Correction of Multispectral Singlet Oxygen Luminescent Dosimetry (MSOLD) for Tissue Optical Properties in Photofrin-Mediated Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
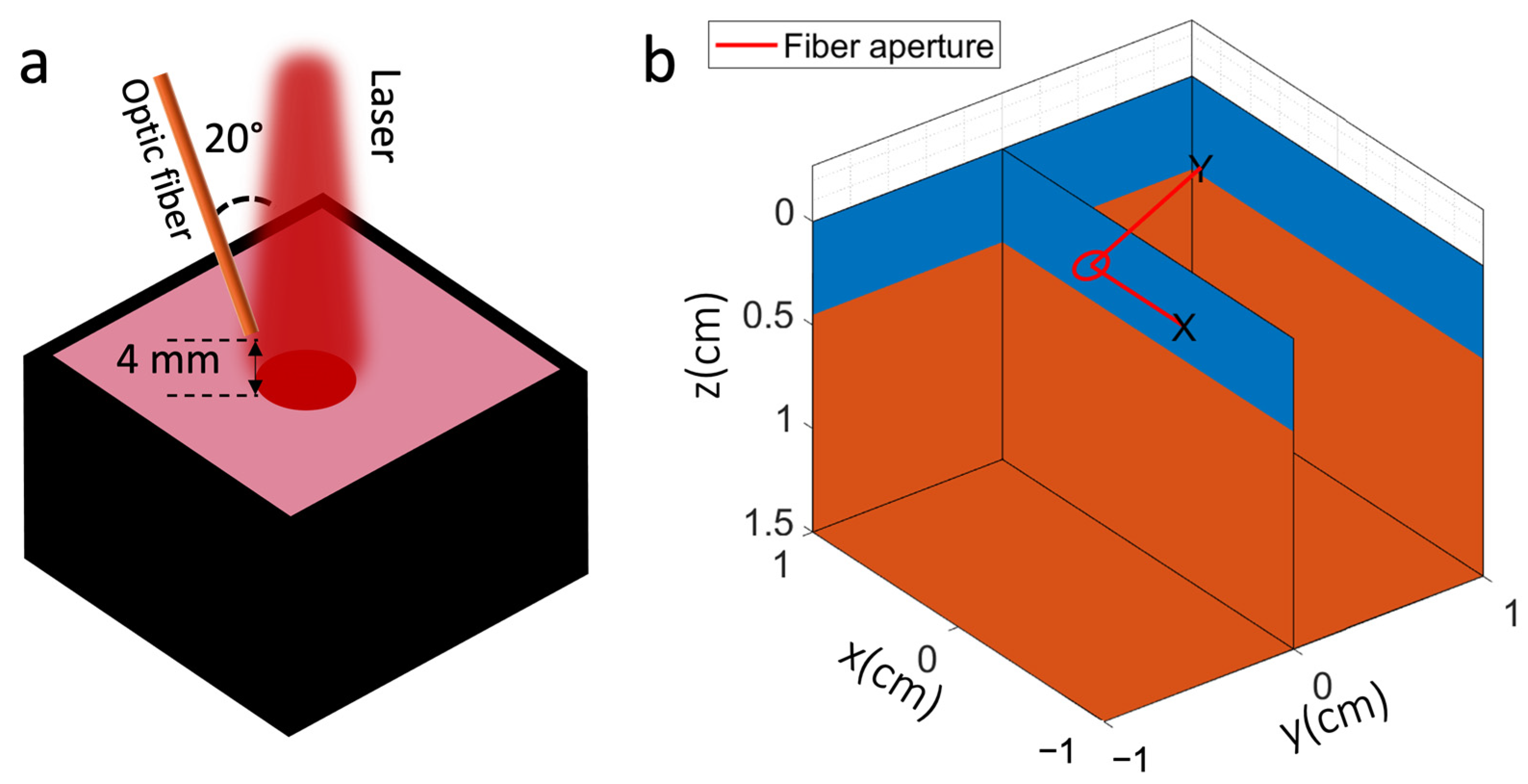
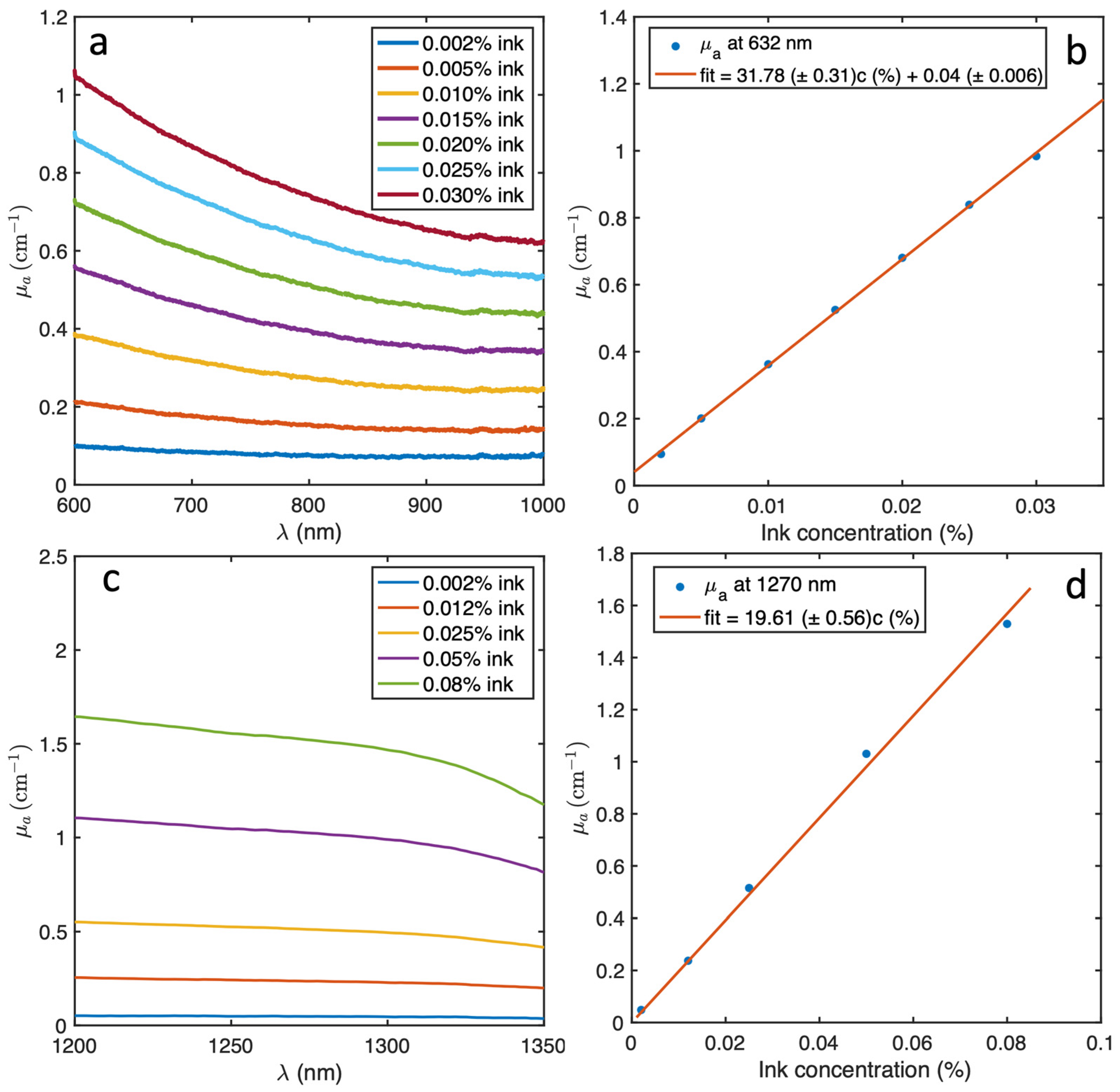
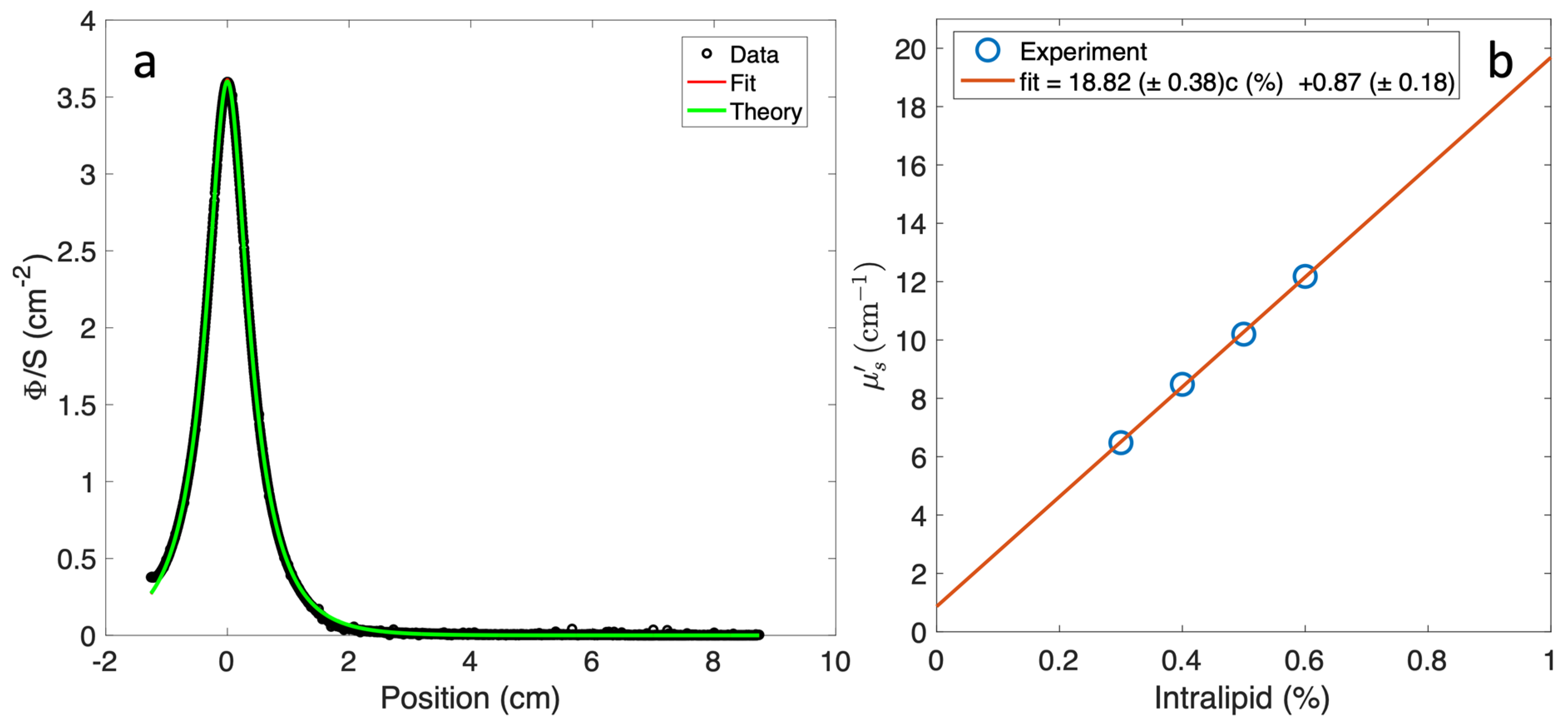
2.1. Optical Properties Measurement for Liquid Phantom
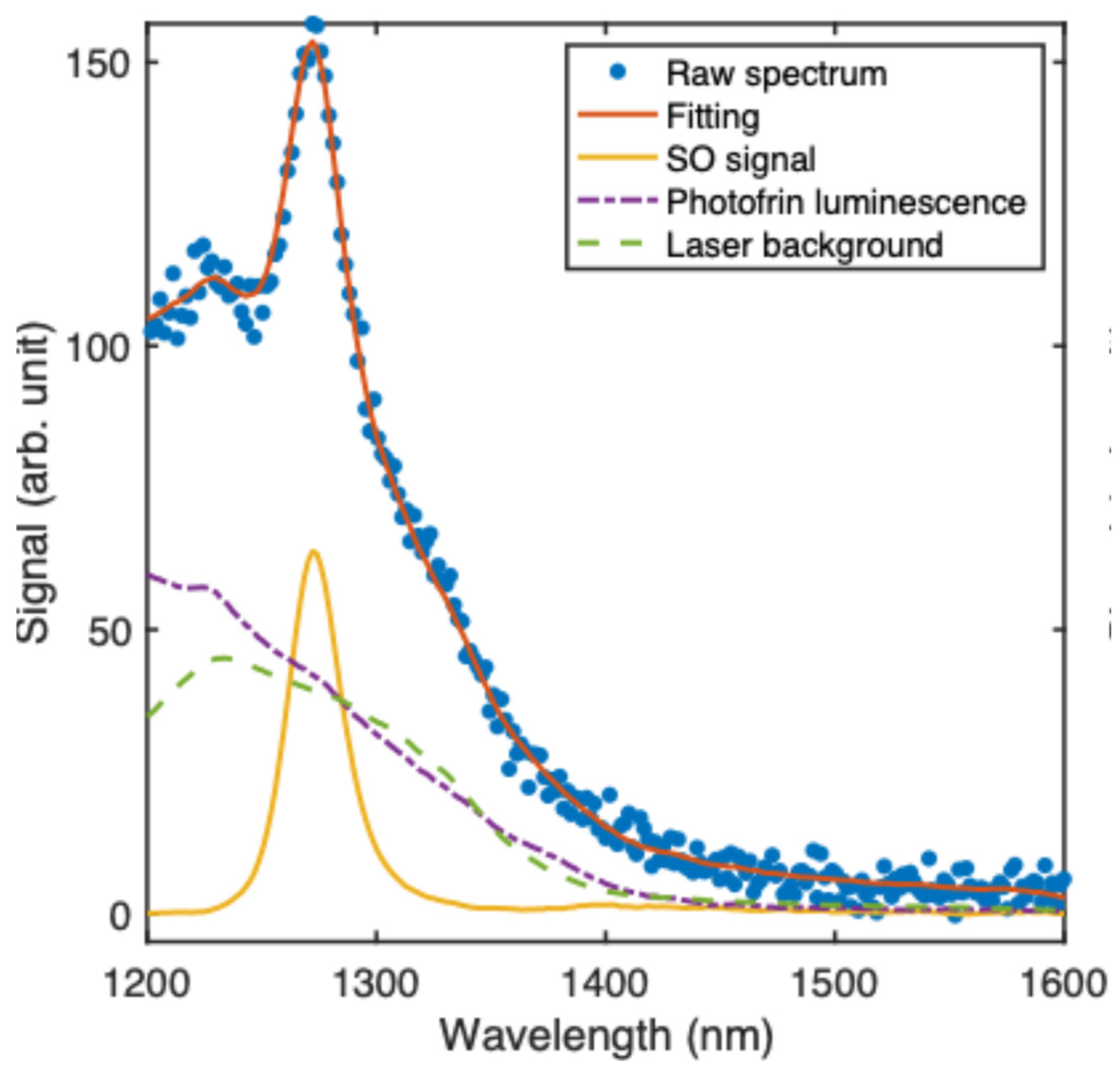
2.2. Experimental Set-Up for Singlet Oxygen Detection
2.3. Monte Carlo Modeling of Singlet Oxygen Signal
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moritz, T.J.; Zhao, Y.; Hinds, M.F.; Gunn, J.R.; Shell, J.R.; Pogue, B.W.; Davis, S.J. Multispectral singlet oxygen and photosensitizer luminescence dosimeter for continuous photodynamic therapy dose assessment during treatment. J. Biomed. Opt. 2020, 25, 063810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Moritz, T.; Hinds, M.F.; Gunn, J.R.; Shell, J.R.; Pogue, B.W.; Davis, S.J. High