Effects of Grape Pomace on Growth Performance, Nitrogen Metabolism, Antioxidants, and Microbial Diversity in Angus Bulls
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Management
2.2. Sample Collection
2.3. Chemical Analysis
2.3.1. Feed Analysis and Nutrient Digestibility
2.3.2. Blood Antioxidant Indicators
2.3.3. Rumen Fermentation Parameters and Feed Fatty Acids
2.3.4. Microbiota Analysis
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Nutrients’ Apparent Digestibility and Nitrogen Metabolism
3.3. Blood Antioxidants
3.4. Ruminal Fermentation Parameters
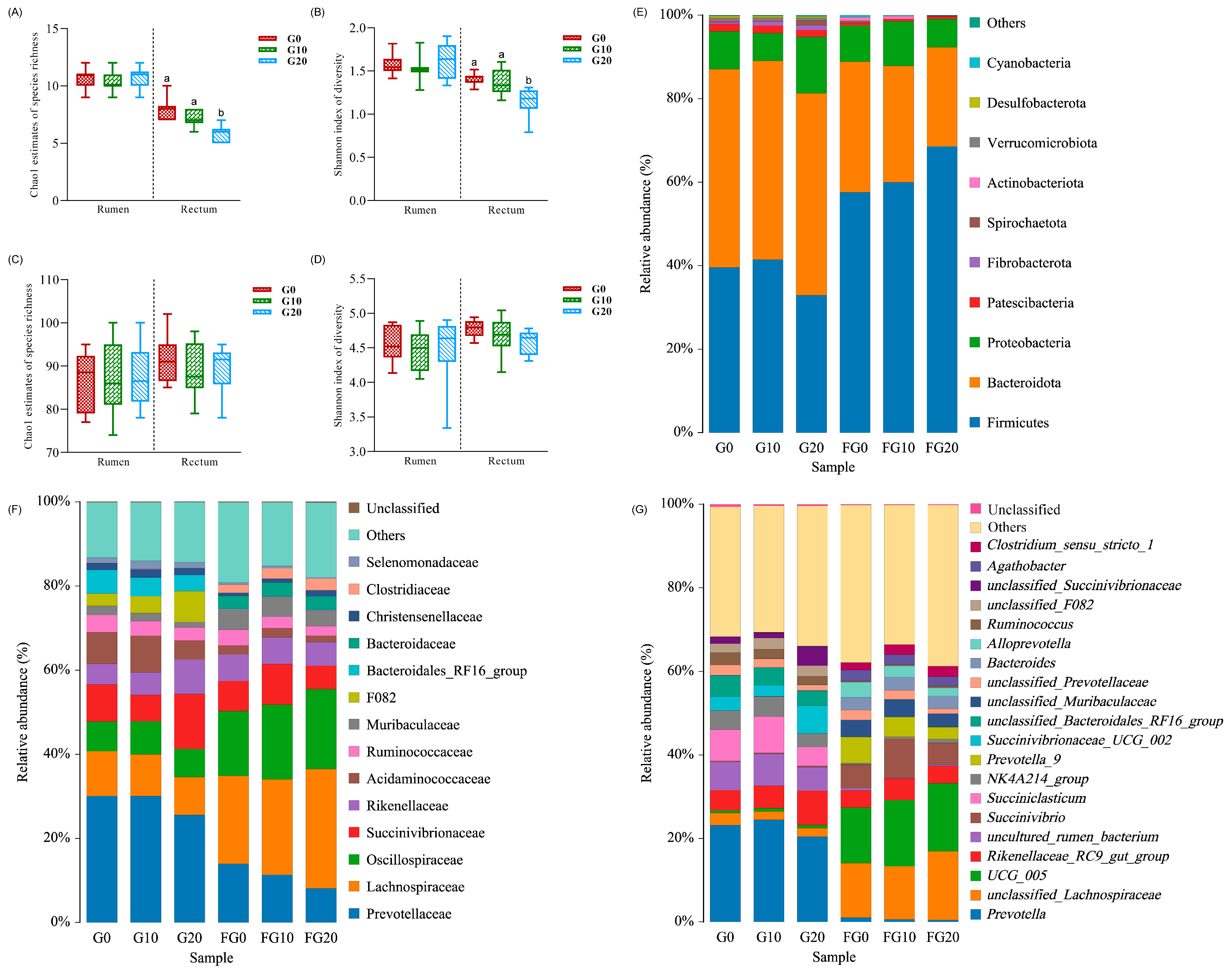
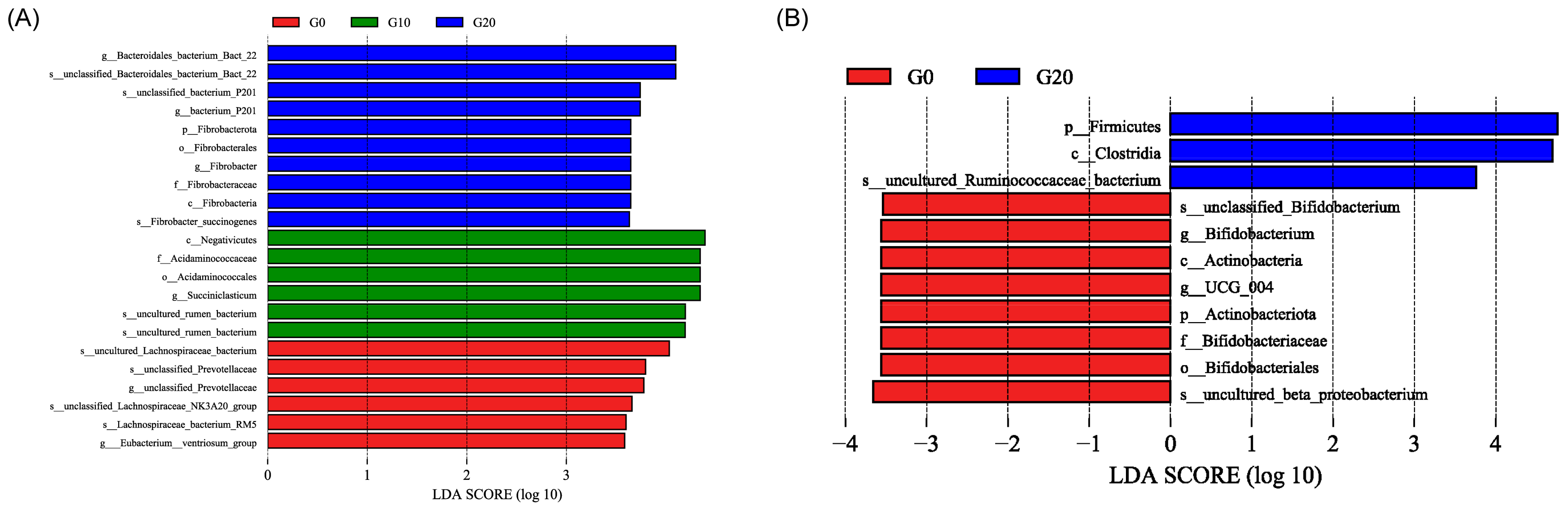
3.5. Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constantin, O.E.; Stoica, F.; Ratu, R.N.; Stanciuc, N.; Bahrim, G.E.; Rapeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.; Cabezudo, I.; Silva-James, N.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Martins Flores, D.R.; Franco Da Fonseca, P.A.; Schmitt, J.; Tonetto, C.J.; Rosado Junior, A.G.; Hammerschmitt, R.K.; Facco, D.B.; Brunetto, G.; Nornberg, J.L. Lambs fed with increasing levels of grape pomace silage: Effects on productive performance, carcass characteristics, and blood parameters. Livest. Sci. 2020, 240, 104169. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Ianni, A.; Luca, A.D.; Martino, C.; Bennato, F.; Marone, E.; Grotta, L.; Cichelli, A.; Martino, G. Dietary Supplementation of Dried Grape Pomace Increases the Amount of Linoleic Acid in Beef, Reduces the Lipid Oxidation and Modifies the Volatile Profile. Animals 2019, 9, 578. [Google Scholar] [CrossRef]
- Voicu, D.; Habeanu, M.; Uta, R.A.; Voicu, I.; Gras, M.A. Effect of the dietary dry grape pomace on the performance and health state of fattening steers. Scientific Papers, Series D. Anim. Sci. 2014, 57, 118–124. [Google Scholar]
- Tayengwa, T.; Chikwanha, O.C.; Dugan, M.E.R.; Mutsvangwa, T.; Mapiye, C. Influence of feeding fruit by-products as alternative dietary fibre sources to wheat bran on beef production and quality of Angus steers. Meat Sci. 2020, 161, 107969. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Reddy, P.R.K.; Elghandour, M.M.M.Y.; Salem, A.Z.M.; Yasaswini, D.; Reddy, P.P.R.; Reddy, A.N.; Hyder, I. Plant secondary metabolites as feed additives in calves for antimicrobial stewardship. Anim. Feed. Sci. Technol. 2020, 264, 114469. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy. Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Mattioli, A.U.; Teslic, N.; Kilmartin, P.A.; Versari, A. Antioxidant activity of commercial food grade tannins exemplified in a wine model. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1761–1774. [Google Scholar] [CrossRef]
