLA2 aggravates neuroinflammation after ischemic stroke via regulating astrocytes-induced M1 microglia. Cell Commun. Signal. CCS 2024, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Shanshan, Y.; Beibei, J.; Li, T.; Minna, G.; Shipeng, L.; Li, P.; Yong, Z. Phospholipase A2 of Peroxiredoxin 6 Plays a Critical Role in Cerebral Ischemia/Reperfusion Inflammatory Injury. Front. Cell. Neurosci. 2017, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Zhou, Y.; Peng, L.; Wu, X.; Li, Y.; Li, Y.; Zhao, Y. Knocking Down Peroxiredoxin 6 Aggravates Cerebral Ischemia-Reperfusion Injury by Enhancing Mitophagy. Neuroscience 2022, 482, 30–42. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Dai, R.; Zhou, X.; Li, X.; Song, X.; Yan, H.; Meng, Q.; Yang, C.; Lin, Q. Protective effect of 4-Methoxy benzyl alcohol on the neurovascular unit after cerebral ischemia reperfusion injury. Biomed. Pharmacother. 2019, 118, 109260. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, J.; Wang, X.; Lei, S.; Wu, X.; Chen, Y.; Wu, J.; Zhao, Y. 4-Hydroxybenzyl alcohol confers neuroprotection through up-regulation of antioxidant protein expression. Neurochem. Res. 2013, 38, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Tian, L.; Liu, J.; Wu, Q.; Wang, N.; Wang, G.; Wang, Y.; Seto, S. Ligustilide ameliorates hippocampal neuronal injury after cerebral ischemia reperfusion through activating PINK1/Parkin-dependent mitophagy. Phytomed. Int. J. Phytother. Phytopharm. 2022, 101, 154111. [Google Scholar] [CrossRef] [PubMed]
- Shichita, T.; Hasegawa, E.; Kimura, A.; Morita, R.; Sakaguchi, R.; Takada, I.; Sekiya, T.; Ooboshi, H.; Kitazono, T.; Yanagawa, T.; et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012, 18, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, P.; Wang, J.; Ye, W.; Ge, X.; Rong, Y.; Ji, C.; Wang, Z.; Bai, J.; Fan, J.; et al. Extracellular vesicles derived from melatonin-preconditioned mesenchymal stem cells containing USP29 repair traumatic spinal cord injury by stabilizing NRF2. J. Pineal Res. 2021, 71, e12769. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, M.E.; Filous, A.R.; Vadala, C.P.; Webb, A.; Pietrzak, M.; Sahenk, Z.; Prüss, H.; Reiser, P.J.; Popovich, P.G.; Arnold, W.D.; et al. Lesion level-dependent systemic muscle wasting after spinal cord injury is mediated by glucocorticoid signaling in mice. Sci. Transl. Med. 2023, 15, eadh2156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, L.L.; Gao, X.; Jiang, D.; Zhong, Z.Q.; Zeng, X.; Rao, Y.; Hu, X.; Li, T.Z.; Li, X.J.; et al. Lentivirus-mediated inhibition of tumour necrosis factor-α improves motor function associated with PRDX6 in spinal cord contusion rats. Sci. Rep. 2015, 5, 8486. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Agrawal, S.K. Regulation of Prdx6 by Nrf2 Mediated Through aiPLA2 in White Matter Reperfusion Injury. Mol. Neurobiol. 2021, 58, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pacheco, V.; Vargas-Medrano, J.; Tran, E.; Nicolas, M.; Price, D.; Patel, R.; Tonarelli, S.; Gadad, B.S. Prognosis and Diagnostic Biomarkers of Mild Traumatic Brain Injury: Current Status and Future Prospects. J. Alzheimer’s Dis. JAD 2022, 86, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Buonora, J.E.; Rhind, S.G.; Hutchison, M.G.; Baker, A.J.; Rizoli, S.B.; Diaz-Arrastia, R.; Mueller, G.P. Blood Biomarkers in Moderate-To-Severe Traumatic Brain Injury: Potential Utility of a Multi-Marker Approach in Characterizing Outcome. Front. Neurol. 2015, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Buonora, J.E.; Yarnell, A.M.; Lazarus, R.C.; Mousseau, M.; Latour, L.L.; Rizoli, S.B.; Baker, A.J.; Rhind, S.G.; Diaz-Arrastia, R.; Mueller, G.P. Multivariate analysis of traumatic brain injury: Development of an assessment score. Front. Neurol. 2015, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Manevich, Y.; Hutchens, S.; Halushka, P.V.; Tew, K.D.; Townsend, D.M.; Jauch, E.C.; Borg, K. Peroxiredoxin VI oxidation in cerebrospinal fluid correlates with traumatic brain injury outcome. Free Radic. Biol. Med. 2014, 72, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Abu Hamdeh, S.; Shevchenko, G.; Mi, J.; Musunuri, S.; Bergquist, J.; Marklund, N. Proteomic differences between focal and diffuse traumatic brain injury in human brain tissue. Sci. Rep. 2018, 8, 6807. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Churchill, N.; Schweizer, T.A.; Rhind, S.G.; Richards, D.; Baker, A.J.; Hutchison, M.G. Blood biomarkers are associated with brain function and blood flow following sport concussion. J. Neuroimmunol. 2018, 319, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buonora, J.E.; Mousseau, M.; Jacobowitz, D.M.; Lazarus, R.C.; Yarnell, A.M.; Olsen, C.H.; Pollard, H.B.; Diaz-Arrastia, R.; Latour, L.; Mueller, G.P. Autoimmune Profiling Reveals Peroxiredoxin 6 as a Candidate Traumatic Brain Injury Biomarker. J. Neurotrauma 2015, 32, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Orge, L.; Lima, C.; Machado, C.; Tavares, P.; Mendonça, P.; Carvalho, P.; Silva, J.; Pinto, M.L.; Bastos, E.; Pereira, J.C.; et al. Neuropathology of Animal Prion Diseases. Biomolecules 2021, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Piñar-Morales, R.; Barrero-Hernández, F.; Aliaga-Martínez, L. Human prion diseases: An overview. Med. Clin. 2023, 160, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Ramosaço, E.; Bajrami, N.; Vyshka, G. A Theoretical Framework on the Biology of Prion Diseases. Acta Inform. Med. 2023, 31, 141–145. [Google Scholar] [PubMed]
