Molecular Mechanism of Natural Food Antioxidants to Regulate ROS in Treating Cancer: A Review
Abstract
1. Introduction
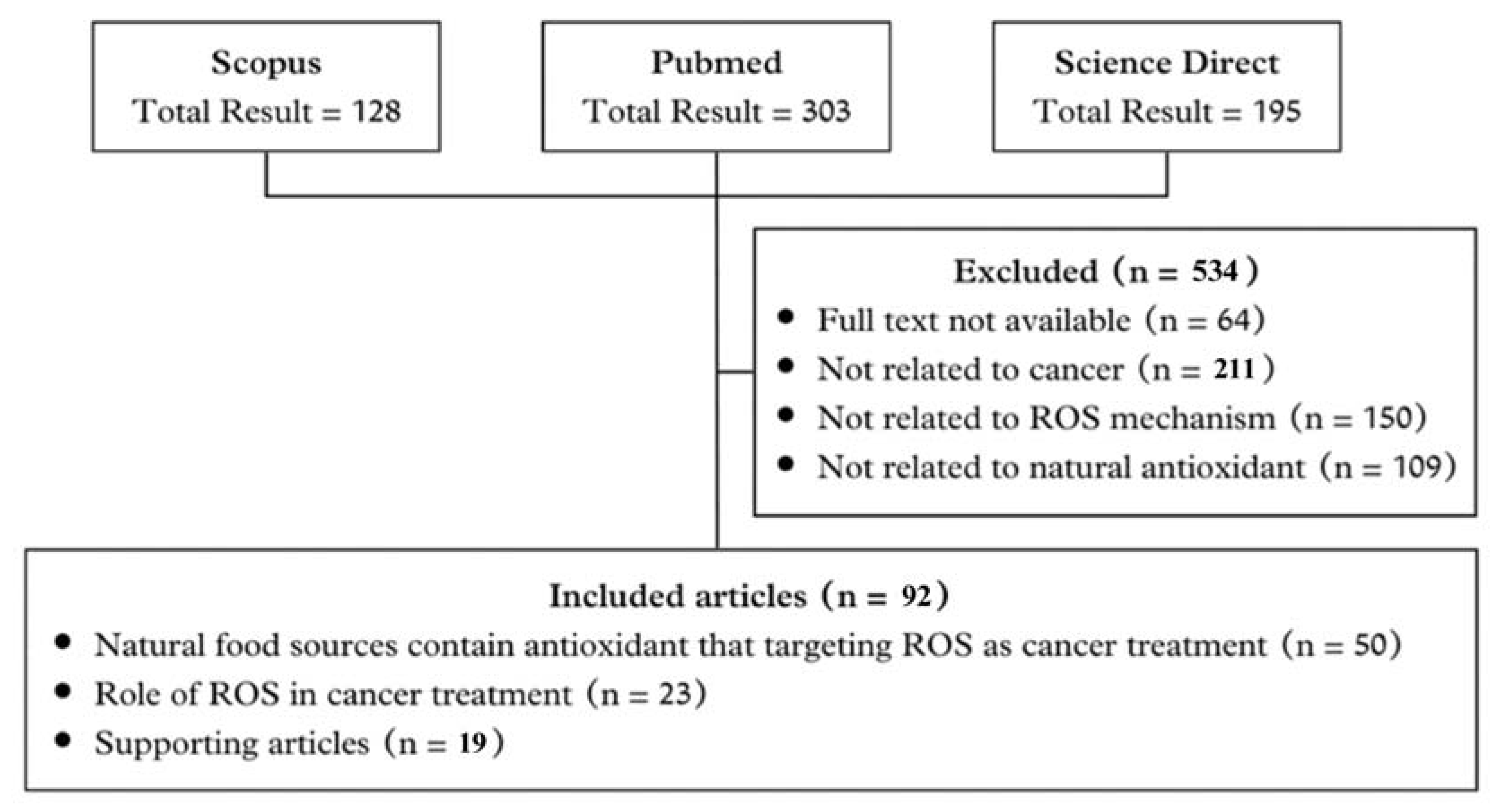
2. The Search Criteria
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Main Categories
3.1. Cancer and Oxidative Stress
3.2. ROS Production in Biological System and Its Sources
3.3. Regulation of ROS Levels
3.4. Role of ROS in Anticancer Therapy
3.5. Natural Antioxidants
4. Conclusions and Recommendation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Bajaj, J.; Diaz, E.; Reya, T. Stem cells in cancer initiation and progression. J. Cell Biol. 2020, 219, e201911053. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Bhat, A.A.; Siveen, K.S.; Kuttikrishnan, S.; Raza, S.S.; Raheed, T.; Jochebeth, A.; Khan, A.Q.; Chawdhery, M.Z.; Haris, M.; et al. Sanguinarine mediated apoptosis in Non-Small Cell Lung Cancer via generation of reactive oxygen species and suppression of JAK/STAT pathway. Biomed. Pharmacother. 2021, 144, 112358. [Google Scholar] [CrossRef] [PubMed]
- Mohany, M.; Al-Zharani, M.; Nasr, F.A.; El-Wetidy, M.S.; Farag, M.; Abdel-Mageed, W.M.; El-Gamal, A.A.; Al-Rejaie, S.S.; Noman, O.M.; Qurtam, A.A.; et al. Persicaline, an alkaloid from Salvadora persica, inhibits proliferation and induces apoptosis and cell-cycle arrest in MCF-7 cells. Open Chem. 2023, 21. [Google Scholar] [CrossRef]
- Carmen, V.-V.; Luis, D.-O.; José, A.M.G.; Ernesto Alanís, G.; José Roberto Villagomez, I.; Esther Ramírez, M.; Manuel Sánchez, G.; María Teresa Sumaya, M.; Zuñiga Pérez, C.; Zuli Calderón, R. The Role of Natural Antioxidants in Cancer Disease. In Oxidative Stress and Chronic Degenerative Diseases; José, A.M.-G., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 16. [Google Scholar]
- Sahoo, B.M.; Banik, B.K.; Borah, P.; Jain, A. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anticancer. Agents Med. Chem. 2022, 22, 215–222. [Google Scholar] [CrossRef]
- Jiao, R.; Liu, Y.; Gao, H.; Xiao, J.; So, K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa Morales, M.E.; Lorenzo, J.M. Plant Extracts Obtained with Green Solvents as Natural Antioxidants in Fresh Meat Products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular Mechanisms and Pathways as Targets for Cancer Prevention and Progression with Dietary Compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Altabbal, S.; Athamnah, K.; Rahma, A.; Wali, A.F.; Eid, A.H.; Iratni, R.; Al Dhaheri, Y. Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals 2023, 16, 450. [Google Scholar] [CrossRef]
- Veiga, A.A.; Irioda, A.C.; Mogharbel, B.F.; Bonatto, S.J.R.; Souza, L.M. Quercetin-Rich Extracts from Onions (Allium cepa) Play Potent Cytotoxicity on Adrenocortical Carcinoma Cell Lines, and Quercetin Induces Important Anticancer Properties. Pharmaceuticals 2022, 15, 754. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Parsa, N. Environmental factors inducing human cancers. Iran. J. Public. Health 2012, 41, 1–9. [Google Scholar] [PubMed]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gao, Y.; Li, H.; Wang, X.; Jin, M.; Shen, Z.; Yang, D.; Zhang, X.; Wei, Z.; Chen, Z.; et al. Association between oxidative stress, mitochondrial function of peripheral blood mononuclear cells and gastrointestinal cancers. J. Transl. Med. 2023, 21, 107. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Amirah, S.R.; Harmonis, J.A.; Ikram, E.H.K. Role of Nuclear Factor Erythroid 2 (Nrf2) in the Recovery of Long COVID-19 Using Natural Antioxidants: A Systematic Review. Antioxidants 2022, 11, 1551. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Caserta, S.; Genovese, S.; Pioggia, G.; Gangemi, S. Gender Differences in Oxidative Stress in Relation to Cancer Susceptibility and Survival. Antioxidants 2023, 12, 1255. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Li, Y.R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic Pathway of Natural Antioxidants, Antioxidant Enzymes and ROS Providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, A.; Arif, U.; Akram, F.; Batool, F.; Hassan, S.; Rashid, B. Abiotic Stress: Interplay Between ROS Production and Antioxidant Machinery, Signaling, and ROS Homeostasis. OBM Genet. 2022, 6, 171. [Google Scholar] [CrossRef]
- Tyagi, M.; Bauri, A.K.; Chattopadhyay, S.; Patro, B.S. Thiol antioxidants sensitize malabaricone C induced cancer cell death via reprogramming redox sensitive p53 and NF-κB proteins in vitro and in vivo. Free Radic. Biol. Med. 2020, 148, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, H.; Wang, Y.; Zheng, A.; Cao, L.; Liu, J. Autophagy Deficiency Leads to Impaired Antioxidant Defense via p62-FOXO1/3 Axis. Oxidative Med. Cell. Longev. 2019, 2019, 2526314. [Google Scholar] [CrossRef]
- Upegui, Y.; Robledo, S.M.; Gil Romero, J.F.; Quiñones, W.; Archbold, R.; Torres, F.; Escobar, G.; Nariño, B.; Echeverri, F. In vivo Antimalarial Activity of α-Mangostin and the New Xanthone δ-Mangostin. Phytother. Res. 2015, 29, 1195–1201. [Google Scholar] [CrossRef]
- Papa, S.; Zazzeroni, F.; Pham, C.; Bubici, C.; Franzoso, G. Linking JNK signaling to NF-κB: A key to survival. J. Cell Sci. 2004, 117, 5197–5208. [Google Scholar] [CrossRef]
- Chen, M.; Qian, C.; Jin, B.; Hu, C.; Zhang, L.; Wang, M.; Zhou, B.; Zuo, W.; Huang, L.; Wang, Y. Curcumin analog WZ26 induces ROS and cell death via inhibition of STAT3 in cholangiocarcinoma. Cancer Biol. Ther. 2023, 24, 2162807. [Google Scholar] [CrossRef]
- Krishnaraj, J.; Yamamoto, T.; Ohki, R. p53-Dependent Cytoprotective Mechanisms behind Resistance to Chemo-Radiotherapeutic Agents Used in Cancer Treatment. Cancers 2023, 15, 3399. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef]
- Tchelebi, L.; Ashamalla, H.; Graves, P.R. Mutant p53 and the response to chemotherapy and radiation. Subcell. Biochem. 2014, 85, 133–159. [Google Scholar] [CrossRef]
- Xu, J.; Patel, N.H.; Gewirtz, D.A. Triangular Relationship between p53, Autophagy, and Chemotherapy Resistance. Int. J. Mol. Sci. 2020, 21, 8991. [Google Scholar] [CrossRef]
- Hu, J.; Cao, J.; Topatana, W.; Juengpanich, S.; Li, S.; Zhang, B.; Shen, J.; Cai, L.; Cai, X.; Chen, M. Targeting mutant p53 for cancer therapy: Direct and indirect strategies. J. Hematol. Oncol. 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015, 16, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 2019, 19, 1224. [Google Scholar] [CrossRef] [PubMed]
