The Role of Potato Glycoside Alkaloids Mediated Oxidative Stress in Inducing Apoptosis of Wolfberry Root Rot Pathogen Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Strain
2.2. Extraction of Potato Glycoside Alkaloids (PGAs)
2.3. Determination of the Concentration for 50% of Maximal Effect (EC50)
2.4. Preparation of Mycelium
2.5. Determination of Antifungal Effect
2.5.1. Determination of Colony Diameter and Biomass
2.5.2. Determination of Sporulation and Spore Germination Rate
2.6. Determination of Metacaspase Enzyme Activity
2.7. Propidium Iodide (PI) Stain
2.8. Determination of ROS Levels
2.8.1. Determination of NADH Oxidase (NOX) and Superoxide Dismutase (SOD) Activities
2.8.2. Determination of Superoxide Anion (O2−) Production Rate and Hydrogen Peroxide (H2O2) Content
2.8.3. Measurement of Intracellular ROS Staining and Fluorescence Intensity
2.9. Determination of Antioxidant Capacity
2.9.1. Determination of Antioxidant Enzyme Activity
2.9.2. Determination of Antioxidant Substance Content
2.9.3. Determination of Total Antioxidant Capacity (T-AOC) and Hydroxyl Radical (·OH) Scavenging Capacity
2.10. Statistical Analysis
3. Results
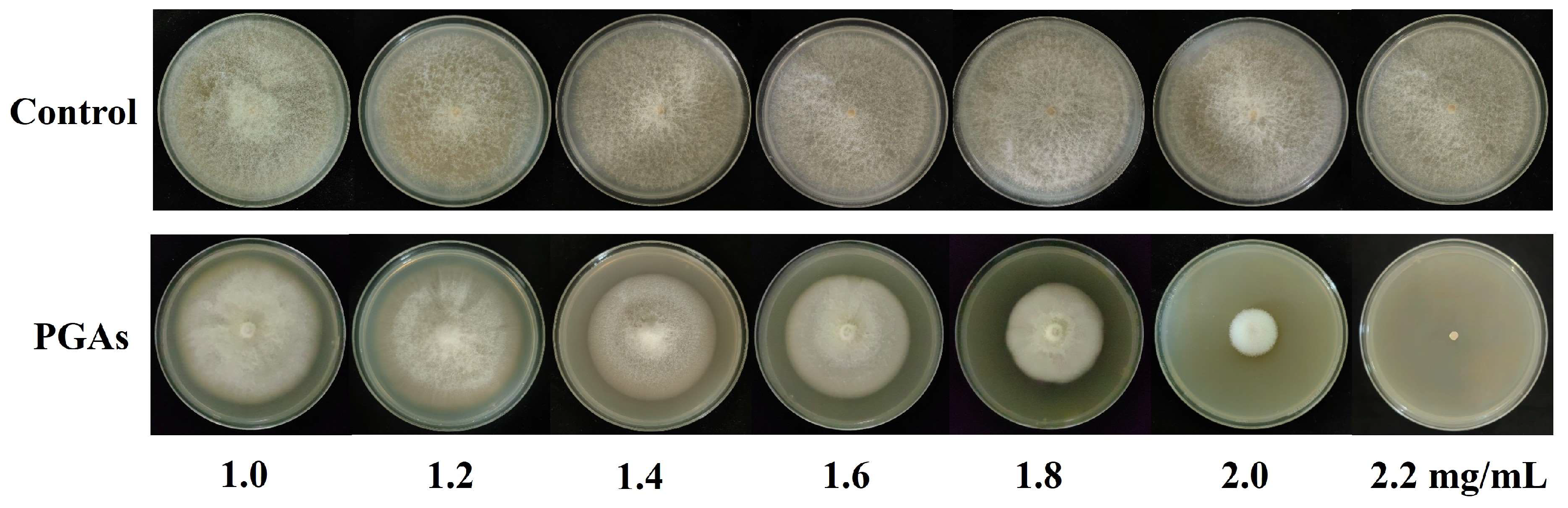
3.1. Determination of the Concentration for 50% of Maximal Effect (EC50) Values
3.2. The Changes of Colony Diameter, Biomass, Sporulation, and Spore Germination Rate of F. solani After PGAs Treatment
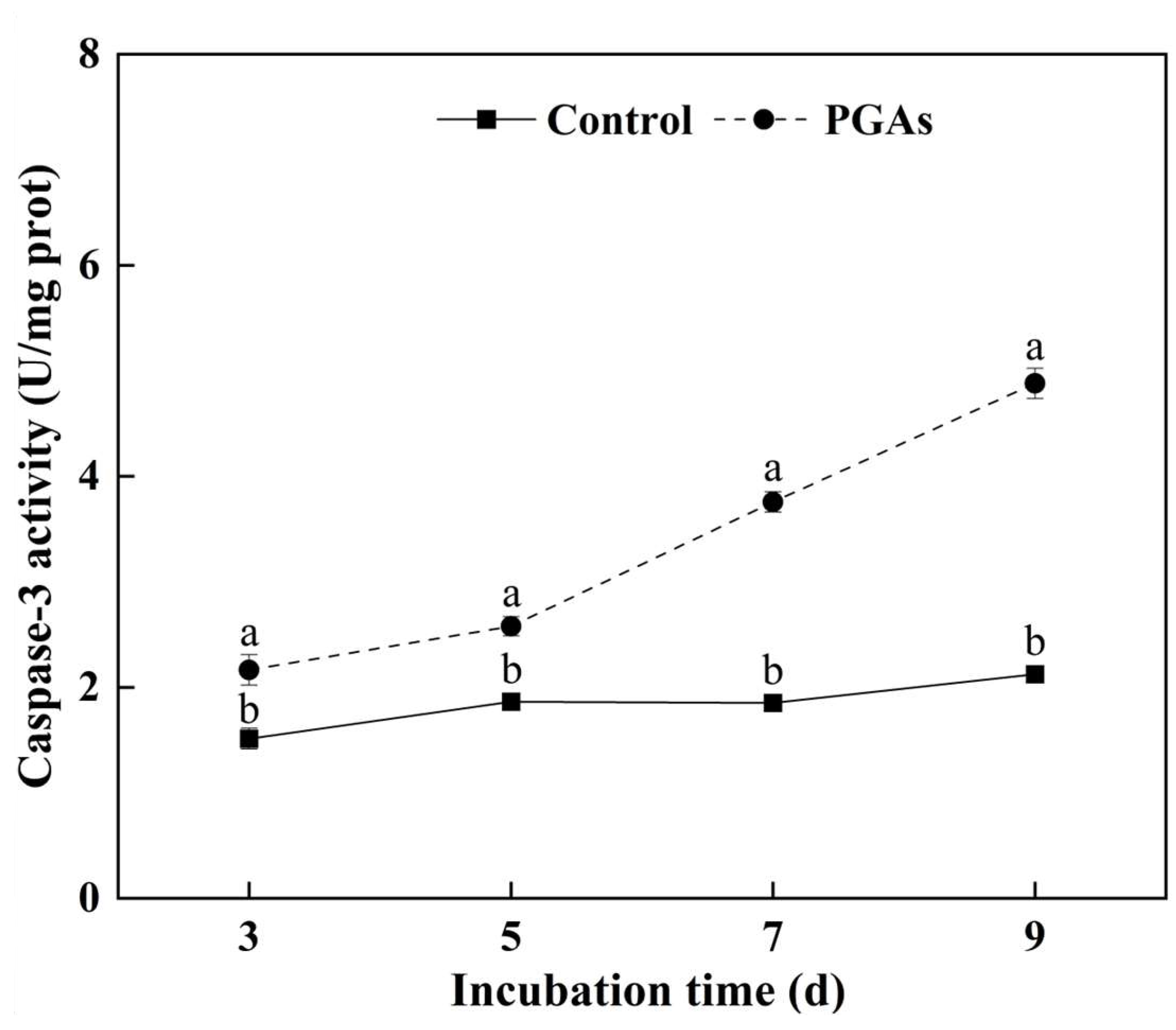
3.3. Changes of Metacaspase Activity
3.4. Propidium Iodide (PI) Staining
3.5. Effect of PGAs on the Level of F. solani Reactive Oxygen Species (ROS)
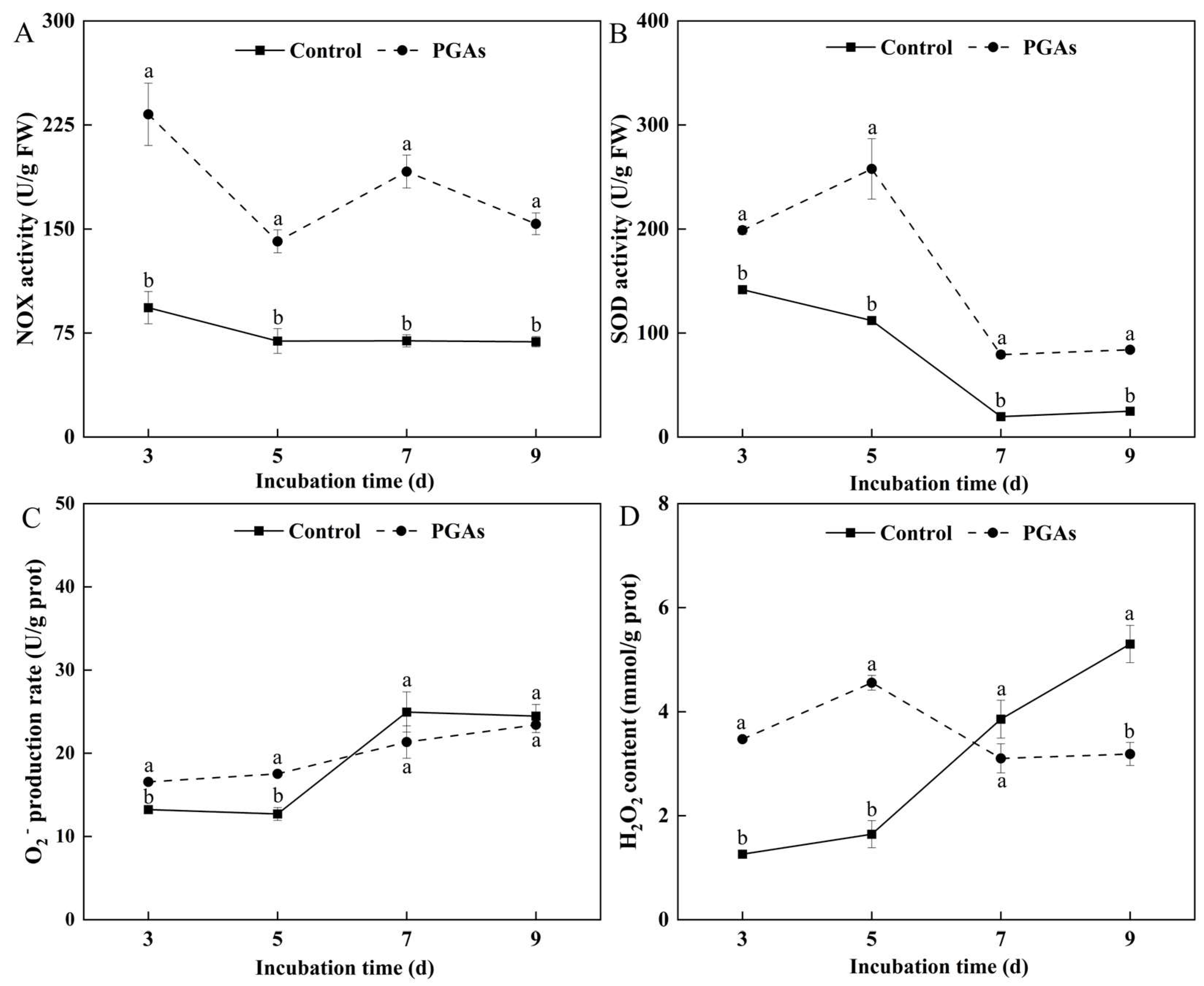
3.5.1. Effects of PGAs on the Activities of NADH Oxidase (NOX) and Superoxide Dismutase (SOD) and the Contents of Superoxide Anion (O2−) and Hydrogen Peroxide (H2O2) in F. solani
