Abstract
Low molecular weight (LMW) thiols, particularly glutathione, play pathogenic roles in various multiorgan diseases. The liver is central for the production and systemic distribution of LMW thiols; thus, it is particularly susceptible to the imbalance of redox status that may determine increased oxidative stress and trigger the liver damage observed in metabolic dysfunction-associated steatotic liver disease (MASLD) models and humans. Indeed, increased LMW thiols at the cellular and extracellular levels may be associated with the severity of MASLD. Here, we present a systematic literature review of recent studies assessing the levels of LMW thiols in MASLD in in vivo and in vitro models and human subjects. Based on the PRISMA 2020 criteria, a search was conducted using PubMed and Scopus by applying inclusion/exclusion filters. The initial search returned 1012 documents, from which 165 eligible studies were selected, further described, and qualitatively analysed. Of these studies, most focused on animal and cellular models, while a minority used human fluids. The analysis of these studies revealed heterogeneity in the methods of sample processing and measurement of LMW thiol levels, which hinder cut-off values for diagnostic use. Standardisation of the analysis and measure of LMW thiol is necessary to facilitate future studies.
1. Introduction
Low-molecular-weight (LMW) thiols, including glutathione (GSH), cysteine (Cys), cysteinyl-glycine (CysGly), and homocysteine (Hcy), are ubiquitous molecules that exert several biological functions. Still, their pivotal role is to preserve redox homeostasis in the cell [1,2]. In particular, LMW thiols may regulate the activity of specific antioxidant enzymes by acting as cofactors and several proteins by establishing covalent bonds with them, thus protecting the cell from oxidative stress [1]. Moreover, LMW thiols may directly donate electrons to oxidants, form complexes with metal ions, and bind xenobiotic agents, thus helping cells in detoxification [1].
Among the LMW thiols, GSH, a tripeptide composed of sequential Glu, Cys, and Gly residues, represents the first line of cellular defence against oxidative stress. Intracellular levels of GSH range from 1 to 15 mM in the cytoplasm and from 10 to 14 mM in mitochondria [3]. In particular, in cultured cells, GSH values were reported to range approximately from 20 to 150 nmol/mg of proteins, whereas the extracellular levels of GSH range between 2 and 20 µM, values often found in total body fluids. Approximately 99% of the intracellular GSH was found in a reduced (GSH) state and 1% in the oxidized form (GSSG). At the same time, different values of extracellular GSH/GSSG ratios were reported in various body fluids [4]. These latter values could represent the benchmark for assessing GSH and GSSG amounts as biomarkers in different pathological conditions, including neurological disorders, type 2 diabetes, and cardiovascular and liver diseases [5,6,7,8]. However, despite heterogeneity in the most appropriate methods for sample pre-treatment and quantification, there is a strong interest in using the GSH/GSSG ratio to assess redox status. Therefore, reported concentrations can vary widely across laboratories [9]. Measurement of GSH and GSSG may be performed by using different approaches (e.g., Ellman’s method unmodified/modified by using an enzymatic recycling procedure and fluorometric and spectrophotometric assays), even though high-performance liquid chromatography (HPLC) coupled with UV or fluorescent detection, and the addition of N-ethylmaleimide earlier during sample preparation to prevent GSH oxidation is also often used [10,11,12,13].
GSH is mainly produced by the liver, which retains the unique ability to synthesise its precursor (i.e., Cys) and provide the principal reservoir for releasing GSH into circulation [6,14]. Besides its role as a GSH precursor, Cys is central to sulphur metabolism, involving Hcy and serine in the synthesis of GSH. Furthermore, GSH serves as a steady source of Cys through the extracellular degradation to form CysGly via the γ-glutamyl cycle, thus playing a pivotal role in regulating the cellular stress response when Cys levels are low [15]. The total extracellular concentration of free Cys is typically maintained at values ranging from 200 to 300 μM [16], while a healthy Hcy level is generally below 14 μM [17]. A large amount of the literature highlighted that different methods, such as fluorescence or electrochemical detection, may be used to detect Cys, CysGly, and Hcy. HPLC remains the most sensitive approach for simultaneously measuring single thiols, at least in body fluids [18].
Several lines of evidence demonstrated that thiol levels were strongly associated with the onset and progression of different pathologies, with the liver playing a pivotal role in controlling the production of Cys, the limiting molecule for GSH synthesis. Therefore, it is unsurprising that LMW thiols may indicate oxidative stress and are pathogenically involved in a multi-organ disease such as non-alcoholic fatty liver disease (NAFLD) [6]. NAFLD term was recently replaced by metabolic dysfunction-associated steatotic liver disease (MASLD), thus including the entire spectrum of liver damages (i.e., metabolic dysfunction-associated steatohepatitis—MASH, and fibrosis) and metabolic derangements associated with this multi-spectrum disease and avoiding the “fatty liver” stigma [19,20]. MASLD, with an estimated global prevalence of around 30% in adults and around 13% in children, represents the most prevalent chronic liver disease and the principal cause of cirrhosis, hepatocellular carcinoma, hepatic-related mortality, and liver transplantation [20,21]. The multifactoriality of MASLD development and progression may be mainly ascribed to a complex network of molecular events that include genetic background, epigenetic mechanisms, gut dysbiosis, lipid dysmetabolism, insulin resistance, inflammation, and oxidative stress [22,23,24].
Studies in experimental in vitro and in vivo models and humans demonstrated that different molecules and proteins implied in the control of the redox status in the liver cells exhibited a causal link with exacerbation of hepato-metabolic damage occurring in MASLD, MASH and its related fibrosis [25]. The role of thiols was also widely investigated with a primary focus on GSH and Hcy, suggesting that changes in these molecules at cellular and extracellular levels (e.g., blood, blood cells, serum, and plasma) may be associated with the disease severity. However, a literature review of the results addressing the amount of LMW thiols in MASLD by considering experimental studies on in vivo and in vitro models and humans is still lacking. As the potential diagnostic and therapeutic role of these thiols in MASLD, with our systematic review, we aimed to fill the gap by providing a summary of data, methods, and statistical significance of studies assessing the amount of LMW thiols in the disease.
2. Materials and Methods
2.1. Search Strategy
The present systematic review followed the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [26]. The protocol for the present systematic review was registered on INPLASY (protocol number: INPLASY2024100096).
The search was performed on PubMed, Embase, and Scopus databases for articles published between 1 January 2019 and 30 June 2024. The following advanced search approach was used for PubMed and Embase: (glutathione OR GSH OR thiols) AND (NAFLD) were searched first, then (glutathione OR GSH OR thiols) AND (MAFLD OR MASLD). English language restriction was applied. The following advanced search approach was used for Scopus: TITLE-ABS-KEY ((glutathione OR GSH OR thiols) AND (NAFLD)) were searched first, then TITLE-ABS-KEY ((glutathione OR GSH OR thiols) AND (MAFLD OR MASLD)). The subject area was limited to the “English” language. Articles retrieved by PubMed and Scopus were then merged. Articles retrieved by PubMed, Scopus, and Embase were subsequently merged.
2.2. Article Screening and Selection
Duplicate records from the database were removed before the first eligibility screening. Next, we excluded all non-peer-reviewed studies (e.g., conference abstracts, dissertations, and other grey literature). Reviews and articles outside the time window were also excluded.
Overall, the articles publishing data describing alterations in GSH redox homeostasis in the presence of MASLD/NAFLD in animal models, cell lines, and human samples were all considered. In particular, articles were screened by title and abstract to identify those relevant to the topic covered in the present review. However, some articles were excluded as GSH, thiols, or oxidative stress were not mentioned in the abstract and/or title. If the abstract mentioned oxidative stress without mentioning GSH/thiols explicitly, the linked article was further screened for the keywords “GSH”, “glutathione”, “thiol”, and “cysteine*” to check whether the text contained data not described explicitly in the abstract. Articles that assessed oxidative stress or GSH metabolism parameters but did not measure GSH, Cys, CysGly, and Hcy were excluded. Articles mentioning GSH and/or thiols as a diet or treatment component were also excluded.
Moreover, the full text of the articles was screened according to the following objective exclusion criteria: absence of explicit values for LMW thiols, missing methods, missing units of measurement, and missing information on the study design. The examination and screening of all the search results were conducted by three independent authors (L.C., A.A., and A.P.).
2.3. Data Extraction
The data extracted from each article were different for experimental models and studies.
Data on the type of the organism (e.g., mice and rats), disease model, length of study, assay methods, biological matrix, and measurements and statistical significance of the amount of GSH (GSH, total glutathione (tGSH), and GSSG) and thiols (Cys, CysGly, and Hcy) were extracted in experimental studies on animal models.
Cellular line type, study length, assay methods, measurements, and statistical significance of the amount of GSH (GSH, tGSH, and GSSG) and thiols (Cys, CysGly, and Hcy) were extracted from experimental studies on cell models.
In human studies, study type, age, number of subjects, assay methods, measurements, and statistical significance of the amount of GSH (GSH, tGSH, and GSSG) and thiols (Cys, CysGly, and Hcy) were captured.
3. Results
3.1. Search Results
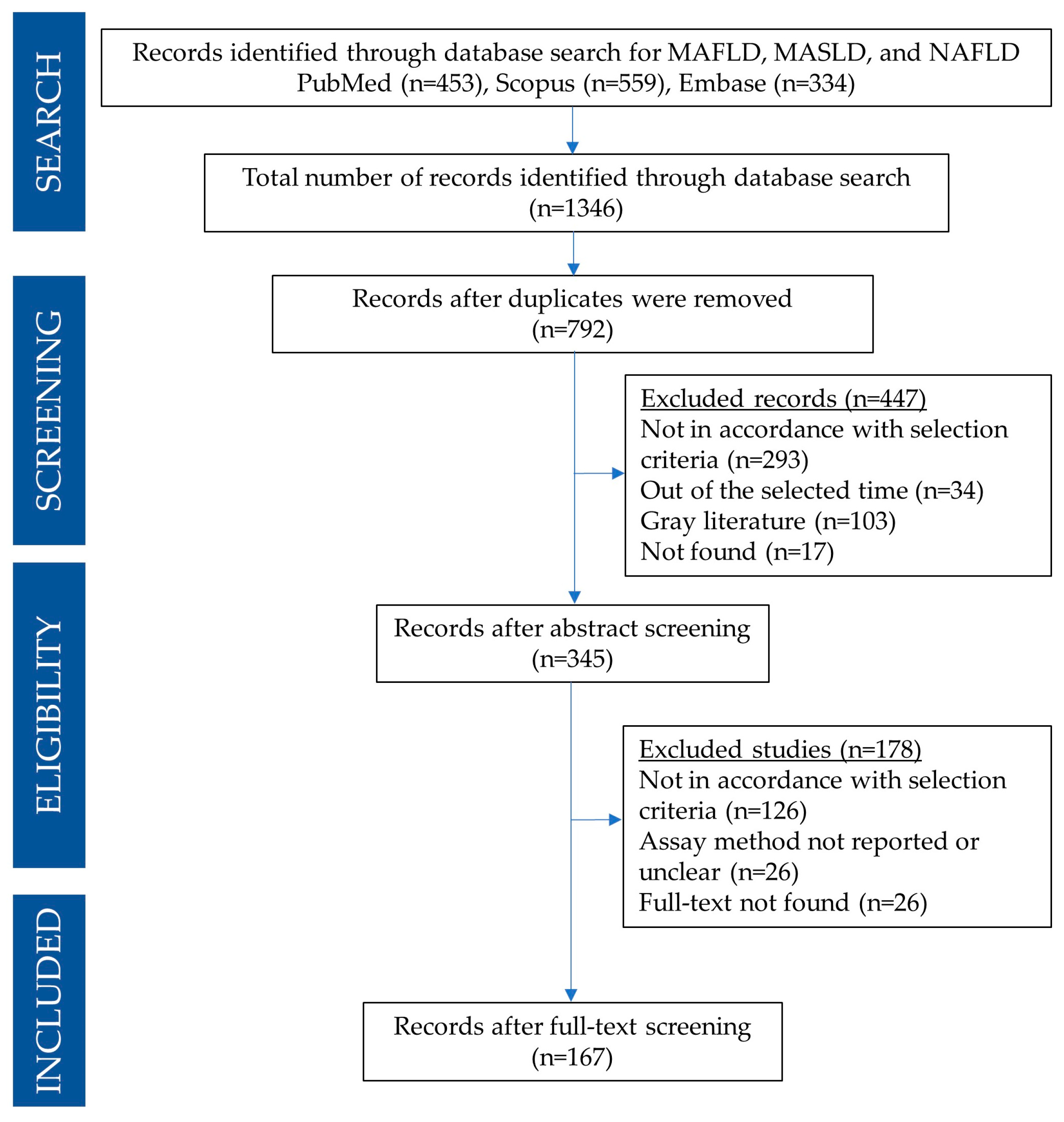
A PRISMA flow diagram detailing the search and selection process for the present systematic review is reported in Figure 1. A total of 1346 articles were found in PubMed, Embase, and Scopus databases. In particular, 23 articles for MAFLD or MASLD query and 430 for NAFLD were found using the PubMed search engine; 24 articles for MAFLD or MASLD and 310 for NAFLD were found using the Embase search engine, and 45 articles for MAFLD or MASLD and 514 for NAFLD were found using Scopus’ search engine). Search results were imported into JabRef for further management [27].
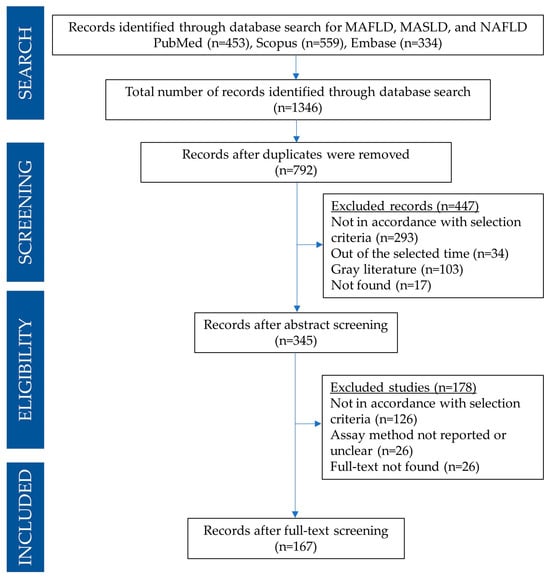
Figure 1.
PRISMA flow diagram detailing the search and selection process applied during the overview.
After removing duplicates, the search amounted to 792 articles. Based on the title and abstract, 447 articles were excluded as they did not align with the focus of this review, following the previously outlined exclusion criteria. Subsequently, the remaining 345 articles were thoroughly manually examined. Based on the analysis of the full text, only 167 articles were found to be appropriate to be included in this systematic review.
3.2. Results Organization
All selected articles were initially categorised into three sections, depending on whether the study design included animal, cell models, or human fluids (tissue/plasma/serum/blood), and summarised in tables in chronological order. Next, the articles were divided into two macro areas depending on whether the molecule of interest was all forms of GSH or other LMW thiols (i.e., Cys, CysGly, and Hcy). Overall, Section 3.3, Section 3.4 and Section 3.5, as well as the included tables, described studies performed in animal models, cell models, and humans, respectively. Rats and mice studies that did not report explicit data and studies using other animals were included in Supplementary Tables.
3.3. Studies Evaluating GSH Levels in Animal Models of MASLD
Data extracted from articles studying all forms of GSH in animal models of MASLD were summarised in chronological order for rats in Table 1 [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] and Supplementary Table S1 [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102] and for mice in Table 2 [103,104,105,106,107,108] and Supplementary Table S2 [109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156]. Additional studies using rabbits, fish, and primates were reported in Supplementary Table S3 [157,158,159,160]. In particular, each of the selected studies was firstly screened against the Critical Appraisal Skills Programme—CASP [161], and only when the article reached a score greater than or equal to 5 over ten questions were considered eligible for a detailed outline and main table.

Table 1.
Studies assessing reduced, oxidized, and total glutathione (GSH, GSSG, tGSH) levels in MASLD rat models.