optical-throughput spectroscopic singlet oxygen and photosensitizer luminescence dosimeter for monitoring of photodynamic therapy. J. Biophotonics 2021, 14, e202100088. [Google Scholar] [CrossRef]
- Yu, T.C.; Davis, S.J.; Scimone, M.T.; Grimble, J.; Maguluri, G.; Anand, S.; Cheng, C.E.; Maytin, E.; Cao, X.; Pogue, B.W.; et al. High Sensitivity Singlet Oxygen Luminescence Sensor Using Computational Spectroscopy and Solid-State Detector. Diagnostics 2023, 13, 3431. [Google Scholar] [CrossRef] [PubMed]
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct Near-infrared Luminescence Detection of Singlet Oxygen Generated by Photodynamic Therapy in Cells In Vitro and Tissues In Vivo. Photochem. Photobiol. 2002, 75, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Kanofsky, J.R. Quenching of singlet oxygen by human plasma. Photochem. Photobiol. 1990, 51, 299–303. [Google Scholar] [CrossRef]
- Hatz, S.; Lambert, J.D.; Ogilby, P.R. Measuring the lifetime of singlet oxygen in a single cell: Addressing the issue of cell viability. Photochem. Photobiol. Sci. 2007, 6, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; McDonald, J.D.; Schuster, G.B. Lifetime of singlet oxygen in solution directly determined by laser spectroscopy. J. Am. Chem. Soc. 1982, 104, 2065–2067. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.; Ogilby, P.R. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef] [PubMed]
- Nonell, S.; Braslavsky, S.E. Time-resolved singlet oxygen detection. Methods Enzymol. 2000, 319, 37–49. [Google Scholar]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C. Photosensitized singlet oxygen generation and detection: Recent advances and future perspectives in cancer photodynamic therapy. J. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Pogue, B.W.; Patterson, M.S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 2006, 11, 041102. [Google Scholar] [CrossRef]
