Abstract
It has been suggested that silymarin (SIL) supplementation has positive effects on cardiovascular health and reduces the risk of cardiometabolic syndrome (CMS). This systematic review and dose–response meta-analysis assessed the impacts of SIL administration on cardiovascular risk factors. A systematic search of multiple databases was performed to identify eligible controlled trials published up to January 2023. The analysis used a random-effects model and included 33 trials with 1943 participants. It was revealed that SIL supplementation led to a notable reduction in serum levels of fasting blood glucose (FBG) (weighted mean difference (WMD): −21.68 mg/dL, 95% CI: −31.37, −11.99; p < 0.001), diastolic blood pressure (DBP) (WMD: −1.25 mmHg; 95% CI: −2.25, −0.26; p = 0.013), total cholesterol (TC) (WMD: −13.97 mg/dL, 95% CI: −23.09, −4.85; p = 0.003), triglycerides (TG) (WMD: −26.22 mg/dL, 95% CI: −40.32, −12.12; p < 0.001), fasting insulin (WMD: −3.76 mU/mL, 95% CI: −4.80, −2.72; p < 0.001), low-density lipoprotein (LDL) (WMD: −17.13 mg/dL, 95% CI: −25.63, −8.63; p < 0.001), and hemoglobin A1C (HbA1c) (WMD: −0.85%, 95% CI: −1.27, −0.43; p < 0.001) in the SIL-treated groups compared to their untreated counterparts. In addition, there were no substantial differences in body mass index (BMI), systolic blood pressure (SBP), C-reactive protein (CRP), body weight, and high-density lipoprotein (HDL) between the two groups. These outcomes suggest that SIL consumption reduces certain CMS risk factors and has favorable impacts on lipid and glycemic profiles with potential hypotensive effects. These findings should be supported by additional trials with larger sample sizes and longer durations.
1. Introduction
Cardiometabolic syndrome (CMS) is a group of risk factors or metabolic disorders that include a combination of systemic arterial hypertension, diabetes mellitus, hyperlipidemia, and central obesity [1]. These metabolic conditions are linked to the onset of atherosclerotic cardiovascular disease (ASCVD) [2,3]. Antidiabetic, antihypertensive, lipid-lowering, and anti-obesity medications are the main focus of routine CMS management [4]. There is a growing interest in complementary and alternative medicine (CAM), such as herbal medicine, to treat metabolic dysfunction due to the persistent degenerative nature and prolonged therapy of CMS, its potential adverse effects, and its considerable economic burden [5]. In addition, herbal medicine has received growing interest in developing better therapeutic strategies for secondary or primary prevention of cardiovascular diseases (CVDs) [6,7]. Evidence supports the beneficial effects of silymarin (SIL) as a medicinal herb on cardiometabolic risk factors [8,9,10] with anti-atherosclerotic actions [11].
Milk thistle (Silybum marianum (L.) Gaertn) is a spiny herb that has been used as a herbal remedy and functional food ingredient for centuries [12,13]. Dried milk thistle seeds were used to extract SIL [14]. Silymarin is a polyphenolic flavonoid with 20–35% fatty acids, 70–80% SIL flavonolignans, and several other polyphenolic ingredients [15]. Silibinin (silybin) is the most prevalent and biologically active flavonoid isomer of SIL [13]. It is most commonly used for medicinal purposes to treat or protect the liver from toxic substances [12,16,17,18]. Several studies have revealed the cardioprotective and anti-obesity properties of SIL, as well as the beneficial impacts of SIL on CMS and lipid profiles [11,19,20,21]. Most of its potential therapeutic effects are related to its antioxidative characteristics [22]. Dietary antioxidant supplements may exert modulatory effects on oxidative stress (OS) [23]. Numerous clinical trials and reviews have been conducted to confirm the antioxidant effects of SIL and the effectiveness of its administration for the treatment of several medical disorders [22,24,25,26,27].
Silymarin may have atheroprotective effects and reduce LDL oxidation, which is a stage in the progression of atherosclerosis [11,28]. It may enhance vascular function [29,30] and have cardioprotective effects [31,32]. It could increase high-density lipoprotein (HDL) levels, but its effect on total cholesterol levels is debatable [33,34]. Furthermore, it has the potential to reduce levels of fasting blood glucose (FBG), triglyceride (TG), blood pressure (BP), and body weight [33,34,35,36]. It may decrease overall OS levels with a specific focus on inhibiting the release of oxidants by the liver [35,37]. The hepatoprotective effects of SL may positively influence various metabolic parameters [11,38]. Specifically, SIL can inhibit the generation of inflammatory agents from the liver, which play a significant role in the development of CVDs [11,39]. Several systematic reviews and meta-analyses have documented the positive impact of SIL on metabolic status [26], type 2 diabetes mellitus (T2DM) [24,40], and the features of CMS in adults [9,41]. However, the impact of SIL supplements on cardiovascular health in various consumer or patient groups has not been thoroughly evaluated, and the results are inconclusive, contradictory, and inconsistent. Therefore, this systematic review and dose–response meta-analysis of trials assessed the impacts of supplementation with SIL on cardiovascular risk factors in adults.
2. Materials and Methods
The systematic review and meta-analysis protocol was registered in the international prospective register of systematic reviews (PROSPERO) with the registration number CRD42023488215. This procedure followed the guidelines outlined in the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist [42].
2.1. Search Strategy
One investigator employed a systematic search using a systematic approach to find potentially relevant studies published until January 2023 in several databases (Scopus, Cochrane Library, PubMed, and Web of Science). Two researchers (V.F. and Y.J.) independently assessed the articles and selected eligible randomized controlled trials (RCTs) with crossover or parallel designs. The search strategy was formulated based on four essential elements in RCTs, encompassing population (adult), intervention/exposure (SIL supplement), comparator/control (placebo or intervention), and outcomes (body mass index (BMI), diastolic blood pressure (DBP), body weight, systolic blood pressure (SBP), serum levels of low-density lipoprotein (LDL), fasting blood glucose (FBG), C-reactive protein (CRP), triglycerides (TG), hemoglobin A1C (HbA1c), total cholesterol (TC), fasting insulin, and high-density lipoprotein (HDL)). The search terms were as follows: (“silymarin” OR “silibinin” OR “silybin”) AND (“Intervention” OR “Intervention Study” OR “Intervention Studies” OR “controlled trial” OR “randomized” OR “randomised” OR “random” OR “randomly” OR “placebo” OR “clinical trial” OR “randomized controlled trial” OR “randomized clinical trial” OR “RCT” OR “blinded” OR “double blind” OR “double blinded” OR “trial” OR “trials” OR “Pragmatic Clinical Trial” OR “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR “parallel” OR “parallel study” OR “parallel trial”) AND (“lipid” OR “glycemic” OR “anthropometric” OR “body weight” OR “SBP” OR “DBP” OR “HbA1c” OR “LDL” OR “HDL” OR “TC” OR “FBG” OR “TG” OR “diastolic blood pressure” OR “systolic blood pressure” OR “body mass index” OR “fasting blood glucose” OR “insulin” OR “hemoglobin A1C” OR “low-density lipoprotein” OR “total cholesterol” OR “C-reactive protein” OR “CRP” OR “high-density lipoprotein” OR “low-density lipoprotein” OR “triglycerides”).
2.2. Eligibility Criteria
The researchers employed EndNote software (version 20) to facilitate the export of articles. Subsequently, two researchers (V.F. and Y.J.) independently checked the abstracts with the titles of the articles and extracted the pertinent data from the eligible full-text articles. Any discrepancies were addressed through collaborative discussions with a third researcher (D.A.L.). The current systematic review and meta-analysis encompassed all trials that investigated the efficacy of supplementation with SIL on body weight, BMI, SBP, DBP, serum levels of FBG, HbA1c, TG, fasting insulin, LDL, CRP, TC, and HDL in the SIL group compared to the placebo group.
Eligible RCTs enrolled adult participants; they had control or placebo groups and presented sufficient data on predetermined outcomes in the SIL-treated and untreated groups at the study endpoint and baseline. Furthermore, SIL was not administered as a multi-component supplement. Studies that did not have the following criteria were omitted from the analysis: uncontrolled trials; observational studies utilizing case–control, cross-sectional, or cohort designs; non-peer-reviewed articles or abstracts; studies that involved pregnant women or individuals under 18 years old; articles that did not report the impact of SIL administration on the specified outcomes compared to the control group; and studies with insufficient data on baseline or follow-up measurements of body weight, BMI, SBP, DBP, serum levels of HDL, fasting insulin, FBG, HbA1c, LDL, TC, TG, and CRP.
2.3. Data Extraction
Two researchers independently extracted the data (S.M. and Y.J.). Information regarding the study’s characteristics (e.g., study design, duration and location of the trial, sample size, first author’s name, publication year, and dosage of SIL supplement), participants’ demographic details (e.g., gender, average age, BMI, and comorbidities), initial and final measurements of specific outcomes, and mean differences in outcome changes between the study’s initial and final measurements. The main outcomes were body weight, BMI, SBP, DBP, and serum levels of TG, fasting insulin, HbA1c, LDL, CRP, FBG, TC, and HDL. In addition, two researchers resolved disagreements and discrepancies through discussions.
2.4. Quality Assessment
Two separate researchers (S.M. and D.A.L.) evaluated the quality of the trials using the Cochrane risk of bias (RoB 2) tool [43]. It determined potential sources of bias, including reporting bias, performance bias, attrition bias, detection bias, and allocation bias. The RoB for each domain was categorized as low, unclear, or high [43].
2.5. Statistical Analysis
This meta-analysis used Stata software (version 17). The impact of SIL was assessed through the calculation of weighted mean differences (WMDs) along with 95% confidence intervals (CIs) to measure the absolute changes in outcomes between the SIL and placebo groups from the study baseline to endpoints. The outcomes were quantified using the mean ± standard deviation (SD) measurement, and the effect size was identified by calculating the mean difference. The subsequent formula was employed to compute the SD change from the SD at the baseline of the trial to the SD at the end of the trial: square root ([SD2baseline + SD2final] − [2 × R × SDbaseline × SDfinal]) [44]. Furthermore, a random-effects model [45] was applied to determine the pooled WMDs. The between-study heterogeneity was assessed using the I2 statistic [46]; I2 values of 50%, 25%, and 75% were categorized as representing moderate, low, and high levels of heterogeneity, respectively [47].
Subgroup analyses were carried out to explore the potential factors contributing to the heterogeneity across trials. The analysis considered factors such as baseline values of serum levels of HDL, TC, FBG, TG, LDL, and CRP, as well as the dosage of SIL (≥400 mg/day vs. <400 mg/day), baseline BMI (obese (≥30 kg/m2) vs. overweight (25–29.9 kg/m2) vs. normal (18.5–24.9 kg/m2)), trial duration (<12 weeks vs. ≥12 weeks), and health status (healthy vs. unhealthy). A leave-one-out sensitivity analysis was applied to assess the effects of individual studies on the analysis. Statistical significance was considered at a level of p < 0.05. In addition, funnel plots and Begg’s [48] and Egger’s tests [49] were utilized to identify possible publication bias. Additionally, the fractional polynomial model was employed to investigate the potential non-linear effects of the duration of the trial (weeks) and the dosage of SIL (mg/day). Meta-regression analysis was implemented to assess the dose–response relationship and potential linear associations between duration trials, SIL dosage, and effect sizes [50].