- López-Pérez, Ó.; Badiola, J.J.; Bolea, R.; Ferrer, I.; Llorens, F.; Martín-Burriel, I. An Update on Autophagy in Prion Diseases. Front. Bioeng. Biotechnol. 2020, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Piconi, G.; Peden, A.H.; Barria, M.A.; Green, A.J.E. Epitope mapping of the protease resistant products of RT-QuIC does not allow the discrimination of sCJD subtypes. PLoS ONE 2019, 14, e0218509. [Google Scholar] [CrossRef] [PubMed]
- Asuni, A.A.; Guridi, M.; Sanchez, S.; Sadowski, M.J. Antioxidant peroxiredoxin 6 protein rescues toxicity due to oxidative stress and cellular hypoxia in vitro, and attenuates prion-related pathology in vivo. Neurochem. Int. 2015, 90, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Reuter, A.; Hüller, P.; Löwer, J.; Wessler, S. Peroxiredoxin 6 promotes upregulation of the prion protein (PrP) in neuronal cells of prion-infected mice. Cell Commun. Signal. CCS 2012, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Antel, J.; Kuhlmann, T. Inflammation in multiple sclerosis: Consequences for remyelination and disease progression. Nat. Rev. Neurol. 2023, 19, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.F.; Hoffman, B.M.; Tyor, W.R. CNS inflammatory demyelinating disorders: MS, NMOSD and MOG antibody associated disease. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2020, 68, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, A.; Mori, M.; Masuda, H.; Ohtani, R.; Uchida, T.; Aoki, R.; Kuwabara, S. Peroxiredoxins are involved in the pathogenesis of multiple sclerosis and neuromyelitis optica spectrum disorder. Clin. Exp. Immunol. 2020, 202, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Jagaraj, C.J.; Parakh, S.; Atkin, J.D. Emerging Evidence Highlighting the Importance of Redox Dysregulation in the Pathogenesis of Amyotrophic Lateral Sclerosis (ALS). Front. Cell. Neurosci. 2020, 14, 581950. [Google Scholar] [CrossRef] [PubMed]
- Maniatis, S.; Äijö, T.; Vickovic, S.; Braine, C.; Kang, K.; Mollbrink, A.; Fagegaltier, D.; Andrusivová, Ž.; Saarenpää, S.; Saiz-Castro, G.; et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 2019, 364, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tang, L.; Li, X.; Fan, F.; Liu, Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020, 476, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Kim, J.H.; Moon, M.J.; Jung, J.H.; Lim, S.Y.; Park, S.H.; Kim, J.H.; Kim, D.G.; Jung, H.W.; Cho, B.K.; et al. Investigation of molecular factors associated with malignant transformation of oligodendroglioma by proteomic study of a single case of rapid tumor progression. J. Cancer Res. Clin. Oncol. 2008, 134, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gollapalli, K.; Ghantasala, S.; Atak, A.; Rapole, S.; Moiyadi, A.; Epari, S.; Srivastava, S. Tissue Proteome Analysis of Different Grades of Human Gliomas Provides Major Cues for Glioma Pathogenesis. Omics J. Integr. Biol. 2017, 21, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Y.; Chen, X.; Zhang, J. The Roles of Peroxiredoxin 6 in Brain Diseases. Mol. Neurobiol. 2021, 58, 4348–4364. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, M. Comprehensive analysis of the expression levels and prognostic values of PRDX family genes in glioma. Neurochem. Int. 2022, 153, 105256. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Czech, T.; Felizardo, M.; Baumgartner, C.; Lubec, G. Aberrant expression of cytoskeleton proteins in hippocampus from patients with mesial temporal lobe epilepsy. Amino Acids 2006, 30, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, X.; Liu, S.; Liu, Y.; Yang, Y.; Yang, H.; Chen, Y.; Chen, L. Differentially expressed proteins underlying childhood cortical dysplasia with epilepsy identified by iTRAQ proteomic profiling. PLoS ONE 2017, 12, e0172214. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.J.; Lee, C.; Kim, J.; Shin, H.S.; Yu, M.H. Proteomic analysis of stargazer mutant mouse neuronal proteins involved in absence seizure. J. Neurochem. 2008, 104, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, D.S.; Kang, T.C. Sp1-Mediated Prdx6 Upregulation Leads to Clasmatodendrosis by Increasing Its aiPLA2 Activity in the CA1 Astrocytes in Chronic Epilepsy Rats. Antioxidants 2022, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Park, H.; Kang, T.C. Peroxiredoxin 6 Regulates Glutathione Peroxidase 1-Medited Glutamine Synthase Preservation in the Hippocampus of Chronic Epilepsy Rats. Antioxidants 2023, 12, 156. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).