In Vitro Protective Effects of a Standardized Extract of Opuntia ficus-indica (L.) Mill. Cladodes and Olea europaea L. Leaves Against Indomethacin-Induced Intestinal Epithelial Cell Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures and Treatments
2.3. TEER Determination
2.4. Fluorescein Permeability
2.5. Cells Lysate Extraction
2.6. Western Blot Analysis
2.7. Real-Time PCR
2.8. Intracellular Total Antioxidant Activity (TAA)
2.9. ROS Measurement by Dichlorodihydro-Fluorescein Diacetate Assay
2.10. Statistical Analysis
3. Results
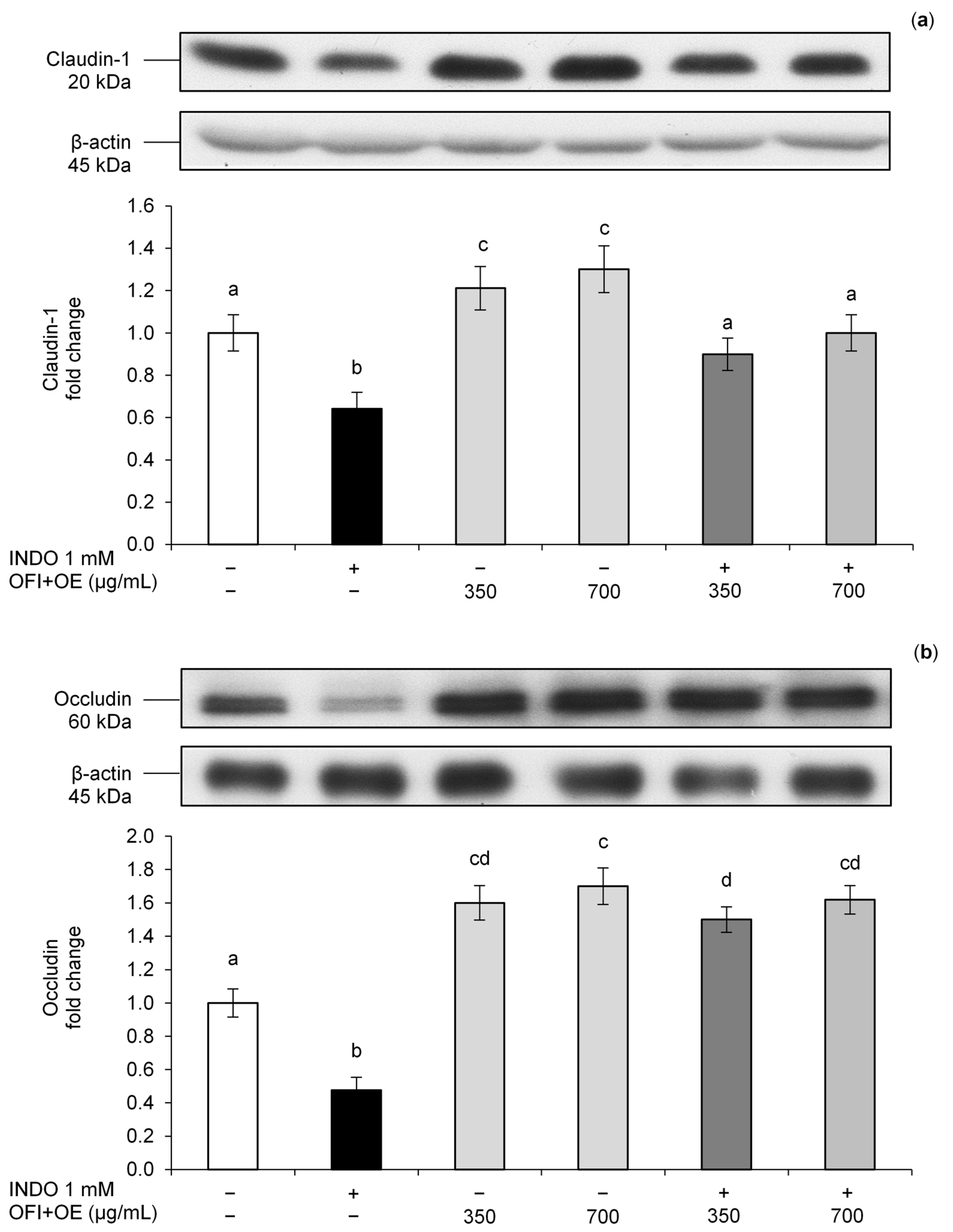
3.1. Protective Effects of OFI+OE on Indomethacin-Induced Intestinal Epithelial Barrier Function Alteration
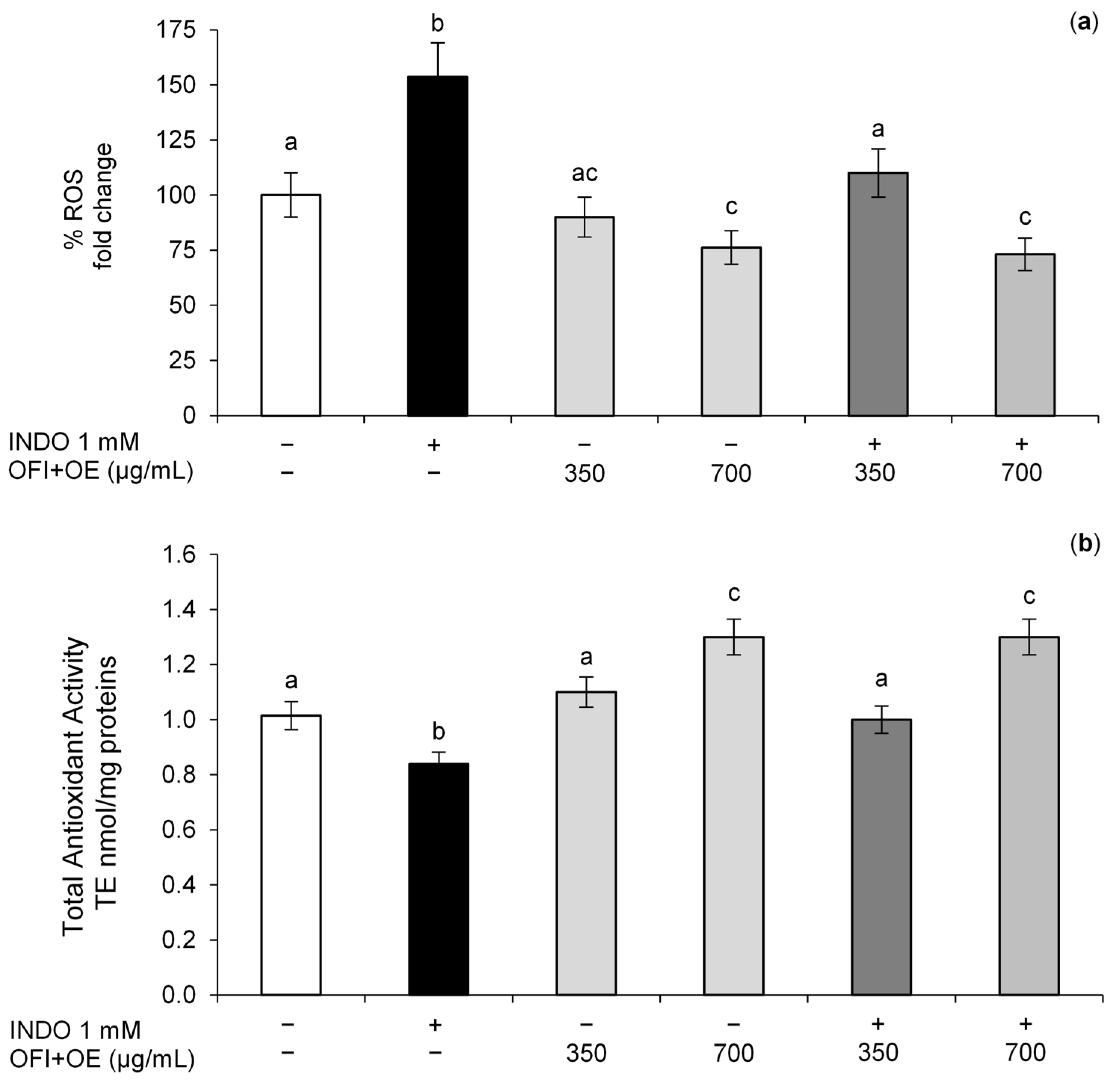
3.2. Protective Effect of OFI+OE on the Intracellular Redox Status Alteration Induced by INDO
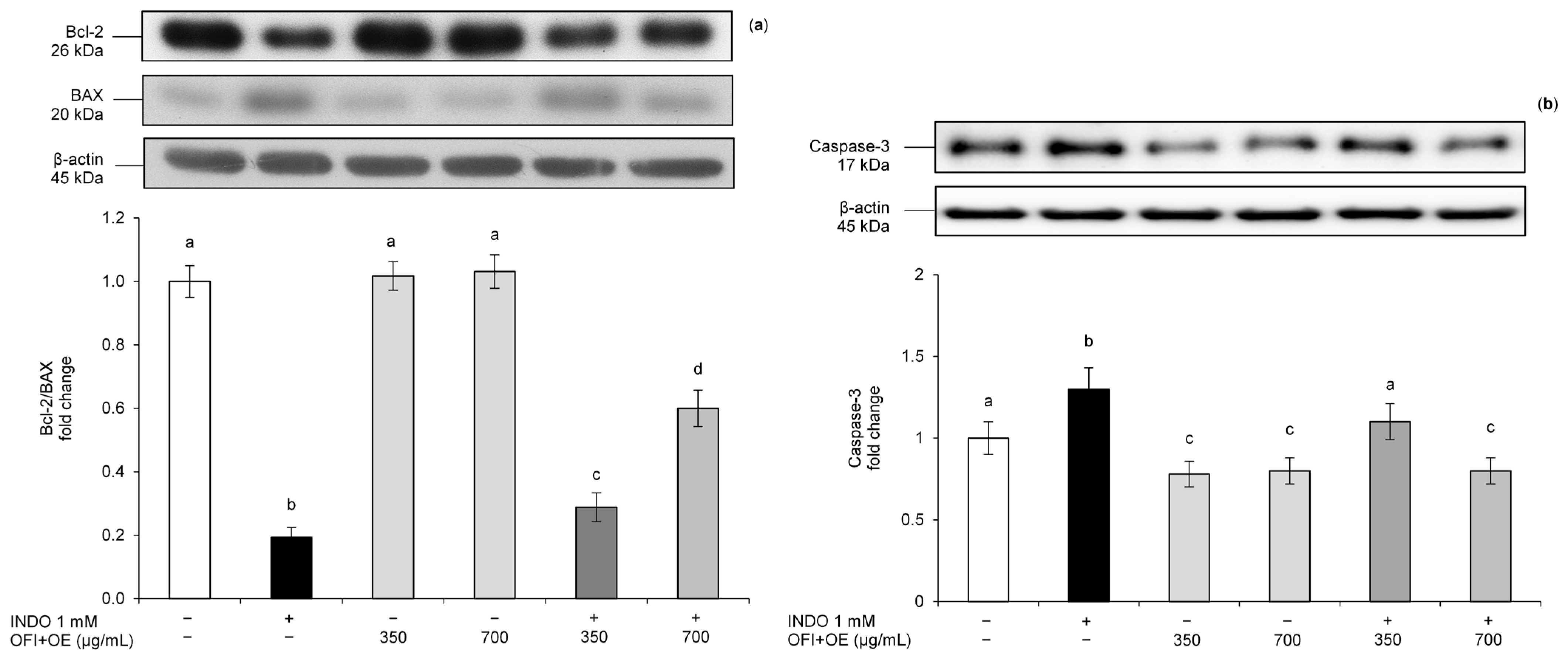
3.3. OFI+OE Effects on Apoptosis Pathway
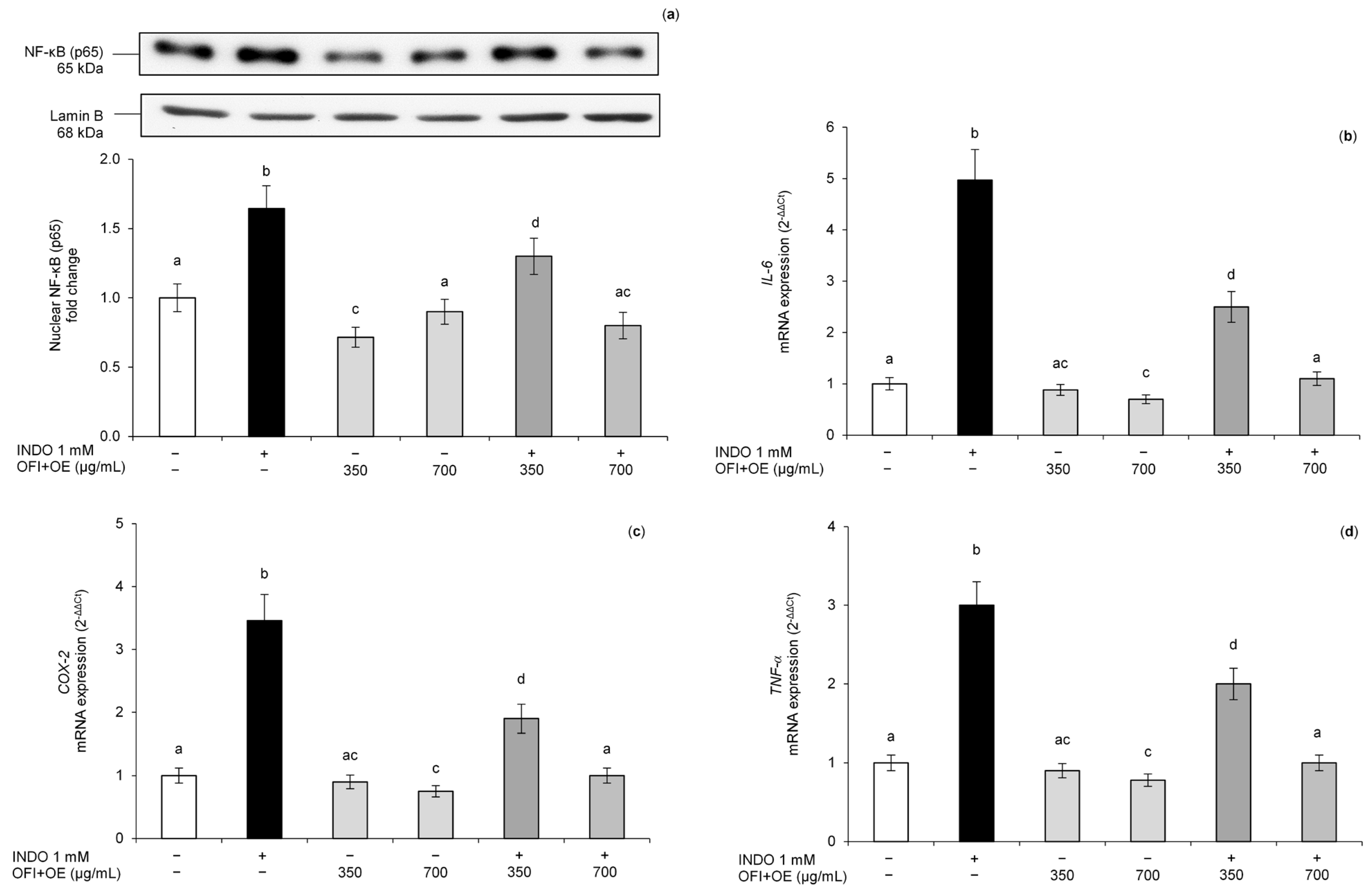
3.4. Protective Effect of OFI+OE on Indomethacin-Induced NF-κB Pathway Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2-azinobis-(3-ethybenzothiazoline-6-sulfonic acid) |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| DCFH-DA | dichloro-dihydro-fluorescein diacetate |
| DMEM | Dulbecco’s modified eagle’s medium |
| DMSO | dimethyl sulfoxide |
| DPBS | Dulbecco’s phosphate-buffered saline |
| ER | endoplasmic reticulum |
| GI | gastrointestinal |
| INDO | indomethacin |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| OFI+OE | Opuntia ficus-indica (L.) Mill. cladodes and Olea europaea L. leaf extract |
| PPIs | proton pump inhibitors |
| ROS | reactive oxygen species |
| TAA | Total Antioxidant Activity |
| TEER | Trans-Epithelial Electrical Resistance |
| TJs | tight junctions |
References
- Scarpignato, C.; Hunt, R.H. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: Clinical picture, pathogenesis, and prevention. Gastroenterol. Clin. N. Am. 2010, 39, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Thakre-Nighot, M.; Blikslager, A.T. Indomethacin induces increase in gastric epithelial tight junction permeability via redistribution of occludin and activation of p38 MAPK in MKN-28 Cells. Tissue Barriers 2016, 4, e1187325. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S. The Pharmacology of Indomethacin. Headache 2016, 56, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, H.; Albayrak, A.; Bilici, M.; Cadirci, E.; Halici, Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 2010, 33, 224–234. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Morales, P.; Gotteland, M. Polyphenols protect the epithelial barrier function of Caco-2 cells exposed to indomethacin through the modulation of occludin and zonula occludens-1 expression. J. Agric. Food Chem. 2013, 61, 5291–5297. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Gotteland, M.; Speisky, H. Protection by apple peel polyphenols against indometacin-induced oxidative stress, mitochondrial damage and cytotoxicity in Caco-2 cells. J. Pharm. Pharmacol. 2010, 62, 943–950. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Lu, C.C.; Yen, G.C. Phytochemicals enhance antioxidant enzyme expression to protect against NSAID-induced oxidative damage of the gastrointestinal mucosa. Mol. Nutr. Food Res. 2017, 61, 1600659. [Google Scholar] [CrossRef]