3.5.2. F. solani ROS Staining
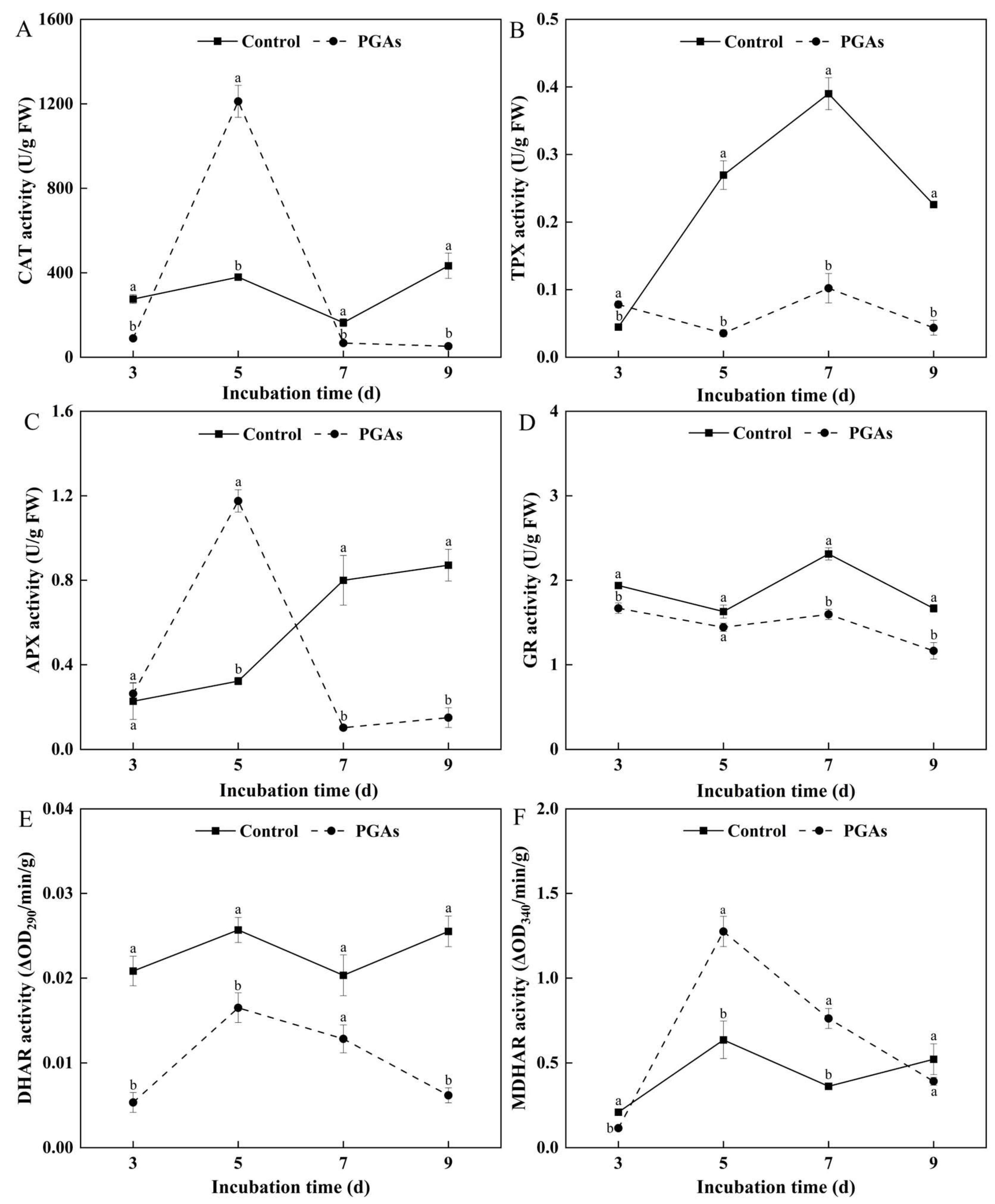
3.6. Effect of PGAs on the Activity of Antioxidant Enzymes of F. solani
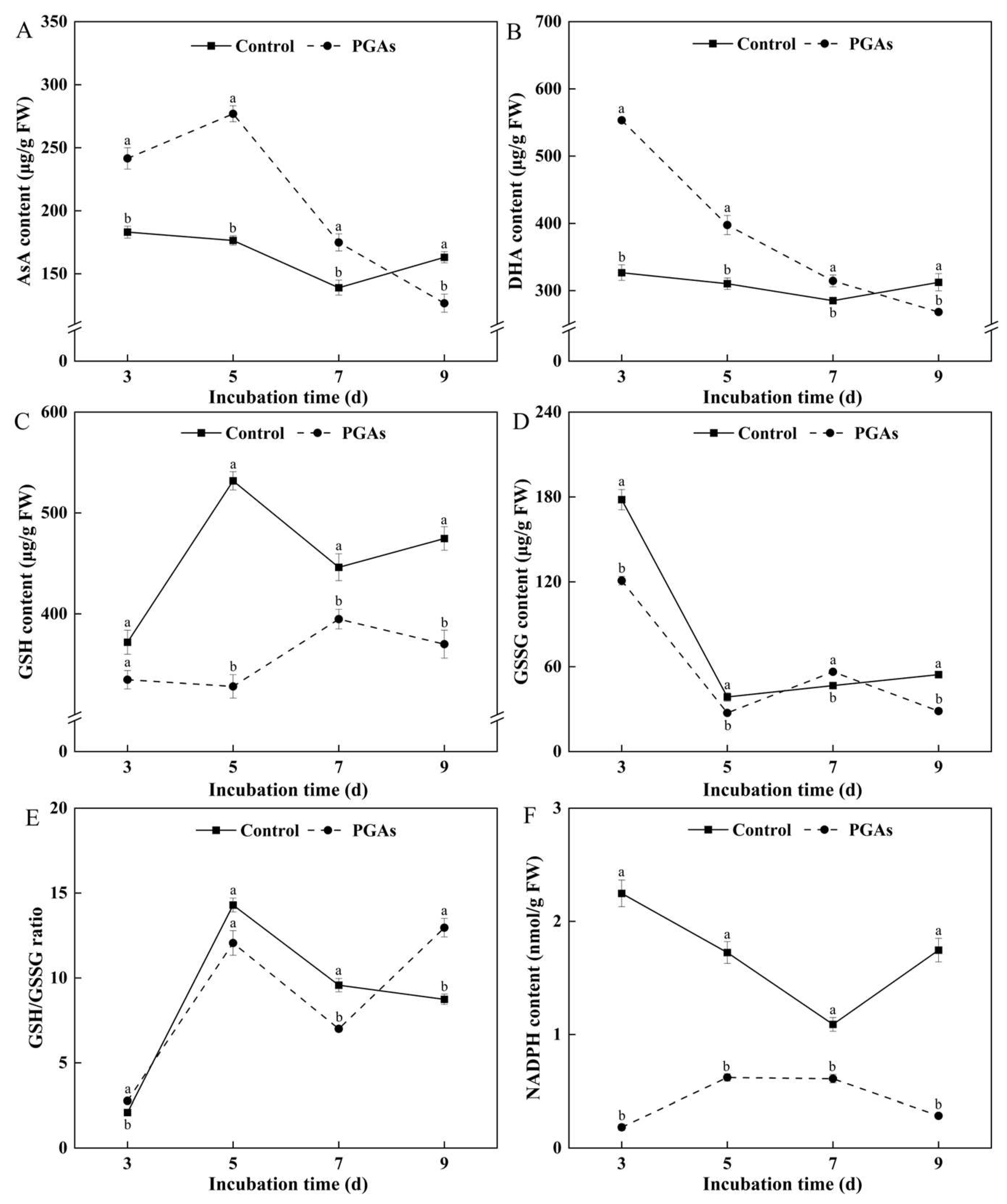
3.7. Effects of PGAs on the Content of Antioxidant Substances of F. solani
3.8. Effect of PGAs on the Antioxidant Capacity of F. solani
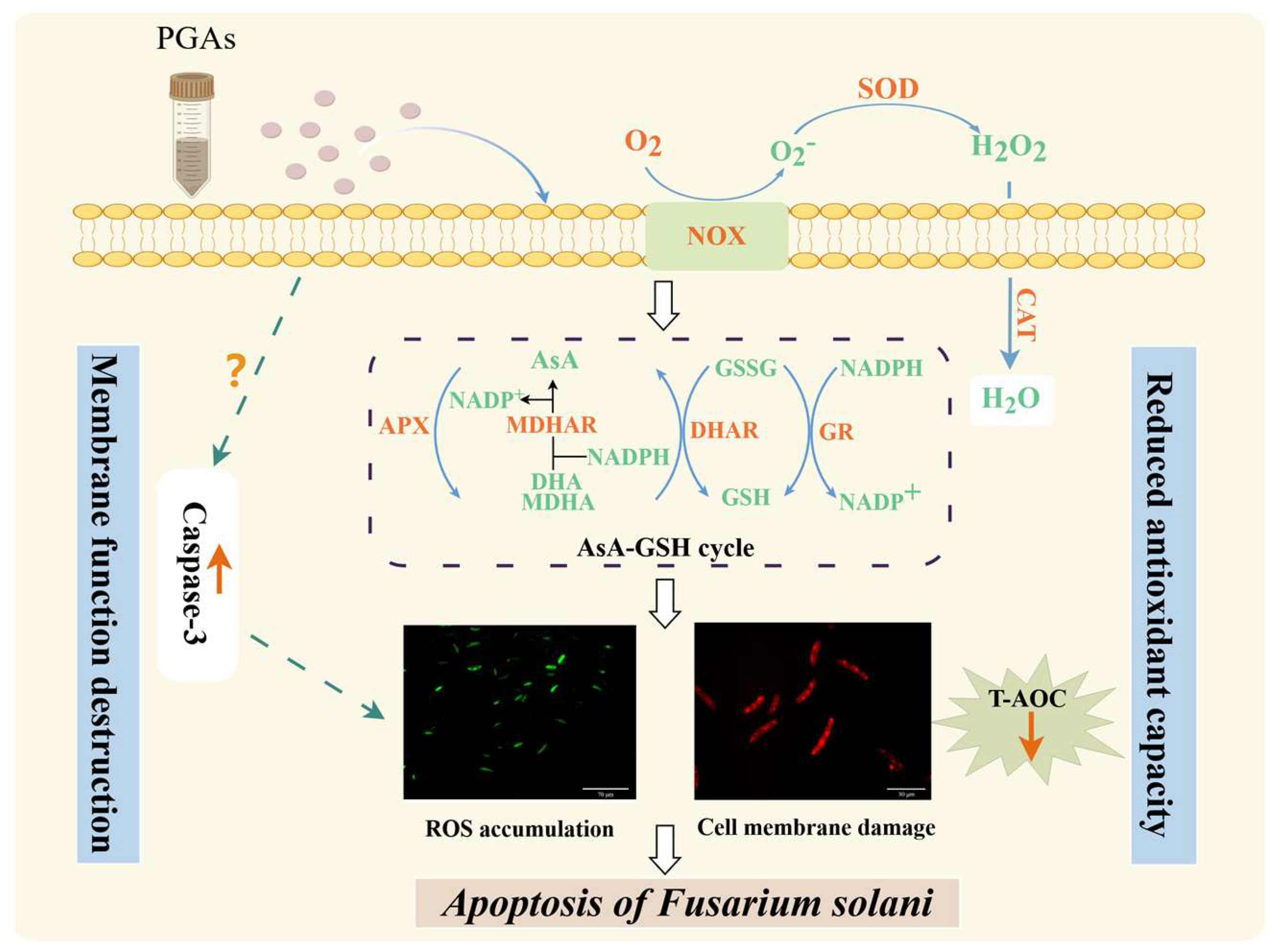
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, R.; Shi, Z.G.; Li, Y.K.; Huang, T.; Cao, Y.L.; An, W.; Zhang, X.Y. Effect of potassiumon the agronomic traits and fruit quality of Goji (Lycium barbarum L.). Sci. Rep. 2024, 14, 21477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Xu, Y.H.; Li, H.X.; An, W.; Yin, Y.; Wang, B.; Wang, L.P.; Wang, B.; Duan, L.Y.; Ren, X.Y.; et al. Metabolite-based genome-wide association studies enable the dissection of the genetic bases of flavonoids, betaine and spermidine in wolfberry (Lycium). Plant Biotechnol. J. 2024, 22, 1435–1452. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.J.; Ma, R.H.; Wang, W.; Thakur, K.; Ma, Y.L.; Khan, M.R.; Zhang, J.G.; Wei, Z.J. Potential biosurfactant and methanol extraction for phenolic active substances from Lycium barbarum fruits and leaves. Ind. Crops Prod. 2024, 212, 118333. [Google Scholar] [CrossRef]
- Bai, L.C.; Li, X.J.; Cao, Y.T.; Song, Z.M.; Ma, K.; Fan, Y.X.; Ma, M.C. Fusarium culmorum and Fusarium equiseti causing root rot disease on Lycium barbarum (Goji Berry) in China. Plant Dis. 2020, 104, 3066. [Google Scholar] [CrossRef]