- Ray, G.; Batra, S.; Shukla, N.K.; Deo, S.; Raina, V.; Ashok, S.; Husain, S.A. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res. Treat. 2000, 59, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Lang, W.H.; Craig, J.; Potts, M.B.; Budhraja, A.; Opferman, J.; Bollinger, J.; Maier, J.; Marsico, T.D.; Rivas, F. Studies of Jatrogossone A as a Reactive Oxygen Species Inducer in Cancer Cellular Models. J. Nat. Prod. 2019, 82, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Lian, X.-D.; Lee, S.-J.; Li, W.-L.; Sun, H.-N.; Jin, M.-H.; Kwon, T. Regulatory effect of peroxiredoxin 1 (PRDX1) on doxorubicin-induced apoptosis in triple negative breast cancer cells. Appl. Biol. Chem. 2022, 65, 63. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, R.; Gaspar, J.M.; Sargsyan, D.; Su, Z.-Y.; Zhang, C.; Gao, L.; Cheng, D.; Li, W.; Wang, C.; et al. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis 2018, 39, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.M.; Zhang, P.Y. Protective effects of curcumin and quercetin during benzo(a)pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1736–1743. [Google Scholar] [PubMed]
- Wulandari, F.; Ikawati, M.; Widyarini, S.; Kirihata, M.; Novitasari, D.; Kato, J.-y.; Meiyanto, E. Tumour-suppressive effects of curcumin analogs CCA-1.1 and Pentagamavunone-1 in colon cancer: In vivo and in vitro studies. J. Adv. Pharm. Technol. Res. 2023, 14, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Novitasari, D.; Kato, J.-Y.; Ikawati, M.; Putri, D.D.P.; Wulandari, F.; Widyarini, S.; Zulfin, U.M.; Salsabila, D.U.; Meiyanto, E. PGV-1 permanently arrests HepG2 cells in M phase and inhibits liver carcinogenesis in DMH-induced rats. J. Appl. Pharm. Sci. 2023, 13, 204–211. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the Risk of Prostate Cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Sithara, T.; Dhanya, B.P.; Arun, K.B.; Sini, S.; Dan, M.; Kokkuvayil Vasu, R.; Nisha, P. Zerumbone, a Cyclic Sesquiterpene from Zingiber zerumbet Induces Apoptosis, Cell Cycle Arrest, and Antimigratory Effects in SW480 Colorectal Cancer Cells. J. Agric. Food Chem. 2018, 66, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, P.; Hsieh, P.F.; Jiang, W.P.; Bau, D.T.; Huang, G.J.; Huang, W.C. Elucidation of scandenolone as anti-cancer activity through impairment of the metabolic and signaling vulnerabilities in prostate cancer. Biomed. Pharmacother. 2023, 164, 114948. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kwon, O.Y.; Lee, S.H. Kaempferide Prevents Photoaging of Ultraviolet-B Irradiated NIH-3T3 Cells and Mouse Skin via Regulating the Reactive Oxygen Species-Mediated Signalings. Antioxidants 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Tie, F.; Ding, J.; Hu, N.; Dong, Q.; Chen, Z.; Wang, H. Kaempferol and Kaempferide Attenuate Oleic Acid-Induced Lipid Accumulation and Oxidative Stress in HepG2 Cells. Int. J. Mol. Sci. 2021, 22, 8847. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, B.H.; Qiu, X.; Wang, H.S.; Zhang, F.; Fang, R.; Wang, X.F.; Cai, S.H.; Du, J.; Bu, X.Z. T63, a new 4-arylidene curcumin analogue, induces cell cycle arrest and apoptosis through activation of the reactive oxygen species-FOXO3a pathway in lung cancer cells. Free Radic. Biol. Med. 2012, 53, 2204–2217. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef]
- Aslan, A.; Gok, O.; Beyaz, S.; Uslu, H.; Erman, F.; Erman, O.; Baspinar, S. Ellagic acid inhibits proinflammatory intermediary manufacture by suppressing NF-κB/Akt, VEGF and activating Nrf-2/Caspase-3 signaling pathways in rat testicular damage: A new way for testicular damage cure and in silico approach. Toxicol. Mech. Methods 2022, 32, 463–476. [Google Scholar] [CrossRef]