- Zhao, J.X.; Li, Q.; Zhang, R.X.; Liu, W.Z.; Ren, Y.S.; Zhang, C.X.; Zhang, J.X. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs. Anim. Feed. Sci. Technol. 2018, 236, 76–85. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zuniga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livest. Sci. 2016, 189, 32–49. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: Effects in Caco-2 cells. Food Biosci. 2020, 35, 100607. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Kokka, E.; Kouka, P.; Terzopoulou, Z.; Gerasopoulos, K.; Stagos, D.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. 2017, 101, e108–e121. [Google Scholar] [CrossRef]
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy. Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef]
- Martin, K.R.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2010, 2, 1–12. [Google Scholar] [CrossRef]
- Vinyard, J.R.; Myers, C.A.; Murdoch, G.K.; Rezamand, P.; Chibisa, G.E. Optimum grape pomace proportion in feedlot cattle diets: Ruminal fermentation, total tract nutrient digestibility, nitrogen utilization, and blood metabolites. J. Anim. Sci. 2021, 99, skab044. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Beef Cattle, 8th ed.; National Academy Press: Washington, DC, USA, 2016. [Google Scholar]
- Li, Y.; Shi, C.; Deng, J.; Cui, Y.; Wang, L.; Zhang, S.; Jiang, W.; Wang, R.; Cao, B.; Su, H. Effects of grape pomace with different processing methods on growth performance, digestion and metabolism and economic benefit of beef cattle. Chin. J. Anim. Nutr. 2022, 34, 5891–5901. [Google Scholar]
- Wang, F. Establishment and Evaluation of Effective Energy Prediction Model in Feeds of Beef Cattle. Ph.D. Thesis, China Agriculture University, Beijing, China, 2016. [Google Scholar]
- Vansoest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Association, O.O.A.C.; Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975. [Google Scholar]
- Zhong, R.Z.; Li, J.G.; Gao, Y.X.; Tan, Z.L.; Ren, G.P. Effects of substitution of different levels of steam-flaked corn for finely ground corn on lactation and digestion in early lactation dairy cows. J. Dairy. Sci. 2008, 91, 3931–3937. [Google Scholar] [CrossRef]
- Silva, L.; Filho, S.; Chizzotti, M.L.; Rotta, P.P.; Braga, J. Creatinine excretion and relationship with body weight of Nellore cattle. Rev. Bras. Zootec. 2013, 41, 807–810. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Mirzaei-Aghsaghali, A.; Maheri-Sis, N.; Mansouri, H.; Razeghi, M.E.; Alipoor, K. Estimation of the nutritive value of grape pomace for ruminant using gas production technique. Afr. J. Biotechnol. 2011, 10, 6251–6256. [Google Scholar]