- Sandell, J.L.; Zhu, T.C. A review of in-vivo optical properties of human tissues and its impact on PDT. J. Biophotonics 2011, 4, 773–787. [Google Scholar] [CrossRef]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef]
- Nilsson, A.M.; Berg, R.; Andersson-Engels, S. Measurements of the optical properties of tissue in conjunction with photodynamic therapy. Appl. Opt. 1995, 34, 4609–4619. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.J.; Marshallsay, L.V.; Ward, A.D. The composition of Photofrin II. J. Photochem. Photobiol. B Biol. 1990, 6, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Lawrence, G.; Kaufman, J.H.; Boyle, D.; Weishaupt, K.R.; Goldfarb, A. Photoradiation in the treatment of recurrent breast carcinoma. J. Natl. Cancer Inst. 1979, 62, 231–237. [Google Scholar]
- Hergert, W.; Wriedt, T. (Eds.) The Mie Theory: Basics and Applications; Springer: Berlin/Heidelberg, Germany, 2012; Volume 169. [Google Scholar]
- Dimofte, A.; Finlay, J.C.; Zhu, T.C. A method for determination of the absorption and scattering properties interstitially in turbid media. Phys. Med. Biol. 2005, 50, 2291–2311. [Google Scholar] [CrossRef]
- Yang, W.; Rastogi, V.; Sun, H.; Sharma, D.; Wilson, B.C.; Hadfield, R.H.; Zhu, T.C. Multispectral singlet oxygen luminescent dosimetry (MSOLD) for Photofrin-mediated photodynamic therapy. Proc. SPIE-Int. Soc. Opt. Eng. 2023, 12359, 1235908. [Google Scholar] [CrossRef]
- Padalkar, M.V.; Pleshko, N. Wavelength-dependent penetration depth of near-infrared radiation into cartilage. Analyst 2015, 140, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Lammertyn, J.; Peirs, A.; De Baerdemaeker, J.; Nicolaı, B. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Marti, D.; Aasbjerg, R.N.; Andersen, R.E.; Hansen, A.K. MCmatlab: An open-source, user-friendly, MATLAB-integrated three-dimensional Monte Carlo light transport solver with heat diffusion and tissue damage. J. Biomed. Opt. 2018, 23, 121622. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical properties of protoporphyrin IX, pyropheophorbide-a, and Photofrin® in different conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, Y.; Li, H.; Chen, H.; Yuan, Z. Recent advances in in situ oxygen-generating and oxygen-replenishing strategies for hypoxic-enhanced photodynamic therapy. Biomater. Sci. 2022, 10, 51–84. [Google Scholar] [CrossRef] [PubMed]
- Torrent, J.; Barrón, V. Diffuse reflectance spectroscopy. In Methods of Soil Analysis Part 5—Mineralogical Methods; Wiley: Hoboken, NJ, USA, 2008; Volume 5, pp. 367–385. [Google Scholar]

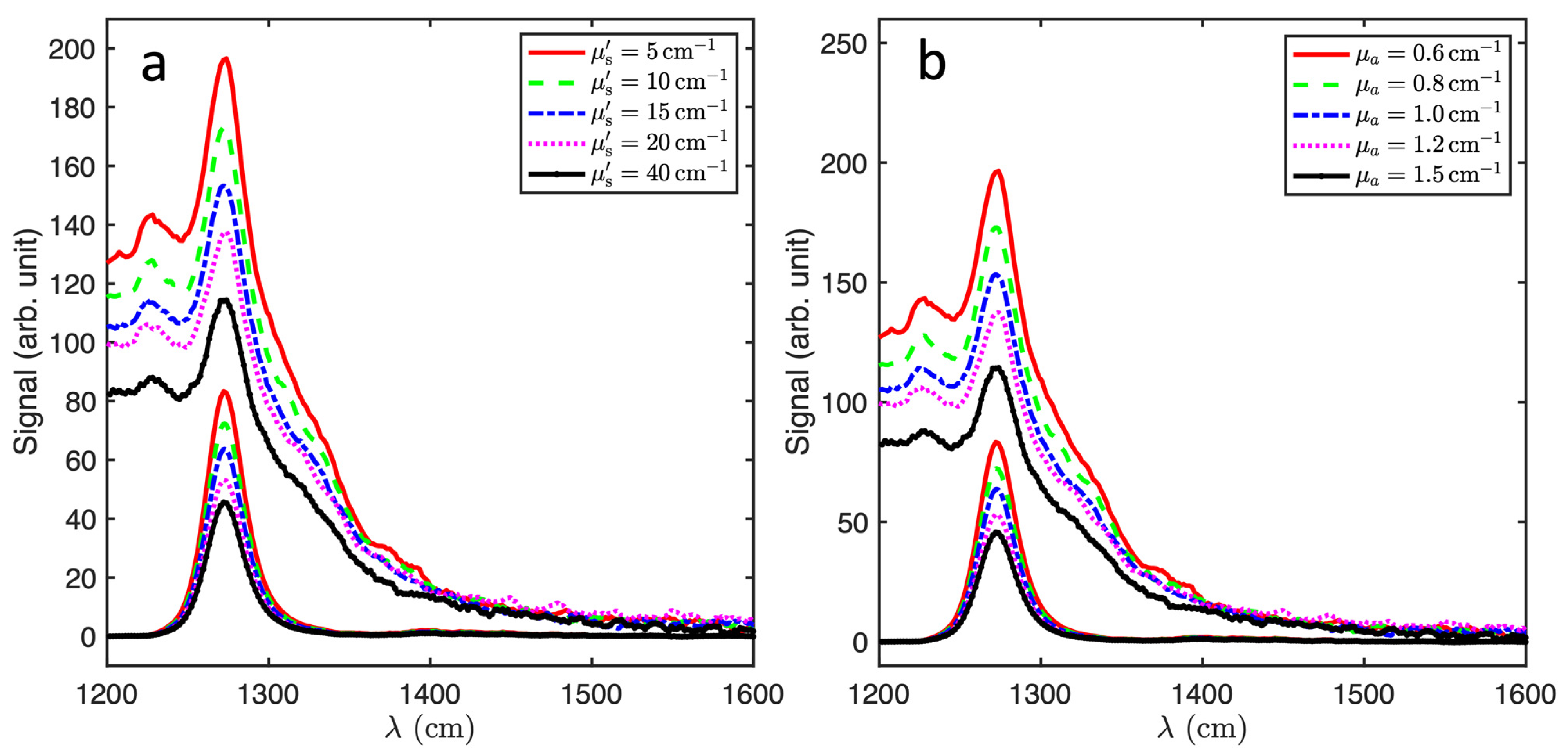