Table 2.
Studies assessing the levels of GSH forms in mice models of MASLD.
3.3.1. Studies on Rat Models
In this sub-section, we summarised the results of studies that evaluated GSH levels in MASLD models established in rats.
In a study by Khalaf et al. [28], the effects of allopurinol, metformin (MET), and vitamin E (VitE), both individually and in combinations, were investigated in a rat model of high-fructose diet (HFruD)-induced MASLD. All treatments, whether administered alone or in combination, restored the reduced hepatic GSH content in the model.
Palladini et al. [29] investigated changes in fatty acid desaturases, D5D, D6D, D9–16D, and D9–18D, and their relationship with oxidative stress on a rat model fed for 3 weeks with a methionine-choline-deficient (MCD) diet, evaluating hepatic oxidative stress parameters, such as reactive oxygen species (ROS) and GSH. In particular, GSH levels were reduced in the MCD group compared to control animals, and interestingly, GSH was positively correlated with D5 and D6.
Induction of hepatic oxidative stress and reduction of GSH levels were also confirmed in a MASLD rat model induced by a high cholesterol and fat-enriched diet (HCHF) [30]. Carvedilol and nicorandil counteracted these effects by lowering NF-kb expression and malondialdehyde (MDA) levels and increasing GSH content and endothelial Nitric oxide synthases (eNOS) expression, proving that vasodilatation could ameliorate MASLD.
Park et al. [31] evaluated the effects of mulberry water extracts (MB) combined with silk amino acids (SA) on Sprague Dawley rats fed with an HFD to induce MASLD. Measured reduced hepatic GSH was significantly lower in the HFD groups, but different ratios of treatments improved the liver damage and the GSH levels. Interestingly, MS1:3 exhibited a higher hepatic GSH content.
In a model where MASLD was achieved by administering 30% fructose in drinking water to rats, Pai et al. [32] demonstrated that the disease model exhibited a marked reduction in hepatic GSH levels with respect to the control group and that most of the hepato-metabolic effects were facilitated by the antioxidant and anti-inflammatory chrysin (chry).
Meng et al. [33] reported that treatment with Cassia semens prevented the histological damage and the reduction of the hepatic levels of GSH in rats with HFD-induced MASLD.
Souza Cruz et al. [34] investigated the long-term effects of ingestion of a 40% sucrose solution on serum and hepatic parameters in male Wistar rats. The study highlighted alteration in the oxidative stress markers, such as the decrease of all forms of GSH, alongside increased fibrotic tissue frequently described in MASLD.
Faheem et al. [35] induced MASLD in an HFD-fed rat model and observed significant hepatoprotective effects, as evidenced by the improved histopathological changes and restoration of oxidative stress markers (e.g., hepatic GSH) after treatment with cranberry.
The levels of LMW thiols were also evaluated in more complex models of MASLD, which combined ovariectomy with an HFHF diet. Ovariectomised (OVX) rats on an HFHF diet had significantly higher hepatic levels of MDA and lower GSH than OVX and the control group [36].
The ameliorative effect of ethanolic extract of garden cress seeds (EEGS) was explored in a rat model by Ibrahim et al. [37]. The study demonstrated that the administration of EEGS had hepatoprotective, antioxidant, and anti-steatosis characteristics in HFD-induced MASLD by restoring the hepatic GSH levels.
Ogunlana et al. [38] reported a significant reduction of GSH levels in the liver of HFD rats compared to the control rats. Still, different treatments, including pioglitazone (PIO), Ruzu herbal bitters (RUZU), and fenofibrate (FENO), counteracted this effect.
Also, MET alone or combined with phosphodiesterase inhibitors protected against hepatic-metabolic damage and restored hepatic GSH levels in rats with HFD-dependent MASLD [39].
The HFD-induced MASLD was also recovered by lycopene (lyc) supplementation. Indeed, Saeed et al. [40] demonstrated that the treatment with lyc hampered the lowering of GSH hepatic levels caused by HFD.
HFD has been linked to an imbalance in the intestinal microbiota, which may contribute to the pathogenesis of MASLD [41]. Moreover, the authors reported that restoration of eubiosis by supplementation with probiotic banana juice (PPBJ) significantly improved liver damage, reduced oxidative stress, and restored GSH to normal levels in the liver of rats fed with HFD.
The liver-brain axis was a further example of cross-talk among organs during MASLD pathogenesis. Jaleel et al. [42] explored the therapeutic effect of melatonin (MEL) on hepato- and neuro-complications in a rat model of MASLD induced by HFHF. The treatment with MEL improved several liver metabolites, neurotransmitters, and liver/brain GSH levels in the HFHF model.
In another model of fructose-induced MASLD, no significant differences in GSH were observed despite the reported hepato-metabolic effects [43].
In an HFD-fed MASLD rat model developed by Fawzy et al. [44], the treatment with eugenol successfully counteracted the histopathological lesions and the alterations of oxidative stress parameters (e.g., GSH hepatic levels).
Mengesha et al. [45] developed a MASLD model by administering a 20% fructose solution to Wistar rats. Owing to its hepatoprotective properties, this model restored dyslipidaemia and steatosis and altered hepatic GSH levels after silymarin (sily) treatment.
Coconut oil (CO) and thermally oxidized CO (TCO) integrated in an HFD and combined with streptozotocin (STZ) injection induced MASLD in rats [46]. Increased levels of hepatic GSH were found in the disease model groups, mainly when the liver tissue was extracted from a high-fat area.
Carvalho et al. [47] investigated how the concentration of fructose and the duration of exposure may influence the histological grading of hepatic microsteatosis and metabolic parameters in rats. Fructose consumption affected redox status, with GSH levels decreasing with increasing concentration and duration of exposure.
In an HFHF-induced MASLD rat model, Abd-Elrazek et al. [48] reported liver damage accompanied by a significant reduction in hepatic GSH levels compared to control rats, which were subsequently restored following treatment with sily, curcumin, and celery extracts.
Zakaria et al. [49] compared the protective and therapeutic effects of orlistat administration in an HFD-fed rat model. As expected, oxidative stress markers, including GSH, worsened after the diet. However, GSH levels were significantly increased in the treatment groups compared to the HFD group.
GSH levels in the hepatic tissues were also evaluated in a model of progressive MASLD induced by MCD in rats. GSH values at different time points were consistently lower than the corresponding controls. Interestingly, there was an inverse correlation between GSH and Fe levels but no correlation with Zn [50].
According to a previously mentioned study [32], two other studies evaluated the effects of chry on models of MASLD in rats. Attia et al. [51] induced MASLD by using HFruD, thus leading to a redox imbalance in the liver, evidenced by GSH depletion and the aggravation of other measured markers significantly improved by chry. Oriquat et al. [52], by obesogenic diet-induced MASLD, reported alterations in liver features and all forms of hepatic GSH, which were partially reverted after treatment with chry.
Reda et al. [53] induced MASLD using an HFD combined with fructose water, which exacerbated oxidative parameters, such as the reduction of hepatic GSH. Treatment with vitamin D improved these effects, thus reducing hepatic inflammation and steatosis.
Another study investigating the effect of natural extracts on HFD-induced MASLD in rats reported that Matricaria pubescens suppressed the hepatic damage and significantly increased GSH levels [54].
3.3.2. Studies on Mice Models
Analogous to rats, mice models of MASLD may be established by using different dietetic regimens, and in the present paragraph, we reported studies that analyse GSH amounts.
Liu et al. [103] established a mice model fed with an HFHC to evaluate the protective effects of 14-deoxy-11,12-didehydroandrographolide (deAND) on MASLD-related liver injury. The disease model revealed increased oxidative stress and liver damage markers, and deAND treatment ameliorated these conditions, as evidenced by hepatic GSH patterns.
Several tea extracts were tested as treatments for the HFD-induced MASLD in a murine model. As expected, some of them may exert multiple actions on liver homeostasis, thus preventing liver impairment and diet-dependent reduction of GSH levels [104].
A daily HFD in combination with ethanol was used by Sukkasem et al. [105] to explore the potential therapeutic effects of hesperidin and myricetin against MASLD in mice. Notably, GSH levels and GSH/GSSG ratio were improved by all treatments.
Kang et al. [106] investigated the pharmacological effects of water chestnut extracts (WC) on a murine model of HFD-induced MASLD. A significant reduction in the content of hepatic GSH was reported in the disease group. However, MASLD and diabetes-related complications were significantly and dose-dependently normalised by oral administration of WC.
Mak et al. [107] reported the effects of the natural compound swietenine (SW) on a diabetic MASLD mouse model induced by HFD combined with STZ. The disease group exhibited altered liver antioxidant markers, notably decreased levels of serum GSH, which were reversed by oral administration of SW.
A further study evaluated the effects of 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, an inhibitor of 5’-adenosine monophosphate-activated protein kinase (AMPK) in a mice model of MASLD induced by HFHF [108]. As described above, this dietetic regimen caused hepato-metabolic dysregulation and lowered GSH plasma levels, which were counteracted by the AMPK inhibitor.
3.4. Studies Evaluating GSH Levels in Cell Models of MASLD
Limited articles study all forms of GSH in models in vitro of MASLD. Most reported primary hepatocytes or hepatocyte-like cell lines treated with fatty acids (e.g., oleic acid and palmitic acid, alone or in combination) to mimic the steatosis in MASLD. In these models, often the authors evaluated the effect of different compounds with antioxidant properties. All these studies were summarised in chronological order in Table 3. We extrapolated the data from 25 studies, which evaluated the effects of pro-steatotic treatments, investigated the effects of some treatments in cell models, and measured GSH and or GSSG [58,66,68,92,144,147,148,151,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178]. However, only the study by Balkrishna et al. [172] explicitly provided the GSH concentrations. In particular, the study evaluated the efficacy of livogrit, a tri-herbal Ayurvedic medicine, as a potential hepatoprotective agent against MASH-related hepatocellular damage, using HepG2 spheroids and rat primary hepatocytes. Results showed that livogrit effectively prevented disease damage by reducing lipid accumulation, ROS production, aspartate transferase release, and nuclear factor kappa B activation while increasing lipolysis, GSH levels, and mitochondrial membrane potential.

Table 3.
Cell studies on GSH involvement in MASLD.
3.5. Studies Evaluating Cys, CysGly, Hcy, and Total Thiols in In Vivo and In Vitro Models of MASLD
Only data from three articles measuring other hepatic LMW thiols, including CysGly, Cys, and Hcy, were extrapolated [110,179,180]. Notably, all studies were conducted in murine models of MASLD, but only one study reported a statistically significant difference between the disease model and the control (Table 4).

Table 4.
Studies on LMW thiols involvement in models of MASLD.
Deng et al. [110] used an omics approach to investigate the impact of PCB-126 on liver metabolites in healthy mice and those with MCD-dependent MASLD. The authors reported that LMW thiol levels were similar between the control and model but found significant alteration when the disease model was treated with PCB-126. Similarly, Luciano-Mateo et al. [179] showed that the hepatic levels of Hcy remained unaltered in the HFD model with respect to control mice, even though CCL2 deficiency may affect the amount of these metabolites.
The third study evaluated the total amount of LMW thiols in the livers of control mice compared to HFHF mice [180]. The study demonstrated a reduction in total thiol concentration in the model, which was restored by treatment with lupeol or MET. Interestingly, lupeol also downregulated the expression of androgen receptors and toll-like receptors 2 and 4 (TLR), thus leading to antioxidant and anti-inflammatory responses.
3.6. Studies Evaluating LMW Thiols in Humans
Our search results revealed that a systematic review and meta-analysis recently reviewed the Hcy levels in human MASLD [181]. Therefore, here, we focused on evaluating observational studies that assessed GSH in plasma samples (Table 5) or clinical trials measuring GSH or other thiols as secondary endpoints in clinical trials conducted on adult and paediatric patients with MASLD (Table 6).

Table 5.
Observational studies evaluating the levels of LMW thiols in human MASLD.

Table 6.
Clinical trials on LMW thiols involvement in human MASLD.
3.6.1. Observational Studies
In particular, Table 5 reports studies investigating the circulating levels of LMW thiols in five observational case-control studies in adults affected by MASLD compared to healthy subjects [182,183,184,185,186,187].
Świderska et al. [182] investigated redox abnormalities in MASLD, focusing on enzymatic and non-enzymatic antioxidants, redox homeostasis, and oxidative damage in 67 patients. Results indicated significantly elevated levels of Cu-Zn-superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), total oxidant status (TOS), advanced glycation end products (AGE), MDA, and DNA/RNA oxidative damage in both MASLD groups compared to controls. Surprisingly, in this study, the levels of the reduced form of GSH were significantly higher in patients with early and advanced MASLD than in controls.
The relationship between systemic oxidative stress, indicated by protein-adjusted serum-free thiol levels, and MASLD was investigated in a large cohort of 5562 patients [183]. The disease was defined using the Fatty Liver Index (FLI) differentiating patients with FLI < 60 from patients with FLI ≥ 60. Results showed that serum-free thiol levels were significantly lower in FLI ≥ 60 than in FLI < 60 individuals. Stratified analyses revealed that the relationship between thiol levels and MASLD varied by gender, hypertension, and hypercholesterolemia, and, additionally, lower thiol levels were strongly linked to an increased risk of all-cause mortality.
Masarone et al. [184] aimed to determine if metabolomic profiles could distinguish between different stages of MASLD (simple steatosis, steatohepatitis, cirrhosis) and controls. Metabolomic signatures were analysed in 69 controls and 144 patients with MASLD. The authors demonstrated that the primary metabolic derangements in the MASLD group included essential and non-essential amino acids, GSH and xanthine, free fatty acids, and short-chain fatty acids and their intermediates. All their pathways are linkable with the known pathophysiologic mechanisms of disease onset and progression.
The cross-sectional study by Arya et al. [185] aimed at comparing oxidative stress markers and antioxidant enzyme activity in 60 patients with MASLD versus 25 healthy individuals, finding significantly higher levels of alanine aminotransferase, MDA, and nitric oxide metabolites in MASLD patients, along with lower total thiol levels and SOD activity compared to the controls. Molecular docking analysis suggested that MDA may deactivate SOD1 by interacting with its active site, indicating that impaired antioxidant defences, particularly through the deactivation of SOD1 by MDA, may play a critical role in the progression of MASLD.
In children with MASLD associated with severe obesity who underwent laparoscopic sleeve gastrectomy (LSG), Pastore et al. [186] hypothesized that an additional factor linked to one-carbon metabolism, that could be related to the recovery of metabolic derangement and histological damage after LSG, could be associated with increased levels of reduced GSH. Accordingly, they found a trend of increase in plasma levels of tGSH and Hcy that correlated with several parameters that ameliorated after LSG in children.
Garcia et al. [187] evaluated the levels of ROS, GSH, and antioxidant enzyme activities in peripheral blood mononuclear cells, and CD4+ and CD8+ T-lymphocytes from patients with MASLD and control healthy subjects. Cells from MASLD patients showed higher ROS levels, increased GPx activity, and lower levels of GR, SOD, and GSH compared to controls. Resistin stimulation further decreased GSH content in blood cells with a major effect on the MASLD group, thus highlighting the key role of resistin in the disruption of redox homeostasis in patients.
3.6.2. Clinical Trials
As shown in Table 6, most of the studies that investigated LMW thiols in humans are clinical trials in which the authors assessed not only the improvement of steatosis but also the amelioration of different anthropometric and metabolic parameters and changes in the oxidative stress circulating biomarkers [95,96,97,98,99,100,101,102].
In a randomized, double-blind, placebo-controlled trial involving children with biopsy-proven MASLD, Nobili et al. [188] aimed to assess the anti-steatogenic effects of a 4-month treatment with VitE and hydroxytyrosol (HXT). In particular, 80 paediatric patients with MASLD were enrolled in two arms: the treatment group receiving HXT and VitE, and the placebo group receiving placebo. Results showed that the treatment with HXT and VitE greatly improved steatosis, insulin resistance, triglyceride levels, and oxidative stress parameters, including GSH and GSSG.