- Chikwanha, O.C.; Raffrenato, E.; Muchenje, V.; Musarurwa, H.T.; Mapiye, C. Varietal differences in nutrient, amino acid and mineral composition and in vitro rumen digestibility of grape (Vitis vinifera) pomace from the Cape Winelands vineyards in South Africa and impact of preservation techniques. Ind. Crops Prod. 2018, 118, 30–37. [Google Scholar] [CrossRef]
- Makmur, M.; Zain, M.; Sholikin, M.M.; Suharlina; Jayanegara, A. Modulatory effects of dietary tannins on polyunsaturated fatty acid biohydrogenation in the rumen: A meta-analysis. Heliyon 2022, 8, e09828. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Fitri, A.; Suwignyo, B.; Elfahmi; Hidayatik, N.; Kumalasari, N.R.; Irawan, A.; Jayanegara, A. The Utilisation of Tannin Extract as a Dietary Additive in Ruminant Nutrition: A Meta-Analysis. Animals 2021, 11, 3317. [Google Scholar] [CrossRef]
- Winkler, A.; Weber, F.; Ringseis, R.; Eder, K.; Dusel, G. Determination of polyphenol and crude nutrient content and nutrient digestibility of dried and ensiled white and red grape pomace cultivars. Arch. Anim. Nutr. 2015, 69, 187–200. [Google Scholar] [CrossRef]
- Abarghuei, M.J.; Rouzbehan, Y.; Alipour, D. The influence of the grape pomace on the ruminal parameters of sheep. Livest. Sci. 2010, 132, 73–79. [Google Scholar] [CrossRef]
- Fahey, G.C.; Mertens, D.R. Regulation of Forage Intake. In Forage Quality, Evaluation, and Utilization; Wiley Press: Hoboken, NJ, USA, 1994. [Google Scholar]
- Kansagara, Y.K.; Savsani, H.H.; Chavda, M.R.; Chavda, J.A.; Belim, S.Y.; Makwana, K.R.; Kansagara, B.K. Rumen microbiota and nutrient metabolism: A review. Bhartiya Krishi Anusandhan Patrika 2022, 37, 320–327. [Google Scholar] [CrossRef]
- Li, X.; Jiao, W.; Zhang, W.; Xu, Y.; Cao, J.; Jiang, W. Characterizing the Interactions of Dietary Condensed Tannins with Bile Salts. J. Agric. Food Chem. 2019, 67, 9543–9550. [Google Scholar] [CrossRef]
- Molosse, V.L.; Deolindo, G.L.; Lago, R.V.P.; Cecere, B.G.O.; Zotti, C.A.; Vedovato, M.; Copetti, P.M.; Fracasso, M.; Morsch, V.M.; Xavier, A.C.H.; et al. The effects of the inclusion of ensiled and dehydrated grape pomace in beef cattle diet: Growth performance, health, and economic viability. Anim. Feed. Sci. Technol. 2023, 302, 115671. [Google Scholar] [CrossRef]
- Grainger, C.; McGinn, S.M.; Clarke, T.; Eckard, R.J.; Beauchemin, K.A.; Auldist, M.J.; Waghorn, G.C. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef]
- De Klein, C.A.M.; Eckard, R.J. Targeted technologies for nitrous oxide abatement from animal agriculture. Aust. J. Exp. Agric. 2008, 48, 14–20. [Google Scholar] [CrossRef]
- Kebreab, E.; France, J.; Mills, J.; Allison, R.; Dijkstra, J. A dynamic model of N metabolism in the lactating dairy cow and an assessment of impact of N excretion on the environment. J. Anim. Sci. 2002, 80, 248–259. [Google Scholar] [CrossRef]
