Protective Effect of Knee Postoperative Fluid on Oxidative-Induced Damage in Human Knee Articular Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Serum Sample Preparation
2.2. Cell Culture and Treatments
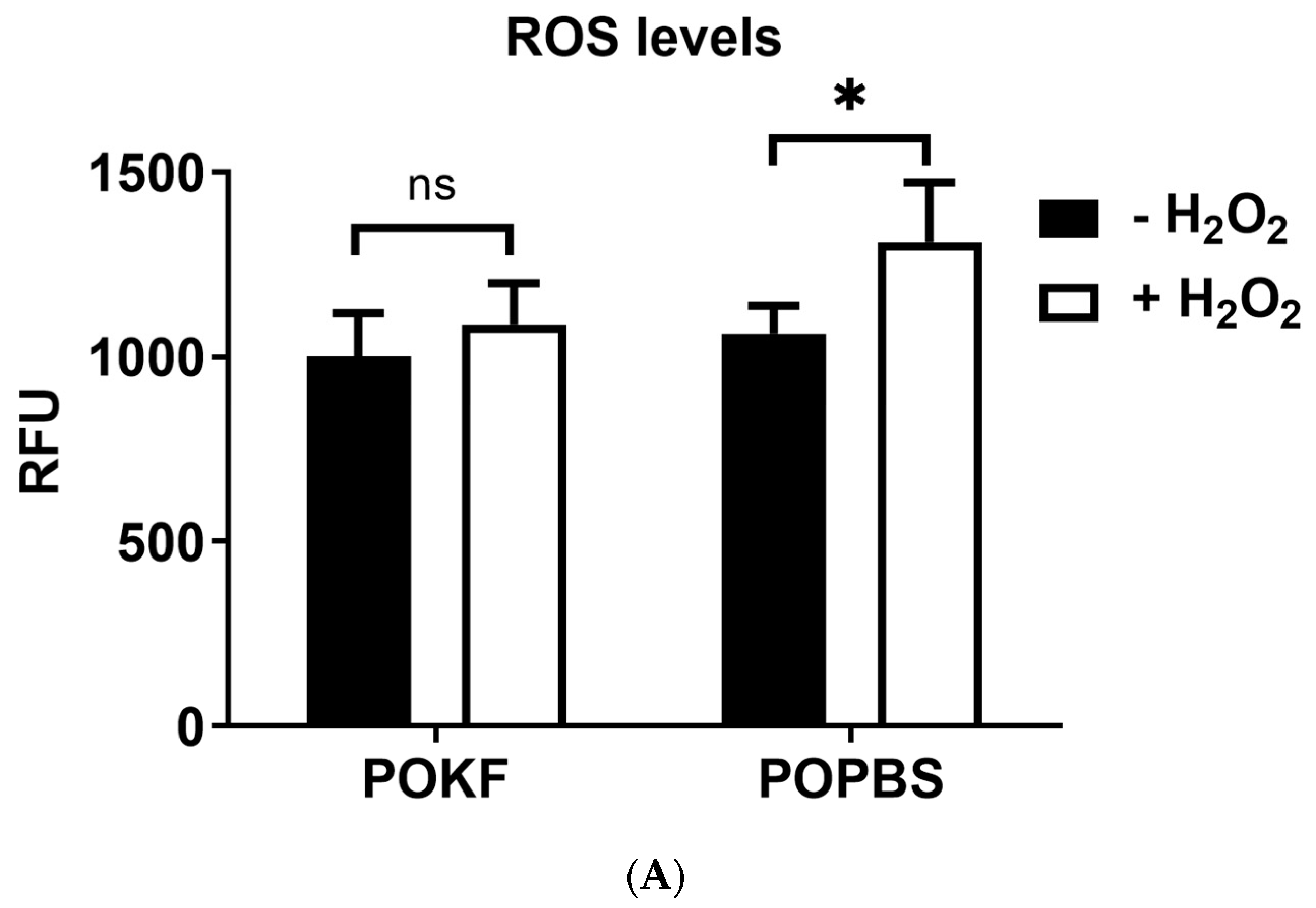
2.3. Determination of Intracellular ROS Levels
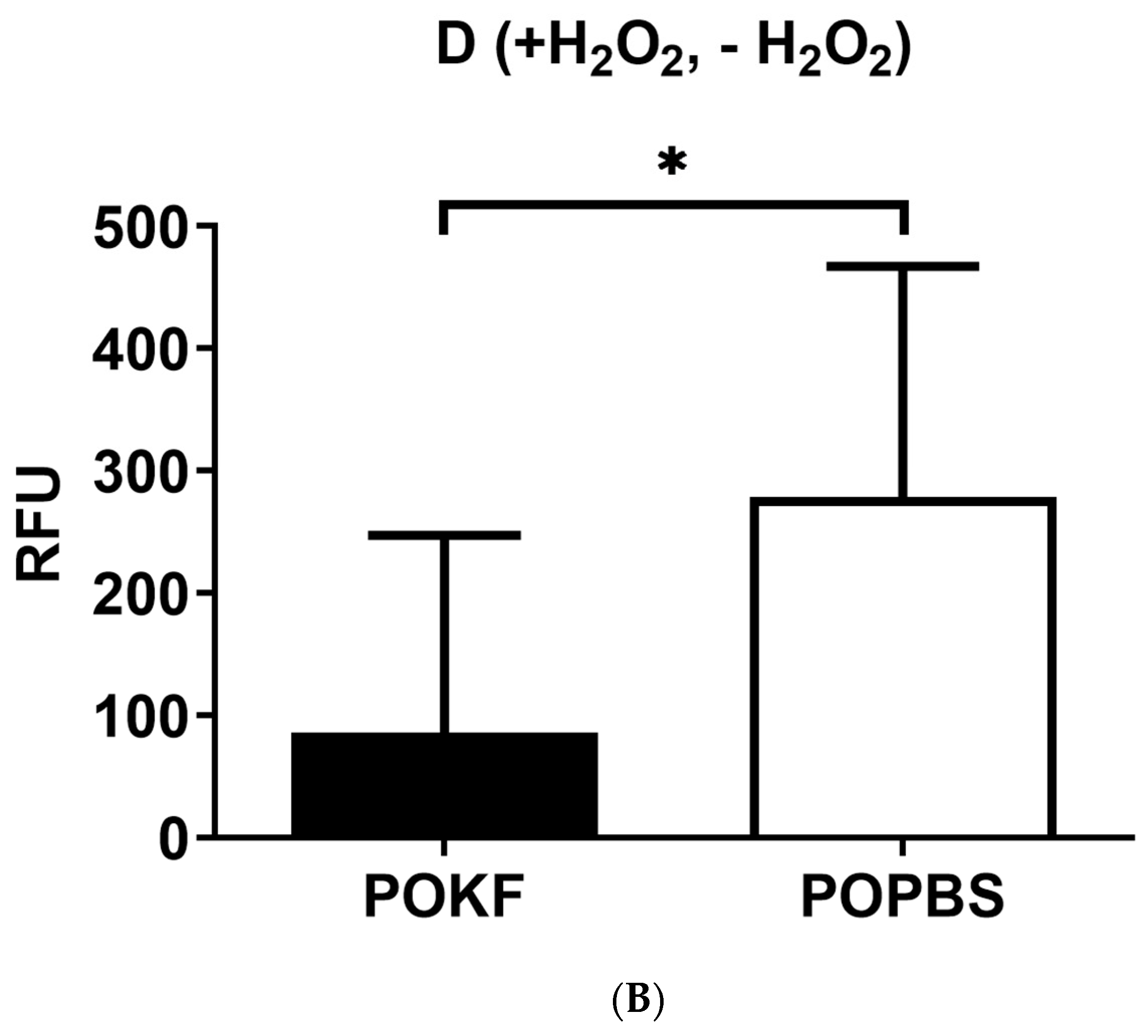
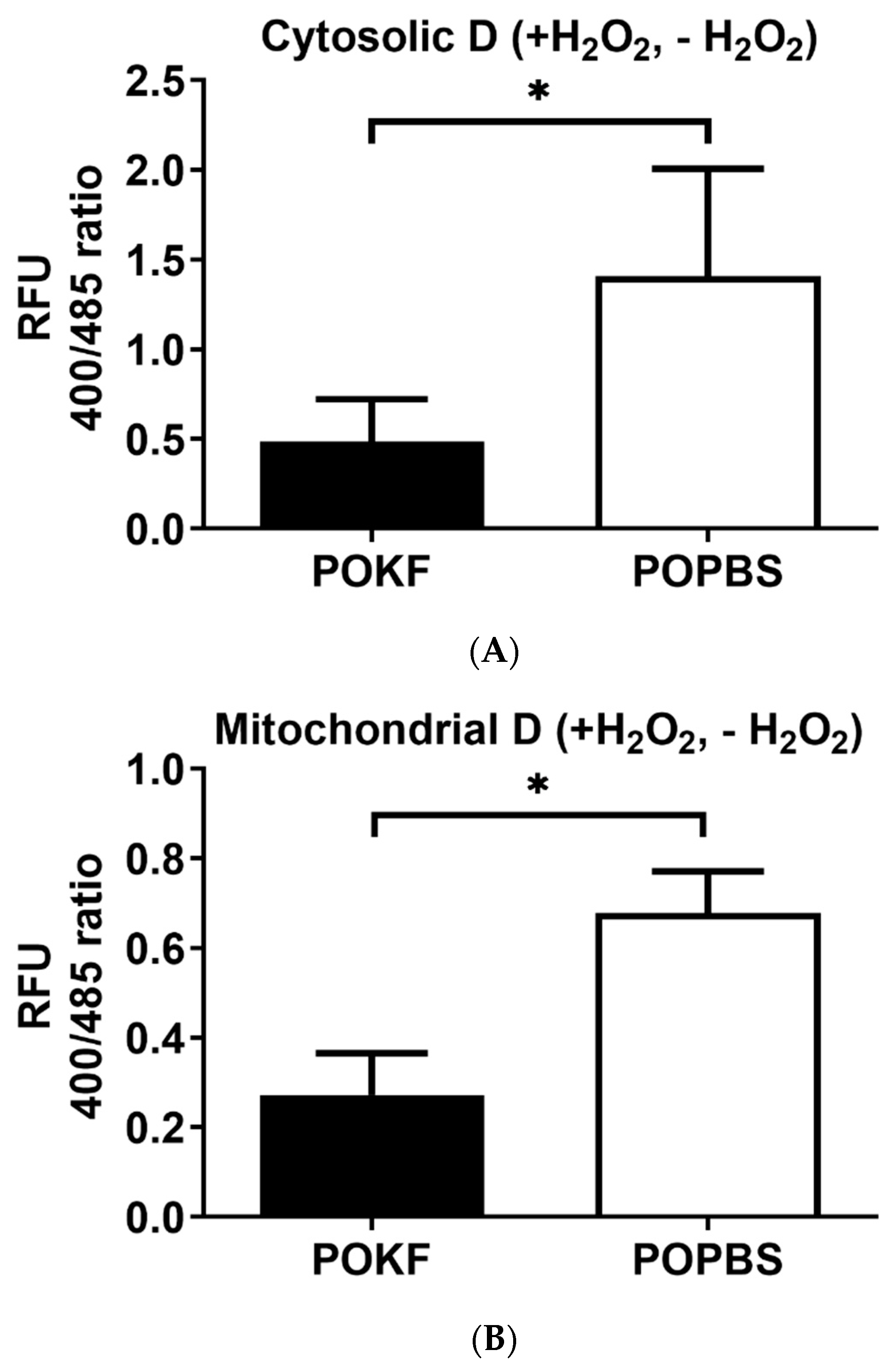
2.4. Determination of Cytosolic and Mitochondrial Redox Status
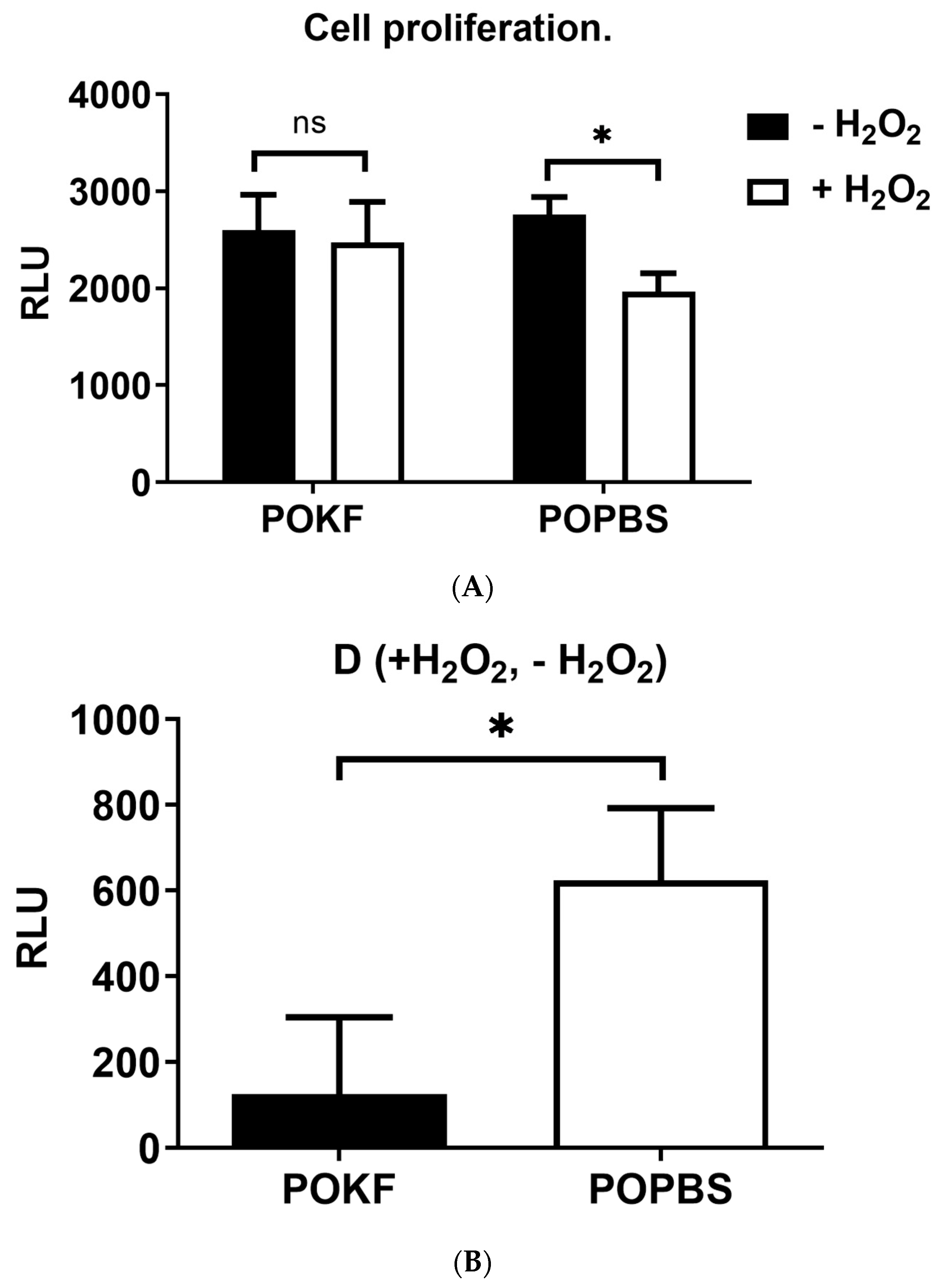
2.5. Determination of Cell Proliferation
2.6. Protein Extraction
2.7. Determination of Superoxide Dismutase (SOD) Activity
2.8. Determination of Catalase Activity
2.9. Determination of Glutathione (GSH) Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Circulating Factors in POKF Protect Chondrocytes from H2O2-Induced Oxidative Stress
3.2. Circulating Factors in POKF Preserve Cytosolic and Mitochondrial Redox Status in Oxidatively Stressed Chondrocytes
3.3. Circulating Factors in POKF Preserve the Proliferative Ability of Oxidatively Stressed Chondrocytes
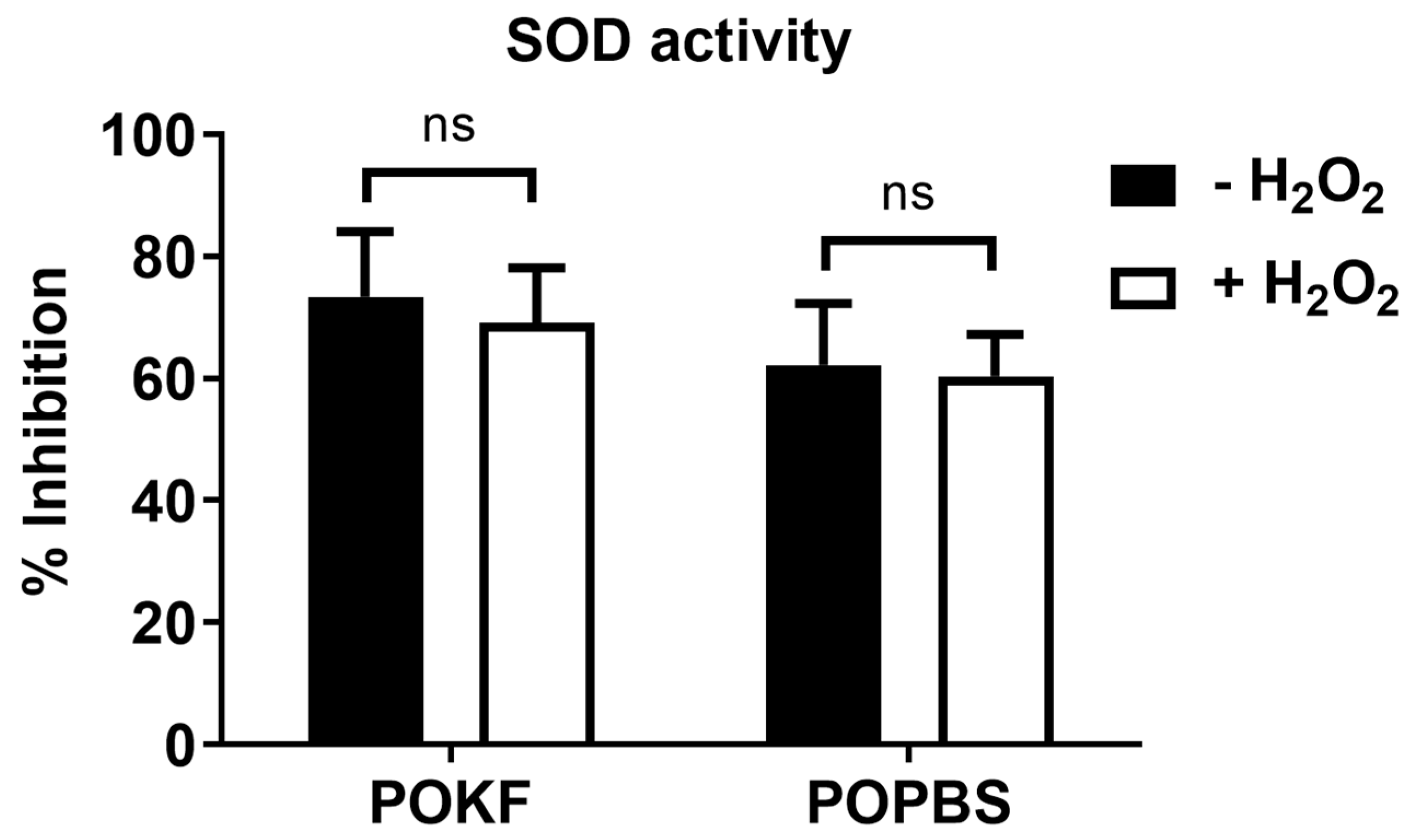
3.4. Soluble Factors in POKF Failed to Increase SOD Activity in Oxidatively Stressed Chondrocytes
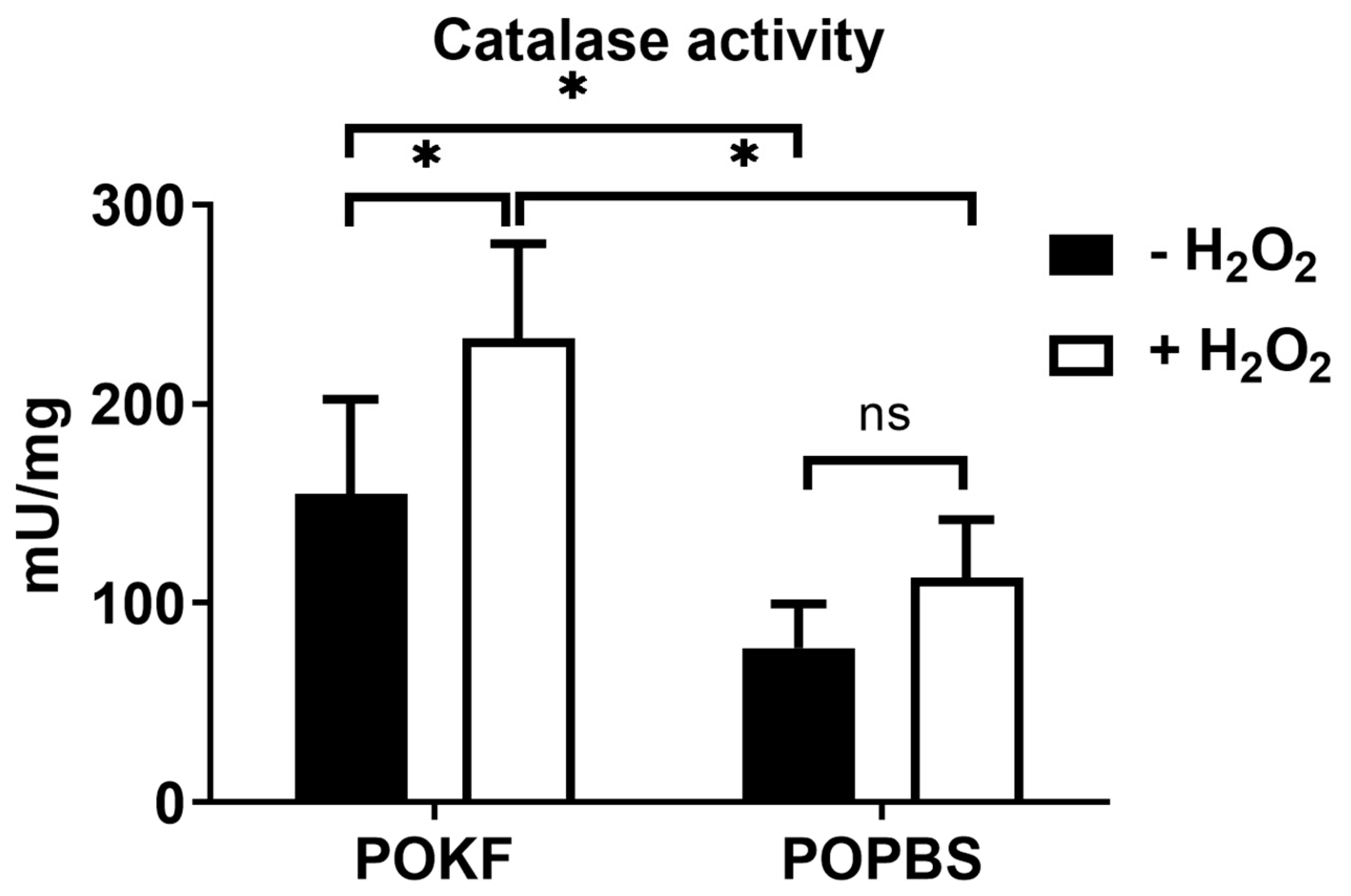
3.5. Soluble Factors in POKF Trigger Catalase Activity in Oxidatively Stressed Chondrocytes
3.6. Soluble Factors in POKF Lower GSSG/GSH Ratio in Oxidatively Stressed Chondrocytes
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melero-Martin, J.; Al-Rubeai, M. In vitro expansion of chondrocytes. Top. Tissue Eng. 2007, 3, 1–37. [Google Scholar]
- Akkiraju, H.; Nohe, A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Cromme, C.; Umlauf, D.; Frank, S.; Pap, T. Molecular mechanisms of cartilage remodelling in osteoarthritis. Int. J. Biochem. Cell Biol. 2010, 42, 1594–1601. [Google Scholar] [CrossRef]
- Bank, R.A.; Bayliss, M.T.; Lafeber, F.P.; Maroudas, A.; Tekoppele, J.M. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem. J. 1998, 330 Pt 1, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; White, R. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J. Biol. Chem. 1980, 255, 217–224. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Cole, A.; Murphy, G.; Bienias, J.L.; Im, H.-J.; Loeser, R.F. Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 1118–1124. [Google Scholar] [CrossRef]
- Lee, R.B.; Wilkins, R.J.; Razaq, S.; Urban, J.P. The effect of mechanical stress on cartilage energy metabolism. Biorheology 2002, 39, 133–143. [Google Scholar]
- Yamazaki, K.; Fukuda, K.; Matsukawa, M.; Hara, F.; Matsushita, T.; Yamamoto, N.; Yoshida, K.; Munakata, H.; Hamanishi, C. Cyclic tensile stretch loaded on bovine chondrocytes causes depolymerization of hyaluronan: Involvement of reactive oxygen species. Arthritis Rheum. 2003, 48, 3151–3158. [Google Scholar] [CrossRef]
- Roos, E.M. Joint injury causes knee osteoarthritis in young adults. Curr. Opin. Rheumatol. 2005, 17, 195–200. [Google Scholar] [CrossRef]