2.6. GARDE Assessment
The certainty of the evidence was evaluated using the GRADE framework (Grading of Recommendations Assessment, Development, and Evaluation) categorized into four levels (moderate, high, very low, and low) [51].
3. Results
3.1. Study Selection
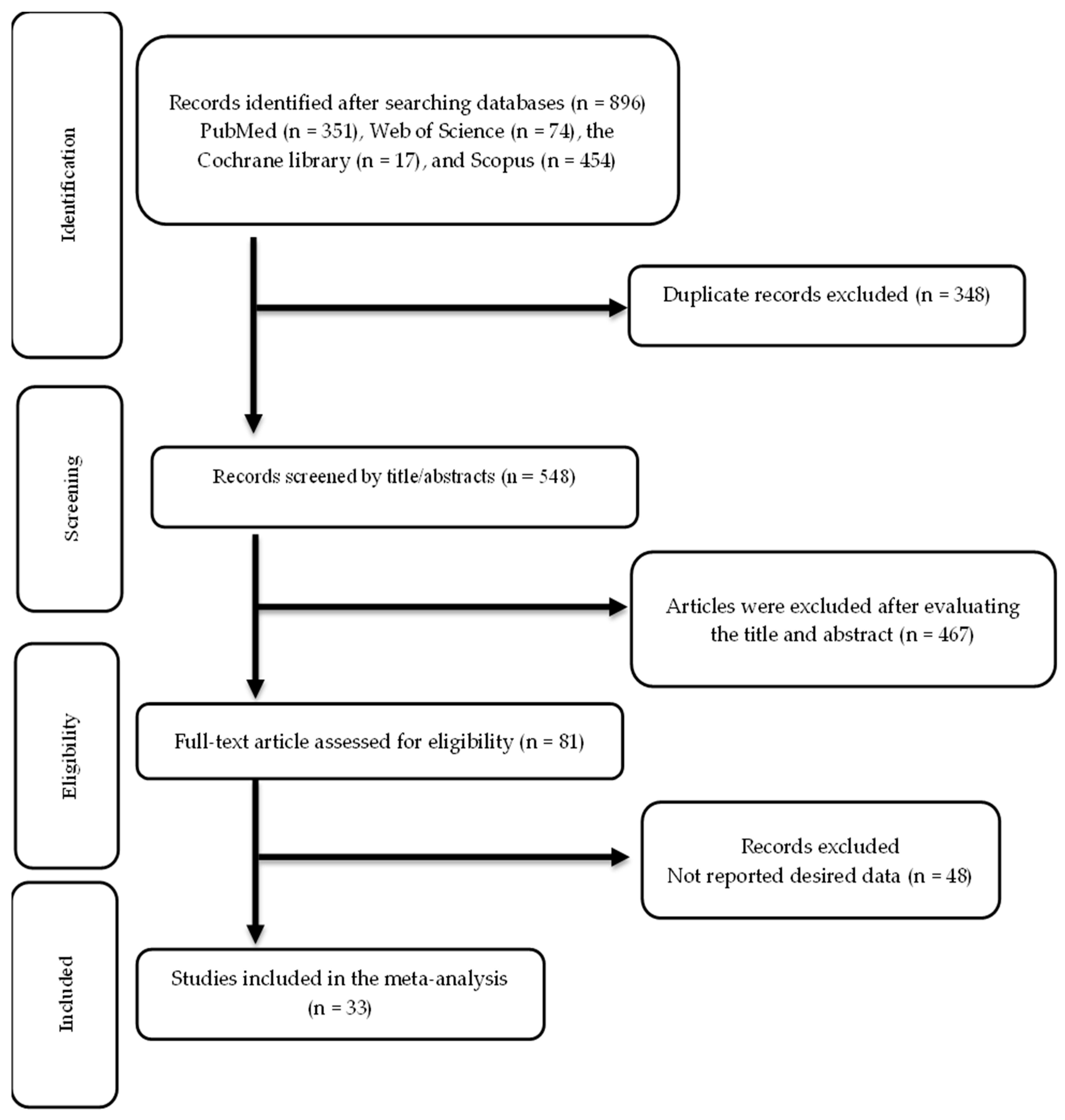
The screening and selection processes for the included trials are illustrated in Figure 1. The first search across multiple databases yielded 896 publications. After eliminating 348 duplicate records, 548 records were reviewed, and 467 were not relevant. The remaining 81 articles were assessed for eligibility, and 33 articles were ultimately included in this meta-analysis [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84].
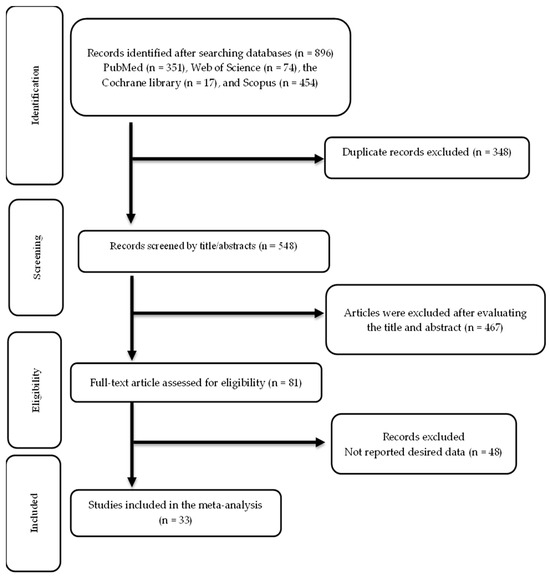
Figure 1.
Flow chart of study selection.
3.2. Study Characteristics
This meta-analysis included 33 RCTs with 1943 participants (978 cases and 982 controls). Characteristics of the included trials are provided in Table 1. The articles were published from 1993 to 2022. Thirty-two studies used a randomized parallel design [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,79,80,81,82,83,84], while one study was a cross-over trial [78]. The trial duration and sample size varied from 2 to 48 weeks and 16 to 100 participants, respectively. The mean age of the participants varied from 20 to 63 years old. The trials were performed in Iran [55,56,59,60,61,62,64,65,67,68,69,70,72,73,75,76,77,79,81,82], Italy [52,53,84], the Czech Republic [54,58], Iraq [57,63,66], Malaysia [74], Egypt [71], Pakistan [80,83], and Australia [78]. They were conducted among healthy individuals [54,72,73,78], patients with cirrhosis [52,53], diabetic nephropathy [64], diabetes mellitus [55,56,57,59,63,65,67,70,71,75,77,79,80,83,84], metabolic syndrome [58], hyperlipidemia [66], β-thalassemia major (β-TM) [61], non-alcoholic fatty liver disease (NAFLD) [60,69,76,82], non-alcoholic steatohepatitis [62,68,74], and coronavirus disease 2019 (COVID-19) [81]. One study was implemented among women [79] and five among men [54,72,73,77,78], whereas the majority of the trials comprised both sexes. The daily intake of SIL supplements varied between 140 and 2100 mg. Supplementary Table S1 presents the RoB assessments of trials.

Table 1.
Characteristic of included studies in meta-analysis.
3.3. Meta-Analysis
3.3.1. Impact of Silymarin Supplementation on Anthropometric Parameters
BMI
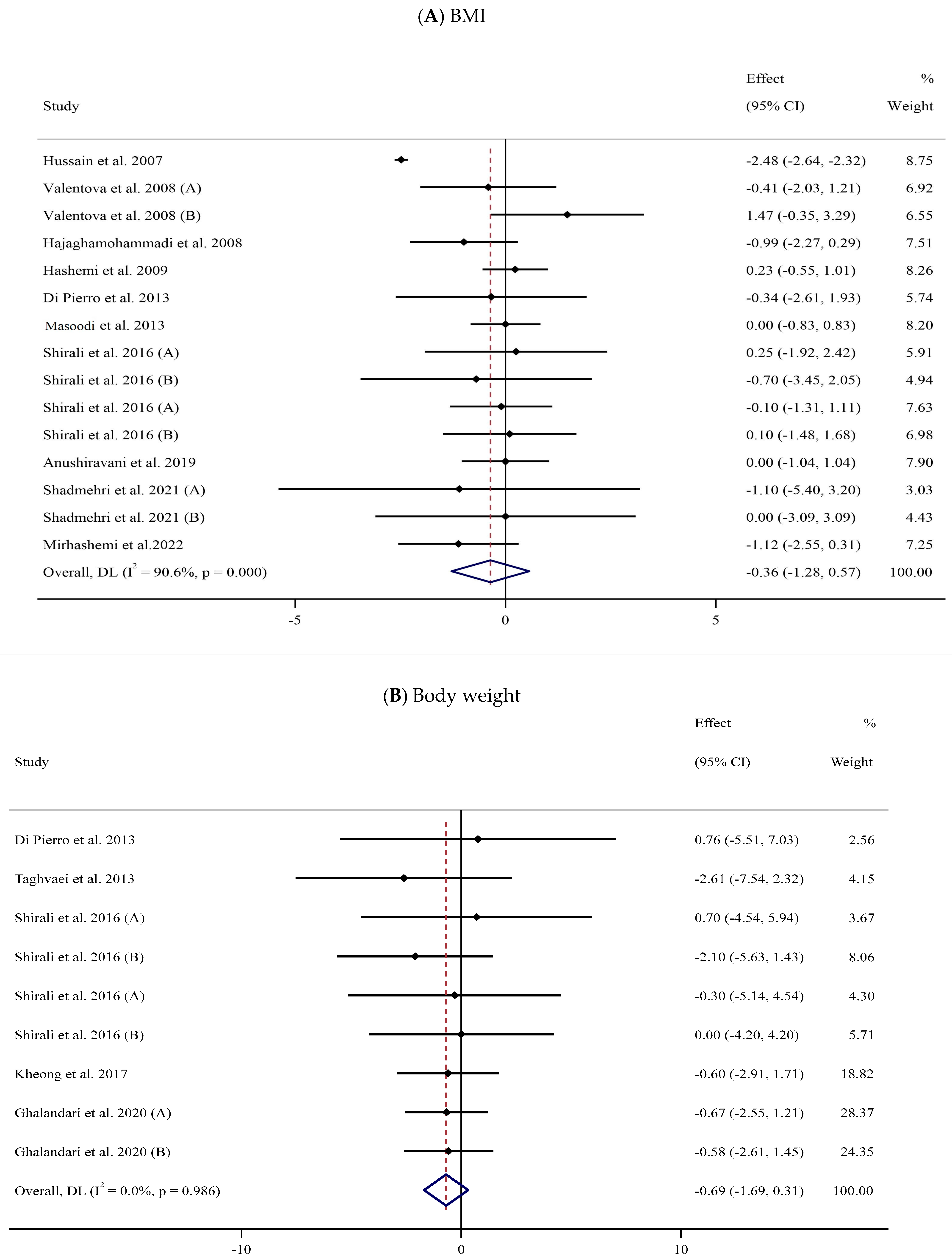
Eleven trials [57,58,60,62,69,72,73,76,79,82,84] with 15 arms and 671 participants (SIL-treated, n = 327; untreated, n = 344) were analyzed to evaluate the impact of SIL supplementation on BMI (Figure 2A). Analysis of the pooled data displayed no considerable differences in BMI between the two groups (WMD: −0.36 kg/m2, 95% CI: −1.28, 0.57; p = 0.447) with substantial heterogeneity (I2 = 90.6%, p < 0.001). The subgroup analysis explored a significant fall in BMI among participants with obesity (Table 2).
