Selenium Nanoparticles Suppressed Oxidative Stress and Promoted Tenocyte Marker Expression in Tendon-Derived Stem/Progenitor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Preparation of SeNPs
2.3. TDSC Isolation
2.4. Alamar Blue Assay
2.5. Immunofluorescence Staining of Ki67, IL-6, Cyclooxygenase-2 (Cox-2), Bax, Nitrotyrosine, and Malonaldehyde (MDA)
2.6. CM-H2DCF-DA Assay
2.7. Catalase Activity Assay
2.8. qRT-PCR
2.9. Statistical Analysis
3. Results
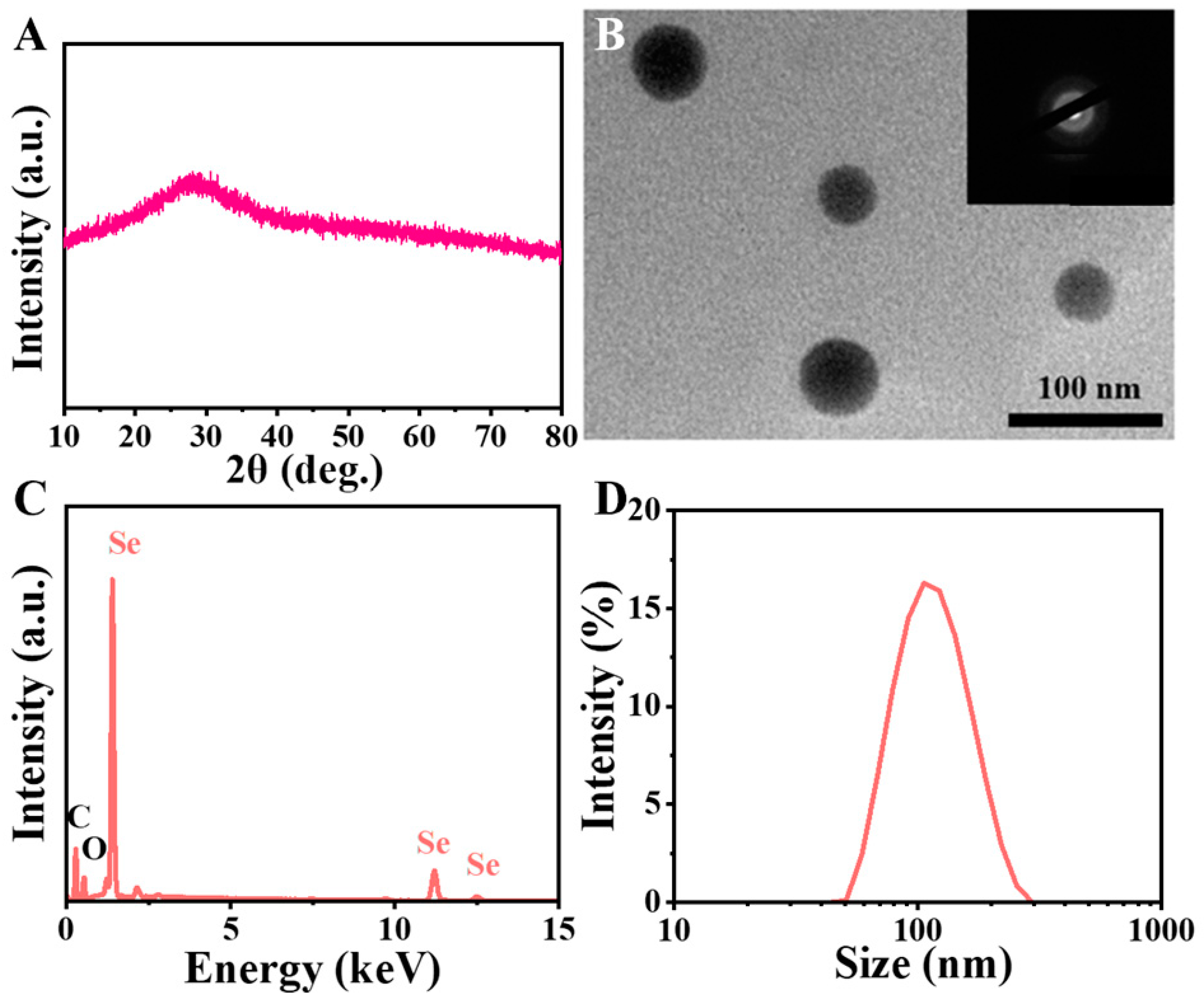
3.1. Characterization of SeNPs
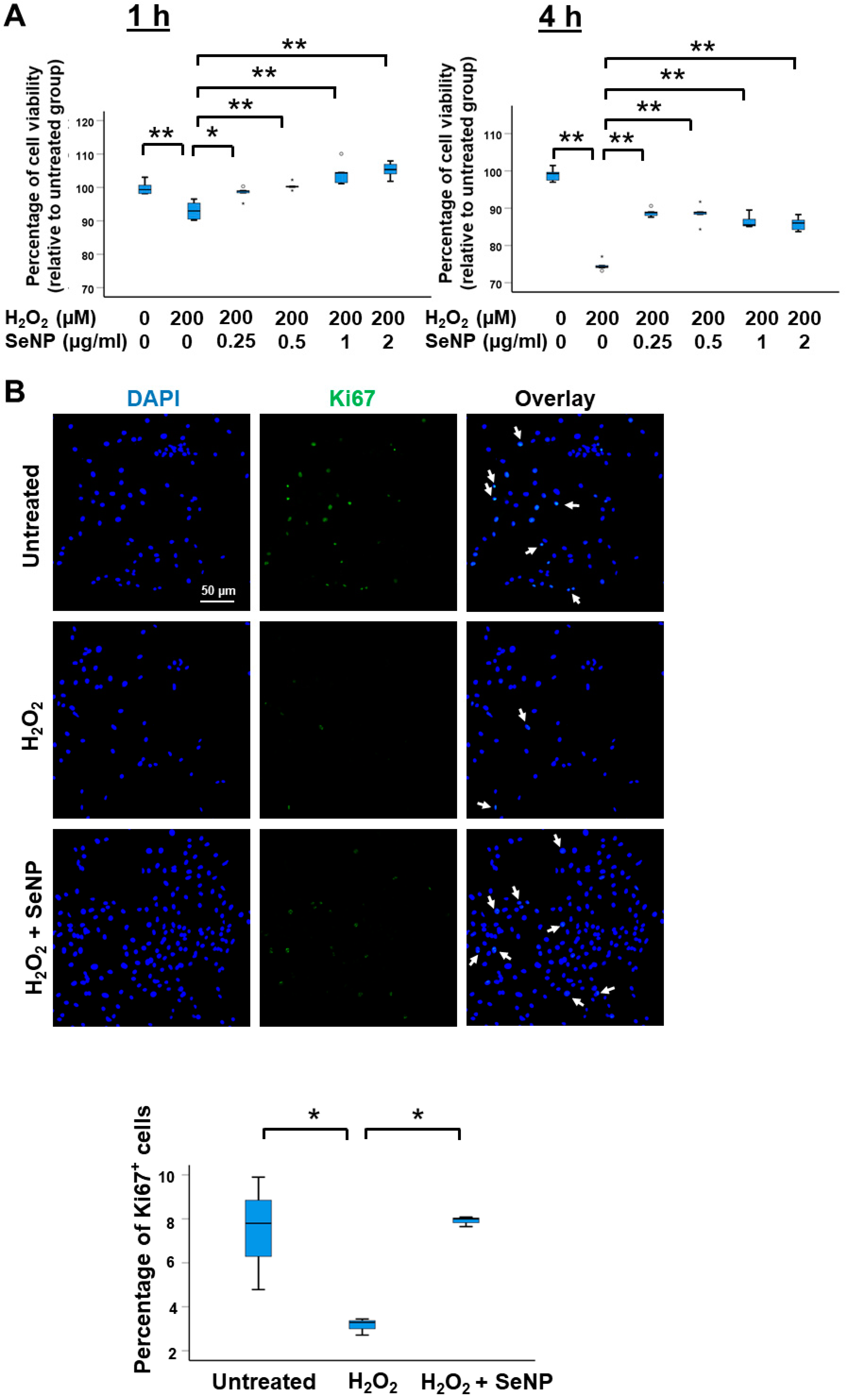
3.2. Viability and Expression of Marker of Proliferation
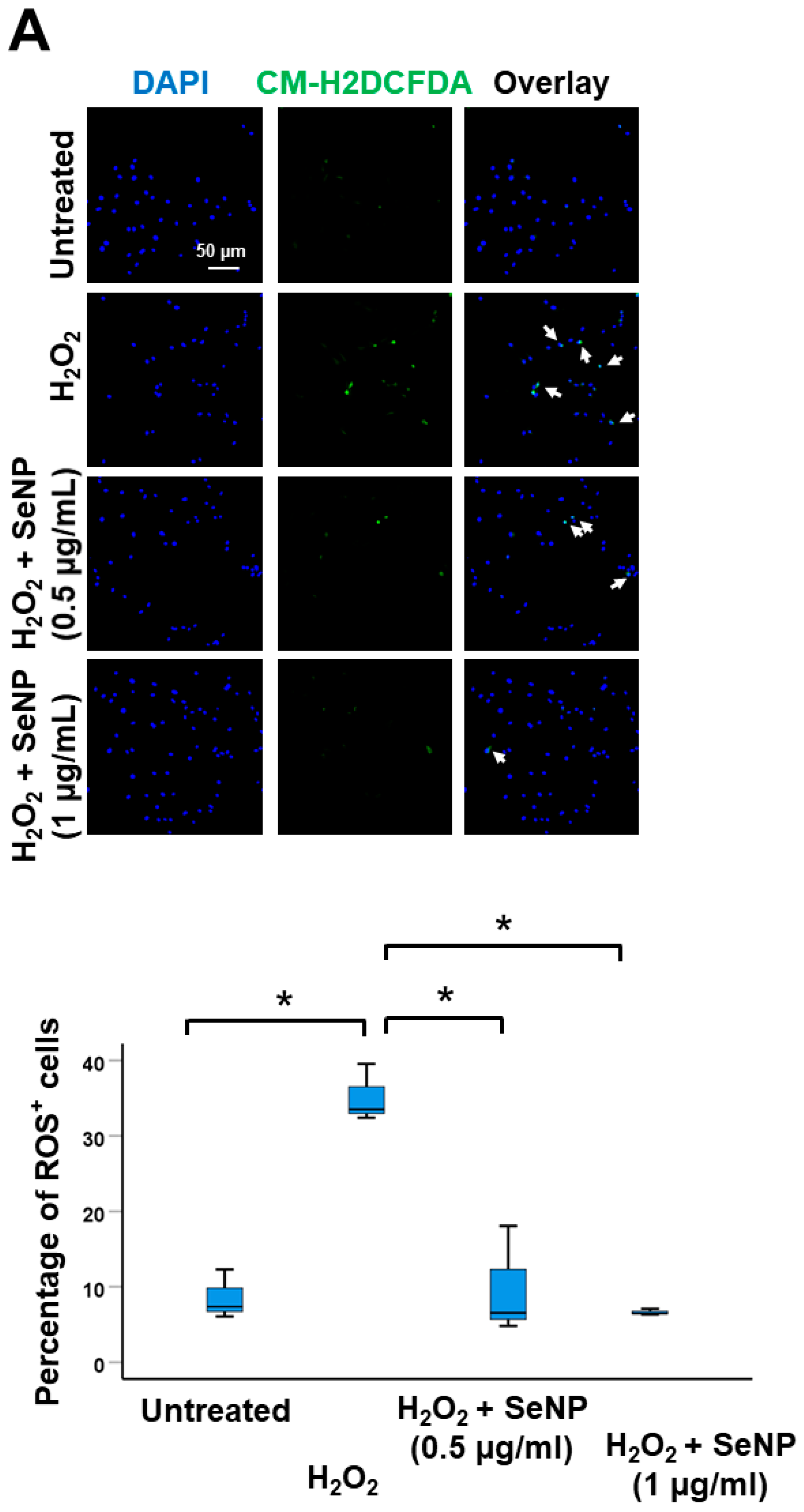
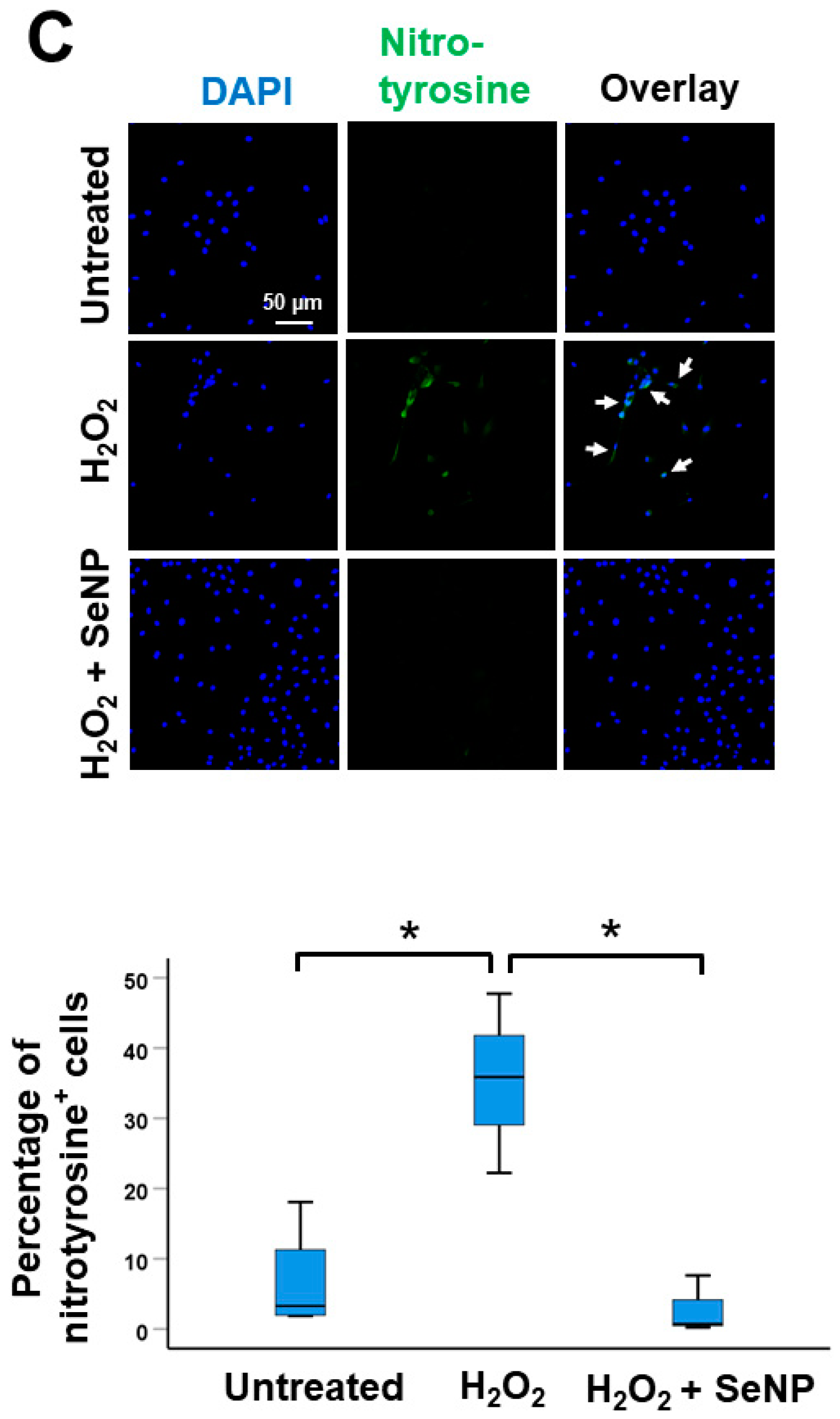
3.3. Oxidative Stress
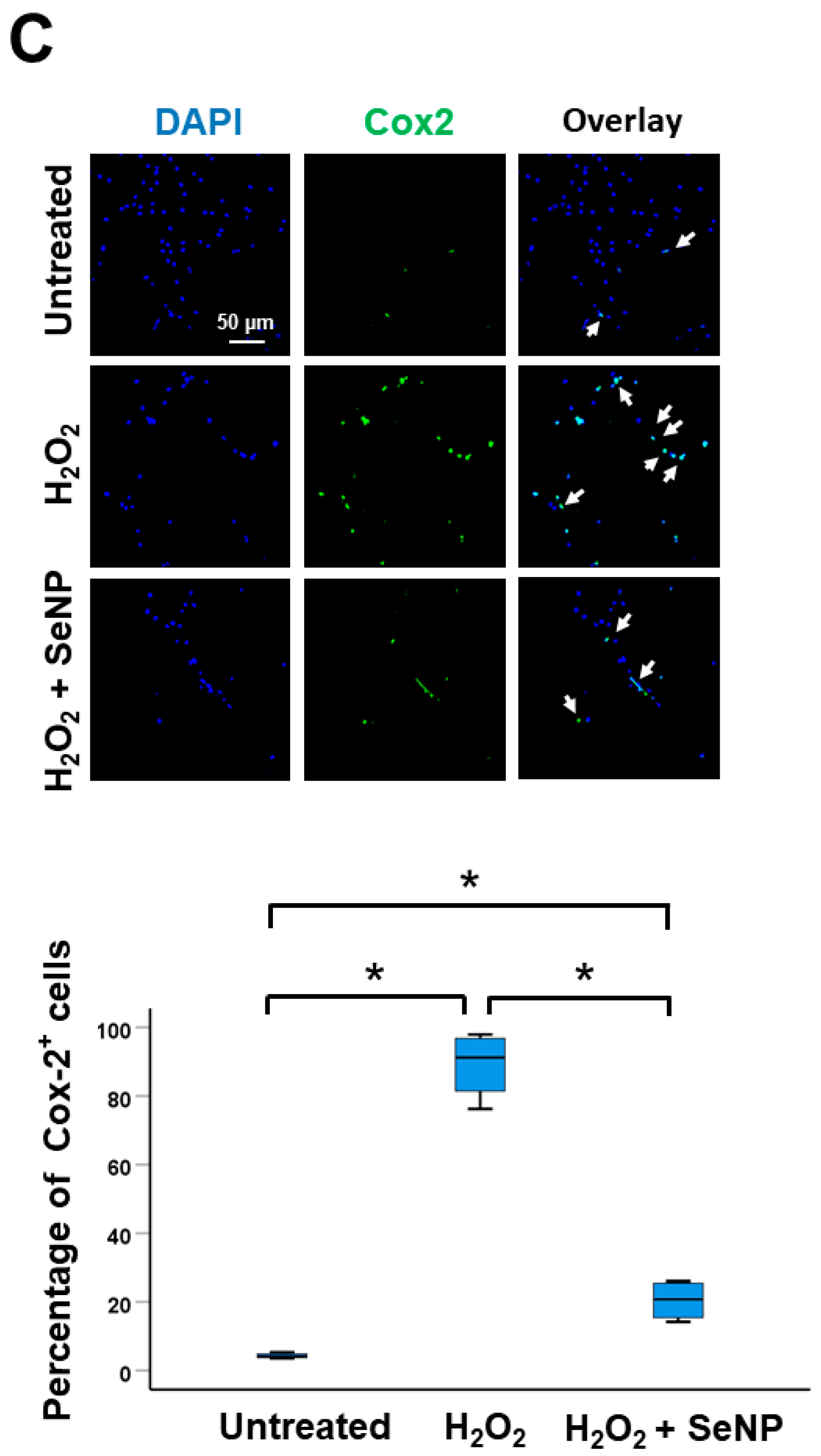
3.4. Expression of Inflammatory Cytokines
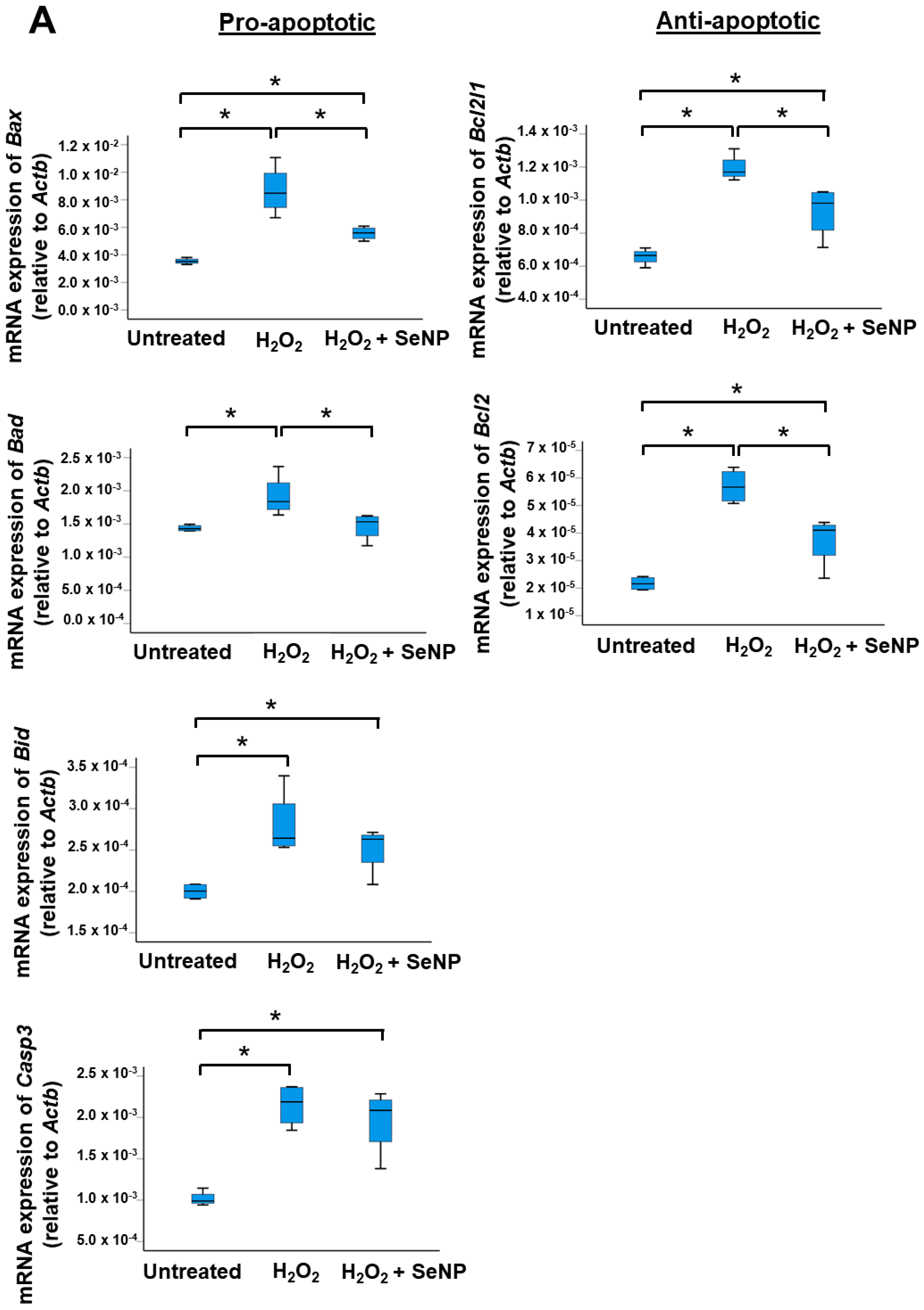
3.5. Apoptosis
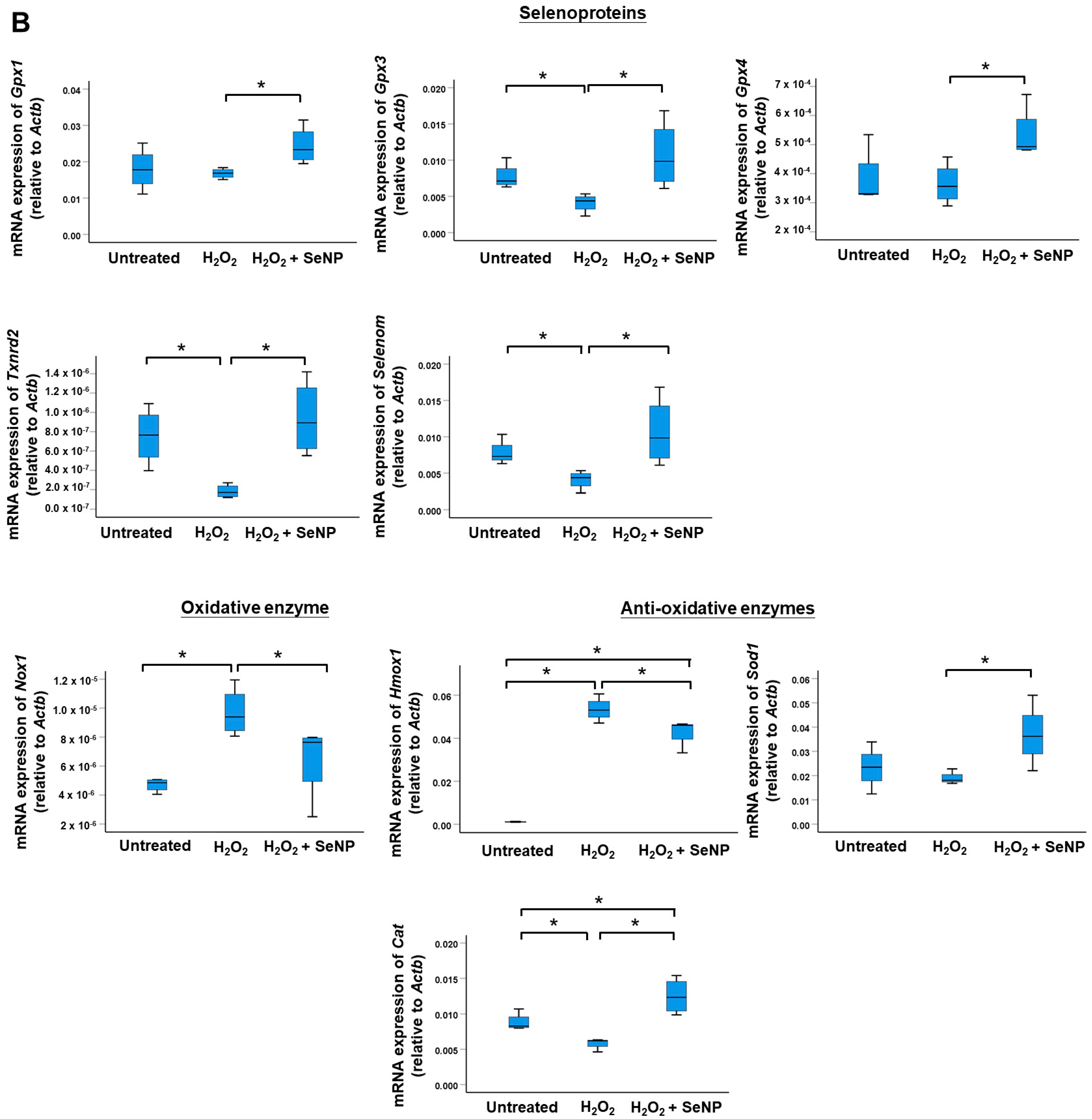
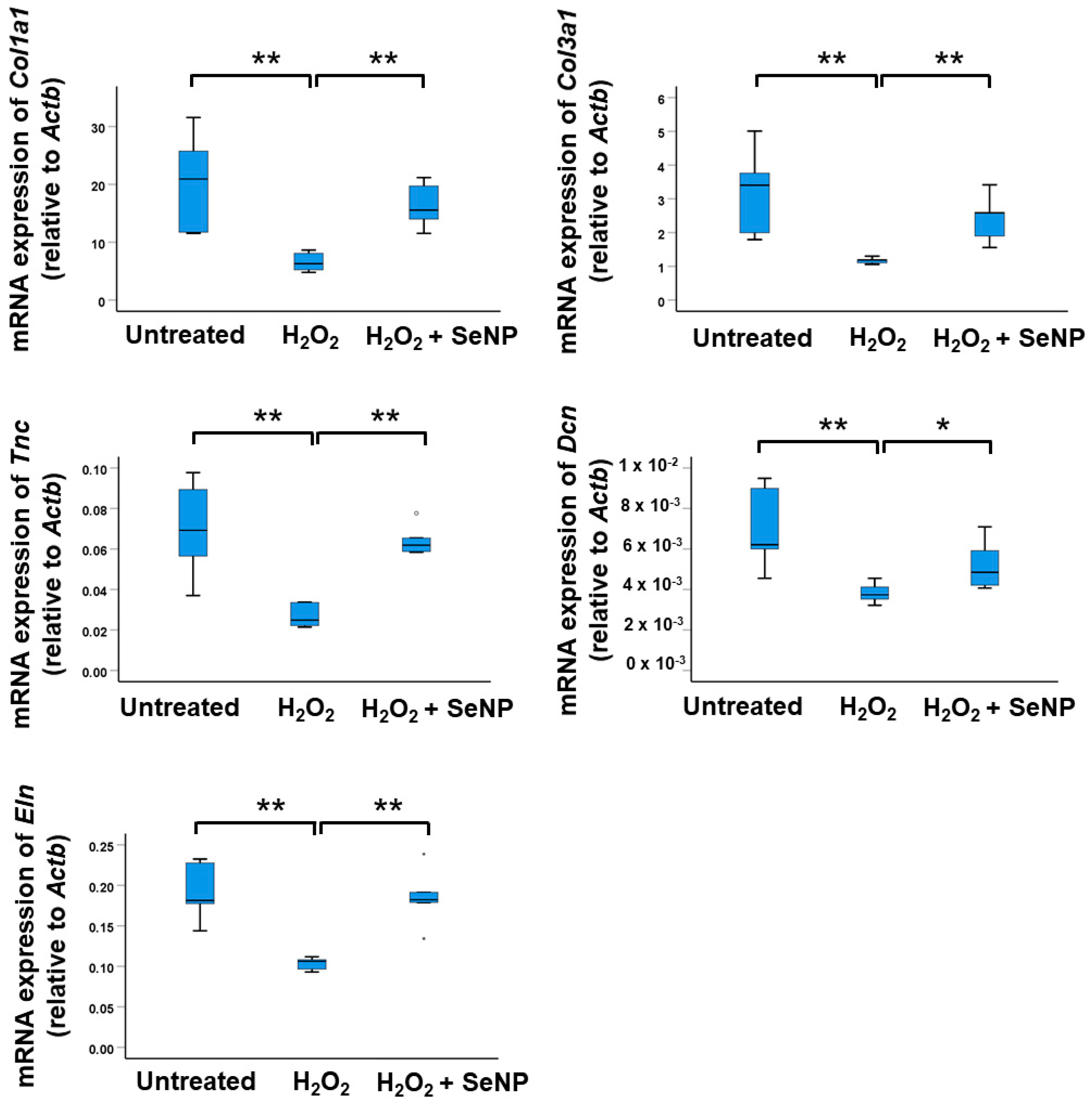
3.6. Expression of Tenocyte Markers
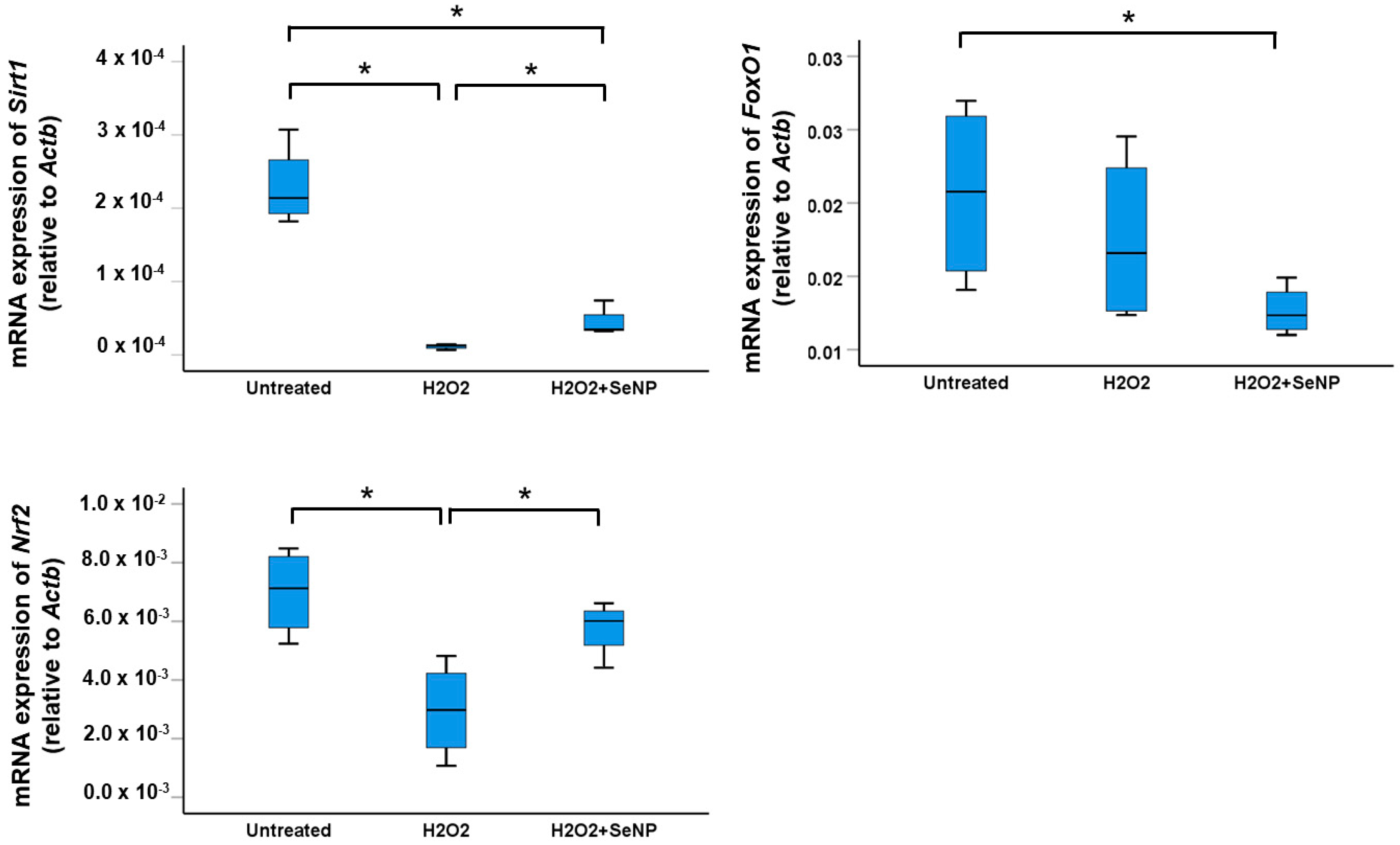
3.7. Expression of Transcription Factors Sirt1, FoxO1, and Nrf2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longo, U.G.; Oliva, F.; Denaro, V.; Maffulli, N. Oxygen species and overuse tendinopathy in athletes. Disabil. Rehabil. 2008, 30, 1563–1571. [Google Scholar] [CrossRef]
- Li, P.; Zhou, H.; Tu, T.; Lu, H. Dynamic exacerbation in inflammation and oxidative stress during the formation of peritendinous adhesion resulted from acute tendon injury. J. Orthop. Surg. Res. 2021, 16, 293. [Google Scholar] [CrossRef]