- Richmond, S.A.; Fukuchi, R.K.; Ezzat, A.; Schneider, K.; Schneider, G.; Emery, C.A. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J. Orthop. Sports Phys. Ther. 2013, 43, 515-B19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L.; Olsen, B.R. Lessons from genetic forms of osteoarthritis for the pathogenesis of the disease. Osteoarthr. Cartil. 2007, 15, 1101–1105. [Google Scholar] [CrossRef]
- Sharma, L.; Song, J.; Felson, D.T.; Shamiyeh, E.; Dunlop, D.D. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 2001, 286, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Watari, T.; Naito, K.; Sakamoto, K.; Kurosawa, H.; Nagaoka, I.; Kaneko, K. Evaluation of the effect of oxidative stress on articular cartilage in spontaneously osteoarthritic STR/OrtCrlj mice by measuring the biomarkers for oxidative stress and type II collagen degradation/synthesis. Exp. Ther. Med. 2011, 2, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, S.; Johnell, O.; Pedersen, N. Genetic contribution to severe osteoarthritis of the hip and knee leading to arthroplasty: A twin study. Arthritis Rheum 2004, 50, S140. [Google Scholar]
- Valdes, A.M.; Spector, T.D. The genetic epidemiology of osteoarthritis. Curr. Opin. Rheumatol. 2010, 22, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Loughlin, J.; Oene, M.V.; Chapman, K.; Surdulescu, G.L.; Doherty, M.; Spector, T.D. Sex and ethnic differences in the association of ASPN, CALM1, COL2A1, COMP, and FRZB with genetic susceptibility to osteoarthritis of the knee. Arthritis Rheum. 2007, 56, 137–146. [Google Scholar] [CrossRef]
- Roughley, P.; Martens, D.; Rantakokko, J.; Alini, M.; Mwale, F.; Antoniou, J. The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur. Cell Mater. 2006, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gleghorn, L.; Ramesar, R.; Beighton, P.; Wallis, G. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am. J. Hum. Genet. 2005, 77, 484–490. [Google Scholar] [CrossRef]
- Henrotin, Y.; Blanco, F.J.; Aigner, T.; Kurz, B. The significance of oxidative stress in articular cartilage ageing and degradation. Curr. Rheumatol. Rev. 2007, 3, 261–274. [Google Scholar] [CrossRef]
- Henrotin, Y.; Bruckner, P.; Pujol, J.-P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Milner, P.; White, R.; Fairfax, T.; Wilkins, R. Oxygen and reactive oxygen species in articular cartilage: Modulators of ionic homeostasis. Pflüg. Arch.-Eur. J. Physiol. 2008, 455, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Tiku, M.L.; Gupta, S.; Deshmukh, D.R. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic. Res. 1999, 30, 395–405. [Google Scholar] [CrossRef]
- Schalkwijk, J.; van den Berg, W.B.; van de Putte, L.B.; Joosten, L.A. Hydrogen peroxide suppresses the proteoglycan synthesis of intact articular cartilage. J. Rheumatol. 1985, 12, 205–210. [Google Scholar]