Another double-blind, placebo-controlled trial evaluated the effects of pinitol supplementation on liver fat content in adults with MASLD. Treatment with low or high doses of pinitol significantly reduced liver fat content and liver enzymes. However, the authors did not find a statistically significant change in GSH levels. Still, they found GPx, pyroglutamic acid, and glutamate significantly decreased after the treatment compared to the placebo group [189].
Maharshi et al. [190], in a pilot study, demonstrated that in adult patients with MASLD, lifestyle standard management (SMT) or SMT combined with H. pylori-eradication therapy (HPET) exhibited after 24 weeks a comparable effect in reducing hepatic steatosis and liver enzymes, even if interestingly only the HPET induced significant increase of serum GSH levels.
A randomized cross-over clinical trial performed on obese children with MASLD demonstrated that a calorie-restricted regimen alone or coupled with lycopene-rich tomato sauce improved steatosis and metabolism, though these effects were more profound in the tomato-supplemented group [191]. Moreover, the authors reported that only tomato supplementation resulted in glycolytic metabolic activation of T-cells and a marked increase in serum GSH.
The effects of VSL#3® probiotic supplementation on cardiovascular risk and liver injury biomarkers in patients with MASLD were investigated by Chong et al. [192] in a randomized, double-blinded, placebo-controlled study. Endothelial function, oxidative stress, inflammation, insulin resistance, and liver injury markers were measured before and after the intervention. No significant changes were observed in the markers of cardiovascular risk, fibrosis indexes, and levels of GSH blood following VSL#3® supplementation.
On the other hand, Yurtdas et al. [193] assessed the impact of the Mediterranean diet (MD) versus a conventional low-fat diet (LFD) in adolescents with obesity and MASLD. Both diets significantly reduced hepatic steatosis, serum transaminase levels, and insulin resistance, while improving inflammation and oxidative stress markers. Specifically, the difference in GSH blood levels between the two groups was significant at the 12-week follow-up, with higher levels in MD vs. LFD.
Tavakoli et al. [194] evaluated oxidative stress markers in MASLD patients diagnosed using abdominal ultrasound before and after treatment with pioglitazone. The results showed that at diagnosis, MASLD patients had significantly higher MDA and thiol levels compared to the control group. However, after three months of treatment with pioglitazone, MDA levels decreased, while thiol levels increased highlighting the role of pioglitazone in reducing these oxidative stress markers.
According to a previous study [193], Quetglas-Llabrés et al. [195] examined the impact of an MD intervention on antioxidant and inflammatory markers in patients with MASLD. Forty adult patients were divided based on their adherence to the MD, and after the intervention, both groups achieved an improved lipid profile characterized by decreases in total cholesterol and triglyceride levels. However, only participants who achieved higher adherence to the MD also exhibited decreased levels of glucose and liver enzymes, and increased GSH blood levels.
4. Discussion
The onset and progression of various pathologies are strongly linked to LMW thiol levels, and the liver is crucial in regulating the production and distribution of most of these molecules (i.e., GSH, Cys, CysGly, and Hcy) to various organs. Consequently, it is unsurprising that alterations in LMW thiols could indicate oxidative stress and play a key role in the pathogenesis of multiorgan diseases, such as MASLD. Several mechanisms have been proposed to explain changes in the levels of LMW thiols in MASLD [6]. GSH metabolism is regulated by the expression/activity of several enzymes (e.g., GSH peroxidase and GSH reductase), and it is susceptible to their gene expression by NRF2 transcription, whose knockout may reduce hepatocellular fat accumulation in experimental models [196]. The serum alteration of Cys and Hcy levels could be influenced directly by the GSH levels or other mechanisms, including the remethylation cycle and epigenetic control [186]. Even though the pathways acting as upstream regulators of LMW thiols may offer plausible therapeutic targets, they still remain poorly explored.
Hence, this systematic review was mainly focused on presenting a comprehensive summary of findings related to the assessment of LMW thiol levels in MASLD by analysing experimental studies conducted in in vivo and in vitro models and human subjects. A qualitative analysis of the included studies was also performed and reported in the next paragraph.
4.1. Qualitative Analysis
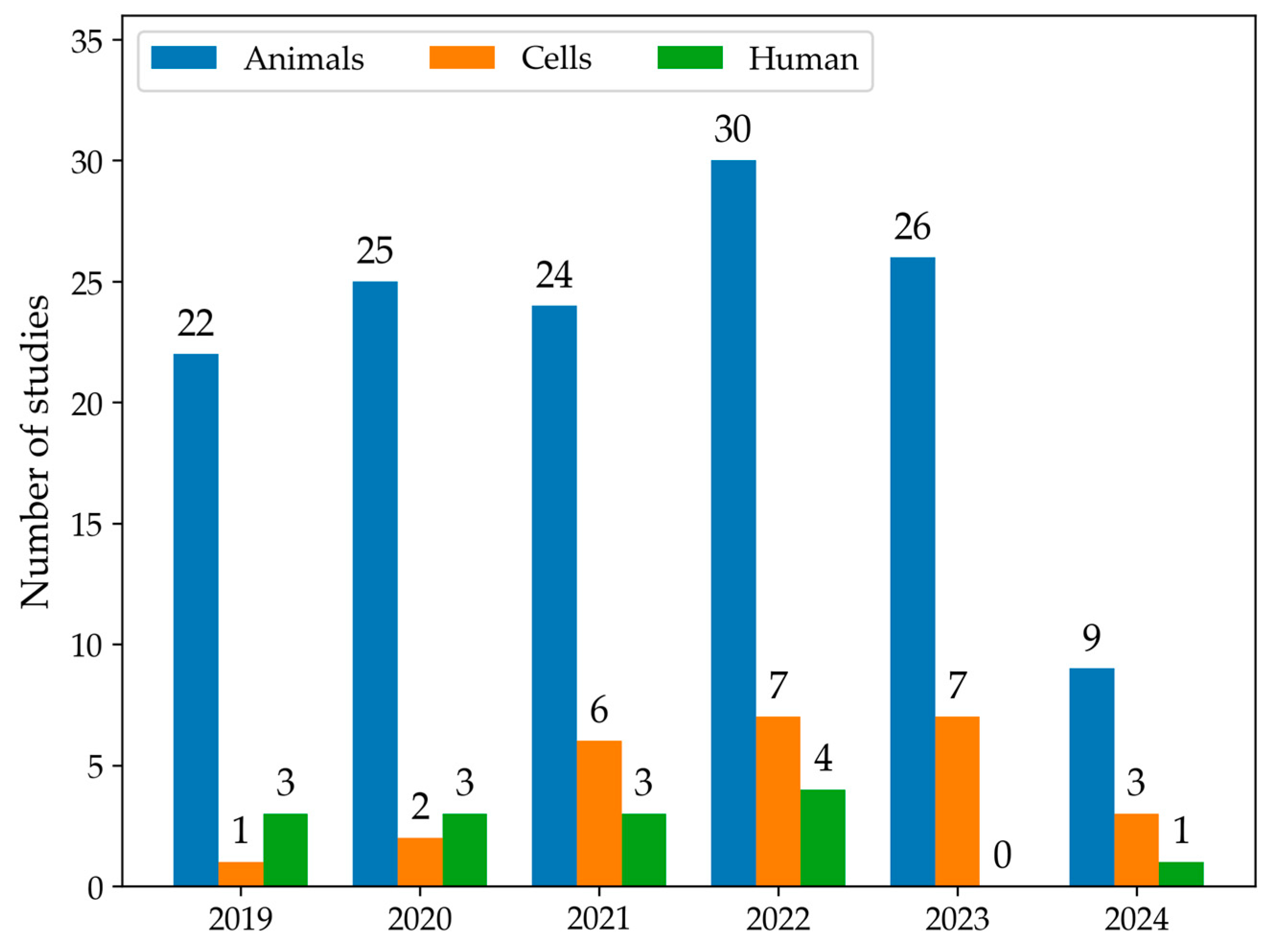
The first qualitative analysis examined the number of articles that discussed data on LMW thiols in MASLD from 2019 to 2024, categorising studies by models or human samples. As shown in Figure 2, the number of eligible articles increased each year. Moreover, animals were the most common experimental MASLD model employed in studies, followed by cellular lines. In 2019, a total of 26 articles were published. Among these articles, 22 used animal models, one used cell models, and three studies were conducted in humans. In 2022, there was an increase in the total number of articles to 41, including 30 articles in animal models, 7 in cell models, and four studies in human samples. Overall, animal models were the most commonly used across all years. The use of cell models showed a gradual increase, particularly from 2021 onward. Human studies remained low throughout the years. The number of articles selected for 2024 only accounts for the year’s first half; therefore, the number is smaller.
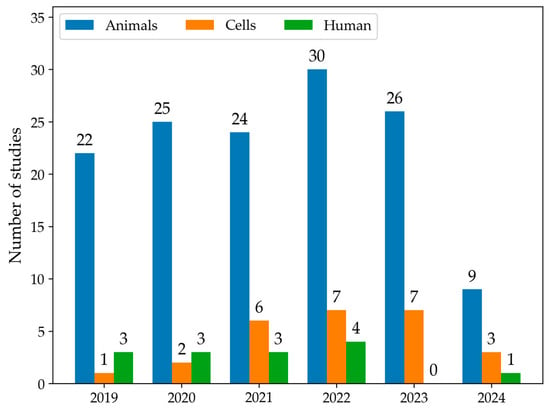
Figure 2.
The histogram summarises the number of studies that measure LMW thiols using animal (blue) or cell models (orange) or conducted in human samples (green) from January 2019 to June 2024.
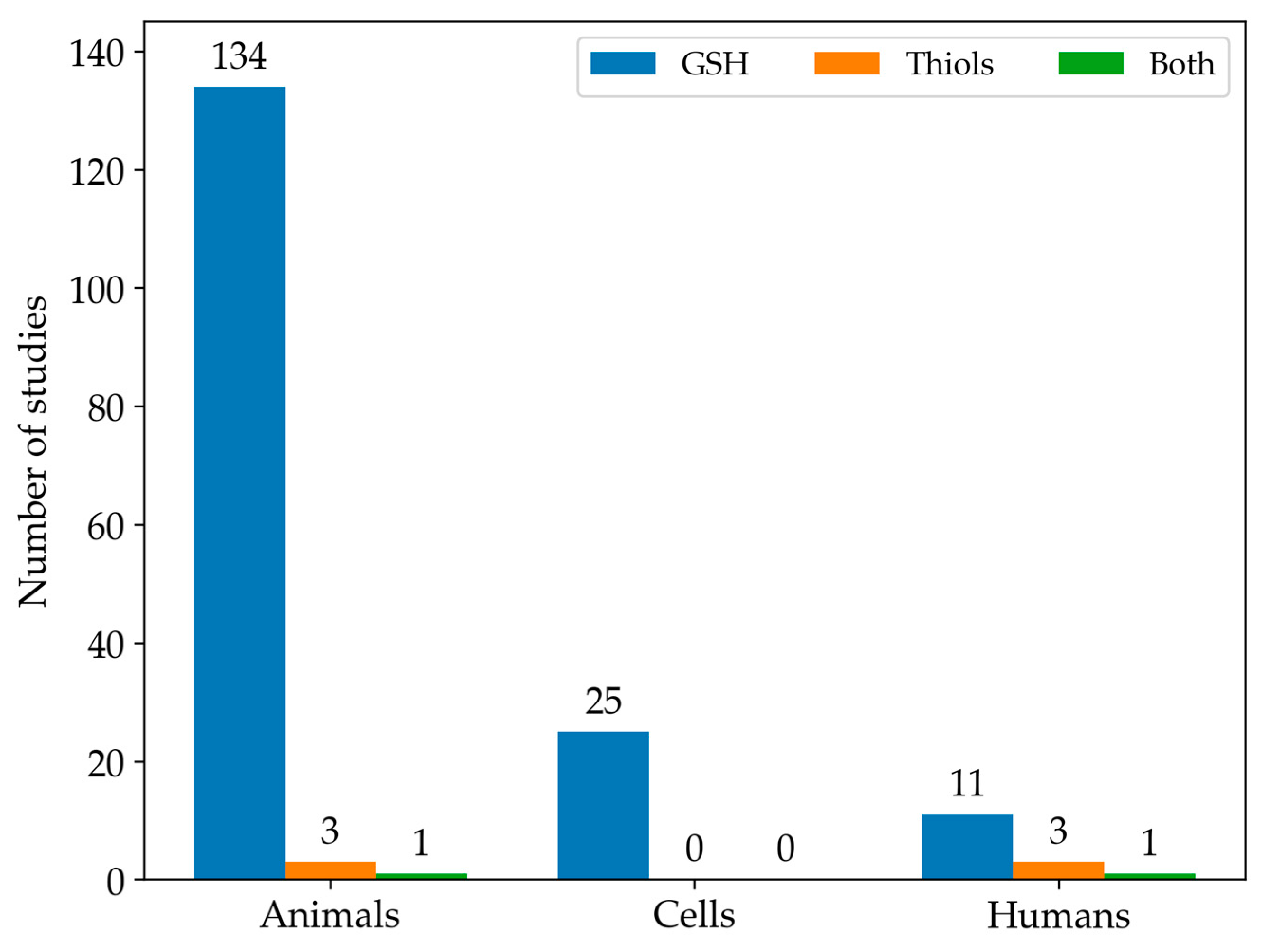
Among the studies we found eligible for this systematic review, GSH was the most commonly analysed LMW thiol. In contrast, only 3% of the studies measured the full spectrum of LMW thiols. In particular, Figure 3 illustrates the number of articles that measured either GSH or all LMW thiols across different experimental types of samples.
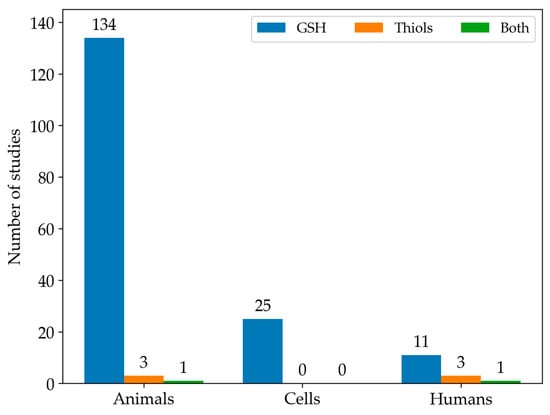
Figure 3.
The histogram shows the distribution of studies focused on GSH levels (blue), other LMW thiol levels (orange), or both (green) in animal, cell, and human models.