- Pan, C.; Yang, K.L.; Erhunmwunsee, F.; Wang, B.; Yang, D.J.; Lu, G.Q.; Liu, M.X.; Li, J.X.; Tian, J. Antifungal activity of perillaldehyde on Fusarium solani and its control effect on postharvest decay of sweet potatoes. J. Fungi 2023, 9, 257. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Chen, W.; Wang, B.; Wang, Y.P.; Li, N.; Li, R.Y.; Yan, Y.K.; Sun, Y.Y.; He, J. Potato glycoside alkaloids exhibit antifungal activity by regulating the tricarboxylic acid cycle pathway of Fusarium solani. Front. Microbiol. 2024, 15, 1390269. [Google Scholar] [CrossRef]
- Benkeblia, N. Potato glycoalkaloids: Occurrence, biological activities and extraction for biovalorisation-a review. Int. J. Food Sci. Technol. 2020, 55, 2305–2313. [Google Scholar] [CrossRef]
- Sun, P.Z.; Cao, B.W.; Han, Y.M. Study on antimicrobial activities of α-solanine against Botrytis cinerea in vitro. Storage Process 2014, 14, 12–15. (In Chinese) [Google Scholar]
- Andrivon, D.; Corbière, R.; Lucas, J.M.; Pasco, C.; Gravoueille, J.M.; Pellé, R.; Dantec, E.; Ellissèche, D. Resistance to late blight and soft rot in six potato progenies and glycoalkaloid contents in the tubers. Am. J. Potato Res. 2003, 80, 125–134. [Google Scholar] [CrossRef]
- Rocha, A.B.O.; Honório, S.L.; Messias, C.L.; Otón, M.; Gómez, P.A. Effect of UV-C radiation and fluorescent light to control postharvest soft rot in potato seed tubers. Sci. Hortic. 2015, 181, 174–181. [Google Scholar] [CrossRef]
- Joshi, J.R.; Yao, L.X.; Charkowski, A.O.; Heuberger, A.L. Metabolites from wild potato inhibit virulence factors of the soft rot and blackleg pathogen Pectobacterium brasiliense. Mol. Plant Microbe Interact. 2021, 34, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Fan, Y.L.; Liu, Y.H.; Qin, S.H. Antifungal activity of potato glycoalkaloids and its potential to control severity of dry rot caused by Fusarium sulphureum. Crop Sci. 2023, 63, 801–811. [Google Scholar] [CrossRef]