- Brandl, A.; Hartmann, A.; Bechmann, V.; Graf, B.; Nerlich, M.; Angele, P. Oxidative stress induces senescence in chondrocytes. J. Orthop. Res. 2011, 29, 1114–1120. [Google Scholar] [CrossRef]
- Tiku, M.; Liesch, J.; Robertson, F. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J. Immunol. 1990, 145, 690–696. [Google Scholar] [CrossRef]
- Mathy-Hartert, M.; Hogge, L.; Sanchez, C.; Deby-Dupont, G.; Crielaard, J.M.; Henrotin, Y. Interleukin-1beta and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: A possible explanation for oxidative stress generation. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2008, 16, 756–763. [Google Scholar] [CrossRef]
- Jallali, N.; Ridha, H.; Thrasivoulou, C.; Underwood, C.; Butler, P.E.M.; Cowen, T. Vulnerability to ROS-induced cell death in ageing articular cartilage: The role of antioxidant enzyme activity. Osteoarthr. Cartil. 2005, 13, 614–622. [Google Scholar] [CrossRef]
- Baker, M.; Feigan, J.; Lowther, D. Chondrocyte antioxidant defences: The roles of catalase and glutathione peroxidase in protection against H2O2 dependent inhibition of proteoglycan biosynthesis. J. Rheumatol. 1988, 15, 670–677. [Google Scholar]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef]
- Alcaraz, M.J.; Megias, J.; Garcia-Arnandis, I.; Clerigues, V.; Guillen, M.I. New molecular targets for the treatment of osteoarthritis. Biochem. Pharmacol. 2010, 80, 13–21. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Activation and dedifferentiation of chondrocytes: Implications in cartilage injury and repair. Ann. Anat.-Anat. Anz. 2009, 191, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments. Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.; van den Berg, W. TGF-beta and osteoarthritis. Osteoarthr. Cartil. 2007, 15, 597–604. [Google Scholar]
- Loeser, R.F.; Pacione, C.A.; Chubinskaya, S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 2188–2196. [Google Scholar] [CrossRef]
- Schmidt, M.; Chen, E.; Lynch, S. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef]
- Wolf, F.; Haug, M.; Farhadi, J.; Candrian, C.; Martin, I.; Barbero, A. A low percentage of autologous serum can replace bovine serum to engineer human nasal cartilage. Eur. Cells Mater. 2008, 15, 1–10. [Google Scholar] [CrossRef]
- Munirah, S.; Ruszymah, B.H.; Samsudin, O.C.; Badrul, A.H.; Azmi, B.; Aminuddin, B.S. Autologous versus pooled human serum for articular chondrocyte growth. J. Orthop. Surg. (Hong Kong) 2008, 16, 220–229. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamamoto, H.; Ogawa, Y.; Yoshida, S.; Kataoka, S.; Majumdar, M.; Morris, E.; Tripple, S.B. Role of apoptosis inhibition in various chondrocyte culture systems. Int. J. Mol. Med. 2003, 11, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.L.; Hassan, M.N.F.b.; Tang, Y.L.; Ng, M.H.; Law, J.X. Feasibility of human platelet lysate as an alternative to foetal bovine serum for in vitro expansion of chondrocytes. Int. J. Mol. Sci. 2021, 22, 1269. [Google Scholar] [CrossRef] [PubMed]
