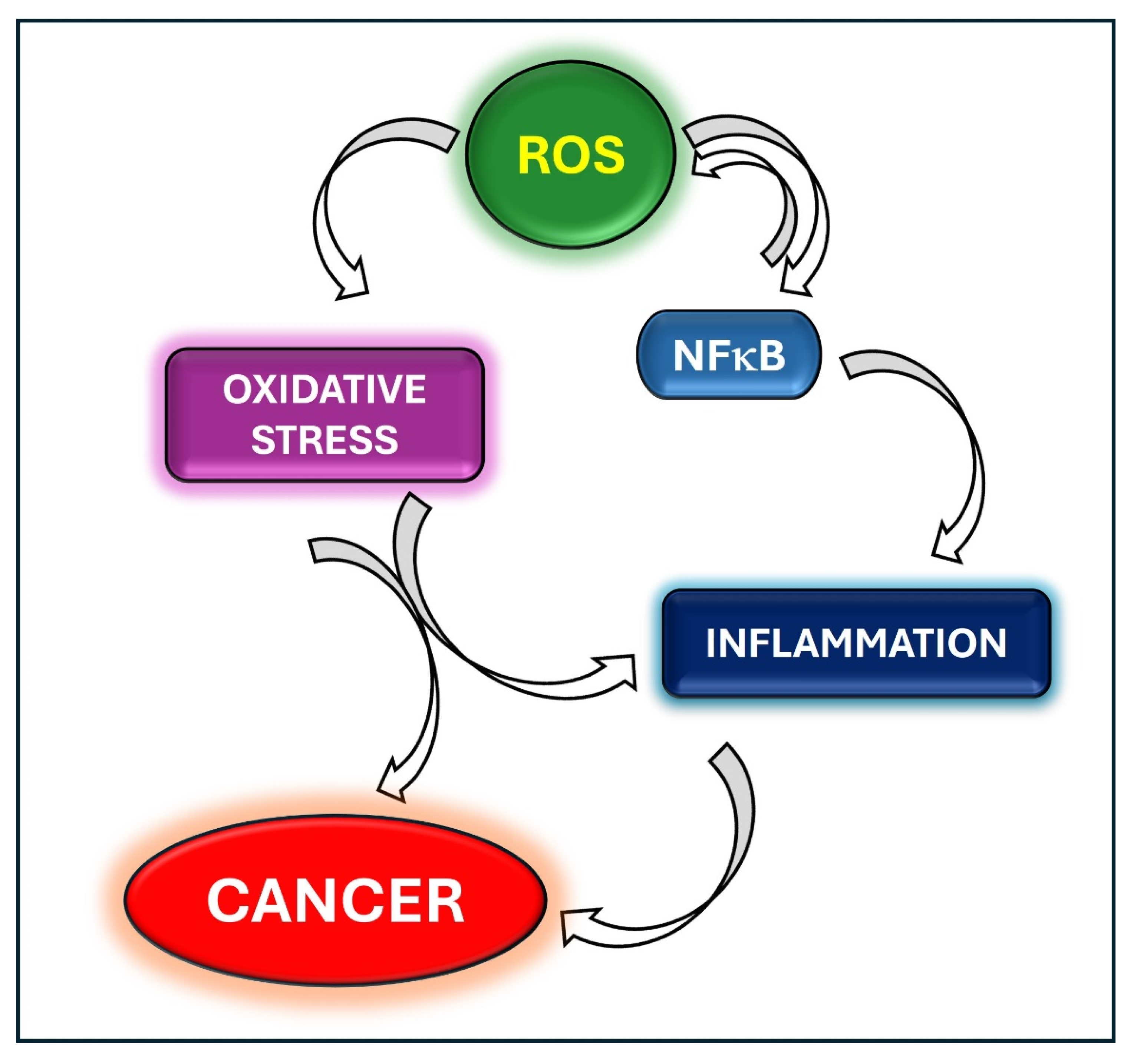
Oxidative Stress and Inflammation in Cancer
Conflicts of Interest
List of Contributions
- Raddatz, A.D.; Furdui, C.M.; Bey, E.A.; Kemp, M.L. Single-Cell Kinetic Modeling of beta-Lapachone Metabolism in Head and Neck Squamous Cell Carcinoma. Antioxidants 2023, 12, 741. https://doi.org/10.3390/antiox12030741.
- Sanhueza, S.; Simon, L.; Cifuentes, M.; Quest, A.F.G. The Adipocyte-Macrophage Relationship in Cancer: A Potential Target for Antioxidant Therapy. Antioxidants 2023, 12, 126. https://doi.org/10.3390/antiox12010126.
- Ortega, M.A.; Fraile-Martinez, O.; Pekarek, L.; Garcia-Montero, C.; Alvarez-Mon, M.A.; Castellanos, A.J.; Garcia-Honduvilla, N.; Bujan, J.; Alvarez-Mon, M.; Saez, M.A.; et al. Oxidative Stress Markers Are Associated with a Poor Prognosis in Patients with Pancreatic Cancer. Antioxidants 2022, 11, 759. https://doi.org/10.3390/antiox11040759.