- Fan, J.; Li, B.R.; Zhang, Q.; Zhao, X.H.; Wang, L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem. Toxicol. 2021, 147, 111896. [Google Scholar] [CrossRef]
- Han, Y.M.; Park, J.M.; Her, S.; Kim, M.S.; Park, Y.J.; Hahm, K.B. Revaprazan prevented indomethacin-induced intestinal damages by enhancing tight junction related mechanisms. Biochem. Pharmacol. 2020, 182, 114290. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Gotteland, M.; Speisky, H. Apple peel polyphenol extract protects against indomethacin-induced damage in Caco-2 cells by preventing mitochondrial complex I inhibition. J. Agric. Food Chem. 2011, 59, 11501–11508. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Lopez-Alarcón, C.; Aliaga, M.E.; Speisky, H. Inhibition of mitochondrial complex I by various non-steroidal anti-inflammatory drugs and its protection by quercetin via a coenzyme Q-like action. Chem. Biol. Interact. 2012, 199, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Pellegatta, G.; Coppo, C.; Savarino, E. Proton pump inhibitors: Use and misuse in the clinical setting. Expert. Rev. Clin. Pharmacol. 2018, 11, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Syer, S.; Denou, E.; de Palma, G.; Vong, L.; McKnight, W.; Jury, J.; Bolla, M.; Bercik, P.; Collins, S.M.; et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 2011, 141, 1314–1322.e1-5. [Google Scholar] [CrossRef] [PubMed]
- Boelsterli, U.A.; Redinbo, M.R.; Saitta, K.S. Multiple NSAID-induced hits injure the small intestine: Underlying mechanisms and novel strategies. Toxicol. Sci. 2013, 131, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; McAlindon, M.E. NSAIDs and the small bowel. Curr. Opin. Gastroenterol. 2018, 34, 175–182. [Google Scholar] [CrossRef]
- Varum, F.J.; McConnell, E.L.; Sousa, J.J.; Veiga, F.; Basit, A.W. Mucoadhesion and the gastrointestinal tract. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 207–258. [Google Scholar] [CrossRef]
- Kumar, R.; Islam, T.; Nurunnabi, M. Mucoadhesive carriers for oral drug delivery. J. Control. Release 2022, 351, 504–559. [Google Scholar] [CrossRef]
- Boonyong, C.; Vardhanabhuti, N.; Jianmongkol, S. Natural polyphenols prevent indomethacin-induced and diclofenac-induced Caco-2 cell death by reducing endoplasmic reticulum stress regardless of their direct reactive oxygen species scavenging capacity. J. Pharm. Pharmacol. 2020, 72, 583–591. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Aref, A.R.; Sethi, G.; Ertas, Y.N.; Wang, L. Natural product/diet-based regulation of macrophage polarization: Implications in treatment of inflammatory-related diseases and cancer. J. Nutr. Biochem. 2024, 130, 109647. [Google Scholar] [CrossRef]