- Glynn, L.G.; Mustafa, A.; Casey, M.; Krawczyk, J.; Blom, J.; Galvin, R.; Hannigan, A.; Dunne, C.P.; Murphy, A.W.; Mallen, C. Platelet-rich plasma (PRP) therapy for knee arthritis: A feasibility study in primary care. Pilot Feasibility Stud. 2018, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, J.; Wang, Y.; He, J.; Chen, L.; Chu, J.; Wu, H. Platelet Rich Plasma in the Repair of Articular Cartilage Injury: A Narrative Review. Cartilage 2022, 13, 19476035221118419. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Ezquerro, F.; Marcon Alfieri, F.; Vilas Boas, L.; Tozetto-Mendoza, T.R.; Chen, J.; Özçakar, L.; Arendt-Nielsen, L.; Rizzo Battistella, L. Serum Levels of Proinflammatory Cytokines in Painful Knee Osteoarthritis and Sensitization. Int. J. Inflamm. 2015, 2015, 329792. [Google Scholar] [CrossRef] [PubMed]
- Mabey, T.; Honsawek, S. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95. [Google Scholar] [CrossRef]
- Manunta, A.F.; Zedde, P.; Cudoni, S.; Caggiari, G.; Pintus, G. Early joint degeneration and antagonism between growth factors and reactive oxygen species. Is non-surgical management possible? Joints 2015, 3, 123–128. [Google Scholar] [CrossRef]
- Pintus, G.; Tadolini, B.; Posadino, A.M.; Sanna, B.; Debidda, M.; Carru, C.; Deiana, L.; Ventura, C. PKC/Raf/MEK/ERK signaling pathway modulates native-LDL-induced E2F-1 gene expression and endothelial cell proliferation. Cardiovasc. Res. 2003, 59, 934–944. [Google Scholar] [CrossRef]
- Giordo, R.; Thuan, D.T.B.; Posadino, A.M.; Cossu, A.; Zinellu, A.; Erre, G.L.; Pintus, G. Iloprost attenuates oxidative stress-dependent activation of collagen synthesis induced by sera from scleroderma patients in human pulmonary microvascular endothelial cells. Molecules 2021, 26, 4729. [Google Scholar] [CrossRef]
- Ramli, I.; Posadino, A.M.; Zerizer, S.; Spissu, Y.; Barberis, A.; Djeghim, H.; Azara, E.; Bensouici, C.; Kabouche, Z.; Rebbas, K. Low concentrations of Ambrosia maritima L. phenolic extract protect endothelial cells from oxidative cell death induced by H2O2 and sera from Crohn’s disease patients. J. Ethnopharmacol. 2023, 300, 115722. [Google Scholar] [CrossRef]
- Posadino, A.M.; Erre, G.L.; Cossu, A.; Emanueli, C.; Eid, A.H.; Zinellu, A.; Pintus, G.; Giordo, R. NADPH-derived ROS generation drives fibrosis and endothelial-to-mesenchymal transition in systemic sclerosis: Potential cross talk with circulating miRNAs. Biomol. Concepts 2022, 13, 11–24. [Google Scholar] [CrossRef]
- Vono, R.; Fuoco, C.; Testa, S.; Pirrò, S.; Maselli, D.; Ferland McCollough, D.; Sangalli, E.; Pintus, G.; Giordo, R.; Finzi, G. Activation of the pro-oxidant PKCβII-p66Shc signaling pathway contributes to pericyte dysfunction in skeletal muscles of patients with diabetes with critical limb ischemia. Diabetes 2016, 65, 3691–3704. [Google Scholar] [CrossRef]