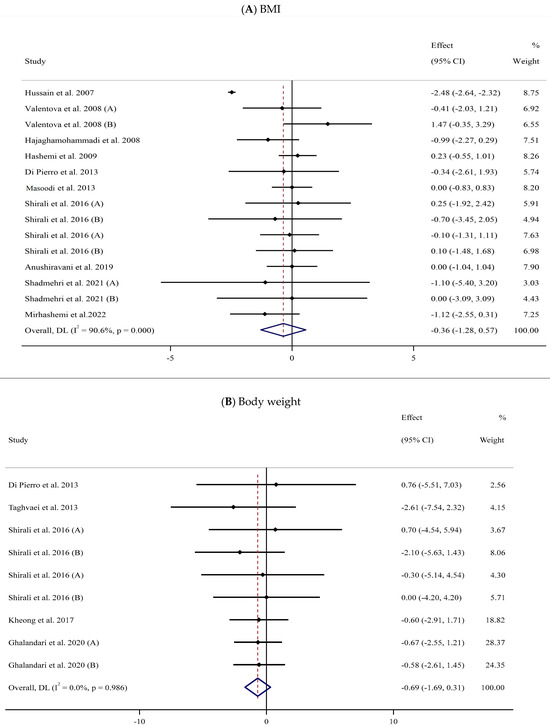
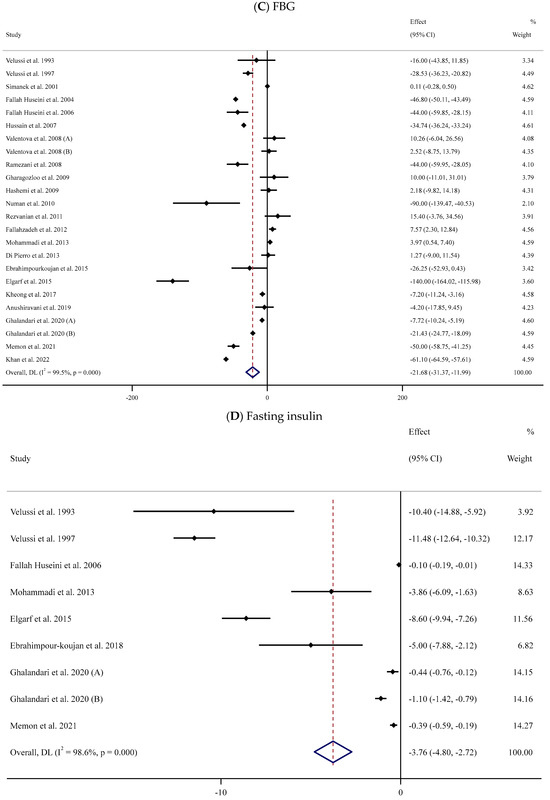
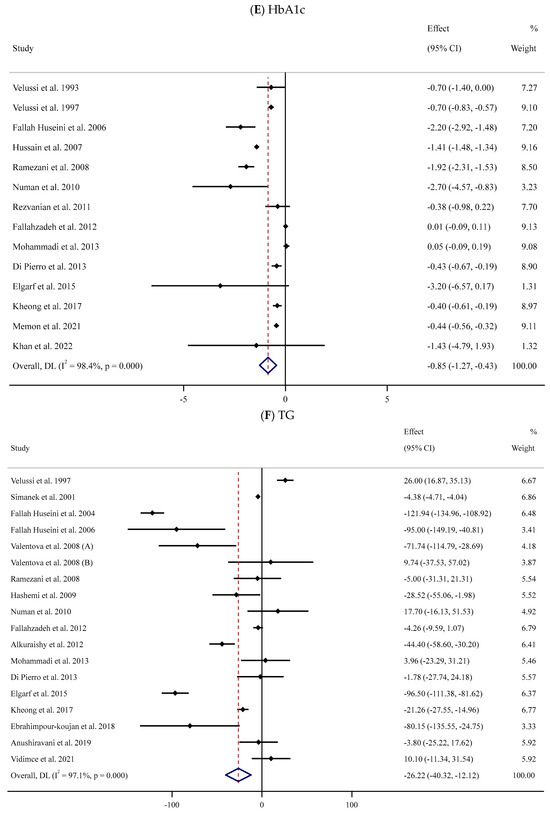
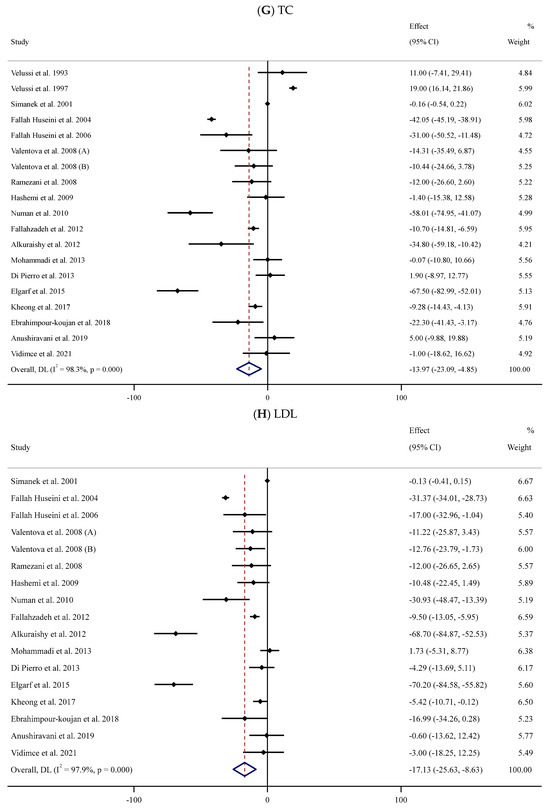
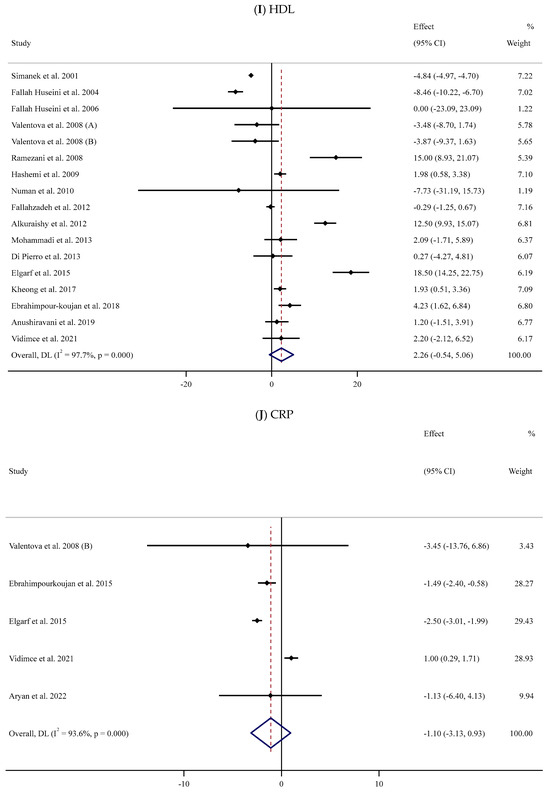
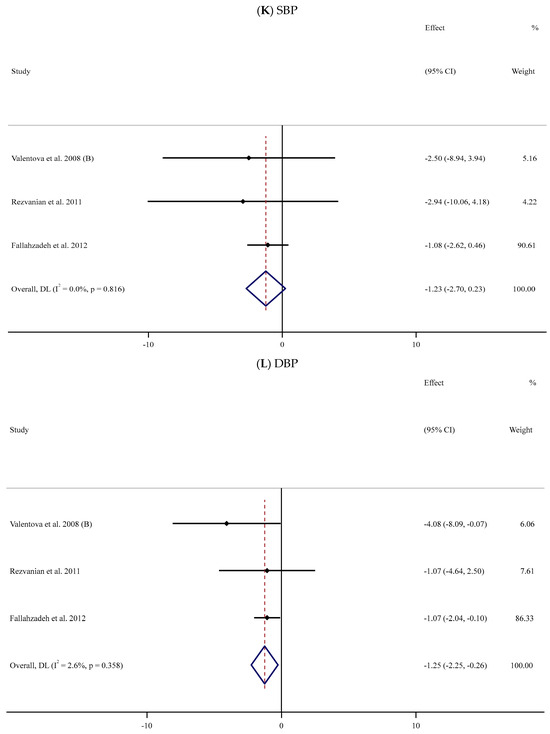
Figure 2.