- Lui, P.P.Y.; Zhang, X.; Yao, S.; Sun, H.; Huang, C. Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy—A Target for Intervention. Int. J. Mol. Sci. 2022, 23, 3571. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Di Carlo, L.; Cocco, G.; Cocco, A.; Sabatini, E.; Salini, V. Oxidative stress and abnormal tendon sonographic features in elite soccer players (a pilot study). Rev. Bras. Orthop. 2021, 56, 432–437. [Google Scholar]
- Li, X.; Su, Z.; Shen, K.; Wang, Q.; Xu, C.; Wang, F.; Zhang, Y.; Jiang, D. Eugenol-preconditioned mesenchymal stem cell-derived extracellular vesicles promote antioxidant capacity of tendon stem cells in vitro and in vivo. Oxid. Med. Cell Longev. 2022, 2022, 3945195. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Yin, Z.; Shen, W.L.; Xie, Y.B.; Zhu, T.; Lu, P.; Cai, Y.Z.; Kong, M.J.; Heng, B.C.; Zhou, Y.T.; et al. Pharmacological regulation of in situ tissue stem cells differentiation for soft tissue calcification treatment. Stem Cells 2016, 34, 1083–1096. [Google Scholar] [CrossRef]
- Brandt, L.; Schubert, S.; Scheibe, P.; Brehm, W.; Franzen, J.; Gross, C.; Burk, J. Tenogenic properties of mesenchymal progenitor cells are compromised in an inflammatory environment. Int. J. Mol. Sci. 2018, 19, 2549. [Google Scholar] [CrossRef]