- Burke, F.; O’Donovan, M.A.; Murphy, J.J.; O’Mara, F.P.; Mulligan, F.J. Effect of pasture allowance and supplementation with maize silage and concentrates differing in crude protein concentration on milk production and nitrogen excretion by dairy cows. Livest. Sci. 2008, 114, 325–335. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, G. Potentials of using dietary plant secondary metabolites to mitigate nitrous oxide emissions from excreta of cattle: Impacts, mechanisms and perspectives. Anim. Nutr. 2022, 9, 327–334. [Google Scholar] [CrossRef]
- Wischer, G.; Greiling, A.; Boguhn, J.; Steingass, H.; Schollenberger, M.; Hartung, K.; Rodehutscord, M. Effects of long-term supplementation of chestnut and valonea extracts on methane release, digestibility and nitrogen excretion in sheep. Animal 2014, 8, 938–948. [Google Scholar] [CrossRef]
- Chibisa, G.E.; Ream, C.; Stevens, A. Effects of feeding ensiled or dried grape pomace on measures of nitrogen utilization in backgrounding cattle. J. Anim. Sci. 2020, 984, 428. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, Q.; Tian, T.; Chang, Y.; Li, Y.; Duan, L.; Li, H.; Wang, S. Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: Involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed. Pharmacother. 2020, 126, 110075. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- de Veras, B.O.; Da Silva, M.V.; Cabral Ribeiro, P.P. Tannic acid is a gastroprotective that regulates inflammation and oxidative stress. Food Chem. Toxicol. 2021, 156, 112482. [Google Scholar] [CrossRef]
- Alhosin, M.; Leon-Gonzalez, A.J.; Dandache, I.; Lelay, A.; Rashid, S.K.; Kevers, C.; Pincemail, J.; Fornecker, L.; Mauvieux, L.; Herbrecht, R.; et al. Bilberry extract (Antho 50) selectively induces redox-sensitive caspase 3-related apoptosis in chronic lymphocytic leukemia cells by targeting the Bcl-2/Bad pathway. Sci. Rep. 2015, 5, 8996. [Google Scholar] [CrossRef]
- Lovendahl, P.; Difford, G.F.; Li, B.; Chagunda, M.G.G.; Huhtanen, P.; Lidauer, M.H.; Lassen, J.; Lund, P. Review: Selecting for improved feed efficiency and reduced methane emissions in dairy cattle. Animal 2018, 122, S336–S349. [Google Scholar] [CrossRef]
- Mizrahi, I.; Jami, E. Review: The compositional variation of the rumen microbiome and its effect on host performance and methane emission. Animal 2018, 122, S220–S232. [Google Scholar] [CrossRef]
- Lin, L.; Xie, F.; Sun, D.; Liu, J.; Zhu, W.; Mao, S. Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model. Microbiome 2019, 7, 83. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, J.; Wang, H.; Degen, A.A.; Gou, N.; Li, S.; Bai, Y.; Shang, Z. The effects of supplementing sweet sorghum with grapeseeds on dry matter intake, average daily gain, feed digestibility and rumen parameters and microbiota in lambs. Anim. Feed. Sci. Technol. 2021, 272, 114750. [Google Scholar] [CrossRef]