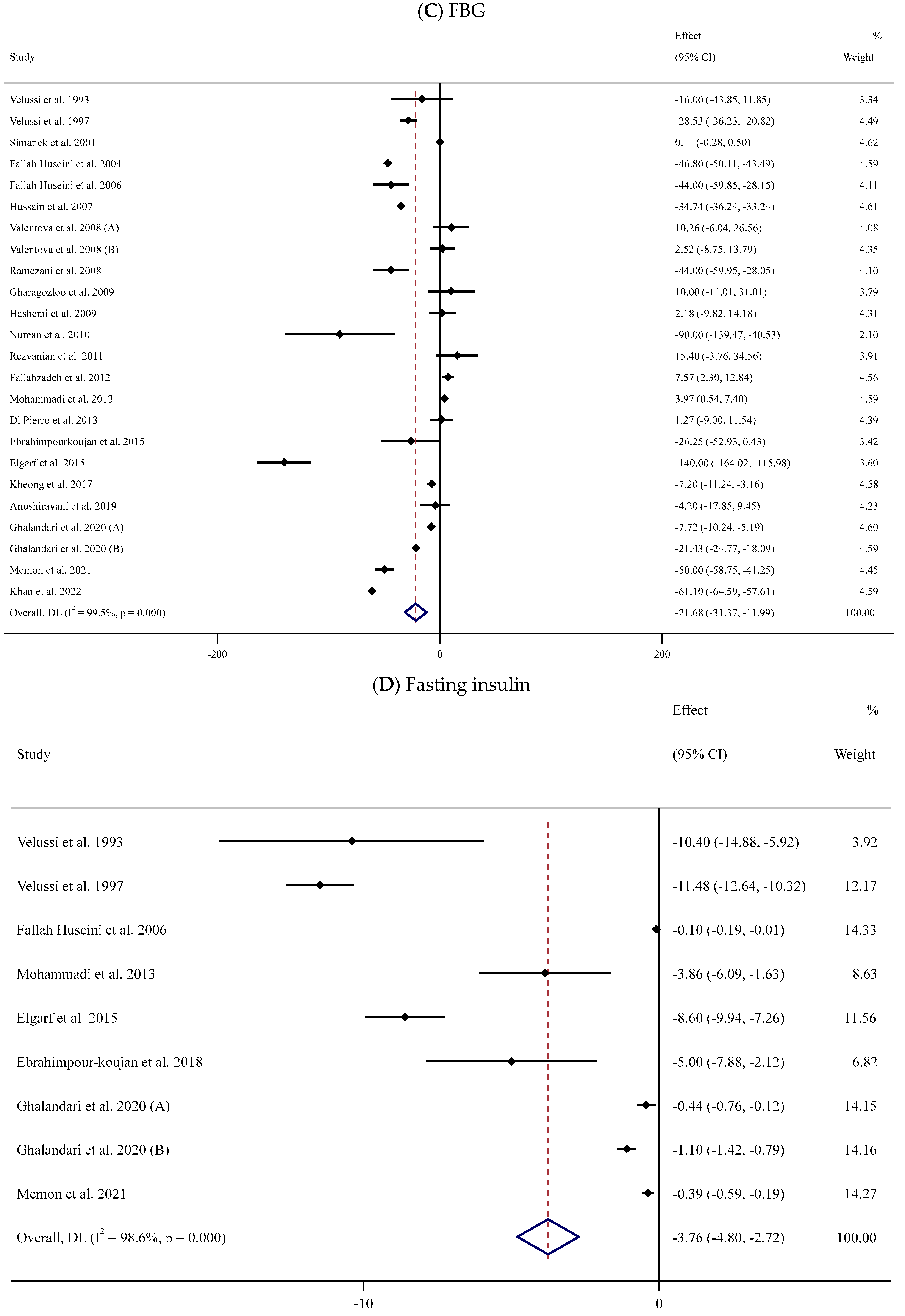
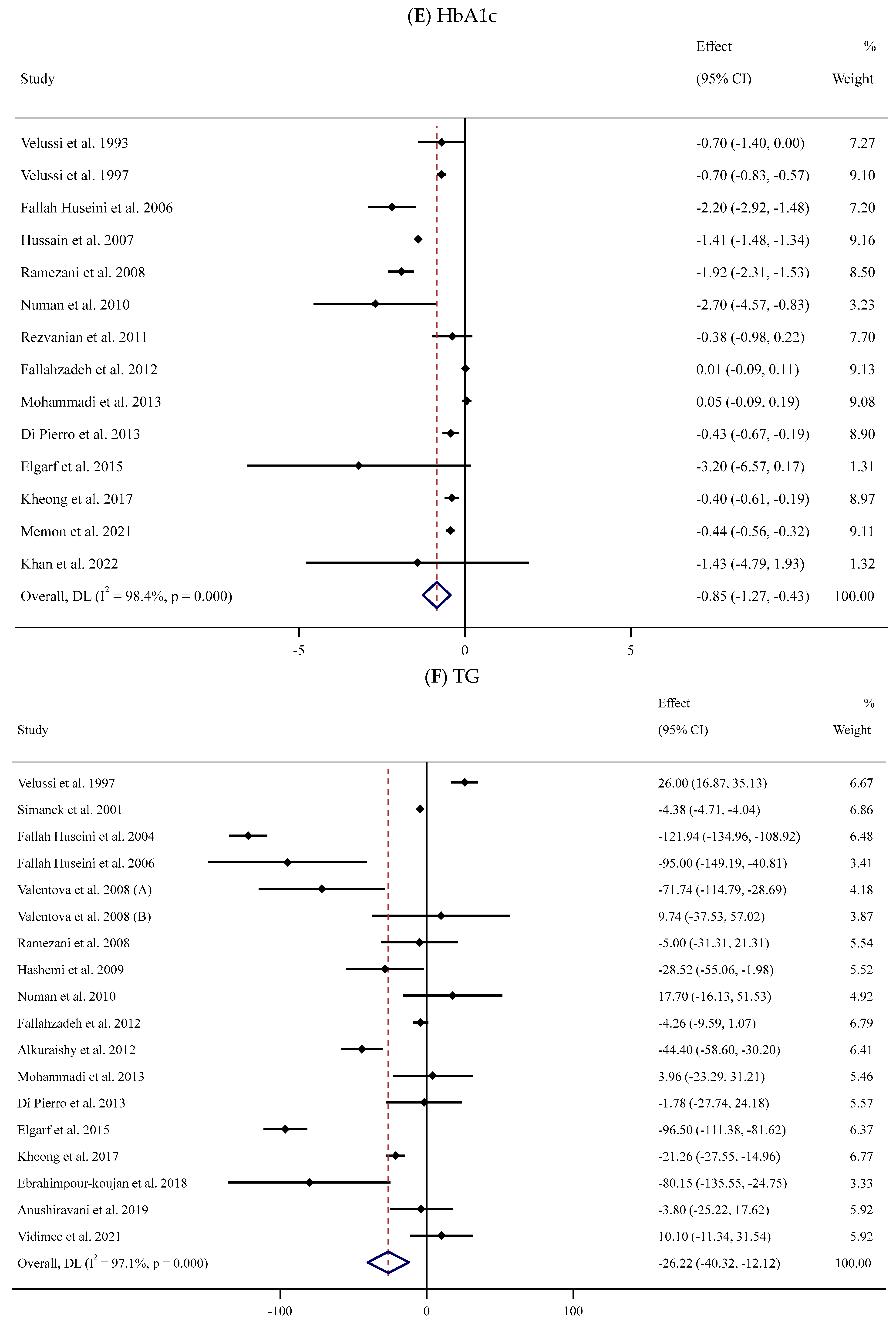
Forest plots for the effect of silymarin supplementation on (A) BMI (kg/m2), (B) body weight (kg), (C) FBG (mg/dL), (D) fasting insulin (mU/mL), (E) HbA1c (%), (F) TG (mg/dL), (G) TC (mg/dL), (H) LDL (mg/dL), (I) HDL (mg/dL), (J) CRP (mg/dL), (K) SBP (mmHg), and (L) DBP (mmHg) [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84]. Horizontal lines represent 95% confidence intervals (CIs). Diamonds represent pooled estimates from random-effects analysis. The effect column comprises weighted mean differences (WMDs) and 95% CIs.

Table 2.
Subgroup analyses of the impacts of silymarin supplementation on cardiovascular risk factors.
Body Weight
The impact of SIL intake on body weight was investigated in six studies [68,72,73,74,77,84] with 325 participants (Table 2). A pooled analysis of nine effect sizes indicated no substantial differences in body weight between the SIL-treated and untreated groups (WMD: −0.69 kg, 95% CI: −1.69, 0.31; p = 0.178, I2 = 0.0%) (Figure 2B).
3.3.2. Impact of Silymarin Supplementation on Glycemic Parameters
FBG
The effect of supplementation with SIL on the concentrations of serum FBG was assessed in 22 trials [52,53,54,55,56,57,58,59,61,62,63,64,65,67,70,71,74,76,77,80,83,84] that involved 1445 participants (719 cases versus 726 controls). The pooled results indicated that SIL effectively decreased serum FBG values in the SIL-treated groups (n = 719) compared to their untreated counterparts (n = 726) (WMD: −21.68 mg/dL, 95% CI: −31.37, −11.99; p < 0.001) with remarkable between-trial heterogeneity (I2 = 99.5%, p < 0.001) (Figure 2C). Subgroup analyses revealed a considerable decrease in serum levels of FBG in the SIL group in comparison to the untreated group in studies involving unhealthy individuals with obesity and baseline serum FBG levels > 126 mg/dL. This reduction was observed in long- and short-term supplementation (<12 and ≥12 weeks) with high or low doses of SIL (≥400 or <400 mg/day) (Table 2).
Fasting Insulin
The association between serum fasting insulin levels and SIL intake was assessed in eight studies [52,53,56,67,71,75,77,80] involving 571 participants (Figure 2D). The meta-analysis revealed that serum fasting insulin concentrations decreased following SIL consumption compared to the untreated group (WMD: −3.76 mU/mL, 95% CI: −4.80, −2.72; p < 0.001); nevertheless, there was substantial heterogeneity between trials (I2 = 98.6%, p < 0.001) (Table 2). Subgroup analyses indicated a notable reduction in serum levels of insulin in the SIL-treated group compared to controls in studies among participants with overweight or obesity, in short-term or long-term supplementation with low or high doses of SIL.
HbA1c
Fourteen studies [52,53,56,57,59,63,64,65,67,71,74,80,83,84] assessed the impact of supplementation with SIL on the serum HbA1c levels in a total of 957 participants (SIL-treated vs. placebo). Meta-analysis of these trials indicated a substantial decline in serum HbA1c levels following SIL intake compared to the untreated group (WMD: −0.85%, 95% CI: −1.27, −0.43; p < 0.001) with a high degree of between-trial heterogeneity (I2 = 98.4%, p < 0.001) (Figure 2E). Subgroup analyses suggested a considerable fall in serum HbA1c concentrations in the SIL group in comparison with the untreated group in trials with short- or long-term duration and high daily doses of SIL among participants with obesity (Table 2).
3.3.3. Impact of Silymarin Supplementation on Lipid Profile
TG
The meta-analysis of 17 RCTs [53,54,55,56,58,59,62,63,65,66,67,71,74,75,76,78,84] with 953 participants (480 cases and 490 controls) indicated a substantial reduction in serum TG levels in the SIL group in comparison with the controls (WMD: −26.22 mg/dL, 95% CI: −40.32, −12.12; p < 0.001). Furthermore, there was considerable heterogeneity among trials (I2 = 97.1%, p < 0.001) (Figure 2F). Similar findings were detected in subgroup analyses among unhealthy participants with normal BMI or obesity and baseline serum TG levels >150 mg/dL after long-term supplementation with high doses of SIL (Table 2).