- Fang, J.N.; Zhou, G.; Zhao, H.F.; Xie, D.D.; Zhang, J.N.; Kües, U.; Xiao, Y.Z.; Fang, Z.M.; Liu, J.J. An apoptosis-inducing factor controls programmed cell death and laccase expression during fungal interactions. Appl. Microbiol. Biotechnol. 2024, 108, 135. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Ding, D.D.; Wang, B.; Wang, Y.P.; Li, N.; Li, R.Y.; Yan, Y.K.; He, J. Effect of potato glycoside alkaloids on energy metabolism of Fusarium solani. J. Fungi 2023, 9, 777. [Google Scholar] [CrossRef]
- Park, S.C.; Yoon, A.M.; Kim, Y.M.; Lee, M.Y.; Lee, J.R. Antifungal action of Arabidopsis thaliana TCP21 via induction of oxidative stress and apoptosis. Antioxidants 2023, 12, 1767. [Google Scholar] [CrossRef]
- Dou, S.W.; Liu, S.Q.; Xu, X.Y.; OuYang, Q.L.; Tao, N.G. Octanal inhibits spore germination of Penicillium digitatum involving membrane peroxidation. Protoplasma 2017, 254, 1539–1545. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Liu, Z.T.; Li, Y.C.; Bi, Y.; Lu, Y.H.; Xie, P.D. Control effect of pyridine-2,6-dipicolinic acid treatment on postharvest black spot of pear fruit and possible antifungal mechanism. Food Sci. 2022, 43, 163–170. (In Chinese) [Google Scholar] [CrossRef]
- Sun, Y.Y.; Wang, Y.; Xu, Y.; Chen, T.; Li, B.Q.; Zhang, Z.Q.; Tian, S.P. Application and mechanism of benzyl-isothiocyanate, a natural antimicrobial agent from cruciferous vegetables, in controlling postharvest decay of strawberry. Postharvest Biol. Technol. 2021, 180, 111604. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, H.; Bi, Y.; He, X.F.; Wang, Y.; Li, Y.C.; Zheng, X.Y.; Prusky, D. Preharvest multiple sprays with sodium nitroprusside promote wound healing of harvested muskmelons by activation of phenylpropanoid metabolism. Postharvest Biol. Technol. 2019, 158, 110988. [Google Scholar] [CrossRef]