- Asai, S.; Otsuru, S.; Candela, M.E.; Cantley, L.; Uchibe, K.; Hofmann, T.J.; Zhang, K.; Wapner, K.L.; Soslowsky, L.J.; Horwitz, E.M.; et al. Tendon progenitor cells in injured tendons have strong chondrogenic potential: The CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells 2014, 32, 3266–3277. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Gromadzinska, J.; Reszka, E.; Bruzelius, K.; Wasowicz, W.; Akesson, B. Selenium and cancer: Biomarkers of selenium status and molecular action of selenium supplements. Eur. J. Nutr. 2008, 47, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Albanes, D. Selenium, vitamin E, and prostate cancer--ready for prime time? J. Natl. Cancer Inst. 1998, 90, 1184–1185. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigo, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Webster, T.J.; Roy, A.K. Cytoprotective effects of cerium and selenium nanoparticles on heat-shocked human dermal fibroblasts: An in vitro evaluation. Int. J. Nanomed. 2016, 11, 1427–1433. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother 2019, 109, 2562572. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lee, N.H.; Patel, K.D.; Jun, S.K.; Park, J.H.; Knowles, J.C.; Kim, H.W.; Lee, H.H.; Lee, J.H. A Study on Myogenesis by Regulation of Reactive Oxygen Species and Cytotoxic Activity by Selenium Nanoparticles. Antioxidants 2021, 10, 1727. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Zhang, M.; Ullah, M.W.; Liu, L.; Zhao, W.; Li, Y.; Ahmed, A.A.Q.; Cheng, H.; Shi, Z.; et al. In Situ Synthesized Selenium Nanoparticles-Decorated Bacterial Cellulose/Gelatin Hydrogel with Enhanced Antibacterial, Antioxidant, and Anti-Inflammatory Capabilities for Facilitating Skin Wound Healing. Adv. Healthc. Mater. 