- Abdu, S.; Juaid, N.; Amin, A.; Moulay, M.; Miled, N. Effects of Sorafenib and Quercetin Alone or in Combination in Treating Hepatocellular Carcinoma: In Vitro and In Vivo Approaches. Molecules 2022, 27, 22. [Google Scholar] [CrossRef]
- Speciale, A.; Saija, A.; Bashllari, R.; Molonia, M.S.; Muscarà, C.; Occhiuto, C.; Cimino, F.; Cristani, M. Anthocyanins As Modulators of Cell Redox-Dependent Pathways in Non-Communicable Diseases. Curr. Med. Chem. 2020, 27, 1955–1996. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Molonia, M.S.; Muscarà, C.; Cristani, M.; Salamone, F.L.; Saija, A.; Cimino, F. An overview on the cellular mechanisms of anthocyanins in maintaining intestinal integrity and function. Fitoterapia 2024, 175, 105953. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F.; et al. The polysaccharide and low molecular weight components of Opuntia ficus indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Mannai, F.; Elhleli, H.; Ammar, M.; Passas, R.; Elaloui, E.; Moussaoui, Y. Green process for fibrous networks extraction from Opuntia (Cactaceae): Morphological design, thermal and mechanical studies. Ind. Crops Prod. 2018, 126, 347–356. [Google Scholar] [CrossRef]
- Mannai, F.; Mechi, L.; Alimi, F.; Alsukaibi, A.K.D.; Belgacem, M.N.; Moussaoui, Y. Biodegradable composite films based on mucilage from Opuntia ficus-indica (Cactaceae): Microstructural, functional and thermal properties. Int. J. Biol. Macromol. 2023, 252, 126456. [Google Scholar] [CrossRef]
- Mannai, F.; Elhleli, H.; Ben Mosbah, M.; Khiari, R.; Nacer, S.N.; Belgacem, M.N.; Moussaoui, Y. Comparative study of conventional and combined ultrasound-assisted methods on the quality of mucilage extracted from Opuntia ficus-indica cladodes. Ind. Crops Prod. 2024, 214, 118566. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Asnam, A.; Bouras, O.; Aouabed, A.; Bourven, I.; Baudu, M. Structuration of biosorbents in the form of reinforced gelled and porous composites based on Opuntia ficus indica (cactus) extract and sodium alginate. J. Water Process Eng. 2022, 46, 102612. [Google Scholar] [CrossRef]
- Elhleli, H.; Mannai, F.; Khiari, R.; Moussaoui, Y. The use of mucilage extracted from Opuntia ficus indica as a microencapsulating shell. J. Serb. Chem. Soc. 2020, 85, 33. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, V.K.; Du, Y.; Aghbashlo, M.; Show, P.L.; Pan, J.; Tabatabaei, M.; Rajaei, A. Potential application of polysaccharide mucilages as a substitute for emulsifiers: A review. Int. J. Biol. Macromol. 2023, 242, 124800. [Google Scholar] [CrossRef]
- Waghmare, R.; Preethi, R.; Moses, J.A.; Anandharamakrishnan, C. Mucilages: Sources, extraction methods, and characteristics for their use as encapsulation agents. Crit. Rev. Food Sci. Nutr. 2022, 62, 4186–4207. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological Actions of Opuntia ficus indica: A Review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Tripodo, M.M.; d’Aquino, A.; Mondello, M.R. Antiulcer activity of Opuntia ficus indica (L.) Mill. (Cactaceae): Ultrastructural study. J. Ethnopharmacol. 