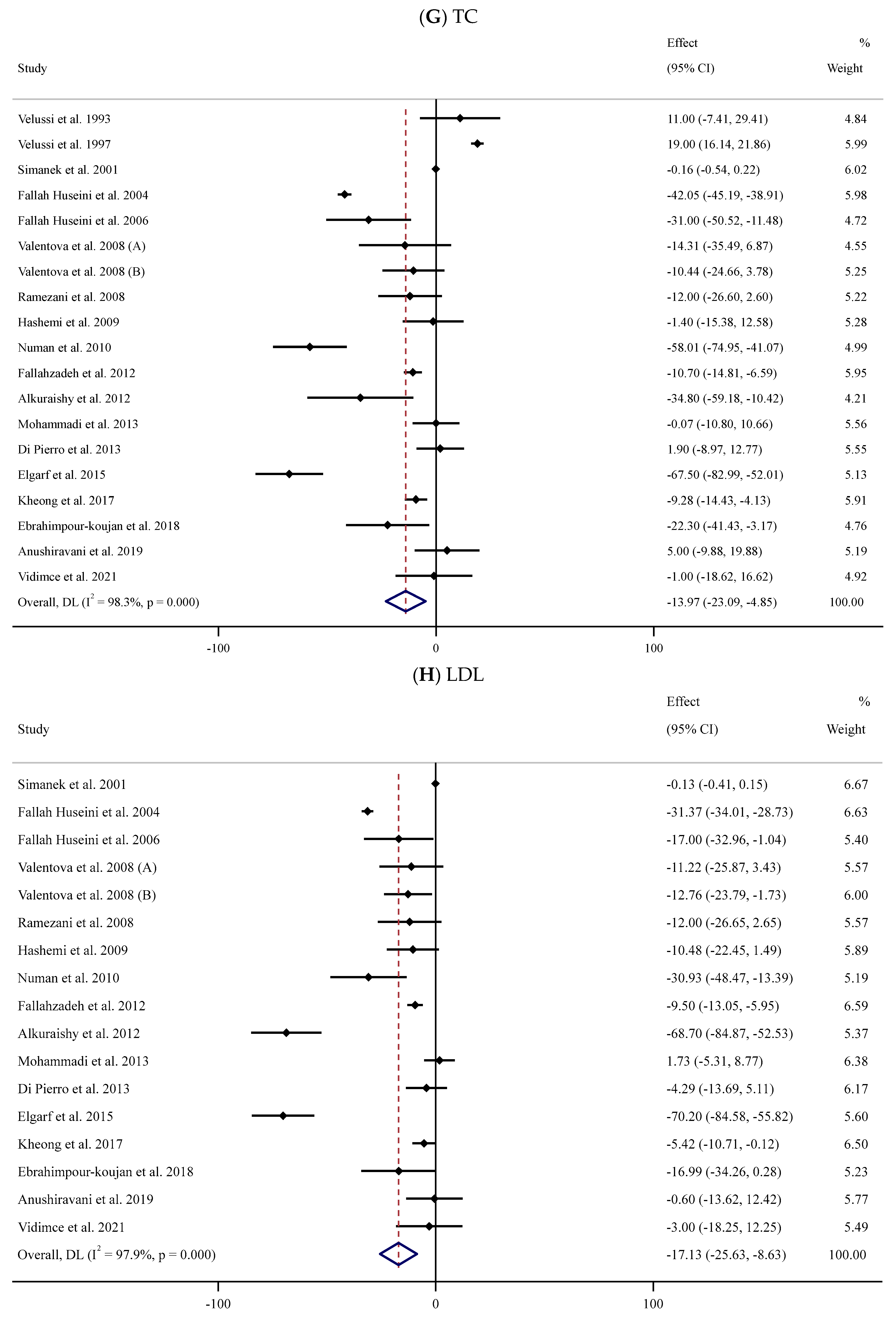
TC
Eighteen trials [52,53,54,55,56,58,59,62,63,65,66,67,71,74,75,76,78,84] with 1013 participants (SIL group vs. controls) explored the impact of SIL administration on serum TC levels (Figure 2G). Pooled analysis showed that SIL consumption caused a significant decline in serum TC levels (WMD: −13.97 mg/dL, 95% CI: −23.09, −4.85; p = 0.003) with substantial between-trial heterogeneity (I2 = 98.3%, p < 0.001). Subgroup analyses indicated a notable reduction in serum TC concentration among unhealthy participants with obesity and baseline serum TC levels >200 or <200 mg/dL after short-term supplementation with high doses of SIL (Table 2).
LDL
Sixteen RCTs [54,55,56,58,59,62,63,65,66,67,71,74,75,76,78,84] with 893 participants (450 cases vs. 460 controls) were included in the meta-analysis to evaluate the impact of SIL consumption on serum LDL levels (SIL group vs. placebo group). Analysis of the pooled effect sizes revealed a significant decline in serum LDL concentration in the SIL-treated group (WMD: −17.13 mg/dL, 95% CI: −25.63, −8.63; p < 0.001) with substantial heterogeneity (I2 = 97.9%, p < 0.001) (Figure 2H). Similar results were revealed based on sub-analyses among overweight unhealthy participants with high or low baseline serum LDL levels during short- or long-term supplementation with high doses of SIL (Table 2).
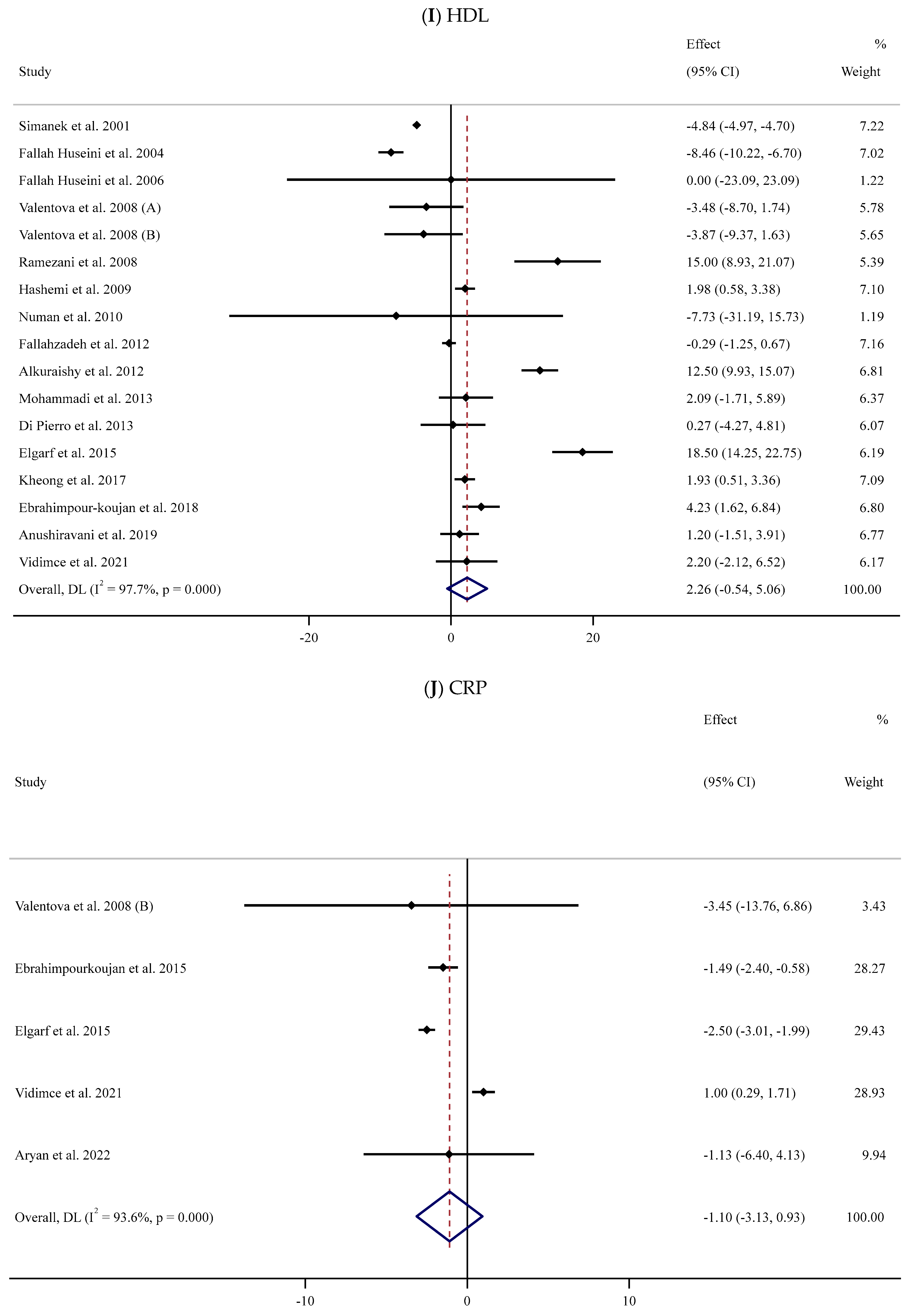
HDL
Sixteen trials [54,55,56,58,59,62,63,65,66,67,71,74,75,76,78,84] were conducted to assess the impact of SIL on serum HDL levels among 893 participants. The meta-analysis revealed that there was no substantial impact of SIL on the HDL level in the SIL-treated group when compared to the control group (WMD: 2.26 mg/dL, 95% CI: −0.54, 5.06; p = 0.114). Considerable heterogeneity between studies was also noted (I2 = 97.7%, p < 0.001) (Figure 2I). Subgroup analyses revealed a substantial reduction in serum HDL levels among participants with normal BMI and high or low baseline serum HDL levels after supplementation with low doses of SIL (Table 2).
3.3.4. Impact of Silymarin Supplementation on Serum CRP
In the meta-analysis of five RCTs [58,70,71,78,81] with 185 participants, SIL consumption had no substantial impact on serum CRP levels (WMD: −1.10 mg/dL, 95% CI: −3.13, 0.93; p = 0.289) (Figure 2J). However, the outcomes showed considerable between-study heterogeneity (I2 = 93.6%, p < 0.001) (Table 2). Subgroup analyses revealed a substantial decline in serum CRP levels among healthy and unhealthy participants with obesity and baseline serum CRP levels >3 mg/L in long-term (≥12 weeks) supplementation with high doses of SIL (Table 2).
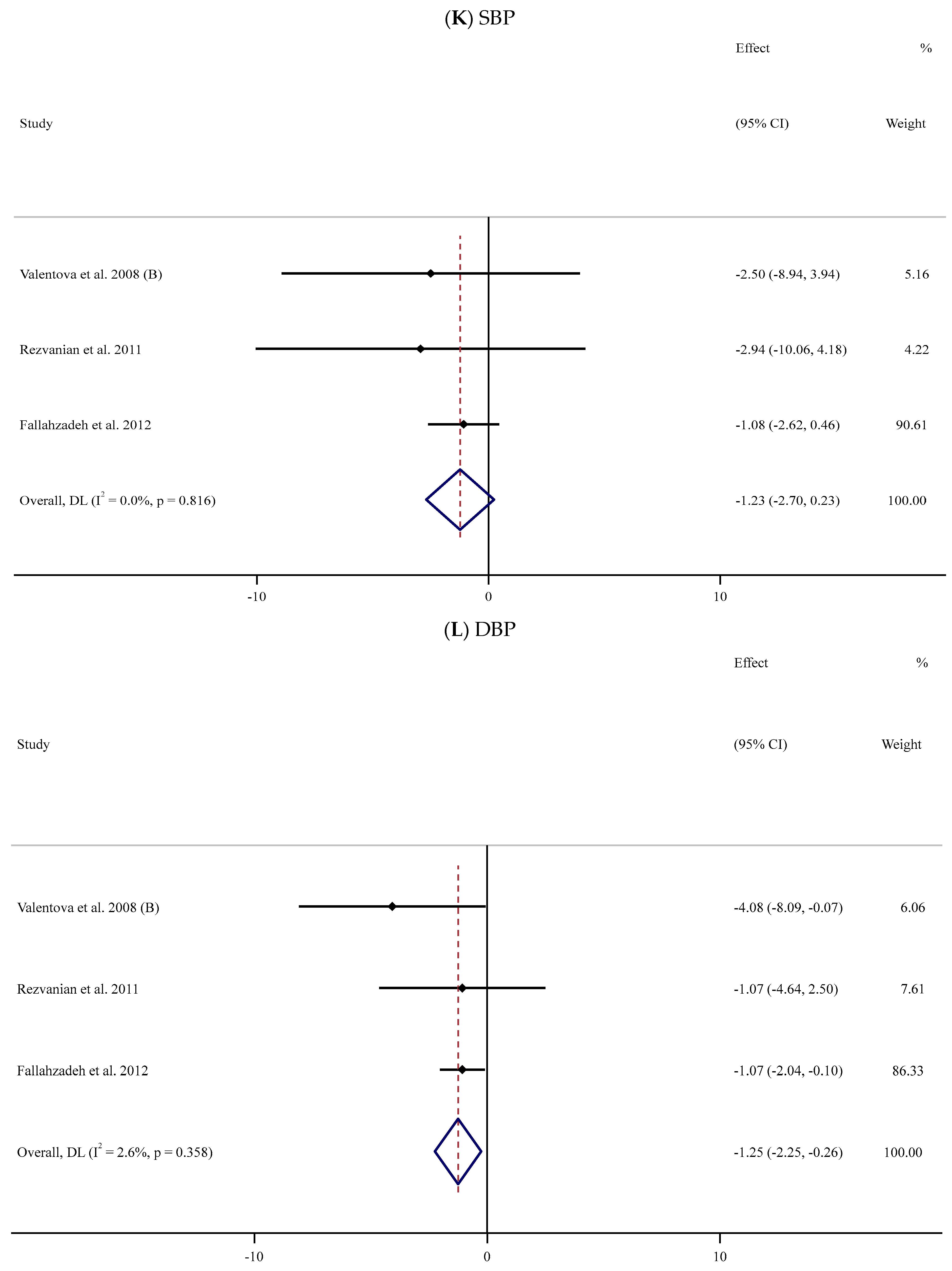
3.3.5. Impact of Silymarin Supplementation on Blood Pressure
SBP and DPB
The impact of supplementation with SIL on BP (SBP and DBP) was analyzed in three trials [58,64,65] involving 150 participants (SIL group, n = 78; control group, n = 72) (Table 2). The pooled data revealed no statistically notable differences in SBP values between the two groups (WMD: −1.23 mmHg, 95% CI: −2.70, 0.23; p = 0.099, I2 = 0%) (Figure 2K); however, a significant reduction in the magnitude of DBP was observed among the SIL-treated participants in comparison with the placebo group (WMD: −1.25 mmHg; 95% CI: −2.25, −0.26; p = 0.013, I2 = 2.6%) (Figure 2L).