2021, 10, e2100402. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P. A practical guide for the isolation and maintenance of stem cells from tendon. Methods Mol. Biol. 2015, 1212, 127–140. [Google Scholar]
- Liu, Y.C.; Wang, H.L.; Huang, Y.Z.; Weng, Y.H.; Chen, R.S.; Tsai, W.C.; Yeh, T.H.; Lu, C.S.; Chen, Y.L.; Lin, Y.W.; et al. Alda-1, an activator of ALDH2, ameliorates Achilles tendinopathy in cellular and mouse models. Biochem. Pharmacol. 2020, 175, 113919. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.; Ye, H.; Liang, W.; Lam, K.K.; Cheng, B.; Lu, Y.; Jiang, C. Nudt21-mediated alternative polyadenylation of HMGA2 3′-UTR impairs stemness of human tendon stem cell. Aging (Albany NY) 2020, 12, 18436–18452. [Google Scholar] [CrossRef]
- Lee, Y.W.; Fu, S.C.; Yeung, M.Y.; Lau, C.M.L.; Chan, K.M.; Hung, L.K. Effects of redox modulation on cell proliferation, viability, and migration in cultured rat and human tendon progenitor cells. Oxid. Med. Cell Longev. 2017, 2017, 8785042. [Google Scholar] [CrossRef]
- Chen, H.; Ge, H.A.; Wu, G.B.; Cheng, B.; Lu, Y.; Jiang, C. Autophagy prevents oxidative stress-induced loss of self-renewal capacity and stemness in human tendon stem cells by reducing ROS accumulation. Cell Physiol. Biochem. 2016, 39, 2227–2238. [Google Scholar] [CrossRef]