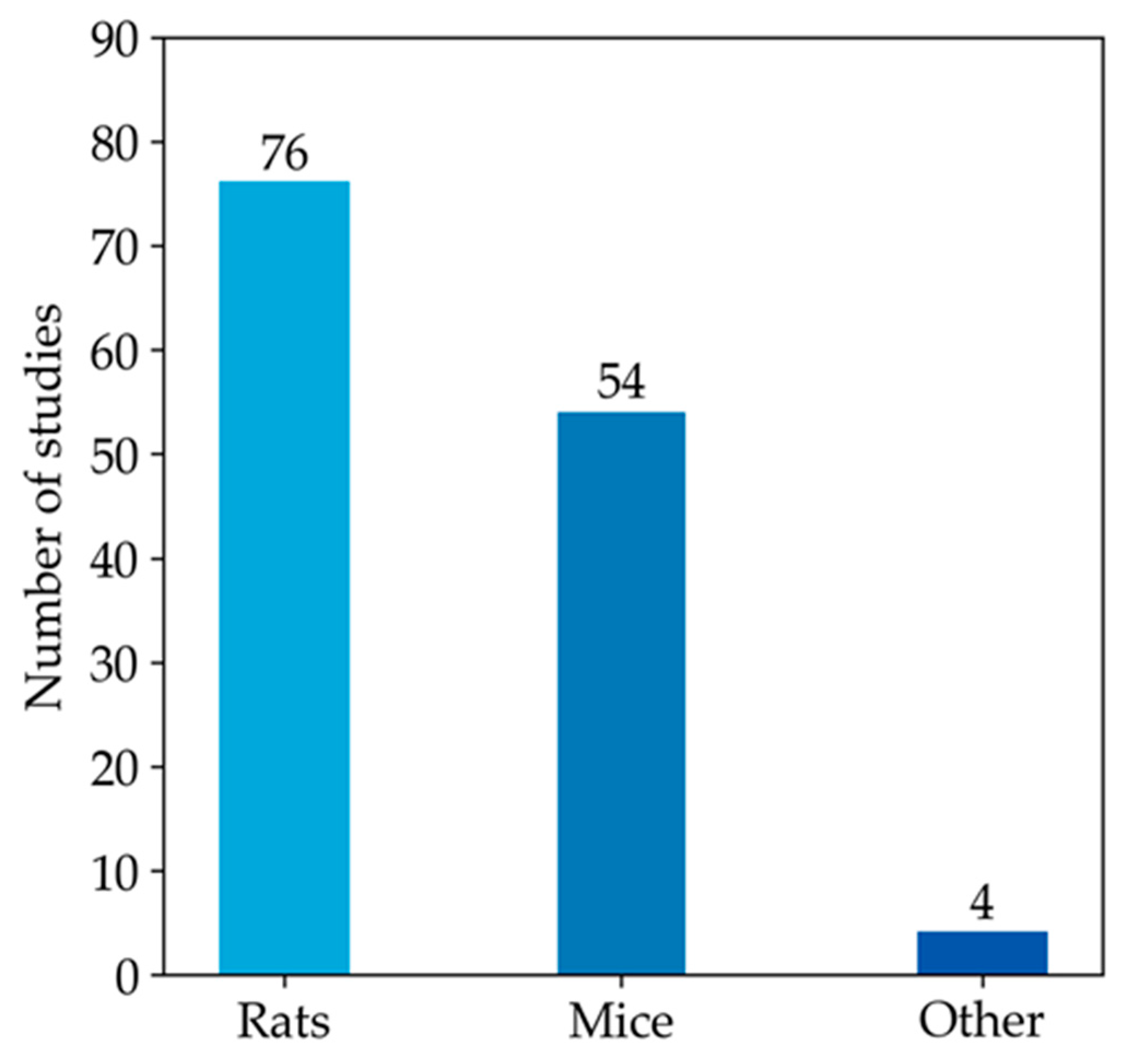
Since most studies focused on the measurement of GSH in animal models, the rest of our qualitative analysis was conducted only on these articles. Among animal studies (Figure 4), rats were the most commonly used animal model, specifically in 76 studies, followed by mice, featured in 54 articles. In contrast, other models (i.e., monkeys, rabbits, and zebrafish) were used much less frequently. As emerged from the evaluation of the animal studies, changes in GSH levels were different in direction and amount. These discrepancies depend on the fact that animal models of MASLD were established using different diets, mainly enriched in lipid or carbohydrate content, but at varied percentages. Moreover, other study design variables, such as the age of animals and length of study, can also influence the outcome of model induction and treatment, thus influencing the amount of GSH in both liver and blood samples [197]. Moreover, GSH was analysed using different types of sample matrices and various methods. Figure 5a reports the number of studies measuring GSH levels in blood samples (i.e., blood cells, plasma, serum) compared to the studies assessing GSH amounts in hepatic tissue. In particular, GSH is mainly measured in hepatic tissue (91.7%), whereas studies estimating the LMW thiol in serum, plasma, or blood are less frequent (5.3%). A few studies analysed GSH in both sample matrices (3.0%).
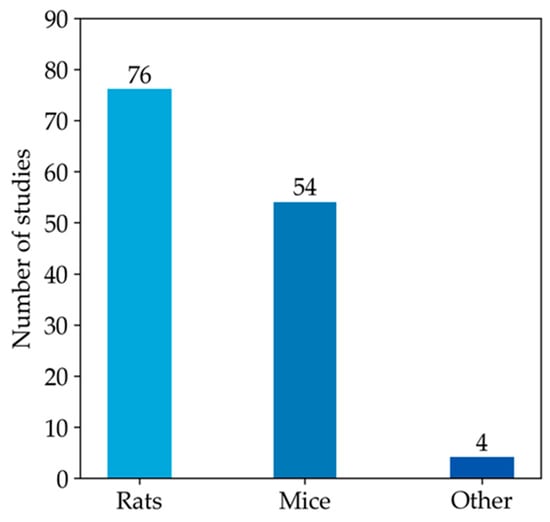
Figure 4.
The histogram represents the number of articles measuring GSH levels in rat or mouse models. The remaining four articles in the ‘other’ category include studies that investigate GSH levels in monkey, rabbit, and zebrafish models.
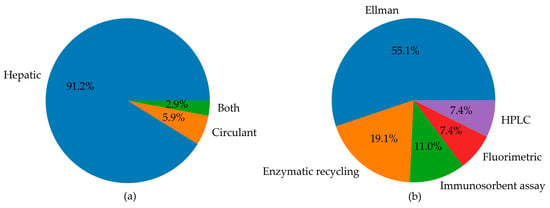
Figure 5.
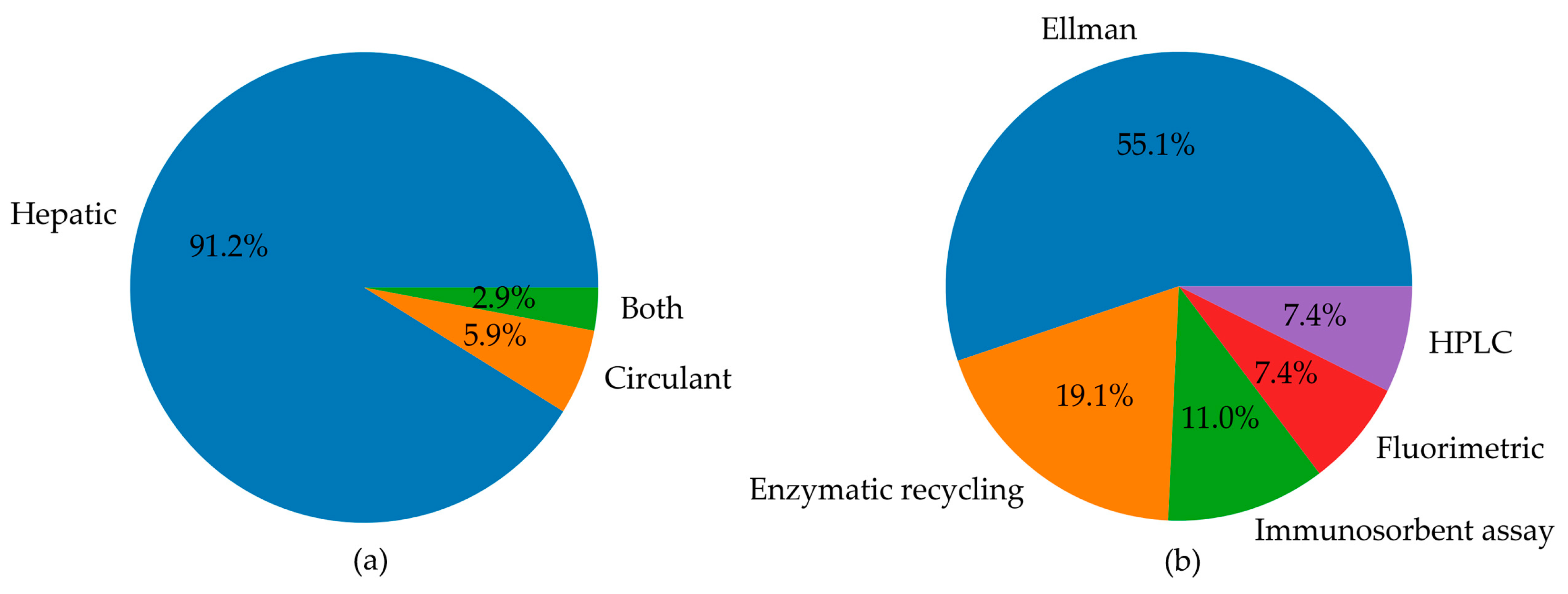
Pie charts summarising (a) the number of studies measuring circulating and hepatic levels of GSH, or both, in animal models; (b) the landscape of methods used for the determination of GSH levels in animal models, including Ellman’s method, enzymatic recycling, immunosorbent assays, fluorimetric analysis, and HPLC.
The distribution of various methods used for GSH level detection was reported in Figure 5b. The pie chart highlights that Ellman’s method was the most commonly used to analyse GSH levels (55.1%). The enzymatic recycling method and immunosorbent assays were also frequently used in 19.1% and 11% of studies, respectively. HPLC and fluorimetric techniques were employed collectively in 14.8% of studies. This latter analysis of methods used in the selected article reveals the pitfalls in the GSH analysis and emphasises the need for standardisation and reproducibility tools. The major weak points in GSH determination are the ease of non-enzymatic GSH autoxidation at pH > 7 and enzymatic conversion of GSH, the first step of which is mediated by GGTs, which exhibit optimal activity at neutral pH. Thus, it is essential to maintain the pH of the media in the acidic range [12]. The measurement of GSH and GSSG in biological samples [9,198] requires thus caution to prevent assay artefacts or data misinterpretation. Accordingly, the following points must be checked to achieve accurate analyses of GSH and GSSG: sample collection, reduction of disulphides, and, if needed, deproteinisation. Not all the literature cited points out this essential step in the methods section. Another critical point in GSH analysis is the method used. Below, we report the methods utilised in the literature cited in this review. Analytical methods that use colourimetric reagents and UV–VIS absorbance detection are inferior in sensitivity but simple compared with electrochemical or fluorometric determination. Ellman’s reagent (5,5′-dithio-(bis-2-nitrobenzoic) acid, DTNB) is widely used for the analysis of thiols in biological samples via the determination of the liberated anion [10].
Tietze [11] published his classical spectrophotometric method in 1969, often called the GR-coupled enzymatic recycling assay or the GSH-recycling assay. The GR-coupled recycling assay is still one of the most widely applied techniques to detect GSH and GSSG due to its simplicity, satisfactory sensitivity, and low cost. Several spectrofluorimetric methods have been developed to analyse GSH, GSSG, and related compounds in different matrices [12]. Several fluorophores were used, such as the dithiolic fluorophore and other rhodamine-based fluorescent probes [199], most reacting with all the cell’s thiol functionalities. The HPLC, coupled with a fluorometric, spectrophotometric, or mass spectrometry detector, has recently been the method of choice for measuring GSH and related thiols in biological samples. The HPLC techniques are rapid, highly specific, sensitive (0.5 pmol order), and reproducible. The simultaneous determination of GSH and other thiols in a single assay may be achieved by the appropriate choice of column, derivatisation, elution protocols, and detection system [12]. Finally, a few papers used an immunosorbent method for GSH assay. This method has not been validated against a gold standard but seems precise and sensitive. Anyway, it only allows reduced GSH determinations.
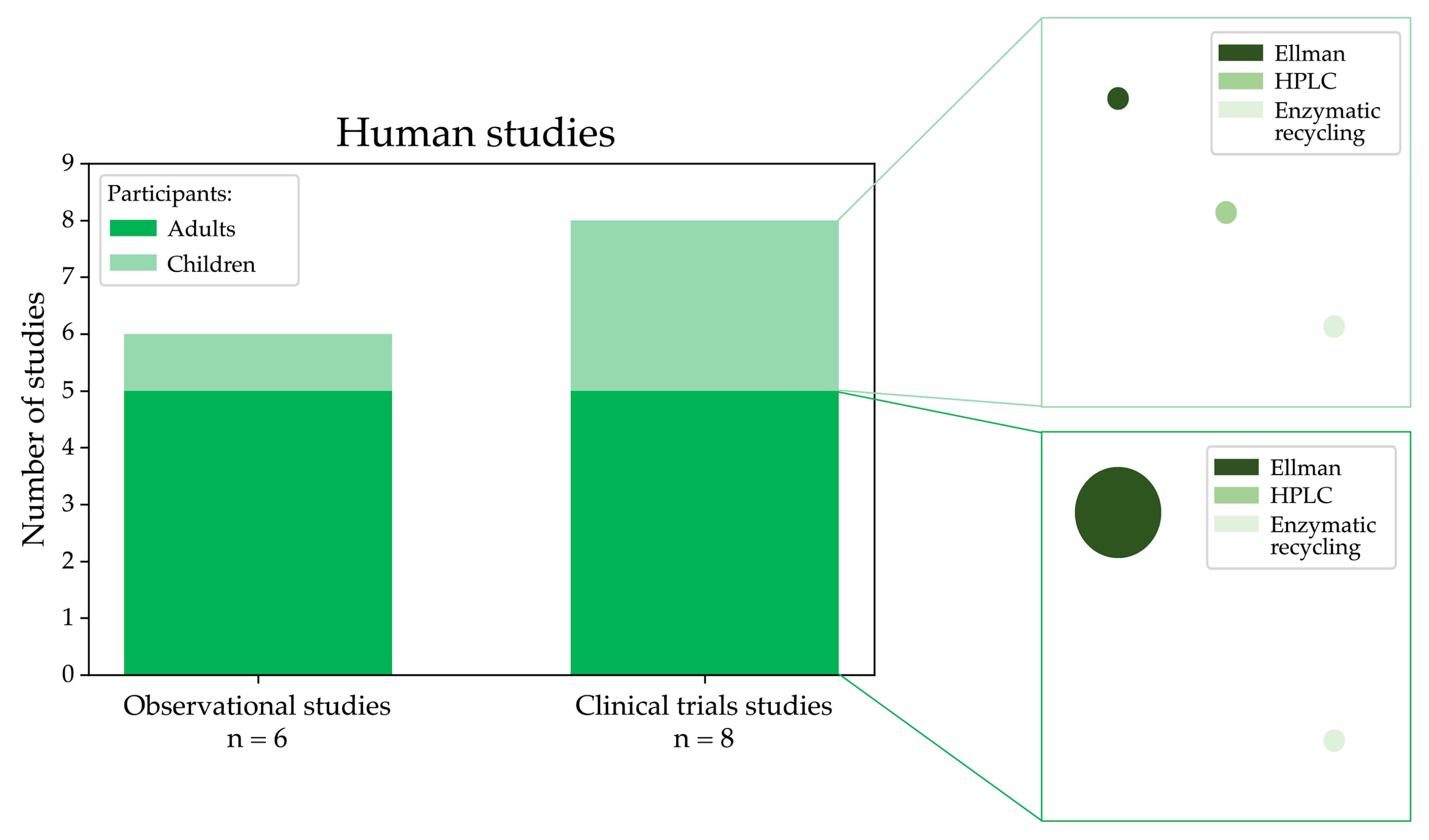
In this systematic review, we also selected 14 studies that analysed LMW thiols using samples of patients affected by MASLD and relative controls. Figure 6 shows the distribution of study types and participant demographics in human studies selected in this review, highlighting that clinical trials and observational studies often focus on adults, with fewer studies involving paediatric populations. As evidenced in the plot, many studies used Ellman’s method for the analysis.
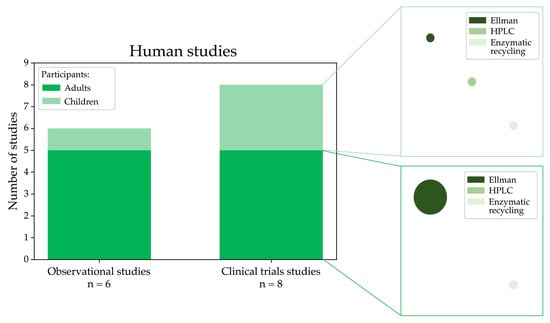
Figure 6.
Graphical visualisation of the number of observational and clinical trials measuring LMW thiols conducted in humans affected by MASLD categorised into adult and paediatric groups and the experimental methods used for the analysis (the circle size is proportional to the number of studies using a specific methodology).