3.4. Publication Bias
Begg’s and Egger’s tests did not display any substantial publication bias for several evaluated outcomes (body weight, DBP, serum levels of TC, HbA1c, TG, and CRP). However, evidence of potential bias was detected in trials assessing the effects of SIL consumption on BMI (Begg’s test, p = 0.018; Egger’s test, p <0.001), SBP (Egger’s test, p = 0.046), serum levels of HDL (Egger’s test, p = 0.002), LDL (Egger’s test, p = 0.022), FBG (Egger’s test, p = 0.036), and insulin (Egger’s test, p = 0.013). Additionally, a visual examination of funnel plots revealed different degrees of asymmetry for all outcomes (Supplementary Figure S1).
3.5. Sensitivity Analysis
Sensitivity analyses revealed that the findings were not influenced by the removal of any specific trial related to body weight, BMI, SBP, and serum levels of insulin, FBG, HbA1c, TG, TC, LDL, and CRP. However, the outcomes related to DBP values changed after excluding two studies [64,65]. In addition, the outcomes related to serum HDL values changed after excluding one study [55].
3.6. GRADE Assessment
The GRADE framework was utilized to evaluate the quality of evidence for all outcomes (Supplementary Table S2). The certainty of evidence related to DBP values was deemed low due to a very serious risk of bias. For the other assessed outcomes, GRADE was rated as very low, primarily attributed to serious risks of imprecision, publication bias, very serious inconsistency, and bias.
3.7. Linear and Non-Linear Dose–Response Associations
No linear (Supplementary Figures S4 and S5) or non-linear (Supplementary Figures S2 and S3) correlation was depicted between the duration of the trial or the dosage of SIL supplement and the alterations in all assessed outcomes.
4. Discussion
This systematic review and dose–response meta-analysis examined the impact of supplementation with SIL on CVD risk factors. The findings suggest that SIL intake may improve certain CMD risk factors by decreasing levels of FBG, fasting insulin, HbA1c, TC, TG, LDL, and DBP. However, it did not significantly affect anthropometric measurements, SBP, or serum CRP levels. Supplementation with SIL demonstrated enhancements in glycemic control, lipid profile, and potential hypotensive effects.
The subgroup analysis revealed a substantial decrease in BMI among individuals with obesity following SIL supplementation compared to their untreated counterparts. It also indicated a notable reduction in FBG levels in trials involving unhealthy participants with obesity and baseline FBG levels >126 mg/dL during both long- and short-term supplementation (<12 and ≥12 weeks) with low or high doses of SIL (≥400 or <400 mg/d). Furthermore, a considerable decline in serum insulin levels was observed among participants with overweight or obesity following short- and long-term supplementation with low or high doses of SIL. It also suggested a significant fall in HbA1c concentration in trials with short- or long-term duration and high doses of SIL among participants with obesity. Additionally, a substantial reduction in TG levels was noted in unhealthy participants with a normal BMI or obesity and baseline serum TG levels below 150 mg/dL after long-term supplementation with high doses of SIL. It indicated a significant decrease in serum TC concentration among unhealthy obese participants during short-term supplementation with high doses of SIL. A notable fall in serum LDL levels was detected in unhealthy overweight participants after receiving high doses of SIL over a short or long period. Furthermore, a considerable reduction in serum HDL levels was depicted in participants with a normal BMI after supplementation with low doses of SIL. In addition, there was a considerable decrease in serum levels of CRP among both healthy and unhealthy participants with obesity and baseline CRP levels >3 mg/L over long-term supplementation with high doses of SIL.
The outcomes of this study regarding serum lipids were in agreement with two previous meta-analyses [41,85]. Similarly, the results related to lipid profiles and glycemic parameters were consistent with those reported in another meta-analysis [26]. However, there were discrepancies when compared with a meta-analysis of seven trials among patients with T2DM, which indicated that SIL did not lead to a substantial decline in serum levels of TC and TG [40]. Furthermore, a meta-analysis comprising five trials demonstrated that SIL could lower FBS and HbA1c levels without affecting the lipid profile of T2DM patients [24]. Although one meta-analysis indicated a positive impact of SIL on HDL levels, the clinical significance of this effect was minimal [41], which aligns with the non-significant increase observed in the present study.
In recent years, there have been extensive investigations into the potential health benefits and cardiovascular-protective properties of SIL and its supplements. Previous studies in animals have indicated that SIL intake may reduce the risk of CVDs [86,87]. Silymarin has been shown to contribute to the restoration of pancreatic function [88]. Animal studies have demonstrated that SIL and its components have the potential to improve glucose regulation in diabetic rats [89,90]. It could promote the restoration of pancreatic function by upregulating insulin and glucagon proteins, leading to the normalization of blood glucose levels and the restoration of insulin serum levels [88]. It has been reported that supplementation with SIL may improve the restoration of Langerhans islet β cells [91], stimulation of beta precursor cells to develop into insulin-producing cells, and reduction of advanced glycation end product (AGE) formation [92]. Silymarin exhibits low water solubility and limited absorption following oral administration [93], potentially leading to insignificant responses in certain studies. Therefore, enhancing the bioavailability of SIL by consuming it with or after a meal may increase its effectiveness [85]. Additionally, evidence suggests that SIL may promote the proliferation of insulin-producing cells [94]. Silymarin was considered safe for human consumption at therapeutic levels and was well tolerated, even at a high dosage of 700 mg administered three times daily over 24 weeks [95].
The positive impacts of SIL on metabolic status are related to its antioxidant effects [28]. In addition, SIL may have beneficial impacts on blood lipids through various mechanisms [9,78,96,97,98,99,100]. Hypertension is a significant factor contributing to CVDs [101]. Existing evidence suggests that the mechanisms through which SIL influences the prevention and management of high BP can be categorized into various groups [102]. Silymarin plays a role in regulating vascular tone and suppressing platelet aggregation, thereby contributing to the regulation of BP in individuals with hypertension [102].
It has been indicated that incorporating antioxidant-rich foods into the diet may enhance the treatment of patients with T2DM [103]. Given the antioxidant properties of SIL, it is hypothesized that it may be particularly effective in improving CVD risk factors; however, further evidence is required to fully support this assertion. The present systematic review and meta-analysis demonstrated a considerable cardioprotective benefit of supplementation with SIL.
It is important to emphasize that in all clinical trials, participants received standard treatment in conjunction with SIL supplementation [41]. Consequently, the specific effects of SIL remain unclear [41]. However, discontinuing standard treatment for ethical reasons was not feasible, and only the synergistic effects were investigated. Furthermore, the stringent regulations governing the production of nutraceuticals and herbal and dietary supplements are less comprehensive than those for pharmaceuticals. This could potentially lead to ineffectiveness in some cases due to the limited examination of effective dosage, bioavailability, and formulation [104]. Since most studies did not provide detailed information on SIL supplements, addressing this aspect was not feasible in the current systematic review and meta-analysis [41].
This first dose–response meta-analysis examined the effect of SIL consumption on CVD risk factors. The selection of trials for the systematic search was not limited by period or language. Moreover, a sufficient number of trials was evaluated in the analysis. Additionally, supplementary analyses including subgroup analyses, publication bias assessments, and sensitivity analyses were conducted. Systematic reviews and meta-analyses are widely regarded as the most reliable forms of clinical evidence [104,105]. However, evidence regarding the efficacy of herbal dietary supplements remains inconclusive [106]. Key concerns related to herbal nutraceuticals include the absence of standardized extracts, ambiguity regarding plant parts, and failure to disclose the type of extract [106]. The absence of rigorous regulations poses challenges for nutraceutical manufacturers in verifying the quality, safety, and effectiveness of their products [106]. Consequently, numerous available products may be ineffective [106].
This meta-analysis encountered various limitations, including significant methodological and clinical heterogeneity among trials. This heterogeneity was evident in the varying trial durations, doses of SIL supplements, participants’ underlying health conditions, and sample sizes. The majority of trials had poor quality with a high risk of bias. Moreover, over half of the trials in this meta-analysis were performed in a single country (Iran), emphasizing the necessity for additional research in various geographical locations to validate positive results across different ethnic populations. In addition, the included trials involved diverse non-intervention or control groups.
5. Conclusions
In summary, the results of this meta-analysis indicated that SIL may mitigate certain cardiometabolic risk factors by reducing FBG, fasting insulin, HbA1c, TC, TG, LDL, and DBP levels. However, it did not significantly affect the anthropometric parameters, SBP values, or serum CRP levels. It demonstrated positive effects on lipid and glycemic profiles with potential hypotensive impacts but did not affect anthropometric or inflammatory parameters. SIL has the potential to be a beneficial adjunctive treatment for enhancing various aspects of chronic CMS. However, the existing data show significant variability and a lack of extensive clinical trials for specific parameters. These findings should be validated by additional well-designed RCTs with larger sample sizes and longer durations to ascertain the clinical efficacy and potential therapeutic application of SIL supplementation in cardiovascular health.
Supplementary Materials
The following are available at https://www.mdpi.com/article/10.3390/antiox13040390/s1.