- Bell, C.; Hawthorne, S. Ellagic acid, pomegranate and prostate cancer—A mini review. J. Pharm. Pharmacol. 2008, 60, 139–144. [Google Scholar] [CrossRef]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.A.; Cardile, V.; Kim, D.H.; Abraham, N.G.; Sorrenti, V. Apoptotic markers in a prostate cancer cell line: Effect of ellagic acid. Oncol. Rep. 2013, 306, 2804–2810. [Google Scholar] [CrossRef]
- Safari, H.; Zabihi, E.; Pouramir, M.; Morakabati, P.; Abedian, Z.; Karkhah, A.; Nouri, H.R. Decrease of intracellular ROS by arbutin is associated with apoptosis induction and downregulation of IL-1β and TNF-α in LNCaP; prostate cancer. J. Food Biochem. 2020, 44, e13360. [Google Scholar] [CrossRef]
- Mileo, A.M.; Di Venere, D.; Mardente, S.; Miccadei, S. Artichoke Polyphenols Sensitize Human Breast Cancer Cells to Chemotherapeutic Drugs via a ROS-Mediated Downregulation of Flap Endonuclease 1. Oxid. Med. Cell Longev. 2020, 2020, 7965435. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chang, C.T.; Gowrisankar, Y.V.; Chen, X.Z.; Lin, H.C.; Yen, H.R.; Yang, H.L. Zerumbone Exhibits Antiphotoaging and Dermatoprotective Properties in Ultraviolet A-Irradiated Human Skin Fibroblast Cells via the Activation of Nrf2/ARE Defensive Pathway. Oxid. Med. Cell Longev. 2019, 2019, 4098674. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Lee, C.-L.; Korivi, M.; Liao, J.-W.; Rajendran, P.; Wu, J.-J.; Hseu, Y.-C. Zerumbone protects human skin keratinocytes against UVA-irradiated damages through Nrf2 induction. Biochem. Pharmacol. 2018, 148, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qiu, X.Y.; Wu, X.; Hu, D.X.; Li, C.Y.; Yu, S.B.; Pan, F.; Chen, X.Q. Piperlongumine suppresses bladder cancer invasion via inhibiting epithelial mesenchymal transition and F-actin reorganization. Biochem. Biophys. Res. Commun. 2017, 494, 165–172. [Google Scholar] [CrossRef]
- Dewangan, J.; Tandon, D.; Srivastava, S.; Verma, A.K.; Yapuri, A.; Rath, S.K. Novel combination of salinomycin and resveratrol synergistically enhances the anti-proliferative and pro-apoptotic effects on human breast cancer cells. Apoptosis 2017, 22, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Preya, U.H.; Lee, K.-T.; Kim, N.J.; Lee, J.-Y.; Jang, D.S.; Choi, J.-H. The natural terthiophene α-terthienylmethanol induces S phase cell cycle arrest of human ovarian cancer cells via the generation of ROS stress. Chem. Biol. Interact. 2017, 272, 72–79. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatt, P.C.; Kaithwas, G.; Rashid, M.; Al-Abbasi, F.; Khan, J.A.; Anwar, F.; Verma, A. α-Mangostin mediated pharmacological modulation of hepatic carbohydrate metabolism in diabetes induced Wistar rat. Beni-Suef Univ. J. Basic. Appl. Sci. 2016, 5, 255–276. [Google Scholar] [CrossRef]
- Oršolić, N.; Kunštić, M.; Kukolj, M.; Gračan, R.; Nemrava, J. Oxidative stress, polarization of macrophages and tumour angiogenesis: Efficacy of caffeic acid. Chem. Biol. Interact. 2016, 256, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mandal, M. Involvement of non-protein thiols, mitochondrial dysfunction, reactive oxygen species and p53 in honey-induced apoptosis. Invest. New Drugs 2010, 28, 624–633. [Google Scholar] [CrossRef]