- Pitta, D.W.; Pinchak, W.E.; Indugu, N.; Vecchiarelli, B.; Sinha, R.; Fulford, J.D. Metagenomic Analysis of the Rumen Microbiome of Steers with Wheat-Induced Frothy Bloat. Front. Microbiol. 2016, 7, 689. [Google Scholar] [CrossRef]
- Effects of brewers’ spent grain protein hydrolysates on gas production, ruminal fermentation characteristics, microbial protein synthesis and microbial community in an artificial rumen fed a high grain diet. J. Anim. Sci. Biotechnol. 2021, 12, 314–327.
- Li, L.; Cheng, S.; Diao, Q.; Fu, T.; Bi, Y.; Wang, A.; Li, M.; Tu, Y. Effects of diets with different NFC/NDF levels on the rumen fermentation parameters and bacterial community in male calves. Acta Vet. Zootech. Sin. 2017, 48, 2347–2357. [Google Scholar]
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Ariaeenejad, S.; Ding, X.; Han, J.; Salekdeh, G.H. Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses. Environ. Res. 2023, 229, 115925. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology—Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Sharma, V.; Smolin, J.; Nayak, J.; Ayala, J.E.; Scott, D.A.; Peterson, S.N.; Freeze, H.H. Mannose Alters Gut Microbiome, Prevents Diet-Induced Obesity, and Improves Host Metabolism. Cell Rep. 2018, 24, 3087–3098. [Google Scholar] [CrossRef]
- Ransom-Jones, E.; Jones, D.L.; McCarthy, A.J.; McDonald, J.E. The Fibrobacteres: An Important Phylum of Cellulose-Degrading Bacteria. Microb. Ecol. 2012, 63, 267–281. [Google Scholar] [CrossRef]
- Holman, D.B.; Gzyl, K.E. A meta-analysis of the bovine gastrointestinal tract microbiota. Fems Microbiol. Ecol. 2019, 95, fiz072. [Google Scholar] [CrossRef]
- Li, Q.S.; Wang, R.; Ma, Z.Y.; Zhang, X.M.; Jiao, J.Z.; Zhang, Z.G.; Ungerfeld, E.M.; Yi, K.L.; Zhang, B.Z.; Long, L.; et al. Dietary selection of metabolically distinct microorganisms drives hydrogen metabolism in ruminants. Isme J. 2022, 16, 2535–2546. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Nan, X.; Cai, M.; Yang, L.; Xiong, B.; Zhao, Y. Synergistic Effects of 3-Nitrooxypropanol with Fumarate in the Regulation of Propionate Formation and Methanogenesis in Dairy Cows In Vitro. Appl. Environ. Microbiol. 2022, 88, e0190821. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Lee, R.; Lu, Q.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.; Finegold, S.M.; et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Choy, Y.Y.; Quifer-Rada, P.; Holstege, D.M.; Frese, S.A.; Calvert, C.C.; Mills, D.A.; Lamuela-Raventos, R.M.; Waterhouse, A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 2014, 5, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Leth, M.L.; Pichler, M.J.; Abou Hachem, M. Butyrate-producing colonic clostridia: Picky glycan utilization specialists. Essays Biochem. 2023, 67, 415–428. [Google Scholar] [PubMed]