Author Contributions
S.M. and D.A.-L. prepared the study protocol. S.M., V.F., Y.J., R.A., F.M. and D.A.-L. were involved in data extraction, search, and screening. Data analysis was conducted by O.A. The initial draft of the manuscript was prepared by S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kelli, H.M.; Kassas, I.; Lattouf, O.M. Cardio metabolic syndrome: A global epidemic. J. Diabetes Metab. 2015, 6, 2–14. [Google Scholar] [CrossRef]
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic syndrome: Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Yanovski, J.A. Long-term drug treatment for obesity: A systematic and clinical review. JAMA 2014, 311, 74–86. [Google Scholar] [CrossRef]
- Baumgartner, S.; Bruckert, E.; Gallo, A.; Plat, J. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis 2020, 311, 116–123. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Christodoulou, E.; Kostomitsopoulos, N.; Valsami, G. The cardiovascular-protective properties of saffron and its potential pharmaceutical applications: A critical appraisal of the literature. Phytother. Res. 2021, 35, 6735–6753. [Google Scholar] [CrossRef]
- Vahabzadeh, M.; Amiri, N.; Karimi, G. Effects of silymarin on metabolic syndrome: A review. J. Sci. Food Agric. 2018, 98, 4816–4823. [Google Scholar] [CrossRef]
- Tajmohammadi, A.; Razavi, B.M.; Hosseinzadeh, H. Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review. Phytother. Res. 2018, 32, 1933–1949. [Google Scholar] [CrossRef]
- Radjabian, T.; Fallah, H.H. Anti-hyperlipidemic and anti-atherosclerotic activities of silymarins from cultivated and wild plants of Silybum marianum L. with different content of flavonolignans. Iran. J. Pharmacol. Ther. 2010, 9, 63–67. [Google Scholar]
- Kadoglou, N.P.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Kostomitsopoulos, N.G.; Tsaroucha, A.K.; Valsami, G. A comprehensive review of the cardiovascular protective properties of Silibinin/Silymarin: A new kid on the block. Pharmaceuticals 2022, 15, 538. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, A.; Schmidt, H.H.J. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [PubMed]
- Marceddu, R.; Dinolfo, L.; Carrubba, A.; Sarno, M.; Di Miceli, G. Milk thistle (Silybum Marianum L.) as a novel multipurpose crop for agriculture in marginal environments: A review. Agronomy 2022, 12, 729. [Google Scholar] [CrossRef]
- Delmas, D. Silymarin and derivatives: From biosynthesis to health benefits. Molecules 2020, 25, 2415. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, A.S.; Singh, N. Silymarin and its role in chronic diseases. Drug Discov. Mother Nat. 2016, 25–44. [Google Scholar]
- Saller, R.; Brignoli, R.; Melzer, J.; Meier, R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Complement. Med. Res. 2008, 15, 9–20. [Google Scholar] [CrossRef]
- Polyak, S.J.; Morishima, C.; Lohmann, V.; Pal, S.; Lee, D.Y.; Liu, Y.; Graf, T.N.; Oberlies, N.H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. USA 2010, 107, 5995–5999. [Google Scholar] [CrossRef]
- Ralli, T.; Kalaiselvan, V.; Tiwari, R.; Shukla, S.; Kholi, K. Clinical and Regulatory Status of Silymarin. Appl. Drug Res. Clin. Trials Regul. Aff. 2021, 8, 104–111. [Google Scholar] [CrossRef]
- Camini, F.C.; Costa, D.C. Silymarin: Not just another antioxidant. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190206. [Google Scholar] [CrossRef]
- Gu, M.; Zhao, P.; Huang, J.; Zhao, Y.; Wang, Y.; Li, Y.; Li, Y.; Fan, S.; Ma, Y.-M.; Tong, Q. Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Front. Pharmacol. 2016, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Phytotherapeutic properties of milk thistle seeds: An overview. J. Adv. Pharm. Educ. Res. 2011, 1, 69–79. [Google Scholar]
- Taleb, A.; Ahmad, K.A.; Ihsan, A.U.; Qu, J.; Lin, N.; Hezam, K.; Koju, N.; Hui, L.; Qilong, D. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. Pharmacother. 2018, 102, 689–698. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Estêvão, M.D.; Morvaridi, M.; Belančić, A.; Mohammadi, S.; Hassani, M.; Heshmati, J.; Ziaei, S. The effect of Conjugated Linoleic Acid intake on oxidative stress parameters and antioxidant enzymes: A systematic review and meta-analysis of randomized clinical trials. Prostaglandins Other Lipid Mediat. 2022, 163, 106666. [Google Scholar] [CrossRef]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Res. 2016, 2016, 5147468. [Google Scholar] [CrossRef]
- Koltai, T.; Fliegel, L. Role of silymarin in cancer treatment: Facts, hypotheses, and questions. J. Evid.-Based Integr. Med. 2022, 27, 2515690X211068826. [Google Scholar] [CrossRef]
- Xiao, F.; Gao, F.; Zhou, S.; Wang, L. The therapeutic effects of silymarin for patients with glucose/lipid metabolic dysfunction: A meta-analysis. Medicine 2020, 99, e22249. [Google Scholar] [CrossRef] [PubMed]
- Fallah, M.; Davoodvandi, A.; Nikmanzar, S.; Aghili, S.; Mirazimi, S.M.A.; Aschner, M.; Rashidian, A.; Hamblin, M.R.; Chamanara, M.; Naghsh, N. Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer. Biomed. Pharmacother. 2021, 142, 112024. [Google Scholar] [CrossRef] [PubMed]
- Surai, P. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, X.; Zhang, H.; Li, Q.; Lu, G.; Zhao, X. Modulatory effect of silymarin on pulmonary vascular dysfunction through HIF-1α-iNOS following rat lung ischemia-reperfusion injury. Exp. Ther. Med. 2016, 12, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Demirci, B.; Dost, T.; Gokalp, F.; Birincioglu, M. Silymarin improves vascular function of aged ovariectomized rats. Phytother. Res. 2014, 28, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lin, H.; Wang, Q.; Hou, J.-W.; Mao, Z.-J.; Li, Y.-G. Protective role of silibinin against myocardial ischemia/reperfusion injury-induced cardiac dysfunction. Int. J. Biol. Sci. 2020, 16, 1972. [Google Scholar] [CrossRef]
- Rao, P.R.; Viswanath, R.K. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp. Clin. Cardiol. 2007, 12, 179. [Google Scholar]
- Poruba, M.; Matuskova, Z.; Kazdová, L.; Oliyarnyk, O.; Malínská, H.; di Angelo, I.T.; Vecera, R. Positive effects of different drug forms of silybin in the treatment of metabolic syndrome. Physiol. Res. 2015, 64, S507. [Google Scholar] [CrossRef] [PubMed]
- Poruba, M.; Kazdová, L.; Oliyarnyk, O.; Malinská, H.; Matusková, Z.; di Angelo, I.T.; Skop, V.; Vecera, R. Improvement bioavailability of silymarin ameliorates severe dyslipidemia associated with metabolic syndrome. Xenobiotica 2015, 45, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Alex, R.; Bellner, L.; Raffaele, M.; Licari, M.; Vanella, L.; Stec, D.E.; Abraham, N.G. Milk thistle seed cold press oil attenuates markers of the metabolic syndrome in a mouse model of dietary-induced obesity. J. Food Biochem. 2020, 44, e13522. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Micheli, L.; Luceri, C.; D’Ambrosio, M.; Cinci, L.; Ghelardini, C.; Bilia, A.R.; Mannelli, L.D.C.; Bergonzi, M.C. Nanostructured lipid carriers for oral delivery of silymarin: Improving its absorption and in vivo efficacy in type 2 diabetes and metabolic syndrome model. Int. J. Pharm. 2019, 572, 118838. [Google Scholar] [CrossRef]
- Prakash, P.; Singh, V.; Jain, M.; Rana, M.; Khanna, V.; Barthwal, M.K.; Dikshit, M. Silymarin ameliorates fructose induced insulin resistance syndrome by reducing de novo hepatic lipogenesis in the rat. Eur. J. Pharmacol. 2014, 727, 15–28. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ashtary-Larky, D.; Asbaghi, O.; Farrokhi, V.; Jadidi, Y.; Mofidi, F.; Mohammadian, M.; Afrisham, R. Effects of silymarin supplementation on liver and kidney functions: A systematic review and dose–response meta-analysis. Phytother. Res. 2024, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Marková, I.; Malinska, H.; Hüttl, M.; Miklánková, D.; Oliyarnyk, O.; Poruba, M.; Racova, Z.; Kazdova, L.; Večeřa, R. The combination of atorvastatin with silymarin enhances hypolipidemic, antioxidant and anti-inflammatory effects in a rat model of metabolic syndrome. Physiol. Res. 2021, 70, 33. [Google Scholar] [CrossRef]
- Hadi, A.; Pourmasoumi, M.; Mohammadi, H.; Symonds, M.; Miraghajani, M. The effects of silymarin supplementation on metabolic status and oxidative stress in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2018, 41, 311–319. [Google Scholar] [CrossRef]
- Soleymani, S.; Ayati, M.H.; Mansourzadeh, M.J.; Namazi, N.; Zargaran, A. The effects of Silymarin on the features of cardiometabolic syndrome in adults: A systematic review and meta-analysis. Phytother. Res. 2022, 36, 842–856. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Berlin, J.A. Publication bias: A problem in interpreting medical data. J. R. Stat. Soc. Ser. A 1988, 151, 419–445. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.N. Interpreting and Visualizing Regression Models Using Stata; Stata Press: College Station, TX, USA, 2012; Volume 558. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Velussi, M.; Cernigoi, A.; Viezzoli, L.; Dapas, F.; Caffau, C.; Zilli, M. Silymarin reduces hyperinsulinemia, malondialdehyde levels, and daily insulin need in cirrhotic diabetic patients. Curr. Ther. Res. 1993, 53, 533–545. [Google Scholar] [CrossRef]
- Velussi, M.; Cernigoi, A.M.; Dapas, F.; Caffau, C.; Zilli, M. Long-term (23 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J. Hepatol. 1997, 26, 871–879. [Google Scholar] [CrossRef]
- Simanek, V.; Skottova, N.; Bartek, J.; Psotova, J.; Kosina, P.; Balejová, L.; Ulrichova, J. Extract from Silybum marianum as a nutraceutical: A double-blind placebo-controlled study in healthy young men. Czech J. Food Sci. 2001, 19, 105–110. [Google Scholar] [CrossRef]
- Huseini, H.F.; Larijani, B.; Fakhrzadeh, H.; Akhondzadeh, S.; Rajabipour, B.; Toliat, T.; Heshmat, R. The clinical trial of silybum marianum seed extract (silymarin) on type ii diabetic patients with hyperlipidemia. J. Diabetes Metab. Disord. 2004, 3, 78. [Google Scholar]
- Huseini, H.F.; Larijani, B.; Heshmat, R.; Fakhrzadeh, H.; Radjabipour, B.; Toliat, T.; Raza, M. The efficacy of Silybum marianum (L.) Gaertn.(silymarin) in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2006, 20, 1036–1039. [Google Scholar] [CrossRef]
- Hussain, S.A.-R. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J. Med. Food 2007, 10, 543–547. [Google Scholar] [CrossRef]
- Valentová, K.; Stejskal, D.; Bartek, J.; Dvořáčková, S.; Křen, V.; Ulrichová, J.; Šimánek, V. Maca (Lepidium meyenii) and yacon (Smallanthus sonchifolius) in combination with silymarin as food supplements: In vivo safety assessment. Food Chem. Toxicol. 2008, 46, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Azarabadi, M.; Abdi, H.; Baher, G.; Huseini, M. The effects of Silybum marianum (L.) Gaertn. seed extract on glycemic control in type II diabetic patient’s candidate for insulin therapy visiting endocrinology clinic in baqiyatallah hospital in the years of 2006. J. Med. Plants 2008, 7, 79–84. [Google Scholar]