- Wang, H.; Sun, N.; Li, X.; Li, K.; Tian, J.; Li, J. Diallyl trisulfide induces osteosarcoma cell apoptosis through reactive oxygen species-mediated downregulation of the PI3K/Akt pathway. Oncol. Rep. 2016, 35, 3648–3658. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Pelicano, H.; Liao, J.; Huang, J.; Feng, L.; Keating, M.J.; Huang, P. Targeting p53-deficient chronic lymphocytic leukemia cells in vitro and in vivo by ROS-mediated mechanism. Oncotarget 2016, 7, 71378–71389. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Torres, Á.; Bort, A.; Morell, C.; Rodríguez-Henche, N.; Díaz-Laviada, I. The pepper’s natural ingredient capsaicin induces autophagy blockage in prostate cancer cells. Oncotarget 2015, 7, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhao, Y.; Jiao, Y.; Shi, T.; Yang, X. ROS-Dependent Mitochondria Molecular Mechanisms Underlying Antitumor Activity of Pleurotus abalonus Acidic Polysaccharides in Human Breast Cancer MCF-7 Cells. PLoS ONE 2013, 8, e64266. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Ho, Y.J.; Lin, J.F.; Yeh, C.W.; Kao, S.H.; Hsu, L.S. Butein inhibits the proliferation of breast cancer cells through generation of reactive oxygen species and modulation of ERK and p38 activities. Mol. Med. Rep. 2012, 6, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Lai, T.Y.; Yang, J.H.; Yang, J.S.; Ma, Y.S.; Weng, S.W.; Chen, Y.Y.; Lin, J.G.; Chung, J.G. Gallic acid induces apoptosis in A375.S2 human melanoma cells through caspase-dependent and -independent pathways. Int. J. Oncol. 2010, 37, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of maceration temperature in red wine vinification on extraction of phenolics from berry skins and seeds of grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 282, 1034–1043. [Google Scholar] [CrossRef]
- Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Perez-Ruiz, A.G.; Luna-Arias, J.P.; García-Sánchez, J.R. Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells is Mediated by Reactive Oxygen Species. Molecules 2020, 25, 1020. [Google Scholar] [CrossRef]
- Cheng, S.-B.; Wu, L.-C.; Hsieh, Y.-C.; Wu, C.-H.; Chan, Y.-J.; Chang, L.-H.; Chang, C.-M.J.; Hsu, S.-L.; Teng, C.-L.; Wu, C.-C. Supercritical Carbon Dioxide Extraction of Aromatic Turmerone from Curcuma longa Linn. Induces Apoptosis through Reactive Oxygen Species-Triggered Intrinsic and Extrinsic Pathways in Human Hepatocellular Carcinoma HepG2 Cells. J. Agric. Food Chem. 2012, 60, 9620–9630. [Google Scholar] [CrossRef]
- Moon, D.O.; Kang, S.H.; Kim, K.C.; Kim, M.O.; Choi, Y.H.; Kim, G.Y. Sulforaphane decreases viability and telomerase activity in hepatocellular carcinoma Hep3B cells through the reactive oxygen species-dependent pathway. Cancer Lett. 2010, 295, 260–266. [Google Scholar] [CrossRef]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef] [PubMed]