2001, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Pergolizzi, S.; Miceli, N.; Monforte, M.T.; Tripodo, M.M. Study on the increment of the production of gastric mucus in rats treated with Opuntia ficus indica (L.) Mill. cladodes. J. Ethnopharmacol. 2002, 83, 229–233. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Miceli, N.; Mondello, M.R.; Taviano, M.F.; Galluzzo, M.; Tripodo, M.M. Opuntia ficus indica (L.) Mill. mucilages show cytoprotective effect on gastric mucosa in rat. Phytother. Res. 2007, 21, 344–346. [Google Scholar] [CrossRef]
- Vázquez-Ramírez, R.; Olguín-Martínez, M.; Kubli-Garfias, C.; Hernández-Muñoz, R. Reversing gastric mucosal alterations during ethanol-induced chronic gastritis in rats by oral administration of Opuntia ficus-indica mucilage. World J. Gastroenterol. 2006, 12, 4318–4324. [Google Scholar] [CrossRef]
- Rizza, L.; Frasca, G.; Nicholls, M.; Puglia, C.; Cardile, V. Caco-2 cell line as a model to evaluate mucoprotective proprieties. Int. J. Pharm. 2012, 422, 318–322. [Google Scholar] [CrossRef]
- Missaoui, M.; D’Antuono, I.; D’Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of Micronutrients, Bioaccessibility and Antioxidant Activity of Prickly Pear Cladodes as Functional Ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Hernández, F.; Andreu-Coll, L.; Bento-Silva, A.; Serra, A.T.; Mena, P.; Legua, P.; Bronze, M.R. Phytochemical Profile of Opuntia ficus-indica (L.) Mill Fruits (cv. ‘Orito’) Stored at Different Conditions. Foods 2022, 11, 160. [Google Scholar] [CrossRef]
- Petruk, G.; Di Lorenzo, F.; Imbimbo, P.; Silipo, A.; Bonina, A.; Rizza, L.; Piccoli, R.; Monti, D.M.; Lanzetta, R. Protective effect of Opuntia ficus-indica L. cladodes against UVA-induced oxidative stress in normal human keratinocytes. Bioorg. Med. Chem. Lett. 2017, 27, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef] [PubMed]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, D.; Janićijević-Hudomal, S.; Ristić, S.; Radonjić, N.V.; Petronijević, N.D.; Piperski, V.; Mitrović, D.M. Attenuation of cold restraint stress-induced gastric lesions by an olive leaf extract. Gen. Physiol. Biophys. 2009, 28, 135–142. [Google Scholar] [PubMed]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-alpha and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Busa, R.; Saija, A.; Speciale, A. Cyanidin-3-O-Glucoside Modulates the In Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells. Mediat. Inflamm. 2017, 2017, 3454023. [Google Scholar] [CrossRef]
- Park, H.Y.; Kunitake, Y.; Hirasaki, N.; Tanaka, M.; Matsui, T. Theaflavins enhance intestinal barrier of Caco-2 Cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1. Biosci. Biotechnol. Biochem. 2015, 79, 130–137. [Google Scholar] [CrossRef]
- Cimino, F.; Speciale, A.; Anwar, S.; Canali, R.; Ricciardi, E.; Virgili, F.; Trombetta, D.; Saija, A. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013, 8, 391–399. [Google Scholar] [CrossRef]