- Allegra, A.; Petrarca, C.; Di Gioacchino, M.; Casciaro, M.; Musolino, C.; Gangemi, S. Modulation of Cellular Redox Parameters for Improving Therapeutic Responses in Multiple Myeloma. Antioxidants 2022, 11, 455. https://doi.org/10.3390/antiox11030455.
- Kim, K.H.; Ki, M.R.; Min, K.H.; Pack, S.P. Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy. Antioxidants 2023, 12, 1048. https://doi.org/10.3390/antiox12051048.
- Maczka, W.; Grabarczyk, M.; Winska, K. Can Antioxidants Reduce the Toxicity of Bisphenol? Antioxidants 2022, 11, 413. https://doi.org/10.3390/antiox11020413.
- Li, S.; Nguyen, T.T.; Ung, T.T.; Sah, D.K.; Park, S.Y.; Lakshmanan, V.K.; Jung, Y.D. Piperine Attenuates Lithocholic Acid-Stimulated Interleukin-8 by Suppressing Src/EGFR and Reactive Oxygen Species in Human Colorectal Cancer Cells. Antioxidants 2022, 11, 530. https://doi.org/10.3390/antiox11030530.
- Di Sano, C.; Lazzara, V.; Durante, M.; D’Anna, C.; Bonura, A.; Dino, P.; Uasuf, C.G.; Pace, E.; Lenucci, M.S.; Bruno, A. The Protective Anticancer Effect of Natural Lycopene Supercritical CO2 Watermelon Extracts in Adenocarcinoma Lung Cancer Cells. Antioxidants 2022, 11, 1150. https://doi.org/10.3390/antiox11061150.
- Abdel-Latif, R.; Fathy, M.; Anwar, H.A.; Naseem, M.; Dandekar, T.; Othman, E.M. Cisplatin-Induced Reproductive Toxicity and Oxidative Stress: Ameliorative Effect of Kinetin. Antioxidants 2022, 11, 863. https://doi.org/10.3390/antiox11050863.
- Torrisi, F.; D’Aprile, S.; Denaro, S.; Pavone, A.M.; Alberghina, C.; Zappala, A.; Giuffrida, R.; Salvatorelli, L.; Broggi, G.; Magro, G.G.; et al. Epigenetics and Metabolism Reprogramming Interplay into Glioblastoma: Novel Insights on Immunosuppressive Mechanisms. Antioxidants 2023, 12, 220. https://doi.org/10.3390/antiox12020220.
- Alarcon-Veleiro, C.; Mato-Basalo, R.; Lucio-Gallego, S.; Vidal-Pampin, A.; Quindos-Varela, M.; Al-Qatarneh, T.; Berrecoso, G.; Vizoso-Vazquez, A.; Arufe, M.C.; Fafian-Labora, J. Study of Ferroptosis Transmission by Small Extracellular Vesicles in Epithelial Ovarian Cancer Cells. Antioxidants 2023, 12, 183. https://doi.org/10.3390/antiox12010183.
References
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Gambardella, J.; Iaccarino, G. Cancer, NFkappaB, and oxidative stress-dependent phenotypes. In Cancer—Oxidative Stress and Dietary Antioxidants, 2nd ed.; Preedy, V.R., Patel, V.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–177. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Hamarsheh, S.; Osswald, L.; Saller, B.S.; Unger, S.; De Feo, D.; Vinnakota, J.M.; Konantz, M.; Uhl, F.M.; Becker, H.; Lubbert, M.; et al. Oncogenic KrasG12D causes myeloproliferation via NLRP3 inflammasome activation. Nat. Commun. 2020, 11, 1659. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of reactive oxygen species in inflammation and cancer. MedComm 2024, 5, e519. [Google Scholar] [CrossRef]
- Yu, W.; Tu, Y.; Long, Z.; Liu, J.; Kong, D.; Peng, J.; Wu, H.; Zheng, G.; Zhao, J.; Chen, Y.; et al. Reactive Oxygen Species Bridge the Gap between Chronic Inflammation and Tumor Development. Oxid. Med. Cell Longev. 2022, 2022, 2606928. [Google Scholar] [CrossRef]
- Glorieux, C.; Liu, S.; Trachootham, D.; Huang, P. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug Discov. 2024, 23, 583–606. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, B.; Peng, J.; Tang, H.; Wang, S.; Peng, S.; Ye, F.; Wang, J.; Ouyang, K.; Li, J.; et al. Inhibition of NF-kappaB signaling unveils novel strategies to overcome drug resistance in cancers. Drug Resist. Updat. 2024, 73, 101042. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Gomez-Quiroz, L.; Arreguin Camacho, L.O.; Pinna, F.; Lee, Y.H.; Kitade, M.; Dominguez, M.P.; Castven, D.; Breuhahn, K.; Conner, E.A.; et al. Curcumin effectively inhibits oncogenic NF-kappaB signaling and restrains stemness features in liver cancer. J. Hepatol. 2015, 63, 661–669. [Google Scholar] [CrossRef]
- Jeong, Y.; Lim, J.W.; Kim, H. Lycopene Inhibits Reactive Oxygen Species-Mediated NF-kappaB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients 2019, 11, 762. [Google Scholar] [CrossRef]