- Rettig, M.B.; Heber, D.; An, J.; Seeram, N.P.; Rao, J.Y.; Liu, H.; Klatte, T.; Belldegrun, A.; Moro, A.; Henning, S.M.; et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic Acid, a Triterpene from Olive, Affects the Antioxidant and Mitochondrial Status of B16F10 Melanoma Cells Grown under Stressful Conditions. Evid. Based Complement. Altern. Med. 2015, 2015, 272457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, X.X.; Yang, S.Y.; Chen, Y.Z. Clinical Success of Drug Targets Prospectively Predicted by In Silico Study. Trends Pharmacol. Sci. 2018, 39, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Hao, Y.; Chen, S.; Jia, G.; Guo, Y.; Zhang, G.; Wang, C.; Cheng, R.; Hu, T.; Zhang, X.; et al. Rutin induces apoptosis via P53 up-regulation in human glioma CHME cells. Transl. Cancer Res. 2019, 8, 2005–2013. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Das, L.; Vinayak, M. Long term effect of curcumin in restoration of tumour suppressor p53 and phase-II antioxidant enzymes via activation of Nrf2 signalling and modulation of inflammation in prevention of cancer. PLoS ONE 2015, 10, e0124000. [Google Scholar] [CrossRef]
- Scuto, M.; Trovato Salinaro, A.; Caligiuri, I.; Ontario, M.L.; Greco, V.; Sciuto, N.; Crea, R.; Calabrese, E.J.; Rizzolio, F.; Canzonieri, V.; et al. Redox modulation of vitagenes via plant polyphenols and vitamin D: Novel insights for chemoprevention and therapeutic interventions based on organoid technology. Mech. Ageing Dev. 2021, 199, 111551. [Google Scholar] [CrossRef]
- Jung, K.-H.; Lee, J.H.; Park, J.W.; Moon, S.-H.; Cho, Y.S.; Choe, Y.S.; Lee, K.-H. Effects of curcumin on cancer cell mitochondrial function and potential monitoring with 18F-FDG uptake. Oncol. Rep. 2016, 35, 861–868. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re308. [Google Scholar] [CrossRef] [PubMed]

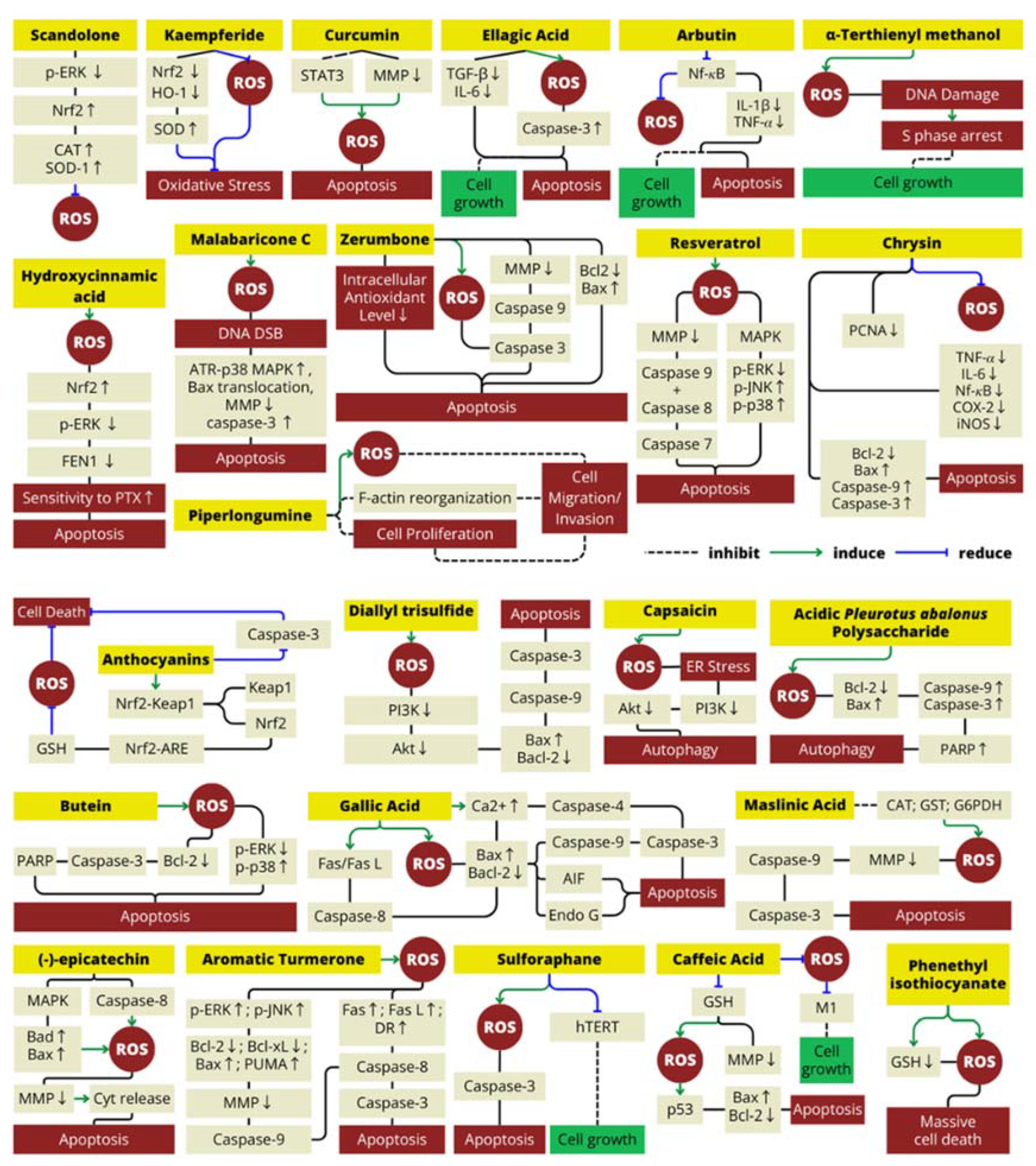
| Natural Antioxidant | Type of Antioxidant | Food Source(s) | Molecular Mechanism | Cancer Line Cell(s) | Reference(s) |
|---|---|---|---|---|---|
| Scandolone | Flavonoid | Cudrania tricuspidata (Chinese mulberry) | Decrease ROS levels in cells by suppressing the protein levels of p-ERK that inhibit Nrf2 expression, increasing the expression levels of Nrf2/HO-1 and elevating the expression levels of CAT and SOD-1 | LNCap and CRPC (prostate cancer cell line) | [53] |
| Kaempferide | Flavonoid | Alpinia officinarum (galangal rhizome) and Hippophae rhamnoides (sea-buckthorn’s fruit and seed) | Reduce oxidative stress by decreasing the levels of Nrf2 and HO-1 expression; increased the SOD content | HepG2 (human liver cancer cell line) | [54,55] |
| Curcumin | Polyphenol | Curcuma longa (turmeric rhizome) | Increase ROS (H2O2) production by reducing mitochondrial membrane potential and inhibiting the activation of STAT3 signaling that induce apoptosis and cell cycle arrest in cancer cells; promoted MAPK (mitogen-activated protein kinase) pathway activation | CCA (human cholangiocarcinoma cell line); human glioblastoma multiforme (GBM) cell line | [32,56,57] |
| Ellagic acid | Polyphenol | Punica granatum (pomegranate fruit) | Inhibit cell growth and induces apoptosis; increase ROS, activate caspase-3; reduce TGF-beta and IL-6 that inhibit cancel cell apoptosis | LNCap and PC3 (prostate cancer cell line) | [58,59,60] |
| Arbutin/hydroquinone-β-d-glucopyranoside | Polyphenol | Arctostaphylos sp. (bearberry) | Reduce ROS levels and decrease proliferative gene that characterized by an increase in apoptosis via reduction in the activation of NF-κB that reduce the expression of downstream genes, including IL-1β and TNF-𝛼 | LNCap (prostate cancer cell line) | [61] |