- Muscarà, C.; Molonia, M.S.; Speciale, A.; Bashllari, R.; Cimino, F.; Occhiuto, C.; Saija, A.; Cristani, M. Anthocyanins ameliorate palmitate-induced inflammation and insulin resistance in 3T3-L1 adipocytes. Phytother. Res. 2019, 33, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seed, B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003, 31, e154. [Google Scholar] [CrossRef] [PubMed]
- Molonia, M.S.; Occhiuto, C.; Muscarà, C.; Speciale, A.; Bashllari, R.; Villarroya, F.; Saija, A.; Cimino, F.; Cristani, M. Cyanidin-3-O-glucoside restores insulin signaling and reduces inflammation in hypertrophic adipocytes. Arch. Biochem. Biophys. 2020, 691, 108488. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Fratantonio, D.; Ferrari, D.; Saija, A.; Cimino, F.; Speciale, A. Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase-3 activation and p21 upregulation. Mol. Med. Rep. 2016, 14, 1397–1403. [Google Scholar] [CrossRef]
- Dehimi, K.; Speciale, A.; Saija, A.; Dahamna, S.; Raciti, R.; Cimino, F.; Cristani, M. Antioxidant and Anti-inflammatory Properties of Algerian Thymelaea microphylla Coss. and Dur. Extracts. Pharmacogn. Mag. 2016, 12, 203–210. [Google Scholar]
- Fratantonio, D.; Speciale, A.; Canali, R.; Natarelli, L.; Ferrari, D.; Saija, A.; Virgili, F.; Cimino, F. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-kappaB and Nrf2 pathways. Biofactors 2017, 43, 54–62. [Google Scholar] [CrossRef]
- Zhu, M.J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Mousavi, T.; Hadizadeh, N.; Nikfar, S.; Abdollahi, M. Drug discovery strategies for modulating oxidative stress in gastrointestinal disorders. Expert Opin. Drug Discov. 2020, 15, 1309–1341. [Google Scholar] [CrossRef]
- Hasegawa, T.; Mizugaki, A.; Inoue, Y.; Kato, H.; Murakami, H. Cystine reduces tight junction permeability and intestinal inflammation induced by oxidative stress in Caco-2 cells. Amino Acids 2021, 53, 1021–1032. [Google Scholar] [CrossRef]
- Lu, X.; Li, C.; Li, C.; Li, P.; Fu, E.; Xie, Y.; Jin, F. Heat-Labile Enterotoxin-Induced PERK-CHOP Pathway Activation Causes Intestinal Epithelial Cell Apoptosis. Front. Cell Infect. Microbiol. 2017, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Wang, L.; Lee, S. Levistolide A Induces Apoptosis via ROS-Mediated ER Stress Pathway in Colon Cancer Cells. Cell Physiol. Biochem. 2017, 42, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.P.; Haslett, C.; Dransfield, I. Recognition of apoptotic cells by phagocytes. Experientia 1996, 52, 10–11, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ghali, M.; Stiban, J. Regulation of ceramide channel formation and disassembly: Insights on the initiation of apoptosis. Saudi J. Biol. Sci. 2015, 22, 760–772. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes. Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Pastene, E.; Vergara, C.; Zapata, M.; Sandoval, C.; Gotteland, M. Stimulation of cytosolic and mitochondrial calcium mobilization by indomethacin in Caco-2 cells: Modulation by the polyphenols quercetin, resveratrol and rutin. Biochim. Biophys. Acta 2012, 1820, 2052–2061. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef]