- Ji, Q.; Ding, Y.H.; Sun, Y.; Zhang, Y.; Gao, H.E.; Song, H.N.; Yang, M.; Liu, X.L.; Zhang, Z.X.; Li, Y.H.; et al. Antineoplastic effects and mechanisms of micheliolide in acute myelogenous leukemia stem cells. Oncotarget 2016, 7, 65012–65023. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Ma, Q.; Wang, Y.; Song, A.L. Withaferin-A Inhibits Growth of Drug-Resistant Breast Carcinoma by Inducing Apoptosis and Autophagy, Endogenous Reactive Oxygen Species (ROS) Production, and Inhibition of Cell Migration and Nuclear Factor kappa B (Nf-kappaB)/Mammalian Target of Rapamycin (m-TOR) Signalling Pathway. Med. Sci. Monit. 2019, 25, 6855–6863. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, B.; Zhang, W.; Wu, X.; Wang, R.; Huang, Y.; Chen, D.; Park, K.; Weimer, B.C.; Shen, Y. Mycoepoxydiene, a fungal polyketide, induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Bioorg. Med. Chem. Lett. 2010, 20, 7054–7058. [Google Scholar] [CrossRef]
- Sorriento, D.; Campanile, A.; Santulli, G.; Leggiero, E.; Pastore, L.; Trimarco, B.; Iaccarino, G. A new synthetic protein, TAT-RH, inhibits tumor growth through the regulation of NFkappaB activity. Mol. Cancer 2009, 8, 97. [Google Scholar] [CrossRef]
- Gambardella, J.; Fiordelisi, A.; Santulli, G.; Ciccarelli, M.; Cerasuolo, F.A.; Sala, M.; Sommella, E.; Campiglia, P.; Illario, M.; Iaccarino, G.; et al. Exploiting GRK2 Inhibition as a Therapeutic Option in Experimental Cancer Treatment: Role of p53-Induced Mitochondrial Apoptosis. Cancers 2020, 12, 3530. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; De Rosa, M.; Sala, M.; Pacelli, R.; Campiglia, P.; Trimarco, B.; Iaccarino, G.; et al. A Novel Small Peptide Inhibitor of NFkappaB, RH10, Blocks Oxidative Stress-Dependent Phenotypes in Cancer. Oxid. Med. Cell Longev. 2018, 2018, 5801807. [Google Scholar] [CrossRef] [PubMed]
- Soprano, M.; Sorriento, D.; Rusciano, M.R.; Maione, A.S.; Limite, G.; Forestieri, P.; D’Angelo, D.; D’Alessio, M.; Campiglia, P.; Formisano, P.; et al. Oxidative Stress Mediates the Antiproliferative Effects of Nelfinavir in Breast Cancer Cells. PLoS ONE 2016, 11, e0155970. [Google Scholar] [CrossRef] [PubMed]
- Arem, H.; Loftfield, E. Cancer Epidemiology: A Survey of Modifiable Risk Factors for Prevention and Survivorship. Am. J. Lifestyle Med. 2018, 12, 200–210. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical implications of chemotherapeutic agent organ toxicity on perioperative care. Biomed. Pharmacother. 2022, 146, 112503. [Google Scholar] [CrossRef]
- Gambardella, J.; Santulli, G.; Fiordelisi, A.; Cerasuolo, F.A.; Wang, X.; Prevete, N.; Sommella, E.; Avvisato, R.; Buonaiuto, A.; Altobelli, G.G.; et al. Infiltrating macrophages amplify doxorubicin-induced cardiac damage: Role of catecholamines. Cell Mol. Life Sci. 2023, 80, 323. [Google Scholar] [CrossRef]
- Dhanyamraju, P.K. Drug resistance mechanisms in cancers: Execution of pro-survival strategies. J. Biomed. Res. 2024, 38, 95–121. [Google Scholar] [CrossRef]
- Lei, Z.N.; Tian, Q.; Teng, Q.X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.S. Understanding and targeting resistance mechanisms in cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wu, X.; Xu, F.; Ma, H.; Wu, M.; Xia, Y. Targeting Ferroptosis Pathway to Combat Therapy Resistance and Metastasis of Cancer. Front. Pharmacol. 2022, 13, 909821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorriento, D. Oxidative Stress and Inflammation in Cancer. Antioxidants 2024, 13, 1403. https://doi.org/10.3390/antiox13111403
Sorriento D. Oxidative Stress and Inflammation in Cancer. Antioxidants. 2024; 13(11):1403. https://doi.org/10.3390/antiox13111403
Chicago/Turabian StyleSorriento, Daniela. 2024. "Oxidative Stress and Inflammation in Cancer" Antioxidants 13, no. 11: 1403. https://doi.org/10.3390/antiox13111403
APA StyleSorriento, D. (2024). Oxidative Stress and Inflammation in Cancer. Antioxidants, 13(11), 1403. https://doi.org/10.3390/antiox13111403