| Malabaricone C | Polyphenol | Myristica malabarica (fruits rinds) | Malabaricone C mediates the formation of ROS-dependent DNA DSB, which activate caspase-3, ATR-p38 MAPK, BAX translocation, and mitochondrial membrane potential collapse | A549 (human lung carcinoma) | [27] |
| Hydroxycinnamic acid derivatives (mono and dicaffeoylquinic acid) | Polyphenol | Cynara scolymus (artichoke head/edible part) | This polyphenol enhances cancer cell sensitivity to PTX (paclitaxel) treatment by decreasing cell proliferation both through the ROS/Nrf2 pathway (elevate ROS level) and downregulation of p-ERK that cause decrease in FEN1 expression | MCF7 and MDA-MB231 (human breast cancer) | [62] |
| Zerumbone | Terpenoid | Zingiber zerumbet rhizomes (Lempuyang) | Decreased antioxidant level and induce ROS-dependent apoptotic effect in both intrinsic and extrinsic apoptosis pathway | SW480 cell lines (human colon cancer) | [52,63,64] |
| Piperlongumine | Alkaloid | Piper longum (long pepper) | Suppresses bladder cancer cell migration/invasion mainly via ROS accumulation, Erk and PKC pathways | Human bladder cancer cell lines (T24, BIU-87, EJ) | [65] |
| Resveratrol | Polyphenol | Vitis sp. (grape skin), Punica granatum (pomegranate), berries, Glycine max (soy beans) and Arachis hypogaea (Peanuts) | Resveratrol act as antioxidant that can suppresses the production of ROS and inhibits COX-2 expression and prostaglandin synthesis; novel combination with salinomycin synergistically enhance the antiproliferative and proapoptotic effect by induce ROS generation | MCF-7 (human breast cancer cell) | [66] |
| A-terthienyl methanol | Terthiophene | Eclipta prostrata (false daisy) | Induce intracellular ROS and reversed NAC mechanism in arresting the S phase induces by α-terthienylmethanol | A2780, SKOV3, OVCAR3, and ES2 (human ovarian cancer cell line) | [67] |
| Chrysin | Polyphenol | Honey, propolis, Passiflora caerulea (blue passionflower) | Scavenged the ROS, inhibited proliferation (downregulation of PCNA), inflammation (downregulation of TNF-𝛼, IL-6, NF-kB, COX-2) and triggered apoptosis (upregulation of bax, caspase-9 and caspase-3). | Renal cell carcinoma | [68] |
| Caffeic acid | Polyphenol | Coffea sp. (Coffee), Olea europaea (olive oil), propolis | Blocks the production of ROS and suppress tumor growth and angiogenesis | Ehrlich ascites tumor | [69] |
| Honey | Depleting non-protein thiol (GSH) level to elevate ROS production and decrease the mitochondrial membrane potential | Colon carcinoma cell lines HCT-15 | [70] | ||
| Diallyl trisulfide | Organosulfur | Allium sp. (garlic) | Induce ROS increase, inhibit PI3K/Akt pathways, and induce apoptosis | Human osteosarcoma cell lines (MG-63 and MNNG/HOS) | [71] |
| Phenethyl isothiocyanate | Isothiocyanate | Brassica oleracea (broccoli, cauliflower, cabbage, kale) | Induce depletion of GSH and cause severe ROS accumulation leading to massive cell death in CCL with p53-deficiency | Chronic lymphocytic leukemia | [72] |
| Capsaicin | Terpenoid | Capsicum sp. | Inhibits the PI3K/Akt/mTOR axe to modulates autophagy by induce ROS generation and trigger ER stress | LNCaP and PC-3 (prostate cancer cell line) | [73] |
| Acidic pleurotus abalonus polysaccharide | Polysaccharide | Pleurotus abalonus (abalone mushroom) | Increase Bax/Bcl-2 ratio, caspase-9/3 activation, and poly(ADP-ribose) polymerase (PARP) degradation by increasing ROS level and cause apoptosis | MCF-7 (human breast cancer cell line) | [74] |