- Lui, P.P. Identity of tendon stem cells--how much do we know? J. Cell. Mol. Med. 2013, 17, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Cheuk, Y.C.; Lee, Y.W.; Chan, K.M. Ectopic chondro-ossification and erroneous extracellular matrix deposition in a tendon window injury model. J. Orthop. Res. 2012, 30, 37–46. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, L.; Zhou, K.; Ding, L.; Zeng, J.; Zhang, W. Anti-Oxidant and Anti-Endothelial Dysfunctional Properties of Nano-Selenium in vitro and in vivo of Hyperhomocysteinemic Rats. Int. J. Nanomed. 2020, 15, 4501–4521. [Google Scholar] [CrossRef] [PubMed]
- Gasparian, A.V.; Yao, Y.J.; Lü, J.; Yemelyanov, A.Y.; Lyakh, L.A.; Slaga, T.J.; Budunova, I.V. Selenium compounds inhibit I kappa B kinase (IKK) and nuclear factor-kappa B (NF-κB) in prostate cancer cells. Mol. Cancer Ther. 2002, 1, 1079–1087. [Google Scholar]
- Nelson, S.M.; Lei, X.; Prabhu, K.S. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J. Nutr. 2011, 141, 1754–1761. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef]
- Fatima, S.; Alfrayh, R.; Alrashed, M.; Alsobaie, S.; Ahmad, R.; Mahmood, A. Selenium Nanoparticles by Moderating Oxidative Stress Promote Differentiation of Mesenchymal Stem Cells to Osteoblasts. Int. J. Nanomed. 2021, 16, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Kang, C.; Oh, Y.B.; Ko, M.H.; Seo, J.H.; Lee, D. Ultrasonographic Imaging and Anti-inflammatory Therapy of Muscle and Tendon Injuries Using Polymer Nanoparticles. Theranostics 2017, 7, 2463–2476. [Google Scholar] [CrossRef]