- Boin, F.; Erre, G.L.; Posadino, A.M.; Cossu, A.; Giordo, R.; Spinetti, G.; Passiu, G.; Emanueli, C.; Pintus, G. Oxidative stress-dependent activation of collagen synthesis is induced in human pulmonary smooth muscle cells by sera from patients with scleroderma-associated pulmonary hypertension. Orphanet J. Rare Dis. 2014, 9, 123. [Google Scholar] [CrossRef]
- Dooley, C.T.; Dore, T.M.; Hanson, G.T.; Jackson, W.C.; Remington, S.J.; Tsien, R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004, 279, 22284–22293. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Nasrallah, G.K.; Posadino, A.M.; Galimi, F.; Capobianco, G.; Eid, A.H.; Pintus, G. Resveratrol-elicited pkc inhibition counteracts nox-mediated endothelial to mesenchymal transition in human retinal endothelial cells exposed to high glucose. Antioxidants 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Nasrallah, G.K.; Al-Jamal, O.; Paliogiannis, P.; Pintus, G. Resveratrol inhibits oxidative stress and prevents mitochondrial damage induced by zinc oxide nanoparticles in zebrafish (Danio rerio). Int. J. Mol. Sci. 2020, 21, 3838. [Google Scholar] [CrossRef] [PubMed]
- Cossu, A.; Posadino, A.M.; Giordo, R.; Emanueli, C.; Sanguinetti, A.M.; Piscopo, A.; Poiana, M.; Capobianco, G.; Piga, A.; Pintus, G. Apricot melanoidins prevent oxidative endothelial cell death by counteracting mitochondrial oxidation and membrane depolarization. PLoS ONE 2012, 7, e48817. [Google Scholar] [CrossRef] [PubMed]
- Fois, A.G.; Posadino, A.M.; Giordo, R.; Cossu, A.; Agouni, A.; Rizk, N.M.; Pirina, P.; Carru, C.; Zinellu, A.; Pintus, G. Antioxidant activity mediates pirfenidone antifibrotic effects in human pulmonary vascular smooth muscle cells exposed to sera of idiopathic pulmonary fibrosis patients. Oxidative Med. Cell. Longev. 2018, 2018, 2639081. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Deiana, L.; Carru, C.; Pintus, G. Coumaric acid induces mitochondrial damage and oxidative-mediated cell death of human endothelial cells. Cardiovasc. Toxicol. 2013, 13, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Fazal, N.; Khawaja, H.; Naseer, N.; Khan, A.J.; Latief, N. Daphne mucronata enhances cell proliferation and protects human adipose stem cells against monosodium iodoacetate induced oxidative stress in vitro. Adipocyte 2020, 9, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mizokami, A.; Shin, M.; Izumi, K.; Konaka, H.; Kadono, Y.; Kitagawa, Y.; Keller, E.T.; Zhang, J.; Namiki, M. SOD3 acts as a tumor suppressor in PC-3 prostate cancer cells via hydrogen peroxide accumulation. Anticancer Res. 2014, 34, 2821–2831. [Google Scholar]
- Sambon, M.; Gorlova, A.; Demelenne, A.; Alhama-Riba, J.; Coumans, B.; Lakaye, B.; Wins, P.; Fillet, M.; Anthony, D.C.; Strekalova, T. Dibenzoylthiamine has powerful antioxidant and anti-inflammatory properties in cultured cells and in mouse models of stress and neurodegeneration. Biomedicines 2020, 8, 361. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: Homeostatic and pathological responses in mammalian cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Bates, E.J.; Johnson, C.C.; Lowther, D.A. Inhibition of proteoglycan synthesis by hydrogen peroxide in cultured bovine articular cartilage. Biochim. Biophys. Acta 1985, 838, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Brun, H.; Clain, E.; Ronot, X.; Adolphe, M. Effects of oxygen-free radicals on proliferation kinetics of cultured rabbit articular chondrocytes. J. Cell. Physiol. 1989, 141, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; van Trieu, N.; Nakamura, H.; Hongo-Masuko, K.; Kato, T.; Nishioka, K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res. Ther. 2005, 7, R380-91. [Google Scholar]
- Baker, M.S.; Feigan, J.; Lowther, D.A. The mechanism of chondrocyte hydrogen peroxide damage. Depletion of intracellular ATP due to suppression of glycolysis caused by oxidation of glyceraldehyde-3-phosphate dehydrogenase. J. Rheumatol. 1989, 16, 7–14. [Google Scholar]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Schittenhelm, D.; Neuss-Radu, M.; Verma, N.; Pink, M.; Schmitz-Spanke, S. ROS and pentose phosphate pathway: Mathematical modelling of the metabolic regulation in response to xenobiotic-induced oxidative stress and the proposed Impact of the gluconate shunt. Free Radic. Res. 2019, 53, 979–992. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front. Physiol. 2021, 12, 398. [Google Scholar] [CrossRef]
- Mao, X.; Fu, P.; Wang, L.; Xiang, C. Mitochondria: Potential targets for osteoarthritis. Front. Med. 2020, 7, 581402. [Google Scholar] [CrossRef]
- Maneiro, E.; Martín, M.A.; de Andres, M.C.; López-Armada, M.J.; Fernández-Sueiro, J.L.; del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003, 48, 700–708. [Google Scholar] [CrossRef]
- Grishko, V.I.; Ho, R.; Wilson, G.L.; Pearsall, A.W., IV. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2009, 17, 107–113. [Google Scholar] [CrossRef]
- Linnane, A.; Ozawa, T.; Marzuki, S.; Tanaka, M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 1989, 333, 642–645. [Google Scholar] [CrossRef]
- Caramés, B.; Lopez-Armada, M.; Cillero-Pastor, B.; Lires-Dean, M.; Lema, B.; Ruiz-Romero, C.; Fuentes, I.; Galdo, F.; Blanco, F. Inhibition of mitochondrial respiratory chain induces an inflammatory response in human articular chondrocytes. In Annals of the Rheumatic Diseases; BMJ Publishing Group: London, UK, 2005. [Google Scholar]
- Cillero-Pastor, B.; Caramés, B.; Lires-Deán, M.; Vaamonde-García, C.; Blanco, F.J.; López-Armada, M.J. Mitochondrial dysfunction activates cyclooxygenase 2 expression in cultured normal human chondrocytes. Arthritis Rheum. 2008, 58, 2409–2419. [Google Scholar] [CrossRef]
- Maneiro, E.; Lopez-Armada, M.; De Andres, M.; Carames, B.; Martin, M.; Bonilla, A.; Del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann. Rheum. Dis. 2005, 64, 388–395. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop. J. 2001, 21, 1. [Google Scholar]