4.2. Limitations of the Study
Potential biases could include the type and number of selected databases used for the search. Only three databases (i.e., PubMed, Embase, and Scopus) were used for this systematic review. However, these databases are recognised as the principal resources for literature recording [200]. Another limitation could be the choice of eligible studies that could have been strongly influenced by several factors, including the availability of complete explicit data, methods, or an inadequate model to resemble MASLD. Moreover, the grey literature exclusion may represent an additional limitation, even though its inclusion could have emphasised the missing data problem. A further limitation of the present study may be represented by manual screening. An automated or AI-based literature screening might enhance the consistency and efficiency of eligible studies in the future.
Finally, the significant heterogeneity and data insufficiency have hampered the performance of a meta-analysis and the ability to obtain a picture of GSH levels in MASLD.
5. Conclusions
LMW thiols, particularly GSH, play pathogenic roles in various diseases. Central to the production and systemic distribution of LMW thiols, the liver is susceptible to oxidative stress that could trigger liver damage, leading to MASLD. In this systematic literature review of recent studies assessing the levels of LMW thiols in MASLD models and human subjects, we highlight heterogeneity in sample processing and measurement of LMW thiol levels, which does not allow to perform a meta-analysis and hinder the establishment of cut-offs that could be used for MASLD diagnosis and stratification. The introduction of standardisation of measuring methods is an imperative step to move forward future studies that unveil the pathogenetic role of these molecules and their translatability into a diagnostic flowchart.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13121461/s1. Table S1. Studies assessing the levels of GSH forms in rat models of MASLD. Table S2. Studies assessing the levels of GSH forms in mice models of MASLD. Table S3. Studies assessing the levels of GSH forms in other models of MASLD.
Author Contributions
Conceptualization, L.C., A.A. and A.P.; methodology, L.C. and A.A.; formal analysis, L.C., F.G., A.A. and A.P.; data curation, L.C. and A.A.; writing—original draft preparation, L.C. and A.A.; review and editing, L.C., A.A., F.G. and A.P.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union—Next Generation EU—NRRP M6C2—Investment 2.1 Enhancement and strengthening of biomedical research in the NHS (CUP number E83C22006360001). This work was also supported by the Italian Ministry of Health with “Current Research funds”.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Vázquez-Meza, H.; Vilchis-Landeros, M.M.; Vázquez-Carrada, M.; Uribe-Ramírez, D.; Matuz-Mares, D. Cellular Compartmentalization, Glutathione Transport and Its Relevance in Some Pathologies. Antioxidants 2023, 12, 834. [Google Scholar] [CrossRef]
- Giustarini, D.; Colombo, G.; Garavaglia, M.L.; Astori, E.; Portinaro, N.M.; Reggiani, F.; Badalamenti, S.; Aloisi, A.M.; Santucci, A.; Rossi, R.; et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017, 112, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Kiyohara, Y.; Kato, I.; Kitazono, T.; Tanizaki, Y.; Kubo, M.; Ueno, H.; Ibayashi, S.; Fujishima, M.; Iida, M. Relationship between plasma glutathione levels and cardiovascular disease in a defined population: The Hisayama study. Stroke 2004, 35, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Dludla, P.V.; Ziqubu, K.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Nkambule, B.B.; Basson, A.K.; Pheiffer, C.; Tiano, L.; Kengne, A.P. Dietary Supplements Potentially Target Plasma Glutathione Levels to Improve Cardiometabolic Health in Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials. Nutrients 2023, 15, 944. [Google Scholar] [CrossRef]
- Giustarini, D.; Tsikas, D.; Colombo, G.; Milzani, A.; Dalle-Donne, I.; Fanti, P.; Rossi, R. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: An elephant in the room. J. Chromatogr. B 2016, 1019, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Nuhu, F.; Gordon, A.; Sturmey, R.; Seymour, A.M.; Bhandari, S. Measurement of Glutathione as a Tool for Oxidative Stress Studies by High Performance Liquid Chromatography. Molecules 2020, 25, 4196. [Google Scholar] [CrossRef]
- Lu, S.C. Dysregulation of glutathione synthesis in liver disease. Liver Res. 2020, 4, 64–73. [Google Scholar] [CrossRef]
- Yu, X.; Long, Y.C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci. Rep. 2016, 6, 30033. [Google Scholar] [CrossRef]
- Bonifácio, V.D.B.; Pereira, S.A.; Serpa, J.; Vicente, J.B. Cysteine metabolic circuitries: Druggable targets in cancer. Br. J. Cancer 2021, 124, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Nolin, T.D.; McMenamin, M.E.; Himmelfarb, J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: Application to studies of oxidative stress. J. Chromatogr. B 2007, 852, 554–561. [Google Scholar] [CrossRef]
- Krag, A.; Rinella, M.E. Steatotic liver disease: A new name to reflect the combined role of alcohol and metabolic dysfunction. Nat. Med. 2024, 30, 933–936. [Google Scholar] [CrossRef]
- Zhang, L.; El-Shabrawi, M.; Baur, L.A.; Byrne, C.D.; Targher, G.; Kehar, M.; Porta, G.; Lee, W.S.; Lefere, S.; Turan, S.; et al. An international multidisciplinary consensus on pediatric metabolic dysfunction-associated fatty liver disease. Med 2024, 5, 797–815.e2. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Wong, V.W.; Zhang, X.; Yu, J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; McCaughan, G.; Grønbæk, H. Role of gut microbiota and immune cells in metabolic-associated fatty liver disease: Clinical impact. Hepatol. Int. 2024, 18, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yan, Z.; Zhong, H.; Luo, R.; Liu, W.; Xiong, S.; Liu, Q.; Liu, M. Gut microbial metabolites in MASLD: Implications of mitochondrial dysfunction in the pathogenesis and treatment. Hepatol. Commun. 2024, 8, e0484. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kopp, O.; Snethlage, C.C.; Schwentker, C. JabRef: BibTeX-based literature management software. TUGboat 2023, 44, 441–447. [Google Scholar] [CrossRef]
- Khalaf, H.M.; Ibrahim, M.A.; Amin, E.F.; Abdel-Tawab Ibrahim, S.; Abdel-Wahab, S.; Fouad, Y.M. Allopurinol potentiates the hepatoprotective effect of metformin and vitamin E in fructose-induced fatty liver in rats. Clin. Exp. Hepatol. 2019, 5, 65–74. [Google Scholar] [CrossRef]
- Palladini, G.; Di Pasqua, L.G.; Berardo, C.; Siciliano, V.; Richelmi, P.; Mannucci, B.; Croce, A.C.; Rizzo, V.; Perlini, S.; Vairetti, M.; et al. Fatty Acid Desaturase Involvement in Non-Alcoholic Fatty Liver Disease Rat Models: Oxidative Stress Versus Metalloproteinases. Nutrients 2019, 11, 799. [Google Scholar] [CrossRef]
- Soliman, G.F.; Rashed, L.A.; Morsi, H.; Ibrahim, W.; Abdallah, H.; Bastawy, N.; Abdel Maksoud, O.M. Interrelation of liver vascularity to non-alcoholic fatty liver through a comparative study of the vasodilator effect of carvedilol or nicorandil in rats. Life Sci. 2019, 222, 175–182. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Qiu, J.Y.; Wu, X. The Combination of Mulberry Extracts and Silk Amino Acids Alleviated HFD-Induced Nonalcoholic Hepatic Steatosis by Improving Hepatic Insulin Signaling and Normalizing Gut Microbiome Dysbiosis in Rats. Evid. Based Complement. Alternat. Med. 2019, 2019, 8063121. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.S.; Juvekar, A.R. Chrysin ameliorates nonalcoholic fatty liver disease in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, Y.; Fang, N.; Guo, Y. Hepatoprotective effects of Cassia semen ethanol extract on non-alcoholic fatty liver disease in experimental rat. Pharm. Biol. 2019, 57, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Souza Cruz, E.M.; Bitencourt de Morais, J.M.; Dalto da Rosa, C.V.; da Silva Simões, M.; Comar, J.F.; de Almeida Chuffa, L.G.; Seiva, F.R.F. Long-term sucrose solution consumption causes metabolic alterations and affects hepatic oxidative stress in Wistar rats. Biol. Open 2020, 9, bio047282. [Google Scholar] [CrossRef] [PubMed]
- Faheem, S.A.; Saeed, N.M.; El-Naga, R.N.; Ayoub, I.M.; Azab, S.S. Hepatoprotective Effect of Cranberry Nutraceutical Extract in Non-alcoholic Fatty Liver Model in Rats: Impact on Insulin Resistance and Nrf-2 Expression. Front. Pharmacol. 2020, 11, 218. [Google Scholar] [CrossRef]
- Witayavanitkul, N.; Werawatganon, D.; Chayanupatkul, M.; Klaikeaw, N.; Sanguanrungsirikul, S.; Siriviriyakul, P. Genistein and exercise modulated lipid peroxidation and improved steatohepatitis in ovariectomized rats. BMC Complement. Med. Ther. 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Shalaby, A.A.; Abdallah, H.M.L.; El-Zohairy, N.F.; Bahr, H.I. Ameliorative Effect of Garden Cress (Lepidium sativum L.) Seeds Ethanolic Extract on HFD-prompted Non-alcoholic Fatty Liver Disease in the Rat Model: Impact on 3-Hydroxy-3-methylglutaryl-Coenzyme A Reductase and Vascular Endothelial Growth Factor. Adv. Anim. Vet. Sci. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Ogunlana, O.O.; Ogunlana, O.E.; Adekunbi, T.S.; Adetuyi, B.O.; Adegboye, B.E.; Iheagwam, F.N. Anti-inflammatory Mechanism of Ruzu Bitters on Diet-Induced Nonalcoholic Fatty Liver Disease in Male Wistar Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 5246725. [Google Scholar] [CrossRef]
- Heeba, G.H.; El-Deen, R.M.; Abdel-Latif, R.G.; Khalifa, M.M.A. Combined treatments with metformin and phosphodiesterase inhibitors alleviate nonalcoholic fatty liver disease in HFD fed rats: A comparative study. Can. J. Physiol. Pharmacol. 2020, 98, 498–505. [Google Scholar] [CrossRef]
- Saeed, N.M.; Mansour, A.M.; Allam, S. Lycopene induces insulin signaling and alleviates fibrosis in experimental model of non-alcoholic fatty liver disease in rats. PharmaNutrition 2020, 14, 100225. [Google Scholar] [CrossRef]
- Konda, P.Y.; Poondla, V.; Jaiswal, K.K.; Dasari, S.; Uyyala, R.; Surtineni, V.P.; Egi, J.Y.; Masilamani, A.J.A.; Bestha, L.; Konanki, S.; et al. Pathophysiology of HFD induced obesity: Impact of probiotic banana juice on obesity associated complications and hepatosteatosis. Sci. Rep. 2020, 10, 16894. [Google Scholar] [CrossRef] [PubMed]
- Abdel Jaleel, G.A.; Al-Awdan, S.A.; Ahmed, R.F.; Ahmed-Farid, O.A.H.; Saleh, D.O. Melatonin regulates neurodegenerative complications associated with NAFLD via enhanced neurotransmission and cellular integrity: A correlational study. Metab. Brain Dis. 2020, 35, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Bingül, İ.; Aydın, A.F.; Küçükgergin, C.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. The effect of 1,25-dihydroxyvitamin D3 on liver damage, oxidative stress, and advanced glycation end products in experimental nonalcoholic- and alcoholic- fatty liver disease. Turk. J. Med. Sci. 2021, 51, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.H.; Saeed, N.M.; El-Sherbiny, D.A.; El-Demerdash, E. Eugenol modulates insulin sensitivity by upregulating insulin receptor substrate-2 in non-alcoholic fatty liver disease in rats. J. Pharm. Pharmacol. 2021, 73, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, T.; Gnanasekaran, N.; Mehare, T. Hepatoprotective effect of silymarin on fructose induced nonalcoholic fatty liver disease in male albino wistar rats. BMC Complement. Med. Ther. 2021, 21, 140. [Google Scholar] [CrossRef]
- Gopinath, V.; Shamsitha, M.K.A.; Penarveettil Nair, V.; Seena, P.; Uppu, R.M.C.; Raghavamenon, A. Thermally Oxidized Coconut Oil as Fat Source in HFD Induces Hepatic Fibrosis in Diabetic Rat Model. Cell Biochem. Biophys. 2021, 79, 629–639. [Google Scholar] [CrossRef]
- Carvalho, L.C.F.; Dias, B.V.; Gomes, S.V.; Carneiro, C.M.; Costa, D.C. Temporal effect of fructose supplementation at different concentrations on hepatic metabolism of Wistar rats. Nutr. Hosp. 2021, 38, 1089–1100. [Google Scholar] [CrossRef]
- Abd-Elrazek, A.M.; Ibrahim, S.R.; El-dash, H.A. The ameliorative effect of Apium graveolens & curcumin against Non-alcoholic fatty liver disease induced by high fructose-high fat diet in rats. Future J. Pharm. Sci. 2022, 8, 26. [Google Scholar] [CrossRef]
- Zakaria, Z.; Othman, Z.A.; Bagi Suleiman, J.; Jalil, N.A.C.; Ghazali, W.S.W.; Mohamed, M. Protective and Therapeutic Effects of Orlistat on Metabolic Syndrome and Oxidative Stress in HFD-Induced Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) in Rats: Role on Nrf2 Activation. Vet. Sci. 2021, 8, 274. [Google Scholar] [CrossRef]
- Palladini, G.; Di Pasqua, L.G.; Cagna, M.; Croce, A.C.; Perlini, S.; Mannucci, B.; Profumo, A.; Ferrigno, A.; Vairetti, M. MCD Diet Rat Model Induces Alterations in Zinc and Iron during NAFLD Progression from Steatosis to Steatohepatitis. Int. J. Mol. Sci. 2022, 23, 6817. [Google Scholar] [CrossRef]
- Attia, H.; Albekairi, N.; Albdeirat, L.; Soliman, A.; Rajab, R.; Alotaibi, H.; Ali, R.; Badr, A. Chrysin Attenuates Fructose-Induced Nonalcoholic Fatty Liver in Rats via Antioxidant and Anti-Inflammatory Effects: The Role of Angiotensin-Converting Enzyme 2/Angiotensin (1-7)/Mas Receptor Axis. Oxid. Med. Cell. Longev. 2022, 2022, 9479456. [Google Scholar] [CrossRef] [PubMed]
- Oriquat, G.; Masoud, I.M.; Kamel, M.A.; Aboudeya, H.M.; Bakir, M.B.; Shaker, S.A. The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways. Molecules 2023, 28, 1734. [Google Scholar] [CrossRef] [PubMed]
- Reda, D.; Elshopakey, G.E.; Albukhari, T.A.; Almehmadi, S.J.; Refaat, B.; Risha, E.F.; Mahgoub, H.A.; El-Boshy, M.E.; Abdelhamid, F.M. Vitamin D3 alleviates nonalcoholic fatty liver disease in rats by inhibiting hepatic oxidative stress and inflammation via the SREBP-1-c/ PPARα-NF-κB/IR-S2 signaling pathway. Front. Pharmacol. 2023, 14, 1164512. [Google Scholar] [CrossRef] [PubMed]
- Chenna, H.; Khelef, Y.; Halimi, I.; Yilmaz, M.A.; Çakir, O.; Djouder, C.; Tarhan, A.; Idoughi, K.; Boumendjel, M.; Boumendjel, A.; et al. Potential Hepatoprotective Effect of Matricaria Pubescens on High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Rats. Chem. Biodivers. 2024, 21, e202302005. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Bakir, M.B.; Salama, M.A.; Refaat, R.; Ali, M.A.; Khalifa, E.A.; Kamel, M.A. Evaluating the therapeutic potential of one-carbon donors in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2019, 847, 72–82. [Google Scholar] [CrossRef]