- Ma, F.; Cheng, L. The sun-exposed peel of apple fruit has higher xanthophyll cycle-dependent thermal dissipation and antioxidants of the ascorbate-glutathione pathway than the shaded peel. Plant Sci. 2003, 165, 819–827. [Google Scholar] [CrossRef]
- Turcsanyi, E.; Lyons, T.; Plochl, M.; Barnes, J. Does ascorbate in the mesophyll cell walls form the first line of defence against ozone? Testing the concept using broad bean (Vicia faba L.). J. Exp. Bot. 2000, 51, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Lu, H.; Yi, X.Q.; Peng, S.M.; Huang, X.H.; Zhang, Y.; He, C.Z. Potential of alpha-(α)-Solanine as a natural inhibitor of fungus causing leaf spot disease in strawberry. Life 2023, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Shlezinger, N.; Goldfinger, N.; Sharon, A. Apoptotic-like programed cell death in fungi: The benefits in filamentous species. Front. Oncol. 2012, 2, 97. [Google Scholar] [CrossRef] [PubMed]
- Häcker, G. Apoptosis in infection. Microbes Infect. 2018, 20, 552–559. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.Z.; Lu, Z.Q.; Sun, C.H.; Zhang, M.; Zhu, A.H.; Peng, X. Perillaldehyde, a promising antifungal agent used in food preservation, triggers apoptosis through a metacaspase-dependent pathway in Aspergillus flavus. J. Agric. Food Chem. 2016, 64, 7404–7413. [Google Scholar] [CrossRef]
- Deng, Y.J.; Chen, Z.; Chen, Y.P.; Wang, J.P.; Xiao, R.F.; Wang, X.; Liu, B.; Chen, M.C.; He, J. Lipopeptide C17 fengycin B exhibits a novel antifungal mechanism by triggering metacaspase-dependent apoptosis in Fusarium oxysporum. J. Agric. Food Chem. 2024, 72, 7943–7953. [Google Scholar] [CrossRef]