- Forman, H.J.; Bernardo, A.; Davies, K.J. What is the concentration of hydrogen peroxide in blood and plasma? Arch Biochem. Biophys. 2016, 603, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Meng, J.; Wang, Z.; Yuan, T.; Qian, H.; Chen, W.; Tong, J.; Xie, Y.; Zhang, Y.; Zhao, J.; et al. Proanthocyanidins Attenuation of H2O2-Induced Oxidative Damage in Tendon-Derived Stem Cells via Upregulating Nrf-2 Signaling Pathway. Biomed. Res. Int. 2017, 2017, 7529104. [Google Scholar] [CrossRef]
- Ng, H.H.; Leo, C.H.; O’Sullivan, K.; Alexander, S.A.; Davies, M.J.; Schiesser, C.H.; Parry, L.J. 1,4-Anhydro-4-seleno-d-talitol (SeTal) protects endothelial function in the mouse aorta by scavenging superoxide radicals under conditions of acute oxidative stress. Biochem. Pharmacol. 2017, 128, 34–45. [Google Scholar] [CrossRef]
- Au, A.; Mojadadi, A.; Shao, J.Y.; Ahmad, G.; Witting, P.K. Physiological Benefits of Novel Selenium Delivery via Nanoparticles. Int. J. Mol.Sci. 2023, 24, 6068. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.; Sunderam, V.; Manjusha, M.; Dlamini, Z.; Lawrance, A.V. Selenium Nanoparticles: A Comprehensive Examination of Synthesis Techniques and Their Diverse Applications in Medical Research and Toxicology Studies. Molecules 2024, 29, 801. [Google Scholar] [CrossRef]