- Abu-Elsaad, N.; El-Karef, A. Protection against nonalcoholic steatohepatitis through targeting IL-18 and IL-1alpha by luteolin. Pharmacol. Rep. 2019, 71, 688–694. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Zou, Y.; Guo, H.; Zhang, B.; Xia, C.; Zhang, H.; Yang, W.; Xu, C. NFκB/Orai1 Facilitates Endoplasmic Reticulum Stress by Oxidative Stress in the Pathogenesis of Non-alcoholic Fatty Liver Disease. Front. Cell. Dev. Biol. 2019, 7, 202. [Google Scholar] [CrossRef]
- Huang, L.; Ding, W.; Wang, M.Q.; Wang, Z.G.; Chen, H.H.; Chen, W.; Yang, Q.; Lu, T.N.; Yang, Q.; He, J.M. Tanshinone IIA ameliorates non-alcoholic fatty liver disease through targeting peroxisome proliferator-activated receptor gamma and toll-like receptor 4. J. Int. Med. Res. 2019, 47, 5239–5255. [Google Scholar] [CrossRef]
- Kumar, D.; Dwivedi, D.K.; Lahkar, M.; Jangra, A. Hepatoprotective potential of 7,8-Dihydroxyflavone against alcohol and high-fat diet induced liver toxicity via attenuation of oxido-nitrosative stress and NF-κB activation. Pharmacol. Rep. 2019, 71, 1235–1243. [Google Scholar] [CrossRef]
- Loza-Medrano, S.S.; Baiza-Gutman, L.A.; Manuel-Apolinar, L.; García-Macedo, R.; Damasio-Santana, L.; Martínez-Mar, O.A.; Sánchez-Becerra, M.C.; Cruz-López, M.; Ibáñez-Hernández, M.A.; Díaz-Flores, M. High fructose-containing drinking water-induced steatohepatitis in rats is prevented by the nicotinamide-mediated modulation of redox homeostasis and NADPH-producing enzymes. Mol. Biol. Rep. 2020, 47, 337–351. [Google Scholar] [CrossRef]
- El-Derany, M.O.; El-Demerdash, E. Pyrvinium pamoate attenuates non-alcoholic steatohepatitis: Insight on hedgehog/Gli and Wnt/β-catenin signaling crosstalk. Biochem. Pharmacol. 2020, 177, 113942. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Amusa, O.A. Sodium acetate-mediated inhibition of histone deacetylase alleviates hepatic lipid dysregulation and its accompanied injury in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2020, 128, 110226. [Google Scholar] [CrossRef]
- AlFaris, N.A.; Alshammari, G.M.; AlTamimi, J.Z.; AlMousa, L.A.; AlKehayez, N.M.; Aljabryn, D.H.; Alagal, R.I.; Yahya, M.A. The protective effect of shrimp cooked in different methods on high-cholesterol- induced fatty liver in rats. Saudi. J. Biol. Sci. 2021, 28, 170–182. [Google Scholar] [CrossRef]
- Açıkel Elmas, M.; Atay, N.; Bingöl Özakpınar, Ö.; Arbak, S.; Kolgazi, M.; Şener, G.; Ercan, F. Morphological evaluation of the effects of exercise on high-fat-diet-induced liver damage in rats. Turk. J. Gastroenterol. 2020, 31, 626–632. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Kravchenko, L.V.; Aksenov, I.V.; Nikitin, N.S.; Guseva, G.V.; Avrenyeva, L.I.; Trusov, N.V.; Balakina, A.S.; Tutelyan, V.A. Lipoic Acid Exacerbates Oxidative Stress and Lipid Accumulation in the Liver of Wistar Rats Fed a Hypercaloric Choline-Deficient Diet. Nutrients 2021, 13, 1999. [Google Scholar] [CrossRef]
- Mu, J.K.; Zi, L.; Li, Y.Q.; Yu, L.P.; Cui, Z.G.; Shi, T.T.; Zhang, F.; Gu, W.; Hao, J.J.; Yu, J.; et al. Jiuzhuan Huangjing Pills relieve mitochondrial dysfunction and attenuate high-fat diet-induced metabolic dysfunction-associated fatty liver disease. Biomed. Pharmacother. 2021, 142, 112092. [Google Scholar] [CrossRef]
- Miah, P.; Mohona, S.B.S.; Rahman, M.M.; Subhan, N.; Khan, F.; Hossain, H.; Sharker, S.M.; Alam, M.A. Supplementation of cumin seed powder prevents oxidative stress, hyperlipidemia and non-alcoholic fatty liver in high fat diet fed rats. Biomed. Pharmacother. 2021, 141, 111908. [Google Scholar] [CrossRef]
- Yu, Y.; Ye, S.M.; Liu, D.Y.; Yang, L.Q. AP39 ameliorates high fat diet-induced liver injury in young rats via alleviation of oxidative stress and mitochondrial impairment. Exp. Anim. 2021, 70, 553–562. [Google Scholar] [CrossRef]
- Al-Harbi, L.N.; Alshammari, G.M.; Al-Dossari, A.M.; Subash-Babu, P.; Binobead, M.A.; Alhussain, M.H.; AlSedairy, S.A.; Al-Nouri, D.M.; Shamlan, G. Beta vulgaris L. (Beetroot) Methanolic Extract Prevents Hepatic Steatosis and Liver Damage in T2DM Rats by Hypoglycemic, Insulin-Sensitizing, Antioxidant Effects, and Upregulation of PPARα. Biology 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.K.; Pandey, S.; Tomar, R.; Sahoo, J.; Kumar, D.; Jangra, A. Therapeutic potential of policosanol in the concurrent management of dyslipidemia and non-alcoholic fatty liver disease. Future J. Pharm. Sci. 2022, 8, 11. [Google Scholar] [CrossRef]
- Sivaraj, R.; Jaikumar, S.; Sengottuvelu, S. Zingiberene attenuates high fat diet–induced non-alcoholic fatty liver disease through suppression of lipogenesis and oxidative stress in rats. Comp. Clin. Pathol. 2022, 31, 201–209. [Google Scholar] [CrossRef]
- Sabir, U.; Irfan, H.M.; Alamgeer; Ullah, A.; Althobaiti, Y.S.; Asim, M.H. Reduction of Hepatic Steatosis, Oxidative Stress, Inflammation, Ballooning and Insulin Resistance After Therapy with Safranal in NAFLD Animal Model: A New Approach. J. Inflamm. Res. 2022, 15, 1293–1316. [Google Scholar] [CrossRef]
- Shatoor, A.S.; Al Humayed, S.; Almohiy, H.M. Astaxanthin attenuates hepatic steatosis in high-fat diet-fed rats by suppressing microRNA-21 via transactivation of nuclear factor erythroid 2-related factor 2. J. Physiol. Biochem. 2022, 78, 151–168. [Google Scholar] [CrossRef]
- Ommati, M.M.; Li, H.; Jamshidzadeh, A.; Khoshghadam, F.; Retana-Márquez, S.; Lu, Y.; Farshad, O.; Nategh Ahmadi, M.H.; Gholami, A.; Heidari, R. The crucial role of oxidative stress in non-alcoholic fatty liver disease-induced male reproductive toxicity: The ameliorative effects of Iranian indigenous probiotics. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 247–265. [Google Scholar] [CrossRef]
- Hazem, R.M.; Ibrahim, A.Z.; Ali, D.A.; Moustafa, Y.M. Dapagliflozin improves steatohepatitis in diabetic rats via inhibition of oxidative stress and inflammation. Int. Immunopharmacol. 2022, 104, 108503. [Google Scholar] [CrossRef]
- Zhang, J.K.; Zhou, X.L.; Wang, X.Q.; Zhang, J.X.; Yang, M.L.; Liu, Y.P.; Cao, J.X.; Cheng, G.G. Que Zui tea ameliorates hepatic lipid accumulation and oxidative stress in high fat diet induced nonalcoholic fatty liver disease. Food Res. Int. 2022, 156, 111196. [Google Scholar] [CrossRef]
- Biao, Y.; Chen, J.; Liu, C.; Wang, R.; Han, X.; Li, L.; Zhang, Y. Protective Effect of Danshen Zexie Decoction Against Non-Alcoholic Fatty Liver Disease Through Inhibition of ROS/NLRP3/IL-1β Pathway by Nrf2 Signaling Activation. Front. Pharmacol. 2022, 13, 877924. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, B.; Deng, H.; Zhang, M.; Qiao, Y.; Liu, Q.; Shi, C.; Li, Y. Isolation and Quantification of the Hepatoprotective Flavonoids From Scleromitron diffusum (Willd.) R. J. Wang With Bio-Enzymatic Method Against NAFLD by UPLC-MS/MS. Front. Pharmacol. 2022, 13, 890148. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S. Exogenous administration of unacylated ghrelin attenuates hepatic steatosis in high-fat diet-fed rats by modulating glucose homeostasis, lipogenesis, oxidative stress, and endoplasmic reticulum stress. Biomed. Pharmacother. 2022, 151, 113095. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.M. Zingerone ameliorates non-alcoholic fatty liver disease in rats by activating AMPK. J. Food Biochem. 2022, 46, e14149. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Elesawy, A.E.; Sobh, M.S.; Elnagar, G.M. Ellagic acid ameliorates high fructose-induced hyperuricemia and non-alcoholic fatty liver in Wistar rats: Focusing on the role of C1q/tumor necrosis factor-related protein-3 and ATP citrate lyase. Life Sci. 2022, 305, 120751. [Google Scholar] [CrossRef]
- Jiayao, Y.; Dongqing, T.; Wei, M.A.; Song, L.; Yan, L.; Lei, S.; Shu, Z.; Chenyu, L.I.; Nianlong, D.U. Protective effects and mechanisms of Lizhong decoction against non-alcoholic fatty liver disease in a rat model. J. Tradit. Chin. Med. 2022, 42, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.H.; Alzaim, I.; El-Mallah, A.; Aly, R.G.; El-Yazbi, A.F.; Wahid, A. Metformin, pioglitazone, dapagliflozin and their combinations ameliorate manifestations associated with NAFLD in rats via anti-inflammatory, anti-fibrotic, anti-oxidant and anti-apoptotic mechanisms. Life Sci. 2022, 308, 120956. [Google Scholar] [CrossRef] [PubMed]
- Vornoli, A.; Vizzarri, F.; Della Croce, C.M.; Grande, T.; Palazzo, M.; Árvay, J.; Pucci, L.; Gabriele, M.; Matteucci, M.; Paolini, M.; et al. The hypolipidemic, anti-inflammatory and antioxidant effect of Kavolì® aqueous extract, a mixture of Brassica oleracea leaves, in a rat model of NAFLD. Food Chem. Toxicol. 2022, 167, 113261. [Google Scholar] [CrossRef]
- Ruan, L.; Wang, G.; Qing Lv, Z.; Li, S.; Liu, Q.; Ren, Y.; Zhang, Q.; Lu, X.; Wu, R.; Jin, Z. The effect of varied exercise intensity on antioxidant function, aortic endothelial function, and serum lipids in rats with non-alcoholic fatty liver disease. Investig. Clin. 2022, 63, 327–343. [Google Scholar] [CrossRef]
- Deng, A.; Liu, F.; Tang, X.; Wang, Y.; Xie, P.; Yang, Q.; Xiao, B. Water extract from artichoke ameliorates high-fat diet-induced non-alcoholic fatty liver disease in rats. BMC Complement. Med. Ther. 2022, 22, 308. [Google Scholar] [CrossRef]
- Alwadani, A.H.; Almasri, S.A.; Aloud, A.A.; Albadr, N.A.; Alshammari, G.M.; Yahya, M.A. The Synergistic Protective Effect of γ-Oryzanol (OZ) and N-Acetylcysteine (NAC) against Experimentally Induced NAFLD in Rats Entails Hypoglycemic, Antioxidant, and PPARα Stimulatory Effects. Nutrients 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Marschner, R.A.; Roginski, A.C.; Ribeiro, R.T.; Longo, L.; Álvares-da-Silva, M.R.; Wajner, S.M. Uncovering Actions of Type 3 Deiodinase in the Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Cells 2023, 12, 1022. [Google Scholar] [CrossRef]
- Felemban, A.H.; Alshammari, G.M.; Yagoub, A.E.A.; Al-Harbi, L.N.; Alhussain, M.H.; Yahya, M.A. Activation of AMPK Entails the Protective Effect of Royal Jelly against High-Fat-Diet-Induced Hyperglycemia, Hyperlipidemia, and Non-Alcoholic Fatty Liver Disease in Rats. Nutrients 2023, 15, 1471. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Lyu, W.; Lin, Z.; Lu, J.; Geng, Y.; Song, L.; Zhang, H. Quinoa Ameliorates Hepatic Steatosis, Oxidative Stress, Inflammation and Regulates the Gut Microbiota in Nonalcoholic Fatty Liver Disease Rats. Foods 2023, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Campos, A.A.; Ramos-Gómez, M.; De Los Ríos-Arellano, E.A.; Ocampo-Anguiano, P.V.; González-Gallardo, A.; Macotela, Y.; García-Gasca, T.; Ahumada-Solórzano, S.M. Bean Leaves Ameliorate Lipotoxicity in Fatty Liver Disease. Nutrients 2023, 15, 2928. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Sójka, M.; Kosmala, M.; Juśkiewicz, J. Prebiotics Together with Raspberry Polyphenolic Extract Mitigate the Development of Nonalcoholic Fatty Liver Diseases in Zucker Rats. Nutrients 2023, 15, 3115. [Google Scholar] [CrossRef] [PubMed]
- Al Jadani, J.M.; Albadr, N.A.; Alshammari, G.M.; Almasri, S.A.; Alfayez, F.F.; Yahya, M.A. Esculeogenin A, a Glycan from Tomato, Alleviates Nonalcoholic Fatty Liver Disease in Rats through Hypolipidemic, Antioxidant, and Anti-Inflammatory Effects. Nutrients 2023, 15, 4755. [Google Scholar] [CrossRef]
- Song, J.; Ren, L.; Ren, Z.; Ren, X.; Qi, Y.; Qin, Y.; Zhang, X.; Ren, Y.; Li, Y. SIRT1-dependent mitochondrial biogenesis supports therapeutic effects of 4-butyl-polyhydroxybenzophenone compounds against NAFLD. Eur. J. Med. Chem. 2023, 260, 115728. [Google Scholar] [CrossRef] [PubMed]
- Atteia, H.H.; AlFaris, N.A.; Alshammari, G.M.; Alamri, E.; Ahmed, S.F.; Albalwi, R.; Abdel-Sattar, S.A. The Hepatic Antisteatosis Effect of Xanthohumol in High-Fat Diet-Fed Rats Entails Activation of AMPK as a Possible Protective Mechanism. Foods 2023, 12, 4214. [Google Scholar] [CrossRef] [PubMed]
- Kılınç, S.; Şahin, P.; Yığman, Z.; Sevgili, A.M. Topiramate’s effects on normal and fatty liver. Drug Chem. Toxicol. 2024, 47, 729–738. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, J.; Han, J.; Lei, Y.; Cao, Z.; Pan, J.; Pan, Z.; Zhang, Z.; Qu, N.; Luo, H.; et al. Tiaogan Jiejiu Tongluo Formula attenuated alcohol-induced chronic liver injury by regulating lipid metabolism in rats. J. Ethnopharmacol. 2023, 317, 116838. [Google Scholar] [CrossRef]
- Sedik, A.A.; Elgohary, R.; Khalifa, E.; Khalil, W.K.B.; Shafey, H.I.; Shalaby, M.B.; Gouida, M.S.O.; Tag, Y.M. Lauric acid attenuates hepato-metabolic complications and molecular alterations in high-fat diet-induced nonalcoholic fatty liver disease in rats. Toxicol. Mech. Methods. 2024, 34, 454–467. [Google Scholar] [CrossRef]
- Sharma, S.; Gali, S.; Kundu, A.; Park, J.H.; Kim, J.S.; Kim, H.S. Tenovin-1, a Selective SIRT1/2 Inhibitor, Attenuates High-fat Diet-induced Hepatic Fibrosis via Inhibition of HSC Activation in ZDF Rats. Int. J. Biol. Sci. 2024, 20, 3334–3352. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Marschner, R.A.; de Freitas, L.B.R.; de Bona, L.R.; Behrens, L.; Pereira, M.H.M.; de Souza, V.E.G.; Leonhard, L.C.; Zanettini, G.; Pinzon, C.E.; et al. Redefining the Role of Ornithine Aspartate and Vitamin E in Metabolic-Dysfunction-Associated Steatotic Liver Disease through Its Biochemical Properties. Int. J. Mol. Sci. 2024, 25, 6839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Chen, H.W.; Lii, C.K.; Jhuang, J.H.; Huang, C.S.; Li, M.L.; Yao, H.T. A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet. Nutrients 2020, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Li, B.Y.; Meng, J.M.; Gan, R.Y.; Xu, X.Y.; Gu, Y.Y.; Wang, X.H.; Li, H.B. Effects of several tea extracts on nonalcoholic fatty liver disease in mice fed with a high-fat diet. Food Sci. Nutr. 2021, 9, 2954–2967. [Google Scholar] [CrossRef]
- Sukkasem, N.; Chatuphonprasert, W.; Jarukamjorn, K. Hesperidin, a novel candidate for the successful treatment of HFD plus ethanol-induced fatty liver disease in mice. J. Physiol. Pharmacol. 2021, 72, 217–224. [Google Scholar] [CrossRef]
- Kang, H.G.; Bashir, K.M.I.; Kim, K.Y.; Shin, S.; Choi, M.W.; Hong, E.J.; Choi, S.H.; Kim, J.W.; Choi, J.S.; Ku, S.K. Evaluation of Dose-Dependent Obesity and Diabetes-Related Complications of Water Chestnut (Fruit of Trapa japonica) Extracts in Type II Obese Diabetic Mice Induced by 45% Kcal HFD. Medicina 2022, 58, 189. [Google Scholar] [CrossRef]