| Butein | Polyphenol | Rhus verniciflua Stokes | Induce ROS generation, inhibited ERK activity, enhanced p38 activation, decreased Bcl-2 expression, triggered the cleavage of pro-caspase-3 and PARP, and caused inhibited cell proliferation in cancer cells | MDA-MB-231 (human breast cancer cell line) | [75] |
| Gallic acid | Polyphenol | Ceratonia siliqua (carob fruit), Vitis vinifera (grape skin and seed), Camellia sinensis (green tea), Fragaria vesca (strawberries), and Musa sp. (bananas) | Induce ROS and intracellular Ca2+ production, decrease mitochondrial membrane potential level, and downregulate Bcl-2 (anti-apoptotic protein) | A375 S2 (human melanoma cell) | [76,77,78] |
| (−)-epicatechin | Polyphenol | Theobroma cacao (cocoa) | Induces ROS production and oxidative damage in cancer cells with upregulating the Bad and Bax that induce the leakage of cytochrome C into the cytoplasm and cause apoptosis | MDA-MB-231 and MCF-7 cell line (breast cancer) | [79] |
| Aromatic Turmerone | Polyphenol | Curcuma longa | Provoke ROS production that can lead to elevate ERK and JNK protein that cause increase bax/bcl-2 ratio and DR4 production. These condition cause cytochrome release and cause apoptosis | Human hepatocellular carcinoma HepG2 cells | [80] |
| Sulforaphane | Isothiocyanate | Brassica oleracea (broccoli and cauliflower) | Elevate ROS level to inhibit the recovery of hTERT expression after downregulating it and to induce ROS-dependent apoptosis in cancer cell | Human hepatocellular carcinoma Hep3B cells | [81] |
| Anthocyanins | Flavonoid | Punica granatum (pomegranate fruit extract) | Elevate the expression of phase II and antioxidant enzymes; act as ROS scavenger by activation of GSH expression; activate antioxidant response element (ARE) upstream of genes; activate Nf-kb inhibitor to induce apoptosis; | LNCaP-AR and LAPC4 cells (prostate cancer cell line) | [82,83] |
| Maslinic acid | Terpenoid | Olea europaea (olive) | Increase ROS level and induce apoptosis in cancer cell via intrinsic pathway | B16F10 melanoma cell line | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muchtaridi, M.; Az-Zahra, F.; Wongso, H.; Setyawati, L.U.; Novitasari, D.; Ikram, E.H.K. Molecular Mechanism of Natural Food Antioxidants to Regulate ROS in Treating Cancer: A Review. Antioxidants 2024, 13, 207. https://doi.org/10.3390/antiox13020207
Muchtaridi M, Az-Zahra F, Wongso H, Setyawati LU, Novitasari D, Ikram EHK. Molecular Mechanism of Natural Food Antioxidants to Regulate ROS in Treating Cancer: A Review. Antioxidants. 2024; 13(2):207. https://doi.org/10.3390/antiox13020207
Chicago/Turabian StyleMuchtaridi, Muchtaridi, Farhah Az-Zahra, Hendris Wongso, Luthfi Utami Setyawati, Dhania Novitasari, and Emmy Hainida Khairul Ikram. 2024. "Molecular Mechanism of Natural Food Antioxidants to Regulate ROS in Treating Cancer: A Review" Antioxidants 13, no. 2: 207. https://doi.org/10.3390/antiox13020207
APA StyleMuchtaridi, M., Az-Zahra, F., Wongso, H., Setyawati, L. U., Novitasari, D., & Ikram, E. H. K. (2024). Molecular Mechanism of Natural Food Antioxidants to Regulate ROS in Treating Cancer: A Review. Antioxidants, 13(2), 207. https://doi.org/10.3390/antiox13020207