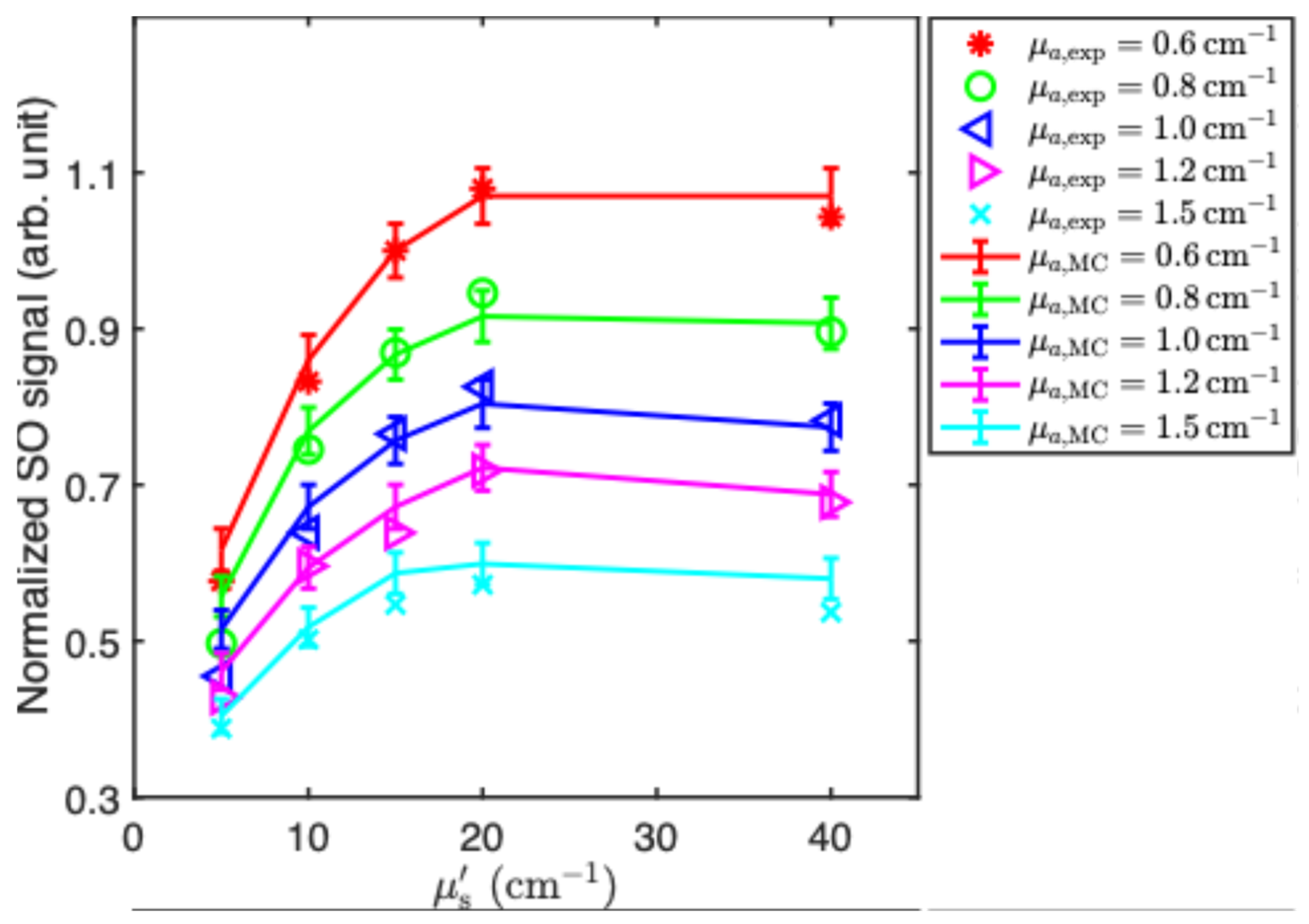
| (cm−1) | (cm−1) | ||||
|---|---|---|---|---|---|
| 0.6 | 0.8 | 1.0 | 1.2 | 1.5 | |
| 5 | 1.22 | 1.36 | 1.47 | 1.64 | 1.87 |
| 10 | 0.88 | 0.99 | 1.13 | 1.28 | 1.46 |
| 15 | 0.76 | 0.87 | 1.0 | 1.13 | 1.29 |
| 20 | 0.71 | 0.83 | 0.94 | 1.05 | 1.26 |
| 40 | 0.71 | 0.84 | 0.98 | 1.10 | 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Johnson, M.; Lu, B.; Sourvanos, D.; Sun, H.; Dimofte, A.; Vikas, V.; Busch, T.M.; Hadfield, R.H.; Wilson, B.C.; et al. Correction of Multispectral Singlet Oxygen Luminescent Dosimetry (MSOLD) for Tissue Optical Properties in Photofrin-Mediated Photodynamic Therapy. Antioxidants 2024, 13, 1458. https://doi.org/10.3390/antiox13121458
Yang W, Johnson M, Lu B, Sourvanos D, Sun H, Dimofte A, Vikas V, Busch TM, Hadfield RH, Wilson BC, et al. Correction of Multispectral Singlet Oxygen Luminescent Dosimetry (MSOLD) for Tissue Optical Properties in Photofrin-Mediated Photodynamic Therapy. Antioxidants. 2024; 13(12):1458. https://doi.org/10.3390/antiox13121458
Chicago/Turabian StyleYang, Weibing, Madelyn Johnson, Baozhu Lu, Dennis Sourvanos, Hongjing Sun, Andreea Dimofte, Vikas Vikas, Theresa M. Busch, Robert H. Hadfield, Brian C. Wilson, and et al. 2024. "Correction of Multispectral Singlet Oxygen Luminescent Dosimetry (MSOLD) for Tissue Optical Properties in Photofrin-Mediated Photodynamic Therapy" Antioxidants 13, no. 12: 1458. https://doi.org/10.3390/antiox13121458
APA StyleYang, W., Johnson, M., Lu, B., Sourvanos, D., Sun, H., Dimofte, A., Vikas, V., Busch, T. M., Hadfield, R. H., Wilson, B. C., & Zhu, T. C. (2024). Correction of Multispectral Singlet Oxygen Luminescent Dosimetry (MSOLD) for Tissue Optical Properties in Photofrin-Mediated Photodynamic Therapy. Antioxidants, 13(12), 1458. https://doi.org/10.3390/antiox13121458