- Mak, K.K.; Zhang, S.; Chellian, J.; Mohd, Z.; Epemolu, O.; Dinkova-Kostova, A.T.; Balijepalli, M.K.; Pichika, M.R. Swietenine Alleviates Nonalcoholic Fatty Liver Disease in Diabetic Mice via Lipogenesis Inhibition and Antioxidant Mechanisms. Antioxidants 2023, 12, 595. [Google Scholar] [CrossRef]
- Krishnan, U.A.; Viswanathan, P.; Venkataraman, A.C. AMPK activation by AICAR reduces diet induced fatty liver in C57BL/6 mice. Tissue Cell. 2023, 82, 102054. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.X.; Pan, W.S.; Khan, F.U.; Qian, C.; Qi-Li, F.R.; Xu, X. Novel hepatoprotective role of Leonurine hydrochloride against experimental non-alcoholic steatohepatitis mediated via AMPK/SREBP1 signaling pathway. Biomed. Pharmacother. 2019, 110, 571–581. [Google Scholar] [CrossRef]
- Deng, P.; Barney, J.; Petriello, M.C.; Morris, A.J.; Wahlang, B.; Hennig, B. Hepatic metabolomics reveals that liver injury increases PCB 126-induced oxidative stress and metabolic dysfunction. Chemosphere 2019, 217, 140–149. [Google Scholar] [CrossRef]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Gao, L.; Ding, T. IDH2 protects against nonalcoholic steatohepatitis by alleviating dyslipidemia regulated by oxidative stress. Biochem. Biophys. Res. Commun. 2019, 514, 593–600. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Carvalho, M.M.; Lage, N.N.; de Souza Paulino, A.H.; Pereira, R.R.; de Almeida, L.T.; da Silva, T.F.; de Brito Magalhães, C.L.; de Lima, W.G.; Silva, M.E.; Pedrosa, M.L.; et al. Effects of açai on oxidative stress, ER stress, and inflammation-related parameters in mice with high fat diet-fed induced NAFLD. Sci. Rep. 2019, 9, 8107. [Google Scholar] [CrossRef]
- Lee, D.H.; Jung, Y.Y.; Park, M.H.; Jo, M.R.; Han, S.B.; Yoon, D.Y.; Roh, Y.S.; Hong, J.T. Peroxiredoxin 6 Confers Protection Against Nonalcoholic Fatty Liver Disease Through Maintaining Mitochondrial Function. Antioxid. Redox. Signal. 2019, 31, 387–402. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef]
- Cui, Y.; Chang, R.; Zhang, T.; Zhou, X.; Wang, Q.; Gao, H.; Hou, L.; Loor, J.J.; Xu, C. Chinese Herbal Formula (CHF03) Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) Through Inhibiting Lipogenesis and Anti-Oxidation Mechanisms. Front. Pharmacol. 2019, 10, 1190. [Google Scholar] [CrossRef]
- Yang, H.; Yang, T.; Heng, C.; Zhou, Y.; Jiang, Z.; Qian, X.; Du, L.; Mao, S.; Yin, X.; Lu, Q. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother. Res. 2019, 33, 3140–3152. [Google Scholar] [CrossRef]
- Qi, J.; Kim, J.W.; Zhou, Z.; Lim, C.W.; Kim, B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation-Mediated Cell Death in Mice. Am. J. Pathol. 2020, 190, 723. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhang, L.; Yuan, Y.; Yu, D. Codonopsis lanceolata polysaccharide CLPS alleviates high fat/high sucrose diet-induced insulin resistance via anti-oxidative stress. Int. J. Biol. Macromol. 2020, 145, 944–949. [Google Scholar] [CrossRef]
- Ke, W.; Wang, P.; Wang, X.; Zhou, X.; Hu, X.; Chen, F. Dietary Platycodon grandiflorus Attenuates Hepatic Insulin Resistance and Oxidative Stress in High-Fat-Diet Induced Non-Alcoholic Fatty Liver Disease. Nutrients 2020, 12, 480. [Google Scholar] [CrossRef]
- Simon, J.; Nuñez-García, M.; Fernández-Tussy, P.; Barbier-Torres, L.; Fernández-Ramos, D.; Gómez-Santos, B.; Buqué, X.; Lopitz-Otsoa, F.; Goikoetxea-Usandizaga, N.; Serrano-Macia, M.; et al. Targeting Hepatic Glutaminase 1 Ameliorates Non-alcoholic Steatohepatitis by Restoring Very-Low-Density Lipoprotein Triglyceride Assembly. Cell Metab. 2020, 31, 605–622.e10. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Y.; Zhao, Y.; Li, M.; Guo, L. Targeting DUSP7 signaling alleviates hepatic steatosis, inflammation and oxidative stress in high fat diet (HFD)-fed mice via suppression of TAK1. Free Radic. Biol. Med. 2020, 153, 140–158. [Google Scholar] [CrossRef]
- Ore, A.; Ugbaja, R.N.; Adeogun, A.I.; Akinloye, O.A. An albino mouse model of nonalcoholic fatty liver disease induced using high-fat liquid “Lieber-DeCarli” diet: A preliminary investigation. Porto Biomed. J. 2020, 5, e071. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Lawrence, L.; Mundkur, L. Novel Combinatorial Regimen of Garcinol and Curcuminoids for Non-alcoholic Steatohepatitis (NASH) in Mice. Sci. Rep. 2020, 10, 7440. [Google Scholar] [CrossRef]
- Bucher, S.; Begriche, K.; Catheline, D.; Trak-Smayra, V.; Tiaho, F.; Coulouarn, C.; Pinon, G.; Lagadic-Gossmann, D.; Rioux, V.; Fromenty, B. Moderate chronic ethanol consumption exerts beneficial effects on nonalcoholic fatty liver in mice fed a high-fat diet: Possible role of higher formation of triglycerides enriched in monounsaturated fatty acids. Eur. J. Nutr. 2020, 59, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Sheng, H.; Bai, Y.F.; Weng, Y.Y.; Fan, X.Y.; Lou, L.J.; Zhang, F. Neohesperidin enhances PGC-1α-mediated mitochondrial biogenesis and alleviates hepatic steatosis in high fat diet fed mice. Nutr. Diabetes 2020, 10, 27. [Google Scholar] [CrossRef]
- Yang, F.; Huang, P.; Shi, L.; Liu, F.; Tang, A.; Xu, S. Phoenixin 14 Inhibits High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Experimental Mice. Drug Des. Dev. Ther. 2020, 14, 3865–3874. [Google Scholar] [CrossRef]
- Hu, D.; Yang, W.; Mao, P.; Cheng, M. Combined Amelioration of Prebiotic Resveratrol and Probiotic Bifidobacteria on Obesity and Nonalcoholic Fatty Liver Disease. Nutr. Cancer 2021, 73, 652–661. [Google Scholar] [CrossRef] [PubMed]
- An, M.Y.; Lee, S.R.; Hwang, H.J.; Yoon, J.G.; Lee, H.J.; Cho, J.A. Antioxidant and Anti-Inflammatory Effects of Korean Black Ginseng Extract through ER Stress Pathway. Antioxidants 2021, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fan, Y.; Loor, J.J.; Liang, Y.; Lv, H.; Sun, X.; Jia, H.; Xu, C. Aloin protects mice from diet-induced non-alcoholic steatohepatitis via activation of Nrf2/HO-1 signaling. Food Funct. 2021, 12, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Huang, R.; Lin, D.; Wang, Y.; Yang, X.; Huang, X.; Zheng, B.; Chen, Z.; Huang, Y.; Wang, X.; et al. Resveratrol Improves Liver Steatosis and Insulin Resistance in Non-alcoholic Fatty Liver Disease in Association with the Gut Microbiota. Front. Microbiol. 2021, 12, 611323. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, B.; Zhang, L.; Jin, X.; Wang, K.; Xu, W.; Zhang, B.; Wang, H. The protective effects of trelagliptin on high-fat diet-induced nonalcoholic fatty liver disease in mice. J. Biochem. Mol. Toxicol. 2021, 35, e22696. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Xie, Z.; Li, E.W.; Yuan, Y.; Fu, Y.; Wang, P.; Zhang, X.; Qiao, Y.; Xu, J.; Hölscher, C.; et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J. Nat. Med. 2021, 75, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Z.; Xu, J.; Zhang, J.; Sun, R.; Zhou, J.; Lu, Y.; Gong, Z.; Huang, J.; Shen, X.; et al. Improving the ameliorative effects of berberine and curcumin combination via dextran-coated bilosomes on non-alcohol fatty liver disease in mice. J. Nanobiotechnology 2021, 19, 230. [Google Scholar] [CrossRef]
- Chen, S.; Che, S.; Li, S.; Ruan, Z. The combined impact of decabromodiphenyl ether and high fat exposure on non-alcoholic fatty liver disease in vivo and in vitro. Toxicology 2021, 464, 153015. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.J.; Tarantino, G.; Iezzi, A.; Alisi, A.; Balsano, C. Copper-catalyzed dicarbonyl stress in NAFLD mice: Protective effects of Oleuropein treatment on liver damage. Nutr. Metab. 2022, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Manna, K.; Das Saha, K. Melatonin Suppresses NLRP3 Inflammasome Activation via TLR4/NF-κB and P2X7R Signaling in High-Fat Diet-Induced Murine NASH Model. J. Inflamm. Res. 2022, 15, 3235–3258. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lian, C.F.; Sun, Q.W.; Wang, T.T.; Liu, Y.Y.; Ye, J.; Gao, L.L.; Yang, Y.F.; Liu, S.N.; Shen, Z.F.; et al. Ramulus Mori (Sangzhi) Alkaloids Alleviate High-Fat Diet-Induced Obesity and Nonalcoholic Fatty Liver Disease in Mice. Antioxidants 2022, 11, 905. [Google Scholar] [CrossRef]
- Arai, N.; Miura, K.; Aizawa, K.; Sekiya, M.; Nagayama, M.; Sakamoto, H.; Maeda, H.; Morimoto, N.; Iwamoto, S.; Yamamoto, H. Probiotics suppress nonalcoholic steatohepatitis and carcinogenesis progression in hepatocyte-specific PTEN knockout mice. Sci. Rep. 2022, 12, 16206. [Google Scholar] [CrossRef]
- Ye, H.; Ma, S.; Qiu, Z.; Huang, S.; Deng, G.; Li, Y.; Xu, S.; Yang, M.; Shi, H.; Wu, C.; et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J. Ethnopharmacol. 2022, 296, 115457. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Song, Y.; Luo, Y.; Song, J.; Li, C.; Yang, S.; Guo, J.; Yu, J.; Zhang, X. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate experimental non-alcoholic steatohepatitis via Nrf2/NQO-1 pathway. Free Radic. Biol. Med. 2022, 192, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhuge, A.; Wang, K.; Xia, J.; Wang, Q.; Han, S.; Shen, J.; Li, L. Obeticholic acid and ferrostatin-1 differentially ameliorate non-alcoholic steatohepatitis in AMLN diet-fed ob/ob mice. Front. Pharmacol. 2022, 13, 1081553. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, H.; Miao, J.; Sheng, Q.; Xu, J.; Gao, Z.; Zhang, X.; Song, Y.; Chen, K. The natural flavone acacetin protects against high-fat diet-induced lipid accumulation in the liver via the endoplasmic reticulum stress/ferroptosis pathway. Biochem. Biophys. Res. Commun. 2023, 640, 183–191. [Google Scholar] [CrossRef]
- Ghrayeb, A.; Agranovich, B.; Peled, D.; Finney, A.C.; Abramovich, I.; Garcia, J.F.; Traylor, J.; Drucker, S.; Fernandes, S.I.; Weissman, N.; et al. Fatty liver-mediated glycine restriction impairs glutathione synthesis and causes hypersensitization to acetaminophen. bioRxiv 2023. [Google Scholar] [CrossRef]
- Abulikemu, A.; Zhao, X.; Xu, H.; Li, Y.; Ma, R.; Yao, Q.; Wang, J.; Sun, Z.; Li, Y.; Guo, C. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox. Biol. 2023, 59, 102569. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Jiang, Y.; Wu, D.; Cai, J.; Jiang, Z.; Zhou, Z.; Liu, L.; Ling, Q.; Wang, Q.; Zhao, G. Atractylodin alleviates nonalcoholic fatty liver disease by regulating Nrf2-mediated ferroptosis. Heliyon 2023, 9, e18321. [Google Scholar] [CrossRef]
- Yuan, X.; Li, L.; Zhang, Y.; Ai, R.; Li, D.; Dou, Y.; Hou, M.; Zhao, D.; Zhao, S.; Nan, Y. Heme oxygenase 1 alleviates nonalcoholic steatohepatitis by suppressing hepatic ferroptosis. Lipids Health Dis. 2023, 22, 99. [Google Scholar] [CrossRef]
- Saha, M.; Das, S.; Manna, K.; Saha, K.D. Melatonin targets ferroptosis through bimodal alteration of redox environment and cellular pathways in NAFLD model. Biosci. Rep. 2023, 43, BSR20230128. [Google Scholar] [CrossRef]
- Spooner, M.H.; Garcia-Jaramillo, M.; Apperson, K.D.; Löhr, C.V.; Jump, D.B. Time course of western diet (WD) induced nonalcoholic steatohepatitis (NASH) in female and male Ldlr-/- mice. PLoS ONE 2023, 18, e0292432. [Google Scholar] [CrossRef]
- You, T.; Li, Y.; Li, B.; Wu, S.; Jiang, X.; Fu, D.; Xin, J.; Huang, Y.; Jin, L.; Hu, C. Caveolin-1 protects against liver damage exacerbated by acetaminophen in non-alcoholic fatty liver disease by inhibiting the ERK/HIF-1α pathway. Mol. Immunol. 2023, 163, 104–115. [Google Scholar] [CrossRef]
- Jiang, T.; Xiao, Y.; Zhou, J.; Luo, Z.; Yu, L.; Liao, Q.; Liu, S.; Qi, X.; Zhang, H.; Hou, M.; et al. Arbutin alleviates fatty liver by inhibiting ferroptosis via FTO/SLC7A11 pathway. Redox. Biol. 2023, 69, 102974. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yue, M.; Cheng, Y.; Sullivan, M.A.; Chen, W.; Yu, H.; Li, F.; Wu, S.; Lv, Y.; Zhai, X.; et al. Naringenin prevents non-alcoholic steatohepatitis by modulating the host metabolome and intestinal microbiome in MCD diet-fed mice. Food Sci. Nutr. 2023, 11, 7826–7840. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Dong, L.; Wang, X.; Qin, Z.; Ma, Y.; Ke, X.; Li, Y.; Wang, Q.; Mi, Y.; Lyu, Q.; et al. Perilipin 5 regulates hepatic stellate cell activation and high-fat diet-induced non-alcoholic fatty liver disease. Anim. Model Exp. Med. 2024, 7, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Y.; Kim, K.; Kwon, H.H.; Leem, J.; Song, J.E. 6-Shogaol Ameliorates Liver Inflammation and Fibrosis in Mice on a Methionine- and Choline-Deficient Diet by Inhibiting Oxidative Stress, Cell Death, and Endoplasmic Reticulum Stress. Molecules 2024, 29, 419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, M.Y.; Wang, G.D.; Lv, Q.Y.; Huang, Y.Q.; Zhang, P.; Wang, W.; Zhang, Y.; Bai, Y.P.; Guo, L.Q. Metformin improves nonalcoholic fatty liver disease in db/db mice by inhibiting ferroptosis. Eur. J. Pharmacol. 2024, 966, 176341. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Wen, J.; Li, G.; Huai, X.; Duan, Y.; Ni, F.; Fu, J.; Li, M.; Li, L.; et al. A novel Alisma orientale extract alleviates non-alcoholic steatohepatitis in mice via modulation of PPARα signaling pathway. Biomed. Pharmacother. 2024, 176, 116908. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Y.M.; Zhang, M.Q.; Han, Y.; Zhang, J.P.; Hu, C.Q. Microarray Expression Profiling and Raman Spectroscopy Reveal Anti-Fatty Liver Action of Berberine in a Diet-Induced Larval Zebrafish Model. Front. Pharmacol. 2020, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Lin, H.; Yan, W.; Deng, G.; Ye, H.; Shi, H.; Wu, C.; Ma, G.; Xu, S.; et al. Limonin Alleviates Non-alcoholic Fatty Liver Disease by Reducing Lipid Accumulation, Suppressing Inflammation and Oxidative Stress. Front. Pharmacol. 2022, 12, 801730. [Google Scholar] [CrossRef]
- Hajinezhad, M.R.; Mokhtarpour, A.; Mirheidari, A. Hypolipidemic and antioxidant effects of aqueous ethanolic extract from Moringa peregrina leaves on high-fat diet-induced fatty liver disease in rabbits. Comp. Clin. Path. 2023, 32, 617–628. [Google Scholar] [CrossRef]
- Qu, P.; Rom, O.; Li, K.; Jia, L.; Gao, X.; Liu, Z.; Ding, S.; Zhao, M.; Wang, H.; Chen, S.; et al. DT-109 ameliorates nonalcoholic steatohepatitis in nonhuman primates. Cell Metab. 2023, 35, 742–757.e10. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP (Qualitative Studies) Checklist. [Online]. 2023. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 1 July 2024).