- Yu, Z.; Li, D.; Sun, H. Herba Origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms. Biomed. Pharmacother. 2023, 161, 114409. [Google Scholar] [CrossRef]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A Survey of Modulation of Gut Microbiota by Dietary Polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]


| Items | G0 | G10 | G20 |
|---|---|---|---|
| Ingredient composition | |||
| Corn silage | 26.08 | 13.05 | 0.00 |
| Rice straw | 11.61 | 11.61 | 11.60 |
| DGP | 0.00 | 10.44 | 20.88 |
| Ground corn | 39.91 | 46.93 | 53.69 |
| Rapeseed meal | 7.22 | 6.69 | 6.49 |
| Soybean meal | 2.16 | 2.17 | 2.14 |
| Wheat bran | 7.82 | 3.91 | 0.00 |
| NaHCO3 | 1.11 | 1.11 | 1.11 |
| NaCl | 0.37 | 0.37 | 0.37 |
| MgO | 0.44 | 0.44 | 0.44 |
| Premix 1 | 3.28 | 3.28 | 3.28 |
| Total | 100 | 100 | 100 |
| Items | DCP 1 | G0 | G10 | G20 |
|---|---|---|---|---|
| Chemical composition | ||||
| CP; % | 11.69 | 11.89 | 11.44 | 12.02 |
| EE; % | 9.80 | 2.13 | 2.28 | 2.39 |
| NDF; % | 42.72 | 26.69 | 27.83 | 29.58 |
| ADF; % | 40.65 | 14.38 | 16.49 | 20.06 |
| ADL; % | 21.24 | 2.43 | 3.88 | 7.88 |
| Ash; % | 11.11 | 8.98 | 9.79 | 10.92 |
| TT; g/kg | 7.98 | 1.46 | 1.71 | 1.86 |
| CT; g/kg | 2.49 | 1.55 | 1.74 | 1.84 |
| TDN 2; %DM | 65.08 | 74.89 | 74.20 | 73.12 |
| DE 2; MJ/kg DM | 9.55 | 12.51 | 12.30 | 11.98 |
| Fatty acids (g/100 g of total fatty acid) | ||||
| 14:0 | 0.06 | 0.08 | 0.07 | 0.07 |
| 16:0 | 7.42 | 9.11 | 8.95 | 8.62 |
| 18:0 | 3.47 | 0.93 | 1.75 | 1.82 |
| 18:1n-9 | 10.99 | 12.89 | 12.93 | 12.97 |
| 18:2n-6 | 55.93 | 28.16 | 34.31 | 37.05 |
| Items | G0 | G10 | G20 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|
| Initial live weights; kg | 580.40 | 580.40 | 580.90 | 7.800 | 1.000 | 0.980 | 0.988 |
| Final live weights; kg | 749.85 | 746.75 | 712.80 | 8.141 | 0.106 | 0.054 | 0.376 |
| ADG; kg | 1.47 a | 1.40 a | 1.14 b | 0.052 | 0.014 | 0.006 | 0.354 |
| FCR | 9.01 b | 9.37 b | 11.62 a | 0.427 | 0.015 | 0.007 | 0.267 |
| Items | G0 | G10 | G20 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|
| Apparent digestibility (%) | |||||||
| DM | 67.48 | 65.89 | 62.73 | 1.526 | 0.449 | 0.218 | 0.810 |
| CP | 67.01 | 60.80 | 59.93 | 1.760 | 0.206 | 0.105 | 0.470 |
| EE | 74.98 a | 73.81 a | 57.60 b | 2.658 | 0.007 | 0.005 | 0.134 |
| NDF | 50.43 | 48.23 | 44.71 | 2.073 | 0.541 | 0.276 | 0.883 |
| ADF | 43.64 | 43.04 | 39.01 | 2.189 | 0.659 | 0.405 | 0.721 |
| Nitrogen metabolism (g/d) | |||||||
| Nitrogen intake | 228.85 | 233.85 | 239.69 | - | - | - | - |
| Fecal nitrogen | 81.94 | 89.40 | 96.64 | 4.434 | 0.432 | 0.200 | 0.991 |