- Yang, F.W.; Mi, J.Q.; Huang, F.; Pienpinijtham, P.; Guo, Y.H.; Cheng, Y.L.; Yao, W.R.; Xie, Y.F. Trans-cinnamaldehyde inhibits Penicillium italicum by damaging mitochondria and inducing apoptosis mechanisms. Food Sci. Hum. Wellness 2022, 11, 975–981. [Google Scholar] [CrossRef]
- Crowley, L.C.; Chojnowski, G.; Waterhouse, N.J. Measuring the DNA content of cells in apoptosis and at different cell-cycle stages by propidium iodide staining and flow cytometry. CSHL 2016, 2016, pdb-prot087247. [Google Scholar] [CrossRef]
- Nescerecka, A.; Hammes, F.; Juhna, T. A pipeline for developing and testing staining protocols for flow cytometry, demonstrated with SYBR Green I and propidium iodide viability staining. J. Microbiol. Methods 2016, 131, 172–180. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Xiao, H.Y.; Zhang, Y.M.; Xie, Y.L. 2-Hydroxy-4-methoxybenzaldehyde, a more effective antifungal aroma than vanillin and its derivatives against Fusarium graminearum, destroys cell membranes, inhibits DON biosynthesis, and performs a promising antifungal effect on wheat grains. Front. Microb. 2024, 15, 1359947. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.; Fieschi, F. NADPH oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.D.; Li, Y.C.; Bi, Y.; Mao, R.Y.; Yang, Y.Y.; Jiang, Q.Q.; Prusky, D. Cellular responses required for oxidative stress tolerance of the necrotrophic fungus Alternaria alternata, causal agent of pear black spot. Microorganisms 2022, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.L.; Zha, Y.Y.; Zhong, Z.; Ruan, Y.M.; Li, Z.W.; Sun, L.L.; Hou, S. Improved detection of reactive oxygen species by DCFH-DA: New insight into self-amplification of fluorescence signal by light irradiation. Sens. Actuators B 2021, 339, 129878. [Google Scholar] [CrossRef]