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P.; Kakkar, P. Berbamine Induced AMPK Activation Regulates mTOR/SREBP-1c Axis and Nrf2/ARE Pathway to Allay Lipid Accumulation and Oxidative Stress in Steatotic HepG2 Cells. Eur. J. Pharmacol. 2020, 882, 173244. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Choi, J.O.; Park, C.; Kim, S.-H.; Kim, D.Y. Water Extract of Artemisia Annua L. Exhibits Hepatoprotective Effects Through Improvement of Lipid Accumulation and Oxidative Stress-Induced Cytotoxicity. J. Med. Food 2020, 23, 1312–1322. [Google Scholar] [CrossRef]
- Drygalski, K.; Siewko, K.; Chomentowski, A.; Odrzygóźdź, C.; Zalewska, A.; Kretowski, A.; Maciejczyk, M. Phloroglucinol Strengthens the Antioxidant Barrier and Reduces Oxidative/Nitrosative Stress in Nonalcoholic Fatty Liver Disease (NAFLD). Oxid. Med. Cell. Longev. 2021, 2021, 8872702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, X.; Gu, Y.; Wang, M.; Guo, S.; Liu, J.; Zhang, X.; Zhao, Z.; Qian, B.; Yan, Y.; et al. Hepatoprotection of Lycii Fructus Polysaccharide against Oxidative Stress in Hepatocytes and Larval Zebrafish. Oxid. Med. Cell. Longev. 2021, 2021, 3923625. [Google Scholar] [CrossRef]
- Xu, Y.; Ke, H.; Li, Y.; Xie, L.; Su, H.; Xie, J.; Mo, J.; Chen, W.; Chen, W. Malvidin-3-O-Glucoside from Blueberry Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Transcription Factor EB-Mediated Lysosomal Function and Activating the Nrf2/ARE Signaling Pathway. J. Agric. Food Chem. 2021, 69, 4663–4673. [Google Scholar] [CrossRef]
- Dhami-Shah, H.; Vaidya, R.; Talwadekar, M.; Shaw, E.; Udipi, S.; Kolthur-Seetharam, U.; Vaidya, A.D.B. Intervention by Picroside II on FFAs Induced Lipid Accumulation and Lipotoxicity in HepG2 Cells. J. Ayurveda Integr. Med. 2021, 12, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, B.; Zhang, Y.; Yan, L.; He, Y.; Hu, L.; Xu, Q.; Li, Q.; Dai, X.; Kuang, Q.; et al. Carminic acid mitigates fructose-triggered hepatic steatosis by inhibition of oxidative stress and inflammatory reaction. Biomed. Pharmacother. 2022, 145, 112404. [Google Scholar] [CrossRef]
- Cheng, X.R.; Tu, P.H.; Dong, W.L.; Yu, B.T.; Xia, S.F.; Muskat, M.N.; Guan, B. Electrophilic thymol isobutyrate from Inula nervosa Wall. (Xiaoheiyao) ameliorates steatosis in HepG2 cells via Nrf2 activation. J. Funct. Foods 2022, 88, 11. [Google Scholar] [CrossRef]
- Gao, W.; Xu, B.; Zhang, Y.; Liu, S.; Duan, Z.; Chen, Y.; Zhang, X. Baicalin Attenuates Oxidative Stress in a Tissue-Engineered Liver Model of NAFLD by Scavenging Reactive Oxygen Species. Nutrients 2022, 14, 541. [Google Scholar] [CrossRef]
- Lan, T.; Hu, Y.; Hu, F.; Li, H.; Chen, Y.; Zhang, J.; Yu, Y.; Jiang, S.; Weng, Q.; Tian, S.; et al. Hepatocyte glutathione S-transferase mu 2 prevents non-alcoholic steatohepatitis by suppressing ASK1 signaling. J. Hepatol. 2022, 76, 407–419. [Google Scholar] [CrossRef]
- Balkrishna, A.; Gohel, V.; Kumari, P.; Manik, M.; Bhattacharya, K.; Dev, R.; Varshney, A. Livogrit Prevents Methionine-Cystine Deficiency Induced Nonalcoholic Steatohepatitis by Modulation of Steatosis and Oxidative Stress in Human Hepatocyte-Derived Spheroid and in Primary Rat Hepatocytes. Bioengineered 2022, 13, 10811–10826. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Xu, K.; Li, Y.; Riaz, F.; Lu, K.; Chen, Q.; Du, X.; Wu, L.; Cao, D.; et al. Cholesterol-induced leucine aminopeptidase 3 (LAP3) upregulation inhibits cell autophagy in pathogenesis of NAFLD. Aging 2022, 14, 3259–3275. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Nair, J.; Sinh, A.; Shivangi; Velpandian, T.; Tripathi, R.; Mathur, R. Guava Leaf Extract Suppresses Fructose Mediated Non-Alcoholic Fatty Liver Disease in Growing Rats. Diabetes Metab. Syndr. Obes. 2022, 15, 2827–2845. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Shan, S.; Yang, D.; Zhang, H.; Sun, Y.; Li, Z. Peonidin-3-O-Glucoside from Purple Corncob Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Mitochondrial and Lysosome Functions to Reduce Oxidative Stress and Inflammation. Nutrients 2023, 15, 372. [Google Scholar] [CrossRef]
- Liu, H.; Yan, J.; Guan, F.; Jin, Z.; Xie, J.; Wang, C.; Liu, M.; Liu, J. Zeaxanthin prevents ferroptosis by promoting mitochondrial function and inhibiting the p53 pathway in free fatty acid-induced HepG2 cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159287. [Google Scholar] [CrossRef]
- Longhitano, L.; Distefano, A.; Musso, N.; Bonacci, P.; Orlando, L.; Giallongo, S.; Tibullo, D.; Denaro, S.; Lazzarino, G.; Ferrigno, J.; et al. (+)-Lipoic Acid Reduces Mitochondrial Unfolded Protein Response and Attenuates Oxidative Stress and Aging in an in Vitro Model of Non-Alcoholic Fatty Liver Disease. J. Transl. Med. 2024, 22, 82. [Google Scholar] [CrossRef]
- Salomone, F.; Pipitone, R.M.; Longo, M.; Malvestiti, F.; Amorini, A.M.; Distefano, A.; Casirati, E.; Ciociola, E.; Iraci, N.; Leggio, L.; et al. SIRT5 rs12216101 T>G variant is associated with liver damage and mitochondrial dysfunction in patients with non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 378. [Google Scholar] [CrossRef]
- Luciano-Mateo, F.; Cabré, N.; Fernández-Arroyo, S.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Rodríguez-Tomàs, E.; Mercado-Gómez, M.; Menendez, J.A.; Camps, J.; Joven, J. Chemokine (C-C motif) ligand 2 gene ablation protects low-density lipoprotein and paraoxonase-1 double deficient mice from liver injury, oxidative stress and inflammation. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 1555–1566. [Google Scholar] [CrossRef]
- Malekinejad, H.; Zeynali-Moghaddam, S.; Rezaei-Golmisheh, A.; Alenabi, A.; Malekinejad, F.; Alizadeh, A.; Shafie-Irannejad, V. Lupeol attenuated the NAFLD and PCOS-induced metabolic, oxidative, hormonal, histopathological, and molecular injuries in mice. Res. Pharm. Sci. 2023, 18, 551–565. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, J.; Dan, L.; Xie, Y.; Sun, Y.; Li, X.; Larsson, S.C. Homocysteine, folate, and nonalcoholic fatty liver disease: A systematic review with meta-analysis and Mendelian randomization investigation. Am. J. Clin. Nutr. 2022, 116, 1595–1609. [Google Scholar] [CrossRef]
- Świderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic. Res. 2019, 53, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; van den Berg, E.H.; Gansevoort, R.T.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; et al. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef]
- Masarone, M.; Troisi, J.; Aglitti, A.; Torre, P.; Colucci, A.; Dallio, M.; Federico, A.; Balsano, C.; Persico, M. Untargeted metabolomics as a diagnostic tool in NAFLD: Discrimination of steatosis, steatohepatitis and cirrhosis. Metabolomics 2021, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Azarmehr, N.; Mansourian, M.; Doustimotlagh, A.H. Inactivation of the superoxide dismutase by malondial-dehyde in the nonalcoholic fatty liver disease: A combined molecular docking approach to clinical studies. Arch. Physiol. Biochem. 2021, 127, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Panera, N.; Mosca, A.; Caccamo, R.; Camanni, D.; Crudele, A.; De Stefanis, C.; Alterio, A.; Di Giovamberardino, G.; De Vito, R.; et al. Changes in Total Homocysteine and Glutathione Levels After Laparoscopic Sleeve Gastrectomy in Children with Metabolic-Associated Fatty Liver Disease. Obes. Surg. 2022, 32, 82–89. [Google Scholar] [CrossRef]
- Garcia, C.C.; Piotrkowski, B.; Baz, P.; Poncino, D.; Benavides, J.; Colombato, L.; Toso, M.L.R.; Yantorno, S.; Descalzi, V.; Gondolesi, G.E.; et al. A Decreased Response to Resistin in Mononuclear Leukocytes Contributes to Oxi-dative Stress in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2022, 67, 3006–3016. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Mosca, A.; Crudele, A.; Zaffina, S.; Denaro, M.; Smeriglio, A.; Trombetta, D. The Antioxidant Effects of Hydroxytyrosol and Vitamin E on Pediatric Nonalcoholic Fatty Liver Disease, in a Clinical Trial: A New Treatment? Antioxid. Redox Signal. 2019, 31, 127–133. [Google Scholar] [CrossRef]
- Lee, E.; Lim, Y.; Kwon, S.W.; Kwon, O. Pinitol consumption improves liver health status by reducing oxidative stress and fatty acid accumulation in subjects with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Nutr. Biochem. 2019, 68, 33–41. [Google Scholar] [CrossRef]
- Maharshi, V.; Gupta, P.; Kumar, V.L.; Upadhyay, A.D.; Das, P.; Yadav, R.; Nayak, B.; Kumar, R.; Shalimar. Effect of Helicobacter pylori-eradication therapy on hepatic steatosis in patients with non-alcoholic fatty liver disease: A randomized-controlled pilot study. Gastroenterol. Rep. 2019, 8, 104–110. [Google Scholar] [CrossRef]
- Negri, R.; Trinchese, G.; Carbone, F.; Caprio, M.G.; Stanzione, G.; di Scala, C.; Micillo, T.; Perna, F.; Tarotto, L.; Gelzo, M.; et al. Randomised Clinical Trial: Calorie Restriction Regimen with Tomato Juice Supplementation Ameliorates Oxidative Stress and Preserves a Proper Immune Surveillance Modulating Mitochondrial Bioenergetics of T-Lymphocytes in Obese Children Affected by Non-Alcoholic Fatty Liver Disease (NAFLD). J. Clin. Med. 2020, 9, 141. [Google Scholar] [CrossRef]
- Chong, P.L.; Laight, D.; Aspinall, R.J.; Higginson, A.; Cummings, M.H. A randomised placebo-controlled trial of VSL#3® probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 144. [Google Scholar] [CrossRef]
- Yurtdaş, G.; Akbulut, G.; Baran, M.; Yılmaz, C. The effects of Mediterranean diet on hepatic steatosis, oxidative stress, and inflammation in adolescents with non-alcoholic fatty liver disease: A randomized controlled trial. Pediatr. Obes. 2022, 17, e12872. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, T.; Zarban, A.; Hooshyar, R.; Salmani, F.; Tajik, H. Improvement of thiol groups and total antioxidant capacity in patients with non-alcoholic fatty liver after treatment with pioglitazone. Arch. Physiol. Biochem. 2022, 128, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Argelich, E.; Casares, M.; Ugarriza, L.; Llompart, I.; Tur, J.A.; Sureda, A. Impact of Adherence to the Mediterranean Diet on Antioxidant Status and Metabolic Parameters in NAFLD Patients: A 24-Month Lifestyle Intervention Study. Antioxidants 2024, 13, 480. [Google Scholar] [CrossRef]
- Li, L.; Fu, J.; Liu, D.; Sun, J.; Hou, Y.; Chen, C.; Shao, J.; Wang, L.; Wang, X.; Zhao, R.; et al. Hepatocyte-specific Nrf2 deficiency mitigates high-fat diet-induced hepatic steatosis: Involvement of reduced PPARγ expression. Redox Biol. 2020, 30, 101412. [Google Scholar] [CrossRef]
- Hunter, H.; de Gracia Hahn, D.; Duret, A.; Im, Y.R.; Cheah, Q.; Dong, J.; Fairey, M.; Hjalmarsson, C.; Li, A.; Lim, H.K.; et al. Weight Loss, Insulin Resistance, and Study Design Confound Results in a Meta-Analysis of Animal Models of Fatty Liver. eLife 2020, 9, e56573. [Google Scholar] [CrossRef]
- Monostori, P.; Wittmann, G.; Karg, E.; Túri, S. Determination of glutathione and glutathione disulfide in biological samples: An in-depth review. J. Chromatogr. B 2009, 877, 3331–3346. [Google Scholar] [CrossRef]
- Camera, E.; Picardo, M. Analytical methods to investigate glutathione and related compounds in biological and pathological processes. J. Chromatogr. B 2002, 781, 181–206. [Google Scholar] [CrossRef]
- Gusenbauer, M.; Haddaway, N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).