- Martin, J.A.; Buckwalter, J.A. The Role of Chondrocyte Senescence in the Pathogenesis of Osteoarthritis and in Limiting Cartilage Repair. J. Bone Jt. Surg. 2003, 85 (Suppl. S2), 106–110. [Google Scholar] [CrossRef]
- Yagi, M.; Endo, K.; Komori, K.; Sekiya, I. Comparison of the effects of oxidative and inflammatory stresses on rat chondrocyte senescence. Sci. Rep. 2023, 13, 7697. [Google Scholar] [CrossRef]
- Cha, B.-H.; Lee, J.-S.; Kim, S.W.; Cha, H.-J.; Lee, S.-H. The modulation of the oxidative stress response in chondrocytes by Wip1 and its effect on senescence and dedifferentiation during in vitro expansion. Biomaterials 2013, 34, 2380–2388. [Google Scholar] [CrossRef]
- Zhuang, C.; Ni, S.; Yang, Z.-C.; Liu, R.-P. Oxidative stress induces chondrocyte apoptosis through caspase-dependent and caspase-independent mitochondrial pathways and the antioxidant mechanism of angelica sinensis polysaccharide. Oxidative Med. Cell. Longev. 2020, 2020, 3240820. [Google Scholar] [CrossRef]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.; Talyor, R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010, 69, 1502–1510. [Google Scholar] [CrossRef]
- Gottfredsen, R.H.; Larsen, U.G.; Enghild, J.J.; Petersen, S.V. Hydrogen peroxide induce modifications of human extracellular superoxide dismutase that results in enzyme inhibition. Redox Biol. 2013, 1, 24–31. [Google Scholar] [CrossRef]
- Chen, B.; He, Q.; Chen, C.; Lin, Y.; Xiao, J.; Pan, Z.; Li, M.; Li, S.; Yang, J.; Wang, F. Combination of curcumin and catalase protects against chondrocyte injury and knee osteoarthritis progression by suppressing oxidative stress. Biomed. Pharmacother. 2023, 168, 115751. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; de Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione–linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef]
- Flohe, L. The fairytale of the GSSG/GSH redox potential. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3139–3142. [Google Scholar] [CrossRef]
- Zhu, S.; Makosa, D.; Miller, B.; Griffin, T.M. Glutathione as a mediator of cartilage oxidative stress resistance and resilience during aging and osteoarthritis. Connect. Tissue Res. 2020, 61, 34–47. [Google Scholar] [CrossRef]
- Carlo, M.D.; Loeser, R.F. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003, 48, 3419–3430. [Google Scholar] [CrossRef]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The protective role of glutathione in osteoarthritis. J. Clin. Orthop. Trauma 2021, 15, 145–151. [Google Scholar] [CrossRef]
- Chen, W.-P.; Xiong, Y.; Hu, P.-F.; Bao, J.-P.; Wu, L.-D. Baicalein Inhibits MMPs Expression via a MAPK-Dependent Mechanism in Chondrocytes. Cell. Physiol. Biochem. 2015, 36, 325–333. [Google Scholar] [CrossRef]
- Panico, A.; Cardile, V.; Santagati, N.A.; Messina, R. Antioxidant and protective effects of Sumac Leaves on chondrocytes. J. Med. Plants Res. 2009, 3, 855–861. [Google Scholar]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum 2008, 58, 2786–2797. [Google Scholar] [CrossRef]