- Li, F.; Du, P.; Yang, W.; Huang, D.; Nie, S.; Xie, M. Polysaccharide from the seeds of Plantago asiatica L. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human Caco-2 cell line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Xiaomeng, R.; Meiqi, L.; Yi, X. A Polysaccharide Isolated from Dictyophora indusiata Promotes Recovery from Antibiotic-Driven Intestinal Dysbiosis and Improves Gut Epithelial Barrier Function in a Mouse Model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Zheng, B.; Yu, Q.; Hou, K.; Wang, H.; Zhao, M.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Ganoderma atrum polysaccharide ameliorates intestinal mucosal dysfunction associated with autophagy in immunosuppressed mice. Food Chem. Toxicol. 2020, 138, 111244. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, J.H.; Bai, G.; Shen, G.S.; Chen, J.; Liu, J.N.; Wang, S.; Liu, X.J. Acanthopanax senticosus polysaccharides-induced intestinal tight junction injury alleviation via inhibition of NF-κB/MLCK pathway in a mouse endotoxemia model. World J. Gastroenterol. 2017, 23, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wu, S.; Li, Z.; Li, J.; Li, X.; Xiang, J.; Ding, H. Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function. Food Funct. 2015, 6, 2568–2577. [Google Scholar] [CrossRef]
- Le Phan, T.H.; Park, S.Y.; Jung, H.J.; Kim, M.W.; Cho, E.; Shim, K.S.; Shin, E.; Yoon, J.H.; Maeng, H.J.; Kang, J.H.; et al. The Role of Processed Aloe vera Gel in Intestinal Tight Junction: An In Vivo and In Vitro Study. Int. J. Mol. Sci. 2021, 22, 6515. [Google Scholar] [CrossRef]
- Guo, Q.; Xiao, X.; Lu, L.; Ai, L.; Xu, M.; Liu, Y.; Goff, H.D. Polyphenol-Polysaccharide Complex: Preparation, Characterization, and Potential Utilization in Food and Health. Annu. Rev. Food Sci. Technol. 2022, 13, 59–87. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.P.; Jin, J.; Huang, L.Y.; Ye, J.H.; Liang, Y.R. Using Defatted Rice Bran as a Bioadsorbent for Carrying Tea Catechins. J. Food Sci. 2015, 80, C2134–C2139. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Ma, Y.; Chen, F.; Zhao, G. Synthesis, characterization, and aqueous self-assembly of octenylsuccinate Oat β-glucan. J. Agric. Food Chem. 2013, 61, 12683–12691. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F.; Tian, W.; Ma, Y.; Li, J.; Zhao, G. Optimization and characterization of curcumin loaded in octenylsuccinate oat β-glucan micelles with an emphasis on degree of substitution and molecular weight. J. Agric. Food Chem. 2014, 62, 7532–7540. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Malfa, G.A.; Di Giacomo, C.; Cardia, L.; Sorbara, E.E.; Mannucci, C.; Calapai, G. A standardized extract of Opuntia ficus-indica (L.) Mill and Olea europaea L. improves gastrointestinal discomfort: A double-blinded randomized-controlled study. Phytother. Res. 2021, 35, 3756–3768. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Algieri, F.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Utrilla, M.P.; Talhaoui, N.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Rodríguez-Cabezas, M.E.; Monteleone, G.; et al. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol. Nutr. Food Res. 2017, 61, 1601066. [Google Scholar] [CrossRef] [PubMed]