- Ryabova, Y.V.; Sutunkova, M.P.; Minigalieva, I.A.; Shabardina, L.V.; Filippini, T.; Tsatsakis, A. Toxicological effects of selenium nanoparticles in laboratory animals: A review. J. Appl. Toxicol. 2024, 44, 4–16. [Google Scholar] [CrossRef]
- Ilhan, I.; Asci, H.; Tepebasi, M.Y.; Imeci, O.B.; Sevuk, M.A.; Temel, E.N.; Ozmen, O. Selenium exerts protective effects on inflammatory cardiovascular damage: Molecular aspects via SIRT1/p53 and Cyt-c/Cas-3 pathways. Mol. Biol. Rep. 2023, 50, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- ALRashdi, B.M.; Hussein, M.M.; Mohammed, R.M.; Abdelhamed, N.W.; Asaad, M.E.; Alruwaili, M.; Alrashidi, S.M.; Habotta, O.A.; Abdel Moneim, A.E.; Ramadan, S.S. Turmeric Extract-loaded Selenium Nanoparticles Counter Doxorubicin-induced Hepatotoxicity in Mice via Repressing Oxidative Stress, Inflammatory Cytokines, and Cell Apoptosis. Anticancer. Agents Med. Chem. 2024, 24, 443–453. [Google Scholar] [CrossRef]
- Rahimi, B.; Panahi, M.; Lotfi, H.; Khalili, M.; Salehi, A.; Saraygord-Afshari, N.; Alizadeh, E. Sodium selenite preserves rBM-MSCs’ stemness, differentiation potential, and immunophenotype and protects them against oxidative stress via activation of the Nrf2 signaling pathway. BMC Complement Med. Ther. 2023, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, R.A.; Abdelghany, A.M.; Samra, Y.A. Rutin and selenium nanoparticles protected against STZ-induced diabetic nephropathy in rats through downregulating Jak-2/Stat3 pathway and upregulating Nrf-2/HO-1 pathway. Eur. J. Pharmacol. 2022, 933, 175289. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.S.; Li, T.L.; Wei, S. Co-modified 3D printed β-tricalcium phosphate with magnesium and selenium promotes bone defect regeneration in ovariectomized rat. J. Mater. Sci. Mater. Med. 2023, 34, 7. [Google Scholar] [CrossRef]
- Amani, H.; Habibey, R.; Shokri, F.; Hajmiresmail, S.J.; Akhavan, O.; Mashaghi, A.; Pazoki-Toroudi, H. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci. Rep. 2019, 9, 6044. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lui, P.P.Y.; Huang, C.; Zhang, X. Selenium Nanoparticles Suppressed Oxidative Stress and Promoted Tenocyte Marker Expression in Tendon-Derived Stem/Progenitor Cells. Antioxidants 2024, 13, 1536. https://doi.org/10.3390/antiox13121536
Lui PPY, Huang C, Zhang X. Selenium Nanoparticles Suppressed Oxidative Stress and Promoted Tenocyte Marker Expression in Tendon-Derived Stem/Progenitor Cells. Antioxidants. 2024; 13(12):1536. https://doi.org/10.3390/antiox13121536
Chicago/Turabian StyleLui, Pauline Po Yee, Caihao Huang, and Xing Zhang. 2024. "Selenium Nanoparticles Suppressed Oxidative Stress and Promoted Tenocyte Marker Expression in Tendon-Derived Stem/Progenitor Cells" Antioxidants 13, no. 12: 1536. https://doi.org/10.3390/antiox13121536
APA StyleLui, P. P. Y., Huang, C., & Zhang, X. (2024). Selenium Nanoparticles Suppressed Oxidative Stress and Promoted Tenocyte Marker Expression in Tendon-Derived Stem/Progenitor Cells. Antioxidants, 13(12), 1536. https://doi.org/10.3390/antiox13121536