- Zheng, S.X.; Mouithys-Mickalad, A.; Deby-Dupont, G.P.; Deby, C.M.; Maroulis, A.P.; Labasse, A.H.; Lamy, M.L.; Crielaard, J.M.; Reginster, J.Y.; Henrotin, Y.E. In vitro study of the antioxidant properties of nimesulide and 4-OH nimesulide: Effects on HRP- and luminol-dependent chemiluminescence produced by human chondrocytes. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2000, 8, 419–425. [Google Scholar] [CrossRef]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Jt. Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef]
- Kurz, B.; Jost, B.; Schünke, M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthr. Cartil. 2002, 10, 119–126. [Google Scholar] [CrossRef]
- Varghese, S.; Theprungsirikul, P.; Sahani, S.; Hwang, N.; Yarema, K.J.; Elisseeff, J.H. Glucosamine modulates chondrocyte proliferation, matrix synthesis, and gene expression. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2007, 15, 59–68. [Google Scholar] [CrossRef]
- Sakata, S.; Hayashi, S.; Fujishiro, T.; Kawakita, K.; Kanzaki, N.; Hashimoto, S.; Iwasa, K.; Chinzei, N.; Kihara, S.; Haneda, M. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J. Orthop. Res. 2015, 33, 359–365. [Google Scholar] [CrossRef]
- Tiku, M.L.; Narla, H.; Jain, M.; Yalamanchili, P. Glucosamine prevents in vitro collagen degradation in chondrocytes by inhibiting advanced lipoxidation reactions and protein oxidation. Arthritis Res. Ther. 2007, 9, R76. [Google Scholar] [CrossRef] [PubMed]
- Jallali, N.; Ridha, H.; Thrasivoulou, C.; Butler, P.; Cowen, T. Modulation of intracellular reactive oxygen species level in chondrocytes by IGF-1, FGF, and TGF-beta1. Connect Tissue Res 2007, 48, 149–158. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordo, R.; Tulasigeri Totiger, S.; Caggiari, G.; Cossu, A.; Manunta, A.F.; Posadino, A.M.; Pintus, G. Protective Effect of Knee Postoperative Fluid on Oxidative-Induced Damage in Human Knee Articular Chondrocytes. Antioxidants 2024, 13, 188. https://doi.org/10.3390/antiox13020188
Giordo R, Tulasigeri Totiger S, Caggiari G, Cossu A, Manunta AF, Posadino AM, Pintus G. Protective Effect of Knee Postoperative Fluid on Oxidative-Induced Damage in Human Knee Articular Chondrocytes. Antioxidants. 2024; 13(2):188. https://doi.org/10.3390/antiox13020188
Chicago/Turabian StyleGiordo, Roberta, Smitha Tulasigeri Totiger, Gianfilippo Caggiari, Annalisa Cossu, Andrea Fabio Manunta, Anna Maria Posadino, and Gianfranco Pintus. 2024. "Protective Effect of Knee Postoperative Fluid on Oxidative-Induced Damage in Human Knee Articular Chondrocytes" Antioxidants 13, no. 2: 188. https://doi.org/10.3390/antiox13020188
APA StyleGiordo, R., Tulasigeri Totiger, S., Caggiari, G., Cossu, A., Manunta, A. F., Posadino, A. M., & Pintus, G. (2024). Protective Effect of Knee Postoperative Fluid on Oxidative-Induced Damage in Human Knee Articular Chondrocytes. Antioxidants, 13(2), 188. https://doi.org/10.3390/antiox13020188