- Ayer, A.; Gourlay, C.W.; Dawes, I.W. Cellular redox homeostasis, reactive oxygen species and replicative ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 2014, 14, 60–72. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.N.; Wan, C.P.; Gan, Z.Y.; Chen, C.Y.; Chen, J. Antofine triggers the resistance against Penicillium italicum in ponkan fruit by driving AsA-GSH cycle and ROS-scavenging system. Front. Microbiol. 2022, 13, 874430. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Shigenaga, T.; Yamauchi, N.; Funamoto, Y.; Shigyo, M. Effects of heat treatment on an ascorbate-glutathione cycle in stored broccoli (Brassica oleracea L.) florets. Postharvest Biol. Technol. 2005, 38, 152–159. [Google Scholar] [CrossRef]
- Ma, Y.H.; Ma, F.W.; Zhang, J.K.; Li, M.J.; Wang, Y.H.; Liang, D. Effects of high temperature on activities and gene expression of enzymes involved in ascorbate-glutathione cycle in apple leaves. Plant Sci. 2008, 175, 761–766. [Google Scholar] [CrossRef]
- Qin, D.; Zhao, L.J.; Gao, Y.G.; Li, F.X.; Li, S.L.; Huo, J.W.; Lou, S.; Liu, P. Effects of fruit thinning on ascorbate-glutathione cycle metabolism in black currants (Ribes nigrum L.). J. For. Res. 2017, 28, 903–908. [Google Scholar] [CrossRef]
- Lee, M.H.; Bostock, R.M. Fruit exocarp phenols in relation to quiescence and development of Monilinia fructicola infections in Prunus spp.: A role for cellular redox? Phytopathology 2007, 97, 269–277. [Google Scholar] [CrossRef]