- Fakhraei, N.; Abdolghaffari, A.H.; Delfan, B.; Abbasi, A.; Rahimi, N.; Khansari, A.; Rahimian, R.; Dehpour, A.R. Protective effect of hydroalcoholic olive leaf extract on experimental model of colitis in rat: Involvement of nitrergic and opioidergic systems. Phytother. Res. 2014, 28, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.S.; Bai, K.L.; Yim, S.H.; Lee, Y.J.; Song, H.J.; Kim, J.H.; Ham, I.; Whang, W.K.; Sohn, U.D. The effect of luteolin-7-O-beta-D-glucuronopyranoside on gastritis and esophagitis in rats. Arch. Pharm. Res. 2006, 29, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Oliverio, M.; Suraci, E.; Greco, M.; Placida, R.; Gervasi, S.; Marasco, R.; Imeneo, M.; Paolino, D.; Tucci, L.; et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients 2017, 9, 391. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef]
- Sottile, F.; Modica, A.; Rosselli, S.; Catania, C.A.; Cavallaro, G.; Lazzara, G.; Bruno, M. Hand-made paper obtained by green procedure of cladode waste of Opuntia ficus indica (L.) Mill. from Sicily. Nat. Prod. Res. 2021, 35, 359–368. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Lal, N.; Kumar, J.; Erdahl, W.E.; Pfeiffer, D.R.; Gadd, M.E.; Graff, G.; Yanni, J.M. Differential effects of non-steroidal anti-inflammatory drugs on mitochondrial dysfunction during oxidative stress. Arch. Biochem. Biophys. 2009, 490, 1–8. [Google Scholar] [CrossRef]
- Han, X.; Fink, M.P.; Yang, R.; Delude, R.L. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock 2004, 21, 261–270. [Google Scholar] [CrossRef] [PubMed]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Pintado, M. In vitro evaluation of the effects of protein–polyphenol–polysaccharide interactions on (+)-catechin and cyanidin-3-glucoside bioaccessibility. Food Funct. 2015, 6, 3444–3453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Interactions between cell wall polysaccharides and polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1808–1831. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, H.; Ilyasoglu-Buyukkestelli, H.; Sogut, E.; Ozyurt, V.H.; Gumus-Bonacina, C.E.; Simsek, S. A review on recent advances of plant mucilages and their applications in food industry: Extraction, functional properties and health benefits. Food Hydrocoll. Health 2023, 3, 100131. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Jilani, H.; Cilla, A.; Barberá, R.; Hamdi, M. Antiproliferative activity of green, black tea and olive leaves polyphenols subjected to biosorption and in vitro gastrointestinal digestion in Caco-2 cells. Food Res. Int. 2020, 136, 109317. [Google Scholar] [CrossRef]
- Logue, S.E.; Cleary, P.; Saveljeva, S.; Samali, A. New directions in ER stress-induced cell death. Apoptosis 2013, 18, 537–546. [Google Scholar] [CrossRef]