- Hajagha, M.A.; Ziaei, A.; Rafiei, R. The efficacy of silymarin in decreasing transaminase activities in non-alcoholic fatty liver disease: A randomized controlled clinical trial. Hepat. Mon. 2008, 8, 191–195. [Google Scholar]
- Gharagozloo, M.; Moayedi, B.; Zakerinia, M.; Hamidi, M.; Karimi, M.; Maracy, M.; Amirghofran, Z. Combined therapy of silymarin and desferrioxamine in patients with β-thalassemia major: A randomized double-blind clinical trial. Fundam. Clin. Pharmacol. 2009, 23, 359–365. [Google Scholar] [CrossRef]
- Hashemi, S.J.; Hajiani, E.; Heydari, S.E. A placebo-controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat. Mon. 2009, 9, 265–270. [Google Scholar]
- Numan, A.T.; Hadi, N.A.; Mohammed, N.S.; Hussain, S.A. Use of silymarine as adjuvant in type 1 diabetes mellitus patients poorly controlled with insulin. J. Fac. Med. Baghdad 2010, 52, 75–79. [Google Scholar] [CrossRef]
- Rezvanian, H.; Kachuei, A.; Mirzapour, A. The Effect of Milk Thistle Extract in the Treatment of Diabetic Nephropathy. J. Isfahan Med. Sch. 2011, 28, 1997–2010. [Google Scholar]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: A randomized, double-blind, placebo-controlled trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef]
- Alkuraishy, H.M.; Alwindy, S. Beneficial effects of silymarin on lipid profile in hyperlipidemic patients: Placebo controlled clinical trail. WebmedCentral Pharmacol. 2012, 3, WMC002966. [Google Scholar]
- Mohammadi, S.; Afkhami Ardacani, M.; Salami, M.; Bolurani, S. Effects of silymarin on insulin resistance and blood lipid profile in first-degree relatives of type 2 diabetic patients. J. Med. Plants 2013, 12, 170–176. [Google Scholar]
- Taghvaei, T.; Bahar, A.; Hosseini, V.; Maleki, I.; Kasrai, M. Efficacy of silymarin on treatment of nonalcoholic steatohepatitis. J. Maz. Univ. Med. Sci. 2013, 23, 164–171. [Google Scholar]
- Masoodi, M.; Rezadoost, A.; Panahian, M.; Vojdanian, M. Effects of silymarin on reducing liver aminotransferases in patients with nonalcoholic fatty liver diseases. Govaresh 2013, 18, 181–185. [Google Scholar]
- Ebrahimpour Koujan, S.; Gargari, B.P.; Mobasseri, M.; Valizadeh, H.; Asghari-Jafarabadi, M. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: A randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine 2015, 22, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Elgarf, A.T.; Mahdy, M.M.; Sabri, N.A. Effect of silymarin supplementation on glycemic control, lipid profile and insulin resistance in patients with type 2 diabetes mellitus. Int. J. Adv. Res. 2015, 3, 812–821. [Google Scholar]
- Shirali, S.; Barari, A.R.; Hosseini, S.A. The Effects of Endurance Training and Administration of Silymarin Supplementation on Oxidative Enzyme of SOD and Heat Shock Proteins 70 in Plasma of Unathletes Men Students. Jundishapur Sci. Med. J. 2016, 14, 703–712. [Google Scholar]
- Shirali, S.; Mashhadi, N.S.; Ashtary-Larky, D.; Safania, T.; Barari, A. Effects of silymarin supplementation on leptin, adiponectin and paraoxanase levels and body composition during exercise: A randomized double-blind placebo controlled clinical trial. Jundishapur J. Nat. Pharm. Prod. 2016, 11, e30044. [Google Scholar] [CrossRef]
- Kheong, C.W.; Mustapha, N.R.N.; Mahadeva, S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1940–1949.e8. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour-Koujan, S.; Gargari, B.P.; Mobasseri, M.; Valizadeh, H.; Asghari-Jafarabadi, M. Lower glycemic indices and lipid profile among type 2 diabetes mellitus patients who received novel dose of Silybum marianum (L.) Gaertn.(silymarin) extract supplement: A Triple-blinded randomized controlled clinical trial. Phytomedicine 2018, 44, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Anushiravani, A.; Haddadi, N.; Pourfarmanbar, M.; Mohammadkarimi, V. Treatment options for nonalcoholic fatty liver disease: A double-blinded randomized placebo-controlled trial. Eur. J. Gastroenterol. Hepatol. 2019, 31, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Ghalandari, K.; Shabani, M.; Khajehlandi, A.; Mohammadi, A. Effect of aerobic training with silymarin consumption on glycemic indices and liver enzymes in men with type 2 diabetes. Arch. Physiol. Biochem. 2023, 129, 76–81. [Google Scholar] [CrossRef]
- Vidimce, J.; Pennell, E.N.; Foo, M.; Shiels, R.G.; Shibeeb, S.; Watson, M.; Bulmer, A.C. Effect of Silymarin Treatment on Circulating Bilirubin and Cardiovascular Disease Risk Factors in Healthy Men: A Single-Blind, Randomized Crossover Trial. Clin. Pharmacol. Drug Dev. 2021, 10, 1156–1165. [Google Scholar] [CrossRef]
- Shadmehri, S.; Aghaei, F.; Mirfallah Lialestani, S.N. The Effect of Silymarin and Pilates Training on Anthropometric Indices, Blood sugar and Some Liver Enzymes in Diabetic Women with Obesity. Sport Physiol. Manag. Investig. 2022, 14, 113–125. [Google Scholar]
- Memon, A.; Siddiqui, S.S.; Ata, M.A.; Shaikh, K.R.; Soomro, U.A.; Shaikh, S. Silymarin improves glycemic control through reduction of insulin resistance in newly diagnosed patients of type 2 diabetes mellitus. Prof. Med. J. 2022, 29, 362–366. [Google Scholar]
- Aryan, H.; Farahani, R.H.; Chamanara, M.; Elyasi, S.; Jaafari, M.R.; Haddad, M.; Sani, A.T.; Ardalan, M.A.; Mosaed, R. Evaluation of the efficacy of oral nano-silymarin formulation in hospitalized patients with COVID-19: A double-blind placebo-controlled clinical trial. Phytother. Res. 2022, 36, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Mirhashemi, S.H.; Hakakzadeh, A.; Yeganeh, F.E.; Oshidari, B.; Rezaee, S.P. Effect of 8 Weeks milk thistle powder (silymarin extract) supplementation on fatty liver disease in patients candidates for bariatric surgery. Metab. Open 2022, 14, 100190. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Salman, M.; Ali, I.; Fatima, N.; Mastoor, M.; Sayed, T.M. Analyze the Efficacy of Silymarin in Treating Newly Diagnosed Cases of Type 2 Diabetes Mellitus by Contrasting its Effects on Glycemic Control and Insulin Resistance. Pak. J. Med. Health Sci. 2022, 16, 365. [Google Scholar] [CrossRef]
- Di Pierro, F.; Putignano, P.; Villanova, N.; Montesi, L.; Moscatiello, S.; Marchesini, G. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin. Pharmacol. Adv. Appl. 2013, 5, 167–174. [Google Scholar] [CrossRef]
- Mohammadi, H.; Hadi, A.; Arab, A.; Moradi, S.; Rouhani, M.H. Effects of silymarin supplementation on blood lipids: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2019, 33, 871–880. [Google Scholar] [CrossRef]
- Stolf, A.M.; Cardoso, C.C.; Acco, A. Effects of silymarin on diabetes mellitus complications: A review. Phytother. Res. 2017, 31, 366–374. [Google Scholar] [CrossRef]
- Alabdan, M.A.J.B.B. Silymarin ameliorates metabolic risk factors and protects against cardiac apoptosis in streptozotocin-induced diabetic rats. Biomed. Biotechnol. 2015, 3, 20–27. [Google Scholar]
- Soto, C.; Mena, R.; Luna, J.; Cerbon, M.; Larrieta, E.; Vital, P.; Uria, E.; Sanchez, M.; Recoba, R.; Barron, H.; et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004, 75, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Bakhshi, A.; Dayati, P.; Abazari, O.; Shahidi, M.; Savaee, M.; Kafi, E.; Rahmanian, M.; Naghib, S.M. Silymarin reduces retinal microvascular damage in streptozotocin-induced diabetic rats. Sci. Rep. 2022, 12, 15872. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.P.; Perez, B.L.; Favari, L.P.; Reyes, J.L. Prevention of alloxan-induced diabetes mellitus in the rat by silymarin. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 119, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Amniattalab, A.; Malekinejad, H.; Rezabakhsh, A.; Rokhsartalab-Azar, S.; Alizade-Fanalou, S. Silymarin: A novel natural agent to restore defective pancreatic β cells in streptozotocin (STZ)-induced diabetic rats. Iran. J. Pharm. Res. IJPR 2016, 15, 493. [Google Scholar] [PubMed]
- Wu, C.-H.; Huang, S.-M.; Yen, G.-C. Silymarin: A novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Antioxid. Redox Signal. 2011, 14, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Kim, T.-S.; Park, J.-H.; Chi, S.-C. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch. Pharmacal Res. 2007, 30, 82–89. [Google Scholar] [CrossRef]
- Soto, C.; Raya, L.; Juárez, J.; Pérez, J.; González, I. Effect of Silymarin in Pdx-1 expression and the proliferation of pancreatic β-cells in a pancreatectomy model. Phytomedicine 2014, 21, 233–239. [Google Scholar] [CrossRef]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Orolin, J.; Večeřa, R.; Jung, D.; Meyer, U.; Škottová, N.; Anzenbacher, P. Hypolipidemic effects of silymarin are not mediated by the peroxisome proliferator-activated receptor alpha. Xenobiotica 2007, 37, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Škottová, N.; Kazdová, L.; Oliyarnyk, O.; Večeřa, R.; Sobolová, L.; Ulrichová, J. Phenolics-rich extracts from Silybum marianum and Prunella vulgaris reduce a high-sucrose diet induced oxidative stress in hereditary hypertriglyceridemic rats. Pharmacol. Res. 2004, 50, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q. Treatment strategy for type 2 diabetes with obesity: Focus on glucagon-like peptide-1 receptor agonists. Clin. Ther. 2017, 39, 1244–1264. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Bagherniya, M.; Fakheran, O.; Reiner, Ž.; Xu, S.; Sahebkar, A. Medicinal plants and bioactive natural compounds as inhibitors of HMG-CoA reductase: A literature review. BioFactors 2020, 46, 906–926. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High blood pressure and cardiovascular disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Keevil, J.G.; Osman, H.E.; Reed, J.D.; Folts, J.D. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J. Nutr. 2000, 130, 53–56. [Google Scholar] [CrossRef]
- Nosratabadi, S.; Ashtary-Larky, D.; Hosseini, F.; Namkhah, Z.; Mohammadi, S.; Salamat, S.; Nadery, M.; Yarmand, S.; Zamani, M.; Wong, A. The effects of vitamin C supplementation on glycemic control in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102824. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef]
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016, 30, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.; Izzo, A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 174, 1177. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).