- Ma, J.Y.; Kong, X.T.; Wang, X.S.; Xu, Y.Y.; Zhao, M.T.; Xie, H.; Si, W.J.; Zhang, Z.X. A dual-emission mitochondria targeting fluorescence probe for detecting hydroxyl radical and its generation induced by cellular activities. J. Mol. Liq. 2024, 406, 125126. [Google Scholar] [CrossRef]
- Chen, F.; Miao, X.; Lin, Z.X.; Xiu, Y.; Shi, L.L.; Zhang, Q.; Liang, D.C.; Lin, S.Z.; He, B. Disruption of metabolic function and redox homeostasis as antibacterial mechanism of Lindera glauca fruit essential oil against Shigella flexneri. Food Control 2021, 130, 108282. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, B.; Chen, W.; Wang, Y.; Zhou, D.; Zhang, M.; Zhang, C.; Li, R.; He, J. The Role of Potato Glycoside Alkaloids Mediated Oxidative Stress in Inducing Apoptosis of Wolfberry Root Rot Pathogen Fungi. Antioxidants 2024, 13, 1537. https://doi.org/10.3390/antiox13121537
Sun Y, Wang B, Chen W, Wang Y, Zhou D, Zhang M, Zhang C, Li R, He J. The Role of Potato Glycoside Alkaloids Mediated Oxidative Stress in Inducing Apoptosis of Wolfberry Root Rot Pathogen Fungi. Antioxidants. 2024; 13(12):1537. https://doi.org/10.3390/antiox13121537
Chicago/Turabian StyleSun, Yuyan, Bin Wang, Wei Chen, Yanbo Wang, Dongdong Zhou, Mengyang Zhang, Chongqing Zhang, Ruiyun Li, and Jing He. 2024. "The Role of Potato Glycoside Alkaloids Mediated Oxidative Stress in Inducing Apoptosis of Wolfberry Root Rot Pathogen Fungi" Antioxidants 13, no. 12: 1537. https://doi.org/10.3390/antiox13121537
APA StyleSun, Y., Wang, B., Chen, W., Wang, Y., Zhou, D., Zhang, M., Zhang, C., Li, R., & He, J. (2024). The Role of Potato Glycoside Alkaloids Mediated Oxidative Stress in Inducing Apoptosis of Wolfberry Root Rot Pathogen Fungi. Antioxidants, 13(12), 1537. https://doi.org/10.3390/antiox13121537