- Shore, G.C.; Papa, F.R.; Oakes, S.A. Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 2011, 23, 143–149. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K.; Kaliora, A.C.; Assimopoulou, A.N.; Papageorgiou, V.P. Inhibitory activity of minor polyphenolic and nonpolyphenolic constituents of olive oil against in vitro low-density lipoprotein oxidation. J. Med. Food 2002, 5, 1–7. [Google Scholar] [CrossRef]
- Scicchitano, S.; Vecchio, E.; Battaglia, A.M.; Oliverio, M.; Nardi, M.; Procopio, A.; Costanzo, F.; Biamonte, F.; Faniello, M.C. The Double-Edged Sword of Oleuropein in Ovarian Cancer Cells: From Antioxidant Functions to Cytotoxic Effects. Int. J. Mol. Sci. 2023, 24, 842. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Giner, R.M. Oleuropein protects against dextran sodium sulfate-induced chronic colitis in mice. J. Nat. Prod. 2013, 76, 1113–1120. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Kitakaze, T.; Makiyama, A.; Yamashita, Y.; Ashida, H. Low dose of luteolin activates Nrf2 in the liver of mice at start of the active phase but not that of the inactive phase. PLoS ONE 2020, 15, e0231403. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 2014, 65, 70–75. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Műzes, G.; Molnár, B.; Tulassay, Z.; Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Baumgart, D.C. Targeting leukocyte migration and adhesion in Crohn’s disease and ulcerative colitis. Inflammopharmacology 2012, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamone, F.L.; Molonia, M.S.; Muscarà, C.; Saija, A.; Cimino, F.; Speciale, A. In Vitro Protective Effects of a Standardized Extract of Opuntia ficus-indica (L.) Mill. Cladodes and Olea europaea L. Leaves Against Indomethacin-Induced Intestinal Epithelial Cell Injury. Antioxidants 2024, 13, 1507. https://doi.org/10.3390/antiox13121507
Salamone FL, Molonia MS, Muscarà C, Saija A, Cimino F, Speciale A. In Vitro Protective Effects of a Standardized Extract of Opuntia ficus-indica (L.) Mill. Cladodes and Olea europaea L. Leaves Against Indomethacin-Induced Intestinal Epithelial Cell Injury. Antioxidants. 2024; 13(12):1507. https://doi.org/10.3390/antiox13121507
Chicago/Turabian StyleSalamone, Federica Lina, Maria Sofia Molonia, Claudia Muscarà, Antonella Saija, Francesco Cimino, and Antonio Speciale. 2024. "In Vitro Protective Effects of a Standardized Extract of Opuntia ficus-indica (L.) Mill. Cladodes and Olea europaea L. Leaves Against Indomethacin-Induced Intestinal Epithelial Cell Injury" Antioxidants 13, no. 12: 1507. https://doi.org/10.3390/antiox13121507
APA StyleSalamone, F. L., Molonia, M. S., Muscarà, C., Saija, A., Cimino, F., & Speciale, A. (2024). In Vitro Protective Effects of a Standardized Extract of Opuntia ficus-indica (L.) Mill. Cladodes and Olea europaea L. Leaves Against Indomethacin-Induced Intestinal Epithelial Cell Injury. Antioxidants, 13(12), 1507. https://doi.org/10.3390/antiox13121507