| Urinary nitrogen | 88.15 | 74.76 | 66.64 | 4.108 | 0.100 | 0.034 | 0.751 |
| Nitrogen retention | 66.18 | 72.10 | 83.36 | 6.160 | 0.540 | 0.288 | 0.840 |
| Nitrogen utilization (%) | 28.68 | 31.99 | 29.51 | 2.079 | 0.805 | 0.880 | 0.523 |
| Items | G0 | G10 | G20 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|
| CAT, U/mL | 13.22 b | 29.39 a | 7.25 b | 3.019 | 0.004 | 0.350 | 0.002 |
| T-AOC, Trolox Mm | 1.00 ab | 1.01 a | 0.96 b | 0.008 | 0.041 | 0.057 | 0.082 |
| SOD, U/mL | 13.77 | 14.34 | 14.06 | 0.260 | 0.681 | 0.654 | 0.456 |
| MDA, nmol/mL | 3.55 | 2.15 | 2.90 | 0.287 | 0.133 | 0.343 | 0.080 |
| ROS, IU/mL | 673.21 | 869.22 | 778.75 | 37.751 | 0.579 | 0.501 | 0.407 |
| Items | G0 | G10 | G20 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|
| pH | 6.36 | 6.57 | 6.42 | 0.048 | 0.181 | 0.573 | 0.080 |
| Ammonia-N; mg/dL | 5.09 | 4.63 | 4.04 | 0.216 | 0.177 | 0.087 | 0.872 |
| VFA; mmol/L | |||||||
| Acetate; mmol/L | 85.78 | 82.41 | 88.60 | 3.921 | 0.823 | 0.777 | 0.581 |
| Propionate; mmol/L | 26.69 | 26.52 | 21.55 | 1.225 | 0.151 | 0.087 | 0.348 |
| Isobutyrate; mmol/L | 0.94 | 0.96 | 1.11 | 0.051 | 0.332 | 0.179 | 0.537 |
| Butyrate; mmol/L | 16.67 | 14.73 | 15.69 | 0.904 | 0.698 | 0.670 | 0.468 |
| Isovalerate; mmol/L | 3.08 | 2.45 | 2.75 | 0.158 | 0.283 | 0.401 | 0.178 |
| Valerate; mmol/L | 1.58 | 1.36 | 1.26 | 0.064 | 0.121 | 0.046 | 0.650 |
| Acetate-to-propionate ratio | 3.27 b | 3.22 b | 4.27 a | 0.175 | 0.015 | 0.014 | 0.102 |
| Total VFA; mmol/L | 134.73 | 128.43 | 130.96 | 5.720 | 0.909 | 0.796 | 0.728 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Shi, C.; Deng, J.; Qiu, X.; Zhang, S.; Wang, H.; Qin, X.; He, Y.; Cao, B.; Su, H. Effects of Grape Pomace on Growth Performance, Nitrogen Metabolism, Antioxidants, and Microbial Diversity in Angus Bulls. Antioxidants 2024, 13, 412. https://doi.org/10.3390/antiox13040412
Li Y, Shi C, Deng J, Qiu X, Zhang S, Wang H, Qin X, He Y, Cao B, Su H. Effects of Grape Pomace on Growth Performance, Nitrogen Metabolism, Antioxidants, and Microbial Diversity in Angus Bulls. Antioxidants. 2024; 13(4):412. https://doi.org/10.3390/antiox13040412
Chicago/Turabian StyleLi, Yingqi, Changxiao Shi, Jiajie Deng, Xinjun Qiu, Siyu Zhang, Huili Wang, Xiaoli Qin, Yang He, Binghai Cao, and Huawei Su. 2024. "Effects of Grape Pomace on Growth Performance, Nitrogen Metabolism, Antioxidants, and Microbial Diversity in Angus Bulls" Antioxidants 13, no. 4: 412. https://doi.org/10.3390/antiox13040412
APA StyleLi, Y., Shi, C., Deng, J., Qiu, X., Zhang, S., Wang, H., Qin, X., He, Y., Cao, B., & Su, H. (2024). Effects of Grape Pomace on Growth Performance, Nitrogen Metabolism, Antioxidants, and Microbial Diversity in Angus Bulls. Antioxidants, 13(4), 412. https://doi.org/10.3390/antiox13040412







